Abstract
This is an update on sarcoidosis, focusing on etiology, diagnosis, and treatment. In the area of etiopathogenesis, we now have a better understanding of the immune response that leads to the disease as well as genetic factors that modify both the risk for the disease and its clinical outcome. Several groups have also identified possible agents as a cause for sarcoidosis. Although none of these potential causes has been definitely confirmed, there is increasing evidence to support that one or more infectious agents may cause sarcoidosis, although this organism may no longer be viable in the patient. The diagnosis of sarcoidosis has been significantly aided by new technology. This includes the endobronchial ultrasound, which has been shown to increase the yield of needle aspiration of mediastinal and hilar lymph nodes. The positive emission tomography scan has proven useful for selecting possible biopsy sites by identifying organ involvement not appreciated by routine methodology. It has also helped in assessing cardiac involvement. The biologic agents, such as the anti–tumor necrosis factor antibodies, have changed the approach to refractory sarcoidosis. There is increasing evidence that the clinician can identify which patient is most likely to benefit from such therapy. As new and more potent antiinflammatory agents have been developed, it is clear that there are other factors that burden the patient with sarcoidosis, including fatigue and sarcoidosis-associated pulmonary hypertension. There have been several recent studies demonstrating treatment options for these problems.
Keywords: mycobacterium, HLA, Löfgren syndrome, infliximab, pulmonary hypertension
Sarcoidosis is a granulomatous disease of unknown etiology that affects people throughout the world (1). Over the past few years, there have been advances in our understanding of sarcoidosis. These include observations about the etiopathogenesis, diagnosis, and treatment of the disease. In this review, we discuss observations in these various areas.
ETIOLOGY AND PATHOGENESIS
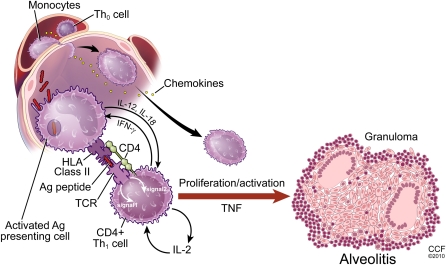
The immunopathogenesis of sarcoidosis is not completely understood, but there has been tremendous progress in the past decade. Most evidence suggests that the development of the disease is similar to other granulomatous diseases of known cause, such as chronic beryllium disease. That is, some antigen(s) enter the host and are phagocytosed by antigen-presenting cells (APCs), predominantly macrophages or dendritic cells. The APCs process the antigen and subsequently present it via human leukocyte antigen (HLA) class II molecules to a restricted set of T-cell receptors on naive T lymphocytes, primarily of the CD4+ class. Induction of the immune response depends on intact cell-mediated immunity, as evidenced by the phenomenon of reactivation sarcoidosis coinciding with immune reconstitution during treatment for HIV (2). The immune reaction begets polarization of the T lymphocytes to a Th1 phenotype, followed by cellular recruitment, proliferation, and differentiation leading to formation of the sarcoid granuloma. A panoply of cytokines and chemokines has been reported in association with sarcoidosis, but the relative importance of most of them is unclear.
The pathogenesis of sarcoidosis seems to involve the interplay of antigen, HLA class II molecules, and T-cell receptors (3). It is likely that specific combinations of these three facets are required for sarcoidosis to develop. If this scenario is correct, the pathophysiology of sarcoidosis depends on genetics that determine specific HLA polymorphisms, exposures in the form of putative antigens, and T-cell responses that may be genetically programmed but may also involve memory from previous antigen exposure. This schema also suggests that there may be multiple causes of sarcoidosis, each requiring a specific arrangement of antigen, HLA molecule, and T-cell receptor (Figure 1).
Figure 1.
Inflammatory response of sarcoidosis with formation of granuloma and subsequent resolution or persistence of disease. HLA = human leukocyte antigen; IFN = interferon; TCR = T cell receptor; TNF = tumor necrosis factor.
Investigation of the genes involved in sarcoidosis has focused on the HLA genes (1). For example, analysis of patients enrolled in a multicenter epidemiologic study of sarcoidosis in the United States (A Case Controlled Etiologic Study of Sarcoidosis) demonstrated that carriage of HLA-DRB1*1101 and HLA-DPB1*0101 alleles are risk factors for the disease (4). A family-based association study in black U.S. patients with sarcoidosis suggested that susceptibility or protection may be associated with certain HLA-DQB1 alleles (5). The phenotype and outcome of sarcoidosis is probably influenced strongly by HLA genes. For example, carriage of HLA-DRB1*03 in Swedish subjects with sarcoidosis is strongly associated with the development of Löfgren syndrome and also with disease resolution (6, 7). Similarly, HLA-DQB1*0201 associates with a good prognosis in Dutch and British patients (8), although it is difficult to ascertain which is the responsible allele because of its strong linkage to HLA-DRB1. These studies have highlighted the issues of population stratification and the need for careful clinical phenotyping in association studies of sarcoidosis.
Genome-wide approaches have identified non-HLA candidate susceptibility genes. For example, a family-based study in affected German families led to the discovery of a mutation in a putative immune regulatory gene, butyrophilin-like 2 (BTNL2), that may explain 23% of the attributable risk in that population (9, 10). Analysis of the same gene in black U.S. subjects failed to reveal any role for BTNL2 but did confirm its association with sarcoidosis in white subjects (11). In contrast, a genome-wide sibling-based microsatellite linkage analysis in 229 families of black U.S. patients most strongly implicated regions in chromosomes 5p and 5q (12). More recently, a genome-wide association study in German subjects suggested a role for mutations in the annexin1 gene (13); however, this observation has not yet been confirmed. Using a different technique of biallelic marker scanning, linkage peaks in chromosomes 12p and 9q were also identified (14). A key aspect of genome-wide studies is the requirement for adequate fine-mapping and functional studies after the initial scan to define the biologic relevance of the findings.
The granuloma in sarcoidosis is characterized by a core of monocyte-derived epithelioid histiocytes and multinucleate giant cells with interspersed CD4+ T lymphocytes. A minority of cells in or near the granuloma are CD8+ T lymphocytes, fibroblasts, regulatory T cells, and B lymphocytes. The T-cell response is biased toward a Th1 phenotype, with important roles for IFN-γ and interleukin-12 (15). A variety of chemokines and cytokines have been associated with the granulomatous response in sarcoidosis, including tumor necrosis factor α (TNF-α) (16, 17). The importance of TNF in sarcoidosis has been validated by studies documenting effectiveness of biologic TNF antagonists in sarcoidosis in treating some patients with sarcoidosis (18).
Rather than focusing on candidate mediators, a hypothesis-free approach to understanding the granulomatous response has been reported recently. Using a bioinformatic analysis, Crouser and colleagues analyzed global gene expression networks in sarcoidosis and control lung tissue and lymph nodes (19). They identified a dominant network regulated by signal transducer and activator of transcription-1 (STAT1) as the most significantly associated with sarcoidosis. Because STAT1 is the signaling target of IFN-γ, this analysis confirmed the importance of the Th1-dominated lymphocyte response. It also allowed identification of novel gene products tightly associated with sarcoidosis, such as interleukin-7 and matrix metalloproteinase 12. A separate study in peripheral blood cells used the same techniques and also implicated STAT-1 signaling pathways as a central feature of sarcoidosis (20). Given the complexity of the immune response in sarcoidosis, the difficulties with animal models and lack of a defined antigen, inductive research designs and bioinformatic techniques may play important roles in future pathophysiologic studies.
Sarcoidosis probably requires exposure to one or more exogenous antigens. Epidemiologic data, including reports of case-clustering, increased susceptibility with certain occupations, and transmissibility via transplant, all support this theory (21, 22). Infectious agents have long been suspected as possible causes of sarcoidosis, but early studies failed to yield convincing support for various organisms. Using molecular techniques, there are now accumulating data suggesting that bacteria, such as mycobacteria or Propionibacterium acnes, may contribute to the disease (23). It is quite possible that the triggering antigen varies depending on ethnicity, geographic location, and individual genetic background.
