Abstract
Rationale: Sex hormones have effects on the left ventricle, but hormonal influences on the right ventricle (RV) are unknown.
Objectives: We hypothesized that sex hormones would be associated with RV morphology in a large cohort free of cardiovascular disease.
Methods: Sex hormones were measured by immunoassay and RV ejection fraction (RVEF), stroke volume (RVSV), mass, end-diastolic volume, and end-systolic volume (RVESV) were measured by cardiac magnetic resonance imaging in 1,957 men and 1,738 postmenopausal women. The relationship between each hormone and RV parameter was assessed by multivariate linear regression.
Measurements and Main Results: Higher estradiol levels were associated with higher RVEF (β per 1 ln[nmol/L], 0.88; 95% confidence interval [CI], 0.32 to 1.43; P = 0.002) and lower RVESV (β per 1 ln[nmol/L], −0.87; 95% CI, −1.67 to −0.08; P = 0.03) in women using hormone therapy. In men, higher bioavailable testosterone levels were associated with higher RVSV (β per 1 ln[nmol/L], 1.97; 95% CI, 0.20 to 3.73; P = 0.03) and greater RV mass and volumes (P ≤ 0.01). Higher dehydroepiandrosterone levels were associated with higher RVSV (β per 1 ln[nmol/L], 1.37; 95% CI, 0.15 to 2.59; P = 0.03) and greater RV mass (β per 1 ln[nmol/L], 0.25; 95% CI, 0.00 to 0.49; P = 0.05) and volumes (P ≤ 0.001) in women.
Conclusions: Higher estradiol levels were associated with better RV systolic function in women using hormone therapy. Higher levels of androgens were associated with greater RV mass and volumes in both sexes.
Keywords: sex, sex hormones, right ventricle
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Female sex is a risk factor for the development of pulmonary hypertension (PH), yet women appear to have better right ventricular (RV) function and improved survival compared to men with PH. Whereas sex hormones have been well studied in left heart failure, the role of sex hormones in RV function is largely unknown.
What This Study Adds to the Field
We have shown that higher levels of estradiol are associated with better RV systolic function in postmenopausal women using hormone therapy. Higher levels of androgens (both testosterone and dehydroepiandrosterone) are associated with greater RV mass and larger RV volumes in men and postmenopausal women, respectively, in cardiovascular disease–free participants.
Sex differences in atherosclerosis and congestive heart failure (CHF) have been well described, with women experiencing a significant lag in the onset of coronary artery disease and lower rates of CHF compared with men (1). Estrogen, testosterone, dehydroepiandrosterone (DHEA), and sex hormone–binding globulin (SHBG) have important, albeit controversial, roles in left ventricular (LV) function and systemic vascular disease, whereas the effects of sex hormones on right ventricular (RV) and pulmonary vascular function are unknown.
Estrogen has protective effects on the pulmonary vasculature and improves RV contractility in animals (2–4). Testosterone has been shown to be a pulmonary vasodilator, yet has unclear effects on pulmonary endothelium (5, 6). Treatment with DHEA prevents pulmonary vascular changes and RV hypertrophy, and prolongs survival in animal models of pulmonary hypertension (PH) (7, 8). SHBG is expressed in myocytes of failing human hearts and in murine fetal lung epithelium, but its role in pulmonary vascular function has not been studied (9, 10).
Despite the beneficial effects of estrogen on the pulmonary vasculature, female sex is the best established clinical risk factor for idiopathic pulmonary arterial hypertension (PAH) (11). Yet, women have higher RV ejection fraction (RVEF) and improved survival compared with men with PAH (12–14). In systemic cardiovascular disease, an individual's estrogen:testosterone balance may be more predictive of disease risk than either hormone alone (15, 16). Last, it is unknown how sex hormones affect the interaction of the RV with the pulmonary vasculature, particularly in individuals with no known (or subclinical) PH.
We examined the cross-sectional association of serum estradiol (E2), bioavailable testosterone (bioT), DHEA, SHBG, and the E2:testosterone ratio (E2:T) with RV structure and function assessed by cardiac magnetic resonance imaging (MRI) in a large cohort of men and postmenopausal women without clinical cardiovascular disease. We hypothesized that higher E2, DHEA, and E2:T and lower testosterone and SHBG levels would be associated with higher RVEF and RV stroke volume (RVSV) and lower RV mass, RV end-diastolic volume (RVEDV), and RV end-systolic volume (RVESV). Preliminary results from this study have been published in abstract form (17).
METHODS
Study Sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study to investigate the prevalence, correlates, and progression of subclinical cardiovascular disease in white, African-American, Hispanic, and Chinese subjects (18). In 2000–2002, MESA recruited 6,814 subjects aged 45–84 years from six U.S. communities: Forsyth County, North Carolina; Northern Manhattan and the Bronx, New York; Baltimore City and Baltimore County, Maryland; St. Paul, Minnesota; Chicago, Illinois; and Los Angeles, California. Exclusion criteria included clinical cardiovascular disease, weight greater than 300 lb, pregnancy, or impediment to long-term participation. The presence of clinical cardiovascular disease was determined at participant screening by questionnaire. Participants were excluded if they answered “yes” to having been diagnosed by a physician with heart attack, stroke, transient ischemic attack, heart failure, angina, current atrial fibrillation, and/or to having undergone any prior cardiovascular procedure. The protocols of MESA and studies described herein were approved by the institutional review boards of all collaborating institutions and the National Heart, Lung, and Blood Institute. The MESA-Right Ventricle Study measured RV morphology in participants eligible for MRI (without metal implants, device or fragment). We included all subjects from MESA-RV with interpretable cardiac MRIs and available sex hormone levels.
Cardiac Magnetic Resonance Imaging Measures
The cardiac MRI protocol has been described elsewhere (19). All imaging was performed on 1.5-T magnets with a four-element phased-array surface coil positioned anteriorly and posteriorly and electrocardiographic gating. Imaging consisted of fast gradient echo cine images with temporal resolution not greater than 50 milliseconds.
Methods for interpretation of LV and RV parameters have been previously reported (19, 20). Briefly, RV image analysis was performed by two independent analysts on Windows workstations using QMASS software (v4.2; Medis, The Netherlands). The endocardial and epicardial borders of the RV were traced manually on short-axis cine images at the end-diastolic and end-systolic phases. Papillary muscles and trabeculae were included in the RV volumes and excluded from RV mass (21). RVEDV and RVESV were calculated using Simpson's rule by summation of areas on each slice multiplied by the sum of slice thickness and image gap. RV mass was determined at the end-diastolic phase as the difference between end-diastolic epicardial and endocardial volumes multiplied by the specific gravity of the heart (1.05 g/cm3) (19). RVSV was calculated by subtracting RVESV from RVEDV. RVEF was calculated by dividing RVSV by RVEDV. The intrareader intraclass correlation coefficient (ICC) from random, blinded rereads of 229 scans for RV mass was 0.94 and for 230 scans was 0.99, 0.95, and 0.89 for RVEDV, RVESV, and RVEF, respectively. The intrareader ICC was 0.96 for RVSV. The interreader ICC from random, blinded rereads of 240 scans for RV mass, RVEDV, RVESV, and RVEF was 0.89, 0.96, 0.94, and 0.80, respectively. The interreader ICC for RVSV was 0.93.
