Abstract
Rationale: Respiratory virus infections are associated with chronic obstructive pulmonary disease (COPD) exacerbations, but a causative relationship has not been proven. Studies of naturally occurring exacerbations are difficult and the mechanisms linking virus infection to exacerbations are poorly understood. We hypothesized that experimental rhinovirus infection in subjects with COPD would reproduce the features of naturally occurring COPD exacerbations and is a valid model of COPD exacerbations.
Objectives: To evaluate experimental rhinovirus infection as a model of COPD exacerbation and to investigate the mechanisms of virus-induced exacerbations.
Methods: We used experimental rhinovirus infection in 13 subjects with COPD and 13 nonobstructed control subjects to investigate clinical, physiologic, pathologic, and antiviral responses and relationships between virus load and these outcomes.
Measurements and Main Results: Clinical data; inflammatory mediators in blood, sputum, and bronchoalveolar lavage; and viral load in nasal lavage, sputum, and bronchoalveolar lavage were measured at baseline and after infection with rhinovirus 16. After rhinovirus infection subjects with COPD developed lower respiratory symptoms, airflow obstruction, and systemic and airway inflammation that were greater and more prolonged compared with the control group. Neutrophil markers in sputum related to clinical outcomes and virus load correlated with inflammatory markers. Virus load was higher and IFN production by bronchoalveolar lavage cells was impaired in the subjects with COPD.
Conclusions: We have developed a new model of COPD exacerbation that strongly supports a causal relationship between rhinovirus infection and COPD exacerbations. Impaired IFN production and neutrophilic inflammation may be important mechanisms in virus-induced COPD exacerbations.
Keywords: disease exacerbation, respiratory tract infections, COPD, rhinovirus
AT A GLANCE COMMENTARY.
Current Scientific Knowledge on the Subject
Respiratory virus infections are associated with chronic obstructive pulmonary disease (COPD) exacerbations, but a causative relationship has not been established and mechanisms of virus-associated exacerbations are undetermined.
What This Study Adds to the Field
We report that experimental rhinovirus infection in subjects with COPD reproduced the clinical, physiologic, and inflammatory features of COPD exacerbations and implicates neutrophilic inflammation and impaired IFN production as important mechanisms of virus-induced COPD exacerbations.
Chronic obstructive pulmonary disease (COPD) is a growing global epidemic and its prevalence is expected to increase markedly in the future (1). The major cause of morbidity and mortality in COPD is acute exacerbations that are associated with impaired quality of life (2, 3), accelerated loss of lung function (4), and enormous health care costs (5). Current therapies are only partially effective, have significant side effects, and may not address the true underlying mechanisms (6, 7). Exacerbations are associated with increased airway inflammation; however, studies of cellular and molecular inflammatory mediators in COPD exacerbations have yielded conflicting results (8–13). This is due to factors such as heterogeneous exacerbation etiologies, variations in time from onset to investigation, effects of treatment, and difficulties with both baseline and acute sampling that are inherent in studies of naturally occurring exacerbations. Respiratory virus infections, most commonly rhinoviruses, can be detected in COPD exacerbations but detection rates vary (11, 13–16) and viruses are found in stable COPD also (14, 17). Therefore a causal relationship between respiratory virus infection and acute exacerbations in patients with COPD has not been established. In healthy individuals rhinovirus infections cause a predominantly upper respiratory tract illness with little evidence of lower respiratory tract involvement (18, 19). In COPD the association of rhinovirus infection with exacerbations is suggestive of increased susceptibility to infection and lower respiratory tract involvement; however, this has not been proven and the mechanisms involved are unknown.
Experimental rhinovirus infection studies in asthma have yielded important insights into disease mechanisms, and have identified candidates for the development of new therapies (19–21). No similar experimental model has existed for studying mechanisms of COPD exacerbations. Such a model could overcome the sources of variation in naturally occurring exacerbations and provide a tool with which to investigate the relationship between virus infection and COPD exacerbations. We previously reported an uncontrolled pilot study investigating experimental infection with low-dose rhinovirus in four patients with COPD; infection induced symptoms consistent with COPD exacerbation and no adverse safety signal was observed (22). In the present study we aimed to demonstrate that this model is valid in larger numbers of subjects with COPD and to perform intensive lower airway sampling to investigate airway inflammation in virus-induced COPD exacerbations. In addition, we also infected a group of smokers without airflow obstruction to investigate mechanisms of susceptibility to virus infection in COPD. Here we report that rhinovirus infection induces the symptomatic, physiologic, and inflammatory features that have been reported in naturally occurring exacerbations, and that infection is associated with neutrophilic inflammation and deficient production of IFN-β in subjects with COPD. Some of the results of this study have been previously reported in abstract form (23–25).
METHODS
Participants
Subjects were recruited from Imperial College Healthcare NHS Trust (St. Mary's Hospital), the Royal Brompton Hospital, local general practices, and by advertisement. Ethics approval was obtained from the local research ethics committee (study number 00/BA/459E) and informed consent was obtained from all subjects. The inclusion/exclusion criteria for the subjects with COPD were identical to those used in our pilot study (22) and a group of control subjects with a similar smoking history but with normal lung function was also recruited (see Table E1 in the online supplement).