Although Mycobacterium tuberculosis does not seem to be the etiologic trigger for sarcoidosis, there is increasing evidence for mycobacteria as a cause of at least some cases of sarcoidosis. A key observation was the finding that the protein mycobacterial catalase-peroxidase (mKatG) was present in sarcoidosis tissue, had the same physicochemical properties as the Kveim-Siltzbach reagent, and was associated with the presence of humoral immunity in the same subjects (24). Subsequent studies have demonstrated a T-cell response to mKatG by the peripheral blood lymphocytes of patients with sarcoidosis (25, 26). Analogous to infection with tuberculosis, even more robust responses to mKatG have been found in T cells obtained by bronchoalveolar lavage from patients with sarcoidosis, but not other lung diseases, and more strongly in active disease (25, 27). Sequence analysis of nucleic acids in granulomas suggests that the putative mycobacterium has closer homology to the M. tuberculosis family rather than to other nontuberculous mycobacteria (28).
Importantly, T-cell responses are not limited to the mKatG protein alone but can also be demonstrated for mycolyl transferase antigen 85A, mycobacterial superoxide dismutase, and early secreted antigen target 6 in the peripheral blood and bronchoalveolar lavage (BAL) (29, 30). One interpretation of these observations is that the agent causing sarcoidosis for some patients may be more than just a single, poorly degradable peptide; these observations do not exclude the possibility of an intact organism as the cause for the disease. However, it is not required that the organism causing sarcoidosis be viable. The Kveim-Siltzbach agent has no observed viable organism present, yet it will induce a granulomatous response in the majority of patients with sarcoidosis. Moller postulated that the antigen(s) from this mycobacterium could be released during the death of the organism, with a complex of host and mycobacterial proteins in response to the infection leading to sarcoidosis (31). He also suggests that the failure to clear these antigen/protein complexes in some patients could lead to chronic disease.
Persistent granulomatous inflammation may in part be due to failure of immune regulatory mechanisms to limit the duration of the inflammatory process. A recent report by Chen and colleagues demonstrated that granulomas in sarcoidosis are characterized by extensive deposition of serum amyloid A protein (32). The amyloid protein is capable of eliciting immune responses and triggering cytokine release through an interaction with toll-like receptor 2. One potential implication of this observation is that immune clearance of the etiologic agent responsible for sarcoidosis might be attenuated by the involvement of amyloid in the granulomatous process. Interestingly, measurement of serum amyloid has been found to correlate with disease activity in pulmonary sarcoidosis (33). The recent development of antigen-specific rodent models of granuloma formation should facilitate further unraveling of the responsible mechanisms (32, 34).
Another immune regulatory mechanism that may be perturbed in sarcoidosis is through the T-lymphocyte. Regulatory T cells (T-reg), normally essential for suppression of cell-mediated immune responses, are expanded in peripheral blood, BAL, and granulomas in patients with sarcoidosis during active disease (35). However, there are data suggesting that the T-reg population in sarcoidosis is either functionally defective or “exhausted,” compared with healthy control subjects (35). CD1d-restricted natural killer T cells (NKT cells) can also limit CD4-mediated immune responses. In a study comparing 60 patients with sarcoidosis to healthy control subjects, NKT cells were markedly reduced in both blood and BAL fluid, except in patients with Löfgren syndrome (36). Because Löfgren syndrome is usually associated with resolving disease, this observation suggests that the loss of NKT cells may allow for persistence of sarcoidosis.
DIAGNOSIS
Although it has been claimed that the method of diagnosis of sarcoidosis has been established (1), the reality is that the diagnosis of sarcoidosis is never secure. The diagnosis of sarcoidosis is arbitrarily made when the statistical likelihood of alternative diagnoses becomes too small to warrant further investigation. There are certain clinical features that are typical of sarcoidosis but there are none that are specific for the diagnosis (18). Therefore, sarcoidosis is a diagnosis of exclusion, and it is impossible to completely exclude alternative diagnoses.
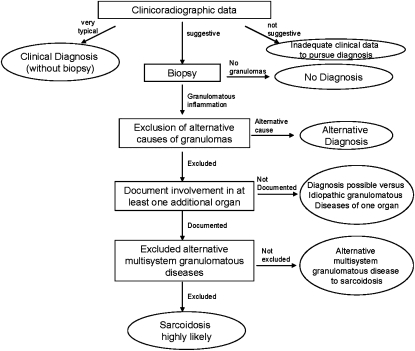
Sarcoidosis is defined as a multisystem granulomatous disorder of unknown cause (1, 18). This usually warrants a tissue biopsy, although in special situations a presumptive diagnosis may be made based on clinicoradiographic findings alone. These situations include the presence of bilateral hilar adenopathy on the chest radiograph in an asymptomatic patient, Löfgren syndrome (erythema nodosum skin rash coupled with bilateral hilar adenopathy on chest radiograph and often fever and arthritis), Heerfordt syndrome (uveitis, parotiditis, and fever), and when a gallium-67 scan reveals uptake in the parotid and lacrimal glands (Panda sign) along with right paratracheal and bilateral hilar uptake (Lambda sign). Likewise, the presence of granulomas alone is also inadequate for the diagnosis of sarcoidosis (18). The diagnosis is established when clinicoradiographic findings are supported by histologic evidence of noncaseating granulomatous inflammation and other causes of granulomas and local reactions have been reasonably excluded (1).
Figure 2 outlines a general diagnostic algorithm for sarcoidosis. Unless one of the special clinical situations previously described is present, the diagnosis usually requires histological confirmation of granulomatous inflammation, exclusion of alternative causes of granulomatous inflammation, evidence of systemic (multiorgan) disease, and exclusion of rare alternative multisystem granulomatous diseases such as Blau syndrome (37) or granulomatous lesions of unknown significance (38).
Figure 2.
An approach to diagnosis of pulmonary sarcoidosis.
The diagnosis of sarcoidosis can be problematic (39). On average, patients have symptoms for more than 3 months before diagnosis and require three or more encounters with health care providers before specific diagnosis (40). Patients with sarcoidosis presenting with pulmonary symptoms often have a relative delay in the diagnosis of sarcoidosis, as these symptoms are nonspecific, and alternative diagnoses, such as asthma or bronchitis, are often considered (19).
The lungs are affected in more than 90% of patients with sarcoidosis. Pulmonary function studies are abnormal in many patients with sarcoidosis (41–43), but there is no diagnostic pattern. Restrictive physiology is usually observed (44, 45). However, airflow obstruction is found in a significant proportion of those patients with abnormal studies (41, 45–47). This may be more common in the African American population than other groups (48). Airflow obstruction may be the result of endobronchial disease (49) or stenosis (50), airway reactivity (49, 51), or airway distortion from parenchymal disease (45, 46). There is only a modest correlation between the FVC and the level of dyspnea reported by the patient (52). A reduction of diffusion capacity may be related not only to restrictive disease but also to sarcoidosis-associated pulmonary hypertension (53, 54).
The chest roentgenogram is the most commonly used radiologic imaging technique to evaluate patients with pulmonary sarcoidosis. In the 1960s, Scadding proposed a staging system of the chest roentgenogram (55). The original staging system has been modified and now includes stage 0: no adenopathy or infiltrates; stage 1: hilar and mediastinal adenopathy alone; stage 2: adenopathy and pulmonary infiltrates; stage 3: pulmonary infiltrates alone; and stage 4: pulmonary fibrosis. It provides general information regarding the prognosis of the pulmonary disease over time. In the original description, it was noted that patients with stage 1 findings had a greater than 90% chance of resolution of their radiographic findings within 2 years, whereas those with stage 3 had resolution in less than one-third of cases in 2 years (55). This difference in resolution of the chest roentgenogram stage at presentation is similar in American (56) or in Japanese (57) patients with sarcoidosis. As discussed later, most studies of treatment of pulmonary sarcoidosis have been shown to be effective only in patients with parenchymal lung disease, stages 2 to 4 (58).
There are problems with the staging system, however. One major issue has been interobserver variability. A recent multicenter study compared the readings of two experienced radiologists to a local expert and found a poor correlation (59). In addition, the agreement between the two radiologists was only fair. Furthermore, although the staging system has a significant correlation with physiologic markers of pulmonary disease, such as decrements in vital capacity, when examined across groups of patients (41, 42), the variation is such that it has limited applicability in individual patient assessments, including treatment decisions. A prospective study of 36 patients compared clinical status and spirometry during flares of pulmonary sarcoidosis to chest roentgenogram findings (60). Approximately one-half of the radiographic readings showed an improvement or no change during a significant exacerbation. Also, the chest roentgenogram stage has been shown to correlate only weakly with the level of dyspnea (52); no significant correlation was demonstrated between chest radiographic findings and 6-minute walk distance (61).
