Abstract
Major depressive disorder has been associated with activation of inflammatory processes as well as with reductions in innate, adaptive and non-specific immune responses. The objective of this study was to evaluate the association between major depression and a disease-relevant immunologic response, namely varicella-zoster virus (VZV)-specific immunity, in elderly adults. A cross-sectional cohort study was conducted in 104 elderly community dwelling adults ≥ 60 years of age who were enrolled in the Depression Substudy of the Shingles Prevention Study, a double blind, placebo-controlled vaccine efficacy trial. Fifty-two subjects had a current major depressive disorder, and 52 age- and sex-matched controls had no history of depression or any mental illness. VZV-specific cell-mediated immunity (VZV-CMI) was measured by VZV responder cell frequency (VZV-RCF) and interferon-γ enzyme-linked immunospot (ELISPOT) assays, and antibody to VZV was measured by an enzyme-linked immunosorbent assay against affinity-purified VZV glycoproteins (gpELISA). VZV-CMI, measured by VZV-RCF, was significantly lower in the depressed group than in the controls (p<0.001), and VZV-RCF was inversely correlated with the severity of depressive symptoms in the depressed patients. In addition, an age-related reduction in VZV-RCF was observed in the depressed patients, but not in the controls. Furthermore, there was a trend for depressive symptom severity to be associated with lower ELISPOT counts. Finally, VZV-RCF was higher in depressed patients treated with antidepressant medications as compared to untreated depressed patients. Since lower levels of VZV-RCF appear to explain the increased risk and severity of herpes zoster observed in older adults, these findings suggest that, in addition to increasing age, depression may increase the risk and severity of herpes zoster.
INTRODUCTION
Herpes zoster, or shingles, is a painful neurocutaneous syndrome caused by reactivation and replication of varicella-zoster virus (VZV) that has remained latent in sensory neurons following varicella(Gilden et al., 2000; Gnann and Whitley, 2002; Hope-Simpson, 1965; Ragozzino et al., 1982). The incidence and severity of herpes zoster increase with advancing age in association with a progressive age-related decline in VZV-specific T cell mediated immunity (VZV-CMI)(Berger et al., 1981; Burke et al., 1982; Levin et al., 1992; Miller, 1980). In the US, the incidence of herpes zoster exceeds 1% per year in persons ≥60 years of age; more than a million new cases occur each year; and one-third of the current population will experience herpes zoster during their lifetime – numbers destined to increase with the increasing age of the population (Donahue et al., 1995; Insinga et al., 2005; Oxman et al., 2005; Ragozzino et al., 1982). VZV-CMI is thought to play a critical role in protecting against herpes zoster and postherpetic neuralgia, and we have found that the magnitude and duration of the boost in VZV-CMI induced by zoster vaccine parallels the clinical effects of the vaccine observed during a large scale efficacy trial, the Shingles Prevention Study (SPS)(Levin et al., 2008; Oxman et al., 2005; Weinberg et al., 2009). In contrast, antibody to VZV does not appear to protect against herpes zoster; levels of antibody to VZV do not decline with increasing age and higher levels of VZV-specific antibody in subjects with herpes zoster in the SPS were correlated with increased disease severity and an increased risk of postherpetic neuralgia (Levin et al., 2008; Weinberg et al., 2009).
Among older adults, risk factors other than increasing age and lower levels of VZV-CMI have not been clearly identified, although psychological stress may play a role. In a retrospective, case-control study of 101 healthy community dwelling older adults, higher numbers of stressful life events were associated with a 2-fold increase in the risk of herpes zoster (Schmader et al., 1990), with similar findings reported in a prospective 8 year follow-up of 2568 adults (Schmader et al., 1998a). Whereas depression is associated with an activation of pro-inflammatory cytokines (Howren et al., 2009), other studies show that depression can reduce innate and adaptive cell-mediated immunity, although findings in the latter are limited(Irwin, 2008; Irwin and Miller, 2007). However, alterations in inflammation and innate immunity appear to be independent of one another, and increases in markers of inflammation are not associated with decreases in innate immunity in depression (Pike and Irwin, 2006).
Few studies have examined virus-specific immune responses in depression (Irwin, 2008; Irwin and Miller, 2007). Nevertheless, given that psychological stress can reduce immune responses to viral challenges (i.e., immunization) (Kiecolt-Glaser et al., 1996; Vedhara et al., 1999) and that psychological stress and depression appear to have similar effects on innate and virus-specific cellular-mediated immunity (CMI) (Irwin et al., 1990; Zorrilla et al., 2001), it was hypothesized that depression might reduce VZV-specific CMI in older adults who are at increased risk for herpes zoster and its complications. In a preliminary study, we reported that VZV-CMI was lower in eleven adults with major depression compared with eleven non-depressed age- and sex-matched controls (Irwin et al., 1998), although the conclusions were constrained by the small sample size and inclusion of only middle-aged adults. In the present study, measures of immunity to VZV were compared in depressed- and non- depressed adults ≥60 years of age, with examination of the effects of depressive symptom severity. Second, given that older age is associated with lower levels of VZV-CMI (Berger et al., 1981; Burke et al., 1982; Levin et al., 1992; Miller, 1980), and that depression and age interact such that depressed patients show a greater decline in non-specific immunity with age as compared to controls (Schleifer et al., 1989), we evaluated the associations between age and depression on VZV-immunity. Finally, the combined effects of depression and antidepressant medications on immunity to VZV were explored, because antidepressant medications appear to alter cellular immune responses in depressed patients (Evans et al., 2008; Tucker et al., 2004).
METHODS
Subject population and study design
Department of Veterans Affairs (VA) Cooperative Study #403: the Shingles Prevention Study (SPS) provided the data presented in this article (Oxman et al., 2005). The SPS was a 22 site, randomized, double-blind, placebo-controlled trial in 38,546 subjects ≥ 60 years of age, who received zoster vaccine or placebo and were subsequently observed for the development of herpes zoster and postherpetic neuralgia.