Serum Sex Hormones
Fasting morning blood samples were drawn and stored using standardized procedures (22). Serum hormone concentrations (nmol/L) were measured in the Steroid Hormone Laboratory at the University of Massachusetts Medical Center (Worcester, MA). E2 was measured via an ultrasensitive radioimmunoassay kit (Diagnostic System Laboratories, Webster, TX). Total testosterone (total T) and DHEA were measured directly with radioimmunoassay kits and SHBG was measured by chemiluminescence enzyme immunometric assay using Immulite kits (Diagnostic Products Corporation, Los Angeles, CA). BioT was calculated from total T and SHBG, using the method described by Södergård (23). Assay quality control has been described elsewhere (24). The intraassay coefficients of variation for E2, total T, DHEA, and SHBG were 10.5, 12.3, 11.2, and 9.0%, respectively.
Other Covariates
Race/ethnicity was self-reported during the baseline examination according to year 2000 U.S. Census criteria as race (white, African-American, etc.) and ethnicity (Hispanic or non-Hispanic). Standard questionnaires were used to ascertain smoking status and level of education. Medication use, including current postmenopausal hormone therapy (HT), testosterone therapy, and DHEA supplement use, was ascertained by medication inventory (25). Self-report was used to determine pre-, peri-, or postmenopausal status. Intentional exercise (MET-min/wk) was assessed by survey. Height was measured to the nearest 0.1 cm with the participant in stocking feet and weight was measured to the nearest pound with the participant in light clothing, using a balanced scale. Resting blood pressure was measured with a Dinamap Monitor PRO 100 (Critikon, Tampa, FL) automated oscillometric device. Hypertension was defined as systolic blood pressure equal to or greater than 140 mm Hg, diastolic blood pressure equal to or greater than 90 mm Hg, or current use of antihypertension medication. Fasting blood samples were drawn and sent to a central laboratory for measurement of glucose and lipids. Presence of diabetes mellitus was based on self-reported physician diagnosis or a fasting glucose value equal to or greater than 126 mg/dl, the latter measured by rate reflectance spectrophotometry (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY). Fasting glucose of 100–125 mg/dl was considered impaired fasting glucose. Spirometry, urine cotinine, and computed tomographic (CT) lung density (percentage of emphysema-like lung) measures were available for 2,406 participants (26, 27).
Statistical Analysis
Continuous variables were expressed as means and standard deviations. Categorical variables were expressed as percentages. Sex hormones were logarithmically transformed, except in the case of E2:T.
Multivariate linear regression was used to assess the relationship of each hormone with each RV parameter. Initial models included age, race/ethnicity, height, weight, waist circumference, and current hormone supplementation. Adjustment for height and weight avoided the assumptions made in indexing the RV measures to certain parameters of body size (e.g., body surface area), while accounting for differences in body size between participants. Models were further adjusted for smoking, diabetes mellitus, impaired glucose tolerance, hypertension and use of antihypertensive medications, systolic and diastolic blood pressure, cholesterol, high-density lipoprotein levels, statin use, intentional exercise, education level, and respective LV parameters (e.g., the model for RVEF was adjusted for LV ejection fraction, the model for RV mass for LV mass, and so forth). Adjustment for LV parameters was performed to account for the contribution of LV abnormalities to RV changes (e.g., increased LV mass causing pulmonary venous hypertension leading to increased RV mass), to account for body size differences, and to examine RV-specific associations. RVSV was not adjusted for LV stroke volume, considering the significant interdependence of these measures.
We performed adjustments for lung function in the subgroup with available lung function measures (n = 2,406). Statistical significance was defined as P < 0.05. As each hormone analysis was considered an independent hypothesis, there was no correction made for multiple comparisons (28). Analyses were performed with STATA 10.0 (StataCorp, College Station, TX).
RESULTS
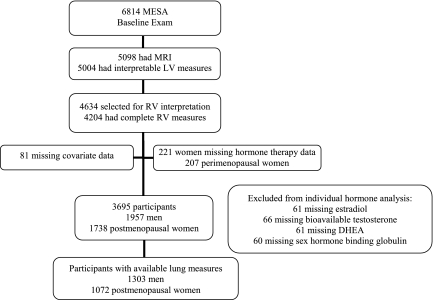
MESA enrolled 6,814 participants of whom 5,098 had cardiac MRIs and 5,004 had interpretable scans (Figure 1). Of these, 4,634 were selected for RV interpretation, and 4,204 had RV measures completed. We excluded women missing HT data (n = 221), those who reported pre- (n = 146), unknown (n = 60), or missing (n = 1) menopausal status, and participants with missing data for covariates (n = 81). The final study sample of 3,695 participants included 1,957 men and 1,738 postmenopausal women, of whom 61 were missing E2 levels, 66 were missing bioT levels, 61 were missing DHEA levels, and 60 were missing SHBG levels.
Figure 1.
Study sample. DHEA = dehydroepiandrosterone; LV = left ventricle; MESA = Multi-Ethnic Study of Atherosclerosis; MRI = magnetic resonance imaging; RV = right ventricle.
Participant characteristics are shown in Table 1 and Table E1 in the online supplement. One-third of women reported current HT use. Women tended to have higher RVEF (72.6 ± 6.0 vs. 68.2 ± 6.2%) and lower RVSV (77.5 ± 16.3 vs. 95.9 ± 20.7 ml), RV mass (18.9 ± 3.6 vs. 23.1 ± 4.4 g), RVEDV (107.2 ± 22.5 vs. 140.9 ± 29.7 ml), and RVESV (29.7 ± 10.0 vs. 45.1 ± 14.1 ml) than men.
TABLE 1.