In terms of diagnosis, a chest roentgenogram demonstrating hilar adenopathy is associated with a relatively rapid diagnosis, as the differential diagnosis is typically narrowed to sarcoidosis, lymphoma, tuberculosis, and fungal infections. The diagnostic approach to mediastinal adenopathy has been greatly aided by the use of the transbronchial needle aspiration (TBNA). The addition of endobronchial ultrasound guidance (EBUS) has increased the ability to biopsy smaller nodes and those not readily accessed by mediastinoscopy (62). In a randomized trial, Tremblay and colleagues compared EBUS to blind TBNA for diagnosis of sarcoidosis (63). The authors found that the yield for granulomas was enhanced by using EBUS (TBNA = 73%, EBUS = 96%). The authors did find that the addition of EBUS increased the time of bronchoscopy by an average of 10 minutes and was associated with a higher percentage of patients sedated with propofol.
The diagnosis of sarcoidosis requires evidence of multisystem disease such that granulomatous inflammation is present in at least two organs (see Figure 2). There are idiopathic granulomatous diseases of individual organs that have a different clinical course or immunopathology than sarcoidosis, such as idiopathic granulomatous hepatitis (64) and idiopathic panuveitis (65). However, the diagnosis of sarcoidosis does not necessarily require histological confirmation in a second organ. For example, the finding of noncaseating granulomas in the liver alone is inadequate for a diagnosis of sarcoidosis; however, the presence of concomitant bilateral hilar adenopathy on chest radiograph is believed to be sufficient evidence of sarcoidosis involvement of a second organ, such that a hilar lymph node or lung biopsy is not required. A consensus of sarcoidosis experts in the A Case Controlled Etiology of Sarcoidosis Study developed clinical criteria for when a second organ can be considered involved with sarcoidosis without a biopsy (this presumes that noncaseating granulomatous inflammation has been histologically confirmed in the “first” organ) (66).
Angiotensin-converting enzyme (ACE) is produced in the epithelioid cell of the sarcoid granuloma (67) and serum ACE levels reflect the total body granuloma burden in sarcoidosis (68). However, the diagnostic and prognostic usefulness of the serum ACE is questionable. In a study of 1,941 patients with sarcoidosis, 1,575 healthy control subjects, and 1,355 patients with other diseases, the sensitivity of an elevated serum ACE for the diagnosis of sarcoidosis was 57%, the specificity 90%, positive predictive value 90%, but negative predictive value only 60% (69). Therefore, this test is not adequately sensitive to be useful for screening for the diagnosis of sarcoidosis. Furthermore, although an elevated serum ACE is fairly specific for the diagnosis, it is still not adequately specific to confirm a diagnosis of sarcoidosis. Other conditions associated with an elevated serum ACE include disseminated tuberculosis, fungal infections, hyperthyroidism, and Gaucher disease (70). These data suggest that the serum ACE level may be used as supportive evidence for or against the diagnosis of sarcoidosis; however, it should not be used in isolation to secure or exclude the diagnosis.
Polymorphisms of the ACE gene may lead to changes in the serum ACE level in both normal control subjects and patients with sarcoidosis. The polymorphism with insertion (I) or deletion (D) of a portion of the gene affects the enzyme activity, with DD having higher serum ACE levels than the II polymorphism (71) However, there is no evidence that these polymorphisms are associated with an increased risk for or protection from sarcoidosis. One study has demonstrated that the DD polymorphism was associated with a higher rate of chronic disease (72). Serial ACE studies have been proposed as a means to follow the course of the disease (73, 74). However, the level of ACE does not correlate with the severity of the disease (75). Corticosteroid therapy has an independent effect on the ACE level, making serial studies of limited value in treated patients (76, 77). Other markers of disease activity, including soluble IL-2 receptor and chitotriosidase (78, 79), may prove more useful markers of activity; however, more information about changes in these markers over time and with therapy are still needed (80).
F-18 fluorodeoxyglucose positron emission tomography (PET) scanning has been found to frequently display positive activity in areas with active granulomatous inflammation from sarcoidosis (81). PET scans are expensive and cannot definitively diagnose sarcoidosis, because a positive result may be the result of malignancy or an alternative inflammatory condition. Therefore, PET scans are not routinely performed in the diagnostic evaluation of sarcoidosis. However, a PET scan can be useful in the diagnosis of sarcoidosis by identifying potential diagnostic biopsy sites and suggesting the presence of disease in relatively inaccessible organs (e.g., heart and brain). In a large prospective trial of the application of PET scans in patients with sarcoidosis, this modality helped identify potential biopsy sites (82). Gadolinium enhancement of nuclear magnetic resonance imaging (MRI) scans may also be similarly useful in detecting areas of sarcoidosis, including relatively inaccessible areas such as the heart and brain (83). It is not clear if either of these expensive tests is superior to gallium-67 scanning, although the latter is cumbersome, requiring the patient to return in 48 hours for reimaging; furthermore, limited data suggest that these newer imaging techniques may be more sensitive than gallium-67 scanning (84).
One area in which PET scanning may have a significant clinical role is in the diagnosis of cardiac sarcoidosis. Cardiac sarcoidosis is potentially life threatening and endomyocardial biopsy, although the diagnostic gold standard, confirms the diagnosis in less than one-quarter of patients with disease (85). PET scanning has been shown to have a specific pattern suggestive of cardiac involvement. Although MRI scanning is also useful for cardiac sarcoidosis, PET scanning can be performed in a patient with a pacemaker or defibrillator, whereas MRI is contraindicated in these situations. Autopsy studies have suggested that cardiac sarcoidosis is often present in asymptomatic patients (86). This has been confirmed in one series in which up to 40% of patients with sarcoidosis were believed to have cardiac sarcoidosis on the basis of either PET or MRI scanning (87). However, it also raises the question of whether the asymptomatic cardiac sarcoidosis detected by an abnormality on PET or MRI requires treatment. Although the number of patients analyzed was small, a recent study by Smedema and associates suggests that such patients have an excellent prognosis without therapy (88).
THERAPY
Treatment of sarcoidosis is usually limited to the symptomatic patient. In most series, about half of patients do not require long-term systemic therapy. In some of those cases, topical therapy, such as fluorinated steroid creams, or corticosteroid injections for skin lesions, or steroid-containing eye drops, are sufficient to control the disease. For pulmonary patients with cough, inhaled corticosteroids may be sufficient to control this symptom (51).
The decision to treat has to be tempered by the lack of understanding about the natural course of the disease. Treatment decisions have to consider that patients may have spontaneous resolution of their disease. In one study of patients with stage 2 or 3 disease observed without therapy for 6 months, 20% of patients had spontaneous improvement of their chest roentgenogram without therapy, whereas 40% deteriorated and were started on corticosteroids (89). Also, once corticosteroid therapy is started, a large number of these patients may require long-term treatment (90, 91). There is limited information on how to predict who will need long-term therapy (91). Finally, the studies to date have not clearly demonstrated that corticosteroids or any other therapy prevents progression or fibrosis (92, 93).
Table 1 lists specific recommendations regarding therapy for pulmonary sarcoidosis. Specific recommendations are listed with levels of evidence of support based on standard criteria for grading evidence (94). The table also lists key references that support the recommendations. A stepwise approach to therapy is recommended (95).
TABLE 1.
TREATMENT OF PULMONARY SARCOIDOSIS
| Chest X-ray stage 0/1 |
| No symptoms |
| No systemic therapy |
| Level 1A (123) |
| Chest X-ray stage 2 to 4 |
| Symptomatic |
| Treat with corticosteroids |
| Level 1A (89, 123) |
| Initial dosage of 20–40 mg prednisone or its equivalent |
| Level 1B (89, 124) |
| Treat for 12–24 mo |
| Level 1C (90, 91, 125) |
| Steroid-sparing alternatives for chronic pulmonary sarcoidosis |
| Methotrexate |
| Dose of 5–15 mg once a week |
| Level 1A (126–128) |
| Folic acid 1 mg/d may reduce toxicity |
| Level 1B (129) |
| Azathioprine 50–200 mg daily |
| Level 1B (130, 131) |
| Leflunomide 10–20 mg daily |
| Level 1B (132) |
| Mycophenolate |
| Level 1C (101, 133, 134) |
| Treatment of refractory sarcoidosis |
| Infliximab intravenously 3–5 mg/kg initially, 2 wk later, then once a month |
| Level 1A (18, 98) |
Level A: At least one double-blind, placebo-controlled trial with positive results with one or more case series supporting the results. Level B: Majority of case series showing positive results. Level C: Case series with mixed reports of effectiveness, or only a small number of cases reported. 1A = strong recommendation; 1B = strong recommendation; 1C = strong recommendation; 2A = weak recommendation; 2B = weak recommendation; 2C = weak recommendation. Scoring level of evidence as proposed by Guyatt and coworkers (94).