Study Design and Experimental Infection Protocol
We aimed to recruit and infect 13 subjects in each group, as such numbers had yielded significant findings in similar studies in asthma (19, 20, 26). At initial screening visits suitability for the study was assessed, informed consent was obtained, and serum neutralizing antibodies to rhinovirus 16 were measured (19). For those entering the study baseline clinical sampling was performed 1–4 weeks before virus inoculation, which was on Study Day 0. Subjects were seen daily on the 9 days immediately after inoculation, on Days 12 and 15, and then weekly until 6 weeks after inoculation. The timeline for clinical measurements and sampling is outlined in Table E2.
Clinical Procedures
Upper and lower respiratory symptom scores.
Daily diary cards of upper and lower respiratory symptoms were commenced at screening and continued until 6 weeks after inoculation. The symptom scores and cold and exacerbation criteria were the same as those used in the pilot study and are described in the expanded Methods in the online supplement and in Table E3.
Pulmonary function.
Spirometry was performed with a Micromedical MicroLab spirometer (MicroMedical, Rochester, UK) according to British Thoracic Society guidelines (27) before and 15 minutes after administration of salbutamol (200 μg) via metered dose inhaler and large volume spacer for pre- and postbronchodilator values. Carbon monoxide diffusion capacity corrected for alveolar volume (Kco) was measured with a Vmax 229 (Viasys Healthcare, Warwickshire, UK) in the pulmonary function laboratory of St. Mary's Hospital, Imperial College Healthcare NHS Trust according to established protocols.
Virus inoculation.
Details regarding the preparation and safety testing of the rhinovirus 16 inoculum used in this study have been published (28). The virus (10 TCID50) was diluted in a total volume of 1 ml of 0.9% saline and inoculated into both nostrils, using an atomizer (no. 286; DeVilbiss Co., Heston, UK).
Nasal lavage, induced sputum, and bronchoscopy.
Sputum was induced by inhalations of hypertonic saline according to European Respiratory Society guidelines (29) and processed according to standard protocols (30). Bronchoscopies were performed according to British Thoracic Society guidelines for research bronchoscopies (31). Details of nasal lavage, sputum, and bronchoalveolar lavage (BAL) processing are provided in the online supplement.
Virus detection.
Rhinovirus and other respiratory viruses were detected by polymerase change reaction (PCR) according to previously established protocols (19). Infection with viruses other than rhinoviruses was excluded by testing nasal lavage by PCR. Details are provided in the online supplement.
Inflammatory mediators.
IL-6, IL-8, neutrophil elastase, and tumor necrosis factor (TNF)-α were measured in sputum and BAL supernatants. IFN-β, IFN-α, and IFN-λ– and IFN-γ–inducible protein 10 (CXCL10) were measured in supernatants from BAL cells cultured ex vivo. All soluble mediators were measured by ELISA; details are provided in the online supplement. Serum C-reactive protein (CRP) and peripheral blood cell counts were measured in the Clinical Biochemistry and Haematology Laboratories of St. Mary's Hospital, Imperial College Healthcare NHS Trust.
Statistical Analysis
Data are presented as mean (± SEM) values for normally distributed data or as median (interquartile range) values for nonparametric data. Data were assessed for normal distribution using the Shapiro-Wilk normality test. Changes from baseline were analyzed by repeated measures analysis of variance (Friedman test for nonparametric data) and, if significant, paired t tests or Wilcoxon matched pairs test. Differences between groups were analyzed by unpaired t tests or Mann-Whitney tests. Correlations between data sets were examined using Pearson's correlation for normally distributed data and Spearman's rank correlation coefficient for nonparametric data. Differences were considered significant for all statistical tests at P values less than 0.05. All reported P values are two-sided. Analysis was performed with GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Thirteen subjects were recruited in each group and inoculated with low-dose rhinovirus 16. In 3 of 26 subjects (2 patients with COPD and 1 control subject) rhinovirus was not detected after inoculation and therefore they were excluded from further analysis. The clinical characteristics of the 23 successfully infected subjects included in the data analysis are shown in Table 1. After inoculation there were no adverse events and no subjects withdrew from the study or required corticosteroids, antibiotics, or hospital admission.
TABLE 1.
CLINICAL CHARACTERISTICS OF THE 23 STUDY SUBJECTS
| Subject Characteristic | Patients with COPD (n = 11) | Control Subjects (n = 12) | P Value (unpaired t test) |
|---|---|---|---|
| Age, yr | 59.6 (47–70) | 48.5 (40–58) | 0.0021 |
| Sex, M/F | 6/5 | 6/6 | NS |
| Smoking history, pack-years* | 48 (20–109) | 34.8 (20–60) | NS |
| Current smokers, no. | 8 | 9 | NS |
| FEV1, L | 1.94 (1.23–2.7) | 3.58 (2.8–4.76) | <0.0001 |
| FEV1, % of predicted normal value | 69.73 (62–78) | 109.5 (90–128) | <0.0001 |
| FEV1/FVC, % | 55.55 (39–69) | 80.33 (73–86) | <0.0001 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; F = female; M = male; NS = not significant.
Values are presented as means (range).
Pack-years, the number of cigarettes smoked per day multiplied by the number of years of smoking.
Clinical Responses to Rhinovirus Infection
Upper respiratory symptoms.