As part of the SPS eligibility requirements (Oxman et al. 2005), subjects were required to give written informed consent and to either have a history of varicella or at least 30 years of residence in the Continental United States. These criteria in persons ≥60 years of age ensured that virtually every subject was already latently infected with VZV (Harpaz et al, 2008). This expectation was supported by the observation that 100% of 1369 SPS subjects who were tested were found to be seropositive for VZV. We excluded immunocompromised persons who might be at risk from the live attenuated zoster vaccine and might not have a normal immunologic response to it. We also excluded persons with other conditions (e.g., chronic pain syndromes, cognitive impairment, severe hearing loss) that would interfere with the evaluation of herpes zoster or compliance with protocol requirements (e.g., monthly automated telephonic response survey calls). Specific exclusion criteria were: a) immunosuppression resulting from disease (e.g., malignancy; HIV infection), corticosteroids (except intermittent topical or inhaled corticosteroid [<800 mcg/day beclomethasonedipropionate or equivalent]), or other immunosuppressive/cytotoxic therapy (cancer chemotherapy or organ transplantation); b) active neoplastic disease (except local skin cancer or other malignancies [e.g., prostate cancer] that are stable in the absence of immunosuppressive/cytotoxic therapy); c) prior herpes zoster (consequently, none of the subjects in the Depression Substudy had a history of herpes zoster); d) prior receipt of varicella vaccine; e) allergic sensitivity to neomycin; f) history of anaphylactoid reaction to gelatin g) significant underlying illness that would be expected to prevent completion of the study (e.g., life-threatening disease likely to limit survival to less than 5 years); h) subjects not ambulatory (i.e., bed-ridden or homebound); i) receipt of immune globulin or any other blood product within 3 months before or planned during the 3–5 year study period; j) receipt of any other immunizations within one month before study vaccination (2 weeks in the case of inactivated influenza vaccines or other non-replicating immunization products [e.g., dT, pneumoccocal vaccine, hepatitis A vaccine, hepatitis B vaccine]), or scheduled within 6 weeks after study vaccination; k) subjects currently receiving antiviral therapy; l) any other condition (e.g., extensive psoriasis, chronic pain syndrome, cognitive impairment, severe hearing loss) that, in the opinion of the investigator, might interfere with the evaluations required by the study m) subject with an intercurrent illness (e.g., urinary tract infection, influenza) that might interfere with the interpretation of the study n) subjects who were female and pre-menopausal (women enrolled in the study were required to be postmenopausal); o) subjects unlikely to adhere to protocol follow-up; p) subjects involved in a conflicting (vaccine or investigational drug) clinical trial.
At two study sites, Denver, Colorado and San Diego, California, where the SPS core immunology laboratories were located, a total of 2850 subjects underwent SPS eligibility evaluation and depression screening between February 2000 and September 2001. Depression screening included completion of an abbreviated version of the Centers for Epidemiological Study of Depression (CES-D) scale (Irwin et al., 1999) and answering two questions about prior episodes of depression and/or treatment for depression. Of this eligible and screened sample, a total of 1395 subjects agreed to participate in the Immunology Substudy of the SPS, which involved assessment of VZV-specific immunity before vaccine or placebo administration, after 6 weeks, and after 1, 2, and 3 years. Once a subject agreed to participate in the Immunology Substudy, nursing coordinators reviewed depression screening information to identify subjects who screened positive for depressive symptoms and/or had a history of depression or depression treatment. In addition, after a subject was identified who was screened positive for depression, another subject who screened negative for depressive symptoms and/or depression history was identified (i.e., a comparison control); this comparison control, who was enrolled concurrently, was identified based on comparable age- (+/− 3 years) and sex criteria. Together, these procedures yielded 341 subjects who screened positive for depression or depression history, and 349 age- and sex comparable subjects who screened negative for depression or depression history (i.e., comparison controls). A total of 212 screened depression positive-, and 219 screened depression negative, comparison controls, agreed to participate in the Depression Substudy. All of these subjects completed clinical and psychiatric assessments including the Structured Clinical Interview for DSM-IV diagnosis (SCID-DSM-IV) (Spitzer et al., 1987); these interviews were completed on the day of entry in >95% of the participants.
Of the 212 persons who fulfilled screening criteria for depressive disorder or history of depression, SCID-DSM-IV interview data resulted in the exclusion of the following subjects: 2 for current alcohol dependence, 15 for current depression not otherwise specified (NOS) that also fulfilled criteria for the alternate label of minor depressive disorder, and 143 for a history of depression who did not fulfill diagnostic criteria for current major depression. Hence, 52 subjects who had current major depression were included in the study. Two of these patients were co-morbid for panic disorder; no other current psychiatric co-morbidities were diagnosed. None of the depressed subjects had a history of bipolar disorder.
Of the 219 persons who did not reach screening criteria for a depressive disorder or history of depression (i.e., comparison controls), 13 subjects were excluded by SCID-DSM-IV interview for past history of an Axis I disorder (e.g., alcohol dependence) and 6 for depression not otherwise specified. Thus, this control group represented a sample of older adults who had never been mentally ill (n=200). To identify 52 comparison controls for inclusion in this study, controls subjects were again matched with the depressed subjects using three criteria: age (+/− 3 years), sex, and Chronic Disease Score (CDS) stratified by quartile; matching procedures were done blind to VZV- immunity status as these immune data were maintained by the Coordinating Center of the SPS, separate from the Depression Substudy. Age, sex, and CDS were used as matching criteria, given the known effects of age- and sex and use of medications and/or medical co-morbidities on cellular immune response. Hence, the present report focuses on the baseline VZV-CMI in the Depression Substudy (n=104; 52 depressed subjects; 52 age-, sex- and CDS comparable) where all measurements of immune response and severity of depression were obtained prior to inoculation in the Shingles Prevention Study. It should be emphasized that all of the Depression Substudy subjects were VZV seropositive before zoster vaccine or placebo vaccination. The Institutional Review Boards of the University of Colorado, University of California, San Diego (UCSD), and University of California, Los Angeles (UCLA) approved the Depression Substudy.
Clinical and psychiatric assessment
To evaluate medical eligibility, a trained nurse performed a medical history. Subjects with exclusionary medical conditions were not entered into the study; for eligible subjects, medical conditions were further classified into the following categories: hypertension, heart disease, chronic lung disease, asthma, diabetes, rheumatoid arthritis, cancer, and neurologic disorder based on history and self-reported medication use. In addition, to provide an measure of medical history that could be summarized as single score, the Chronic Disease Score (CDS) questionnaire, a tabulation of medication usage, was administered as an interview by a nurse coordinator (von Korff et al., 1992). In addition, participants were instructed to bring their medication bottles and prescriptions to the study appointment, and the nurse coordinator recorded all medications used over the previous six months. Because the CDS does not include psychotropic or analgesic medications, this measure provides an estimate of medical history independent of psychiatric and pain-related symptoms.
