Abstract
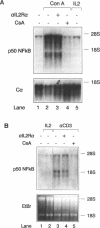
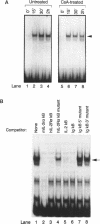
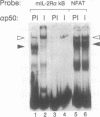
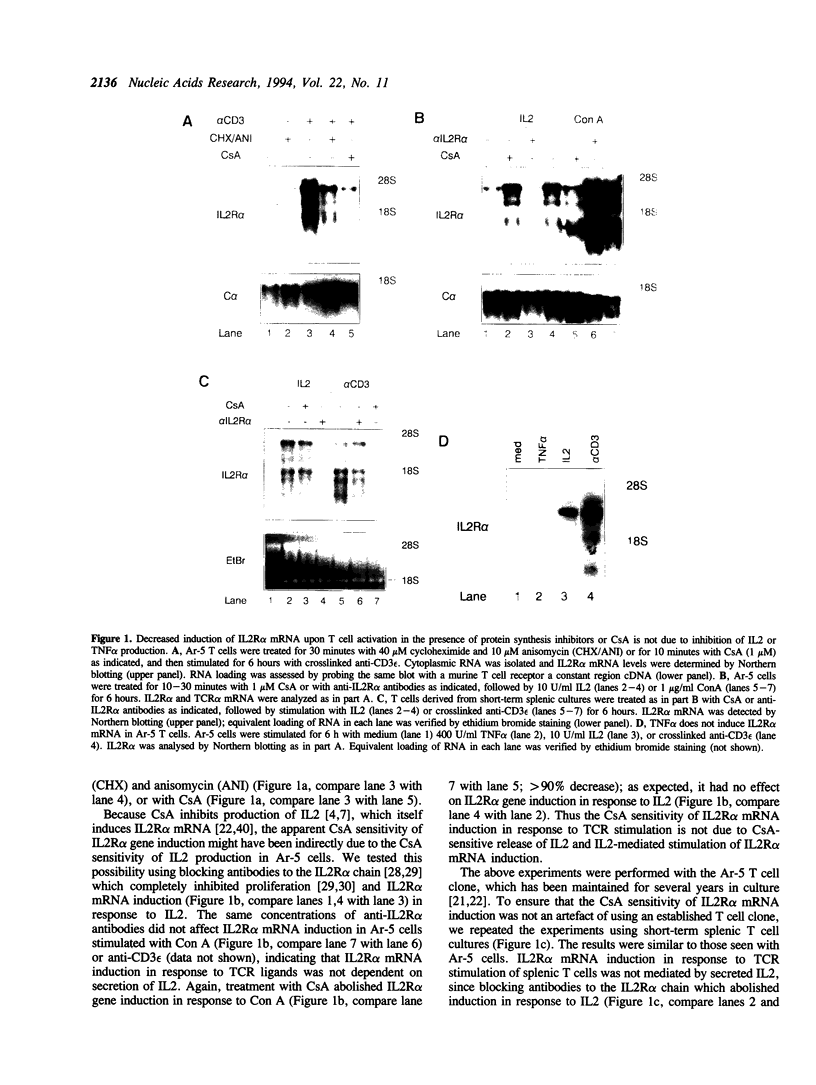
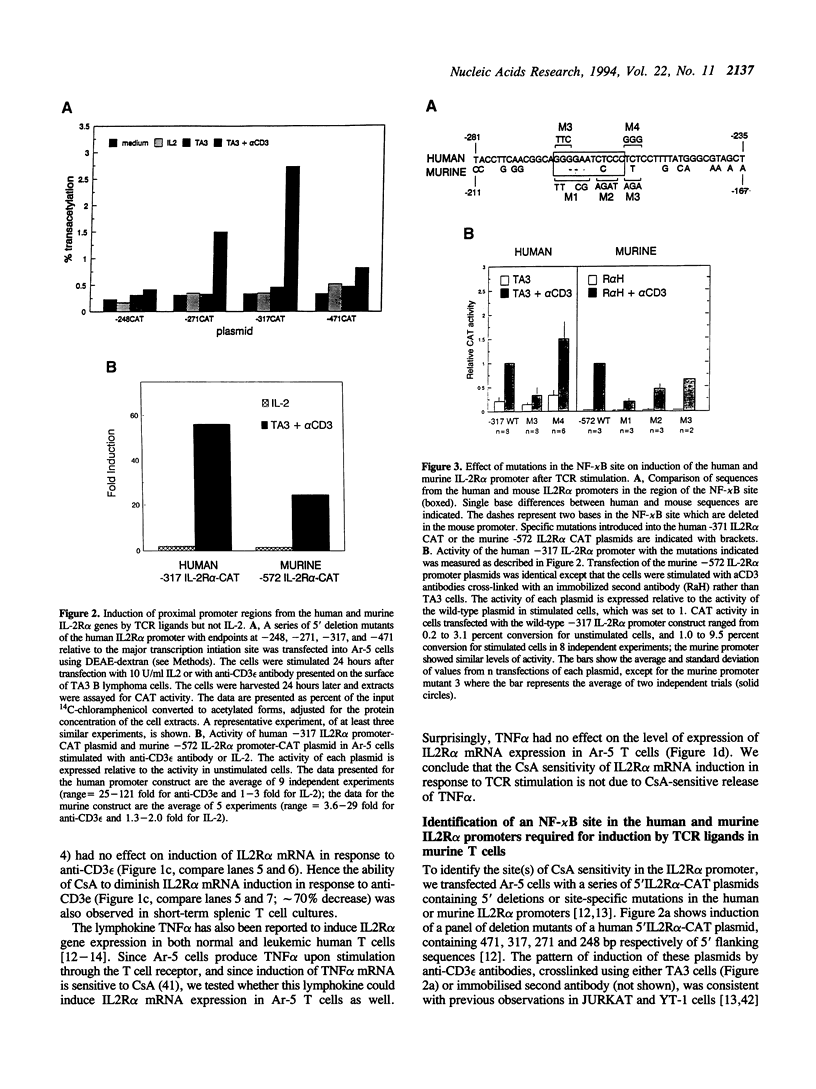
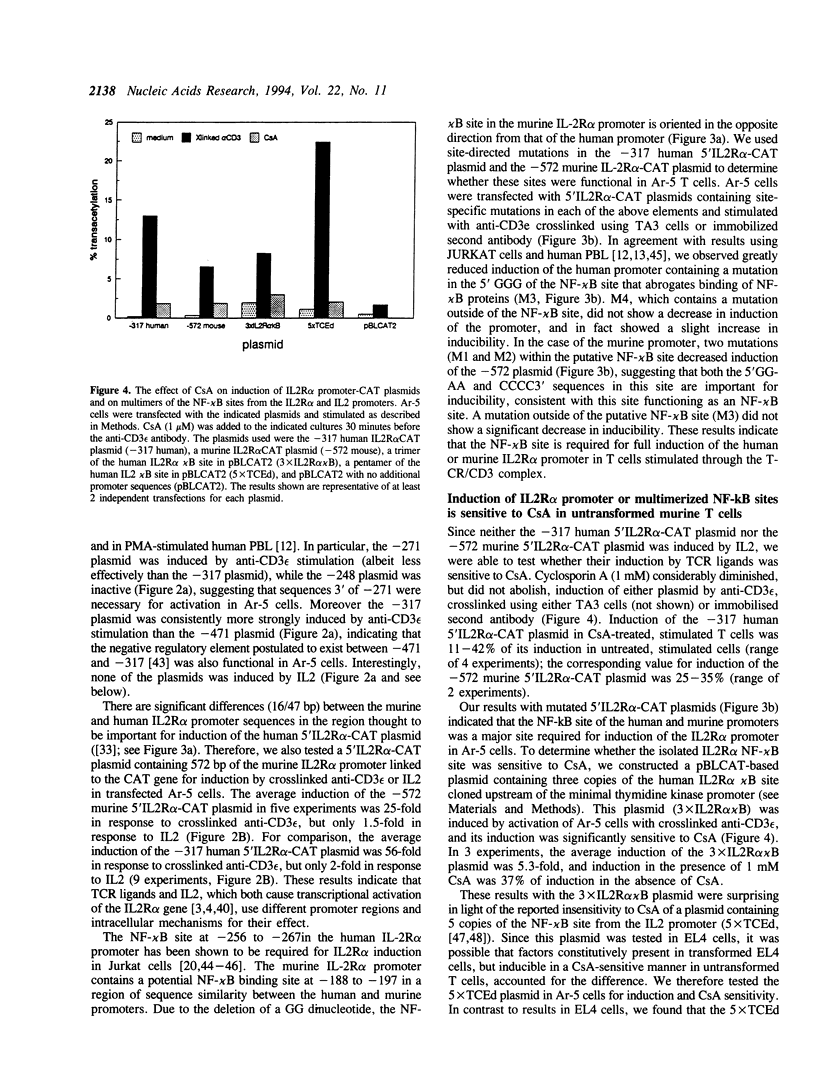
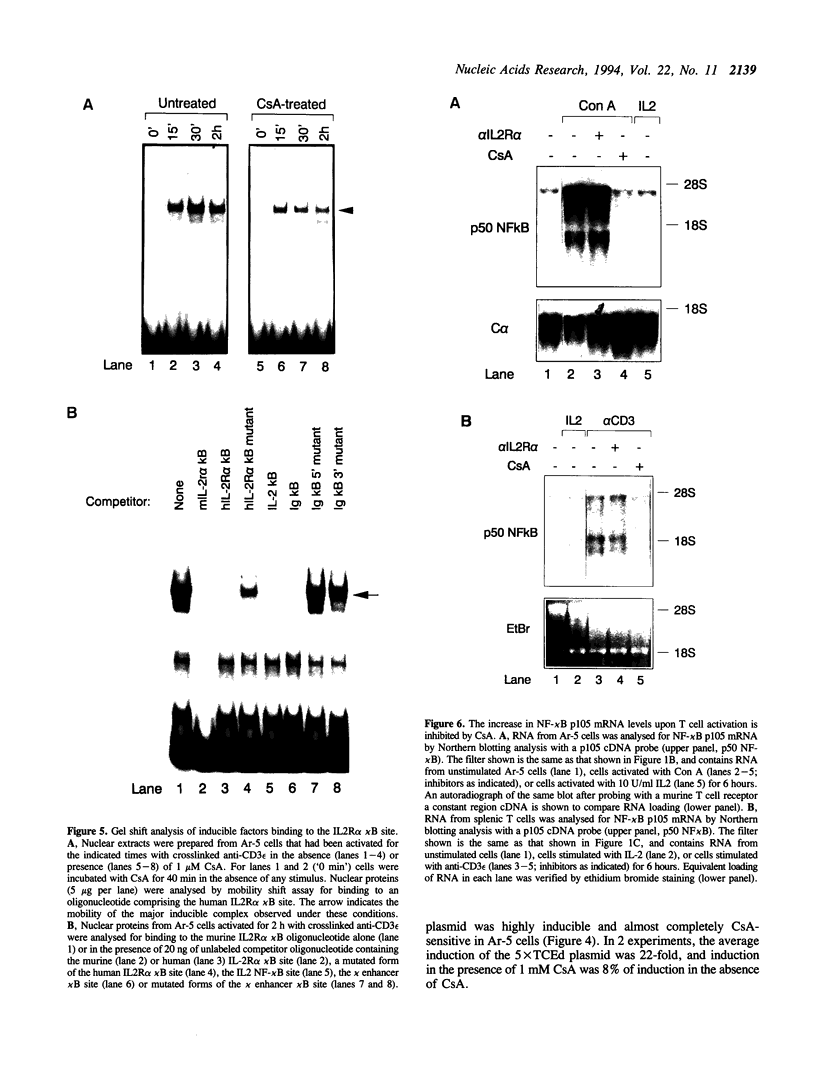
We have investigated the characteristics of IL2R alpha gene induction in untransformed murine T cells. Induction of IL2R alpha mRNA by TCR/CD3 ligands in a murine T cell clone and in short-term splenic T cell cultures was inhibited by protein synthesis inhibitors and by CsA. This result was contrary to previous observations in JURKAT T leukemia cells and human peripheral blood T cells, suggesting a difference in the mechanisms of IL2R alpha gene induction in these different cell types. The CsA sensitivity of IL2R alpha mRNA induction represented a direct effect on the TCR/CD3 response, and was not due to CsA-sensitive release of the lymphokines IL2 or tumour necrosis factor alpha (TNF alpha) and consequent lymphokine-mediated induction of IL2R alpha mRNA. The NF-kappa B site of the IL2R alpha promoter was essential for gene induction through the TCR/CD3 complex, and the induction of reporter plasmids containing multimers of this site was significantly inhibited by CsA. Northern blotting analysis indicated that while the p65 subunit of NF-kappa B was constitutively expressed and not appreciably induced upon T cell activation, mRNA for the p105 precursor of p50 NF-kappa B was induced in response to TCR/CD3 stimulation and this induction was sensitive to CsA. Electrophoretic mobility shift assays and antiserum against the p50 subunit of NF-kappa B indicated that p50 was a component of the inducible nuclear complex that bound to the IL2R alpha kappa B site. Appearance of the kB-binding proteins was insensitive to CsA at early times after activation (approximately 15 min), but was partially sensitive to CsA at later times. Based on these results, we propose that the NF-kappa B site of the IL2R alpha promoter mediates at least part of the CsA sensitivity of IL2R alpha gene induction in untransformed T cells, possibly because de novo synthesis of p105 NF-kappa B is required for sustained IL2R alpha expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baeuerle P. A., Baltimore D. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science. 1988 Oct 28;242(4878):540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Böhnlein E., Hoffman J. A., Bogerd H. P., Dixon E. P., Franza B. R., Greene W. C. Activation of the interleukin-2 receptor alpha gene: regulatory role for DNA-protein interactions flanking the kappa B enhancer. New Biol. 1989 Oct;1(1):83–92. [PubMed] [Google Scholar]