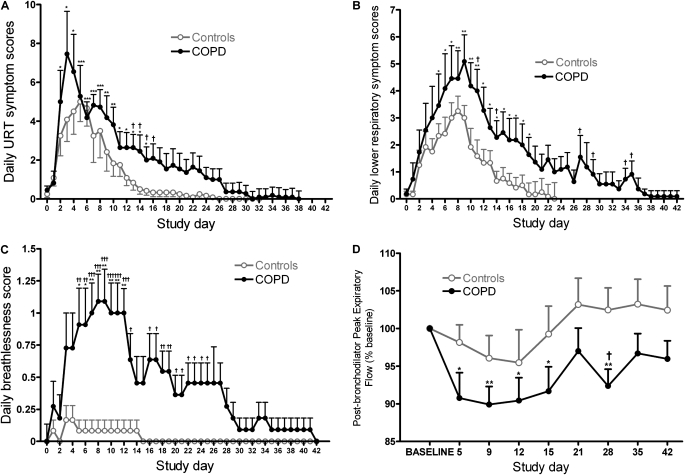
Twenty-one of the 23 subjects fulfilled the symptom criteria for a clinical cold (see the online supplement). The remaining two subjects (one in each group) had the subjective sensation of a cold but did not fulfill the other two symptom criteria; however, both had virologic evidence of rhinovirus infection. Daily upper respiratory symptom scores were significantly increased above baseline in the COPD group on Days 2–14 postinoculation and on Days 1–12 in the control subjects (Figure 1A) and were significantly greater in the COPD group compared with the control subjects on Days 13–16.
Figure 1.
Symptom scores and lung function during experimental rhinovirus infection. The time course for daily symptom scores is shown for (A) total daily upper respiratory tract (URT) symptoms, (B) total daily lower respiratory symptoms, and (C) breathlessness. (D) The time course of postbronchodilator peak expiratory flow as a percentage of baseline (all values represent means ± SEM). *P < 0.05 versus baseline; **P < 0.01 versus baseline; †P < 0.05, patients with chronic obstructive pulmonary disease (COPD) versus control subjects; ††P < 0.01, patients with COPD versus control subjects; †††P < 0.001, patients with COPD versus control subjects.
Lower respiratory symptoms.
Ten of the 11 infected subjects with COPD fulfilled the symptom criteria for a COPD exacerbation. Daily total lower respiratory symptom scores were increased significantly over baseline on Days 5–19 in the COPD group and on Days 3–9 in the control subjects (Figure 1B). Daily lower respiratory symptoms were significantly greater in the COPD group compared with control subjects on Days 11, 14, 27, 29, 34, and 35. When the individual symptoms were analyzed daily breathlessness scores were increased over baseline on Days 5–12 in the COPD group, whereas breathlessness did not increase significantly in the control subjects (Figure 1C). Daily breathlessness scores were significantly higher in the COPD group than in control subjects on Days 5 through 25, except for Days 14 and 15.
Lung function.
Postbronchodilator peak expiratory flow (PEF) fell significantly from baseline in the COPD group, but no significant reductions were observed in the control subjects (Figure 1D), and on Day 28 PEF was significantly lower in the COPD group compared with the control subjects (92.36 ± 2.23 vs. 102.5 ± 2.95%; P = 0.014). The nadir of PEF occurred on Day 9 and the mean absolute fall in PEF at this time point in the COPD group was 121 L/minute. Kco (% baseline) on Day 12 fell significantly from baseline in the COPD group (100 vs. 93.82 ± 1.52; P = 0.0027) but not in the control subjects (100 vs. 96.18 ± 2.15; P = 0.1) There were small falls from baseline in FEV1, FVC, and the FEV1/FVC ratio that were of similar magnitude in both groups but were not statistically significant.
Inflammatory Responses to Rhinovirus Infection
Blood.
There was a significant increase in blood neutrophils on Day 15 compared with baseline in the COPD group; however, no significant change in blood cell counts was observed in the control group. There were significant increases from baseline in serum CRP on Day 5 in both the COPD and control groups, and CRP was increased above baseline on Day 9 only in the COPD group. There were no significant differences between groups in blood neutrophils or CRP (Table E4).
Sputum.
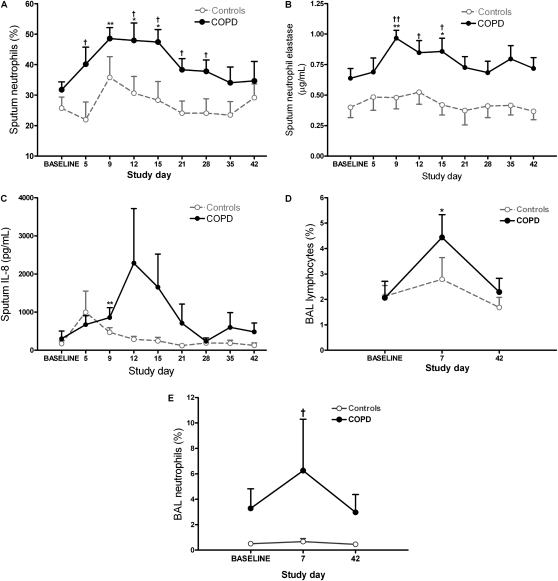
Adequate sputum samples (<20% squamous cells) were obtained from 10 of 11 subjects with COPD and from 11 of 12 control subjects. Percent sputum neutrophils increased significantly over baseline on Days 5, 9, 12, and 15 in the subjects with COPD, whereas there was no significant increase in sputum neutrophils in the control subjects (Figure 2A). Sputum neutrophil percentages were significantly higher in the COPD group compared with the control subjects on Days 5, 12, 15, 21, and 28. Sputum supernatant neutrophil elastase levels were significantly increased over baseline on Days 9 and 15, as were IL-8 levels on Day 9, in the subjects with COPD, whereas no significant increases were observed in the control subjects (Figures 2B and 2C). Sputum neutrophil elastase levels were significantly higher in subjects with COPD compared with control subjects on Days 9–15. There were no statistically significant changes from baseline in sputum supernatant levels of IL-6 or TNF-α in either group, and no statistically significant differences in sputum IL-6, IL-8, or TNF-α between groups (Table E4).
