Abstract
Rationale: Cross-sectional studies have reported inconsistent findings for the association between recreational swimming pool attendance and asthma and allergic diseases in childhood.
Objectives: To examine whether swimming in infancy and childhood was associated with asthma and allergic symptoms at age 7 and 10 years in a UK longitudinal population-based birth cohort, the Avon Longitudinal Study of Parents and Children.
Methods: Data on swimming were collected by questionnaire at 6, 18, 38, 42, 57, 65, and 81 months. Data on rhinitis, wheezing, asthma, eczema, hay fever, asthma medication, and potential confounders were collected through questionnaires at 7 and 10 years. Spirometry and skin prick testing were performed at 7 to 8 years. Data for analysis were available for 5,738 children.
Measurements and Main Results: At age 7 years, more than 50% of the children swam once per week or more. Swimming frequency did not increase the risk of any evaluated symptom, either overall or in atopic children. Children with a high versus low cumulative swimming pool attendance from birth to 7 years had an odds ratio of 0.88 (95% confidence interval, 0.56–1.38) and 0.50 (0.28–0.87), respectively, for ever and current asthma at 7 years, and a 0.20 (0.02–0.39) standard deviation increase in the forced midexpiratory flow. Children with asthma with a high versus low cumulative swimming had an odds ratio for current asthma at 10 years of 0.34 (0.14–0.80).
Conclusions: This first prospective longitudinal study suggests that swimming did not increase the risk of asthma or allergic symptoms in British children. Swimming was associated with increased lung function and lower risk of asthma symptoms, especially among children with preexisting respiratory conditions.
Keywords: Avon Longitudinal Study of Parents and Children; pediatric; epidemiology, prospective; irritants
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
There is inconsistent evidence from cross-sectional studies for an association between swimming pool attendance and the risk of asthma in childhood.
What This Study Adds to the Field
This prospective longitudinal study on the topic, following 5,738 British children from birth until age 10 years, suggests that swimming does not increase the risk of asthma. On the contrary, swimming appears to be associated with higher lung function and fewer respiratory symptoms, particularly among children with asthma.
In recent years, several epidemiological studies have suggested that attending chlorinated swimming pools during childhood is a risk factor for developing asthma and other allergic diseases (1). The underlying hypothesis is that exposure to disinfectants and disinfection by-products in the swimming pool (probably trichloramine, a strong irritant [2]) may cause a detrimental effect in the airways of children with a consequent increased risk of developing asthma (3). It was previously shown that chronic exposure to the irritant environment of indoor swimming pools was associated with higher prevalence of respiratory symptoms among lifeguards (4). The prevalence of asthma among elite swimmers is also higher than among other elite athletes (1, 5). However, it has been argued that reverse causation may explain these findings, because swimming is a well-tolerated and recommended sport for people with asthma (1).
Epidemiological studies conducted in Belgium have found an increased risk of childhood asthma related to both indoor and outdoor swimming pool attendance (6–9). A recent study conducted in Ireland among 121 boys (10) found a significant association between asthma and the number of years attending pools, but not with the frequency of attendance. Studies conducted in Germany (11, 12), Italy (13), and Spain (14) did not find an increased risk of asthma among children attending swimming pools. Despite the conflicting results, there is agreement on the complexity of the potential role of swimming in asthma etiology and the important public health implications (1, 15–17). Asthma is among the most common chronic diseases in children (18), and swimming is one of the most practiced sports in western countries (19), where sedentarism and obesity are increasing, especially among children (20). In August 2007, a multidisciplinary group of experts evaluated the evidence on childhood asthma and swimming pools to establish future research agendas (17). Several shortcomings in the current literature were identified in the area of exposure assessment and the characterization of asthma (17). Currently available studies used a cross-sectional design with a retrospective assessment of swimming pool attendance, which could have led to recall bias and exposure misclassification. The possibility of reverse causation has been identified as another limitation of previous studies, highlighting the need for longitudinal epidemiological studies (14–17), including the use of data in existing prospective birth cohorts (17).
The Avon Longitudinal Study of Parents and Children (ALSPAC) in the United Kingdom has followed from birth more than 5,700 children with prospectively collected data on swimming and respiratory symptoms and measurements. Therefore, this study represents a unique opportunity to assess the risk of childhood asthma associated with swimming pool attendance in childhood. The aim of our study is to examine whether swimming at different periods during early childhood is associated with the prevalence of asthma and allergic symptoms at 7 and 10 years of age.
METHODS
Study Design and Population
The population-based ALSPAC study recruited 14,541 pregnant women resident in Avon, UK, with expected delivery dates between 1 April 1991 and 31 December 1992, resulting in a cohort of 14,062 live births (21). Ethical approval was obtained from the ALSPAC Law and Ethics Committee and the Local Research Ethics Committees. The study protocol has been previously described (22), and further details are on the ALSPAC Web site (http://www.alspac.bris.ac.uk).
