Abstract
There are numerous studies reporting on the effects of inhalation anaesthesia in cells of exposed individuals but not much is known about the ability of isoflurane (ISF) to induce oxidative DNA damage. However, surgery is often associated with a temporary perioperative immunological alteration, and some volatile anaesthetics seem to contribute to a transient lymphocytopenia after surgery. We conducted a study to evaluate a possible genotoxic effect, including oxidative DNA damage, and apoptosis in peripheral lymphocytes of 20 patients American Society of Anaesthesiologists physical status I undergoing minor elective surgery lasting at least 120 min, under anaesthesia with ISF. We also investigated the expression of several genes in blood cells. Blood samples were collected at three time points: before anaesthesia (T1), 2 h after the beginning of anaesthesia (T2) and on the first post-operative day (T3). General DNA damage and oxidised bases (Fpg and endo III-sites) in blood lymphocytes were evaluated using the comet assay. Lymphocytes were phenotyped and apoptosis was evaluated by flow cytometry. In addition, expressions of hOGG1 and XRCC1, genes involved in DNA repair, and BCL2, a gene related to apoptosis, were assessed by quantitative real-time polymerase chain reaction. Results showed no statistically significant difference in the level of DNA damage and oxidised bases among the three sampling times. Anaesthesia with ISF did not increase the percentage of cells in early or late apoptosis in cytotoxic or helper T lymphocytes. Lower hOGG1 and BCL2 expressions were detected at T3 in comparison to the other two previous time points, and there was significantly lower expression of XRCC1 at T3 in relation to T2. In conclusion, the exposure to ISF did not result in genotoxicity and cytotoxicity in lymphocytes and in toxicogenomic effect in leukocytes, although DNA repair and apoptosis-related genes were down-regulated on the first post-operative day.
Introduction
DNA is continuously exposed to a variety of biological, chemical and physical agents, which may alter its structure, modifying its function (1). Among exogenous compounds, anaesthetic gases, commonly used in general anaesthesia procedures, have attracted attention because of concern about their potential for having genotoxic effects (2–4). Worldwide, ∼100 million people every year undergo surgery, with the majority of patients receiving inhalation general anaesthesia (5). Isoflurane (ISF) and sevoflurane are the most widely used inhaled halogenated anaesthetics. Due to its low metabolism rate and low blood-gas partition coefficient, which decreases its induction and recovery times, the introduction of ISF [2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane] in clinical practice represented a significant advance for inhalation anaesthesia (6). In humans, ISF is metabolised by Phase 1 enzyme cytochrome P450 (CYP2E1), generating a reactive trifluoroacetyl ester. ISF, an ether compound, has a structure similar to that of some non-anaesthetic carcinogens, including chloromethyl methyl ether (7).
Despite of the observation that increased levels of DNA damage has been reported in cells sampled from operating room personnel occupationally exposed to trace concentrations of anaesthetic gases (8–12), the genotoxicity of ISF is still controversial. Negative results were obtained in the Ames reverse mutation test using Salmonella typhimurium strain TA 1535, with and without metabolic activation (13), and in Drosophila melanogaster sex-linked recessive lethal assay (14). Conversely, ISF genotoxicity has been reported in lymphocytes in vitro (15,16) and in animals exposed to the anaesthetic (17). Positive results were also detected in patients undergoing invasive surgery (2,18). Jaloszynski et al. (15) have hypothesized that polyfluorinated anaesthetics, such as ISF and halothane, can alkylate N-7 position of purines. Furthermore, these same authors believe that anaesthetic genotoxicity might also be due to their metabolic oxidation or reduction, giving rise to reactive metabolites and reactive oxygen species (ROS). It is well known that oxidative DNA damage can be repaired, especially by the base excision repair system, which include the genes hOGG1 (human 8-oxoguanine DNA glycosylase 1) and XRCC1 (X-ray cross-complementing group 1 protein) (19,20). However, it is not known about the interference of anaesthetics on the expressions of such genes.
Evidence of immune system dysfunctions has been frequently reported after surgery, which can lead to profound, but transient, depletion of all types of lymphocytes (21). Although the mechanism underlying the decrease of the number of immunological cells is still not clear, it may result from lymphocytes being induced to enter into apoptosis (22). It has been reported that halogenated anaesthetics in vitro cause lymphocytopenia due to apoptosis via up-regulation of caspase-3 (23). Similarly, elevated rates of apoptosis 24 h after invasive surgery under ISF or nitrous oxide (N2O) anaesthesia have been observed in cultured T lymphocytes (24,25). In contrast, it has been reported that ISF protected cardiomyocytes and reduced the level of apoptosis induced by hydrogen peroxide and hypoxia in rodents (26).
In the literature, there is no information about the ISF genotoxicity in healthy young patients undergoing non-invasive surgeries. Furthermore, it remains controversial the interference of possible confounding factors, such as age, physical status of the patients and type of surgery on the genotoxic potential of the anaesthetic. Therefore, the present study aimed to evaluate general DNA damage and specifically oxidised purines and pyrimidines induced by ISF in lymphocytes of patients undergoing minor surgeries. Additionally, it was also investigated whether the exposure to the anaesthetic could interfere with the immune system and also with the expression of DNA repair (hOGG1 and XRCC1) and apoptosis (BCL2)-related genes.