- Ballard D. W., Böhnlein E., Lowenthal J. W., Wano Y., Franza B. R., Greene W. C. HTLV-I tax induces cellular proteins that activate the kappa B element in the IL-2 receptor alpha gene. Science. 1988 Sep 23;241(4873):1652–1655. doi: 10.1126/science.241.4873.1652. [DOI] [PubMed] [Google Scholar]
- Banerji S. S., Parsons J. N., Tocci M. J. The immunosuppressant FK-506 specifically inhibits mitogen-induced activation of the interleukin-2 promoter and the isolated enhancer elements NFIL-2A and NF-AT1. Mol Cell Biol. 1991 Aug;11(8):4074–4087. doi: 10.1128/mcb.11.8.4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours V., Villalobos J., Burd P. R., Kelly K., Siebenlist U. Cloning of a mitogen-inducible gene encoding a kappa B DNA-binding protein with homology to the rel oncogene and to cell-cycle motifs. Nature. 1990 Nov 1;348(6296):76–80. doi: 10.1038/348076a0. [DOI] [PubMed] [Google Scholar]
- Brabletz T., Pietrowski I., Serfling E. The immunosuppressives FK 506 and cyclosporin A inhibit the generation of protein factors binding to the two purine boxes of the interleukin 2 enhancer. Nucleic Acids Res. 1991 Jan 11;19(1):61–67. doi: 10.1093/nar/19.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Böhnlein E., Ballard D. W., Bogerd H., Peffer N. J., Lowenthal J. W., Greene W. C. Induction of interleukin-2 receptor-alpha gene expression is regulated by post-translational activation of kappa B specific DNA binding proteins. J Biol Chem. 1989 May 25;264(15):8475–8478. [PubMed] [Google Scholar]
- Ceredig R., Lowenthal J. W., Nabholz M., MacDonald H. R. Expression of interleukin-2 receptors as a differentiation marker on intrathymic stem cells. Nature. 1985 Mar 7;314(6006):98–100. doi: 10.1038/314098a0. [DOI] [PubMed] [Google Scholar]
- Cross S. L., Halden N. F., Lenardo M. J., Leonard W. J. Functionally distinct NF-kappa B binding sites in the immunoglobulin kappa and IL-2 receptor alpha chain genes. Science. 1989 Apr 28;244(4903):466–469. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Drogula C., Krönke M., Waldmann T. A., Greene W. C. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Freimuth W. W., Depper J. M., Nabel G. J. Regulation of the IL-2 receptor alpha-gene. Interaction of a kappa B binding protein with cell-specific transcription factors. J Immunol. 1989 Nov 1;143(9):3064–3068. [PubMed] [Google Scholar]
