Abstract
The study of human pulmonary immunity against Mycobacterium tuberculosis (M.tb) provides a unique window into the biological interactions between the human host and M.tb within the broncho-alveolar microenvironment, the site of natural infection. Studies of bronchoalveolar cells (BACs) and lung tissue evaluate innate, adaptive, and regulatory immune mechanisms that collectively contribute to immunological protection or its failure. In aerogenically M.tb–exposed healthy persons lung immune responses reflect early host pathogen interactions that may contribute to sterilization, the development of latent M.tb infection, or progression to active disease. Studies in these persons may allow the identification of biomarkers of protective immunity before the initiation of inflammatory and disease-associated immunopathological changes. In healthy close contacts of patients with tuberculosis (TB) and during active pulmonary TB, immune responses are compartmentalized to the lungs and characterized by an exuberant helper T-cell type 1 response, which as suggested by recent evidence is counteracted by local suppressive immune mechanisms. Here we discuss how exploring human lung immunity may provide insights into disease progression and mechanisms of failure of immunological protection at the site of the initial host–pathogen interaction. These findings may also aid in the identification of new biomarkers of protective immunity that are urgently needed for the development of new and the improvement of current TB vaccines, adjuvant immunotherapies, and diagnostic technologies. To facilitate further work in this area, methodological and procedural approaches for bronchoalveolar lavage studies and their limitations are also discussed.
Keywords: tuberculosis, bronchoalveolar lavage, alveolar macrophages, innate immunity, interferon gamma release assays
Tuberculosis (TB) is a lung disease that was expected to afflict almost 10 million people in 2010, thus remaining a preeminent global public health problem that is inextricably linked to poverty, HIV coinfection, dynamic population migration, and adaptations of Mycobacterium tuberculosis (M.tb) strains to specific host populations (1). This situation has been worsened by an increasing incidence of TB cases caused by multidrug and extensively drug-resistant M.tb strains (2). Although improvements in political commitment, poverty alleviation, and universal HIV-related care are expected to reduce the global TB burden, sustained disease eradication will depend on the development of new antituberculous drugs, vaccines, diagnostic tools, and immunotherapeutic interventions. The development and improvement of these modalities, however, require a better understanding of what comprises protective human antimycobacterial immunity as data from animal studies frequently cannot reliably be extrapolated to humans.
The components and mechanisms of antimycobacterial protective immunity are still poorly understood. This is particularly true for human lungs, the most common site of M.tb infection. Innate lung immune mechanisms can be assumed to be responsible for sterilizing immunity in those healthy TB contacts in whom there is no evidence of T-cell sensitization despite significant concurrent aerogenic M.tb exposure. Lung immunity research in such persons will likely reveal new biomarkers of protective immunity.
Here we provide a clinically oriented focus to human lung antimycobacterial immunity that will appeal to the clinician scientist who proposes to undertake studies exploring the immunopathogenesis of TB. We also discuss the challenges and caveats that lung immunity studies pose, and the opportunities provided to decipher protective and susceptibility immune mechanisms.
TUBERCULOSIS LUNG IMMUNITY RESEARCH: LOGISTICAL AND TECHNICAL ASPECTS
Given the potential risks to volunteers and the complexity of the procedure, lung immunity research is governed by complex regulatory, management, and scientific considerations. Methodological approaches to maximize the safety of research bronchoscopies and to ensure a high yield and quality of biological material obtained are outlined in Tables 1, 2, and 3. Although risk to the operator for developing active TB is minimal, airborne infection control with recommended administrative, environmental, and personal protection measures, including use of 0.5-μm pore size face masks, and regular screening (annual chest radiography and immunodiagnostic tests such as tuberculin skin test and IFN-γ release assay [IGRA]) of research workers should be employed to prevent the acquisition of latent M.tb infection (LTBI) and active TB (3). Human research using bronchoscopy and bronchoalveolar lavage (BAL) requires expensive equipment (well-equipped bronchoscopy suites and postbronchoscopy monitoring facilities), a complex skill set (trained bronchoscopists, anesthesiologists, and nurses), and a supportive institutional environment. The number of institutions in high TB burden settings that can support this type of research is still low. Short funding cycles and the difficulty in supporting international study sites from investigator-initiated grants pose additional obstacles.
TABLE 1.
CLINICAL CRITERIA PRECLUDING BRONCHOSCOPY AND RECOMMENDED INVESTIGATION BEFORE INITIATING BRONCHOSCOPIC EVALUATION
| Criterion | Examples |
|---|---|
| Conditions and comorbidities that will likely exclude study candidates from research-based bronchoscopic evaluation | • Respiratory diseases such as uncontrolled asthma, severe hypoxia, hypercapnia, etc. |
| • Upper airway and oral cavity infections | |
| • Lidocaine allergy | |
| • Severe chronic liver and chronic kidney diseases | |
| • Alcohol abuse | |
| Factors that confound interpretation of data obtained from bronchoscopic studies | • Immunocompromising conditions (diabetes, HIV-1 infection, chronic kidney or liver diseases) |
| • Illicit drug use | |
| • Immunosuppressive medication | |
| • Tobacco smoking | |
| Preprocedural examinations and diagnostic tests that should be undertaken in the appropriate study subject before research bronchoscopy | • Anterior/posterior and lateral chest radiographs |
| • Physical examination | |
| • Venipuncture (blood cell count) | |
| • Respiratory function tests (FEV1 and FVC) |
TABLE 2.
CLINICAL CONSIDERATIONS WHEN PERFORMING RESEARCH-RELATED BRONCHOALVEOLAR LAVAGE PROCEDURES
| Factors relevant to approvals by institutional or ethical review boards: |
| • Increase in procedure time, BAL volumes, and resulting changes in adverse event/risk profiles, compared with clinically indicated BALs, need to be acknowledged in study protocols and consent forms |
| Before the BAL procedure: |
| • Obtain signed study consent before sedative medication |
| • Confirm lack of recent upper respiratory tract or oral cavity infection |
| • Assess chest radiographs—localize lung pathology or confirm lack thereof |
| • Establish intravenous access (for blood sampling and emergency interventions) |
| • Calculate maximally permissible volumes of sprays and gels based on the total permitted lidocaine dose of 4.5 mg/kg body weight* |
| During the BAL procedure: |
| • Have an anesthesiologist or CPR-trained person available |
| • Administer conscious sedation at operator discretion (e.g., midazolam and/or fentanyl) |
| • Monitor use of lidocaine in sprays and gels during the procedure—stop procedure once a total of 4.5 mg/kg body weight has been used |
| • Continously monitor heart rate, blood pressure, and O2 saturation |
| • Provide supplemental O2 via nasal prongs |
| • Stop BAL procedure at a sustained O2 saturation < 88% |
| After the BAL procedure: |
| • Observe study subject for a minimum of 1 h |
| • Monitor heart rate, blood pressure, O2 saturation |
| • Do not permit eating or drinking until swallow reflexes have recovered |
| • Discuss driving safety |
| • Provide health care personnel contact information and encourage return to the clinic, at any time, should symptoms such as dyspnea, fever, cough, hemoptysis, or thoracic pain be noted |
| • Appropriately decontaminate bronchoscopes to prevent nosocomial infection |
Definition of abbreviations: BAL = bronchoalveolar lavage; CPR = cardiopulmonary resuscitation.
Modified from: British Thoracic Society Bronchoscopy Guidelines Committee, Subcommittee of the Standards of Care Committee of British Thoracic Society. The British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001;56(Suppl I):i1–i21.
TABLE 3.
TECHNICAL FACTORS IMPACTING ON BRONCHOALVEOLAR CELL YIELD
| BAL fluid: |
| • Use sterile 0.9% sodium chloride (NaCl) at room temperature |
| • Total instillation volumes vary between 100 and 300 ml |
| Choice of BAL location: |
| • One or two lung segments (up to 150 ml each) can be lavaged—in patients with TB, e.g., radiographically affected and unaffected lung areas and in healthy persons, two adjacent segments |
| Approach to harvesting the BAL fluid: |
| • Using 60-ml syringes and three-way valves for manual instillation and suction of the BAL fluid provides an exquisitely sensitive approach to increasing the yield by reducing airway collapse (70–85% yield of instilled saline fluid from healthy persons, less from persons with TB) |
| • Alternatively, use lowest possible wall suction strength to minimize airway collapse and cellular damage, and preserve optimal immunological function of the BACs |
| • Maximal yields of BAL fluid are obtained from the right middle lobe and from the lower lobes. In the upper lobes aspirated volumes are unpredictable and substantially reduced |
Definition of abbreviations: BACs = bronchoalveolar cells; BAL = bronchoalveolar lavage; TB = tuberculosis.
