Abstract
Rationale: Mechanisms leading to obstructive sleep apnea syndrome (OSAS) in obese children are not well understood.
Objectives: The aim of the study was to determine anatomical risk factors associated with OSAS in obese children as compared with obese control subjects without OSAS.
Methods: Magnetic resonance imaging was used to determine the size of upper airway structure, and body fat composition. Paired analysis was used to compare between groups. Mixed effects regression models and conditional multiple logistic regression models were used to determine whether body mass index (BMI) Z-score was an effect modifier of each anatomic characteristic as it relates to OSAS.
Measurements and Main Results: We studied 22 obese subjects with OSAS (12.5 ± 2.8 yr; BMI Z-score, 2.4 ± 0.4) and 22 obese control subjects (12.3 ± 2.9 yr; BMI Z-score, 2.3 ± 0.3). As compared with control subjects, subjects with OSAS had a smaller oropharynx (P < 0.05) and larger adenoid (P < 0.01), tonsils (P < 0.05), and retropharyngeal nodes (P < 0.05). The size of lymphoid tissues correlated with severity of OSAS whereas BMI Z-score did not have a modifier effect on these tissues. Subjects with OSAS demonstrated increased size of parapharyngeal fat pads (P < 0.05) and abdominal visceral fat (P < 0.05). The size of these tissues did not correlate with severity of OSAS and BMI Z-score did not have a modifier effect on these tissues.
Conclusions: Upper airway lymphoid hypertrophy is significant in obese children with OSAS. The lack of correlation of lymphoid tissue size with obesity suggests that this hypertrophy is caused by other mechanisms. Although the parapharyngeal fat pads and abdominal visceral fat are larger in obese children with OSAS we could not find a direct association with severity of OSAS or with obesity.
Keywords: lymphoid hypertrophy, MRI, obese children
AT A GLANCE COMMENTARY.
Current Scientific Knowledge on the Subject
The pathophysiology of obstructive sleep apnea syndrome (OSAS) in obese children is not well established. It is not known what anatomical changes occur and contribute to OSAS in this population.
What This Study Adds to the Field
This study enhances several new important aspects of the anatomical changes in the upper airway and body fat composition contributing to OSAS in obese children.
It is estimated that the prevalence of obstructive sleep apnea syndrome (OSAS) in obese children is much higher than the 1–4% reported in the general pediatric population (1). This is based on several observations. A large epidemiological study of children between 2 and 18 years of age noted that children with obesity had the highest risk to develop OSAS, with an odds ratio of 4.5 (2). Other studies support this finding: the occurrence of OSAS in unselected obese children undergoing polysomnography was 46% (3), and in morbidly obese children the reported rate was 55% (4). Last, when studying obese children referred for evaluation of OSAS, 59% had the disorder (5). It seems that children with metabolic syndrome, which is closely associated with obesity, are at particular risk for developing OSAS (6, 7). However, the anatomical and functional correlates leading to OSAS in obese children, and why some have the disorder whereas others do not, are not well understood and have not been studied systematically.
Several investigators emphasize the role of adenoid and tonsillar hypertrophy in the pathophysiology of the disorder in obese children (8–12). In addition, the American Academy of Pediatrics recommends considering adenotonsillectomy as the first treatment modality for obese children with OSAS (13). However, after adenotonsillectomy, residual OSAS continues in a significant number of children (8–12), suggesting that anatomical and/or functional factors may continue to restrict and/or allow restriction of the upper airway and lead to OSAS in these children.
In obese adults with OSAS, adenoid and tonsillar hypertrophy are not usually considered a risk factor. However, other soft tissues such as the soft palate, lateral pharyngeal walls, tongue, and parapharyngeal fat pads have been found to be larger and to restrict the upper airway (14–17). In addition, increased abdominal visceral fat has been shown to be associated with OSAS (18, 19), possibly by lowering lung volumes and promoting a smaller and more collapse-prone upper airway (20). Similar alterations in upper airway structure and body fat composition noted in obese adults should be considered in obese children with OSAS.
In this study, using magnetic resonance imaging (MRI), we performed a quantitative analysis of anatomical factors associated with OSAS in obese 8- to 17-year-old children. We hypothesized that the size of lymphoid tissues surrounding the upper airway is larger in the obese OSAS group as compared with obese control subjects, as found in young nonobese children with OSAS (21). Our secondary analysis examined other possible anatomical causes that have been previously noted in obese adults to determine whether these may play a role in the pathogenesis of the disorder in obese children, as well. Portions of this study have been presented previously in abstract form at the 2010 American Thoracic Society International Conference, May 14–19, 2010, New Orleans, Louisiana (22).