Figure 2.
Airway inflammatory cells and soluble mediators during experimental rhinovirus infection. Shown is the time course of (A) percentage neutrophils, (B) neutrophil elastase, and (C) IL-8 in induced sputum. (D and E) Lymphocytes and neutrophils, respectively, in bronchoalveolar lavage (BAL). All values represent means ± SEM. *P < 0.05 versus baseline; **P < 0.01 versus baseline; †P < 0.05, patients with chronic obstructive pulmonary disease (COPD) versus control subjects; ††P < 0.01, patients with COPD versus control subjects.
BAL.
BAL was not obtained from one subject with COPD at the infection bronchoscopy. There were no significant differences in cell viability or differential cell count between the two groups at baseline. There was a significant increase in BAL lymphocyte percentage from baseline to infection (Figure 2D) in the COPD group but not in the control subjects. There was no significant change between baseline and infection in BAL neutrophils; however, BAL neutrophils at infection were significantly higher in the COPD group compared with the control subjects (Figure 2E). There was a significant increase from baseline to infection in BAL fluid IL-6 levels in the COPD group, but not in the control subjects, whereas BAL fluid neutrophil elastase, IL-8, and TNF-α did not increase significantly in either group. There were no significant differences between groups in terms of BAL lymphocytes or BAL fluid levels of neutrophil elastase, IL-6, IL-8, or TNF-α (Table E5).
Virologic Responses
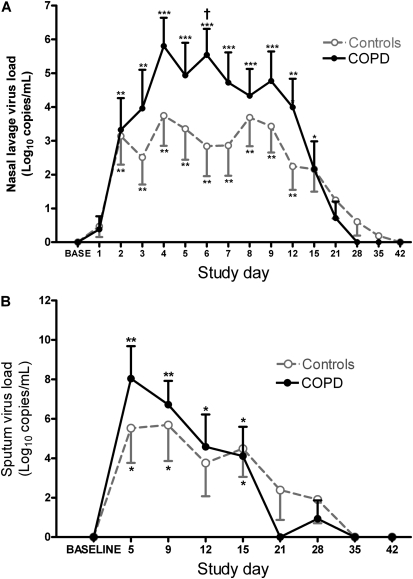
Infection with rhinovirus was confirmed by PCR in all 23 subjects. After inoculation with rhinovirus 16, nasal lavage virus load (log10 copies/ml) showed a rapid rise in both groups commencing approximately 48 hours after inoculation in a manner typical of an acute infection (Figure 3A and Table E6). Virus load peaked on Days 4–8 in nasal lavage and in sputum on Day 5, remaining significantly elevated over baseline up to Day 15. Nasal lavage virus load was 1–2 logs higher in the COPD group compared with the control subjects from Day 3 to Day 12, and this difference was statistically significant on Day 6. Sputum virus load was also 1–3 logs higher in the COPD group on Days 5 and 9, but this was not statistically significant (Figure 3B). In the COPD group, peak virus load was 1–2 logs (not significantly) higher in nasal lavage (7.0 ± 0.58, 5.85 ± 0.47; P = 0.14), sputum (9.44 ± 0.95, 8.53 ± 0.34; P = 0.41), and BAL (6.26 ± 0.63, 5.09 ± 0.24; P = 0.14).
Figure 3.
Virus load in nasal lavage and sputum. The time course of virus load, measured by quantitative polymerase chain reaction, is shown in (A) nasal lavage and (B) sputum (values in both panels represent means ± SEM). *P < 0.005 versus baseline; **P < 0.01 versus baseline; ***P < 0.001 versus baseline; †P < 0.05, patients with chronic obstructive pulmonary disease (COPD) versus control subjects.
Relationships between Virus Load, Inflammatory Markers, and Clinical Outcomes
In the subjects with COPD there were strong correlations between sputum neutrophil elastase levels and clinical outcomes: peak increase in sputum neutrophil elastase correlated with peak total lower respiratory symptom scores (r = 0.62, P = 0.044), peak breathlessness scores (r = 0.62, P = 0.042), and maximal fall in PEF (r = −0.8, P = 0.003) and FEV1/FVC ratio (r = −0.68, P = 0.021). Peak sputum neutrophil numbers also correlated with fall in PEF (r = −0.77, P = 0.0092).