In the laboratory, specialized expertise and resources, including a category 3 containment facility, may be required for research work with bronchoalveolar cell (BAC) material. Careful study and experimental design including optimal cell utilization are crucial as BAC material can be limited, and variable in quality and quantity, between subjects despite attempts to use standardized BAL procedures. Obtaining accurate differential cell counts and biomarker detection by flow cytometry can be challenging given the high autofluorescence of alveolar macrophages. Furthermore, BAL fluid biomarker data must be normalized to control for the variable amount of starting material, variability in yields between study subjects, and an approximately 200- to 300-fold dilution of the biomarker of interest. How to accurately normalize the data (protein levels, number of cells, ratio of BAL to serum urea, etc.) remains a conundrum. Although computer-assisted tomographic scans of the lungs are desirable to accurately localize diseased segments including identification of those with cavities, such scans add to the complexity and cost of investigations and the radiation exposure may have ethical implications. Disease localization is frequently possible using a posterior–anterior and lateral chest radiograph. In addition, transbronchial biopsies or lung epithelial biopsies may provide insights into cellular and molecular innate and adaptive immune processes in the respiratory epithelium and local lymphatic tissue.
Taken together, the obstacles to lung immunity research are considerable (but not insurmountable), and explain the relative scarcity of human lung immunity studies in persons with TB and, particularly, in M.tb-exposed healthy persons (4, 5).
TRANSMISSION DYNAMICS AND HOST IMMUNITY
M.tb infection in the majority of cases occurs by inhalation of infectious M.tb-containing aerosolized droplet nuclei that are released from persons with active pulmonary TB. In rare cases, M.tb-containing aerosolized droplet nuclei can also be derived from contact with diseased animals or exposure to laboratory-generated aerosol. Aerogenic M.tb transmission hinges on multiple factors including (1) the intensity of the contact, which is influenced by the exposure duration and physical distance within a shared air space; (2) the mycobacterial load in the respiratory secretions of the TB index person; and (3) the immune status (HIV-1 serostatus, diabetes, malnutrition, immunosuppressive [anti–tumor necrosis factor-α; Reference 6] therapies, etc.) of TB index and contact person. Other poorly studied factors such as volume and rheological characteristics of the sputum and cough strength (7–9) may affect the M.tb transmission efficacy. To complicate matters, genetic differences between M.tb strains affect immune escape mechanisms and transmission efficacy (10–14).
INSIGHTS INTO PULMONARY IMMUNITY
General Considerations
The respiratory tract and the bronchoalveolar spaces represent a unique immunological compartment where tissue-specific cells and interactions shape the first-line response to inhaled M.tb. These interactions and how they are interlinked with the life cycle of M.tb, including the spectrum of M.tb infection (15, 16), and the resulting impact on currently available immunodiagnostic tests are outlined in Figures 1 and 2. Humoral factors such as surfactant proteins A–D and other effector molecules from the respiratory epithelium alter the activation status of alveolar macrophages and the uptake of and responses to M.tb (see below, Humoral Immunity). Cell contact–dependent interactions of type I and type II epithelial cells with macrophages and dendritic cells regulate and limit inflammatory processes within the alveolar microenvironment via multiple mechanisms including CD200/CD200 receptor, IL-10, and transforming growth factor (TGF)-β (17) (Figure 2). Similarly, regional immune responses are under the control of local dendritic cell (DC) subsets and the potent T-cell–inhibitory activity of lung macrophages (17).
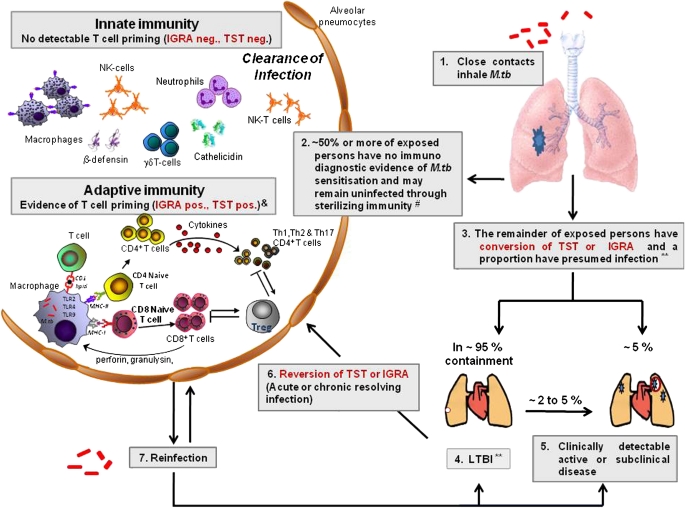
Figure 1.
Spectrum of tuberculosis (TB) infection, life cycle of Mycobacterium tuberculosis (M.tb), and the immunopathogenesis of pulmonary tuberculosis. Mycobacteria are inhaled into the lung alveoli (1). Here the infection may be cleared (2) by sterilizing innate or adaptive immune mechanisms (these mechanisms may determine the results of immunodiagnostic tests such as the tuberculin skin test [TST] and IFN-γ release assay [IGRA]). In the remainder (3) the infection may progress to latent M.tb infection (LTBI) (4), or in a small percentage to active TB (5). Some individuals with positive immunodiagnostic tests may, after a transient period of positivity, revert to negative and they presumably may have “acute or chronic resolving infection” (6). Those who have cleared their infection (2) may become reinfected and, depending on prevailing host immunity, may clear their infection (2) or progress to LTBI (4) or active disease (5). **The proportion of persons with positive immunodiagnostic tests who have LTBI is unclear. #The proportion of individuals who remain presumably uninfected is variable and will depend on host genetics, strain type, transmission dynamics, and several factors that may attenuate pulmonary-specific host immunity including HIV coinfection, diabetes, malnutrition, immunosuppressive therapy, and so on. &The transiency of these responses and the proportion in whom they manifest remain unclear. NK = natural killer; TLR2, 4, and 9 = Toll-like receptors 2, 4, and 9, respectively; Treg = regulatory T cells.
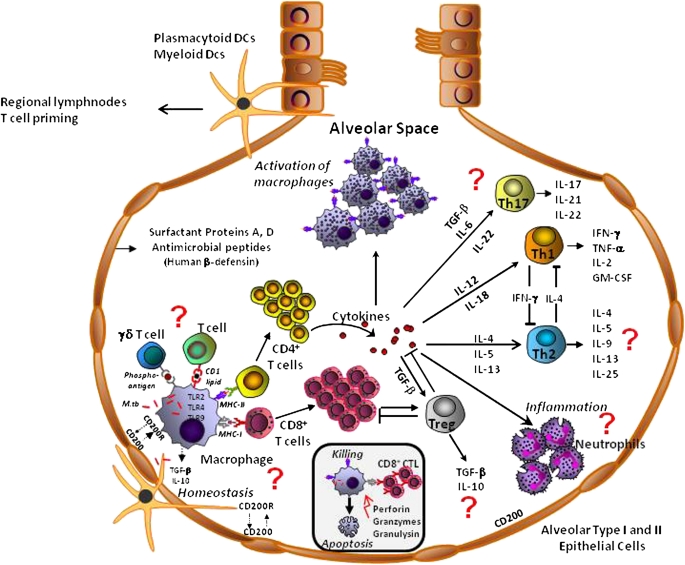
Figure 2.
Presumed cellular and humoral components of adaptive and innate antimycobacterial immunity in the bronchoalveolar spaces. Many of the cellular interactions have not been shown in the bronchoalveolar cells. Cell subpopulations and cytokines that have not been studied in human bronchoalveolar cells are indicated by a question mark (?). Interactions with epithelial cells can be assumed to be far more complex and diversified and humoral factors that may play an important role in the bronchoalveolar spaces have been omitted. DCs = dendritic cells; GM-CSF = granulocyte-macrophage colony-stimulating factor; M.tb = Mycobacterium tuberculosis; TGF-β = transforming growth factor-β; Th1, 2, and 17 = helper T-cell types 1, 2, and 17, respectively; TNF-α = tumor necrosis factor-α; Treg = regulatory T cells.
BACs are strikingly different from autologous peripheral blood mononuclear cells regarding functional capabilities and phenotype. Alveolar macrophages infected with M.tb in vitro are more potent producers of tumor necrosis factor (TNF)-α (18) and of chemokines (19) than are blood monocytes. M.tb uptake, accessory function, and infectability of alveolar macrophages are greater than that of peripheral blood–derived monocytes. Major differences also exist in the cell subset composition between BACs and whole blood (Table 4). In healthy persons 90–95% of BACs are macrophages with 5–10% lymphocytes, with occasional (<1%) DCs, neutrophils, basophils, or eosinophils (20–25).
TABLE 4.
AVERAGE PROPORTIONS OF CELLULAR SUBSETS IN BRONCHOALVEOLAR AND PERIPHERAL BLOOD COMPARTMENTS OF HEALTHY PERSONS
| Bronchoalveolar Cells |
Whole Blood |
||
|---|---|---|---|
| Cell Type | Proportion (%) | Cell Type | Proportion (%) |
| Macrophages | 90–95 | Monocytes | 0–10 |
| Lymphocytes | 5–10 | Lymphocytes | 15–45 |
| Neutrophils | <1 | Neutrophils | 50–70 |
| Eosinophils | <1 | Eosinophils | 0–6 |
| Basophils | <1 | Basophils | 0–2 |
| Dendritic cells | <1 | Dendritic cells | <1 |
As new diagnostic and vaccination approaches begin to interrogate local lung immunity, there is increased recognition of the compartmentalized nature of lung immune responses. This has implications for the study and development of new diagnostic tools including IGRAs for the diagnosis of sputum smear–negative TB, using BACs rather than blood cells (Reference 26; and see below, Immune-based Diagnosis of M.tb Infection and Active TB in the Lungs), and the assessment of novel antituberculous vaccines administered by aerosol immunization. Exploratory preclinical studies of the immunogenicity and safety of such TB vaccines are underway (Reference 27; and see below, Implications for TB Vaccinology and Immunotherapy).