METHODS
The study was approved by the Committee of Clinical Investigations at Albert Einstein College of Medicine (Bronx, NY). Informed consent was obtained from each subject and or/parent. Sequential obese subjects (body mass index [BMI] > 95th percentile for age and sex) (23) were recruited from the adolescent, endocrine, and general pediatric obesity clinics at the Children's Hospital at Montefiore (Bronx, NY). Subjects had normal development, intact adenoid and tonsils, and did not have a known metabolic or endocrine disorder other than prediabetes or type 2 diabetes. Determination of OSAS or control was made after overnight polysomnography in our hospital. Control subjects were matched to subjects with OSAS by age, sex, height, weight, BMI Z-score, Tanner stage, and ethnicity.
Overnight Polysomnography
Overnight polysomnography (XLTEK, Oakville, ON, Canada) was performed at the Sleep Disorders Center at the Children's Hospital at Montefiore. Sleep staging and scoring of arousals were performed according to standard criteria (24) by one blinded scorer. OSAS was determined if the obstructive apnea index was greater than 1/hour or if the apnea–hypopnea index (AHI) was greater than or equal to 5/hour (25) (for details on methodology see the online supplement).
Magnetic Resonance Imaging
MRI studies were performed in the Department of Radiology at the Children's Hospital at Montefiore within 4 weeks of recruitment and after polysomnography using a 16-channel Philips 3.0-tesla Achieva Quasar TX scanner (Philips Medical Systems, Best, The Netherlands). Subjects were awake during the period of imaging and were monitored continuously by pulse oximetry and direct observation. (For details of MRI methodology, sequences, and image processing see the online supplement).
Image Analysis
Image analysis was performed using Amira software (Version 4.1.1; Visage Imaging GmbH). Volumetric measurements were performed on each slice, using intensity threshold after normalization.
Upper airway structure.
The volumes of the following structures were determined:
Airway: The upper airway was subdivided to the following segments: Nasopharynx, defined as the region located superior to the level of the soft palate and continuous anteriorly, through the choanae, with the nasal cavities; oropharynx, defined as the region located between the level of the soft palate and the larynx, communicating anteriorly with the oral cavity, and having the posterior one-third of the tongue as its anterior border; and hypopharynx, defined as the region posterolateral to the larynx, and communicating with the cavity of the larynx through the auditus and including the pyriform recesses and the valleculae
Lymphoid tissues: Adenoid, combined palatine tonsils, combined retropharyngeal nodes (defined as lymph nodes located between the internal carotid arteries from the base of the skull to the hyoid bone), and the combined deep cervical lymph nodes (defined as level II nodes, located along the internal jugular vein from base of the skull to the level of the hyoid bone)
Tongue (including the genioglossus and geniohyoid muscles)
Soft palate
Mandible
Head, neck, and abdomen fat composition.
Fat tissues of the head and neck included parapharyngeal and subcutaneous fat, and in the abdomen the visceral and subcutaneous fat compartments.
Data Analysis
Statistical analysis was conducted using SPSS version 18. Means and standard deviations were used to summarize continuous variables. For comparisons between the groups for demographics, polysomnography, and MRI data, we used two-tailed paired t tests, Wilcoxon signed rank tests, or McNemar's tests. Pearson correlations were derived between AHI and BMI Z-score and each anatomical variable. Mixed-effects regression models using AHI as a dependent variable, and conditional multiple logistic regression models with OSAS versus non-OSAS as the dependent variable, were derived to determine whether BMI Z-score was an effect modifier of each anatomic characteristic as it relates to OSAS. A P value less than 0.05 was considered significant.
RESULTS
Twenty-two obese children with OSAS (mean age, 12.5 ± 2.8 yr [range, 8.6–17.8 yr]; mean BMI Z-score, 2.4 ± 0.4) were compared with 22 obese control subjects without OSAS (mean age, 12.3 ± 2.9 yr [range, 8.5–17.8 yr]; mean BMI Z-score, 2.3 ± 0.3). There were no significant differences in demographic, anthropometric, and Tanner stage characteristics between groups (Table 1). One subject in the OSAS group had glucose intolerance and one had type 2 diabetes. None of the control subjects had prediabetes or type 2 diabetes.
TABLE 1.