Materials and methods
Subjects
The Ethical Committee for Human Research from Botucatu Medical School—Universidade Estadual Paulista (581/2006—Botucatu, São Paulo, Brazil) approved the protocol used in the present study. After signing the informed consent, all the patients answered a detailed questionnaire about their lifestyle, health status and previous exposure to environmental pollutants. Patients with a disease, smokers, alcoholics and those who were under medication or antioxidant supplement or who had received radiation were excluded. Twelve male and eight female non-smoker adults classified by the American Society of Anaesthesiologists (ASA) as physical status I patients (healthy patient, with no disease other than surgical abnormality and with no systemic disturbances) with normal body mass index (23.5 ± 3.5 kg/m2), aged from 18 to 45 years (25.1 ± 6.8 years) and scheduled for elective minor otorhinological surgery lasting at least 120 min (140.6 ± 35.8 min), at Botucatu Medical School Hospital, were enrolled in this study. Propofol (160 ± 46.7 mg), fentanyl (462 ± 151.3 μg) and rocuronium (44.4 ± 7.1 mg) were used during anaesthesia.
General anaesthesia procedure
Standard clinical monitoring was performed: electrocardiogram, peripheral oxygen saturation (SpO2), non-invasive arterial pressure (systolic and diastolic), end-tidal CO2 (PETCO2) and ISF and monitoring of neuromuscular blockade.
All patients were premedicated in the operating room with intravenous (IV) benzodiazepine midazolam (3 mg; Roche, São Paulo, Brazil). Anaesthesia was induced with opioid fentanyl (5 μg/kg IV; Janssen, São Paulo, Brazil), hypnotic agent propofol (2 mg/kg IV; Astra Zeneca, Milan, Italy) and rocuronium bromide (0.6 mg/kg IV; Organon, Oss, Holland), which is a neuromuscular blocker that was given to facilitate orotracheal intubation. The lungs were mechanically ventilated with 40% oxygen (0.8 l/min) in air (1.2 l/min). ISF (Abbott, Rio de Janeiro, Brazil) at 1.0 minimum alveolar concentration (1.2%) was administered by inhalation. Adequacy of anaesthesia during maintenance was assessed by haemodynamic responses, and additional doses of fentanyl (2 μg/kg) and rocuronium (0.2 mg/kg) were used when necessary, if the patients were judged to be inadequately anaesthetised.
The neuromuscular block was reversed with neostigmine (30 μg/kg IV; União Química, São Paulo, Brazil) and atropine (10 μg/kg IV; Ariston, São Paulo, Brazil) at the end of surgery. Ondansetron (8 mg IV; Cristália, São Paulo, Brazil) was utilised for antiemesis. Dipyrone (1 g; Hoechst, Rio de Janeiro, Brazil) and tramadol (100 mg IV; Pfizer, São Paulo, Brazil) were used for post-operative analgesia, at the end of the surgery. If necessary, dipyrone (1 g IV) was used on the first post-operative day.
Blood sampling
Venous blood samples from all patients undergoing inhalation anaesthesia were drawn at three time points: before premedication and anaesthesia (T1), 2 h after the beginning of anaesthesia (T2) and on the first post-operative day (T3). Blood was collected in sodium heparin tubes (10 ml) for immediate lymphocytes isolation and in PAXgene Blood RNA Tubes (Qiagen/PreAnalytiX, Hombrechtikon, Switzerland) for RNA stabilisation. These tubes were kept at room temperature (RT) for 12 h and then placed into a freezer maintained at −20°C until RNA isolation.
Chemicals
Ethidium bromide, HEPES and bovine serum albumin were purchased from Sigma (St Louis, MO, USA); normal and low melting point agaroses, EDTA and Tris from Invitrogen (Carlsbad, CA, USA); hydrogen peroxide (H2O2), H3BO3, NaCl, NaOH, KCl, HCl, NaHCO3, KH2PO4 and Na2HPO4 from Merck (Germany); Ficoll–Paque® from GE (Sweden); dimethylsulfoxide from Mallinckrodt (Mexico); Triton X-100 from J. T. Baker (Phillipsburg, NJ, USA); annexin labelled with fluorescein isothiocyanate (FITC) and annexin V buffer, 7-amino-actinomycin D (7-AAD), phycoerythrin (PE)-labelled monoclonal antibodies anti-hCD4+ and anti-hCD8+ were purchased from Becton Dickinson (San Jose, CA, USA) and endonuclease III (endo III) and formamidopyrimidine DNA glycosylase (Fpg) from New England Biolabs (Ipswich, MA, USA).
Lymphocyte isolation
Lymphocytes were isolated in Ficoll–Paque® gradients. Samples of peripheral blood (2 ml) were mixed with 2 ml of phosphate-buffered saline (PBS), layered over 3 ml of Ficoll and centrifuged at 1100 × g for 30 min, at RT. The lymphocyte layer was removed, mixed with 4 ml PBS, centrifuged at 400 × g for 15 min and the cell pellet was resuspended in PBS (27). Lymphocytes were used for comet assay and flow cytometry.
Alkaline comet assay
The protocol used followed the general procedures used by Singh et al. (28) and Tice et al. (29), with some modifications. Every step was carried out under indirect light. Slides were coded and blindly analysed. Volumes of 10 μl of fresh lymphocytes (∼1 to 5 × 104 cells/μl) were added to 120 μl of 0.5% low melting point agarose at 37°C. The mixtures were layered onto slides precoated with 1.5% normal agarose, covered with a coverslip and left for 5 min at 4°C to solidify the agarose. Afterwards, the coverslips were carefully removed and the slides immersed, overnight, into a cold lysis solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris at pH 10, with 1% Triton X-100 and 10% dimethylsulfoxide added fresh). To evaluate oxidative DNA damage, slides were washed in PBS for 5 min and washed again (3 × 5 min each) in a buffer ×1 (40 mM HEPES, 100 mM KCl, 0.2 mg/ml bovine serum albumin and 0.5 mM EDTA at pH 8). Slides were incubated at 37°C for 30 min in a moist and dry chamber, with 100 μl of Fpg and endo III (1:1000) that recognises oxidised purine and pyrimidine lesions, respectively, and with 100 μl of enzyme buffer only (control). Subsequently, the slides were left for 15 min at 4°C and then the coverslips were removed. Afterwards, the slides were exposed to a freshly prepared alkaline buffer (1 mM EDTA, 300 mM NaOH at pH > 13) in a horizontal electrophoresis tank. After a 40-min DNA unwinding period, electrophoresis was conducted at 0.8 V/cm and 300 mA for 30 min. Following 15 min neutralisation with 0.4 M Tris (pH 7.5), the slides were fixed in absolute ethanol and then stored at 4°C. Lymphocytes sampled at the three time points from exposed patients were also treated with hydrogen peroxide (H2O2) at 100 μM for 30 min at 4°C to evaluate the sensitivity of these cells to oxidative DNA damage.