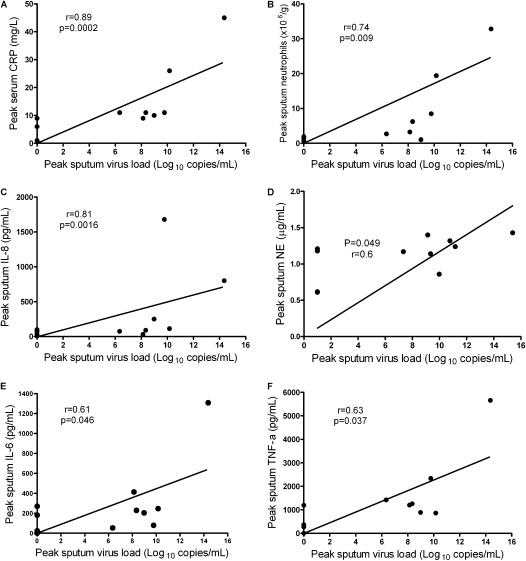
Peak sputum virus load in subjects with COPD correlated positively with each of peak serum CRP levels (r = 0.89, P = 0.0002), peak sputum neutrophil numbers (r = 0.74, P = 0.009), and peak sputum supernatant levels of IL-8 (r = 0.81, P = 0.0016), IL-6 (r = 0.61, P = 0.046), neutrophil elastase (r = 0.60, P = 0.049), and TNF-α (r = 0.63, P = 0.037) (Figure 4). BAL virus load in subjects with COPD correlated positively with BAL fluid levels of neutrophil elastase (r = 0.86, P = 0.0013), IL-8 (r = 0.65, P = 0.042), and IL-6 (r = 0.67, P = 0.034). There were no significant correlations between virus load and inflammatory markers in the control subjects.
Figure 4.
Correlations between sputum virus load and inflammatory markers. Relationships between sputum virus load and inflammatory markers in subjects with chronic obstructive pulmonary disease (COPD) are shown. There were significant correlations between peak sputum virus load and (A) peak serum C-reactive protein (CRP), (B) peak sputum neutrophils, (C) peak sputum IL-8, (D) peak sputum neutrophil elastase (NE), (E) peak sputum IL-6, and (F) peak sputum tumor necrosis factor (TNF)-α.
Impaired IFN Production in Subjects with COPD
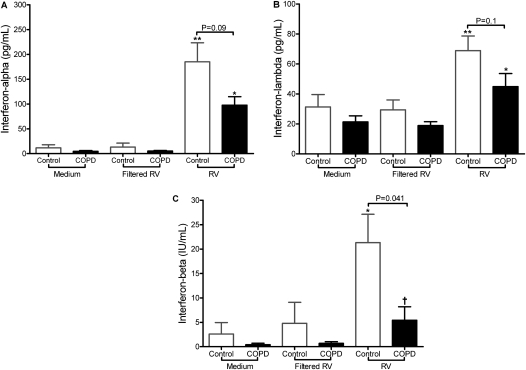
Sufficient BAL cells for ex vivo culture were obtained at the baseline bronchoscopy from 10 control subjects and 7 subjects with COPD. These cells were more than 95% macrophages and were incubated with live rhinovirus, medium alone, or virus inoculum from which virus had been removed by molecular weight filtration (20). In BAL cells infected with live rhinovirus induction of both IFN-α and IFN-λ release into supernatants was impaired by approximately 50% in the COPD group, but differences between the groups were not statistically significant (Figures 5A and 5B). In BAL cells from the control subjects rhinovirus infection induced significant increases in IFN-β compared with filtered virus, but not in the subjects with COPD, and IFN-β levels induced by infection were significantly higher in the control subjects compared with the COPD group (Figure 5C). The deficient IFN induction by rhinovirus in COPD was also accompanied by deficient induction of the interferon-stimulated gene (ISG) CXCL10. Virus infection induced significant up-regulation of IL-8 in BAL cells from both groups (data not shown).
Figure 5.
Interferon responses in bronchoalveolar lavage (BAL) cells. Cells obtained by BAL were infected ex vivo with rhinovirus 16 (RV) and (A) IFN-α, (B) IFN-λ, and (C) IFN-β were measured in supernatants 48 hours postinfection (all values represent means and SEM). *P < 0.05 versus filtered virus; **P < 0.01 versus filtered virus; †P < 0.05, patients with chronic obstructive pulmonary disease (COPD) versus control subjects. Results of comparisons between COPD and control groups are indicated above the relevant brackets.
DISCUSSION
In this study we report that experimental rhinovirus infection in subjects with COPD induced symptoms, airflow obstruction, and neutrophilic inflammation of significant severity and duration. Virus load in sputum correlated strongly with inflammatory markers and IFN-β production by airway macrophages was impaired in COPD. These data suggest that experimental rhinovirus infection is a valid human model of COPD exacerbations and a novel tool with which to further investigate mechanisms of virus-induced exacerbations.
There is no universally accepted definition of a COPD exacerbation; however, exacerbations are normally defined by acute increases in lower respiratory symptoms above normal daily variability (5) and are associated with increased airflow obstruction (32). After rhinovirus infection subjects with COPD reported significantly increased lower respiratory symptoms above their baseline symptoms and 10 of 11 fulfilled the predetermined criteria for an acute exacerbation that was based on accepted criteria from studies of naturally occurring exacerbations (2, 33–35). The control subjects also developed lower respiratory symptoms, but these were of lesser severity and duration than for the subjects with COPD. Only the subjects with COPD developed significant increases in breathlessness, suggesting this is the key symptom that differentiates a COPD exacerbation from uncomplicated acute viral bronchitis, and this is supported by data from naturally occurring rhinovirus infections (36). Furthermore, we documented falls in PEF and Kco, which compare with the changes in lung function seen in naturally occurring exacerbations (8, 37). There was a temporal relationship between virus detection in the respiratory tract and the onset of symptoms and airflow obstruction, and virus clearance was followed by clinical recovery. Therefore this study provides the first direct experimental evidence that rhinovirus infection precipitates the symptomatic and physiological changes in COPD that define an acute exacerbation.