With the introduction of biologic agents capable of blocking TNF, the treatment of sarcoidosis has been changed (96). In a randomized double-blind placebo-controlled trial, infliximab, a chimeric monoclonal antibody to TNF, was shown to be superior to placebo in treating pulmonary sarcoidosis (18). Additional analysis of these patients found that the drug may also be useful in treating extrapulmonary manifestations of the disease (97).
There are some conditions in which anti-TNF agents are particularly effective (Table 2) (96). These include patients with an FVC of 70% or less (18, 98) or a reticulonodular infiltrate on chest roentgenogram (59). Lupus pernio is a chronic facial cutaneous lesion in sarcoidosis that is often refractory to routine treatment. In a retrospective analysis of a large group of patients, infliximab was superior to all other agents in controlling the disease (99). Neurologic disease can also be refractory to conventional treatments. Again, infliximab has been reported as successful in treating patients with neurosarcoidosis who have failed other therapies (100, 101). A recent post hoc analysis of a trial of infliximab for pulmonary sarcoidosis found that an elevated C-reactive protein was associated with significantly better response to treatment (102).
TABLE 2.
FACTORS ASSOCIATED WITH AN ENHANCED RESPONSE TO ANTI–TUMOR NECROSIS FACTOR THERAPY
| FVC < 70% |
| ATS dyspnea : 1 |
| Disease > 2 yr |
| Significant extrapulmonary disease |
| Lupus pernio |
| CNS |
| Elevated C-reactive protein |
Definition of abbreviations: ATS = American Thoracic Society; CNS = central nervous system.
Although these studies support the usefulness of anti-TNF agents in treating sarcoidosis, there have been reports of a sarcoid-like reaction occurring in patients receiving anti-TNF biologic agents for conditions other than sarcoidosis (103, 104). In one series, the incidence of this complication was estimated at 1/2,800 patients treated (104). The mechanism causing this reaction is unclear; however, these observations stress that sarcoidosis is a complex immunologic reaction and modulation of one cytokine is unlikely to resolve all aspects of the disease (105).
In most series, significant fatigue was reported in more than half of patients studied (107). The level of fatigue is similar in studies comparing European to U.S. patients, even though the clinical features and treatment of these two groups was different (108). Fatigue was demonstrated to be more severe in patients with pulmonary disease plus extrapulmonary disease than in those with pulmonary disease alone (109). Fatigue can affect overall patient health. In one study, the severity of fatigue correlated inversely with the 6-minute walk distance (61). In some patients, treatment with anti-TNF antibodies may improve fatigue (110), but there are patients with persistent fatigue despite anti-TNF therapy.
Wagner and colleagues were the first to report that methylphenidate could treat sarcoidosis-associated fatigue (111). In a subsequent double-blind, placebo-controlled crossover trial on 10 patients, Lower and colleagues found that d-methylphenidate was superior to placebo in treating sarcoidosis-associated fatigue (112). In that study, all but one patient was on systemic therapy for sarcoidosis and still had significant fatigue.
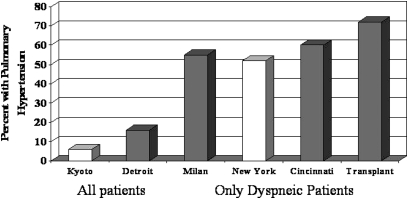
Sarcoidosis-associated pulmonary hypertension (SAPH) has been reported by several groups (113). Figure 3 demonstrates the frequency of pulmonary hypertension reported over the past few years. Two studies examined the frequency of pulmonary hypertension in consecutive patients with sarcoidosis seen at an individual clinic (114, 115). The prevalence of pulmonary hypertension was 5 to 15%. Several studies examined only patients with symptomatic sarcoidosis. In such populations, the prevalence of pulmonary hypertension was greater than 50% (53, 54, 116), with the highest rate noted in patients listed for lung transplantation (117).
Figure 3.
Rate of pulmonary hypertension in patients with sarcoidosis in various series across the world. The studies from Kyoto (114) and Detroit (115) examined a cohort of all patients with sarcoidosis. The studies from Milan (116), New York (53), and Cincinnati (54) were of patients with pulmonary symptoms referred for evaluation for pulmonary hypertension. Patients referred for lung transplant had the highest rate of pulmonary hypertension (117). The studies marked by open bars used echocardiography to determine pulmonary hypertension. The solid bar indicates those studies that used right-sided heart catheterization to determine pulmonary hypertension.
There are several potential causes of pulmonary hypertension, including interstitial lung disease, pulmonary vascular disease, pulmonary veno-occlusion, compression of pulmonary arteries by adenopathy, and left ventricular diastolic dysfunction (118). These different mechanisms may respond to different treatments and be associated with a different clinical outcome. In comparing patients with sarcoidosis with pulmonary hypertension due to left ventricular diastolic dysfunction to pulmonary hypertension alone, there was a significant difference in survival. Patients with SAPH without left ventricular dysfunction had significantly worse survival, with a median survival of 3 years (119).
There have been several reports of treatment of SAPH. The prostacyclin epoprostenol had been reported to be successful in long-term management of some patients with severe disease (120). In an open-label trial of the inhaled prostacyclin iloprost, more than half the patients who completed 16 weeks of therapy had either hemodynamic or exercise improvement with treatment. For the group, there was a significant improvement in quality of life, as measured by the Saint George Respiratory Questionnaire (120). The oral agents sildenafil and bosentan have also been used to treat SAPH. In the largest series reported to date, clinicians were able to successfully treat more than half of their patients with one or more of these agents (122).
In the treatment of sarcoidosis, the assessment of response to therapy has been the subject of increasing investigation. Although some studies have demonstrated an improvement in FVC with therapy (18), an improvement in the chest roentgenogram with therapy may be more specific (59). For extrapulmonary disease, a composite score of physician assessment has been proposed (97). This allows one to analyze patients with multiple manifestations of disease. However, this system does not allow assessment of any specific organ in isolation, which may be more responsive and/or most important to the patient. Two scores for cutaneous sarcoidosis have been recently reported. One incorporates blinded readings of photographs of skin lesions (99). The other provides a systemic score of the degree of erythema, induration, and desquamation as well as the percent of predefined areas involved. This sarcoidosis activity and severity index (SASI) was developed to mimic the scoring system for psoriasis. This scoring system has been validated for chronic facial sarcoidosis and has little interobserver variability (106).
CONCLUSIONS
Although there has been progress in sarcoidosis over the past few years, much is still unknown. Several important questions remain to be answered. These include whether there is one or more than one agent leading to the disease and what the important genes are that increase susceptibility to the disease and shape the clinical outcome of the individual patient. Although diagnostic strategies and management of sarcoidosis have improved, determining the causes and populations at risk for the disease would not only enhance the diagnosis and treatment but possibly aid in prevention.
Supported by National Institutes of Health grant HL081538 (D.A.C.).
Originally Published in Press as DOI:10.1164/rccm.201006-0865CI on October 29, 2010
Author Disclosure: R.P.B. received consultancy fees from Centocor for less than $1,000 and received sponsored grants from Centocor, Celgene, Actelion, and Rob Pierce for $10,001–$50,000 each. D.A.C. received lecture fees from Takeda Corporation for $1,001–$5,000 and received sponsored grants from Takeda Corporation, Actelion, Ortho-McNeil, and Hollister Corporation for $10,001–$50,000 each, Bard Corporation for $50,001–$100,000, and National Institutes of Health for more than $100,001. M.A.J. received consultancy fees from Pulmonary Reviews for less than $1,000, served on the advisory board for Centocor for less than $1,000, and received lecture fees from Boehringer-Ingelheim and Pfizer for $5,001–$10,000 each. M.A.J. received sponsored grants from Centocor and Gilead for more than $100,001.