The slides were stained with 50 μl ethidium bromide (20 μg/ml) and analysed in a fluorescent microscope at ×400 magnification. Images from 100 nucleoids (two replicates) per each treatment/time point/patient were scored using the Comet Assay II Image System (Perceptive Instruments, Haverhill, Suffolk, UK). Tail intensity (%DNA in tail) was used to estimate the extent of DNA damage.
Phenotyping and apoptosis detection
T-lymphocytes phenotyping and assessment of apoptosis were performed in a FACSCalibur Flow Cytometer (BD Biosciences). The percentage of cells in early apoptosis was quantified using the annexin V-FITC method, which detects the phosphatidylserine externalised in the early phases of apoptosis (annexin-V+/7-AAD−); cells in late apoptosis were quantified based on the presence of annexin-V+/7-AAD+ and viable cells were also analysed (annexin-V−/7-AAD−). The annexin-V+ is an important marker of early apoptosis because it happens before DNA fragmentation (30).
Freshly obtained lymphocytes were distributed into aliquots (∼1 × 106 cells/ml) and individually incubated: one tube was mixed with isoton, a saline solution with no antibodies (autofluorescence), vortexed and incubated at RT, in the dark, for 15 min; other aliquots, following PE-labelled monoclonal antibodies anti-CD4+ and anti-CD8+, were phenotyped to differentiate CD4+ T cells and CD8+ T cells. Lymphocytes were mixed with 100 μl of annexin V-binding buffer (diluted 1:10 with distilled water), 5 μl of annexin V-FITC, 5 μl of 7-AAD and 10 μl of anti-CD4 or anti-CD8, vortexed and incubated for 15 min at RT in the dark. Afterwards, a 400 μl aliquot of annexin V-binding buffer ×1 was added to each tube, samples were vortexed and the flow cytometry analysis was performed. Gating on lymphocytes (10 000 events were acquired for each sample) by forward and side scatter (cell size versus granulosity) for defining the target population. Data were analysed by the Cell Quest and FACScomp Programs.
Analysis of hOGG1, XRCC1 and BCL-2 mRNA by quantitative real-time polymerase chain reaction
RNA was isolated with the PAXgene Blood RNA Kit according to the manufacturer’s protocol (Qiagen/PreAnalytiX). The concentration of total RNA was determined by spectrophotometer, and each sample was assessed for purity by absorbance (A260/A280 nm between 1.9 and 2.1). Samples integrity was assessed by 1.5% agarose gel using Tris/borate/EDTA buffer. RNA was reverse-transcribed complementary DNA (cDNA) with a High Capacity cDNA Reverse Transcription kit according to the manufacturer’s protocol (Applied Biosystems—ABI, Foster City, CA, USA). The reactions were firstly incubated at 25°C for 10 min and 37°C for 120 min and then at 4°C. Samples were placed into a freezer at −20°C until polymerase chain reaction (PCR).
Quantitative real-time PCR was performed using Taqman FAM–MGB probes and primers, ordered as inventoried from ABI for the genes hOGG1 (assay ID Hs00213454_m1), XRCC1 (assay ID Hs00959834_m1) and BCL2 (assay ID Hs00608023_m1). After comparing candidate genes for the endogenous control, β-actin (ACTB) was selected for this study. For amplification, TaqMan Universal PCR Master mix (ABI) was used and a final of 10 μl volume was used for each reaction. Thermal cycling and real-time detection of the fluorescence were carried out in an ABI Prism 7500 FAST using the following amplification parameters: denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec. Negative control (no cDNA) in duplicate was added in each plate to ensure no contamination. For the control, a pool of cDNA sample from healthy subjects was used. All controls were run simultaneously with the test samples throughout the experiments. Cycle threshold (Ct) values were determined as the cycle where ROX-normalised fluorescence over background was significantly above background levels. Fold induction was calculated using the formula 2−ΔΔCt (31). In the pool samples (control), relative quantification (RQ) for hOGG1 varied from 0.8 to 1.2; for XRCC1 from 0.8 to 1.3 and for BCL2 from 0.7 to 1.1. Based on these data, genes were considered up-regulated when RQ from patients were ≥1.5; down-regulation was considered when RQ < 0.5.
Statistical analysis
The non-parametric Friedman test was used to compare the extent of DNA damage, the frequency of viable cells and apoptosis and the extent of gene expression (data expressed as median and first and third quartiles) at the three blood sampling times. When two variables were compared, the Mann–Whitney test was performed. Values with P < 0.05 were considered statistically significant.
Results
Inhalation anaesthesia with ISF was not genotoxic
General anaesthesia with the ISF at 1.0 minimum alveolar concentration did not induce DNA single- and double-strand breaks and alkali-labile sites in lymphocytes, as measured by the comet assay, when evaluated 2 h after the beginning of anaesthesia (T2) or on the first post-operative day (T3). Similar results (T1 = T2 = T3) were found when Fpg and endo III were used to detect oxidised purines and pyrimidines, respectively (Table I).