The nature of the inflammatory response in COPD exacerbations remains controversial and there is no single cellular or molecular inflammatory marker that defines an exacerbation (9, 11, 38). Neutrophilic and eosinophilic inflammation has been reported as related to virus infection in some studies (11) but not others (39), and others have reported that virus infection is not associated with pulmonary or systemic inflammatory markers (12, 13, 40). Therefore the relationship between virus infection and airway inflammation in COPD exacerbations is unclear. We report that rhinovirus infection was temporally associated with a significant and sustained neutrophil influx and increased levels of neutrophil elastase and IL-8 in the sputum of subjects with COPD. There were no increases in sputum eosinophils after infection, but our subjects were all nonatopic and therefore likely to have low sputum eosinophils (41). There were strong correlations between sputum neutrophil elastase levels and severity of symptoms and airflow obstruction exclusively in subjects with COPD. Neutrophil elastase has been related to exacerbation severity in bacterial exacerbations (42) and our data implicate it as a key mediator of virus-induced exacerbations also. In addition, virus load correlated significantly with neutrophil numbers and inflammatory mediators in serum, sputum, and BAL. In BAL, lymphocytes and IL-6 were both also significantly increased after rhinovirus infection and further studies will be needed to investigate the importance of these and other inflammatory cells and mediators in COPD exacerbations. Therefore these data are the first to directly link virus infection of the lower respiratory tract to lower airway inflammation in COPD and strongly support a causative role for virus infection.
It is not known whether patients with COPD are more susceptible to respiratory virus infections. Although frequency of infection was similar between the two groups, we demonstrated increased severity and duration of respiratory symptoms, greater lung function impairment, and increased airway inflammation in subjects with COPD compared with smokers without airflow obstruction. Daily and peak virus loads in each of nasal lavage, sputum, and BAL were consistently 1–3 logs higher in subjects with COPD, and although these differences were not statistically significant other than on Day 6 the consistency of these findings throughout the acute infection and in three different clinical samples adds weight to their validity. A key constituent of innate immune responses to virus infection is the production of IFNs by infected cells. We have previously shown that IFN-β and IFN-λ production is impaired in asthma (20, 21) and herein we report impaired BAL cell production of IFN-β in response to virus infection in subjects with COPD and trends to reduced IFN-α and IFN-λ. As IFN-β is an early and essential component of antirhinoviral immunity (21) this may be an important mechanism underlying increased severity of rhinovirus infection in COPD. A report of in vitro rhinovirus infection of epithelial cells from patients with COPD described higher virus load but no differences in IFN production (43). Therefore other mechanisms may also be involved in impaired antiviral immune responses to virus infection in COPD.
We have established experimental rhinovirus infection as a valid model of COPD exacerbation and therefore this provides an important new tool for studying exacerbations. This model has major advantages as it permits investigations of exacerbations in a controlled manner with a single etiological agent, and facilitates longitudinal sampling, allowing investigation of temporal and quantitative relationships between virus infection, inflammatory mediators, and biological and physiological markers in a manner not possible with cross-sectional studies of naturally occurring exacerbations. The evidence we provide linking rhinovirus infection to COPD exacerbations should stimulate research into both existing and novel antiviral therapies as potential therapies in COPD (44). Therapies targeting neutrophilic inflammation (45) and neutrophil elastase inhibitors (46) or augmentation of IFN-β may also have potential in the treatment or prevention of COPD exacerbations. This model will also be a useful tool to assist in testing the efficacy of new therapies.
Limitations of our study include the relatively small numbers of subjects and the higher mean age of the COPD group. As this is the first study to perform experimental rhinovirus infection in subjects with COPD and smokers there were no data on which to base power calculations. The data from this study can be used to design further experimental infection studies and it is possible that with larger numbers of subjects statistically significant differences in virus load and IFN-α and IFN-λ will become apparent. We examined the data for correlations between age and clinical, inflammatory, and virologic outcomes and IFN production and none were found; however, further studies are required to replicate these findings in greater numbers of subjects and with subject groups more closely matched for age. The neutrophil counts and cytokine levels in the COPD group were relatively low compared with previously published studies (9, 47). This may be due to differences between our subjects (all Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage II, mean age 60 yr, and no comorbidities) and the subjects recruited in other clinical studies of patients with COPD (most including many at GOLD stage III and IV). Therefore it is probable that we selected a population of patients with COPD with mild airway inflammation. However, it is likely that the effects of rhinovirus infection in patients with more severe COPD are even greater than those seen in our subjects.
In conclusion, we have shown that experimental rhinovirus infection induces the clinical features of COPD exacerbations, that neutrophilic airway inflammation is an important mechanism of virus-induced exacerbations, and that deficient production of IFN-β may contribute to increased susceptibility to virus infection in COPD. This model will facilitate research into mechanisms of COPD exacerbations, thereby boosting efforts to develop new approaches for the prevention and treatment of exacerbations of COPD.
Supplementary Material
Acknowledgments
The authors thank the study participants for their unfailing commitment and enthusiasm, and the staff of the Chest and Allergy Clinic, the Pulmonary Function Laboratory, and the Endoscopy Unit at Imperial College Healthcare NHS Trust, St. Mary's Hospital for their help in this study.