PARTICIPANT CHARACTERISTICS
| Sex |
||
|---|---|---|
| Men | Women | |
| Number of participants | 1,957 | 1,738 |
| Demographics | ||
| Age, yr | 61.5 ± 10.1 | 64.0 ± 9.1 |
| Race/ethnicity, % | ||
| White | 38.6 | 40.2 |
| African-American | 25.2 | 26.2 |
| Hispanic | 23.5 | 21.1 |
| Chinese | 12.8 | 12.4 |
| Education, % | ||
| <High school | 15.5 | 18.9 |
| High school | 15.7 | 22.0 |
| <College (>high school) | 26.0 | 30.3 |
| ≥College | 42.8 | 28.8 |
| Anthropometrics | ||
| Height, cm | 173.4 ± 7.7 | 159.7 ± 7.1 |
| Weight, kg | 83.0 ± 14.8 | 71.8 ± 15.3 |
| Body mass index, kg/m2 | 27.5 ± 4.1 | 28.1 ± 5.5 |
| Waist circumference, cm | 98.2 ± 11.4 | 96.1 ± 14.5 |
| Comorbid factors | ||
| Hypertension, % | 40.9 | 49.8 |
| Systolic blood pressure, mm Hg | 125.1 ± 18.9 | 128.3 ± 23.1 |
| Diastolic blood pressure, mm Hg | 74.9 ± 9.3 | 69.3 ± 10.4 |
| Diabetes mellitus, % | ||
| Normal | 71.6 | 76.6 |
| Impaired fasting glucose | 15.7 | 12.0 |
| Untreated diabetes | 2.9 | 2.2 |
| Treated diabetes | 9.8 | 9.2 |
| Total cholesterol, mg/dl | 188.1 ± 33.8 | 202.1 ± 35.2 |
| High-density lipoprotein, mg/dl | 45.0 ± 11.5 | 57.1 ± 15.6 |
| Statin use, % | 13.8 | 17.6 |
| Smoking status | ||
| Never-smoker, % | 42.6 | 60.8 |
| Former smoker, % | 43.4 | 28.7 |
| Current smoker, % | 14.1 | 10.6 |
| Pack-years, among ever-smokers | 15.4 ± 26.7 | 13.1 ± 21.9 |
| Serum sex hormone levels | ||
| Estradiol, nmol/L | 0.1 ± 0.0 | 0.1 ± 0.2 |
| Hormone therapy users | 0.3 ± 0.2 | |
| Hormone therapy nonusers | 0.1 ± 0.1 | |
| Bioavailable testosterone, nmol/L | 5.5 ± 2.1 | 0.3 ± 0.3 |
| DHEA, nmol/L | 14.2 ± 7.3 | 11.6 ± 6.4 |
| Sex hormone–binding globulin, nmol/L | 43.9 ± 18.7 | 77.6 ± 55.2 |
| Estradiol:testosterone ratio | 0.01 ± 0.03 | 0.23 ± 0.63 |
| Hormone therapy user | 0.50 ± 1.01 | |
| Hormone therapy nonuser | 0.10 ± 0.16 | |
| Hormone supplementation | ||
| Hormone therapy, % | 0.0 | 33.1 |
| Testosterone compounds, % | 0.7 | 0.0 |
| DHEA supplement, % | 0.01 | 0.01 |
| RV measures | ||
| RVEF, % | 68.2 ± 6.2 | 72.6 ± 6.0 |
| RVSV, ml | 95.9 ± 20.7 | 77.5 ± 16.3 |
| RV mass, g | 23.1 ± 4.4 | 18.9 ± 3.6 |
| RVEDV, ml | 140.9 ± 29.7 | 107.2 ± 22.5 |
| RVESV, ml | 45.1 ± 14.1 | 29.7 ± 10.0 |
Definition of abbreviations: DHEA = dehydroepiandrosterone; RV = right ventricle; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction; RVESV = right ventricular end-systolic volume; RVSV = right ventricular stroke volume.
Data are shown as mean ± standard deviation or as a percentage.
Estradiol
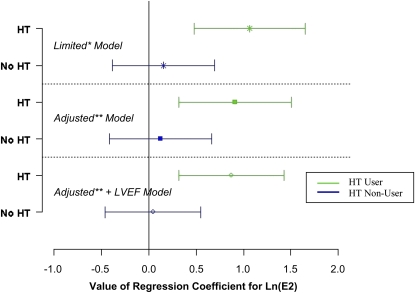
Among women, there were significant or borderline significant interactions between E2 and HT for several RV measures (RVEF, P = 0.02; RV mass, P = 0.09; RVESV, P = 0.10); the E2 analyses were therefore stratified by HT use. Higher levels of E2 were associated with higher RVEF in women using HT (Figure 2 and Table 2). This association persisted in the fully adjusted model and after adjustment for LVEF, suggesting that exogenous E2 was associated with RV systolic function independent of any effects on the LV. There was a 2% difference in RVEF across quintiles of E2 levels (data not shown). There was no association between E2 and RVEF in HT nonusers or in men (Table 2).
Figure 2.
Natural log–transformed estradiol [ln(E2)] parameter estimates with 95% confidence intervals for right ventricular ejection fraction (RVEF) stratified by hormone therapy use in limited and adjusted regression models (women only). *Adjusted for age, race/ethnicity, height, weight, waist circumference. **Adjusted for age, race/ethnicity, height, weight, waist circumference, smoking (status and pack-years), diabetes mellitus, impaired glucose intolerance, hypertension, use of antihypertensive medications, systolic and diastolic blood pressure, cholesterol, high density lipoprotein levels, statin use, intentional exercise, and level of education. HT = hormone therapy; LV = left ventricle.
TABLE 2.
ASSOCIATIONS BETWEEN ESTRADIOL AND RIGHT VENTRICULAR MEASURES IN LIMITED AND ADJUSTED MODELS AMONG MEN AND WOMEN, BASED ON HORMONE THERAPY USE
| Men (n = 1,927) |
Women: HT Users (n = 567) |
Women: HT Nonusers (n = 1,140) |
||||
|---|---|---|---|---|---|---|
| β (95% CI)* | P Value | β (95% CI) | P Value | β (95% CI) | P Value | |
| RVEF, % | ||||||
| Limited† | −0.23 (−0.97 to 0.50) | 0.54 | 1.07 (0.49 to 1.65) | < 0.001 | 0.15 (−0.39 to 0.69) | 0.58 |
| Adjusted‡ | −0.26 (−1.00 to 0.48) | 0.49 | 0.91 (0.32 to 1.50) | 0.003 | 0.13 (−0.41 to 0.67) | 0.65 |
| Adjusted + LVEF | −0.35 (−1.02 to 0.32) | 0.30 | 0.88 (0.32 to 1.43) | 0.002 | 0.05 (−0.46 to 0.55) | 0.86 |
| RVSV, ml | ||||||
| Limited | −1.72 (−3.92 to 0.47) | 0.12 | 1.04 (−0.29 to 2.37) | 0.13 | −0.31 (−1.53 to 0.91) | 0.61 |
| Adjusted | −1.25 (−3.43 to 0.93) | 0.26 | 0.57 (−0.76 to 1.90) | 0.40 | −0.29 (−1.50 to 0.93) | 0.40 |
| RV mass, g | ||||||
| Limited | −0.16 (−0.60 to 0.29) | 0.49 | 0.08 (−0.20 to 0.37) | 0.57 | −0.25 (−0.51 to 0.01) | 0.06 |
| Adjusted | −0.08 (−0.53 to 0.37) | 0.72 | 0.02 (−0.27 to 0.30) | 0.90 | −0.24 (−0.50 to 0.02) | 0.07 |
| Adjusted + LV mass | −0.02 (−0.44 to 0.39) | 0.91 | 0.11 (−0.16 to 0.38) | 0.42 | −0.20 (−0.44 to 0.05) | 0.12 |
| RVEDV, ml | ||||||
| Limited | −2.21 (−5.20 to 0.76) | 0.15 | −0.08 (−1.83 to 1.67) | 0.93 | −0.71 (−2.31 to 0.89) | 0.38 |
| Adjusted | −1.47 (−4.44 to 1.50) | 0.33 | −0.50 (−2.26 to 1.25) | 0.57 | −0.64 (−2.24 to 0.96) | 0.43 |
| Adjusted + LVEDV | 1.17 (−1.03 to 3.38) | 0.30 | 0.17 (−1.13 to 1.47) | 0.80 | 0.57 (−0.62 to 1.76) | 0.35 |
| RVESV, ml | ||||||
| Limited | −0.49 (−1.99 to 1.01) | 0.52 | −1.12 (−1.99 to −0.26) | 0.01 | −0.40 (−1.19 to 0.39) | 0.32 |
| Adjusted | −0.22 (−1.73 to 1.29) | 0.77 | −1.07 (−1.94 to −0.21) | 0.02 | −0.36 (−1.15 to 0.44) | 0.38 |
| Adjusted + LVESV | 0.50 (−0.84 to 1.83) | 0.47 | −0.87 (−1.67 to −0.08) | 0.03 | 0.02 (−0.71 to 0.75) | 0.95 |
Definition of abbreviations: CI = confidence interval; HT = hormone therapy; LV = left ventricle; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; RV = right ventricle; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction; RVESV = right ventricular end-systolic volume; RVSV = right ventricular stroke volume.