References
- 1.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, Du BR, Eklund A, Kitaichi M, Lynch J, Rizzato G, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:149–173. [PubMed] [Google Scholar]
- 2.Calabrese LH, Kirchner E, Shrestha R. Rheumatic complications of human immunodeficiency virus infection in the era of highly active antiretroviral therapy: emergence of a new syndrome of immune reconstitution and changing patterns of disease. Semin Arthritis Rheum 2005;35:166–174. [DOI] [PubMed] [Google Scholar]
- 3.Moller DR, Chen ES. Genetic basis of remitting sarcoidosis: triumph of the trimolecular complex? Am J Respir Cell Mol Biol 2002;27:391–395. [DOI] [PubMed] [Google Scholar]
- 4.Rossman MD, Thompson B, Frederick M, Maliarik M, Iannuzzi MC, Rybicki BA, Pandey JP, Newman LS, Magira E, Beznik-Cizman B, et al. HLA-DRB1*1101: a significant risk factor for sarcoidosis in blacks and whites. Am J Hum Genet 2003;73:720–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rybicki BA, Maliarik MJ, Poisson LM, Sheffer R, Chen KM, Major M, Chase GA, Iannuzzi MC. The major histocompatibility complex gene region and sarcoidosis susceptibility in African Americans. Am J Respir Crit Care Med 2003;167:444–449. [DOI] [PubMed] [Google Scholar]
- 6.Grunewald J, Eklund A. Lofgren's syndrome: human leukocyte antigen strongly influences the disease course. Am J Respir Crit Care Med 2009;179:307–312. [DOI] [PubMed] [Google Scholar]
- 7.Grunewald J, Brynedal B, Darlington P, Nisell M, Cederlund K, Hillert J, Eklund A. Different HLA-DRB1 allele distributions in distinct clinical subgroups of sarcoidosis patients. Respir Res. 2010;11:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato H, Grutters JC, Pantelidis P, Mizzon AN, Ahmad T, Van Houte AJ, Lammers JW, van den Bosch JM, Welsh KI, du Bois RM. HLA-DQB1*0201: a marker for good prognosis in British and Dutch patients with sarcoidosis. Am J Respir Cell Mol Biol 2002;27:406–412. [DOI] [PubMed] [Google Scholar]
- 9.Schurmann M, Reichel P, Muller-Myhsok B, Schlaak M, Muller-Quernheim J, Schwinger E. Results from a genome-wide search for predisposing genes in sarcoidosis. Am J Respir Crit Care Med 2001;164:840–846. [DOI] [PubMed] [Google Scholar]
- 10.Valentonyte R, Hampe J, Huse K, Rosenstiel P, Albrecht M, Stenzel A, Nagy M, Gaede KI, Franke A, Haesler R, et al. Sarcoidosis is associated with a truncating splice site mutation in BTNL2. Nat Genet 2005;37:357–364. [DOI] [PubMed] [Google Scholar]
- 11.Rybicki BA, Walewski JL, Maliarik MJ, Kian H, Iannuzzi MC. The BTNL2 gene and sarcoidosis susceptibility in African Americans and Whites. Am J Hum Genet 2005;77:491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iannuzzi MC, Iyengar SK, Gray-McGuire C, Elston RC, Baughman RP, Donohue JF, Hirst K, Judson MA, Kavuru MS, Maliarik MJ, et al. Genome-wide search for sarcoidosis susceptibility genes in African Americans. Genes Immun 2005;6:509–518. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann S, Franke A, Fischer A, Jacobs G, Nothnagel M, Gaede KI, Schurmann M, Muller-Quernheim J, Krawczak M, Rosenstiel P, et al. Genome-wide association study identifies ANXA11 as a new susceptibility locus for sarcoidosis. Nat Genet 2008;40:1103–1106. [DOI] [PubMed] [Google Scholar]
- 14.Fischer A, Nothnagel M, Schurmann M, Muller-Quernheim J, Schreiber S, Hofmann S. A genome-wide linkage analysis in 181 German sarcoidosis families using clustered bi-allelic markers. Chest 2010;138:151–157. [DOI] [PubMed] [Google Scholar]
- 15.Moller DR, Forman JD, Liu MC, Noble PW, Greenlee BM, Vyas P, Holden DA, Forrester JM, Lazarus A, Wysocka M, et al. Enhanced expression of IL-12 associated with Th 1 cytokine profiles in active pulmonary sarcoidosis. J Immunol 1996;156:4952–4960. [PubMed] [Google Scholar]
- 16.Ziegenhagen MW, Rothe E, Zissel G, Muller-Quernheim J. Exagerated TNFalpha release of alveolar macrophages in corticosteroid resistant sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2002;19:185–190. [PubMed] [Google Scholar]
- 17.Baughman RP, Strohofer SA, Buchsbaum J, Lower EE. Release of tumor necrosis factor by alveolar macrophages of patients with sarcoidosis. J Lab Clin Med 1990;115:36–42. [PubMed] [Google Scholar]
- 18.Baughman RP, Drent M, Kavuru M, Judson MA, Costabel U, Du BR, Albera C, Brutsche M, Davis G, Donohue JF, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795–802. [DOI] [PubMed] [Google Scholar]
- 19.Crouser ED, Culver DA, Knox KS, Julian MW, Shao G, Abraham S, Liyanarachchi S, Macre JE, Wewers MD, Gavrilin MA, et al. Gene expression profiling identifies MMP-12 and ADAMDEC1 as potential pathogenic mediators of pulmonary sarcoidosis. Am J Respir Crit Care Med 2009;179:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenbaum JT, Pasadhika S, Crouser ED, Choi D, Harrington CA, Lewis JA, Austin CR, Diebel TN, Vance EE, Braziel RM, et al. Hypothesis: sarcoidosis is a STAT1-mediated disease. Clin Immunol 2009;132:174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundar KM, Carveth HJ, Gosselin MV, Beatty PG, Colby TV, Hoidal JR. Granulomatous pneumonitis following bone marrow transplantation. Bone Marrow Transplant 2001;28:627–630. [DOI] [PubMed] [Google Scholar]
- 22.Milman N, Andersen CB, Burton CM, Iversen M. Recurrent sarcoid granulomas in a transplanted lung derive from recipient immune cells. Eur Respir J 2005;26:549–552. [DOI] [PubMed] [Google Scholar]
- 23.Eishi Y, Suga M, Ishige I, Kobayashi D, Yamada T, Takemura T, Takizawa T, Koike M, Kudoh S, Costabel U, et al. Quantitative analysis of mycobacterial and propionibacterial DNA in lymph nodes of Japanese and European patients with sarcoidosis. J Clin Microbiol 2002;40:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song Z, Marzilli L, Greenlee BM, Chen ES, Silver RF, Askin FB, Teirstein AS, Zhang Y, Cotter RJ, Moller DR. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 2005;201:755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen ES, Wahlstrom J, Song Z, Willett MH, Wiken M, Yung RC, West EE, McDyer JF, Zhang Y, Eklund A, et al. T cell responses to mycobacterial catalase-peroxidase profile a pathogenic antigen in systemic sarcoidosis. J Immunol 2008;181:8784–8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drake WP, Dhason MS, Nadaf M, Shepherd BE, Vadivelu S, Hajizadeh R, Newman LS, Kalams SA. Cellular recognition of Mycobacterium tuberculosis ESAT-6 and KatG peptides in systemic sarcoidosis. Infect Immun 2007;75:527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oswald-Richter KA, Culver DA, Hawkins C, Hajizadeh R, Abraham S, Shepherd BE, Jenkins CA, Judson MA, Drake WP. Cellular responses to mycobacterial antigens are present in bronchoalveolar lavage fluid used in the diagnosis of sarcoidosis. Infect Immun 2009;77:3740–3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen SS, Evans W, Carlisle J, Hajizadeh R, Nadaf M, Shepherd BE, Pride DT, Johnson JE, Drake WP. Superoxide dismutase A antigens derived from molecular analysis of sarcoidosis granulomas elicit systemic Th-1 immune responses. Respir Res 2008;9:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlisle J, Evans W, Hajizadeh R, Nadaf M, Shepherd B, Ott RD, Richter K, Drake W. Multiple Mycobacterium antigens induce interferon-gamma production from sarcoidosis peripheral blood mononuclear cells. Clin Exp Immunol 2007;150:460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajizadeh R, Sato H, Carlisle J, Nadaf MT, Evans W, Shepherd BE, Miller RF, Kalams SA, Drake WP. Mycobacterium tuberculosis Antigen 85A induces Th-1 immune responses in systemic sarcoidosis. J Clin Immunol 2007;27:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moller DR. Potential etiologic agents in sarcoidosis. Proc Am Thorac Soc 2007;4:465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen ES, Song Z, Willett MH, Heine S, Yung RC, Liu MC, Groshong SD, Zhang Y, Tuder RM, Moller DR. Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am J Respir Crit Care Med 2010;181:360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothkrantz-Kos S, Dieijen-Visser MP, Mulder PG, Drent M. Potential usefulness of inflammatory markers to monitor respiratory functional impairment in sarcoidosis. Clin Chem 2003;49:1510–1517. [DOI] [PubMed] [Google Scholar]