Supported by an unrestricted grant from GlaxoSmithKline, a Medical Research Council Clinical Research Fellowship (to S.D.M.), British Medical Association H.C. Roscoe Fellowships (to S.D.M. and P.M.), British Lung Foundation/Severin Wunderman Family Foundation Lung Research Program grant P00/2, Wellcome Trust grant 083567/Z/07/Z for the Centre for Respiratory Infection, an Imperial College and National Institute for Health Research Biomedical Research Centre funding scheme, a grant from Pfizer UK, and a European Respiratory Society fellowship (to M.C.). Spirometers were provided by Micro Medical Ltd, Rochester, UK.
This article has an online supplement, which is available from the issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201006-0833OC on October 1, 2010
Author Disclosure: M.C. received advisory board fees from Chiesi Farmaceutici for $1,001–$5,000 and lecture fees from Boehringer Ingelheim and Merck Sharp & Dohme (for up to $1,000 each), AstraZeneca, and GlaxoSmithKline (for $1,001–$5,000 each). M.G.E. received a sponsored grant from FAMRI for more than $100,001. M.J. is a full-time employee of GlaxoSmithKline. S.J. received consultancy fees from AstraZeneca, Centocor, Sanofi-Pasteur, and Synairgen for $10,001–$50,000 each, and serves on the advisory board of AstraZeneca, Centocor, Sanofi-Pasteur, and Synairgen for $1,001–$5,000 each. S.J. received sponsored grants from AstraZeneca, Centocor, and Sanofi-Pasteur for more than $100,001, and from Synairgen for $50,001–$100,000. S.J. owns stock in Synairgen for $10,001–$50,000. O.M.K. served on the advisory board of Nycomed for less than $1,000. A.P. has received consultancy fees from Chiesi Farmaceutici for $5,001–$10,000, and from GlaxoSmithKline and AstraZeneca for $1,001–$5,000 each. A.P. served on the advisory board of Mundipharma, UCB, AstraZeneca, Nycomed, Italy, and GlaxoSmithKline for $1,001–$5,000 each, and Chiesi Farmaceutici for $5,001–$10,000. A.P. received lecture fees from Chiesi Farmaceutici for $10,001–$50,000, and from GlaxoSmithKline, AstraZeneca, Merck Sharp & Dohme, Boehringer Ingelheim, Novartis, Italy, and Pfizer for $1,001–$5,000 each. A.P. received sponsored grants from Chiesi Farmaceutici and Merck Sharp & Dohme for $10,001–$50,000 each, and from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline for $50,001–$100,000 each. V.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. T.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. V.L.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L. Slater does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L. Stanciu does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Samet JM, Wipfli HL. Globe still in grip of addiction. Nature 2010;463:1020–1021. [DOI] [PubMed] [Google Scholar]
- 2.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418–1422. [DOI] [PubMed] [Google Scholar]
- 3.Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med 2008;177:396–401. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS; GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276. [DOI] [PubMed] [Google Scholar]
- 6.Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007;356:775–789. [DOI] [PubMed] [Google Scholar]
- 7.Walters JA, Gibson PG, Wood-Baker R, Hannay M, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2009; (1):CD001288. [DOI] [PubMed] [Google Scholar]
- 8.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax 2000;55:114–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aaron SD, Angel JB, Lunau M, Wright K, Fex C, Le Saux N, Dales RE. Granulocyte inflammatory markers and airway infection during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:349–355. [DOI] [PubMed] [Google Scholar]
- 10.Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, Macnee W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005;60:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006;173:1114–1121. [DOI] [PubMed] [Google Scholar]
- 12.Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006;173:71–78. [DOI] [PubMed] [Google Scholar]
- 13.Pant S, Walters EH, Griffiths A, Wood-Baker R, Johns DP, Reid DW. Airway inflammation and anti-protease defences rapidly improve during treatment of an acute exacerbation of COPD. Respirology 2009;14:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Macallum P, Meade TW, Jeffries DJ, Johnston SL, Wedzicha JA. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;164:1618–1623. [DOI] [PubMed] [Google Scholar]
- 15.Beckham JD, Cadena A, Lin J, Piedra PA, Glezen WP, Greenberg SB, Atmar RL. Respiratory viral infections in patients with chronic, obstructive pulmonary disease. J Infect 2005;50:322–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchinson AF, Ghimire AK, Thompson MA, Black JF, Brand CA, Lowe AJ, Smallwood DM, Vlahos R, Bozinovski S, Brown GV, et al. A community-based, time-matched, case–control study of respiratory viruses and exacerbations of COPD. Respir Med 2007;101:2472–2481. [DOI] [PubMed] [Google Scholar]
- 17.Rohde G, Wiethege A, Borg I, Kauth M, Bauer TT, Gillissen A, Bufe A, Schultze-Werninghaus G. Respiratory viruses in exacerbations of chronic obstructive pulmonary disease requiring hospitalisation: a case–control study. Thorax 2003;58:37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet 2002;359:831–834. [DOI] [PubMed] [Google Scholar]
- 19.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci USA 2008;105:13562–13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-λ production in asthma exacerbations. Nat Med 2006;12:1023–1026. [DOI] [PubMed] [Google Scholar]
- 21.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005;201:937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mallia P, Message SD, Kebadze T, Parker HL, Kon OM, Johnston SL. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations [abstract]: a pilot study. Respir Res 2006;7:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallia P, Message S, Laza-Stanca V, Contoli M, Kebadze T, Johnston SL. Neutrophil adhesion molecules in an experimental model of rhinovirus-induced COPD exacerbations [abstract]. Thorax 2005;60:102. [Google Scholar]
- 24.Mallia P, Contoli M, Message S, Laza-Stanca V, Johnston SL. Lung function and sputum neutrophil changes in an experimental model of virus-induced COPD exacerbations. Abstract presented at the European Respiratory Society Annual Congress. September 17–21, 2005, Copenhagen, Denmark, p. S1718.