Per ln(nmol/L) increase in estradiol.
Adjusted for age, race/ethnicity, height, weight, waist circumference.
Adjusted for age, race/ethnicity, height, weight, waist circumference, smoking (status and pack-years), diabetes mellitus, impaired glucose tolerance, hypertension, use of antihypertensive medications, systolic and diastolic blood pressure, cholesterol, high density lipoprotein levels, statin use, intentional exercise, and level of education.
Similarly, a higher E2 level was associated with lower RVESV in HT users, corresponding to a 6% difference across quintiles of E2 levels (data not shown), but not in HT nonusers or in men. This association persisted after adjustment for LVESV (Table 2). In the subset of women with available data (n = 1,072), adjustment for lung function variables did not affect the results (Table E2).
Bioavailable Testosterone
Higher levels of bioT were associated with larger RVSV, greater RV mass, and larger RVEDV and RVESV in men but not in women (Table 3). There was a 4% difference in RVSV, a 3% difference in RV mass, and a 1 and 3% difference in RVEDV and RVESV, respectively, across quintiles of bioT levels (data not shown). These associations persisted after adjustment for respective LV measures (Table 3) and after adjustment for lung function (n = 1,303) (Table E3). Similar associations were seen between total T and RVSV, RV mass, and RV volumes, and results were unchanged when participants taking testosterone supplementation (n = 27) were excluded from analysis (data not shown).
TABLE 3.
ASSOCIATIONS BETWEEN BIOAVAILABLE TESTOSTERONE AND RIGHT VENTRICULAR MEASURES IN LIMITED AND ADJUSTED MODELS, BY SEX
| Men (n = 1,925) |
Women (n = 1,696) |
|||
|---|---|---|---|---|
| β (95% CI)* | P Value | β (95% CI) | P Value | |
| RVEF, % | ||||
| Limited† | −0.37 (−0.97 to 0.23) | 0.23 | −0.15 (−0.51 to 0.20) | 0.40 |
| Adjusted‡ | −0.36 (−0.95 to 0.24) | 0.25 | −0.01 (−0.38 to 0.36) | 0.97 |
| Adjusted + LVEF | −0.40 (−0.94 to 0.14) | 0.15 | 0.02 (−0.33 to 0.37) | 0.92 |
| RVSV, ml | ||||
| Limited | 1.42 (−0.37 to 3.21) | 0.12 | −0.66 (−1.47 to 0.14) | 0.11 |
| Adjusted | 1.97 (0.20 to 3.73) | 0.03 | −0.04 (−0.88 to 0.79) | 0.92 |
| RV mass, g | ||||
| Limited | 0.40 (0.03 to 0.76) | 0.03 | −0.03 (−0.20 to 0.15) | 0.77 |
| Adjusted | 0.49 (0.13 to 0.85) | 0.01 | 0.08 (−0.10 to 0.26) | 0.38 |
| Adjusted + LV mass | 0.44 (0.10 to 0.77) | 0.01 | 0.04 (−0.13 to 0.21) | 0.66 |
| RVEDV, ml | ||||
| Limited | 2.90 (0.47 to 5.33) | 0.02 | −0.70 (−1.75 to 0.36) | 0.20 |
| Adjusted | 3.71 (1.31 to 6.11) | 0.001 | −0.05 (−1.15 to 1.06) | 0.93 |
| Adjusted + LVEDV | 2.43 (0.64 to 4.21) | 0.01 | 0.13 (−0.69 to 0.94) | 0.78 |
| RVESV, ml | ||||
| Limited | 1.48 (0.26 to 2.70) | 0.02 | −0.03 (−0.55 to 0.49) | 0.91 |
| Adjusted | 1.74 (0.53 to 2.96) | 0.01 | −0.01 (−0.55 to 0.54) | 0.98 |
| Adjusted + LVESV | 1.63 (0.55 to 2.71) | 0.001 | −0.01 (−0.51 to 0.49) | 0.96 |
Definition of abbreviations: CI = confidence interval; LV = left ventricle; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; RV = right ventricle; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction; RVESV = right ventricular end-systolic volume; RVSV = right ventricular stroke volume.
Per ln(nmol/L) increase in bioavailable testosterone.
Adjusted for age, race/ethnicity, height, weight, waist circumference, and testosterone supplementation (in men).
Adjusted for age, race/ethnicity, height, weight, waist circumference, testosterone supplementation (in men), smoking (status and pack-years), diabetes mellitus, impaired glucose tolerance, hypertension, use of antihypertensive medications, systolic and diastolic blood pressure, cholesterol, high density lipoprotein levels, statin use, intentional exercise, and level of education.
Dehydroepiandrosterone
In women, higher levels of DHEA were associated with decreased RVEF, greater RV mass, and larger volumes (Table 4). There was a 3% difference in RVEF, a 1% difference in RV mass, and 3 and 6% differences in RVEDV and RVESV, respectively, across quintiles of DHEA (data not shown). There appeared to be similar associations between DHEA and RVEF, RV mass, and RVESV in men, although these were not statistically significant. Similar associations were seen in the subset of participants with available measures of lung function (Table E4). Results were unchanged when participants taking DHEA supplementation (n = 36) were excluded and, among women, effect estimates were unchanged when adjusted for HT (data not shown).