BAL procedures generally sample approximately 1 million alveoli, the walls of which contain TB-specific granulomas. Although BACs do not reflect the three-dimensional granuloma structure (unless done in concert with transbronchial biopsies), they permit the evaluation of antigen and epitope specificity, and functional assessments such as M.tb growth inhibition. The possibility to assess lung-protective immunity in M.tb-exposed but healthy persons lends particular importance to BAC research. Nevertheless, a limitation is the inability to reliably distinguish the various stages of M.tb infection.
What Studies of Pulmonary Immunity Can Reveal Compared with Peripheral Blood
The presence of TB implies preceding failure of innate and adaptive immune responses. Immune responses are compartmentalized to the radiographically affected lung areas with a strongly enhanced M.tb antigen–specific helper T-cell type 1 (Th1) cytokine profile (28), and chemokine production (19). By contrast, systemic (blood) immunity shows a pattern of immunosuppression (29). Compared with healthy control subjects, BAC responses in persons with TB are characterized by increased numbers and proportions of alveolar lymphocytes (lymphocytic alveolitis) and immature macrophages (immature macrophage alveolitis) (28, 30). The majority of the M.tb antigen–specific alveolar T cells are activated, proliferating, CD4+ and CD8+, αβ-TCR bearing (28, 30), and with a memory phenotype (31). These cells express high levels of IFN-γ, TNF-α, and IL-12 (28, 31–33), both constitutively and during in vitro recall stimulation with M.tb antigens.
Because Th1 immunity is a proven requirement for M.tb control, the presence of enhanced local pulmonary Th1 immunity appeared irreconcilable, for many years, with the uncontrolled bacterial replication and progressive tissue pathology during active untreated pulmonary TB. M.tb-induced host immune evasion and findings of regulatory immune mechanisms associated with suppressive cytokines (IL-4, IL-10, TGF-β) in BAC samples (31, 34, 35) and induced sputum (36) have resolved this conundrum. Thus, suppressive immune mechanisms are operative in the bronchoalveolar spaces during pulmonary TB.
Insights from Histopathology
Histopathological assessments of lung granuloma material from persons with preexisting, often multidrug-resistant, TB have shed light on the three-dimensional structure and spatial interrelationship of immune cells in M.tb-induced granulomas. Granulomatous tissue reactions in the lungs are of variable maturity and reflect the variability in receptor expression and cytokine profiles. Laser dissection analyses of gene expression and surface marker analysis by flow cytometry allow limited functional studies.
INNATE IMMUNITY
Insights from Studies of Healthy Contacts of Patients with TB
Epidemiological evidence suggests that innate immune mechanisms control M.tb infection in humans. A significant proportion of heavily aerogenically M.tb-exposed healthy contacts of persons with TB appear to eradicate M.tb infection without inducing measurable T-cell sensitization (37) (persistently negative tuberculin skin test [TST] or IGRAs; see Figure 1). Immunological studies combined with longitudinal monitoring of such persons thus can be expected to aid in the search for biomarkers of protective immunity for TB vaccine and immunotherapy development efforts. However, such studies would need to consider the complexities of M.tb transmission under natural circumstances, including the relationship of immune responses to the intensity and duration of the aerogenic exposure and the genetic characteristics of the transmitted M.tb strain(s).
Despite their potential importance, there are hardly any data about lung immunity in aerogenically M.tb-exposed healthy household contacts. In the only two published studies addressing this topic, household contacts who had shared room air (>3 mo) before study inclusion with persons with untreated smear- and culture-positive TB were examined (4, 5). Compartmentalized immune responses were found in the contacts with increased frequencies of PPD- and M.tb Ag85A/B–specific BACs, but not in autologous peripheral blood mononuclear cells, or compared with BACs or peripheral blood mononuclear cells of healthy community control subjects (4, 5). Another major finding was the measurable M.tb growth restriction in alveolar macrophages conferred by unexpanded ex vivo CD8+ T cells from M.tb-exposed household contacts, but not from unexposed healthy subjects (4). We have ongoing BAL-related studies in African cohorts of healthy household contacts, exposed to subjects with smear-positive TB, with and without immunodiagnostic evidence of presumed latent M.tb infection. The objective is to identify biomarkers and cellular pathways associated with sterilizing immunity.
Unique Cellular and Humoral Innate Immunity in the Bronchoalveolar Spaces
On aerosol inhalation into the lungs, M.tb interacts with a wide variety of pattern recognition receptors (Table 5). M.tb is believed to be taken up by alveolar macrophages and intraepithelial dendritic cells and to interact with respiratory epithelial cells (alveolar types I and II [38]). Polymorphonuclear neutrophils can be found in BACs during TB (30, 39), and are believed to be the first cells recruited to the local site of M.tb entry, where they can be activated by M.tb products (40, 41) to express a range of receptors and a vast arsenal of antimicrobial effector molecules (42, 43) with an ability to restrict mycobacterial growth in vitro (42, 44) and participate in macrophage activation (45, 46). Their exact role in antimycobacterial immunity, particularly in the lungs, requires further clarification.
TABLE 5.
INNATE IMMUNITY: CELL TYPES, RECEPTORS AND EFFECTOR MECHANISMS
| Receptors involved in M.tb uptake and signaling |
| • Complement receptor-3 (CR3) |
| • C-type lectin Dectin-1 |
| • Fc receptors |
| • Scavenger receptors |
| • Chemokine receptors |
| • Mannose receptors |
| • DC-SIGN |
| • Adenosine receptor |
| • Toll-like receptors (TLRs) 2, 4, and 9 |
| • Nucleotide oligomerization domains (NODs) |
| M.tb TLR agonists |
| • Lipoarabinomannan (TLR2) |
| • Heat shock proteins 65 and 71 (TLR2, TLR4) |
| • M.tb DNA (TLR9) |
| TLR-mediated effector mechanisms |
| • NF-kB activation |
| • Antimicrobial peptides |
| • iNOS, nitric oxide |
| • Proinflammatory cytokines |
| • IL-12, IL-18 |
| Lung collectin–mediated host mechanisms |
| • NF-kB activation |
| • Expression of TLR2 and TLR4 (surfactant protein A [SP-A]) |
| • Increase in M.tb adherence and phagocytosis (SP-A) |
| • Decrease in phagocytosis (SP-D) |
| • Limitation of intracellular growth of M.tb (SP-D) |
| • Increased expression of phagocytic receptors, scavenger receptor A, and mannose receptor |
Definition of abbreviations: DC-SIGN = dendritic cell–specific intercellular adhesion molecule-3–grabbing nonintegrin; iNOS = inducible nitric oxide synthase; M.tb = Mycobacterium tuberculosis; NF-kB = nuclear factor-κB.
Dendritic cells and other local bronchoalveolar cell subsets (αβ and γδ T cells, natural killer cells, and natural killer T cells) as well as locally active soluble effector molecules (nitric oxide, inducible nitric oxide synthase, surfactants, antimicrobial peptides) participate in early innate immunity and initiation of adaptive immune mechanisms (Figure 1). M.tb activates at least two pattern recognition receptors: the Toll-like receptors (TLRs) and the nucleotide oligomerization domain–like receptors (47). In the appropriate context (antigen, cytokine milieu, additional inflammatory cells) engagement of TLRs may be immunostimulatory or immunosuppressive. Stimulation of TLR2, TLR4, and/or TLR9 (48–51) on monocytes, alveolar macrophages, and dendritic cells is a key step in initiating innate antimycobacterial host resistance (Table 5) and contributes to chemokine release and local accumulation of multiple cell types (42). TLRs also induce the release of antimicrobial effector molecules such as human β-defensin (52) from alveolar epithelial type II cells (38), which can be infected with M.tb. TLR engagement can also mediate potent immunosuppressive responses, which may drive host immune evasion. M.tb glycosylphosphatidylinositol anchor phosphatidyl-myo-inositol hexamannosides PIM6 and PIM2, for example, strongly inhibit TLR4 and myeloid differentiation protein-88–mediated release of nitric oxide, cytokines, and chemokines, including TNF-α and IL-12 (53). Similarly, ESAT-6 (early secreted antigenic target 6-kD protein) from M.tb inhibits activation of transcription factor NF (nuclear factor)-kB and IFN-regulatory factors after binding of ESAT-6 to TLR2 (54) and M.tb 19-kD lipoprotein inhibits IFN-γ–induced major histocompatibility complex class II (MHC-II) expression by a mechanism involving TLRs (55, 56). M.tb further induces TLR2-dependent inhibition of MHC-II trans-activator expression, MHC class II molecule expression, and antigen presentation (57). Autophagy is characterized by the sequestration of bacterial products in multimembrane autophagic vesicles, which fuse with lysosomes. Th1-mediated autophagy, which is attenuated by IL-4, is thought to be an important innate mycobactericidal mechanism (58–60). Studies characterizing the role of autophagy in the BAC compartment are lacking.