- Ghosh S., Baltimore D. Activation in vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B. Nature. 1990 Apr 12;344(6267):678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Gifford A. M., Riviere L. R., Tempst P., Nolan G. P., Baltimore D. Cloning of the p50 DNA binding subunit of NF-kappa B: homology to rel and dorsal. Cell. 1990 Sep 7;62(5):1019–1029. doi: 10.1016/0092-8674(90)90276-k. [DOI] [PubMed] [Google Scholar]
- Glimcher L. H., Hamano T., Asofsky R., Sachs D. H., Pierres M., Samelson L. E., Sharrow S. O., Paul W. E. IA mutant functional antigen-presenting cell lines. J Immunol. 1983 May;130(5):2287–2294. [PubMed] [Google Scholar]
- Goldfeld A. E., McCaffrey P. G., Strominger J. L., Rao A. Identification of a novel cyclosporin-sensitive element in the human tumor necrosis factor alpha gene promoter. J Exp Med. 1993 Oct 1;178(4):1365–1379. doi: 10.1084/jem.178.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann H. P., Remy R., Scheidereit C., van Loon A. P. Maintenance of NF-kappa B activity is dependent on protein synthesis and the continuous presence of external stimuli. Mol Cell Biol. 1991 Jan;11(1):259–266. doi: 10.1128/mcb.11.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyos B., Ballard D. W., Böhnlein E., Siekevitz M., Greene W. C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989 Apr 28;244(4903):457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- Jain J., Valge-Archer V. E., Sinskey A. J., Rao A. The AP-1 site at -150 bp, but not the NF-kappa B site, is likely to represent the major target of protein kinase C in the interleukin 2 promoter. J Exp Med. 1992 Mar 1;175(3):853–862. doi: 10.1084/jem.175.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson C., McCaffrey P. G., Rao A., Sen R. Physiologic activation of T cells via the T cell receptor induces NF-kappa B. J Immunol. 1991 Jul 15;147(2):416–420. [PubMed] [Google Scholar]
- Kang S. M., Tran A. C., Grilli M., Lenardo M. J. NF-kappa B subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992 Jun 5;256(5062):1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Greene W. C. Sequential expression of genes involved in human T lymphocyte growth and differentiation. J Exp Med. 1985 Jun 1;161(6):1593–1598. doi: 10.1084/jem.161.