Measurements and Data Collection
Outcomes.
Reported symptoms were ascertained through questions similar to those used by the International Study of Asthma and Allergies in Children. Current symptoms (in the last 12 mo) included wheezing, asthma, eczema, hay fever, nasal, nasal and ocular (at 6.7 and 10 yr) and asthma medication (at 7.6 yr). Having ever had asthma at 7.6 years and having ever had eczema and hay fever at 10 years was also collected. Atopy was determined by a skin prick test at age 7 to 8 years. Lung function and bronchial hyperresponsiveness were measured at approximately 8 years. FEV1, FVC, and forced midexpiratory flow were measured by spirometry and converted to sex-, age-, and height-adjusted standard deviation units (23). The rapid methacholine challenge test was performed to measure bronchial hyperresponsiveness (24).
Swimming.
Ever swimming before age 4 years was estimated from questionnaires at 6, 18, 38, and 42 months. Swimming from age 4 to 7 years was summarized in a score based on the swimming frequency during school term periods at 57, 65, and 81 months (4.7, 5.4, and 6.7 yr, respectively). The answers “rarely or not at all,” “once a month,” “once a week,” and “more than once a week” were assigned, respectively, 0, 1, 2, or 3 points. The scores for each period were summed into an overall score: low (0–2 points), medium (3–4 points), and high (5–9 points). A combined score (0–7 yr) distinguished extreme categories: lowest exposed (never swimming before age 4 yr and 4–7 yr swimming score = 0) and highest exposed (ever swimming before age 4 yr and 4–7 yr swimming score > 4).
Confounders.
Sex, birth weight, number of siblings, atopy, maternal education, maternal and paternal social class, maternal age at delivery, maternal asthma, allergy and hay fever, contact with pets, hours of TV watching, exposure to environmental tobacco smoke in several periods, and body mass index (at 7 yr) were considered. Atopy was also considered as a potential effect modifier.
See the online supplement for details on variable definitions and clinical measurements.
Statistical Analysis
Of the 14,062 live births, 13,988 were alive at 1 year. After excluding children in a triplet or quadruplet for confidentiality and missing observations on all swimming variables or outcomes at 7 years, 8,750 children remained. Because atopy was included in the final models, children with missing atopy were further excluded, leaving 5,738 for the final analysis. Missing values in outcomes and covariates (see methods and Table E1 in the online supplement) led to varying sample sizes in the different models.
RESULTS
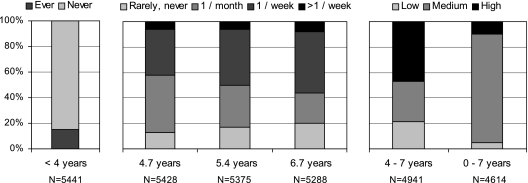
Twelve percent of mothers had ever had asthma, and 21.4% of children were positive to the skin prick test (Table 1). Twenty percent of the children had ever had asthma at 7 years of age (Table 2). The prevalence of hay fever and nasal symptoms increased from age 7 to age 10 years. Swimming before 4 years of age was reported in 14.2% of children (Figure 1). Between 4 and 7 years old, around 50% of children had attended pools at least once a week, whereas around 20% never or very rarely had done so. Forty-seven percent of the children had a high swimming score between age 4 and 7 years, whereas only 10% had a high overall swimming score (Figure 1).
TABLE 1.
CHARACTERISTICS OF THE STUDY POPULATION
| N | % | N Total | |
|---|---|---|---|
| Child characteristics | |||
| Sex, male | 2,908 | 50.7% | 5,738 |
| Birth weight, g, median, percentiles 25, 75 | 3,450 | 3,120, 3,770 | 5,670 |
| Body mass index at 7 yr, kg, median, percentiles 25, 75 | 15.8 | 14.9, 17.0 | 5,693 |
| Environmental exposures | |||
| Any older brothers at age 1.5 yr | 3,197 | 55.7% | 5,738 |
| Weekly contact with pets at age 2 or 4.5 yr* | 4,585 | 82.5% | 5,555 |
| Exposure to environmental tobacco smoke at 6 mo† | 1,676 | 29.8% | 5,620 |
| TV watching at 5.4 yr, > 2 h during weekdays | 506 | 9.5% | 5,348 |
| Maternal characteristics | |||
| Age at delivery, yr, median, percentiles 25, 75 | 29 | 26, 32 | 5,738 |
| Higher education‡ | 2,498 | 44.3% | 5,638 |
| Social class I, II | 2,110 | 42.9% | 4,923 |
| Asthma | 651 | 11.7% | 5,564 |
| Allergy | 2,577 | 46.4% | 5,548 |
| Hay fever | 1,750 | 31.8% | 5,498 |
| Clinical evaluation at 7–8 yr | |||
| Positive skin prick test | 1,226 | 21.4% | 5,738 |
| Lung function | Median | Percentiles 25, 75 | N |
| Standard deviation scores adjusted for height, age, and sex | |||
| FVC | −0.01 | −0.62, 0.66 | 4,708 |
| FEV1 | 0.01 | −0.65, 0.66 | 4,636 |
| FEV1:FVC ratio | 0.89 | 0.85, 0.93 | 4,636 |
| Forced midexpiratory flow (FEF25–75) | −0.04 | −0.68, 0.64 | 4,708 |
| Bronchial hyperresponsiveness§ | 0.10 | −1.33, 1.05 | 3,117 |
N = 5,738.