Table I.
DNA single- and double-strand breaks, alkali-labile sites (DNA damage) and oxidised bases (oxidative DNA damage) evaluated by comet assay in lymphocytes from patients exposed to surgery and ISF among the time points evaluated
| Tail intensity (%) |
P value | |||
| T1 | T2 | T3 | ||
| DNA damage | 19.0 (12.7–24.6) | 17.8 (11.1–28.1) | 18.5 (16.7–21.3) | 0.58 |
| Oxidised purinesa | 31.8 (21.7–38.6) | 29.6 (21.0–39.9) | 22.9 (21.3–29.9) | 0.31 |
| Oxidised pyrimidinesb | 22.3 (13.0–27.4) | 21.8 (15.5–26.9) | 14.5 (9.4–21.1) | 0.10 |
T1: before anaesthesia; T2: 2 h after the beginning of anaesthesia and T3: on the first post-operative day. Data, obtained from 20 subjects, are expressed as median and quartiles.
Determined as Fpg-sensitive sites.
Determined as endo III-sensitive sites.
Hydrogen peroxide-induced DNA damage in lymphocytes
Table II shows the results of DNA damage in lymphocytes sampled at the three time points, when challenged in vitro with H2O2. Significant increase of damage (P < 0.05) was observed in T1, T2 and T3, when compared to the control (without treatment), but with no statistical difference among the three sampling times.
Table II.
DNA damage (%tail intensity) in lymphocytes of patients under anaesthesia with ISF collected at three time points and in vitro exposed to hydrogen peroxide
| Tail Intensity (%) |
|||
| T1 | T2 | T3 | |
| Lymphocytesa | 2.6 (1.3–3.8) | 2.6 (1.5–6.7) | 2.4 (1.4–3.5) |
| H2O2b | 10.3 (4.6–12.8)* | 6.9 (2.7–7.8)* | 5.5 (2.9–9.4)* |
T1: before anaesthesia; T2: 2 h after the beginning of anaesthesia and T3: on the first post-operative day. Data, obtained from 20 subjects, are expressed as median and quartiles.
Without treatment with H2O2 (negative control).
H2O2 treatment: 100 μM (30 min at 4°C).
*P < 0.05: H2O2 × lymphocytes.
General anaesthesia did not induce apoptosis in T lymphocytes
The percentage of viable, early and late apoptotic helper T (CD4+) and cytotoxic T cells (CD8+) from all the patients indicated that inhalation anaesthesia with ISF was not cytotoxic in lymphocytes (Table III). No statistically significant (P > 0.05) difference in the frequency of early apoptosis annexin-V+/7-AAD) was detected among the three sampling times (T1, T2 and T3) in both subtypes of T lymphocytes.
Table III.
Percentage of viable, early and late apoptotic helper and cytotoxic T lymphocytes from patients undergoing anaesthesia with ISF
| T lymphocytes | CD4+ |
CD8+ |
||||
| T1 | T2 | T3 | T1 | T2 | T3 | |
| Viable | 91.9 (89.3–93.7) | 91.7 (88.1–93.2) | 93.1 (91.2–94.4) | 91.9 (87.3–95.6) | 92.1 (88.5–94.2) | 93.4 (91.4–95.3) |
| Early apoptosis | 7.0 (4.8–9.8) | 7.4 (5.5–11.0) | 5.5 (4.2–7.9) | 7.9 (3.9–12.1) | 7.0 (5.4–9.9) | 5.3 (4.2–8.1) |
| Late apoptosis | 1.0 (0.7–1.2) | 0.9 (0.6–1.3) | 1.0 (0.6–1.5) | 0.2 (0.1–0.3) | 0.2 (0.2–0.4) | 0.2 (0.1–0.2) |
T1: before anaesthesia; T2: 2 h after the beginning of anaesthesia and T3: on the first post-operative day. Data, obtained from 20 subjects, are expressed as median and quartiles; P > 0.05.
Quantitative real-time PCR
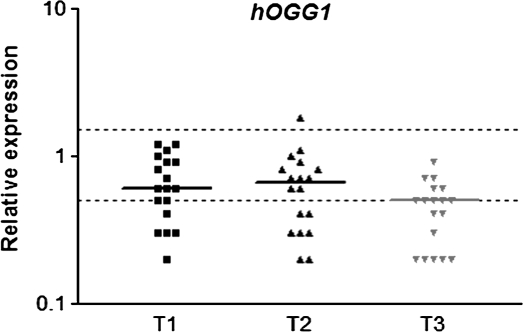
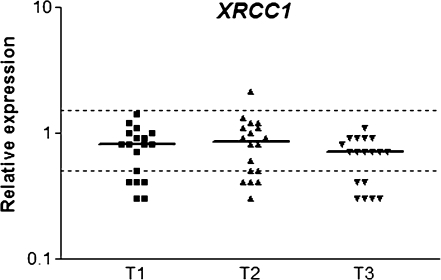
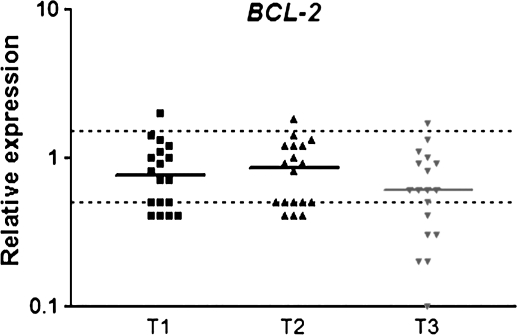
Expression of hOGG1 and XRCC1 in peripheral leukocytes from patients undergoing general anaesthesia with ISF is showed in Figures 1 and 2, respectively. Significant down-regulation of hOGG1 was observed on the first day after surgery (T3), when compared to T1 and T2. XRCC1 also showed down-regulation at T3 (P < 0.05) but only in relation to T2. Similarly to hOGG1, the anti-apoptotic gene BCL2 was down-regulated (P < 0.05) on the first post-operative day (T3) in comparison to T1 and T2, but no difference was detected between the first two sampling times (Figure 3).