The SCID-DSM-IV(First et al., 1996) was administered by trained doctoral level psychiatric nurses. Interview data were presented in a weekly diagnostic consensus teleconference that included at least two psychiatric nurses, a board-eligible psychologist, and a board- certified psychiatrist (MRI). The meeting was shared across sites to minimize diagnostic drift and maintain a consistent approach to resolving diagnostic ambiguities. Over the course of the study, periodic viewing and scoring of videotaped interviews were implemented to maintain reliability and criterion validity. Information about the clinical characteristics of the major depressive episode was obtained, including duration of current episode, number of prior episodes, and medication treatment history. Depression severity was assessed by administration of the Beck Depression Inventory (BDI) (Beck et al., 1996). The Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality (Buysse et al., 1989).
Blood samples were obtained by venipuncture between 8:00 and 10:00 AM on the same day that the clinical and psychiatric assessments were completed in >95% of the Depression Substudy sample; RCF assays were performed and PBMC frozen for ELISPOT assays within 2 hours of sample acquisition by a technician blind to the subjects’ group allocation.
Immunologic assessments
VZV-specific responder cell frequency (RCF) assay, interferon-γ–enzyme-linked immunospot (ELISPOT) assay, and enzyme-linked immunosorbent assay against affinity-purified VZV glycoproteins (gpELISA) were performed as described elsewhere (Hayward et al., 1994; Irwin et al., 1998; Irwin et al., 2007; Weinberg et al., 2009), and as described here briefly.
Responder cell frequency (RCF) assay
For RCF, the frequency of circulating VZV-specific CD4+ T cells was measured by adding a limiting dilution step to a lymphoproliferative assay (Hayward et al., 1994; Irwin et al., 1998; Oxman et al., 2005; Weinberg et al., 2009). Twofold dilutions of peripheral blood mononuclear cells (PBMCs) between 50,000 and 1563 cells/well were added to 24 replicate microtiter wells containing VZV-infected human lung fibroblast lysate or 24 replicate microtiter wells containing mock-infected control antigens. Responding wells were defined as VZV-stimulated wells with tritiatedthymidine–measured proliferation at least 3 standard deviations (SDs) above the median for the 24 mock-stimulated wells at the same PBMC concentration or 3-fold higher than that median if it was <100 counts per minute. On the basis of the Poisson distribution, the RCF was defined as the cell concentration at which 37% of wells were nonresponders and was expressed as the number of VZV-responding cells per 105PBMCs. The analytic sensitivity of this test has been conventionally considered ≥1 responding cell/105PBMCs. A mathematical model predicted a sensitivity of ≥0.2 responding cells/105PBMCs. Statistical analyses using either of these thresholds showed similar results. Here, we report the results of analyses that used the conservative threshold of 1 responding cell/105PBMCs. Values below this limit were recorded as 0.5 responding cell/105PBMCs to minimize the potential for introducing biases due to biologically insignificant assay variability.
ELISPOT assay
ELISPOT assay was used to measure the frequency of VZV specific interferon-γ–producing PBMCs, expressed as spot forming cells (SFCs) per 106PBMCs as previously validated. (Smith et al., 2001) Previously cryopreserved PBMCs at 500,000 cells/well were stimulated in triplicate wells with VZV lysate, mock-infected control, or phytohemagglutinin. After overnight incubation, wells were stained for γ interferon and SFCs were counted with an ImmunoSpot Analyzer (Cellular Technology). Valid assays were defined as those with ≥500 phytohemagglutinin-stimulated SFCs/106PBMCs. VZV-specific SFCs were calculated as SFCs in VZV-stimulated wells minus SFC0s in control-stimulated wells. Values <1 SFC/106PBMCs were recorded as 0.5 SFC/106 PBMCs(Smith et al., 2001).
Glycoprotein ELISA (gpELISA)
VZV-gpELISA was used to measure the VZV-specific antibody concentration. (Hammond et al., 2006) This assay measures antibodies against affinity-purified VZV glycoproteins. At baseline, all subjects had titers 15 gpELISA units/mL, the level considered seroprotective against varicella in varicella vaccine recipients.(Li et al., 2002). ELISPOT assay and gpELISA were performed at Merck Research Laboratories, and RCF assay was performed at Denver and San Diego.
Statistical Analyses
Analyses were carried out with SPSS for Windows, version 15. The distribution of VZV-RCF, ELISPOT, and gpELISA data was skewed, and thus analyses for these variables employed natural log transformations. Differences between depressed and non-depressed (control) subjects in background and clinical variables, as well as VZV-RCF, ELISPOT counts, and gpELISA titers, were evaluated using t-tests; differences in categorical variables were analyzed using chi-square tests. Pearson correlation coefficients were used to assess the association between background and clinical variables and VZV-RCF, ELISPOT, and gpELISA. Variables that were associated (p<0.10) with both major depression and VZV-RCF, ELISPOT, or gpELISA levels were selected as covariates, and analysis of covariance was used to test the independent association between depression diagnosis and each of these indices of VZV-specific immunity, controlling for significant covariates (p<0.10), with evaluation of the effects of age, depressive symptom severity, and their interaction on depression diagnosis given prior findings that such factors interact to influence immunity in depression (Schleifer et al., 1989). Exploratory analyses using ANOVA and ANCOVA models evaluated differences in VZV-RCF between non-depressed controls and depressed patients, stratified by the use of antidepressant medications.