It has been difficult to convincingly demonstrate that human macrophages by themselves can efficiently kill M.tb as M.tb-induced immune evasion mechanisms interfere with antimicrobial macrophage effector mechanisms. However, in the presence of autologous CD8+ T cells, control of M.tb growth by alveolar macrophages can be identified (4). Further work is required to delineate how M.tb and its products attenuate macrophage effector mechanisms in the context of lung immunology.
The distinctiveness of the bronchoalveolar space as an immunological compartment is further evidenced by findings of TLR expression differences between autologous alveolar macrophages and peripheral monocytes (61), and the production of collectins such as surfactant proteins A and D (SP-A and SP-D) (e.g., by alveolar type II epithelial cells) that have a multitude of important effects on local lung immune responses to M.tb (62–64). SP-A regulates the expression of TLR2 and TLR4 and the signaling via these receptors in macrophages, decreases the phosphorylation of a key regulator of NF-kB activity, and is responsible for diminished TNF-α secretion in response to TLR ligands. SP-A also reduces the phosphorylation of TLR signaling proteins including members of the mitogen-activated protein kinase family (62). SP-A mediates adherence of M.tb to and increases phagocytic uptake of M.tb by human alveolar macrophages (65) as a result of up-regulated mannose receptor activity on alveolar macrophages. SP-D, in contrast, reduces the phagocytosis of M.tb, which in part is due to a direct interaction between M.tb and SP-D (66), and limits intracellular growth of M.tb by facilitating phagosome–lysosome fusion (63).
Alveolar macrophages express lower TLR2, comparable TLR4, and higher TLR9 levels than autologous monocytes. Lung-specific interrelationships between surfactant and TLR function (Table 5) may have important repercussions for safety and efficacy of antituberculous vaccines (with or without adjuvants) that may be delivered in future into the respiratory system as aerosols.
ADVANCES IN OUR UNDERSTANDING OF ADAPTIVE IMMUNITY
It has become clear that there is complex plasticity and interplay between established (Th1 and Th2) and emerging helper T-cell subsets (regulatory T cells, Th17, and more recently Th9) and cytokines in the immunopathogenesis of human TB.
Adaptive Cellular Immunity
CD4+ T cells.
A crucial role for CD4+ T cells in immunity against M.tb is indisputable and confirmed by published evidence including the effects of HIV-1–induced CD4+ T-cell depletion on susceptibility to M.tb infection and TB development (67) (Figure 2). HIV coinfection is associated with about 15% of all current global TB cases.
CD8+ T cells.
The role of CD8+ T cells in antimycobacterial immunity is still ill-defined, and yet evidence of their role in the containment and clearance of M.tb infection is emerging (4, 68–73) (Figure 2). Murine studies have provided evidence that M.tb antigen–specific CD8+ T cells can be elicited by respiratory mucosal immunization. These airway luminal T cells display an activated effector memory phenotype that is different from that found in peripheral tissues, proliferate continuously in an antigen-dependent manner, and are maintained even in the absence of peripheral T-cell recruitment (74).
Th17 and IL-23.
Th17 cells (Figure 2), a distinct subset of helper T cells, are of particular importance to the induction of lung immunity through their production of unique cytokines such as IL-17, IL-21, and IL-22, which stimulate defensin production, recruit neutrophils and monocytes to the site of inflammation, and are involved in the early phase of host defense. In human TB, IL-23 mRNA–expressing cells are sequestered in the lungs; however, levels of IL-17 mRNA are comparable with those of healthy control subjects (75). IL-17 has been implicated in the down-regulation of signaling lymphocyte activation molecule (SLAM) expression, cAMP-responsive element–binding protein (CREB) phosphorylation, and thus IFN-γ production in individuals with TB (76). In the murine model, IL-17 is counteracted during mycobacterial infection by IFN-γ, which serves to limit an IL-17–producing T-cell population (77). By contrast, IL-23 is essential for an accelerated immune response, early cessation of bacterial growth, and the establishment of an IL-17–producing CD4+ T-cell population in the murine lung. Depletion of IL-17 during challenge reduces chemokine expression and accumulation of IFN-γ–producing CD4+ T cells in murine lungs (78).
Regulatory T cells.
Regulatory T cells confer modulatory effects on tissue damage from autoreactive and overexuberant immune responses (Figure 2 and Table 6). The role of regulatory T cells (deleterious, beneficial, bystander) in human antimycobacterial immunity is ill-defined given the extensive immunopathology and lung damage in human TB (79–82). CD4+CD25highFoxP3+ T lymphocytes suppress human T-cell (82, 83) and γδ T-cell (84) M.tb antigen–specific IFN-γ release in vitro. Individuals with advanced TB, who lack PPD reactivity (TST negative), are characterized by Tr1 cells (a CD4+ regulatory T cell subpopulation) that constitutively produce IL-10 (85) and inhibit M.tb antigen–specific T-cell responses (80, 85). There are hardly any data about the presence of regulatory T cells and active involvement in controlling local effector immunity and inflammation in the lungs (82). A CD8+ lymphocyte activation gene-3 (LAG-3)+CD25+FoxP3+ regulatory T-cell subset was identified in TB granulomas and M.tb-infected humans that suppresses T cells partly through the secretion of CC chemokine ligand-4 (CCL4, macrophage inflammatory protein-1α) (86).
TABLE 6.
NEW CONCEPTS AND PARADIGMS, IN ADDITION TO HELPER T CELL TYPES 1 AND 2, ASSOCIATED WITH HUMAN T-CELL IMMUNOLOGY
| Th17 and IL-22: |
| • IL-22–driven IL-17–producing CD4+ T cells |
| • Characterized by the transcription factors STAT3, RORγt, RORα, and RORC2 |
| • Role in pulmonary TB undefined |
| Regulatory T cells: |
| • Naturally occurring CD4+ regulatory T cells |
| • Inducible/adaptive Tr1 (IL-10) CD4+ regulatory T cells |
| • Inducible/adaptive Th3 (IL-10, TGF-β) CD4+ regulatory T cells |
| • CD8+ lymphocyte activation gene-3 (LAG-3)+CD25+FoxP3+ |
| Regulatory T-cell markers: |
| • CD25+ and transcription factor FoxP3+ |
| • IL-7Rα chain (CD127−) |
| • LAG-3+ |
| Conditions, mediators, and effects of regulatory T cells: |
| • Cell–cell contact |
| • IL-10, TGF-β, CTLA4 |
| • CCL4 |
| • Suppression of M.tb antigen–specific IFN-γ release |
| • Suppression of M.tb antigen–specific cell proliferation |
| • Cytolysis (granzyme, granulysin, and perforin) |
| • Induction of DCs to produce immunosuppressive molecules; e.g., IDO |
| Th5 and Th9 cells: |
| • IL-4–independent, IL-33–driven, IL-5–producing CD4+ T cells (Th5) |
| • TGF-β–driven, IL-9–producing CD4+ T cells (Th9) |
| • Role in TB, if any, requires clarification |
Definition of abbreviations: CCL4 = CC chemokine ligand-4; CTLA4 = cytotoxic T-lymphocyte antigen-4; DCs = dendritic cells; IDO = indoleamine 2,3-dioxygenase; IL-7Rα chain = interleukin-7 receptor α chain; M.tb = Mycobacterium tuberculosis; RORγt, RORα, and RORC2 = retinoic acid receptor–related orphan receptor γt, α, and C2; STAT3 = signal transducer and activator of transcription-3; TB = tuberculosis; TGF-β = transforming growth factor-β; Th3, Th17, Th5, Th9, and Th17 = helper T cell types 3, 5, 9, and 17; Tr1, regulatory T cell type 1.
Regulatory T cells have been shown to prevent eradication of M.tb in a murine model by suppressing an otherwise efficient CD4+ T-cell response (87). However, the role and effect of regulatory T cells on mycobacterial survival, particularly in human BAC models, remain unclear. Emerging data also highlight the involvement of regulatory T cell and Th17 subsets in the initiation and augmentation of airway inflammation in other respiratory diseases including asthma (88, 89), chronic obstructive pulmonary disease (90), idiopathic pulmonary fibrosis (91), and sarcoidosis (92). However, the roles of these Th subsets (deleterious or favorable in a specific clinical, environmental, and host susceptibility context) remain unclear.
Th9 and other Th2-like subsets.
TGF-β, which drives Th17 cells and inducible regulatory T cells, acts as a regulatory switch that “reprograms” helper T type 2 cells to switch to IL-9 secretion or, in combination with IL-4, drives the differentiation of “TH-9” cells directly (93, 94). A subpopulation of IL-4–independent, IL-33–driven, IL-5–producing CD4+ T cells has been described (95). The role of these helper T-cell subsets in the pathogenesis of pulmonary TB, if any, requires clarification (Table 6).
Humoral Immunity
Humoral immunity is thought to be less important in antimycobacterial defense and its role is practically unknown in the human lung. However, this notion is controversial. Antibodies to M.tb antigens have been implicated in resistance to M.tb dissemination (96) and enhancement of down-regulatory cytokines (97).