Fig. 1.
Relative expression of hOGG1 DNA repair gene in blood cells of patients under general anaesthesia with ISF. T1: before anaesthesia; T2: 2 h after the beginning of anaesthesia and T3: on the first post-operative day. The horizontal bars indicate median RQ in each sampling time; dotted lines indicate the limits of RQ 0.5 and RQ 1.5. Data were obtained from 18 subjects. P < 0.05: (T1 = T2) > T3.
Fig. 2.
Relative expression of XRCC1 DNA repair gene in blood cells of patients under general anaesthesia with ISF. T1: before anaesthesia; T2: 2 h after the beginning of anaesthesia and T3: on the first post-operative day. The horizontal bars indicate median RQ in each sampling time; dotted lines indicate the limits of RQ 0.5 and RQ 1.5. Data were obtained from 18 subjects. P < 0.05: T3 < T2.
Fig. 3.
Relative expression of anti-apoptotic BCL2 gene in blood cells of patients under general anaesthesia with ISF. T1: before anaesthesia; T2: 2 h after the beginning of anaesthesia and T3: on the first post-operative day. The horizontal bars indicate median RQ in each sampling time; dotted lines indicate the limits of RQ 0.5 and RQ 1.5. Data were obtained from 18 subjects. P < 0.05: (T1 = T2) > T3.
Discussion
The mechanisms related to the secondary effects of anaesthesia in patients undergoing surgery are still not completely understood. Thus, we evaluated the extent of DNA strand breaks, alkali-labile sites and oxidative damage and frequency of apoptosis in lymphocytes and gene expression in blood cells from individuals under ISF anaesthesia.
The lack of genotoxicity observed in the present study was in accordance with those negative results found when ISF was tested in S.typhimurium and D.melanogaster assays (13,14). Similarly, no increase of sister chromatid exchanges has been reported in Chinese hamster ovary (CHO) and lung (CHL) cells (32,33) and also in lymphocytes from patients under minor orthopaedic surgery (34). These data are in accordance with the International Agency for Research on Cancer, which classifies volatile anaesthetics as not classifiable as to their carcinogenicity to humans (Group 3) (35). However, positive ISF genotoxicity has been also described. This anaesthetic, at concentrations of 1 and 10 mM, was able to induce DNA damage in lymphocytes in vitro although the lesions have been completely repaired after 60 min (15). Increased DNA damage has been also detected in some organs of rats exposed to 1% ISF, with no evident association between genotoxicity and lipid and protein oxidation (17).
In patients classified as ASA I and II (patients with mild systemic disease and disturbance due to surgical condition) aged from 22 to 66 years, submitted to invasive abdominal surgeries under ISF (1 and 1.5%) anaesthesia, increased DNA damage has been observed in lymphocytes collected 60 and 120 min after the beginning of anaesthesia and also at the day after anaesthesia (2,18). Differently from our results, the positive findings might be related to the recruited patients and/or type of surgery. Literature shows that some confounding factors, such as associated diseases (ASA II) and old age, can increase the level of DNA damage and genomic instability (36–39). In our study, strict criteria for selecting patients were used. All patients enrolled were young adults classified as ASA I, with normal body mass index and with no other associated disease or systemic disturbances. Additionally, all the patients were no smokers or alcohol, drugs and antioxidant supplements consumers. Our data were only obtained from individuals submitted to elective non-invasive (tympanoplasty or septoplasty) surgery, and all the drugs used during anaesthesia and for the post-operative analgesia have not been reported as genotoxics or cytotoxics (40,41). It is known that open surgery, such as abdominal ones, is more traumatic and usually associated with increased inflammatory response (42). As previously suggested (15), ISF can be metabolised into reactive products or generate ROS, which might reach DNA, causing damage. However, it must be considered that only 0.2% of the anaesthetic is metabolised (7). Thus, for the first time, our findings showed that ISF anaesthesia did not induce oxidative DNA damage as recognised by Fpg and endo III enzymes in the comet assay and did not alter the extent of DNA damage induced by the in vitro treatment with H2O2.
It is well known that healthy individuals differ in their intrinsic capability to repair genetic damage, mainly because of DNA repair gene polymorphisms or by alterations in the gene expression pattern (43). On the other hand, it has been demonstrated that inflammatory processes can also inactivate some repair enzymes (44). We have previously observed that patients submitted to surgery under ISF anaesthesia showed increased pro-inflammatory cytokine interleukin-6 concentrations 120 min after the beginning of anaesthesia, with more pronounced increase on the first post-operative day (M. A. Mazoti, M. G. Braz, M. A. Golim, L. G. Braz, N. H. Dias, J. R. Braz, D. M. Salvadori, D. Fecchio, in preparation). Therefore, the expression of hOGG1, which removes 8-oxoguanine, a well-known mutagenic lesion (45), and XRCC1, which is involved in the repair of single-strand breaks and base damage (46,47), was down-regulated on the first post-operative day possibly due to the inflammatory status of the patient. Nevertheless, it was not possible to directly associate gene expression profile with the amount of DNA lesions because the first was investigated in whole blood cells and damage was measured only in lymphocytes. Thus, the down-regulation of XRCC1 and hOGG1 might not necessarily reflect a decreased DNA repair ability.