RESULTS
Subject Characteristics
The controls and depressed subjects were similar in demographic characteristics including age, gender, ethnicity, educational level, and marital and employment status. (Table 1) In addition, similar levels of medical co-morbidity were found in the controls and depressed patients with similar rates of hypertension (31% vs 36%), heart disease (19.2% vs. 19.2%), chronic lung disease (2% vs 2%); asthma (2% vs. 2%); and diabetes (6% vs. 6%), with no reported rheumatoid arthritis, cancer, or neurologic disorder in the two groups. Additionally, medication usage across various medical co-morbidities was comparable for the two groups as indicated by scores on the Chronic Disease Scale for regular prescription medication use. As indicated by the frequency of medical co-morbidities, common medications were antihypertensive and cardiovascular medications including diuretics and beta-blocker agents, and oral hypoglycemic medication for the control of diabetes. The depressed subjects reported significantly more depressive symptoms as measured by the Beck Depression Inventory, as well as significantly greater disturbances in sleep quality, as measured by the Pittsburgh Sleep Quality Index. There were only two current tobacco smokers in this sample, both in the depressed group.
Table 1.
Demographic Variables, Chronic Disease Scores, Severity of Depressive Symptoms, Sleep Quality, and VZV-Immunity in Depressed Patients and Controls.
| Variables | Control (n = 52) |
Major Depression (n = 52) |
Depression Effect |
|||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | |
| Age (yrs.) | 68.4 | 6.1 | 68.4 | 5.7 | 0.05 | 1.0 |
| Chronic Disease Score | 2.0 | 2.2 | 2.0 | 2.3 | 0.04 | 0.97 |
| Depressive Symptoms (BDI Scores) | 2.7 | 2.5 | 16.5 | 8.5 | 10.9 | <0.001 |
| Sleep Quality (PSQI Scores) | 3.6 | 2.9 | 9.0 | 3.6 | 8.3 | <0.001 |
| VZV-RCF (log) | 1.0 | 0.3 | 0.7 | 0.5 | 3.2 | 0.002 |
| VZV-ELISPOT (log) | 1.6 | 0.7 | 1.4 | 0.8 | 0.8 | 0.23 |
| VZV gpELISA (log) | 2.4 | 0.4 | 2.5 | 0.4 | 0.5 | 0.31 |
| N | % | N | % | χ2 | P | |
| Gender (female) | 31 | 60 | 31 | 60 | 0.0 | 1.00 |
| Ethnicity (Euro-American) | 48 | 92 | 49 | 94 | 0.2 | 0.50 |
| Educational level (college graduate) | 26 | 50 | 22 | 42 | 0.6 | 0.28 |
| Marital status (married) | 32 | 62 | 26 | 50 | 1.4 | 0.16 |
| Employment status (employed) | 22 | 42 | 18 | 35 | 0.7 | 0.27 |
The depressed subjects reported on average 1.9 lifetime episodes of major depression, with 54% (n= 28) reporting no prior episodes. The average duration of the current major depressive episode was 26.2 months. Treatment with antidepressant medications was current in 55% (n=29) of the depressed persons, with use of selective serotonin reuptake inhibitors predominating (n=28); one subject reported treatment with a tricyclic antidepressant. No depressed patient was being treated with a mood stabilizer or anti-psychotic medication.
Varicella-zoster virus-specific immunity and depression
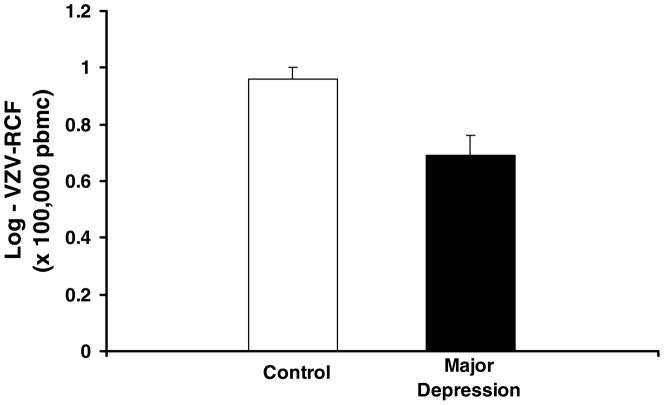
The depressed subjects had lower levels of VZV-RCF as compared to controls (t = 3.2, p = 0.002) (Figure 1; Table 1). However, neither ELISPOT counts nor gpELISA titers differed between the two groups (Table 1).
Figure 1.
Varicella-zoster virus specific responder cell frequency (VZV-RCF) at baseline in elderly subjects with major depressive disorder (n=52) compared to age- and sex- matched controls (n=52). VZV-RCF was significantly lower in the depressed group than the controls (t = 3.2, p = 0.002). Mean ± SEM.
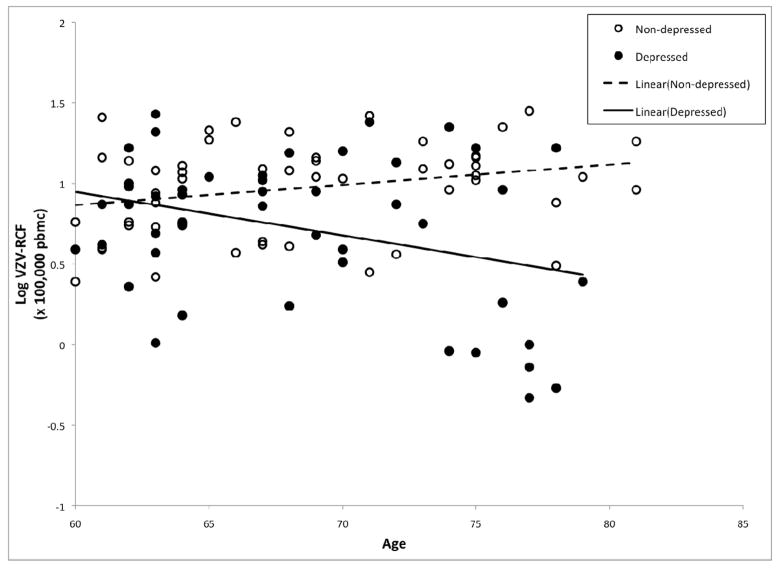
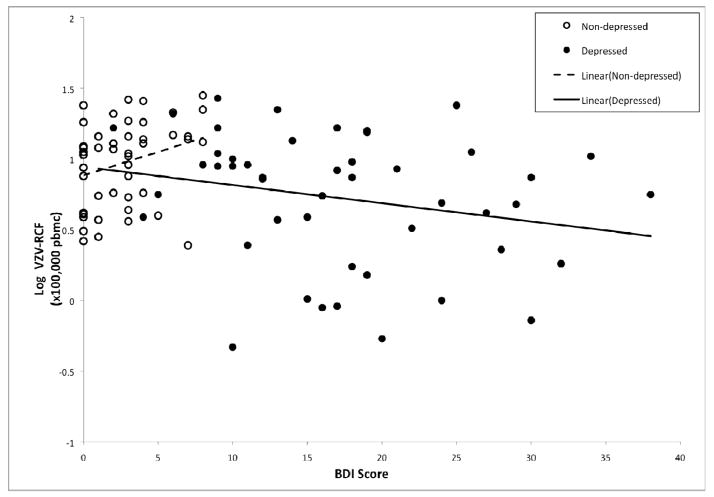