THE SEARCH FOR BIOMARKERS OF PROTECTIVE IMMUNITY
Only about 5 to 10% of persons with a reactive TST (presumed M.tb-infected persons) develop postprimary TB or progress to reactivation TB during their lifetime, providing evidence of protective innate and adaptive immunity in the majority of persons (98). Biomarkers may provide prognostic information about responses to therapeutic interventions or vaccination in clinical trials (99) but have, with few exceptions (sputum markers), been studied in the peripheral blood compartment only. Thus studies of the early stages of M.tb immunity (e.g., in household contacts), particularly in the lungs, hold promise to identify markers that will distinguish protective immunity from immunopathology.
Clearly, neither the induction of single cytokines (IFN-γ, TNF, IL-2) nor that of single effector molecules (granzyme, nitric oxide, granulysin) can predict immunological protection from progression to active TB. Polyfunctional T-cell responses have been suggested to predict protection in vaccine trials; however, they have not been assessed in the context of natural infection in human lungs. In one study using peripheral blood, in more than 5,000 newborns bacillus Calmette-Guérin–induced protective immunity did not correlate with polyfunctional T-cell function (100). The appropriate choice of study populations, study time points, and sites from which biological material is harvested will be crucial for translational biomarker research study design.
IMMUNE ESCAPE MECHANISMS AND SUBVERSION OF THE HOST IMMUNE RESPONSE
Mycobacterium tuberculosis adaptations to the human host have resulted in a multitude of mycobacterial strategies to bypass host immune responses and promote survival of M.tb, and development of latency. Impairments of macrophage and T-cell effector functions, T-cell memory development, and subversive Th2-like cytokines (101, 102) contribute to the observed immunopathology during M.tb infection and TB. During TB there is evidence for the presence of suppressive immune mechanisms that are active locally in the lungs (36).
IMPLICATIONS FOR TB VACCINOLOGY AND IMMUNOTHERAPY
Aerosol delivery of novel TB vaccines as liquid or spray-dried powder aerosols into the lungs may become a possibility in future (27, 103), although the immunosuppressive environment in healthy lungs may represent a barrier to effective aerogenic vaccine take. Preclinical studies supported by the Aeras Global TB Vaccine Foundation (Rockville, MD), however, have provided evidence that aerosol delivery of an anti-TB vaccine candidate may induce lung immune responses that exceed those in blood, and those found after intramuscular or intradermal vaccination (27). Aerosol immunization, thus, may become a promising approach to enhance distinctive protective immune responses locally. Evaluation of protective site-specific immunity by locally delivered vaccines will have to involve assessments of site-specific immune responses (e.g., BACs) as peripheral blood samples may not provide the required sensitivity. This notion was confirmed recently in a murine study in which vaccine-induced airway luminal memory CD8+ T cells were capable of sustained self-renewal in the absence of peripheral T-cell recruitment (74). Thus, aerosol immunizations against TB will require efficacy evaluation with repeat BAL studies. Because BAL procedures are impractical in field trials beyond phase IIB, new approaches to assess antigen-specific immune responses in respiratory material will have to be found. Such studies will have to consider vaccine-induced suppressive immune mechanisms. For example, heparin-binding hemagglutinin (HBHA)–specific responses resistant to suppression by regulatory T cells and differentially modulated responses, compared with peripheral blood responses, have been detected in the BAL fluid of patients with TB (104). Last, BAL responses have been shown to be useful to evaluate the response to local immunotherapy with nebulized IFN-γ (105–107).
IMMUNE-BASED DIAGNOSIS OF M.tb INFECTION AND ACTIVE TB IN THE LUNGS
A lung-oriented approach to TB diagnosis is an attractive diagnostic strategy for smear-negative TB when using BAL fluid or induced sputum. The identification of antigen-specific cytokine production as a marker of existing T-cell sensitization (M.tb infection, active disease or previous exposure) can be accomplished by enzyme-linked immunospot (ELISPOT) assays, ELISAs or flow cytometry. The ELISPOT and ELISA approaches have been standardized into commercial kit formats (T-SPOT.TB [Oxford Immunotec Ltd., Marlborough, MA] and QuantiFERON-TB Gold In-Tube [Cellestis Ltd, Carnegie, Victoria, Australia], respectively). The ELISPOT assay is more sensitive but labor-intensive (108).
Contacts of patients with TB are at increased risk for M.tb infection and progression to active TB. BAL-based identification of persons at risk for transition from LTBI to active TB, although desirable, is not feasible given the low rates of progression to active TB. However, one may speculate that assessments in BACs of helper T-cell cytokine ratios, recognition of M.tb stage–specific antigens, or frequencies of T-cell subpopulations (e.g., CD8+ cytotoxic T lymphocytes) in future may aid in identifying healthy contacts at risk for progression to TB.
M.tb antigen–specific BAL responses may be useful for the rapid diagnosis of TB when smear microscopy is unhelpful. Indeed, in contrast to pleural mononuclear cell responses (109) and in keeping with good discriminatory value when using cells from the cerebrospinal fluid (110), RD1 M.tb antigen–specific quantitative T-cell responses represent an accurate tool for the rapid immunodiagnosis of smear-negative TB, using BACs in high (111) and low burden settings (26). This is likely related to cell trafficking dynamics specific to the different compartments. Thus, unlike the CD4+ cell/CD8+ cell ratio (112), the antigen-specific BAL ELISPOT assay has high specificity for the diagnosis of TB compared with other respiratory diseases including sarcoidosis (111, 113). The high specificity suggests that more frequent exposure to M.tb aerosol in high-burden settings does not confound the assay. One drawback of using BACs for TB diagnosis is the significant rate of inconclusive results for several reasons (outlined in Reference 111). However, BAC responses were not confounded by presumed LTBI (positive blood but not BAC RD1 IGRA) again highlighting the dynamic and compartmentalized nature of the immune response to M.tb. Although HBHA responses have been detected in BAL fluid (104), studies have found limited diagnostic utility of this antigen when using pleural mononuclear cells (109) or bronchoalveolar lavage cells (111). However, the effect of using methylated HBHA from Mycobacterium bovis BCG compared with that obtained from Mycobacterium smegmatis requires a head-to-head comparison. Although we established proof-of-concept that rapid immunodiagnosis of TB is possible when using cells from induced sputum, we also confirmed that this approach was not clinically useful when using the RD1–specific ELISPOT assay. This was primarily due to the high proportion of inconclusive results (114) related to the low numbers of lymphocytes isolated during sputum induction. This limits the utility of sputum antigen–specific T-cell responses, similar to the findings in peripheral blood (115) for the monitoring of disease activity in patients with pulmonary TB.
Thus, although rapid immunodiagnosis of TB with IGRAs, at least when using BACs, is feasible in clinical practice the technique and diagnostic platform will require refinement and improvement if new biomarkers and antigens are to be efficiently evaluated. Alternative diagnostic approaches using pulmonary samples have used nucleic acid amplification tests and antigen detection (116). More recently, breath tests for the detection of TB-specific exhaled volatile organic compounds have been explored for their field-friendly diagnostic potential (117).
CONCLUSIONS AND FUTURE DIRECTIONS
Although logistically and technically challenging, respiratory and BAC studies provide access to a rarely studied immunological compartment and invaluable insights into mycobactericidal immunity at the site of infection. Early innate and adaptive protective immune mechanisms can be assessed in BACs from healthy aerogenically M.tb–exposed persons. Assessments of the impact of inhaled particulate matter (e.g., from tobacco smoke or indoor or outdoor air pollution) on antimycobacterial immune cell functions and of the induction of protective effects from inhaled TB vaccines represent other important areas in which respiratory immune cell studies may prove indispensable. However, given the logistical drawbacks of bronchoscopy, alternative approaches to assessing vaccine-induced antigen-specific lung mucosal immunity (e.g., via induced sputum, exhaled breath condensate, or nasal washings) are needed.
We believe that research into lung immunity may one day explain how respiratory epithelial cells regulate immune responses and execute antimycobacterial effector functions in the alveolar microenvironment. Several other important questions including whether local pulmonary homeostatic immune mechanisms (17) contribute to the attenuation of M.tb-specific host immunity, and whether regulatory T cells may be responsible for increased susceptibility to M.tb, are likely to be answerable only through human lung immunity research.
A greater focus on the lung compartment, including the use of systems biology approaches, will likely yield interesting answers to some of these crucial questions. The notion that lung cell studies may be unnecessary because BACs derive ultimately from the blood compartment, however, is a dangerous oversimplification.
Supported by 1R21ES016928-02 and ES005022 (S.K.S.) and by an MRC Career Development Award, an NRF/SARChI award, the EDCTP, and the European Commission (EU-FP7) (K.D.).