Cat, dog, or any furry pet.
During the weekend.
Maternal higher education: a level or degree (studying at least until age 18 yr).
Mean of least squares dose–response slope. Percentage decline in FEV1 per μmol methacholine.
TABLE 2.
PREVALENCE OF REPORTED RESPIRATORY SYMPTOMS AT APPROXIMATELY 7 AND 10 YEARS OF AGE
| Age 7 yr |
Age 10 yr |
|||||
|---|---|---|---|---|---|---|
| N | % | N total | N | % | N total | |
| Current asthma | 632 | 11.4 | 5,537 | 546 | 11.4 | 4,770 |
| Current wheezing | 598 | 10.8 | 5,545 | 487 | 10.2 | 4,778 |
| Current asthma medicine | 762 | 13.8 | 5,526 | |||
| Current eczema | 956 | 17.3 | 5,534 | 754 | 15.8 | 4,774 |
| Current hay fever | 494 | 8.9 | 5,520 | 741 | 15.5 | 4,766 |
| Current nasal problems | 708 | 12.9 | 5,495 | 868 | 18.6 | 4,660 |
| Current nasal-ocular problems | 285 | 5.2 | 5,493 | 516 | 11.1 | 4,650 |
| Ever asthma* | 1,109 | 20.2 | 5,498 | |||
| Ever hay fever | 990 | 21.3 | 4,651 | |||
| Ever eczema | 1,560 | 33.7 | 4,626 | |||
N = 5,738. Current indicates symptoms in the last 12 mo.
Doctor diagnosed.
Figure 1.
Percentage of reported swimming at different ages (N = 5,738).
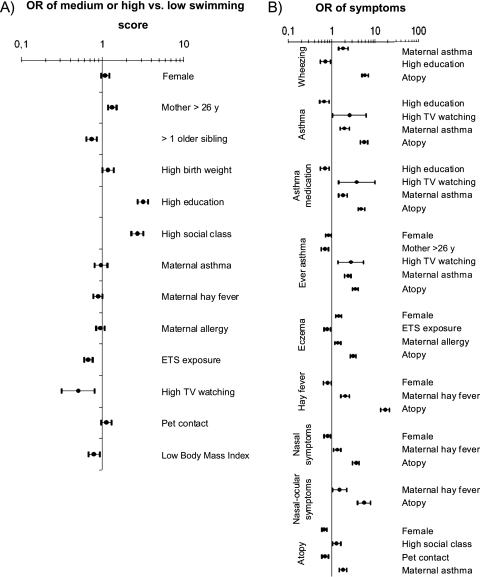
Higher social class and maternal education were associated with a higher frequency of swimming (Figure 2A). A decreased frequency of swimming was associated with having siblings, exposure to environmental tobacco smoke, high TV watching, and low body mass index. A high maternal social class or education was associated with more atopy but with fewer asthma symptoms (Figure 2B). Girls had more eczema and lower prevalence rates of asthma, hay fever, and atopy. Hours of TV watching as well as atopy, maternal asthma, maternal hay fever, and maternal allergy were also predictors of symptoms.
Figure 2.
Association (A) between confounders and swimming at age 4 to 7 years and (B) between confounders and symptoms at 7 or 8 years. Odds ratio (OR) with 95% confidence intervals (N = 5,738). Adjusted by maternal education, except social class. ETS = environmental tobacco smoke.