ANCOVA analyses were used to evaluate whether the effects of depression on VZV-RCF are attributable to depression diagnosis, severity of depressive symptoms, and/or the interaction of depression diagnosis and severity of depressive symptoms, taking into account the additional effects of age, sex, medical co-morbidity (as indexed by CDS scores), and sleep quality. In addition, given prior findings that age is associated with VZV-RCF, and that age and depression interact to predict lower levels of cellular immunity (Schleifer et al., 1989), we also evaluated the interaction between age and depression diagnosis within this ANCOVA model. As shown in Table 2, VZV-RCF was significantly associated with sex (F(1,88)=13.73, p<0.001) and depression diagnosis (F(1,88)=10.16, p=0.002). There was a trend for older individuals to have lower VZV-RCF (F(1,88)=3.53, p<.07). In addition, there was a significant interaction between age and depression diagnosis (F(1,88)=10.77, p=0.001), in which VZV-RCF was lower with increasing age in the depressed group but not in the controls (Figure 2). Furthermore, there was a trend for an interaction between depressive symptom severity and depression diagnosis (F(1,88)=3.20, p=0.08) (Figure 3).Additional analyses were conducted in which anti-hypertensive medications use was included in the ANCOVA model; there was no main effect of anti-hypertensive medication use(F(1,87)=.24, p=0.51) nor interaction of antihypertensive medication use and depression diagnosis(F(1,87)=1.09, p=0.21) on VZV-RCF.
Table 2.
Predictors of VZV-RCF in depressed patients and controls
| Predictors | F | Ba | P |
|---|---|---|---|
| Age (years) | 3.53 | −0.032 | 0.07 |
| Sex (male vs. female) | 13.73 | −.276 | 0.001 |
| Medication usage, Chronic Disease Score | 0.17 | 0.007 | 0.69 |
| Sleep quality, PSQI scores | 0.40 | −0.007 | 0.54 |
| Depressive symptom severity, BDI scores | 1.05 | −.008 | 0.31 |
| Depression diagnosis | 10.16 | −2.684a | 0.002 |
| Age × depression diagnosis | 10.77 | 0.039a | 0.001 |
| Depressive symptom severity × depression diagnosis | 3.20 | 0.037a | 0.08 |
For control group
Figure 2.
Varicella-zoster virus specific responder cell frequency (VZV-RCF) differentially related to age for those with major depressive disorder (●) and controls (○).
Figure 3.
Varicella-zoster virus specific responder cell frequency (VZV-RCF) differentially related to depressive symptoms (BDI total score) for those with major depressive disorder (●) and controls (○).
Similar ANCOVA analyses were performed for ELISPOT and gpELISA. Of the former, there was only a main effect of gender (F(1,72)=4.07, p<.05) and a trend for depressive symptoms (F(1,72)=3.55;p<.07); none of the predictors reported above were significantly associated with gpELISA.
Effect of antidepressant medication use
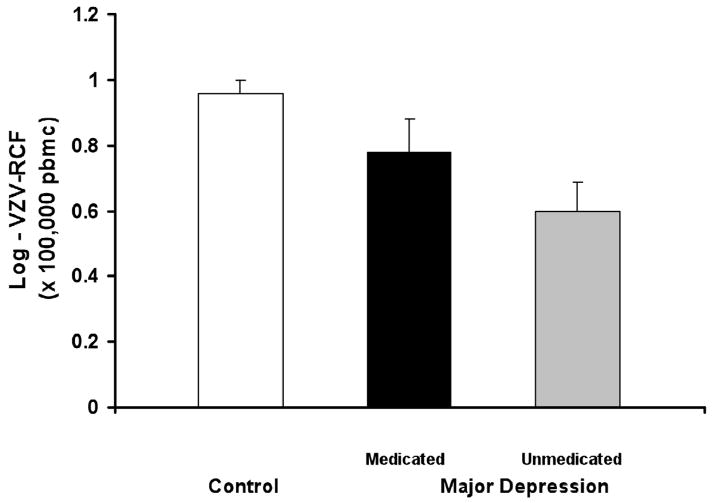
Because the use of antidepressant medications (e.g., selective serotonin reuptake inhibitors) has been associated with alterations of innate and adaptive cellular immune responses (Evans et al., 2008; Tucker et al., 2004), exploratory analyses were conducted in which the depressed patients were stratified on the basis of current use (n= 29) or non-use (n = 23) of antidepressant medication. None of the control subjects were using antidepressant medications. VZV-RCF differed across the three groups (F(2,103)= 7.6; p < 0.001; Figure 3). Depressed subjects not on antidepressants had the lowest mean level of VZV-RCF, which was significantly lower than the mean level of VZV-RCF in the controls (t = 4.0, p < 0.001), with a trend toward having a lower mean level of VZV-RCF than the depressed subjects who were taking antidepressant medications (t = 2.0, p = 0.06). The two depressed groups were similar in age, ethnicity, educational level, employment status, and chronic disease scores (all p> 0.30). Interestingly, depressive symptom severity was similar in the medicated and non-medicated depressed groups (15.7 ± 9.1 and 17.5 ± 7.8, respectively; t = 0.7, p = 0.46).Additional ANCOVA analyses were used to evaluate the effects of antidepressant medication use on VZV-RCF in the depressed group only (i.e., none of the controls were receiving antidepressant medications) taking into account the additional effects of age, sex, medical co-morbidity (as indexed by CDS scores), depressive symptoms, and sleep quality. Within the depression group only, VZV-RCF was related to age (F(1,40)=10.4; p<0.01) and sex(F(1,40)=5.0; p<0.05), with a trend for antidepressant medication use (F(1,40)2.8; p=0.10), but not with chronic disease scores (F(1,40)=0.8; p=0.39), depressive symptom severity (F(1,40)=0.8; p=0.39), nor sleep quality (F(1,40)=0.64; p=0.43). There was no interaction between antidepressant medication status and depressive symptom severity on VZV-RCF.