Originally Published in Press as DOI: 10.1164/rccm.201006-0963PP on November 12, 2010
Author Disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Dye C, Williams BG. The population dynamics and control of tuberculosis. Science 2010;328:856–861. [DOI] [PubMed] [Google Scholar]
- 2.Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L, Willcox P, John MA, Reubenson G, Govindasamy D, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet 2010;375:1798–1807. [DOI] [PubMed] [Google Scholar]
- 3.Shenoi SV, Escombe AR, Friedland G. Transmission of drug-susceptible and drug-resistant tuberculosis and the critical importance of airborne infection control in the era of HIV infection and highly active antiretroviral therapy rollouts. Clin Infect Dis 2010;50:S231–S237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carranza C, Juarez E, Torres M, Ellner JJ, Sada E, Schwander SK. Mycobacterium tuberculosis growth control by lung macrophages and CD8 cells from patient contacts. Am J Respir Crit Care Med 2005;173:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwander SK, Torres M, Carranza CC, Escobedo D, Tary-Lehmann M, Anderson P, Toossi Z, Ellner JJ, Rich EA, Sada E. Pulmonary mononuclear cell responses to antigens of Mycobacterium tuberculosis in healthy household contacts of patients with active tuberculosis and healthy controls from the community. J Immunol 2000;165:1479–1485. [DOI] [PubMed] [Google Scholar]
- 6.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med 2001;345:1098–1104. [DOI] [PubMed] [Google Scholar]
- 7.Fennelly KP, Martyny JW, Fulton KE, Orme IM, Cave DM, Heifets LB. Cough-generated aerosols of Mycobacterium tuberculosis: a new method to study infectiousness. Am J Respir Crit Care Med 2004;169:604–609. [DOI] [PubMed] [Google Scholar]
- 8.Bates JH. Transmission and pathogenesis of tuberculosis. Clin Chest Med 1980;1:167–174. [PubMed] [Google Scholar]
- 9.Loudon RG, Bumgarner LR, Lacy J, Coffman GK. Aerial transmission of mycobacteria. Am Rev Respir Dis 1969;100:165–171. [DOI] [PubMed] [Google Scholar]
- 10.Reed MB, Domenech P, Manca C, Su H, Barczak AK, Kreiswirth BN, Kaplan G, Barry CE III. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 2004;431:84–87. [DOI] [PubMed] [Google Scholar]
- 11.Manca C, Tsenova L, Barry CE, Bergtold A, Freeman S, Haslett PA, Musser JM, Freedman VH, Kaplan G. Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol 1999;162:6740–6746. [PubMed] [Google Scholar]
- 12.Sinsimer D, Huet G, Manca C, Tsenova L, Koo MS, Kurepina N, Kana B, Mathema B, Marras SA, Kreiswirth BN, et al. The phenolic glycolipid of Mycobacterium tuberculosis differentially modulates the early host cytokine response but does not in itself confer hypervirulence. Infect Immun 2008;76:3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, Barry C, Kaplan G. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak–Stat pathway. J Interferon Cytokine Res 2005;25:694–701. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Gong J, Yang Z, Samten B, Cave MD, Barnes PF. Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. J Infect Dis 1999;179:1213–1217. [DOI] [PubMed] [Google Scholar]
- 15.Dheda K, Schwander SK, Zhu B, van Zyl-Smit RN, Zhang Y. The immunology of tuberculosis: from bench to bedside. Respirology 2010;15:433–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barry CE III, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, Schnappinger D, Wilkinson RJ, Young D. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009;7:845–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol 2008;8:142–152. [DOI] [PubMed] [Google Scholar]
- 18.Hirsch CS, Ellner JJ, Russell DG, Rich EA. Complement receptor–mediated uptake and tumor necrosis factor-α–mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J Immunol 1994;152:743–753. [PubMed] [Google Scholar]
- 19.Sadek MI, Sada E, Toossi Z, Schwander SK, Rich EA. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol 1998;19:513–521. [DOI] [PubMed] [Google Scholar]
- 20.Laviolette M. Lymphocyte fluctuation in bronchoalveolar lavage fluid in normal volunteers. Thorax 1985;40:651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merchant RK, Schwartz DA, Helmers RA, Dayton CS, Hunninghake GW. Bronchoalveolar lavage cellularity: the distribution in normal volunteers. Am Rev Respir Dis 1992;146:448–453. [DOI] [PubMed] [Google Scholar]
- 22.Ettensohn DB, Jankowski MJ, Duncan PG, Lalor PA. Bronchoalveolar lavage in the normal volunteer subject. I. Technical aspects and intersubject variability. Chest 1988;94:275–280. [DOI] [PubMed] [Google Scholar]
- 23.Hunninghake GW, Gadek JE, Kawanami O, Ferrans VJ, Crystal RG. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol 1979;97:149–206. [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds HY, Fulmer JD, Kazmierowski JA, Roberts WC, Frank MM, Crystal RG. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest 1977;59:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lommatzsch M, Bratke K, Bier A, Julius P, Kuepper M, Luttmann W, Virchow JC. Airway dendritic cell phenotypes in inflammatory diseases of the human lung. Eur Respir J 2007;30:878–886. [DOI] [PubMed] [Google Scholar]
- 26.Jafari C, Thijsen S, Sotgiu G, Goletti D, Benitez JA, Losi M, Eberhardt R, Kirsten D, Kalsdorf B, Bossink A, et al. Bronchoalveolar lavage enzyme-linked immunospot for a rapid diagnosis of tuberculosis: a Tuberculosis Network European Trials Group study. Am J Respir Crit Care Med 2009;180:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song K, Bolton DL, Wilson RL, Camp JV, Bao S, Mattapallil JJ, Herzenberg LA, Herzenberg LA, Andrews CA, Sadoff JC, et al. Genetic immunization in the lung induces potent local and systemic immune responses. Proc Natl Acad Sci USA 2010;107:22213–22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwander SK, Torres M, Sada E, Carranza C, Ramos E, Tary-Lehmann M, Wallis RS, Sierra J, Rich EA. Enhanced responses to Mycobacterium tuberculosis antigens by human alveolar lymphocytes during active pulmonary tuberculosis. J Infect Dis 1998;178:1434–1445. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R, et al. Depressed T-cell interferon-γ responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis 1999;180:2069–2073. [DOI] [PubMed] [Google Scholar]
- 30.Schwander SK, Sada E, Torres M, Escobedo D, Sierra JG, Alt S, Rich EA. T lymphocytic and immature macrophage alveolitis in active pulmonary tuberculosis. J Infect Dis 1996;173:1267–1272. [DOI] [PubMed] [Google Scholar]
- 31.Herrera MT, Torres M, Nevels D, Perez-Redondo CN, Ellner JJ, Sada E, Schwander SK. Compartmentalized bronchoalveolar IFN-γ and IL-12 response in human pulmonary tuberculosis. Tuberculosis (Edinb) 2009;89:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson DS, Ying S, Taylor IK, Wangoo A, Mitchell DM, Kay AB, Hamid Q, Shaw RJ. Evidence for a Th1-like bronchoalveolar T-cell subset and predominance of interferon-γ gene activation in pulmonary tuberculosis. Am J Respir Crit Care Med 1994;149:989–993. [DOI] [PubMed] [Google Scholar]
- 33.Law KF, Jagirdar J, Weiden MD, Bodkin M, Rom WN. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am J Respir Crit Care Med 1996;153:1377–1384. [DOI] [PubMed] [Google Scholar]
- 34.Fletcher HA, Owiafe P, Jeffries D, Hill P, Rook GA, Zumla A, Doherty TM, Brookes RH. Increased expression of mRNA encoding interleukin (IL)-4 and its splice variant IL-4δ2 in cells from contacts of Mycobacterium tuberculosis, in the absence of in vitro stimulation. Immunology 2004;112:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dheda K, Chang JS, Breen RA, Haddock JA, Lipman MC, Kim LU, Huggett JF, Johnson MA, Rook GA, Zumla A. Expression of a novel cytokine, IL-4δ2, in HIV and HIV–tuberculosis co-infection. AIDS 2005;19:1601–1606. [DOI] [PubMed] [Google Scholar]
- 36.Almeida AS, Lago PM, Boechat N, Huard RC, Lazzarini LC, Santos AR, Nociari M, Zhu H, Perez-Sweeney BM, Bang H, et al. Tuberculosis is associated with a down-modulatory lung immune response that impairs Th1-type immunity. J Immunol 2009;183:718–731. [DOI] [PubMed] [Google Scholar]
- 37.Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2008;8:359–368. [DOI] [PubMed] [Google Scholar]
- 38.Bermudez LE, Goodman J. Mycobacterium tuberculosis invades and replicates within type II alveolar cells. Infect Immun 1996;64:1400–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, Cho SN, Via LE, Barry CE III. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 2010;137:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faldt J, Dahlgren C, Karlsson A, Ahmed AM, Minnikin DE, Ridell M. Activation of human neutrophils by mycobacterial phenolic glycolipids. Clin Exp Immunol 1999;118:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neufert C, Pai RK, Noss EH, Berger M, Boom WH, Harding CV. Mycobacterium tuberculosis 19-kDa lipoprotein promotes neutrophil activation. J Immunol 2001;167:1542–1549. [DOI] [PubMed] [Google Scholar]