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang A. A., Novak K. D., Kang S. M., Bruhn K., Lenardo M. J. Interaction between NF-kappa B- and serum response factor-binding elements activates an interleukin-2 receptor alpha-chain enhancer specifically in T lymphocytes. Mol Cell Biol. 1993 Apr;13(4):2536–2545. doi: 10.1128/mcb.13.4.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou H. C., Baltimore D. Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol. 1993 Jun;5(3):477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Ballard D. W., Bogerd H., Böhnlein E., Greene W. C. Tumor necrosis factor-alpha activation of the IL-2 receptor-alpha gene involves the induction of kappa B-specific DNA binding proteins. J Immunol. 1989 May 1;142(9):3121–3128. [PubMed] [Google Scholar]
- Lowenthal J. W., Ballard D. W., Böhnlein E., Greene W. C. Tumor necrosis factor alpha induces proteins that bind specifically to kappa B-like enhancer elements and regulate interleukin 2 receptor alpha-chain gene expression in primary human T lymphocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2331–2335. doi: 10.1073/pnas.86.7.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Böhnlein E., Ballard D. W., Greene W. C. Regulation of interleukin 2 receptor alpha subunit (Tac or CD25 antigen) gene expression: binding of inducible nuclear proteins to discrete promoter sequences correlates with transcriptional activation. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4468–4472. doi: 10.1073/pnas.85.12.4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila P. S., Ullman K. S., Fiering S., Emmel E. A., McCutcheon M., Crabtree G. R., Herzenberg L. A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO J. 1990 Dec;9(13):4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey P. G., Jain J., Jamieson C., Sen R., Rao A. A T cell nuclear factor resembling NF-AT binds to an NF-kappa B site and to the conserved lymphokine promoter sequence "cytokine-1". J Biol Chem. 1992 Jan 25;267(3):1864–1871. [PubMed] [Google Scholar]
- Molitor J. A., Walker W. H., Doerre S., Ballard D. W., Greene W. C. NF-kappa B: a family of inducible and differentially expressed enhancer-binding proteins in human T cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10028–10032. doi: 10.1073/pnas.87.24.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Ghosh S., Liou H. C., Tempst P., Baltimore D. DNA binding and I kappa B inhibition of the cloned p65 subunit of NF-kappa B, a rel-related polypeptide. Cell. 1991 Mar 8;64(5):961–969. doi: 10.1016/0092-8674(91)90320-x. [DOI] [PubMed] [Google Scholar]