DISCUSSION
The markedly increased risk for herpes zoster and its complications observed among older adults is associated with an age-related decline in VZV-CMI, as exemplified by declining VZV-RCF. This study demonstrated that VZV-RCF was further reduced in older persons with current major depressive disorder compared with non-depressed age-, sex-, and CDS comparable controls. Furthermore, age interacted with depression diagnosis, and an age-related reduction of VZV-RCF was observed in the depressed patients but not in the controls; a finding that is consistent with prior work by Schleifer et al.(1989). Moreover, severity of depressive symptoms appeared to be associated with the extent of the reduction in VZV-RCF in the depressed patients, but not in the controls, in whom depressive symptoms were uncommon. Similarly, the severity of depressive symptoms also appeared to be associated with lower γ-interferon ELISPOT counts, another index of VZV-CMI, although that relationship did not reach statistical significance. Finally, in the depressed group, VZV-RCF was lowest in depressed subjects who were not receiving treatment with antidepressant medications, an effect that could not be accounted for by differences in depressive symptom severity. This may have been because the subjects who were on antidepressant treatment but were still symptomatic for depression were refractory to treatment.
These immunologic findings suggest that depressed adults may be at increased risk for herpes zoster compared with non-depressed adults of the same age. Higher levels of VZV-CMI, measured by VZV-RCF, have been shown to be a robust predictor of protection against the occurrence and severity of herpes zoster and its complications, particularly the development of postherpetic neuralgia (Weinberg et al., 2009). We previously reported that serial measurements of VZV-RCF were higher in subjects who did not develop herpes zoster than in those who did, and that higher levels of VZV-RCF prior to the onset of herpes zoster were predictive of higher levels of VZV-RCF during the first week after herpes zoster onset (Weinberg et al., 2009). Importantly, higher levels of VZV-RCF in the first week after the onset of herpes zoster were correlated with reduced herpes zoster severity and with a reduced risk of postherpetic neuralgia (Weinberg et al., 2009). Furthermore, it was observed that subjects who developed herpes zoster during the SPS follow-up period developed and maintained higher levels of VZV-RCF than subjects who had not developed herpes zoster (Weinberg et al., 2009), which provides a potential explanation for the clinical observation that one episode of herpes zoster reduces the risk that the afflicted individual will suffer a subsequent attack(Yawn et al., 2007).
The findings that depression is associated with reduced levels of VZV-RCF may have broader implications for the risk of other infectious diseases. VZV–RCF measures primarily VZV-specific memory T-cells (CD41 CD45RO1 T-cells)(Hayward, 2001), and the association between depression and lower numbers of circulating VZV-specific memory T cells, as suggested by our observation of lower levels of VZV-RCF in depressed patients, may extend to memory T-cells specific for antigens of other pathogens that cause disease in older adults, such as influenza viruses and Mycobacteria. However, few studies have examined the association between depression and infectious disease and/or disease-relevant immunologic endpoints. Rather, studies have characterized the effects of psychological stress, as opposed to depression, on these outcomes(Irwin and Miller, 2007). For example, psychological stress is associated with an attenuated immune response to influenza vaccines in older adults (Kiecolt-Glaser et al., 1996; Vedhara et al., 1999).Additionally psychological stress is associated with infectious disease such as activation of genital herpes (Cohen et al., 1999). Furthermore, HIV infection shows a highly variable course, and depression, bereavement, and maladaptive coping responses to stress (including the stress of HIV infection itself) have all been shown to predict the rate of immune system decay in HIV patients (Cruess et al., 2005) and to predict mortality in the case of social stress (Cole et al., 2003).
Although previous studies demonstrated that VZV-RCF and ELISPOT counts were modestly correlated with each other (Levin et al., 2008; Weinberg et al., 2009), we found that only VZV-RCF was reduced in subjects with depression, although there was a trend for ELISPOT counts to be negatively associated with depressive symptom severity. This difference may reflect a selective effect of depression on a broad spectrum of VZV-specific memory T-cells, which are not measured by the γ-interferon ELISPOT assay.
In contrast to VZV-RCF, VZV antibody levels were not reduced in depressed subjects. Psychological stress and depression have been found to be associated with a reactivation of Herpesviruses, including Epstein Barr virus, herpes simplex viruses, and VZV (Glaser and Kiecolt-Glaser, 2005; Glaser et al., 1993; Schmader et al., 1998a; Schmader et al., 1998b; Schmader et al., 1990). When viral reactivation is coupled with low levels of CMI, the virus is not fully contained and the proliferating virus results in an antigenic stimulus that can increase titers of antibody to the reactivated virus. We speculate that subjects with depression who had lower levels of VZV-specific memory T-cells may have been more likely to develop herpes zoster when VZV reactivated, to have experienced VZV replication, and thus to develop elevated levels of antibodies to VZV. However, because individuals with prior herpes zoster were not eligible for the SPS, we excluded subjects in whom reactivations of VZV would have resulted in higher levels of antibodies to VZV. In other words, subjects eligible for the present study did not have prior episodes of herpes zoster, which would have resulted in increased levels of antibodies to VZV. Additionally, we and others have shown that VZV antibody levels are not highly correlated with levels of VZV-CMI (Levin et al., 2008). For example, with aging there is a decrease in VZV-CMI, whereas VZV antibody titers remain relatively intact (Oxman and Alani, 1993). Moreover, in subjects receiving immunization for herpes zoster, there was no correlation between levels of antibodies to VZV and VZV-CMI at baseline or after immunization, possibly due to the fact that B cells and T cells respond to different VZV epitopes(Weinberg et al., 2009).