- 42.Korbel DS, Schneider BE, Schaible UE. Innate immunity in tuberculosis: myths and truth. Microbes Infect 2008;10:995–1004. [DOI] [PubMed] [Google Scholar]
- 43.Kisich KO, Higgins M, Diamond G, Heifets L. Tumor necrosis factor α stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect Immun 2002;70:4591–4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martineau AR, Newton SM, Wilkinson KA, Kampmann B, Hall BM, Nawroly N, Packe GE, Davidson RN, Griffiths CJ, Wilkinson RJ. Neutrophil-mediated innate immune resistance to mycobacteria. J Clin Invest 2007;117:1988–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Persson YA, Blomgran-Julinder R, Rahman S, Zheng L, Stendahl O. Mycobacterium tuberculosis–induced apoptotic neutrophils trigger a pro-inflammatory response in macrophages through release of heat shock protein 72, acting in synergy with the bacteria. Microbes Infect 2008;10:233–240. [DOI] [PubMed] [Google Scholar]
- 46.Tan BH, Meinken C, Bastian M, Bruns H, Legaspi A, Ochoa MT, Krutzik SR, Bloom BR, Ganz T, Modlin RL, et al. Macrophages acquire neutrophil granules for antimicrobial activity against intracellular pathogens. J Immunol 2006;177:1864–1871. [DOI] [PubMed] [Google Scholar]
- 47.Liu PT, Modlin RL. Human macrophage host defense against Mycobacterium tuberculosis. Curr Opin Immunol 2008;20:371–376. [DOI] [PubMed] [Google Scholar]
- 48.Bulut Y, Michelsen KS, Hayrapetian L, Naiki Y, Spallek R, Singh M, Arditi M. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. J Biol Chem 2005;280:20961–20967. [DOI] [PubMed] [Google Scholar]
- 49.Reiling N, Holscher C, Fehrenbach A, Kroger S, Kirschning CJ, Goyert S, Ehlers S. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J Immunol 2002;169:3480–3484. [DOI] [PubMed] [Google Scholar]
- 50.Bafica A, Scanga CA, Feng CG, Leifer C, Cheever A, Sher A. TLR9 regulates Th1 responses and cooperates with TLR2 in mediating optimal resistance to Mycobacterium tuberculosis. J Exp Med 2005;202:1715–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pompei L, Jang S, Zamlynny B, Ravikumar S, McBride A, Hickman SP, Salgame P. Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J Immunol 2007;178:5192–5199. [DOI] [PubMed] [Google Scholar]
- 52.Rivas-Santiago B, Schwander SK, Sarabia C, Diamond G, Klein-Patel ME, Hernandez-Pando R, Ellner JJ, Sada E. Human β-defensin 2 is expressed and associated with Mycobacterium tuberculosis during infection of human alveolar epithelial cells. Infect Immun 2005;73:4505–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doz E, Rose S, Court N, Front S, Vasseur V, Charron S, Gilleron M, Puzo G, Fremaux I, Delneste Y, et al. Mycobacterial phosphatidylinositol mannosides negatively regulate host Toll-like receptor 4, MyD88-dependent proinflammatory cytokines, and TRIF-dependent co-stimulatory molecule expression. J Biol Chem 2009;284:23187–23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pathak SK, Basu S, Basu KK, Banerjee A, Pathak S, Bhattacharyya A, Kaisho T, Kundu M, Basu J. Direct extracellular interaction between the early secreted antigen ESAT-6 of Mycobacterium tuberculosis and TLR2 inhibits TLR signaling in macrophages. Nat Immunol 2007;8:610–618. [DOI] [PubMed] [Google Scholar]
- 55.Pai RK, Convery M, Hamilton TA, Boom WH, Harding CV. Inhibition of IFN-γ–induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J Immunol 2003;171:175–184. [DOI] [PubMed] [Google Scholar]
- 56.Noss EH, Pai RK, Sellati TJ, Radolf JD, Belisle J, Golenbock DT, Boom WH, Harding CV. Toll-like receptor 2–dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J Immunol 2001;167:910–918. [DOI] [PubMed] [Google Scholar]
- 57.Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for Toll-like receptors. Nat Rev Microbiol 2010;8:296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deretic V, Delgado M, Vergne I, Master S, De HS, Ponpuak M, Singh S. Autophagy in immunity against Mycobacterium tuberculosis: a model system to dissect immunological roles of autophagy. Curr Top Microbiol Immunol 2009;335:169–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kumar D, Nath L, Kamal MA, Varshney A, Jain A, Singh S, Rao KV. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell 2010;140:731–743. [DOI] [PubMed] [Google Scholar]
- 60.Singh SB, Davis AS, Taylor GA, Deretic V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 2006;313:1438–1441. [DOI] [PubMed] [Google Scholar]
- 61.Juarez E, Nunez C, Sada E, Ellner JJ, Schwander SK, Torres M. Differential expression of Toll-like receptors on human alveolar macrophages and autologous peripheral monocytes. Respir Res 2010;11:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S, Schlesinger LS. Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol 2008;180:7847–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB, Voelker DR, Crouch EC, Schlesinger LS. Surfactant protein D increases fusion of Mycobacterium tuberculosis–containing phagosomes with lysosomes in human macrophages. Infect Immun 2006;74:7005–7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuroki Y, Takahashi M, Nishitani C. Pulmonary collectins in innate immunity of the lung. Cell Microbiol 2007;9:1871–1879. [DOI] [PubMed] [Google Scholar]
- 65.Gaynor CD, McCormack FX, Voelker DR, McGowan SE, Schlesinger LS. Pulmonary surfactant protein A mediates enhanced phagocytosis of Mycobacterium tuberculosis by a direct interaction with human macrophages. J Immunol 1995;155:5343–5351. [PubMed] [Google Scholar]
- 66.Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate–lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol 1999;163:312–321. [PubMed] [Google Scholar]
- 67.Daley CL, Small PM, Schecter GF, Schoolnik GK, McAdam RA, Jacobs WR Jr, Hopewell PC. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus: an analysis using restriction-fragment-length polymorphisms. N Engl J Med 1992;326:231–235. [DOI] [PubMed] [Google Scholar]
- 68.Tan JS, Canaday DH, Boom WH, Balaji KN, Schwander SK, Rich EA. Human alveolar T lymphocyte responses to Mycobacterium tuberculosis antigens: role for CD4+ and CD8+ cytotoxic T cells and relative resistance of alveolar macrophages to lysis. J Immunol 1997;159:290–297. [PubMed] [Google Scholar]
- 69.Jacobsen M, Detjen AK, Mueller H, Gutschmidt A, Leitner S, Wahn U, Magdorf K, Kaufmann SH. Clonal expansion of CD8+ effector T cells in childhood tuberculosis. J Immunol 2007;179:1331–1339. [DOI] [PubMed] [Google Scholar]
- 70.Kamath AB, Woodworth J, Xiong X, Taylor C, Weng Y, Behar SM. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J Exp Med 2004;200:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Canaday DH, Wilkinson RJ, Li Q, Harding CV, Silver RF, Boom WH. CD4+ and CD8+ T cells kill intracellular Mycobacterium tuberculosis by a perforin and Fas/Fas ligand–independent mechanism. J Immunol 2001;167:2734–2742. [DOI] [PubMed] [Google Scholar]
- 72.Lewinsohn DA, Heinzel AS, Gardner JM, Zhu L, Alderson MR, Lewinsohn DM. Mycobacterium tuberculosis-specific CD8+ T cells preferentially recognize heavily infected cells. Am J Respir Crit Care Med 2003;168:1346–1352. [DOI] [PubMed] [Google Scholar]
- 73.Woodworth JS, Wu Y, Behar SM. Mycobacterium tuberculosis–specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J Immunol 2008;181:8595–8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jeyanathan M, Mu J, McCormick S, Damjanovic D, Small CL, Shaler C, Kugathasan K, Xing Z. Mechanisms of airway luminal anti-tuberculosis memory. Am J Respir Crit Care Med 2010;181:862–872. [DOI] [PubMed] [Google Scholar]
- 75.Dheda K, Chang JS, Lala S, Huggett JF, Zumla A, Rook GA. Gene expression of IL17 and IL23 in the lungs of patients with active tuberculosis. Thorax 2008;63:566–568. [DOI] [PubMed] [Google Scholar]
- 76.Pasquinelli V, Townsend JC, Jurado JO, Alvarez IB, Quiroga MF, Barnes PF, Samten B, Garcia VE. IFN-γ production during active tuberculosis is regulated by mechanisms that involve IL-17, SLAM, and CREB. J Infect Dis 2009;199:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cruz A, Khader SA, Torrado E, Fraga A, Pearl JE, Pedrosa J, Cooper AM, Castro AG. Cutting edge: IFN-γ regulates the induction and expansion of IL-17–producing CD4 T cells during mycobacterial infection. J Immunol 2006;177:1416–1420. [DOI] [PubMed] [Google Scholar]
- 78.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 2007;8:369–377. [DOI] [PubMed] [Google Scholar]