TABLE 4.
ASSOCIATIONS BETWEEN DEHYDROEPIANDROSTERONE AND RIGHT VENTRICULAR MEASURES IN LIMITED AND ADJUSTED MODELS, BY SEX
| Men (n = 1,927) |
Women (n = 1,696) |
|||
|---|---|---|---|---|
| β (95% CI)* | P Value | β (95% CI) | P Value | |
| RVEF, % | ||||
| Limited† | −0.19 (−0.83 to 0.44) | 0.55 | −0.54 (−1.07 to −0.01) | 0.05 |
| Adjusted‡ | −0.19 (−0.82 to 0.45) | 0.56 | −0.54 (−1.08 to 0.00) | 0.05 |
| Adjusted + LVEF | −0.24 (−0.82 to 0.33) | 0.40 | −0.44 (−0.94 to 0.07) | 0.09 |
| RVSV, ml | ||||
| Limited | 0.16 (−1.73 to 2.05) | 0.87 | 0.77 (−0.42 to 1.97) | 0.21 |
| Adjusted | 0.55 (−1.32 to 2.42) | 0.56 | 1.37 (0.15 to 2.59) | 0.03 |
| RV mass, g | ||||
| Limited | 0.08 (−0.30 to 0.47) | 0.67 | 0.21 (−0.04 to 0.47) | 0.10 |
| Adjusted | 0.13 (−0.25 to 0.52) | 0.51 | 0.36 (0.10 to 0.62) | 0.01 |
| Adjusted + LV mass | 0.19 (−0.17 to 0.55) | 0.31 | 0.25 (0.00 to 0.49) | 0.05 |
| RVEDV, ml | ||||
| Limited | 0.87 (−1.69 to 3.43) | 0.51 | 2.00 (0.43 to 3.57) | 0.01 |
| Adjusted | 1.39 (−1.16 to 3.94) | 0.29 | 2.80 (1.20 to 4.40) | 0.001 |
| Adjusted + LVEDV | 1.30 (−0.59 to 3.19) | 0.18 | 2.28 (1.09 to 3.46) | <0.001 |
| RVESV, ml | ||||
| Limited | 0.71 (−0.57 to 2.00) | 0.28 | 1.23 (0.45 to 2.01) | 0.001 |
| Adjusted | 0.84 (−0.45 to 2.13) | 0.20 | 1.43 (0.63 to 2.22) | <0.001 |
| Adjusted + LVESV | 0.92 (−0.23 to 2.10) | 0.12 | 1.28 (0.55 to 2.00) | 0.001 |
Definition of abbreviations: CI = confidence interval; DHEA = dehydroepiandrosterone; LV = left ventricle; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; RV = right ventricle; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction; RVESV = right ventricular end-systolic volume; RVSV = right ventricular stroke volume.
Per ln(nmol/L) increase in DHEA.
Adjusted for age, race/ethnicity, height, weight, waist circumference, and DHEA supplementation.
Adjusted for age, race/ethnicity, height, weight, waist circumference, DHEA supplementation, smoking (status and pack-years), diabetes mellitus, impaired glucose tolerance, hypertension, use of antihypertensive medications, systolic and diastolic blood pressure, cholesterol, high density lipoprotein levels, statin use, intentional exercise, and level of education.
Sex Hormone–binding Globulin
Among women and possibly men, higher levels of SHBG were associated with higher RVSV in limited models, but these associations were attenuated and not statistically significant when fully adjusted for all covariates (Table 5). Similarly, among men there was an association between higher SHBG levels and greater RV mass (P = 0.05) that did not persist in multivariate analysis. Results were unchanged when adjusted for HT (in women) and testosterone supplementation (in men). In the smaller subgroup of participants with lung function measures, there were no significant associations seen in men or women but effect estimates were unchanged after adjustment for these measures (Table E5).
TABLE 5.
ASSOCIATIONS BETWEEN SEX HORMONE–BINDING GLOBULIN AND RIGHT VENTRICULAR MEASURES IN LIMITED AND ADJUSTED MODELS, BY SEX
| Men (n = 1,928) |
Women (n = 1,707) |
|||
|---|---|---|---|---|
| β (95% CI)* | P Value | β (95% CI) | P Value | |
| RVEF, % | ||||
| Limited† | 0.08 (−0.69 to 0.85) | 0.85 | 0.38 (−0.12 to 0.87) | 0.14 |
| Adjusted‡ | 0.12 (−0.68 to 0.91) | 0.78 | 0.20 (−0.34 to 0.75) | 0.47 |
| Adjusted + LVEF | 0.03 (−0.68 to 0.74) | 0.93 | 0.09 (−0.42 to 0.60) | 0.73 |
| RVSV, ml | ||||
| Limited | 1.96 (−0.34 to 4.25) | 0.09 | 1.16 (0.05 to 2.27) | 0.04 |
| Adjusted | 0.64 (−1.69 to 2.97) | 0.59 | 0.08 (−1.15 to 1.31) | 0.90 |
| RV mass, g | ||||
| Limited | 0.46 (−0.01 to 0.93) | 0.05 | 0.09 (−0.14 to 0.33) | 0.44 |
| Adjusted | 0.17 (−0.31 to 0.65) | 0.48 | −0.09 (−0.35 to 0.17) | 0.51 |
| Adjusted + LV mass | 0.16 (−0.28 to 0.61) | 0.47 | −0.03 (−0.28 to 0.21) | 0.78 |
| RVEDV, ml | ||||
| Limited | 2.90 (−0.20 to 6.01) | 0.07 | 1.03 (−0.43 to 2.50) | 0.17 |
| Adjusted | 0.78 (−2.40 to 3.95) | 0.63 | −0.18 (−1.80 to 1.44) | 0.83 |
| Adjusted + LVEDV | −0.13 (−2.47 to 2.22) | 0.92 | −0.42 (−1.62 to 0.77) | 0.49 |
| RVESV, ml | ||||
| Limited | 0.95 (−0.61 to 2.51) | 0.23 | −0.13 (−0.85 to 0.60) | 0.73 |
| Adjusted | 0.14 (−1.47 to 1.75) | 0.86 | −0.26 (−1.07 to 0.54) | 0.52 |
| Adjusted + LVESV | −0.07 (−1.49 to 1.36) | 0.93 | −0.09 (−0.82 to 0.65) | 0.82 |
Definition of abbreviations: CI = confidence interval; LV = left ventricle; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; RV = right ventricle; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction; RVESV = right ventricular end-systolic volume; RVSV = right ventricular stroke volume.
Per ln(nmol/L) increase in sex hormone–binding globulin.
Adjusted for age, race/ethnicity, height, weight, and waist circumference.