As crude and adjusted models gave very similar results, only the latter are reported in the tables. After adjusting for confounders, swimming was not associated with ever asthma, but it was associated with a lower prevalence of current asthma and current asthma medication at 7 years of age (Table 3). No significant association was observed between swimming and the prevalence of current wheezing, eczema, hay fever (Table 3), nasal symptoms, and nasal and ocular symptoms at 7 or at 10 years, atopy, having ever had eczema, and hay fever at age 10 years (Table E2). To detect differences of the effect of swimming on asthma by previous respiratory conditions, we stratified the analysis by ever wheezing before 3.5 years (Table 4). The protective effect of swimming on asthma medication and current asthma was only seen among children who wheezed before the age of 3.5 years, whereas it disappeared among never wheezers before age 3.5 years. Swimming was protective for current asthma at 10 years among children ever having had asthma at 7 years of age. Swimming was associated with a higher FVC, FEV1, FEV1:FVC ratio, and forced midexpiratory flow at age 8 years (Table 5), indicating that children who had swum more frequently tended to have a better lung function. No significant association was seen between swimming pool attendance and bronchial hyperresponsiveness. To further control for socioeconomic status, we stratified the main results by maternal education and observed similar risk estimates, indicating no effect modification by socioeconomic status (Table E3).
TABLE 3.
ASSOCIATION BETWEEN SWIMMING IN DIFFERENT AGE PERIODS AND EVER ASTHMA, CURRENT SYMPTOMS, AND ATOPIC STATUS AT APPROXIMATELY 7 AND 10 YEARS OF AGE
| Age 7 yr |
Age 10 yr |
||||||
|---|---|---|---|---|---|---|---|
| Swimming | OR* | 95% CI | N | OR* | 95% CI | N | |
| Ever asthma | |||||||
| Age < 4 yr | Never | 1 | 4440 | ||||
| Ever | 1.01 | 0.81–1.26 | |||||
| Age 4–7 yr | Low | 1 | 4425 | ||||
| Medium | 1.09 | 0.88–1.35 | |||||
| High | 1.07 | 0.87–1.32 | |||||
| Age 0–7 yr | Lowest | 1 | 4193 | ||||
| Highest | 0.88 | 0.56–1.38 | |||||
| Current asthma | |||||||
| Age < 4 yr | Never | 1 | 4,751 | 1 | 3,876 | ||
| Ever | 0.73 | 0.55–0.98 | 0.90 | 0.67–1.21 | |||
| Age 4–7 yr | Low | 1 | 4,481 | 1 | 4,147 | ||
| Medium | 1.00 | 0.76–1.31 | 0.78 | 0.59–1.05 | |||
| High | 0.97 | 0.75–1.25 | 0.91 | 0.69–1.19 | |||
| Age 0–7 yr | Lowest | 1 | 4,243 | 1 | 3,497 | ||
| Highest | 0.50 | 0.28–0.87 | 0.61 | 0.32–1.15 | |||
| Current wheezing | |||||||
| Age < 4 yr | Never | 1 | 4,757 | 1 | 3,885 | ||
| Ever | 0.83 | 0.63–1.11 | 0.95 | 0.70–1.29 | |||
| Age 4–7 yr | Low | 1 | 4,488 | 1 | 3,670 | ||
| Medium | 0.93 | 0.70–1.24 | 0.90 | 0.64–1.26 | |||
| High | 1.12 | 0.86–1.46 | 1.06 | 0.77–1.45 | |||
| Age 0–7 yr | Lowest | 1 | 4,249 | 1 | 3,504 | ||
| Highest | 0.58 | 0.33–1.02 | 0.60 | 0.31–1.14 | |||
| Current asthma medication | |||||||
| Age < 4 yr | Never | 1 | 4,511 | ||||
| Ever | 0.70 | 0.53–0.93 | |||||
| Age 4–7 yr | Low | 1 | 4,437 | ||||
| Medium | 0.87 | 0.68–1.12 | |||||
| High | 0.97 | 0.76–1.23 | |||||
| Age 0–7 yr | Lowest | 1 | 4,204 | ||||
| Highest | 0.58 | 0.35–0.98 | |||||
| Current eczema | |||||||
| Age < 4 yr | Never | 1 | 4,784 | 1 | 4,430 | ||
| Ever | 0.93 | 0.75–1.16 | 1.07 | 0.85–1.34 | |||
| Age 4–7 yr | Low | 1 | 4,448 | 1 | 4,181 | ||
| Medium | 0.95 | 0.76–1.20 | 0.88 | 0.69–1.12 | |||
| High | 1.12 | 0.91–1.38 | 0.99 | 0.79–1.23 | |||
| Age 0–7 yr | Lowest | 1 | 4,267 | 1 | 3,977 | ||
| Highest | 0.66 | 0.42–1.02 | 0.86 | 0.53–1.39 | |||
| Current hay fever | |||||||
| Age < 4 yr | Never | 1 | 4,726 | 1 | 3,828 | ||
| Ever | 1.16 | 0.85–1.56 | 1.05 | 0.81–1.36 | |||
| Age 4–7 yr | Low | 1 | 4,451 | 1 | 3,617 | ||
| Medium | 1.17 | 0.84–1.63 | 0.78 | 0.58–1.05 | |||
| High | 1.17 | 0.86–1.60 | 1.00 | 0.76–1.31 | |||
| Age 0–7 yr | Lowest | 1 | 4210 | 1 | 3,454 | ||
| Highest | 1.70 | 0.82–3.52 | 0.85 | 0.46–1.56 | |||
Definition of abbreviations: CI = confidence interval; OR = odds ratio.