- Pomerantz J. L., Mauxion F., Yoshida M., Greene W. C., Sen R. A second sequence element located 3' to the NF-kappa B-binding site regulates IL-2 receptor-alpha gene induction. J Immunol. 1989 Dec 15;143(12):4275–4281. [PubMed] [Google Scholar]
- Randak C., Brabletz T., Hergenröther M., Sobotta I., Serfling E. Cyclosporin A suppresses the expression of the interleukin 2 gene by inhibiting the binding of lymphocyte-specific factors to the IL-2 enhancer. EMBO J. 1990 Aug;9(8):2529–2536. doi: 10.1002/j.1460-2075.1990.tb07433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A., Faas S. J., Cantor H. Activation specificity of arsonate-reactive T cell clones. Structural requirements for hapten recognition and comparison with monoclonal antibodies. J Exp Med. 1984 Feb 1;159(2):479–494. doi: 10.1084/jem.159.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman D. G., Toledano M. B., Leonard W. J. Sp1 represses IL-2 receptor alpha chain gene expression. New Biol. 1990 Jul;2(7):642–647. [PubMed] [Google Scholar]
- Ruben S., Poteat H., Tan T. H., Kawakami K., Roeder R., Haseltine W., Rosen C. A. Cellular transcription factors and regulation of IL-2 receptor gene expression by HTLV-I tax gene product. Science. 1988 Jul 1;241(4861):89–92. doi: 10.1126/science.2838905. [DOI] [PubMed] [Google Scholar]
- Schmidt A., Hennighausen L., Siebenlist U. Inducible nuclear factor binding to the kappa B elements of the human immunodeficiency virus enhancer in T cells can be blocked by cyclosporin A in a signal-dependent manner. J Virol. 1990 Aug;64(8):4037–4041. doi: 10.1128/jvi.64.8.4037-4041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986 Aug 29;46(5):705–716. doi: 10.1016/0092-8674(86)90346-6. [DOI] [PubMed] [Google Scholar]
- Serfling E., Barthelmäs R., Pfeuffer I., Schenk B., Zarius S., Swoboda R., Mercurio F., Karin M. Ubiquitous and lymphocyte-specific factors are involved in the induction of the mouse interleukin 2 gene in T lymphocytes. EMBO J. 1989 Feb;8(2):465–473. doi: 10.1002/j.1460-2075.1989.tb03399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin-2: inception, impact, and implications. Science. 1988 May 27;240(4856):1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Greene W. C. The same 50-kDa cellular protein binds to the negative regulatory elements of the interleukin 2 receptor alpha-chain gene and the human immunodeficiency virus type 1 long terminal repeat. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8526–8530. doi: 10.1073/pnas.86.21.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Matsunami N., Kanamori H., Ishida N., Shimizu A., Yaoita Y., Nikaido T., Honjo T. The human IL-2 receptor gene contains a positive regulatory element that functions in cultured cells and cell-free extracts. J Biol Chem. 1987 Apr 15;262(11):5079–5086. [PubMed] [Google Scholar]
- Ten R. M., Paya C. V., Israël N., Le Bail O., Mattei M. G., Virelizier J. L., Kourilsky P., Israël A. The characterization of the promoter of the gene encoding the p50 subunit of NF-kappa B indicates that it participates in its own regulation. EMBO J. 1992 Jan;11(1):195–203. doi: 10.1002/j.1460-2075.1992.tb05042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tocci M. J., Matkovich D. A., Collier K. A., Kwok P., Dumont F., Lin S., Degudicibus S., Siekierka J. J., Chin J., Hutchinson N. I. The immunosuppressant FK506 selectively inhibits expression of early T cell activation genes. J Immunol. 1989 Jul 15;143(2):718–726. [PubMed] [Google Scholar]
- Toledano M. B., Roman D. G., Halden N. F., Lin B. B., Leonard W. J. The same target sequences are differentially important for activation of the interleukin 2 receptor alpha-chain gene in two distinct T-cell lines. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1830–1834. doi: 10.1073/pnas.87.5.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman K. S., Northrop J. P., Verweij C. L., Crabtree G. R. Transmission of signals from the T lymphocyte antigen receptor to the genes responsible for cell proliferation and immune function: the missing link. Annu Rev Immunol. 1990;8:421–452. doi: 10.1146/annurev.iy.08.040190.002225. [DOI] [PubMed] [Google Scholar]
- Valge-Archer V. E., de Villiers J., Sinskey A. J., Rao A. Transformation of T lymphocytes by the v-fos oncogene. J Immunol. 1990 Dec 15;145(12):4355–4364. [PubMed] [Google Scholar]
- Valge V. E., Wong J. G., Datlof B. M., Sinskey A. J., Rao A. Protein kinase C is required for responses to T cell receptor ligands but not to interleukin-2 in T cells. Cell. 1988 Oct 7;55(1):101–112. doi: 10.1016/0092-8674(88)90013-x. [DOI] [PubMed] [Google Scholar]
- Wong J. G., Rao A. Mutants in signal transduction through the T-cell antigen receptor. J Biol Chem. 1990 Mar 15;265(8):4685–4693. [PubMed] [Google Scholar]