Adjusted for age, race/ethnicity, height, weight, waist circumference, smoking (status and pack-years), diabetes mellitus, impaired glucose tolerance, hypertension, use of antihypertensive medications, systolic and diastolic blood pressure, cholesterol, high density lipoprotein levels, statin use, intentional exercise, and level of education.
Estradiol:Testosterone Ratio
There was a suggestion of an association between higher E2:T and lower RV volumes in men only (Table E6). For example, a 1-unit increase in E2:T was associated with an 18.98-ml decrement in RVESV after multivariate adjustment (95% CI, –37.19 to −0.77 ml; P = 0.04), although this association was attenuated after adjustment for LVESV (−15.08; 95% CI, −31.22 to 1.07 ml; P = 0.07). Similar associations were seen in the models for RVEDV, although not significant, among men with available lung function measures (Table E7).
DISCUSSION
Serum sex hormones were associated with RV structure and function in an ethnically and racially diverse cohort without clinical cardiovascular disease. Higher levels of E2 were associated with higher RVEF and lower RVESV in postmenopausal women using HT (but not in HT nonusers or men), and higher testosterone levels were associated with greater RV mass and larger RV volumes in men (but not in women). Contrary to our hypothesis, higher levels of DHEA were associated with greater RV mass and larger volumes in women and possibly in men. These associations were similar to those seen with testosterone in men, suggesting an androgenic effect. Higher levels of SHBG may have been associated with higher RVSV among women and possibly men in limited models only. With E2, bioT, and DHEA, most of the results remained significant after adjustment for the respective LV measures, implying RV-specific or RV-disproportionate associations, as well as after adjustment for measures of lung function (in a smaller subset), implying unique associations between sex hormones and the RV independent of pulmonary effects. To our knowledge, this is the only study of sex hormones and RV structure and function assessed by cardiac MRI.
Although some of the effect sizes seem small, they are comparable to those seen in the LV related to active smoking and diabetes mellitus (29). In severe PAH, intravenous epoprostenol improves RVEF by only 4% while leading to significant improvements in exercise capacity, functional status, and survival (30, 31). In the normal RV (with a lower “signal:noise ratio” compared with that seen in disease), similar or smaller differences may have important physiological effects.
We have shown that higher E2 levels in HT users were associated with higher RVEF and lower RVESV. In men, higher E2:T was possibly associated with lower RV volumes. High estrogen/low testosterone states have been associated with a lower risk of cardiovascular disease in aging men (15, 16). Estrogen has been associated with elevated markers of angiogenesis and heart neovascularization, and human ventricular myocardium contains functional estrogen receptors (ERs) (32, 33). Cardiac neovascularization promotes collateral blood flow, which may promote beneficial remodeling and improve RV systolic function (34). There are several possible explanations as to why these associations (in women) were demonstrated only in HT users. A 1-ln increment in E2 in HT users may have different biological implications than a 1-ln increment in nonusers, either because of greater E2 levels (as seen here) or variance in HT users, altered or unmeasured metabolites, or protein–receptor interactions. HT up-regulates ER tissue expression and may lead to altered E2 sensitivity among HT users (35). The appearance of this association in HT users only may be explained by unmeasured differences between users and nonusers, although they had similar baseline characteristics (36).
We have shown that bioT levels were associated with greater RV mass and larger RV volumes, independent of these LV measures. Testosterone increases the myocardial inflammatory response, promotes cardiac remodeling, and increases LV mass in animals (37, 38). On the other hand, epidemiologic studies suggest testosterone deficiency is associated with worse cardiovascular outcomes among men, and there is some evidence that testosterone supplementation improves functional capacity in androgen-deficient men with CHF (39, 40). The association between androgens (both bioT and DHEA) and greater RV mass and volumes here may not be detrimental per se, as has been proposed with exercise-induced increases in LV mass in trained athletes and in RV mass in MESA participants (41). In fact, higher levels of both bioT (in men) and DHEA (in women) were associated with higher RVSV. Ultimately, maintaining androgen balance may be most important for cardiopulmonary health.
The associations of DHEA with greater RV mass and volumes contradict extensive animal and preclinical data demonstrating that DHEA prevents or reverses PH and improves vascular remodeling (7, 8, 42). Similarly, previous epidemiologic studies have shown lower levels of DHEA associated with increased cardiovascular risk (43). It has been proposed that DHEA may have important intracellular signaling effects independent of hormonal or steroid action (42). DHEA stimulates both nitric oxide and endothelin-1, two important but antagonistic mediators in PH (44). As such, DHEA may have more complex effects on the pulmonary vasculature and RV than previously appreciated. As our findings relate to RV morphology, it is possible that DHEA has pleiotropic effects on the pulmonary vasculature and the RV. It has been shown that extremes (low or high levels) and variability in serum DHEA predict mortality in older adults more so than a single measurement (45). Thus, it is possible that baseline measurement may not accurately capture DHEA trajectory and therefore disease risk. The sulfate ester of DHEA (DHEA-S), known to have higher and more stable serum levels than DHEA, was not measured in this study and may have been more informative.
Although the effects were modest, there was a suggestion that higher levels of SHBG were associated with higher RVSV. In left heart failure, higher levels of SHBG are associated with poor outcomes and we have found associations with subclinical atherosclerosis (46, 47). Whether SHBG has unique effects on the pulmonary vasculature and the RV is unknown.
Our study has several limitations. Because this is a cross-sectional observational study, no conclusions can be drawn about causality. However, the MESA cohort offers a unique opportunity to generate hypotheses about hormonal influences on the RV, given (1) it is population based, (2) it includes female and minority participants, and (3) to our knowledge, it is the largest study of RV structure and function assessed by cardiac MRI to date. Cardiac MRI is considered the “gold standard” for assessment of the RV, and RV measures have been found to be highly accurate and reproducible in normal individuals (and in those with heart failure) (48). We included postmenopausal women; the results may not apply to younger, premenopausal women. Measurement of hormones was performed only at baseline, although single measurements of sex hormones are reliable and reproducible over several years in postmenopausal women (49). Although hemodynamic data would have allowed for hypothesis generation about the associations between sex hormones and pulmonary vascular function specifically, these measurements were not available, nor would they have been feasible in a cohort of this size.
We included women taking HT given (1) the potential for selection bias if excluded, as it has been well documented that women taking HT are fundamentally different (e.g., lower cardiovascular risk, higher socioeconomic status) than those who are not (36), (2) E2 levels regardless of source (i.e., endogenous or exogenous) may directly affect RV structure and function, and (3) available data on HT use and related covariates allowed for adjustment for potential unmeasured confounders. A small number of individuals were receiving either testosterone or DHEA supplementation; analyses excluding such individuals provided identical results. The relationship of E2 with RV measures depended on HT use, justifying this approach and indicating an important interaction for further study. Unmeasured or residual confounding of our results is still possible.