DEMOGRAPHICS
| OSAS (n = 22) | Control Subjects (n = 22) | P Value | |
|---|---|---|---|
| Age, yr | 12.5 ± 2.8 | 12.3 ± 2.9 | NS |
| Sex | M, 14; F, 8 | M, 14; F, 8 | NS |
| Height, cm | 158.5 ± 17.7 | 158.8 ± 16.3 | NS |
| Weight, kg | 89.8 ± 35.2 | 84.6 ± 29.8 | NS |
| BMI, kg/m2 | 34.6 ± 8.3 | 32.4 ± 6.9 | NS |
| BMI Z-score | 2.4 ± 0.4 | 2.3 ± 0.3 | NS |
| Tanner stage | 3.4 ± 1.7 | 3.3 ± 1.6 | NS |
| Ethnicity (AA/Hispanic/W/other) | 12/8/1/1 | 12/7/3/0 | NS |
Definition of abbreviations: AA = African American; BMI = body mass index; F = female; M = male; NS = not significant; OSAS = obstructive sleep apnea syndrome; W = white.
Data shown represent means ± SD.
Polysomnography
Subjects with OSAS exhibited mild–severe airway obstruction during sleep as compared with control subjects, having normal polysomnographic data (Table 2). There were significant differences between groups with respect to the following: AHI, 16.3 ± 12.8 versus 1.1 ± 1.1 events/hour (P < 0.001); SpO2 (oxygen saturation as measured by pulse oximetry) nadir, 85.2 ± 6.1 versus 94.2 ± 2.9% (P < 0.001); and arousal index, 15.7 ± 12.1 versus 8.0 ± 4.2 events/hour (P < 0.05).
TABLE 2.
POLYSOMNOGRAPHY
| OSAS (n = 22) | Control Subjects (n = 22) | P Value | |
|---|---|---|---|
| Total sleep time, h | 6.3 ± 1.5 | 6.2 ± 0.7 | NS |
| Sleep efficiency, % | 78.5 ± 16.5 | 84.3 ± 8.2 | NS |
| Apnea index, events/h | 3.1 ± 5.4 | 0.2 ± 0.4 | NS |
| Apnea–hypopnea index | 16.3 ± 12.8 | 1.1 ± 1.1 | <0.001 |
| Baseline SpO2, % | 98.8 ± 1.4 | 98.5 ± 1.2 | NS |
| SpO2 nadir, % | 85.2 ± 6.1 | 94.2 ± 2.9 | <0.001 |
| Baseline ETco2, mm Hg | 38.7 ± 7.7 | 41.2 ± 2.5 | NS |
| Peak ETco2, mm Hg | 50.1 ± 4.4 | 47.6 ± 3.9 | NS |
| Arousal index, events/h | 15.7 ± 12.1 | 8.0 ± 4.2 | <0.05 |
Definition of abbreviations: ETco2 = end-tidal carbon dioxide; NS = not significant; OSAS = obstructive sleep apnea syndrome; SpO2 = oxygen saturation as measured by pulse oximetry;
Data shown represent means ± SD.
Magnetic Resonance Imaging
Upper airway structure.
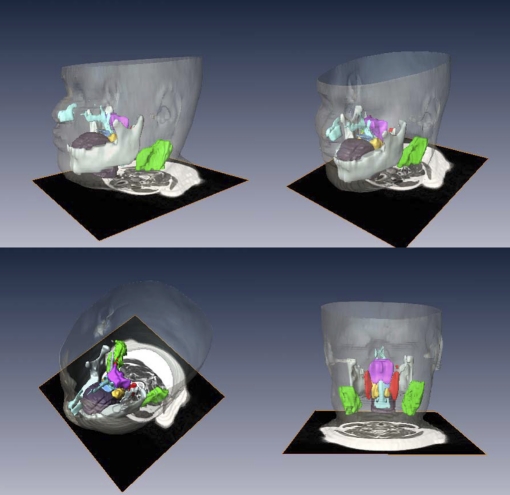
Volumetric analysis based on 3-mm slice thickness axial images of the upper airway structure is shown in Table 3. A three-dimensional depiction in four different views of the upper airway tissues of a subject with OSAS is presented graphically in Figure 1 (dynamic illustrations are shown in the online supplement as E Movie 1.1 and E Movie 1.2).
TABLE 3.