N = 5,738. Current indicates symptoms in the last 12 mo.
Adjusting variables per model: Ever asthma: maternal education, maternal asthma, birth weight, maternal age, TV watching, atopy; Current asthma: maternal asthma, education, atopy; Wheezing: maternal asthma, social class, atopy; Asthma medication: maternal asthma, atopy, education, TV watching; Eczema: maternal allergy, atopy and sex. Hay fever: maternal hay fever, social class, atopy.
Bold indicates P < 0.05.
TABLE 4.
ASSOCIATION BETWEEN ASTHMA AND HIGHEST VERSUS LOWEST SWIMMING SCORE BETWEEN AGES 0 TO 7 YEARS IN THE OVERALL POPULATION AND BY PREVIOUS RESPIRATORY CONDITIONS
| Wheezing before 3.5 yr |
||||
|---|---|---|---|---|
| All Children | Yes (44.3%) | No (55.7%) | Interaction P Value | |
| Ever asthma at 7 yr | ||||
| OR* | 0.88 | 0.59 | 1.63 | 0.061 |
| 95% CI | 0.56–1.38 | 0.33–1.04 | 0.62–4.26 | |
| N | 4,193 | 1,806 | 2,416 | |
| Current asthma medication at 7 yr | ||||
| OR† | 0.58 | 0.35 | 1.88 | 0.036 |
| 95% CI | 0.35–0.98 | 0.18–0.67 | 0.51–6.85 | |
| N | 4,204 | 1,816 | 2,457 | |
| Current asthma at 7 yr | ||||
| OR† | 0.50 | 0.35 | 1.27 | 0.157 |
| 95% CI | 0.28–0.87 | 0.18–0.69 | 0.33–4.91 | |
| N | 4,243 | 1,815 | 2,468 | |
| Current asthma at 10 yr | ||||
| OR† | 0.61 | 0.39 | 0.57 | 0.802 |
| 95% CI | 0.32–1.15 | 0.19–0.81 | 0.21–1.58 | |
| N | 3,497 | 1,701 | 2,267 | |
| Ever Asthma at 7 yr |
||||
| All Children |
Yes (20.2%) |
No (79.8%) |
||
| Current asthma at 10 yr | ||||
| OR† | 0.61 | 0.34 | 0.94 | 0.213 |
| 95% CI | 0.32–1.15 | 0.14–0.80 | 0.32–2.73 | |
| N | 3,497 | 750 | 3,160 | |
For definition of abbreviations see Table 3.
N = 5,738.
Adjusted for atopy, maternal asthma, education, and age.
Adjusted for atopy, maternal asthma and education.
Bold indicates P < 0.05.
TABLE 5.
ASSOCIATION (ADJUSTED LINEAR REGRESSION COEFICIENT) BETWEEN SWIMMING AT DIFFERENT AGE PERIODS AND LUNG FUNCTION AND AIRWAY RESPONSIVENESS AT 8 YEARS, EXPRESSED AS STANDARD DEVIATION SCORES ADJUSTED FOR HEIGHT, AGE, AND SEX
| Swimming | Mean Difference | 95% CI | N | ||
|---|---|---|---|---|---|
| FVC | |||||
| Age < 4 yr | Never | 0 (Reference) | 4,395 | ||
| Ever | −0.05 | −0.13 | 0.03 | ||
| Age 4–7 yr | Low | 0 (Reference) | 4,060 | ||
| Medium | −0.01 | −0.09 | 0.08 | ||
| High | 0.08 | 0.00 | 0.16 | ||
| Age 0–7 yr | Lowest | 0 (Reference) | 3,831 | ||
| Highest | 0.05 | −0.13 | 0.23 | ||
| FEV1 | |||||
| Age < 4 yr | Never | 0 (Reference) | 4,318 | ||
| Ever | −0.03 | −0.11 | 0.05 | ||
| Age 4–7 yr | Low | 0 (Reference) | 3,997 | ||
| Medium | 0.05 | −0.04 | 0.13 | ||
| High | 0.10 | 0.02 | 0.18 | ||
| Age 0–7 yr | Lowest | 0 (Reference) | 3,771 | ||
| Highest | 0.14 | −0.05 | 0.32 | ||
| FEV1:FVC | |||||
| Age < 4 yr | Never | 0 (Reference) | 4,383 | ||
| Ever | 0.00 | −0.00 | 0.01 | ||
| Age 4–7 yr | Low | 0 (Reference) | 4,050 | ||
| Medium | 0.01 | 0.00 | 0.01 | ||
| High | 0.00 | −0.00 | 0.01 | ||
| Age 0–7 yr | Lowest | 0 (Reference) | 3,821 | ||
| Highest | 0.01 | −0.00 | 0.02 | ||
| FEF25–75 | |||||
| Age < 4 yr | Never | 0 (Reference) | 4,419 | ||
| Ever | 0.04 | −0.04 | 0.12 | ||
| Age 4–7 yr | Low | 0 (Reference) | 4,081 | ||
| Medium | 0.10 | 0.02 | 0.19 | ||
| High | 0.09 | 0.01 | 0.17 | ||
| Age 0–7 yr | Lowest | 0 (Reference) | 3,851 | ||
| Highest | 0.20 | 0.02 | 0.39 | ||
| Bronchial hyperresponsiveness* | |||||
| Age < 4 yr | Never | 0 (Reference) | 2,848 | ||
| Ever | 0.08 | −0.08 | 0.25 | ||