In our study, we also evaluated the frequency of cells in early and late apoptosis in patients exposed to ISF during minor surgery. Data showed no increase of apoptosis in either CD4+ or CD8+ non-cultured T cells, suggesting no important effect of this type of anaesthesia on the immune system. Contrarily, in major surgery, the inflammatory stress response and the post-operative immunosupression after inhalation anaesthesia seem to be characterised by peripheral T-cell lymphopenia and leucocytosis (48,49). It has been demonstrated that ISF, only at high concentrations (up to 2.5%) and long-term in vitro treatment, is able to induce dose- and time-dependent apoptosis in lymphocytes (23). Additionally, ASA I and ASA II patients undergoing invasive surgeries (aortic femoral bypass, aneurysmectomy and cholecystectomy) under ISF and nitrous oxide (N2O) anaesthesia exhibited higher frequencies of CD4+ and CD8+ apoptosis, when assessed by the 7-AAD 24 h (but not 96 h) after surgery (24). Similarly, cancer patients undergoing surgery with ISF and N2O anaesthesia presented increased frequency of apoptosis in cultured lymphocytes obtained 2 h after the beginning of the surgery (22). However, it was not possible to determine whether the effect was caused by ISF or by N2O. So, N2O was never used in the patients in the present study in order to avoid possible DNA damage and because of its immunosuppressive activity (3,50).
Down-regulation of pro-apoptotic p53 and anti-apoptotic protein Bcl-2 has been reported 1 day after the surgery in patients under anaesthesia with ISF (24). Similarly, we also detected low expression of this anti-apoptotic gene transcript on the first post-operative day. Perhaps, BCL2 is repressed 1 day after surgery to allow damaged cells to undergo apoptosis. Aravindan et al. (51) have found reduced expression of death receptors, BCL2, TP53 and ATM genes in renal cells after 2% ISF, showing an inhibition of apoptosis in rats. These authors have suggested that ISF has anti-apoptotic activity mediated via both extrinsic and intrinsic signalling pathways. It is believed that short period under anaesthesia with ISF might provide cytoprotection via preconditioning, whereas prolonged exposures produce direct cytotoxicity (52). It has been also demonstrated that exposure to ISF enhances Bcl-2 protein in rodent cardiomyocytes in response to hypoxia, while H2O2 decreases Bcl-2 production (26).
In conclusion, the current study supports the observation that general anaesthesia maintained with ISF does not induce DNA strand breaks, alkali-labile sites or oxidative damage, neither increases the percentage of apoptotic CD4+ and CD8+ in lymphocytes or changes genes expression in blood cells in ASA I patients undergoing elective non-invasive surgery. Nevertheless, DNA repair and apoptosis-related genes were down-regulated on the first post-operative day.
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; Process 06/59625-6). M.G.B. received a PhD fellowship from FAPESP (06/58847-5) and J.G. was granted with a scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico.
Acknowledgments
The authors are grateful to MSc João Paulo C Marcondes [Universidade Estadual Paulista (UNESP)] and to MSc Shadia M Ihlaseh (UNESP) for quantitative real-time PCR analysis advice. We also thank Dr Raymond R. Tice (National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA) for the critical reading of the manuscript.
Conflict of interest statement: None declared.
References
- 1.Halliwell B. Free radicals and antioxidants: a personal view. Nutr. Rev. 1994;52:253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 2.Karabiyik L, Sardas S, Polat U, Kocabas NA, Karakaya AE. Comparison of genotoxicity of sevoflurane and isoflurane in human lymphocytes studied in vivo using the comet assay. Mutat. Res. 2001;492:99–107. doi: 10.1016/s1383-5718(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 3.Alleva R, Tomasetti M, Solenghi MD, Stagni F, Gamberini F, Bassi A, Fornasari PM, Fanelli G, Borghi B. Lymphocyte DNA damage precedes DNA repair or cell death after orthopaedic surgery under general anaesthesia. Mutagenesis. 2003;18:423–428. doi: 10.1093/mutage/geg013. [DOI] [PubMed] [Google Scholar]
- 4.Szyfter K, Szulc R, Mikstacki A, Stachecki I, Rydzanicz M, Jaloszynski P. Genotoxicity of inhalation anaesthetics: DNA lesions generated by sevoflurane in vitro and in vivo. J. Appl. Genet. 2004;45:369–374. [PubMed] [Google Scholar]
- 5.Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anaesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Eger EI., 2nd New inhaled anaesthetics. Anesthesiology. 1994;80:906–922. doi: 10.1097/00000542-199404000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Martin JL, Jr, Njoku DB. Metabolism and toxicity of inhaled anaesthetics. In: Miller RD, editor. Miller's Anaesthesia. 6th edn. Pennsylvania, PA, USA: Elsevier; 2005. p. 262. [Google Scholar]
- 8.Bilban M, Jakopin CB, Ogrinc D. Cytogenetic tests performed on operating room personnel (the use of anaesthetic gases) Int. Arch. Occup. Environ. Health. 2005;78:60–64. doi: 10.1007/s00420-004-0579-1. [DOI] [PubMed] [Google Scholar]