- 34.Swaisgood CM, Oswald-Richter K, Moeller SD, Klemenc JM, Ruple LM, Farver CF, Drake JM, Culver DA, Drake WP. Development of a sarcoidosis murine lung granuloma model using Mycobacterium sodA. Am J Respir Cell Mol Biol (In press) [DOI] [PMC free article] [PubMed]
- 35.Taflin C, Miyara M, Nochy D, Valeyre D, Naccache JM, Altare F, Salek-Peyron P, Badoual C, Bruneval P, Haroche J, et al. FoxP3+ regulatory T cells suppress early stages of granuloma formation but have little impact on sarcoidosis lesions. Am J Pathol 2009;174:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho LP, Urban BC, Thickett DR, Davies RJ, McMichael AJ. Deficiency of a subset of T-cells with immunoregulatory properties in sarcoidosis. Lancet. 2005;365:1062–1072. [DOI] [PubMed] [Google Scholar]
- 37.Manouvrier-Hanu S, Puech B, Piette F, Boute-Benejean O, Desbonnet A, Duquesnoy B, Farriaux JP. Blau syndrome of granulomatous arthritis, iritis, and skin rash: a new family and review of the literature. Am. J Med Genet 1998;76:217–221. [PubMed] [Google Scholar]
- 38.Brinker H, Pederson NT. Immunologic marker patterns in granulomatous lymph node lesions. Histopathology 1989;15:495–503. [DOI] [PubMed] [Google Scholar]
- 39.Judson MA. The diagnosis of sarcoidosis. Clin Chest Med 2008;29:415–427, viii. [DOI] [PubMed] [Google Scholar]
- 40.Judson MA, Thompson BW, Rabin DL, Steimel J, Knatterud GL, Lackland DT, Rose C, Rand CS, Baughman RP, Teirstein AS. The diagnostic pathway to sarcoidosis. Chest 2003;123:406–412. [DOI] [PubMed] [Google Scholar]
- 41.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager HJ, Bresnitz EA, DePalo L, Hunninghake G, Iannuzzi MC, Johns CJ, et al; A Case Control Etiologic Study of Sarcoidosis (ACCESS) Research Group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164:1885–1889. [DOI] [PubMed] [Google Scholar]
- 42.Loddenkemper R, Kloppenborg A, Schoenfeld N, Grosser H, Costabel U. Clinical findings in 715 patients with newly detected pulmonary sarcoidosis–results of a cooperative study in former West Germany and Switzerland. WATL Study Group. Wissenschaftliche Arbeitsgemeinschaft fur die Therapie von Lungenkrankheitan. Sarcoidosis Vasc Diffuse Lung Dis 1998;15:178–182. [PubMed] [Google Scholar]
- 43.Pietinalho A, Ohmichi M, Hiraga Y, Lofroos AB, Selroos O. The mode of presentation of sarcoidosis in Finland and Hokkaido, Japan. A comparative analysis of 571 Finnish and 686 Japanese patients. Sarcoidosis 1996;13:159–166. [DOI] [PubMed] [Google Scholar]
- 44.Keir G, Wells AU. Assessing pulmonary disease and response to therapy: which test? Semin Respir Crit Care Med 2010;31:409–418. [DOI] [PubMed] [Google Scholar]
- 45.Hansell DM, Milne DG, Wilsher ML, Wells AU. Pulmonary sarcoidosis: morphologic associations of airflow obstruction at thin-section CT. Radiology 1998;209:697–704. [DOI] [PubMed] [Google Scholar]
- 46.Handa T, Nagai S, Fushimi Y, Miki S, Ohta K, Niimi A, Mishima M, Izumi T. Clinical and radiographic indices associated with airflow limitation in patients with sarcoidosis. Chest 2006;130:1851–1856. [DOI] [PubMed] [Google Scholar]
- 47.Harrison BD, Shaylor JM, Stokes TC, Wilkes AR. Airflow limitation in sarcoidosis–a study of pulmonary function in 107 patients with newly diagnosed disease. Respir Med 1991;85:59–64. [DOI] [PubMed] [Google Scholar]
- 48.Sharma OP, Johnson R. Airway obstruction in sarcoidosis. A study of 123 nonsmoking black American patients with sarcoidosis. Chest 1988;94:343–346. [DOI] [PubMed] [Google Scholar]
- 49.Shorr AF, Torrington KG, Hnatiuk OW. Endobronchial involvement and airway hyperreactivity in patients with sarcoidosis. Chest 2001;120:881–886. [DOI] [PubMed] [Google Scholar]
- 50.Chambellan A, Turbie P, Nunes H, Brauner M, Battesti JP, Valeyre D. Endoluminal stenosis of proximal bronchi in sarcoidosis: bronchoscopy, function, and evolution. Chest 2005;127:472–481. [DOI] [PubMed] [Google Scholar]
- 51.Baughman RP, Iannuzzi MC, Lower EE, Moller DR, Balkissoon RC, Winget DB, Judson MA. Use of fluticasone in acute symptomatic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2002;19:198–204. [PubMed] [Google Scholar]
- 52.Yeager H, Rossman MD, Baughman RP, Teirstein AS, Judson MA, Rabin DL, Iannuzzi MC, Rose C, Bresnitz EA, DePalo L, et al. Pulmonary and psychosocial findings at enrollment in the ACCESS study. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:147–153. [PubMed] [Google Scholar]
- 53.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest 2005;128:1483–1489. [DOI] [PubMed] [Google Scholar]
- 54.Baughman RP, Engel PJ, Meyer CA, Barrett AB, Lower EE. Pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006;23:108–116. [PubMed] [Google Scholar]
- 55.Scadding JG. Prognosis of intrathoracic sarcoidosis in England. BMJ 1961;4:1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Judson MA, Baughman RP, Thompson BW, Teirstein AS, Terrin ML, Rossman MD, Yeager H Jr, McLennan G, Bresnitz EA, DePalo L, et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis 2003;20:204–211. [PubMed] [Google Scholar]
- 57.Nagai S, Shigematsu M, Hamada K, Izumi T. Clinical courses and prognoses of pulmonary sarcoidosis. Curr Opin Pulm Med 1999;5:293–298. [DOI] [PubMed] [Google Scholar]
- 58.Baughman RP, Selroos O. Evidence-based approach to the treatment of sarcoidosis. In: Gibson PG, Abramson M, Wood-Baker R, Volmick J, Hensley M, Costabel U, editors. Evidence-based respiratory medicine. Malden, MA: Blackwell Publishing Ltd.; 2005. pp. 491–508.
- 59.Baughman RP, Shipley R, Desai S, Drent M, Judson MA, Costabel U, du Bois RM, Kavuru M, Schlenker-Herceg R, Flavin S, et al. Changes in chest roentgenogram of sarcoidosis patients during a clinical trial of infliximab therapy: comparison of different methods of evaluation. Chest 2009;136:526–535. [DOI] [PubMed] [Google Scholar]
- 60.Judson MA, Gilbert GE, Rodgers JK, Greer CF, Schabel SI. The utility of the chest radiograph in diagnosing exacerbations of pulmonary sarcoidosis. Respirology 2008;13:97–102. [DOI] [PubMed] [Google Scholar]
- 61.Baughman RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest 2007;132:207–213. [DOI] [PubMed] [Google Scholar]
- 62.Garwood S, Judson MA, Silvestri G, Hoda R, Fraig M, Doelken P. Endobronchial ultrasound for the diagnosis of pulmonary sarcoidosis. Chest 2007;132:1298–1304. [DOI] [PubMed] [Google Scholar]
- 63.Tremblay A, Stather DR, Maceachern P, Khalil M, Field SK. A randomized controlled trial of standard vs endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected sarcoidosis. Chest 2009;136:340–346. [DOI] [PubMed] [Google Scholar]
- 64.Israel HL, Goldstein RA. Hepatic granulomatosis and sarcoidosis. Ann Intern Med 1973;79:669–678. [DOI] [PubMed] [Google Scholar]
- 65.Herbort CP, Rao NA, Mochizuki M. International criteria for the diagnosis of ocular sarcoidosis: results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm 2009;17:160–169. [DOI] [PubMed] [Google Scholar]
- 66.Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H Jr; ACCESS Research group. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:75–86. [PubMed] [Google Scholar]
- 67.Silverstein E, Pertschuk LP, Friedland J. Immunofluorescent localization of angiotensin converting enzyme in epithelioid and giant cells of sarcoidosis granulomas. Proc Natl Acad Sci USA 1979;76:6646–6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Studdy PR, Bird R, Neville E, James DG. Biochemical findings in sarcoidosis. J Clin Pathol 1980;33:528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Studdy PR, James DG. The specificity and sensitivity of serum angiotensin-converting enzyme in sarcoidosis and other diseases. In: Chretien J, Marsac J, Saltiel JC, editors. Sarcoidosis. Paris: Pergamon Press; 1983. pp. 332–344.