| Age 4–7 yr | Low | 0 (Reference) | 2,677 | ||
| Medium | −0.04 | −0.22 | 0.14 | ||
| High | 0.05 | −0.12 | 0.21 | ||
| Age 0–7 yr | Lowest | 0 (Reference) | 2,584 | ||
| Highest | 0.03 | −0.33 | 0.40 | ||
Definition of abbreviations: CI = confidence interval; FEF25–75 = forced midexpiratory flow.
N = 5,738. Adjusting variable per model: FVC: body mass index, birth weight; FEV1: body mass index, birth weight, environmental tobacco smoke, atopy; Ratio FEV1:FVC: body mass index, sex, atopy; FEF25–75: older siblings, birth weight, atopy; Bronchial hyperresponsiveness: sex, maternal asthma, maternal hay fever, atopy.
Mean of least squares dose–response slope (% decline in FEV1 per μmol methacholine).
Bold indicates P < 0.05.
Before adjusting the models for atopic status, we performed stratified analyses and confirmed that atopy was not an effect modifier (Table E4). Swimming did not increase the risk of any respiratory symptom among atopic children, as defined by the skin prick test. Among atopic children, those in the highest overall swimming category also had less current asthma at age 7 years compared with those in the lowest swimming category (OR = 0.41; 95% confidence interval, 0.17–0.99).
Finally, to detect possible reverse causation or health-related selection, we analyzed the association between wheezing before 3.5 years and swimming later on, as well as the association between symptoms at 7 years and the frequency of swimming at 8.6 years. There was no association with any swimming variable (Table E5), indicating that respiratory and allergic symptoms did not affect the probability of attending swimming pools later in life.
DISCUSSION
This large prospective birth cohort study indicated that reported swimming did not increase the risk of asthma, atopy, or any respiratory and allergic symptom in British children. On the contrary, swimming was associated with increased lung function and with a decreased prevalence of current asthma among children with previous respiratory conditions. In addition, no evidence of reverse causation was detected.
The results of this study are in accordance with the previous cross-sectional studies performed outside Belgium, where no significant positive association between pool attendance and ever having asthma (11, 12, 14) or hay fever (11, 12) was reported. In Germany, swimming also did not increase the risk of eczema (11, 12) although it did in Spain (14). Differences in the results of asthma risk between studies conducted in Belgium or in other countries may reflect true differences or may relate to methodological aspects. There are several possible explanations for real different effects among areas. First, different patterns of swimming pool attendance in children resulting in different cumulative exposures. Second, there may be differences in the level of trichloramine or other irritants in the swimming pools. Third, uncontrolled confounding variables (e.g., physical activity) may be different. Finally, there may be differences in the presence and extent of reverse causation (i.e., children with asthma attending or avoid swimming pools). In this study, the prevalence of swimming was very high and an extreme exposure category was created, and an undetected real effect is unlikely. Regarding methodological differences, the studies with negative results (11, 12, 14) are based on large and population-based samples, whereas the studies with positive results are not (6–9).