- 9.Eroglu A, Celep F, Erciyes N. A comparison of sister chromatid exchanges in lymphocytes of anesthesiologists to nonanesthesiologists in the same hospital. Anesth. Analg. 2006;102:1573–1577. doi: 10.1213/01.ane.0000204298.42159.0e. [DOI] [PubMed] [Google Scholar]
- 10.Rozgaj R, Kasuba V, Brozovic G, Jazbec A. Genotoxic effects of anaesthetics in operating theatre personnel evaluated by the comet assay and micronucleus test. Int. J. Hyg. Environ. Health. 2009;212:11–17. doi: 10.1016/j.ijheh.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Wiesner G, Schiewe-Langgartner F, Lindner R, Gruber M. Increased formation of sister chromatid exchanges, but not of micronuclei, in anaesthetists exposed to low levels of sevoflurane. Anaesthesia. 2008;63:861–864. doi: 10.1111/j.1365-2044.2008.05498.x. [DOI] [PubMed] [Google Scholar]
- 12.Wrońska-Nofer T, Palus J, Krajewski W, Jajte J, Kucharska M, Stetkiewicz J, Wasowicz W, Rydzyński K. DNA damage induced by nitrous oxide: study in medical personnel of operating rooms. Mutat. Res. 2009;666:39–43. doi: 10.1016/j.mrfmmm.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Baden JM, Kelley M, Wharton RS, Hitt BA, Simmon VF, Mazze RI. Mutagenicity of halogenated ether anaesthetics. Anesthesiology. 1977;46:346–350. doi: 10.1097/00000542-197705000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Kundomal YR, Baden JM. Mutagenicity of inhaled anaesthetics in Drosophila melanogaster. Anesthesiology. 1985;62:305–309. doi: 10.1097/00000542-198503000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Jaloszynski P, Kujawski M, Wasowicz M, Szulc R, Szyfter K. Genotoxicity of inhalation anaesthetics halothane and isoflurane in human lymphocytes studied in vitro using the comet assay. Mutat. Res. 1999;439:199–206. doi: 10.1016/s1383-5718(98)00195-8. [DOI] [PubMed] [Google Scholar]
- 16.Hoerauf KH, Schrögendorfer KF, Wiesner G, Gruber M, Spacek A, Kress HG, Rüdiger HW. Sister chromatid exchange in human lymphocytes exposed to isoflurane and nitrous oxide in vitro. Br. J. Anaesth. 1999;82:268–270. doi: 10.1093/bja/82.2.268. [DOI] [PubMed] [Google Scholar]
- 17.Kim H, Oh E, Im H, et al. Oxidative damages in the DNA, lipids, and proteins of rats exposed to isofluranes and alcohols. Toxicology. 2006;220:169–178. doi: 10.1016/j.tox.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Sardas S, Karabiyik L, Aygun N, Karakaya AE. DNA damage evaluated by the alkaline comet assay in lymphocytes of humans anaesthetized with isoflurane. Mutat. Res. 1998;418:1–6. doi: 10.1016/s1383-5718(98)00099-0. [DOI] [PubMed] [Google Scholar]
- 19.Janssen K, Schlink K, Gotte W, Hippler B, Kaina B, Oesch F. DNA repair activity of 8-oxoguanine DNA glycosylase 1 (OGG1) in human lymphocytes is not dependent on genetic polymorphism Ser326/Cys326. Mutat. Res. 2001;486:207–216. doi: 10.1016/s0921-8777(01)00096-9. [DOI] [PubMed] [Google Scholar]
- 20.Lei YC, Hwang SJ, Chang CC, Kuo HW, Luo JC, Chang MJ, Cheng TJ. Effects on sister chromatid exchange frequency of polymorphisms in DNA repair gene XRCC1 in smokers. Mutat. Res. 2002;519:93–101. doi: 10.1016/s1383-5718(02)00127-4. [DOI] [PubMed] [Google Scholar]
- 21.Espanol T, Todd GB, Soothill JF. The effect of anaesthesia on the lymphocyte response to phytohaemagglutinin. Clin. Exp. Immunol. 1974;18:73–79. [PMC free article] [PubMed] [Google Scholar]
- 22.Oka M, Hirazawa K, Yamamoto K, Iizuka N, Hazama S, Suzuki T, Kobayashi N. Induction of Fas-mediated apoptosis on circulating lymphocytes by surgical stress. Ann. Surg. 1996;223:434–440. doi: 10.1097/00000658-199604000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuoka H, Kurosawa S, Horinouchi T, Kato M, Hashimoto Y. Inhalation anaesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology. 2001;95:467–472. doi: 10.1097/00000542-200112000-00028. [DOI] [PubMed] [Google Scholar]
- 24.Delogu G, Moretti S, Antonucci A, Marcellini S, Masciangelo R, Famularo G, Signore L, De Simone C. Apoptosis and surgical trauma: dysregulated expression of death and survival factors on peripheral lymphocytes. Arch. Surg. 2000;135:1141–1147. doi: 10.1001/archsurg.135.10.1141. [DOI] [PubMed] [Google Scholar]
- 25.Delogu G, Moretti S, Famularo G, Marcellini S, Santini G, Antonucci A, Marandola M, Signore L. Mitochondrial perturbations and oxidant stress in lymphocytes from patients undergoing surgery and general anaesthesia. Arch. Surg. 2001;136:1190–1196. doi: 10.1001/archsurg.136.10.1190. [DOI] [PubMed] [Google Scholar]
- 26.Jamnicki-Abegg M, Weihrauch D, Pagel PS, Kersten JR, Bosnjak ZJ, Warltier DC, Bienengraeber MW. Isoflurane inhibits cardiac myocyte apoptosis during oxidative and inflammatory stress by activating Akt and enhancing Bcl-2 expression. Anesthesiology. 2005;103:1006–1014. doi: 10.1097/00000542-200511000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Braz MG, Favero Salvadori DM. Influence of endogenous and synthetic female sex hormones on human blood cells in vitro studied with comet assay. Toxicol. In Vitro. 2007;21:972–976. doi: 10.1016/j.tiv.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Singh NP, Mccoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 29.Tice RR, Andrews PW, Hirai O, Singh NP. The single cell gel (SCG) assay: an electrophoretic technique for the detection of DNA damage in individual cells. Adv. Exp. Med. Biol. 1991;283:157–164. doi: 10.1007/978-1-4684-5877-0_17. [DOI] [PubMed] [Google Scholar]