- 70.Lieberman J, Nosal A, Schlessner A, Sastre-Foken A. Serum angiotensin-converting enzyme for diagnosis and therapeutic evaluation of sarcoidosis. Am Rev Respir Dis 1979;120:329–335. [DOI] [PubMed] [Google Scholar]
- 71.Tomita H, Ina Y, Sugiura Y, Sato S, Kawaguchi H, Morishita M, Yamamoto M, Ueda R. Polymorphism in the angiotensin-converting enzyme (ACE) gene and sarcoidosis. Am J Respir Crit Care Med 1997;156:255–259. [DOI] [PubMed] [Google Scholar]
- 72.Pietinalho A, Furuya K, Yamaguchi E, Kawakami Y, Selroos O. The angiotensin-converting enzyme DD gene is associated with poor prognosis in Finnish sarcoidosis patients. Eur Respir J 1999;13:723–726. [DOI] [PubMed] [Google Scholar]
- 73.Lieberman J, Schleissner LA, Nosal A, Sastre A, Mishkin FS. Clinical correlations of serum angiotensin-converting enzyme (ACE) in sarcoidosis. A longitudinal study of serum ACE, 67gallium scans, chest roentgenograms, and pulmonary function. Chest 1983;84:522–528. [DOI] [PubMed] [Google Scholar]
- 74.DeRemee RA, Rohrbach MS. Serum angiotensin-converting enzyme activity in evaluating the clinical course of sarcoidosis. Ann Intern Med 1980;92:361–365. [DOI] [PubMed] [Google Scholar]
- 75.Pietinalho A, Ohmichi M, Lofroos AB, Hiraga Y, Selroos O. The prognosis of sarcoidosis in Finland and Hokkaido, Japan. A comparative five-year study of biopsy-proven cases. Sarcoidosis Vasc Diffuse Lung Dis 2000;17:158–166. [PubMed] [Google Scholar]
- 76.Gronhagen-Riska C, Selroos O, Niemisto M. Angiotensin converting enzyme. V. Serum levels as monitors of disease activity in corticosteroid-treated sarcoidosis. Eur J Respir Dis 1980;61:113–122. [PubMed] [Google Scholar]
- 77.Baughman RP, Ploysongsang Y, Roberts RD, Srivastava L. Effects of sarcoid and steroids on angiotensin-converting enzyme. Am Rev Respir Dis 1983;128:631–633. [DOI] [PubMed] [Google Scholar]
- 78.Bargagli E, Bianchi N, Margollicci M, Olivieri C, Luddi A, Coviello G, Grosso S, Rottoli P. Chitotriosidase and soluble IL-2 receptor: comparison of two markers of sarcoidosis severity. Scand J Clin Lab Invest 2008;68:479–483. [DOI] [PubMed] [Google Scholar]
- 79.Ziegenhagen MW, Rothe ME, Schlaak M, Muller-Quernheim J. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J 2003;21:407–413. [DOI] [PubMed] [Google Scholar]
- 80.Bargagli E, Mazzi A, Rottoli P. Markers of inflammation in sarcoidosis: blood, urine, BAL, sputum, and exhaled gas. Clin Chest Med 2008;29:445–458, (viii.). [DOI] [PubMed] [Google Scholar]
- 81.Braun JJ, Kessler R, Constantinesco A, Imperiale A. 18F-FDG PET/CT in sarcoidosis management: review and report of 20 cases. Eur J Nucl Med Mol Imaging 2008;35:1537–1543. [DOI] [PubMed] [Google Scholar]
- 82.Teirstein AS, Machac J, Almeida O, Lu P, Padilla ML, Iannuzzi MC. Results of 188 whole-body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest 2007;132:1949–1953. [DOI] [PubMed] [Google Scholar]
- 83.Nunes H, Brillet PY, Valeyre D, Brauner MW, Wells AU. Imaging in sarcoidosis. Semin Respir Crit Care Med 2007;28:102–120. [DOI] [PubMed] [Google Scholar]
- 84.Braun JJ, Imperiale A, Riehm S, Veillon F. Imaging in sinonasal sarcoidosis: CT, MRI, (67)Gallium scintigraphy and (18)F-FDG PET/CT features. J Neuroradiol 2009;73:172–181. [DOI] [PubMed] [Google Scholar]
- 85.Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J 1999;138:299–302. [DOI] [PubMed] [Google Scholar]
- 86.Silverman KJ, Hutchins GM, Bulkley BH. Cardiac sarcoid: a clinicopathologic study of 84 unselected patients with systemic sarcoidosis. Circulation 1978;58:1204–1211. [DOI] [PubMed] [Google Scholar]
- 87.Mehta D, Lubitz SA, Frankel Z, Wisnivesky JP, Einstein AJ, Goldman M, Machac J, Teirstein A. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest 2008;133:1426–1435. [DOI] [PubMed] [Google Scholar]
- 88.Smedema JP, Snoep G, van Kroonenburgh MP, van Geuns RJ, Dassen WR, Gorgels AP, Crijns HJ. Cardiac involvement in patients with pulmonary sarcoidosis assessed at two university medical centers in the Netherlands. Chest 2005;128:30–35. [DOI] [PubMed] [Google Scholar]
- 89.Gibson GJ, Prescott RJ, Muers MF, Middleton WG, Mitchell DN, Connolly CK, Harrison BD. British Thoracic Society Sarcoidosis study: effects of long term corticosteroid treatment. Thorax 1996;51:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gottlieb JE, Israel HL, Steiner RM, Triolo J, Patrick H. Outcome in sarcoidosis. The relationship of relapse to corticosteroid therapy. Chest 1997;111:623–631. [DOI] [PubMed] [Google Scholar]
- 91.Baughman RP, Judson MA, Teirstein A, Yeager H, Rossman M, Knatterud GL, Thompson B. Presenting characteristics as predictors of duration of treatment in sarcoidosis. QJM 2006;99:307–315. [DOI] [PubMed] [Google Scholar]
- 92.Paramothayan NS, Lasserson TJ, Jones PW. Corticosteroids for pulmonary sarcoidosis. Cochrane Database Syst Rev 2005;CD001114. [DOI] [PMC free article] [PubMed]
- 93.Paramothayan S, Lasserson T, Walters EH. Immunosuppressive and cytotoxic therapy for pulmonary sarcoidosis. Cochrane Database Syst Rev 2003;CD003536. [DOI] [PubMed]
- 94.Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, Raskob G, Lewis SZ, Schunemann H. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest 2006;129:174–181. [DOI] [PubMed] [Google Scholar]
- 95.Baughman RP, Costabel U, du Bois RM. Treatment of sarcoidosis. Clin Chest Med 2008;29:533–548. [DOI] [PubMed] [Google Scholar]
- 96.Baughman RP, Lower EE, Drent M. Inhibitors of tumor necrosis factor (TNF) in sarcoidosis: who, what, and how to use them. Sarcoidosis Vasc Diffuse Lung Dis 2008;25:76–89. [PubMed] [Google Scholar]
- 97.Judson MA, Baughman RP, Costabel U, Flavin S, Lo KH, Kavuru MS, Drent M. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J 2008;31:1189–1196. [DOI] [PubMed] [Google Scholar]
- 98.Rossman MD, Newman LS, Baughman RP, Teirstein A, Weinberger SE, Miller WJ, Sands BE. A double-blind, randomized, placebo-controlled trial of infliximab in patients with active pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2006;23:201–208. [PubMed] [Google Scholar]
- 99.Stagaki E, Mountford WK, Lackland DT, Judson MA. The treatment of lupus pernio: results of 116 treatment courses in 54 patients. Chest 2009;135:468–476. [DOI] [PubMed] [Google Scholar]
- 100.Sodhi M, Pearson K, White ES, Culver DA. Infliximab therapy rescues cyclophosphamide failure in severe central nervous system sarcoidosis. Respir Med 2009;103:268–273. [DOI] [PubMed] [Google Scholar]