This is the first longitudinal study with prospectively collected data on the association between swimming pool attendance and childhood asthma. The use of questionnaires not originally designed to answer the specific research question under study led to potential exposure misclassification and absence of data on confounders, such as physical activity. The data on swimming during the first years of life were obtained indirectly through open questions and therefore pool attendance before 4 years of age has likely been underestimated. The effect on the results is difficult to foresee, because we ignore whether the exposure misclassification had been differential or nondifferential. However, a global interpretation of results shows a consistent pattern for different exposure periods, suggesting that findings for the earliest period are not spurious. The questionnaires referred to swimming instead of swimming pool attendance, but given the weather characteristics in the United Kingdom, it is reasonable to assume that answers refer mainly to indoor swimming pool attendance. Although there are no empirical data to confirm this, the majority of swimming pools in the area have probably been chlorinated during the study period, according to local authorities and the World Health Organization data showing that chlorine is the most common disinfectant used in swimming pools (2). The lack of quantitative data on irritants in the swimming pools is a drawback that prevents the evaluation of dose–responses and makes the comparison with other study settings difficult. Selection bias in the initial sample may affect the external validity of our findings. There was a considerable loss to follow-up, which, as in most cohort studies, was greater in children from less advantaged backgrounds, probably leading to an overestimation of the swimming prevalence (Table E6). However, because socioeconomic status was not an effect modifier, the validity of results in the analyzed data set was not compromised. Data on other environmental exposures that could affect respiratory health (e.g., air pollution) were not available to check for effect modification or confounding. On the other hand, the available information on the health outcomes was very accurate, with clinical measurements and validated questions on asthma and allergic symptoms at 7 and at 10 years. The strengths of using data from a population-based and longitudinal study are relevant. The prospective nature of the data collection reduced the probability of recall bias. It also allowed us to look for the first time at temporal relationships between swimming and allergic and respiratory symptoms at different points in time during childhood, showing that in this population there was no evidence of reverse causation. Along with the prospective design, the large sample size was an advantage that allowed us to analyze associations in subgroups of children with different previous respiratory conditions.
Similar to a previous study in Spain (14), an inverse association between swimming and asthma symptoms, but not with ever asthma, was found in the overall population of this British cohort. This appeared to be driven by the subgroup of children with asthma or early wheezing children. Because we could only use TV watching as a proxy for sedentarism, we cannot disentangle whether this protective effect is caused by swimming per se or by other physical activities related to a more active and healthier lifestyle. Recent studies are providing evidence that people with asthma may benefit from swimming training as reflected in the clinical measures of disease severity (1, 25, 26). To our knowledge, this is the first longitudinal and population-based study showing that swimming is associated with fewer asthma symptoms among children with asthma, after discarding a “healthy-swimmer effect.” Although physical fitness in childhood may prevent asthma development in young adulthood (27), our data do not seem to indicate that swimming can prevent asthma development in children. If our results are further confirmed, swimming would not only be a safe sport for people with asthma (26) but also may help control asthma symptoms. These results cannot be extrapolated to swimming pool workers and elite swimmers, which are populations at risk of developing adverse respiratory outcomes deserving further research.
In conclusion, this first large longitudinal study suggests that swimming was not associated with ever asthma or atopy in British children. Swimming was associated with increased lung function and with lower prevalence of asthma symptoms, especially among children with preexisting respiratory conditions. Findings indicate no reverse causation, but confounding by concurrent physical activity or selection bias cannot be ruled out. More large studies with improved exposure assessment, especially during the first years of life, conducted in different settings are required to confirm these results, because they entail important public health implications.
Supplementary Material
Acknowledgments
The authors thank the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Supported by UK Medical Research Council grant 74882, Wellcome Trust grant 076467, and the University of Bristol, providing core support for ALSPAC; a predoctoral fellowship from the Spanish Health Ministry FI06/00651 (L.F.-B.); and contracts funded by the Instituto de Salud Carlos III, Spanish Ministry of Health and Consumption CP06/00341 (C.M.V.) and 01/3058 (J.-P.Z.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201005-0761OC on October 1, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Goodman M, Hays S. Asthma and swimming: a meta-analysis. J Asthma 2008;45:639–647. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Guidelines for safe recreational water environments [accessed 12 November 2010]. Volume 2. Swimming pools and similar environments. World Health Organization, Geneva, 2006. Available from: http://www.who.int/water_sanitation_health/bathing/srwe2full.pdf