UPPER AIRWAY STRUCTURE AND BODY COMPOSITION: VOLUMETRIC MEASUREMENTS
| OSAS (n = 22) | Control Subjects (n = 22) | P Value | |
|---|---|---|---|
| Airway | |||
| Nasopharynx | 8.7 ± 3.5 | 9.8 ± 2.8 | NS |
| Oropharynx | 3.5 ± 1.7 | 4.9 ± 2.0 | <0.05 |
| Hypopharynx | 3.2 ± 1.3 | 3.5 ± 1.5 | NS |
| Lymphoid tissues | |||
| Adenoid | 10.5 ± 4.4 | 7.1 ± 3.6 | <0.01 |
| Tonsils | 10.1 ± 3.9 | 7.8 ± 2.5 | <0.05 |
| Retropharyngeal nodes | 4.7 ± 2.4 | 3.1 ± 1.6 | <0.05 |
| Deep cervical nodes | 32.0 ± 11.9 | 26.8 ± 5.9 | NS |
| Other tissues | |||
| Soft palate | 8.6 ± 2.9 | 8.6 ± 1.8 | NS |
| Tongue | 88.1 ± 17.0 | 89.7 ± 31.8 | NS |
| Mandible | 63.1 ± 12.7 | 64.9 ± 18.5 | NS |
| Regional body fat composition | |||
| Head and neck subcutaneous fat | 483.2 ± 170.1 | 420.1 ± 111.0 | NS |
| Neck parapharyngeal fat | 10.6 ± 4.7 | 8.4 ± 2.3 | <0.05 |
| Abdominal subcutaneous fat | 6,939.5 ± 3,115.0 | 5,916.9 ± 2,289.2 | NS |
| Abdominal visceral fat | 1,434.9 ± 459.6 | 1,101.4 ± 423.7 | <0.05 |
Definition of abbreviations: NS = not significant; OSAS = obstructive sleep apnea syndrome.
Data shown represent mean cubic centimeters ± SD.
Figure 1.
Upper airway, soft tissues, and mandibular reconstructions. Head and neck surface rendering with three-dimensional reconstructions of the upper airway, soft tissues, and mandible of a subject with obstructive sleep apnea syndrome in various views: lateral (top left), anterior oblique (top right), superior oblique (bottom left) and posterior (bottom right). Airway (light blue), tongue (brown), mandible (white), soft palate (blue), tonsils (yellow), adenoid (magenta), retropharyngeal nodes (red), and deep cervical nodes (green).
Airway: We noted a significantly smaller oropharyngeal airway in the OSAS group in comparison with the control group, 3.5 ± 1.7 versus 4.9 ± 2.0 cm3 (P < 0.05). No significant differences were noted with respect to the volume of either the nasopharynx or hypopharynx.
Lymphoid tissues: Except for the deep cervical nodes, all lymphoid tissues surrounding the upper airway were significantly larger in OSAS as compared with control subjects: the mean adenoid volume was 10.5 ± 4.4 versus 7.1 ± 3.6 cm3 (P < 0.01), the mean palatine tonsil volume was 10.1 ± 3.9 versus 7.8 ± 2.5 cm3 (P < 0.05), and the mean volume of the retropharyngeal lymph nodes was 4.7 ± 2.4 versus 3.1 ± 1.6 cm3 (P < 0.05). No significant differences were noted in the size of the tongue, the soft palate, or the mandible.
Regional fat distribution in neck and abdomen.
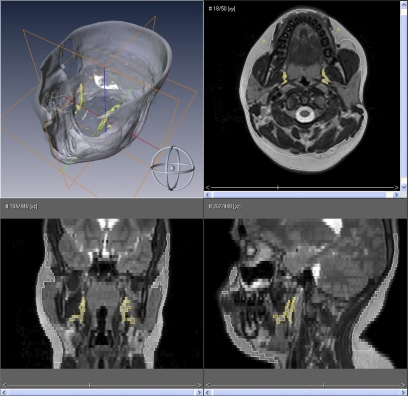
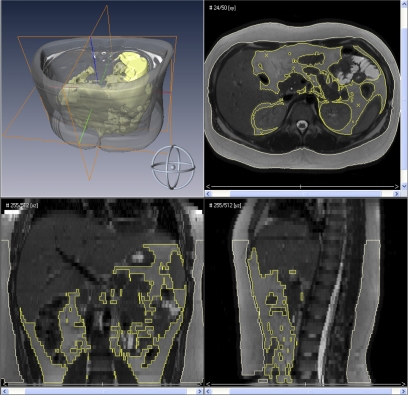
Body fat composition is shown in Table 3. A three-dimensional depiction and axial, coronal, and sagittal views of the head and neck and abdomen are presented graphically in Figure 2 and Figure 3, respectively (respective dynamic illustrations are shown in the online supplement as E Movie 2 and E Movie 3). Accordingly, in the head and neck region, subjects with OSAS had significantly larger parapharyngeal fat pads, 10.6 ± 4.7 versus 8.4 ± 2.3 cm3 (P < 0.05), but no difference in head and neck subcutaneous fat. Similarly, subjects with OSAS had significantly more abdominal visceral fat, 1,434.9 ± 459.6 versus 1,101.4 ± 423.7 cm3 (P < 0.05), but no difference in abdominal subcutaneous fat.