- 79.Ribeiro-Rodrigues R, Resende CT, Rojas R, Toossi Z, Dietze R, Boom WH, Maciel E, Hirsch CS. A role for CD4+CD25+ T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol 2006;144:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delgado JC, Tsai EY, Thim S, Baena A, Boussiotis VA, Reynes JM, Sath S, Grosjean P, Yunis EJ, Goldfeld AE. Antigen-specific and persistent tuberculin anergy in a cohort of pulmonary tuberculosis patients from rural Cambodia. Proc Natl Acad Sci USA 2002;99:7576–7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, Barnes PF. Interleukin-10 downregulates Mycobacterium tuberculosis–induced Th1 responses and CTLA-4 expression. Infect Immun 1996;64:913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med 2006;173:803–810. [DOI] [PubMed] [Google Scholar]
- 83.Hougardy JM, Place S, Hildebrand M, Drowart A, Debrie AS, Locht C, Mascart F. Regulatory T cells depress immune responses to protective antigens in active tuberculosis. Am J Respir Crit Care Med 2007;176:409–416. [DOI] [PubMed] [Google Scholar]
- 84.Li L, Wu CY. CD4+ CD25+ Treg cells inhibit human memory gammadelta T cells to produce IFN-γ in response to M. tuberculosis antigen ESAT-6. Blood 2008;111:5629–5636. [DOI] [PubMed] [Google Scholar]
- 85.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, Berezovskaya A, Rousset D, Reynes JM, Goldfeld AE. IL-10–producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest 2000;105:1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Joosten SA, van Meijgaarden KE, Savage ND, de Boer T, Triebel F, van der Wal A, de Heer E, Klein MR, Geluk A, Ottenhoff TH. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci USA 2007;104:8029–8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kursar M, Koch M, Mittrucker HW, Nouailles G, Bonhagen K, Kamradt T, Kaufmann SH. Cutting edge: regulatory T cells prevent efficient clearance of Mycobacterium tuberculosis. J Immunol 2007;178:2661–2665. [DOI] [PubMed] [Google Scholar]
- 88.McGuirk P, Higgins SC, Mills KH. The role of regulatory T cells in respiratory infections and allergy and asthma. Curr Allergy Asthma Rep 2010;10:21–28. [DOI] [PubMed] [Google Scholar]
- 89.Durrant DM, Metzger DW. Emerging roles of T helper subsets in the pathogenesis of asthma. Immunol Invest 2010;39:526–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lane N, Robins RA, Corne J, Fairclough L. Regulation in chronic obstructive pulmonary disease: the role of regulatory T-cells and Th17 cells. Clin Sci (Lond) 2010;119:75–86. [DOI] [PubMed] [Google Scholar]
- 91.Kotsianidis I, Nakou E, Bouchliou I, Tzouvelekis A, Spanoudakis E, Steiropoulos P, Sotiriou I, Aidinis V, Margaritis D, Tsatalas C, et al. Global impairment of CD4+CD25+FOXP3+ regulatory T cells in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2009;179:1121–1130. [DOI] [PubMed] [Google Scholar]
- 92.Gerke AK, Hunninghake G. The immunology of sarcoidosis. Clin Chest Med 2008;29:379–390. (vii.). [DOI] [PubMed] [Google Scholar]
- 93.Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, Martin B, Wilhelm C, Stockinger B. Transforming growth factor-β “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat Immunol 2008;9:1341–1346. [DOI] [PubMed] [Google Scholar]
- 94.Beriou G, Bradshaw EM, Lozano E, Costantino CM, Hastings WD, Orban T, Elyaman W, Khoury SJ, Kuchroo VK, Baecher-Allan C, et al. TGF-β induces IL-9 production from human Th17 cells. J Immunol 2010;185:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kurowska-Stolarska M, Kewin P, Murphy G, Russo RC, Stolarski B, Garcia CC, Komai-Koma M, Pitman N, Li Y, Niedbala W, et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J Immunol 2008;181:4780–4790. [DOI] [PubMed] [Google Scholar]
- 96.Costello AM, Kumar A, Narayan V, Akbar MS, Ahmed S, Abou-Zeid C, Rook GA, Stanford J, Moreno C. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis? Trans R Soc Trop Med Hyg 1992;86:686–692. [DOI] [PubMed] [Google Scholar]
- 97.Glatman-Freedman A. Advances in antibody-mediated immunity against Mycobacterium tuberculosis: implications for a novel vaccine strategy. FEMS Immunol Med Microbiol 2003;39:9–16. [DOI] [PubMed] [Google Scholar]
- 98.Comstock GW, Livesay VT, Woolpert SF. The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol 1974;99:131–138. [DOI] [PubMed] [Google Scholar]
- 99.Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, Zumla A. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet 2010;375:1920–1937. [DOI] [PubMed] [Google Scholar]
- 100.Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, et al.: South African Tuberculosis Vaccine Initiative. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis, following BCG vaccination of newborns. Am J Respir Crit Care Med 2010;182:1073–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dheda K, Chang JS, Breen RA, Kim LU, Haddock JA, Huggett JF, Johnson MA, Rook GA, Zumla A. In vivo and in vitro studies of a novel cytokine, interleukin 4δ2, in pulmonary tuberculosis. Am J Respir Crit Care Med 2005;172:501–508. [DOI] [PubMed] [Google Scholar]
- 102.Rook GA, Dheda K, Zumla A. Immune responses to tuberculosis in developing countries: implications for new vaccines. Nat Rev Immunol 2005;5:661–667. [DOI] [PubMed] [Google Scholar]
- 103.Barker LF, Brennan MJ, Rosenstein PK, Sadoff JC. Tuberculosis vaccine research: the impact of immunology. Curr Opin Immunol 2009;21:331–338. [DOI] [PubMed] [Google Scholar]
- 104.Place S, Verscheure V, de San N, Hougardy JM, Schepers K, Dirix V, Dediste A, Michel O, Drowart A, Allard SD, et al. Heparin-binding, hemagglutinin-specific IFN-γ synthesis at the site of infection during active tuberculosis in humans. Am J Respir Crit Care Med 2010;182:848–854. [DOI] [PubMed] [Google Scholar]
- 105.Dawson R, Condos R, Tse D, Huie ML, Ress S, Tseng CH, Brauns C, Weiden M, Hoshino Y, Bateman E, et al. Immunomodulation with recombinant interferon-γ1b in pulmonary tuberculosis. PLoS ONE 2009;4:e6984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Condos R, Raju B, Canova A, Zhao BY, Weiden M, Rom WN, Pine R. Recombinant γ interferon stimulates signal transduction and gene expression in alveolar macrophages in vitro and in tuberculosis patients. Infect Immun 2003;71:2058–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Condos R, Hull FP, Schluger NW, Rom WN, Smaldone GC. Regional deposition of aerosolized interferon-γ in pulmonary tuberculosis. Chest 2004;125:2146–2155. [DOI] [PubMed] [Google Scholar]
- 108.Dheda K, van Zyl SR, Badri M, Pai M. T-cell interferon-γ release assays for the rapid immunodiagnosis of tuberculosis: clinical utility in high-burden vs. low-burden settings. Curr Opin Pulm Med 2009;15:188–200. [DOI] [PubMed] [Google Scholar]
- 109.Dheda K, van Zyl-Smit RN, Sechi LA, Badri M, Meldau R, Meldau S, Symons G, Semple PL, Maredza A, Dawson R, et al. Utility of quantitative T-cell responses versus unstimulated interferon-γ for the diagnosis of pleural tuberculosis. Eur Respir J 2009;34:1118–1126. [DOI] [PubMed] [Google Scholar]
- 110.Patel VB, Singh R, Connolly C, Coovadia Y, Peer AK, Parag P, Kasprowicz V, Zumla A, Ndung'u T, Dheda K. Cerebrospinal T-cell responses aid in the diagnosis of tuberculous meningitis in a human immunodeficiency virus– and tuberculosis-endemic population. Am J Respir Crit Care Med 2010;182:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dheda K, van Zyl-Smit RN, Meldau R, Meldau S, Symons G, Khalfey H, Govender N, Rosu V, Sechi LA, Maredza A, et al. Quantitative lung T cell responses aid the rapid diagnosis of pulmonary tuberculosis. Thorax 2009;64:847–853. [DOI] [PubMed] [Google Scholar]
- 112.Kantrow SP, Meyer KC, Kidd P, Raghu G. The CD4/CD8 ratio in BAL fluid is highly variable in sarcoidosis. Eur Respir J 1997;10:2716–2721. [DOI] [PubMed] [Google Scholar]
- 113.Horster R, Kirsten D, Gaede KI, Jafari C, Strassburg A, Greinert U, Kalsdorf B, Ernst M, Lange C. Antimycobacterial immune responses in patients with pulmonary sarcoidosis. Clin.Respir.J. 2009;3:229–238. [DOI] [PubMed] [Google Scholar]
- 114.Cashmore TJ, Peter JG, van Zyl-Smit RN, Semple PL, Maredza A, Meldau R, Zumla A, Nurse B, Dheda K. Feasibility and diagnostic utility of antigen-specific interferon-γ responses for rapid immunodiagnosis of tuberculosis using induced sputum. PLoS ONE 2010;5:e10389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Adetifa IM, Ota MO, Walther B, Hammond AS, Lugos MD, Jeffries DJ, Donkor SA, Adegbola RA, Hill PC. Decay kinetics of an interferon γ release assay with anti-tuberculosis therapy in newly diagnosed tuberculosis cases. PLoS ONE 2010;5:e12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dheda K, Davids V, Lenders L, Roberts T, Meldau R, Ling D, Brunet L, van Zyl SR, Peter J, Green C, et al. Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS ONE 2010;5:e9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Phillips M, Basa-Dalay V, Bothamley G, Cataneo RN, Lam PK, Natividad MP, Schmitt P, Wai J. Breath biomarkers of active pulmonary tuberculosis. Tuberculosis (Edinb) 2010;90:145–151. [DOI] [PubMed] [Google Scholar]