The mechanism(s) that account for the reduction of VZV-RCF in depressed elderly adults are unknown, although our finding that treatment with SSRI tends to be associated with partial normalization of lower levels of VZV-RCF observed in depressed subjects suggests that alterations in the activity of central serotoninergic pathways may play a role. As noted, SSRI treatment was not associated with differences in depressive symptom severity. Our observations are consistent with those of Evans et al., who found that treatment with the SSRI, citalopram, enhanced natural killer cell activity in HIV-infected subjects, independent of effects on depressive symptom severity (Evans et al., 2008). Other data suggest that SSRI-treatment is associated with a normalization of inflammatory cytokine levels (e.g.. IL-1β) in patients with post-traumatic stress disorder (Tucker et al., 2004). Alternatively, we and others have found that sleep disturbance, prevalent in persons with depressive disorders, has a robust and independent effect on innate immune and inflammatory responses (Irwin et al., 1996; Irwin et al., 2006; Irwin et al., 2008; O’Connor et al., 2009), and increases the risk of viral infections (Cohen et al., 2009). Indeed, in the present study, severity of neurovegetative symptoms and poor sleep quality were associated with reduced levels of VZV-RCF.
Further research is needed to evaluate the possible relationship between depression and the risk of herpes zoster. This will require a large number of subjects because of the relatively low annual incidence of herpes zoster (approximately 10–12 cases per 1,000 persons per year in persons ≥60 years of age (Oxman et al., 2005). However, the finding that the well-documented age-related reduction in VZV-RCF was further reduced by depression in elderly individuals has important public health implications. The elderly are already prioritized for zoster vaccination because of their relatively low levels of VZV-CMI and their associated increased risk for herpes zoster and its complications(Harpaz et al., 2008; Oxman). The clinical efficacy of zoster vaccine is correlated with its capacity to boost VZV-specific CMI, and vaccine recipients with higher baseline levels of VZV-CMI have been shown to achieve higher absolute levels of VZV-CMI in response to vaccination. Thus, depressed elderly adults may have a diminished capacity to develop effective levels of VZV-CMI in response to zoster vaccine, and might require immunization with a higher potency vaccine or, possibly, with a multi-dose schedule. Further analyses of results from the Depression Substudy will address this hypothesis by comparing the magnitude and durability of the VZV-CMI response to zoster vaccine in our depressed and non-depressed subjects.
Figure 4.
Varicella-zoster virus specific responder cell frequency (VZV-RCF) at baseline in elderly subjects with major depressive disorder stratified on the basis of current use of antidepressant medication (n= 29) or not (n = 23) vs. controls.. VZV-RCF differed across the three groups (F = 7.6; df = 2, 103; p < 0.001) with depressed subjects not on antidepressants having lower levels as compared to controls (t = 4.0, p < 0.001), and the depressed subjects who were taking antidepressant medications (t = 2.0, p = 0.06). Mean ± SEM.
Acknowledgments
This work was supported by MH 55253 and in part by VA Cooperative Study Program, Office of Research and Development, Department of Veterans Affairs; a grant from Merck & Co., Inc. to the VA Cooperative Studies Program; NIH grants: T32-MH19925;; HL 079955; AG 026364; CA 10014152; CA116778; RR00827; P30-AG028748. Additional support is acknowledged from the General Clinical Research Centers Program; the UCLA Cousins Center for Psychoneuroimmunology at the Semel Institute for Neurosciences; the UCLA Older Americans Independence Center Inflammatory Biology Core; and the James R. and Jesse V. Scott Fund for Shingles Research
Footnotes
The authors have no financial interest related to the outcome of this research, and have no potential conflicts of interest. Dr. Levin receives research funds, consultation fees, and royalties from intellectual property from Merck and Co.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory manual. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berger R, Florent G, Just M. Decrease of the lymphoprolifertive response to varicella-zoster virus antigen in the aged. Infection and Immunity. 1981;32:24–27. doi: 10.1128/iai.32.1.24-27.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune response to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–293. [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cohen F, Kemeny ME, Kearney KA, Zegans LS, Neuhaus JM, Conant MA. Persistent stress as a predictor of genital herpes recurrence. Arch Intern Med. 1999;159:2430–2436. doi: 10.1001/archinte.159.20.2430. [DOI] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Arch Intern Med. 2009;169:62–67. doi: 10.1001/archinternmed.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Kemeny ME, Fahey JL, Zack JA, Naliboff BD. Psychological risk factors for HIV pathogenesis: mediation by the autonomic nervous system. Biol Psychiatry. 2003;54:1444–1456. doi: 10.1016/s0006-3223(02)01888-7. [DOI] [PubMed] [Google Scholar]
- Cruess DG, Douglas SD, Petitto JM, Have TT, Gettes D, Dube B, Cary M, Evans DL. Association of resolution of major depression with increased natural killer cell activity among HIV-seropositive women. Am J Psychiatry. 2005;162:2125–2130. doi: 10.1176/appi.ajp.162.11.2125. [DOI] [PubMed] [Google Scholar]
- Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–1609. [PubMed] [Google Scholar]
- Evans DL, Lynch KG, Benton T, Dube B, Gettes DR, Tustin NB, Lai JP, Metzger D, Douglas SD. Selective serotonin reuptake inhibitor and substance P antagonist enhancement of natural killer cell innate immunity in human immunodeficiency virus/acquired immunodeficiency syndrome. Biol Psychiatry. 2008;63:899–905. doi: 10.1016/j.biopsych.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition, Version 2.0. New York State Psychiatric Institute; New York, New York: 1996. [Google Scholar]
- Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000;342:635–645. doi: 10.1056/NEJM200003023420906. [DOI] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearson GR, Bonneau RH, Esterling BA, Atkinson C, Kiecolt-Glaser JK. Stress and the memory T-cell response to the Epstein-Barr virus in healthy medical students. Health Psychology. 1993;12:435–442. doi: 10.1037//0278-6133.12.6.435. [DOI] [PubMed] [Google Scholar]