Figure 2.
Head and neck fat composition. Surface rendering of the head and neck with three-dimensional reconstructions of the subcutaneous fat (gray) and parapharyngeal fat pads (yellow) of a subject with obstructive sleep apnea syndrome (top left), midtonsillar axial view (top right), coronal view (bottom left), and sagittal view (bottom right).
Figure 3.
Abdominal fat composition. Surface rendering of the abdomen with three-dimensional reconstructions of the subcutaneous fat (gray) and visceral fat (yellow) of a subject with obstructive sleep apnea syndrome (top left), axial view (top right), coronal view (bottom left), and sagittal view (bottom right).
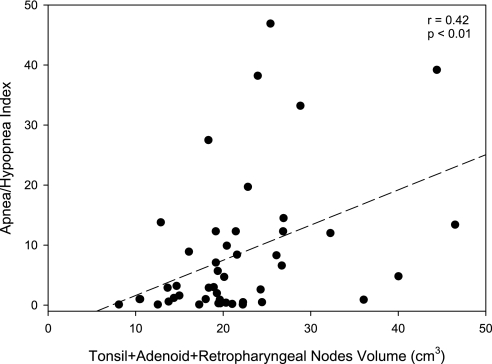
Correlations between AHI and BMI Z-score and each of the anatomical variables are shown in Table 4. We noted a significant negative correlation between AHI and oropharyngeal size. A positive correlation was noted between AHI and the size of adenoid, tonsils, and retropharyngeal nodes with no significant correlation with BMI Z-score. An illustration of the correlation between the AHI and the combined volume of the tonsils, adenoid, and retropharyngeal nodes is shown in Figure 4 (r = 0.42, P < 0.01). No significant correlations between the AHI and the size of the soft palate, tongue, mandible, and body fat composition were noted. BMI Z-score correlated significantly with the size of the tongue and mandible, and body fat tissues except abdominal visceral fat. Mixed-effects regression models and conditional multiple logistic regression models did not demonstrate that BMI Z-score was an effect modifier of anatomic characteristics as they relate to AHI or to OSAS versus subjects without OSAS.
TABLE 4.
CORRELATION COEFFICIENTS BETWEEN UPPER AIRWAY STRUCTURE AND BODY COMPOSITION VERSUS OBSTRUCTIVE SLEEP APNEA SEVERITY AND BODY MASS INDEX
| AHI (n = 44) |
BMI Z-Score (n = 44) |
|||
|---|---|---|---|---|
| r | P Value | r | P Value | |
| Airway | ||||
| Nasopharynx | −0.261 | NS | 0.346 | <0.05 |
| Oropharynx | −0.297 | <0.05 | 0.076 | NS |
| Hypopharynx | −0.122 | NS | 0.075 | NS |
| Lymphoid tissues | ||||
| Adenoid | 0.359 | <0.05 | −0.165 | NS |
| Tonsils | 0.307 | <0.05 | −0.070 | NS |
| Retropharyngeal nodes | 0.371 | <0.05 | 0.072 | NS |
| Deep cervical nodes | 0.02 | NS | 0.404 | <0.01 |
| Other tissues | ||||
| Soft palate | −0.181 | NS | 0.053 | NS |
| Tongue | −0.153 | NS | 0.325 | <0.05 |
| Mandible | −0.114 | NS | 0.292 | <0.05 |
| Body fat | ||||
| Head and neck subcutaneous fat | 0.255 | NS | 0.649 | <0.001 |
| Neck parapharyngeal fat | 0.038 | NS | 0.526 | <0.01 |
| Abdominal subcutaneous fat | 0.287 | NS | 0.682 | <0.001 |
| Abdominal visceral fat | 0.153 | NS | 0.272 | NS |
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; NS = not significant.
Figure 4.
Correlation between apnea–hypopnea index and combined upper airway lymphoid size (cm3): r = 0.42, P < 0.01.
DISCUSSION
Our study demonstrated several anatomical characteristics differentiating obese children with OSAS and obese children without OSAS. These include the increased size of upper airway lymphoid tissues and increased size of the parapharyngeal fat pads and abdominal visceral fat, noted in children with OSAS. However, our results did not demonstrate that variation in our subjects' BMI Z-score was an effect modifier on the above anatomical characteristics or severity of OSAS in these subjects.
To maximize the statistical power of comparisons between the two groups our study used matched pairs. We matched each obese subject with OSAS by age, height, weight, BMI Z-score, sex, and ethnicity with an obese control. Such matching serves to minimize the effect of confounding factors related to demographics and anthropometrics, which can be highly variable in children during growth and development.