- 101.Moravan M, Segal BM. Treatment of CNS sarcoidosis with infliximab and mycophenolate mofetil. Neurology 2009;72:337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sweiss NJ, Barnathan ES, Lo K, Judson MA, Baughman RP. C-reactive protein predicts response to infliximab in patients with chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2010;27:49–56. [PubMed] [Google Scholar]
- 103.Almodovar R, Izquierdo M, Zarco P, Javier QF, Mazzucchelli R, Steen B. Pulmonary sarcoidosis in a patient with ankylosing spondylitis treated with infliximab. Clin Exp Rheumatol 2007;25:99–101. [PubMed] [Google Scholar]
- 104.Daien CI, Monnier A, Claudepierre P, Constantin A, Eschard JP, Houvenagel E, Samimi M, Pavy S, Pertuiset E, Toussirot E, et al. Sarcoid-like granulomatosis in patients treated with tumor necrosis factor blockers: 10 cases. Rheumatology (Oxford) 2009;48:883–886. [DOI] [PubMed] [Google Scholar]
- 105.Sweiss NJ, Baughman RP. Tumor necrosis factor inhibition in the treatment of refractory sarcoidosis: slaying the dragon? J Rheumatol 2007;34:2129–2131. [PubMed] [Google Scholar]
- 106.Baughman RP, Judson MA, Teirstein A, Lower EE, Lo K, Schlenker-Herceg R, Barnathan ES. Chronic facial sarcoidosis including lupus pernio: clinical description and proposed scoring systems. Am J Clin Dermatol 2008;9:155–161. [DOI] [PubMed] [Google Scholar]
- 107.de Kleijn WP, de Vries J, Lower EE, Elfferich MD, Baughman RP, Drent M. Fatigue in sarcoidosis: a systematic review. Curr Opin Pulm Med 2009;15:499–506. [DOI] [PubMed] [Google Scholar]
- 108.de Kleijn WPE, Elfferich MDP, de Vries J, Jonker GJ, Lower EE, Baughman RP, King TE Jr, Drent M. Fatigue in sarcoidosis: American versus Dutch patients. Sarcoidosis Vasc Diffuse Lung Dis 2009;26:92–97. [PubMed] [Google Scholar]
- 109.Gvozdenovic BS, Mihailovic-Vucinic V, Ilic-Dudvarski A, Zugic V, Judson MA. Differences in symptom severity and health status impairment between patients with pulmonary and pulmonary plus extrapulmonary sarcoidosis. Respir Med 2008;102:1636–1642. [DOI] [PubMed] [Google Scholar]
- 110.Elfferich MD, Nelemans PJ, Ponds RW, de Vries J, Wijnen PA, Drent M. Everyday cognitive failure in sarcoidosis: the prevalence and the effect of anti-TNF-alpha treatment. Respiration 2010;80:212–219. [DOI] [PubMed] [Google Scholar]
- 111.Wagner MT, Marion SD, Judson MA. The effects of fatigue and treatment with methylphenidate on sustained attention in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2005;22:235. [PubMed] [Google Scholar]
- 112.Lower EE, Harman S, Baughman RP. Double-blind, randomized trial of dexmethylphenidate hydrochloride for the treatment of sarcoidosis-associated fatigue. Chest 2008;133:1189–1195. [DOI] [PubMed] [Google Scholar]
- 113.Diaz-Guzman E, Farver C, Parambil J, Culver DA. Pulmonary hypertension caused by sarcoidosis. Clin Chest Med 2008;29:549–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Handa T, Nagai S, Miki S, Fushimi Y, Ohta K, Mishima M, Izumi T. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest 2006;129:1246–1252. [DOI] [PubMed] [Google Scholar]
- 115.Bourbonnais JM, Samavati L. Clinical predictors of pulmonary hypertension in sarcoidosis. Eur Respir J 2008;32:296–302. [DOI] [PubMed] [Google Scholar]
- 116.Rizzato G, Pezzano A, Sala G, Merlini R, Ladelli L, Tansini G, Montanari G, Bertoli L. Right heart impairment in sarcoidosis: haemodynamic and echocardiographic study. Eur J Respir Dis 1983;64:121–128. [PubMed] [Google Scholar]
- 117.Shorr AF, Helman DL, Davies DB, Nathan SD. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur Respir J 2005;25:783–788. [DOI] [PubMed] [Google Scholar]
- 118.Nunes H, Humbert M, Capron F, Brauner M, Sitbon O, Battesti JP, Simonneau G, Valeyre D. Pulmonary hypertension associated with sarcoidosis: mechanisms, haemodynamics and prognosis. Thorax 2006;61:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Baughman RP, Engel PJ, Taylor L, Lower EE. Survival in sarcoidosis associated pulmonary hypertension: the importance of hemodynamic evaluation. Chest 2010;138:1078–1085. [DOI] [PubMed] [Google Scholar]
- 120.Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest 2006;130:1481–1488. [DOI] [PubMed] [Google Scholar]
- 121.Baughman RP, Judson MA, Lower EE, Highland K, Kwon S, Craft N, Engel PJ. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis 2009;26:110–120. [PubMed] [Google Scholar]
- 122.Barnett CF, Bonura EJ, Nathan SD, Ahmad S, Shlobin OA, Osei K, Zaiman AL, Hassoun PM, Moller DR, Barnett SD, et al. Treatment of sarcoidosis-associated pulmonary hypertension: a two-center experience. Chest 2009;135:1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pietinalho A, Lindholm A, Haahtela T, Tukiainen P, Selroos O. Inhaled budesonide for treatment of pulmonary sarcoidosis: results of a double-blind, placebo-controlled, multicentre study. Eur Respir J 1996;9:406s. [Google Scholar]
- 124.McKinzie BP, Bullington WM, Mazur JE, Judson MA. Efficacy of short-course, low-dose corticosteroid therapy for acute pulmonary sarcoidosis exacerbations. Am J Med Sci 2010;339:1–4. [DOI] [PubMed] [Google Scholar]
- 125.Johns CJ, Michele TM. The clinical management of sarcoidosis: a 50-year experience at the Johns Hopkins hospital. Medicine 1999;78:65–111. [DOI] [PubMed] [Google Scholar]
- 126.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis 2000;17:60–66. [PubMed] [Google Scholar]
- 127.Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995;155:846–851. [PubMed] [Google Scholar]
- 128.Vucinic VM. What is the future of methotrexate in sarcoidosis? A study and review. Curr Opin Pulm Med 2002;8:470–476. [DOI] [PubMed] [Google Scholar]
- 129.Morgan SL, Baggott JE, Vaughn WH, Austin JS, Veitch TA, Lee JY, Koopman WJ, Krundieck CL, Alarcon GS. Supplementation with folic acid during methotrexate therapy for rheumatoid arthritis. Ann Intern Med 1994;121:833–841. [DOI] [PubMed] [Google Scholar]
- 130.Muller-Quernheim J, Kienast K, Held M, Pfeifer S, Costabel U. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J 1999;14:1117–1122. [DOI] [PubMed] [Google Scholar]
- 131.Lewis SJ, Ainslie GM, Bateman ED. Efficacy of azathioprine as second-line treatment in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:87–92. [PubMed] [Google Scholar]
- 132.Baughman RP, Lower EE. Leflunomide for chronic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 2004;21:43–48. [DOI] [PubMed] [Google Scholar]
- 133.Kouba DJ, Mimouni D, Rencic A, Nousari HC. Mycophenolate mofetil may serve as a steroid-sparing agent for sarcoidosis. Br J Dermatol 2003;148:147–148. [DOI] [PubMed] [Google Scholar]
- 134.Moudgil A, Przygodzki RM, Kher KK. Successful steroid-sparing treatment of renal limited sarcoidosis with mycophenolate mofetil. Pediatr Nephrol 2006;21:281–285. [DOI] [PubMed] [Google Scholar]