- 30.Van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.White AE, Takehisa S, Eger EI, 2nd, Wolff S, Stevens WC. Sister chromatid exchanges induced by inhaled anaesthetics. Anesthesiology. 1979;50:426–430. doi: 10.1097/00000542-197905000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Trudnowski RJ, Mehta MP, Rucinski M. Evaluation of the mutagenic potential of enflurane and isoflurane by sister chromatid exchange. J. Med. 1987;18:55–60. [PubMed] [Google Scholar]
- 34.Husum B, Wulf HC, Niebuhr E, Kyst A, Valentin N. Sister chromatid exchanges in lymphocytes of humans anaesthetized with isoflurane. Br. J. Anaesth. 1984;56:559–564. doi: 10.1093/bja/56.6.559. [DOI] [PubMed] [Google Scholar]
- 35.IARC. (1987) International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risks to Humans Overall Evaluations of Carcinogenicity. An updating of IARC Monographs, Vol. 1–42 (Suppl. 7) IARC, Lyon, France. [PubMed] [Google Scholar]
- 36.Fenech M. Chromosomal damage rate, aging, and diet. Ann. N. Y. Acad. Sci. 1998;20:23–36. doi: 10.1111/j.1749-6632.1998.tb09889.x. [DOI] [PubMed] [Google Scholar]
- 37.Botto N, Masetti S, Petrozzi L, Vassalle C, Manfredi S, Biagini A, Andreassi MG. Elevated levels of oxidative DNA damage in patients with coronary artery disease. Coron. Artery Dis. 2002;13:269–274. doi: 10.1097/00019501-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Yildiz A, Gur M, Yilmaz R, Demirbag R, Celik H, Aslan M, Koçyigit A. Lymphocyte DNA damage and total antioxidant status in patients with white-coat hypertension and sustained hypertension. Turk. Kardyol. Dern. Ars. 2008;36:231–238. [PubMed] [Google Scholar]
- 39.Piperakis SM, Kontogianni K, Karanastasi G, Iakovidou-Kritsi Z, Piperakis MM. The use of comet assay in measuring DNA damage and repair efficiency in child, adult, and old age populations. Cell Biol. Toxicol. 2009;25:65–71. doi: 10.1007/s10565-007-9046-6. [DOI] [PubMed] [Google Scholar]
- 40.Braz MG, Magalhães MR, Salvadori DM, Ferreira AL, Braz LG, Sakai E, Braz JR. Evaluation of DNA damage and lipoperoxidation of propofol in patients undergoing elective surgery. Eur. J. Anaesthesiol. 2009;26:654–660. doi: 10.1097/eja.0b013e328329b12c. [DOI] [PubMed] [Google Scholar]
- 41.Brambilla G, Martelli A. Genotoxicity and cariconogenicity studies of analgesics, anti-inflammatory drugs and antipyretics. Pharmacol. Res. 2009;60:1–17. doi: 10.1016/j.phrs.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Sido B, Teklote JR, Hartel M, Friess H, Büchler MW. Inflammatory response after abdominal surgery. Best Pract. Res. Clin. Anaesthesiol. 2004;18:439–454. doi: 10.1016/j.bpa.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Cornetta T, Festa F, Testa A, Cozzi R. DNA damage repair and genetic polymorphisms: assessment of individual sensitivity and repair capacity. Int. J. Radiat. Oncol. Biol. Phys. 2006;66:537–545. doi: 10.1016/j.ijrobp.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 44.Tudek B. Base excision repair modulation as a risk factor for human cancers. Mol. Aspects Med. 2007;28:258–275. doi: 10.1016/j.mam.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Moriya M. Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted GC-TA transversions in simian kidney cells. Proc. Natl Acad. Sci. USA. 1993;90:1122–1126. doi: 10.1073/pnas.90.3.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson LH, Brookman KW, Jones NJ, Allen SA, Carrono AV. Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol. Cell. Biol. 1990;10:6160–6171. doi: 10.1128/mcb.10.12.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kubota Y, Nash RA, Klungland A, Schar P, Barnes DE, Lindahl T. Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. EMBO J. 1996;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- 48.Slade MS, Simmons RL, Yunis E, Greenberg LJ. Immunodepression after major surgery in normal patients. Surgery. 1975;78:363–372. [PubMed] [Google Scholar]
- 49.Rem J, Brandt MR, Kehlet H. Prevention of postoperative lymphopenia and granulocytosis by epidural analgesia. Lancet. 1980;1:283–284. doi: 10.1016/s0140-6736(80)90780-1. [DOI] [PubMed] [Google Scholar]
- 50.Schneemilch CE, Hachenberg T, Ansorge S, Ittenson A, Bank U. Effects of different anaesthetic agents on immune cell function in vitro. Eur. J. Anaesthesiol. 2005;22:616–623. doi: 10.1017/s0265021505001031. [DOI] [PubMed] [Google Scholar]
- 51.Aravindan N, Cata JP, Hoffman L, Dougherty PM, Riedel KJ, Shaw AD. Effects of isoflurane, pentobarbital, and urethane on apoptosis and apoptotic signal transduction in rat kidney. Acta Anaesthesiol. Scand. 2006;50:1229–1237. doi: 10.1111/j.1399-6576.2006.01102.x. [DOI] [PubMed] [Google Scholar]
- 52.Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG. The common inhalational anaesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–252. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]