When considering the various mechanisms leading to OSAS in obese children one should initially consider whether these may be related to an augmented effect of known causative factors resulting from their obesity and/or a distinct underlying pathophysiology of the disorder distinguishing it from OSAS in nonobese children. Because obesity is characterized by extensive somatic growth, we postulated, as previously described in younger nonobese children with OSAS (21), that older obese children with OSAS will have an excess of upper airway lymphoid tissues compared with obese subjects without OSAS.
In this study, in addition to comparing the size of the adenoid and tonsils, we have compared the difference in retropharyngeal nodes, which may also contribute to reduced airway volume. The retropharyngeal nodes are defined as lymph nodes located between the internal carotid arteries from the base of the skull to the hyoid bone. The afferents of these nodes drain the pharyngeal nasal cavity, adenoid, paranasal sinuses, and the auditory tubes and their efferents pass to the deep cervical lymph nodes. Because of the proximity of the retropharyngeal nodes to the nasopharynx and oropharynx, and because no rigid anatomic barrier lies between these nodes and the upper airway, the retropharyngeal nodes may contribute directly to reduction of the volume of the upper airway when they are enlarged. This is the first study to examine how these nodes contribute to airway restriction. Although we have focused in this study on obese children, these nodes may play a similar role in nonobese children with OSAS as well.
Several important relationships that enhance our understanding about the pathophysiology of OSAS in obese children have been observed in our study: first, the larger size of the adenoid (48%), tonsils (29%), and retropharyngeal nodes (54%) in the obese OSAS group, which is not offset by other soft tissue volumes being reduced, could explain the 28% reduction in oropharyngeal size, as compared with control subjects. It should be noted that the oropharynx loses 1.4 cm3 whereas lymphatic tissue gains 7.3 cm3; however, not all the lymphatic tissue actually impinges into the oropharyngeal space. This difference, measured during wakefulness, may predispose to upper airway obstruction during sleep. The oropharyngeal airway (velopharynx) has been identified to be an area of vulnerability to airway collapse in both children (26, 27) and adults (28, 29). The velopharynx in children has also been described as the “overlap region” (27, 30), because it is where the lower portion of the adenoid and upper poles of the tonsils overlap with each other and the soft palate. Second, the size of each of the upper airway lymphoid tissues significantly correlates with severity of OSAS whereas BMI Z-score did not have an effect modifier on these tissues. This suggests that lymphoid proliferation as it relates to OSAS is independent of obesity and could possibly be linked to other conditions such as local or systemic inflammation described in nonobese children with OSAS (31–33) as well as in obese children with the disorder (34). Third, the soft palate, tongue, and mandible did not differ in size between groups or correlate with OSAS severity. However, the tongue and mandible did correlate with BMI Z-score. Thus, these tissues may not contribute to OSAS in obese children as has been reported in adults (16, 35). It is possible that these tissues develop as risk factors later in life and contribute to the adult form of OSAS.
Regarding body fat composition, we found significant differences between groups. On average, children with OSAS had a 28 and 30% increase in size of the parapharyngeal fat pads and abdominal visceral fat, respectively. However, we could not demonstrate a significant correlation between severity of OSAS and alterations in body fat composition, or establish BMI Z-score as an effect modifier on these tissues. These findings could reflect the nature of our design in that only obese subjects within a narrow range of BMI Z-score (median, 2.4; range, 1.7–3.1) were included. Thus, it would be important to confirm these findings in a larger scale study.
Nevertheless, the finding of increased parapharyngeal fat pad size contributing to airway restriction in subjects with OSAS in this study is similar to that noted in obese adults with OSAS (14, 17), but not in nonobese adults (15) or children with the disorder (21). Similarly, increased abdominal visceral fat in our subjects with OSAS parallels the observation in obese children with metabolic syndrome and OSAS that used an indirect anthropometric measure of waist circumference (6). Thus, increased visceral fat in the neck and body has several important clinical implications in relation to OSAS. Visceral fat can directly affect chest wall and upper airway mechanics by reducing functional residual capacity, making such subjects more vulnerable to hypoxemia during sleep (20). Such a reduction of lung volume could also predispose to airway collapse by reducing tracheal traction and airway stability (36). In addition, visceral obesity is strongly associated with the proinflammatory and prothrombotic conditions that increase risk for insulin resistance, type 2 diabetes, atherosclerosis, and OSAS (37). On the other hand, studies have demonstrated that OSAS can independently induce metabolic syndrome by decreasing insulin sensitivity in both animal and humans (34, 38–40).