- 3.Bernard A. Chlorination products: emerging links with allergic diseases. Curr Med Chem 2007;14:1771–1782. [DOI] [PubMed] [Google Scholar]
- 4.Massin N, Bohadana AB, Wild P, Hery M, Toamain JP, Hubert G. Respiratory symptoms and bronchial responsiveness in lifeguards exposed to nitrogen trichloride in indoor swimming pools. Occup Environ Med 1998;55:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levesque B, Duchesne JF, Gingras S, Lavoie R, Prud'Homme D, Bernard E, Boulet LP, Ernst P. The determinants of prevalence of health complaints among young competitive swimmers. Int Arch Occup Environ Health 2006;80:32–39. [DOI] [PubMed] [Google Scholar]
- 6.Bernard A, Carbonnelle S, de Burbure C, Michel O, Nickmilder M. Chlorinated pool attendance, atopy, and the risk of asthma during childhood. Environ Health Perspect 2006;114:1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernard A, Carbonnelle S, Dumont X, Nickmilder M. Infant swimming practice, pulmonary epithelium integrity, and the risk of allergic and respiratory diseases later in childhood. Pediatrics 2007;119:1095–1103. [DOI] [PubMed] [Google Scholar]
- 8.Bernard A, Nickmilder M, Voisin C. Outdoor swimming pools and the risks of asthma and allergies during adolescence. Eur Respir J 2008;32:979–988. [DOI] [PubMed] [Google Scholar]
- 9.Bernard A, Nickmilder M, Voisin C, Sardella A. Impact of chlorinated swimming pool attendance on the respiratory health of adolescents. Pediatrics 2009;124:1110–1118. [DOI] [PubMed] [Google Scholar]
- 10.Cotter A, Ryan CA. The pool chlorine hypothesis and asthma among boys. Ir Med J 2009;102:79–82. [PubMed] [Google Scholar]
- 11.Kohlhammer Y, Doring A, Schafer T, Wichmann HE, Heinrich J. Swimming pool attendance and hay fever rates later in life. Allergy 2006;61:1305–1309. [DOI] [PubMed] [Google Scholar]
- 12.Schoefer Y, Zutavern A, Brockow I, Schafer T, Kramer U, Schaaf B, Herbarth O, von Berg A, Wichmann HE, Heinrich J. Health risks of early swimming pool attendance. Int J Hyg Environ Health 2008;211:367–373. [DOI] [PubMed] [Google Scholar]
- 13.Carraro S, Pasquale MF, Da Fre M, Rusconi F, Bonetto G, Zanconato S, Baraldi E. Swimming pool attendance and exhaled nitric oxide in children. J Allergy Clin Immunol 2006;118:958–960. [DOI] [PubMed] [Google Scholar]
- 14.Font-Ribera L, Kogevinas M, Zock JP, Nieuwenhuijsen MJ, Heederik D, Villanueva CM. Swimming pool attendance and risk of asthma and allergic symptoms in children. Eur Respir J 2009;34:1304–1310. [DOI] [PubMed] [Google Scholar]
- 15.Spivey A. Widening the pool of factors: studies needed to assess asthma-swimming link. Environ Health Perspect 2009;117:A162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uyan ZS, Carraro S, Piacentini G, Baraldi E. Swimming pool, respiratory health, and childhood asthma: should we change our beliefs? Pediatr Pulmonol 2009;44:31–37. [DOI] [PubMed] [Google Scholar]
- 17.Weisel CP, Richardson SD, Nemery B, Aggazzotti G, Baraldi E, Blatchley ER III, Blount BC, Carlsen KH, Eggleston PA, Frimmel FH, et al. Childhood asthma and environmental exposures at swimming pools: state of the science and research recommendations. Environ Health Perspect 2009;117:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connell EJ. The burden of atopy and asthma in children. Allergy 2004;59:7–11. [DOI] [PubMed] [Google Scholar]
- 19.Vaz de Almeida MD, Graça P, Afonso C, D'Amicis A, Lappalainen R, Damkjaer S. Physical activity levels and body weight in a nationally representative sample in the European Union. Public Health Nutr 1999;2:105–113. [DOI] [PubMed] [Google Scholar]
- 20.Hardy LR, Harrell JS, Bell RA. Overweight in children: definitions, measurements, confounding factors, and health consequences. J Pediatr Nurs 2004;19:376–384. [DOI] [PubMed] [Google Scholar]
- 21.Maitra A, Sherriff A, Northstone K, Strachan D, Henderson AJ. Maternal age of menarche is not associated with asthma or atopy in prepubertal children. Thorax 2005;60:810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golding J, Pembrey M, Jones R. ALSPAC–the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol 2001;15:74–87. [DOI] [PubMed] [Google Scholar]
- 23.Chinn S, Rona RJ. Height and age adjustment for cross sectional studies of lung function in children aged 6–11 years. Thorax 1992;47:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan K, Salome C, Woolcock AJ. Rapid method for measurement of bronchial responsiveness. Thorax 1983;38:760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JS, Hung WP. The effects of a swimming intervention for children with asthma. Respirology 2009;14:838–842. [DOI] [PubMed] [Google Scholar]
- 26.Weisgerber M, Webber K, Meurer J, Danduran M, Berger S, Flores G. Moderate and vigorous exercise programs in children with asthma: safety, parental satisfaction, and asthma outcomes. Pediatr Pulmonol 2008;43:1175–1182. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen F, Lambrechtsen J, Siersted HC, Hansen HS, Hansen NC. Low physical fitness in childhood is associated with the development of asthma in young adulthood: the Odense schoolchild study. Eur Respir J 2000;16:866–870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.