We have shown that serum E2 in the setting of HT is associated with better RV systolic function in postmenopausal women and that higher levels of androgens (bioT and DHEA) are associated with larger RVs in men and postmenopausal women without cardiovascular disease. The estrogen:testosterone balance may be particularly informative in men and further study is needed to define the role of SHBG in pulmonary vascular and RV function. The associations seen for E2 and DHEA, in particular, contradict what is known about pulmonary vascular disease, suggesting that certain hormones may have pleiotropic actions for the RV and the pulmonary vasculature.
Supplementary Material
Acknowledgments
This manuscript has been reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications. Significant comments have been incorporated before submission for publication. The authors thank the other investigators, staff, and participants in the MESA and MESA-Lung Studies for their valuable contributions. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org.
Supported by National Institutes of Health R01-HL086719, R01-HL074406, RO1-HL074338, R01-HL077612, and N01-HC95159 through N01-HC95169.
This article has an online supplement, which is available from the issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201007-1027OC on October 19, 2010
Author Disclosure: C.E.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.O. received $1,000–$5,000 from the SWHR-ISIS Fund for serving on a scientific advisory group to develop research projects in areas of need for research into sex differences in CVD, $50,001–$100,000 from BMS in industry-sponsored grants as an investigator in a multicenter trial, and more than $100,001 in NIH grants for investigator-sponsored studies and cohort studies. D.A.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. H.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.G.B. received more than $100,001 from the NIH and more than $100,001 from the EPA in sponsored grants. E.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.R.C. received more than $100,001 from the NIH in grant funding; M.R.B does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. C.J. received more than $100,001 from the NHLBI/NIH in sponsored grants as an NHLBI contract (MESA). R.A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.C.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.K. received $1,001–$5,000 from Gilead and $1,001–$5,000 from Novartis in consultancy fees, $1,001–$5,000 from Bayer, and $1,001–$5,000 from Gilead for serving on an advisory board, $10,001–$50,000 from Gilead for serving on steering committees, $10,001–$50,000 from Gilead and $10,001–$50,000 from Pfizer for serving on a grant review committee, $1,001–$5,000 from Gilead and $1,001–$5,000 from Actelion in nonpromotional lecture fees, $10,001–$50,000 from Actelion, $10,001–$50,000 from Gilead, $10,001–$50,000 from United Therapeutics, $10,001–$50,000 from Lung Rx, and $10,001–$50,000 from Pfizer in industry-sponsored grants as conference support, $50,001–$100,000 from Pfizer as a collaborator on an institutional grant, has received gratis study drugs from Merck and Bayer for an NIH-funded grant, $50,001–$100,000 from Actelion and $50,001–$100,000 from Gilead for contracted research, more than $100,001 from the NIH, and $1,001–$5,000 from the American Lung Association and up to $1,000 from the NIH for serving on review committees.
References
- 1.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001;161:996–1002. [DOI] [PubMed] [Google Scholar]
- 2.Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol 2001;280:L88–L97. [DOI] [PubMed] [Google Scholar]
- 3.Giuberti K, Pereira RB, Bianchi PR, Paigel AS, Vassallo DV, Stefanon I. Influence of ovariectomy in the right ventricular contractility in heart failure rats. Arch Med Res 2007;38:170–175. [DOI] [PubMed] [Google Scholar]
- 4.Lahm T, Patel KM, Crisostomo PR, Markel TA, Wang M, Herring C, Meldrum DR. Endogenous estrogen attenuates pulmonary artery vasoreactivity and acute hypoxic pulmonary vasoconstriction: the effects of sex and menstrual cycle. Am J Physiol Endocrinol Metab 2007;293:E865–E871. [DOI] [PubMed] [Google Scholar]
- 5.Rowell KO, Hall J, Pugh PJ, Jones TH, Channer KS, Jones RD. Testosterone acts as an efficacious vasodilator in isolated human pulmonary arteries and veins: evidence for a biphasic effect at physiological and supra-physiological concentrations. J Endocrinol Invest 2009;32:718–723. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Wu S, Wei H, Zhou K, Ruan Y, Lai W. Effects of sex hormones and their balance on the proliferation of rat vascular endothelial cells. Horm Res Paediatr 2002;58:16–20. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet S, Dumas-de-La-Roque E, Begueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci USA 2003;100:9488–9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homma N, Nagaoka T, Karoor V, Imamura M, Taraseviciene-Stewart L, Walker LA, Fagan KA, McMurtry IF, Oka M. Involvement of RhoA/Rho kinase signaling in protection against monocrotaline-induced pulmonary hypertension in pneumonectomized rats by dehydroepiandrosterone. Am J Physiol Lung Cell Mol Physiol 2008;295:L71–L78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becchis M, Sullivan PM, Ordronneau P, Petrusz P, Joseph DR. Distribution of immunoreactive androgen-binding protein/sex hormone–binding globulin in tissues of the fetal rat. Steroids 1996;61:392–400. [DOI] [PubMed] [Google Scholar]
- 10.Schock HW, Herbert Z, Sigusch H, Figulla HR, Jirikowski GF, Lotze U. Expression of androgen-binding protein (ABP) in human cardiac myocytes. Horm Metab Res 2006;38:225–229. [DOI] [PubMed] [Google Scholar]
- 11.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010;137:376–387. [DOI] [PubMed] [Google Scholar]
- 12.Kawut SM, Al-Naamani N, Agerstrand C, Berman Rosenzweig E, Rowan C, Barst RJ, Bergmann S, Horn EM. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest 2009;135:752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benza RL, Miller DP, Gomberg-Maitland M, Frantz RP, Foreman AJ, Coffey CS, Frost A, Barst RJ, Badesch DB, Elliott CG, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010;122:164–172. [DOI] [PubMed] [Google Scholar]
- 14.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010;122:156–163. [DOI] [PubMed] [Google Scholar]
- 15.Arnlov J, Pencina MJ, Amin S, Nam BH, Benjamin EJ, Murabito JM, Wang TJ, Knapp PE, D'Agostino RB Sr, Bhasin S, et al. Endogenous sex hormones and cardiovascular disease incidence in men. Ann Intern Med 2006;145:176–184. [DOI] [PubMed] [Google Scholar]
- 16.Tivesten A, Mellstrom D, Jutberger H, Fagerberg B, Lernfelt B, Orwoll E, Karlsson MK, Ljunggren O, Ohlsson C. Low serum testosterone and high serum estradiol associate with lower extremity peripheral arterial disease in elderly men: the MROS Study in Sweden. J Am Coll Cardiol 2007;50:1070–1076. [DOI] [PubMed] [Google Scholar]