Although we noted that one subject in the OSAS group had glucose intolerance and one had type 2 diabetes, and none of the control subjects had evidence of prediabetes or type 2 diabetes, our study was not designed to correlate between OSAS, anatomical measures, and the biomarkers of metabolic syndrome, such as insulin resistance, dyslipidemia, inflammation, and so on. Therefore, we are limited in drawing conclusions regarding this possible association. Nevertheless, we suggest that the two groups emerging from the present study with similar BMI Z-score and that were a priori selected according to the existence or nonexistence of OSAS, present distinct phenotypes of childhood obesity: the first, with marked visceral adiposity, upper airway lymphoid hypertrophy, and OSAS, and a second with less profound visceral adiposity, smaller upper airway lymphoid tissues, and no evidence of OSAS. We speculate that the first phenotype is more prone to develop metabolic syndrome and its sequelae because OSAS was shown to independently lead to the disorder in both children and adults (6, 34, 38).
In summary, this study noted generalized overgrowth of upper airway lymphoid tissues in obese children with OSAS, restricting their upper airway. However, because surgical modalities, particularly adenotonsillectomy, in obese children often do not completely ameliorate OSAS (8, 11, 12), a prospective randomized control trial evaluating outcomes of this approach versus other treatment alternatives such as noninvasive ventilation and weight management would be helpful. Such a study should also include the metabolic outcomes linked to obesity and OSAS, such as insulin resistance and metabolic syndrome.
Supplementary Material
Acknowledgments
The authors thank the children and families who participated in the study; and Micaela Jackson, the study coordinator.
Supported by grant HD-053693 from the National Institutes of Health.
This article has an online supplement, which is available from the issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201008-1249OC on October 8, 2010
Author Disclosure: R.A. received an RO1 grant from the NIH. S.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.R. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. U.I.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.W.-R. was on the Board or Advisory Board for d-Life (up to $1,000). M.L.L. received grant support from Repligen Corporation ($10,001–$50,000), Elan Pharmaceutical Company ($1,001–$5,000), and the NIH (more than $100,001). D.M.W. received grant support from Yeshiva University ($10,001–$50,000). J.M.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med 1999;159:1527–1532. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol 1996;21:176–183. [DOI] [PubMed] [Google Scholar]
- 4.Kalra M, Inge T, Garcia V, Daniels S, Lawson L, Curti R, Cohen A, Amin R. Obstructive sleep apnea in extremely overweight adolescents undergoing bariatric surgery. Obes Res 2005;13:1175–1179. [DOI] [PubMed] [Google Scholar]
- 5.Silvestri JM, Weese-Mayer DE, Bass MT, Kenny AS, Hauptman SA, Pearsall SM. Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol 1993;16:124–129. [DOI] [PubMed] [Google Scholar]
- 6.Redline S, Storfer-Isser A, Rosen CL, Johnson NL, Kirchner HL, Emancipator J, Kibler AM. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med 2007;176:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhulst SL, Schrauwen N, Haentjens D, Rooman RP, Van Gaal L, De Backer WA, Desager KN. Sleep-disordered breathing and the metabolic syndrome in overweight and obese children and adolescents. J Pediatr 2007;150:608–612. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for severe obstructive sleep apnea in children. Int J Pediatr Otorhinolaryngol 2004;68:1375–1379. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell RB, Kelly J. Adenotonsillectomy for obstructive sleep apnea in obese children. Otolaryngol Head Neck Surg 2004;131:104–108. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell RB, Kelly J. Outcome of adenotonsillectomy for obstructive sleep apnea in obese and normal-weight children. Otolaryngol Head Neck Surg 2007;137:43–48. [DOI] [PubMed] [Google Scholar]
- 11.Tauman R, Gulliver TE, Krishna J, Montgomery-Downs HE, O'Brien LM, Ivanenko A, Gozal D. Persistence of obstructive sleep apnea syndrome in children after adenotonsillectomy. J Pediatr 2006;149:803–808. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharjee R, Kheirandish-Gozal L, Spruyt K, Mitchell RB, Promchiarak J, Simakajornboon N, Kaditis AG, Splaingard D, Splaingard M, Brooks LJ, et al. Adenotonsillectomy outcomes in treatment of obstructive sleep apnea in children: a multicenter retrospective study. Am J Respir Crit Care Med 2010;182:676–683. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics. Clinical practice guideline: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2002;109:704–712. [DOI] [PubMed] [Google Scholar]