- 25.Mallia P, Message S, Contoli M, Gray K, Kebadze T, Laza-Stanca V, Johnston SL. An experimental model of virus-induced COPD exacerbation [abstract]. Thorax 2006;61(Suppl. 2) ii28.
- 26.Lemanske RF Jr, Dick EC, Swenson CA, Vrtis RF, Busse WW. Rhinovirus upper respiratory infection increases airway hyperreactivity and late asthmatic reactions. J Clin Invest 1989;83:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.British Thoracic Society, Association of Respiratory Technicians and Physiologists. Guidelines for the measurement of respiratory function: recommendations of the British Thoracic Society and the Association of Respiratory Technicians and Physiologists. Respir Med 1994;88:165–194. [PubMed] [Google Scholar]
- 28.Bardin PG, Sanderson G, Robinson BS, Holgate ST, Tyrrell DA. Experimental rhinovirus infection in volunteers. Eur Respir J 1996;9:2250–2255. [DOI] [PubMed] [Google Scholar]
- 29.Pizzichini E, Pizzichini MM, Leigh R, Djukanovi R, Sterk PJ. Safety of sputum induction. Eur Respir J Suppl 2002;37:9s–18s. [DOI] [PubMed] [Google Scholar]
- 30.Donaldson GC, Seemungal TA, Patel IS, Bhowmik A, Wilkinson TM, Hurst JR, Macallum PK, Wedzicha JA. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 2005;128:1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.British Thoracic Society. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax 2001;56:i1–i21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000;161:1608–1613. [DOI] [PubMed] [Google Scholar]
- 33.Leidy NK, Rennard SI, Schmier J, Jones MK, Goldman M. The breathlessness, cough, and sputum scale: the development of empirically based guidelines for interpretation. Chest 2003;124:2182–2191. [DOI] [PubMed] [Google Scholar]
- 34.Calverley PM, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C; TRial of Inhaled STeroids ANd long-acting beta2 agonists study group. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003;361:449–456. [DOI] [PubMed] [Google Scholar]
- 35.Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;179:369–374. [DOI] [PubMed] [Google Scholar]
- 36.Wald TG, Shult P, Krause P, Miller BA, Drinka P, Gravenstein S. A rhinovirus outbreak among residents of a long-term care facility. Ann Intern Med 1995;123:588–593. [DOI] [PubMed] [Google Scholar]
- 37.Parker CM, Voduc N, Aaron SD, Webb KA, O'Donnell DE. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Respir J 2005;26:420–428. [DOI] [PubMed] [Google Scholar]
- 38.Wilkinson TM, Hurst JR, Perera WR, Wilks M, Donaldson GC, Wedzicha JA. Effect of interactions between lower airway bacterial and rhinoviral infection in exacerbations of COPD. Chest 2006;129:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohde G, Borg I, Wiethege A, Kauth M, Jerzinowski S, An Duong Dinh T, Bauer TT, Bufe A, Schultze-Werninghaus G. Inflammatory response in acute viral exacerbations of COPD. Infection 2008;36:427–433. [DOI] [PubMed] [Google Scholar]
- 40.Bozinovski S, Hutchinson A, Thompson M, Macgregor L, Black J, Giannakis E, Karlsson AS, Silvestrini R, Smallwood D, Vlahos R, Irving LB, Anderson GP. Serum amyloid A is a biomarker of acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:269–278. [DOI] [PubMed] [Google Scholar]
- 41.Belda J, Leigh R, Parameswaran K, O'Byrne PM, Sears MR, Hargreave FE. Induced sputum cell counts in healthy adults. Am J Respir Crit Care Med 2000;161:475–478. [DOI] [PubMed] [Google Scholar]
- 42.Sethi S, Wrona C, Eschberger K, Lobbins P, Cai X, Murphy TF. Inflammatory profile of new bacterial strain exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:491–497. [DOI] [PubMed] [Google Scholar]
- 43.Schneider D, Ganesan S, Comstock AT, Meldrum CA, Mahidhara R, Goldsmith AM, Curtis JL. Martinez FJ, Hershenson MB, Sajjan U, et al. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;182:332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayden FG, Herrington DT, Coats TL, Kim K, Cooper EC, Villano SA, Liu S, Hudson S, Pevear DC, Collett M, et al.; Pleconaril Respiratory Infection Study Group. Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double-blind, randomized, placebo-controlled trials. Clin Infect Dis 2003;36:1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685–694. [DOI] [PubMed] [Google Scholar]
- 46.Voynow JA, Fischer BM, Malarkey DE, Burch LH, Wong T, Longphre M, Ho SB, Foster WM. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell Mol Physiol 2004;287:L1293–L1302. [DOI] [PubMed] [Google Scholar]
- 47.Singh D, Edwards L, Tal-Singer R, Rennard S. Sputum neutrophils as a biomarker in COPD: findings from the ECLIPSE Study. Respir Res 2010;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.