- 17.Ventetuolo C, Ouyang P, Bluemke D, Tandri H, Barr R, Bagiella E, Bristow M, Johnson C, Kizer J, Lima J, et al. Sex hormones and the right ventricle: the MESA-Right Ventricle Study [abstract]. Am J Respir Crit Care Med 2009;179:A4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacobs DR, Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 19.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JAC, et al. Cardiovascular function in Multi-Ethnic Study of Atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol 2006;186:S357–S365. [DOI] [PubMed] [Google Scholar]
- 20.Chahal H, Johnson C, Tandri H, Jain A, Hundley WG, Barr RG, Kawut SM, Lima JA, Bluemke DA. Relation of cardiovascular risk factors to right ventricular structure and function as determined by magnetic resonance imaging (results from the Multi-Ethnic Study of Atherosclerosis). Am J Cardiol 2010;106:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel-Claussen J, Finn JP, Gomes AS, Hundley GW, Jerosch-Herold MP, Pearson G, Sinha SP, Lima JAC, Bluemke DA. Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr 2006;30:426–432. [DOI] [PubMed] [Google Scholar]
- 22.Cushman M, Cornell E, Howard P, Bovill E, Tracy R. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem 1995;41:264–270. [PubMed] [Google Scholar]
- 23.Södergård R, Bäckström T, Shanbhag V, Carstensen H. Calculation of free and bound fractions of testosterone and estradiol-17β to human plasma proteins at body temperature. J Steroid Biochem 1982;16:801–810. [DOI] [PubMed] [Google Scholar]
- 24.Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab 2007;92:1289–1295. [DOI] [PubMed] [Google Scholar]
- 25.Psaty B, Lee M, Savage PJ, Rutan GH, German PS, Lyles M; Cardiovascular Health Study Collaborative Research Group. Assessing the use of medication in the elderly: methods and initial experience in the Cardiovascular Health Study. J Clin Epidemiol 1992;45:683–692. [DOI] [PubMed] [Google Scholar]
- 26.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JAC, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med 2010;362:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction. Ann Intern Med 2010;152:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46. [PubMed] [Google Scholar]
- 29.Heckbert SR, Post W, Pearson GDN, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol 2006;48:2285–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roeleveld RJ, Vonk-Noordegraaf A, Marcus JT, Bronzwaer JGF, Marques KMJ, Postmus PE, Boonstra A. Effects of epoprostenol on right ventricular hypertrophy and dilatation in pulmonary hypertension. Chest 2004;125:572–579. [DOI] [PubMed] [Google Scholar]
- 31.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996;334:296–301. [DOI] [PubMed] [Google Scholar]
- 32.Iwakura A, Shastry S, Luedemann C, Hamada H, Kawamoto A, Kishore R, Zhu Y, Qin G, Silver M, Thorne T, et al. Estradiol enhances recovery after myocardial infarction by augmenting incorporation of bone marrow–derived endothelial progenitor cells into sites of ischemia-induced neovascularization via endothelial nitric oxide synthase–mediated activation of matrix metalloproteinase-9. Circulation 2006;113:1605–1614. [DOI] [PubMed] [Google Scholar]
- 33.Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science 2005;308:1583–1587. [DOI] [PubMed] [Google Scholar]
- 34.Handoko ML, de Man FS, Happe CM, Schalij I, Musters RJP, Westerhof N, Postmus PE, Paulus WJ, van der Laarse WJ, Vonk-Noordegraaf A. Opposite effects of training in rats with stable and progressive pulmonary hypertension. Circulation 2009;120:42–49. [DOI] [PubMed] [Google Scholar]
- 35.Donaldson C, Eder S, Baker C, Aronovitz MJ, Weiss AD, Hall-Porter M, Wang F, Ackerman A, Karas RH, Molkentin JD, et al. Estrogen attenuates left ventricular and cardiomyocyte hypertrophy by an estrogen receptor–dependent pathway that increases calcineurin degradation. Circ Res 2009;104:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Before use of estrogen replacement therapy, are users healthier than nonusers? Am J Epidemiol 1996;143:971–978. [DOI] [PubMed] [Google Scholar]
- 37.Nahrendorf M, Frantz S, Hu K, von zur Muhlen C, Tomaszewski M, Scheuermann H, Kaiser R, Jazbutyte V, Beer S, Bauer W, et al. Effect of testosterone on post-myocardial infarction remodeling and function. Cardiovasc Res 2003;57:370–378. [DOI] [PubMed] [Google Scholar]
- 38.Kłapcińska B, Jagsz S, Sadowska-Krępa E, Górski J, Kempa K, Langfort L. Effects of castration and testosterone replacement on the antioxidant defense system in rat left ventricle. J Physiol Sci 2008;58:173–177. [DOI] [PubMed] [Google Scholar]
- 39.Khaw K, Dowsett M, Folkerd E, Bingham S, Wareham N, Luben R, Welch A, Day N. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) prospective population study. Circulation 2007;116:2694–2701. [DOI] [PubMed] [Google Scholar]
- 40.Malkin CJ, Pugh PJ, West JN, van Beek EJR, Jones TH, Channer KS. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J 2006;27:57–64. [DOI] [PubMed] [Google Scholar]
- 41.Aaron C, Tandri H, Barr R, Johnson C, Bagiella E, Chahal H, Jain A, Kizer J, Lima J, Bluemke D, et al. Physical activity and right ventricular structure and function: the MESA-Right Ventricle Study. Am J Respir Crit Care Med 2011;183:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JRB, Michelakis ED. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the AKT/GSK3-β/NFAT axis. Circulation 2009;120:1231–1240. [DOI] [PubMed] [Google Scholar]
- 43.Ponikowska B, Jankowska EA, Maj J, Wegrzynowska-Teodorczyk K, Biel B, Reczuch K, Borodulin-Nadzieja L, Banasiak W, Ponikowski P. Gonadal and adrenal androgen deficiencies as independent predictors of increased cardiovascular mortality in men with type II diabetes mellitus and stable coronary artery disease. Int J Cardiol 2009;21:21. [DOI] [PubMed] [Google Scholar]
- 44.Formoso G, Chen H, Kim JA, Montagnani M, Consoli A, Quon MJ. Dehydroepiandroesterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase and mitogen-activated protein kinase–dependent pathways in vascular endothelium. Mol Endocrinol 2006;20:1153–1163. [DOI] [PubMed] [Google Scholar]
- 45.Cappola AR, O'Meara ES, Guo W, Bartz TM, Fried LP, Newman AB. Trajectories of dehydroepiandrosterone sulfate predict mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 2009;64A:1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guder G, Frantz S, Bauersachs J, Allolio B, Ertl G, Angermann CE, Stork S. Low circulating androgens and mortality risk in heart failure. Heart 2010;96:504–509. [DOI] [PubMed] [Google Scholar]
- 47.Ouyang P, Vaidya D, Dobs A, Golden SH, Szklo M, Heckbert SR, Kopp P, Gapstur SM. Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis 2009;204:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J 2004;147:218–223. [DOI] [PubMed] [Google Scholar]
- 49.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3 year period. Cancer Epidemiol Biomarkers Prev 1995;4:649–654. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.