- 14.Horner RL, Mohiaddin RH, Lowell DG, Shea SA, Burman ED, Longmore DB, Guz A. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J 1989;2:613–622. [PubMed] [Google Scholar]
- 15.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing: significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 1995;152:1673–1689. [DOI] [PubMed] [Google Scholar]
- 16.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med 2003;168:522–530. [DOI] [PubMed] [Google Scholar]
- 17.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis 1993;148:462–466. [DOI] [PubMed] [Google Scholar]
- 18.Vgontzas AN. Does obesity play a major role in the pathogenesis of sleep apnoea and its associated manifestations via inflammation, visceral adiposity, and insulin resistance? Arch Physiol Biochem 2008;114:211–223. [DOI] [PubMed] [Google Scholar]
- 19.Makino S, Fujiwara M, Suzukawa K, Handa H, Fujie T, Ohtaka Y, Komatsu Y, Aoki Y, Maruyama H, Terada Y, et al. Visceral obesity is associated with the metabolic syndrome and elevated plasma retinol binding protein-4 level in obstructive sleep apnea syndrome. Horm Metab Res 2009;41:221–226. [DOI] [PubMed] [Google Scholar]
- 20.Hoffstein V, Zamel N, Phillipson EA. Lung volume dependence of pharyngeal cross-sectional area in patients with obstructive sleep apnea. Am Rev Respir Dis 1984;130:175–178. [DOI] [PubMed] [Google Scholar]
- 21.Arens R, McDonough JM, Costarino AT, Mahboubi S, Tayag-Kier CE, Maislin G, Schwab RJ, Pack AI. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2001;164:698–703. [DOI] [PubMed] [Google Scholar]
- 22.Arens R, Sin SH, Wootton DM, McDonough JM, Rieder J, Khan U, Lipton M, Shifteh K. Upper airway structure in obese children with and without OSAS. ATS 2010 International Conference, New Orleans, Louisiana [abstract]. Am J Respir Crit Care Med 2010;181:A2433. [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11 2002;246:1–190. [PubMed] [Google Scholar]
- 24.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007.
- 25.Muzumdar H, Arens R. Diagnostic issues in pediatric obstructive sleep apnea. Proc Am Thorac Soc 2008;5:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isono S, Shimada A, Utsugi M, Konno A, Nishino T. Comparison of static mechanical properties of the passive pharynx between normal children and children with sleep-disordered breathing. Am J Respir Crit Care Med 1998;157:1204–1212. [DOI] [PubMed] [Google Scholar]
- 27.Arens R, McDonough JM, Corbin AM, Rubin NK, Carroll ME, Pack AI, Liu J, Udupa JK. Upper airway size analysis by magnetic resonance imaging of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2003;167:65–70. [DOI] [PubMed] [Google Scholar]
- 28.Suratt PM, Dee P, Atkinson RL, Armstrong P, Wilhoit SC. Fluoroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis 1983;127:487–492. [DOI] [PubMed] [Google Scholar]
- 29.Ryan CF, Love LL. Mechanical properties of the velopharynx in obese patients with obstructive sleep apnea. Am J Respir Crit Care Med 1996;154:806–812. [DOI] [PubMed] [Google Scholar]
- 30.Fregosi RF, Quan SF, Kaemingk KL, Morgan WJ, Goodwin JL, Cabrera R, Gmitro A. Sleep-disordered breathing, pharyngeal size and soft tissue anatomy in children. J Appl Physiol 2003;95:2030–2038. [DOI] [PubMed] [Google Scholar]
- 31.Goldbart AD, Goldman JL, Veling MC, Gozal D. Leukotriene modifier therapy for mild sleep-disordered breathing in children. Am J Respir Crit Care Med 2005;172:364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma C-reactive protein levels among children with sleep-disordered breathing. Pediatrics 2004;113:e564–e569. [DOI] [PubMed] [Google Scholar]
- 33.Gozal D, Serpero LD, Sans Capdevila O, Kheirandish-Gozal L. Systemic inflammation in non-obese children with obstructive sleep apnea. Sleep Med 2008;9:254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med 2008;177:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwab RJ, Pasirstein M, Kaplan L, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Family aggregation of upper airway soft tissue structures in normal subjects and patients with sleep apnea. Am J Respir Crit Care Med 2006;173:453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van de Graaff WB. Thoracic traction on the trachea: mechanisms and magnitude. J Appl Physiol 1991;70:1328–1336. [DOI] [PubMed] [Google Scholar]
- 37.Schafer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest 2002;122:829–839. [DOI] [PubMed] [Google Scholar]
- 38.Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 2002;165:670–676. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O'Donnell CP, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 2005;97:698–706. [DOI] [PubMed] [Google Scholar]
- 40.Arens R, Muzumdar H. Childhood obesity and obstructive sleep apnea syndrome. J Appl Physiol 2010;108:436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.