Abstract
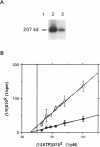
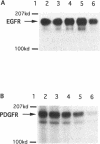
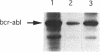
Protein tyrosine kinases play key roles in cellular physiology. Specific inhibitors of these enzymes are important laboratory tools and may prove to be novel therapeutic agents. In this report we describe a new class of tyrosine kinase inhibitor, synthetic oligodeoxynucleotides (ODNs). An ODN is described which specifically inhibits p210bcr-abl tyrosine kinase autophosphorylation in vitro with a Ki of 0.5 microM. Inhibition is non-competitive with respect to ATP. The effects upon inhibitory activity of ODN structure modifications are described. The inhibition described is not mediated by classical antisense mechanisms and represents an example of the recently recognized aptameric properties of ODNs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock L. C., Griffin L. C., Latham J. A., Vermaas E. H., Toole J. J. Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature. 1992 Feb 6;355(6360):564–566. doi: 10.1038/355564a0. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Veillette A., Schwartz A. M., DeSeau V., Rosen N. Activation of pp60c-src protein kinase activity in human colon carcinoma. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2251–2255. doi: 10.1073/pnas.84.8.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Lewis I., Sanghera J. S., Pelech S. L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis-activated myelin basic protein kinase. J Biol Chem. 1991 Aug 15;266(23):15180–15184. [PubMed] [Google Scholar]
- Di Marco E., Pierce J. H., Aaronson S. A., Di Fiore P. P. Mechanisms by which EGF receptor and TGF alpha contribute to malignant transformation. Nat Immun Cell Growth Regul. 1990;9(3):209–221. [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Druker B. J., Mamon H. J., Roberts T. M. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989 Nov 16;321(20):1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- Gao W. Y., Hanes R. N., Vazquez-Padua M. A., Stein C. A., Cohen J. S., Cheng Y. C. Inhibition of herpes simplex virus type 2 growth by phosphorothioate oligodeoxynucleotides. Antimicrob Agents Chemother. 1990 May;34(5):808–812. doi: 10.1128/aac.34.5.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gishizky M. L., Witte O. N. Initiation of deregulated growth of multipotent progenitor cells by bcr-abl in vitro. Science. 1992 May 8;256(5058):836–839. doi: 10.1126/science.1375394. [DOI] [PubMed] [Google Scholar]
- Grosveld G., Verwoerd T., van Agthoven T., de Klein A., Ramachandran K. L., Heisterkamp N., Stam K., Groffen J. The chronic myelocytic cell line K562 contains a breakpoint in bcr and produces a chimeric bcr/c-abl transcript. Mol Cell Biol. 1986 Feb;6(2):607–616. doi: 10.1128/mcb.6.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullick W. J. Prevalence of aberrant expression of the epidermal growth factor receptor in human cancers. Br Med Bull. 1991 Jan;47(1):87–98. doi: 10.1093/oxfordjournals.bmb.a072464. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M. Protein kinase catalytic domain sequence database: identification of conserved features of primary structure and classification of family members. Methods Enzymol. 1991;200:38–62. doi: 10.1016/0076-6879(91)00126-h. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinase classification. Methods Enzymol. 1991;200:3–37. doi: 10.1016/0076-6879(91)00125-g. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein modification: phosphorylation on tyrosine residues. Curr Opin Cell Biol. 1989 Dec;1(6):1168–1181. doi: 10.1016/s0955-0674(89)80068-7. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp B. E., Pearson R. B., House C. M. Pseudosubstrate-based peptide inhibitors. Methods Enzymol. 1991;201:287–304. doi: 10.1016/0076-6879(91)01026-x. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Witte O. N. Detection of c-abl tyrosine kinase activity in vitro permits direct comparison of normal and altered abl gene products. Mol Cell Biol. 1985 Nov;5(11):3116–3123. doi: 10.1128/mcb.5.11.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Marshall W. S., Caruthers M. H. Phosphorodithioate DNA as a potential therapeutic drug. Science. 1993 Mar 12;259(5101):1564–1570. doi: 10.1126/science.7681216. [DOI] [PubMed] [Google Scholar]
- Maxwell S. A., Kurzrock R., Parsons S. J., Talpaz M., Gallick G. E., Kloetzer W. S., Arlinghaus R. B., Kouttab N. M., Keating M. J., Gutterman J. U. Analysis of P210bcr-abl tyrosine protein kinase activity in various subtypes of Philadelphia chromosome-positive cells from chronic myelogenous leukemia patients. Cancer Res. 1987 Mar 15;47(6):1731–1739. [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Baltimore D. The noncatalytic src homology region 2 segment of abl tyrosine kinase binds to tyrosine-phosphorylated cellular proteins with high affinity. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):627–631. doi: 10.1073/pnas.88.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer S. E., Stull J. T., Wastila W. B., Thompson B. Assay of cyclic AMP by protein kinase activation. Methods Enzymol. 1974;38:66–73. doi: 10.1016/0076-6879(74)38012-3. [DOI] [PubMed] [Google Scholar]
- Montminy M. Trying on a new pair of SH2s. Science. 1993 Sep 24;261(5129):1694–1695. doi: 10.1126/science.8397444. [DOI] [PubMed] [Google Scholar]
- Pendergast A. M., Gishizky M. L., Havlik M. H., Witte O. N. SH1 domain autophosphorylation of P210 BCR/ABL is required for transformation but not growth factor independence. Mol Cell Biol. 1993 Mar;13(3):1728–1736. doi: 10.1128/mcb.13.3.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast A. M., Muller A. J., Havlik M. H., Maru Y., Witte O. N. BCR sequences essential for transformation by the BCR-ABL oncogene bind to the ABL SH2 regulatory domain in a non-phosphotyrosine-dependent manner. Cell. 1991 Jul 12;66(1):161–171. doi: 10.1016/0092-8674(91)90148-r. [DOI] [PubMed] [Google Scholar]
- Powis G. Signalling targets for anticancer drug development. Trends Pharmacol Sci. 1991 May;12(5):188–194. doi: 10.1016/0165-6147(91)90545-4. [DOI] [PubMed] [Google Scholar]
- Sartor O., Sameshima J. H., Robbins K. C. Differential association of cellular proteins with family protein-tyrosine kinases. J Biol Chem. 1991 Apr 5;266(10):6462–6466. [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Tritton T. R., Hickman J. A. How to kill cancer cells: membranes and cell signaling as targets in cancer chemotherapy. Cancer Cells. 1990 Apr;2(4):95–105. [PubMed] [Google Scholar]
- Tuerk C., MacDougal S., Gold L. RNA pseudoknots that inhibit human immunodeficiency virus type 1 reverse transcriptase. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6988–6992. doi: 10.1073/pnas.89.15.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Weber W., Bertics P. J., Gill G. N. Immunoaffinity purification of the epidermal growth factor receptor. Stoichiometry of binding and kinetics of self-phosphorylation. J Biol Chem. 1984 Dec 10;259(23):14631–14636. [PubMed] [Google Scholar]
- Yaish P., Gazit A., Gilon C., Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988 Nov 11;242(4880):933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]