- Gnann JW, Jr, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med. 2002;347:340–346. doi: 10.1056/NEJMcp013211. [DOI] [PubMed] [Google Scholar]
- Hammond O, Wang Y, Green T, Antonello J, Kuhn R, Motley C, Stump P, Rich B, Chirmule N, Marchese RD. The optimization and validation of the glycoprotein ELISA assay for quantitative varicella-zoster virus (VZV) antibody detection. J Med Virol. 2006;78:1679–1687. doi: 10.1002/jmv.20754. [DOI] [PubMed] [Google Scholar]
- Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57:1–30. quiz CE32–34. [PubMed] [Google Scholar]
- Hayward AR. In vitro measurement of human T cell responses to varicella zoster virus antigen. Arch Virol Suppl. 2001:143–149. doi: 10.1007/978-3-7091-6259-0_15. [DOI] [PubMed] [Google Scholar]
- Hayward AR, Zerbe GO, Levin MJ. Clinical application of responder cell frequency estimates with four years of follow up. J Immunol Methods. 1994;170:27–36. doi: 10.1016/0022-1759(94)90242-9. [DOI] [PubMed] [Google Scholar]
- Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Insinga RP, Itzler RF, Pellissier JM, Saddier P, Nikas AA. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med. 2005;20:748–753. doi: 10.1111/j.1525-1497.2005.0150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch Intern Med. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Irwin M, Costlow C, Williams H, Artin KH, Chan CY, Stinson DL, Levin MJ, Hayward AR, Oxman MN. Cellular immunity to varicella-zoster virus in patients with major depression. J Infect Dis. 1998;178(Suppl 1):S104–108. doi: 10.1086/514272. [DOI] [PubMed] [Google Scholar]
- Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- Irwin M, Patterson T, Smith TL, Caldwell C, Brown SA, Gillin JC, Grant I. Reduction of immune function in life stress and depression. Biol Psychiatry. 1990;27:22–30. doi: 10.1016/0006-3223(90)90016-u. [DOI] [PubMed] [Google Scholar]
- Irwin MR. Human psychoneuroimmunology: 20 years of discovery. Brain Behav Immun. 2008;22:129–139. doi: 10.1016/j.bbi.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Oxman MN. Augmenting immune responses to varicella zoster virus in older adults: a randomized, controlled trial of Tai Chi. J Am Geriatr Soc. 2007;55:511–517. doi: 10.1111/j.1532-5415.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, Martinez-Maza O, Cole S. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R, Gravenstein S, Malarkey WB. Chronic stress alters the immune response to influenza virus vaccine in older adults. Proc Natl Acad Sci. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MJ, Murray M, Rotbart HA, Zerbe GO, White CJ, Hayward AR. Immune response of elderly individuals to a live-attenuated varicella vaccine. J Infect Dis. 1992;166:253–259. doi: 10.1093/infdis/166.2.253. [DOI] [PubMed] [Google Scholar]
- Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, Caulfield MJ, Irwin MR, Smith JG, Clair J, Chan IS, Williams H, Harbecke R, Marchese R, Straus SE, Gershon A, Weinberg A. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008;197:825–835. doi: 10.1086/528696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Chan IS, Matthews H, Heyse JF, Chan CY, Kuter BJ, Kaplan KM, Vessey SJ, Sadoff JC. Inverse relationship between six week postvaccination varicella antibody response to vaccine and likelihood of long term breakthrough infection. Pediatr Infect Dis J. 2002;21:337–342. doi: 10.1097/00006454-200204000-00014. [DOI] [PubMed] [Google Scholar]
- Miller AE. Selective decline in cellular immune response to varicella-zoster in the elderly. Neurology. 1980;30:582–587. doi: 10.1212/wnl.30.6.582. [DOI] [PubMed] [Google Scholar]
- O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: Effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxman MN. Zoster vaccine: current status and future prospects. Clin Infect Dis. 51:197–213. doi: 10.1086/653605. [DOI] [PubMed] [Google Scholar]
- Oxman MN, Alani R. Varicella and Herpes Zoster. In: Fitzpatrick TB, et al., editors. Dermatology in General Medicine. McGraw-Hill Book Co; New York: 1993. pp. 2543–2572. [Google Scholar]
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, Weinberg A, Boardman KD, Williams HM, Zhang JH, Peduzzi PN, Beisel CE, Morrison VA, Guatelli JC, Brooks PA, Kauffman CA, Pachucki CT, Neuzil KM, Betts RF, Wright PF, Griffin MR, Brunell P, Soto NE, Marques AR, Keay SK, Goodman RP, Cotton DJ, Gnann JW, Jr, Loutit J, Holodniy M, Keitel WA, Crawford GE, Yeh SS, Lobo Z, Toney JF, Greenberg RN, Keller PM, Harbecke R, Hayward AR, Irwin MR, Kyriakides TC, Chan CY, Chan IS, Wang WW, Annunziato PW, Silber JL. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- Pike JL, Irwin MR. Dissociation of inflammatory markers and natural killer cell activity in major depressive disorder. Brain Behav Immun. 2006;20:169–174. doi: 10.1016/j.bbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Ragozzino MW, Melton LJ, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine. 1982;61:310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- Schleifer SJ, Keller SE, Bond RN, Cohen J, Stein M. Major depressive disorder and immunity: role of age, sex, severity, and hospitalization. Arch Gen Psychiatry. 1989;46:81–87. doi: 10.1001/archpsyc.1989.01810010083011. [DOI] [PubMed] [Google Scholar]
- Schmader K, George LK, Burchett BM, Hamilton JD, Pieper CF. Race and stress in the incidence of herpes zoster in older adults. J Am Geriatr Soc. 1998a;46:973–977. doi: 10.1111/j.1532-5415.1998.tb02751.x. [DOI] [PubMed] [Google Scholar]
- Schmader K, George LK, Burchett BM, Pieper CF. Racial and psychosocial risk factors for herpes zoster in the elderly. J Infect Dis. 1998b;178(Suppl 1):S67–70. doi: 10.1086/514254. [DOI] [PubMed] [Google Scholar]
- Schmader KE, Studenski S, MacMillan J, Grufferman S, Cohen HJ. Are stressful life events risk factors for herpes zoster? Am J Geriatr Soc. 1990;38:1188–1195. doi: 10.1111/j.1532-5415.1990.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Smith JG, Liu X, Kaufhold RM, Clair J, Caulfield MJ. Development and validation of a gamma interferon ELISPOT assay for quantitation of cellular immune responses to varicella-zoster virus. Clin Diagn Lab Immunol. 2001;8:871–879. doi: 10.1128/CDLI.8.5.871-879.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M. Structure clinical interview for DSM-IIIR (SCID) Biometrics Research; New York: 1987. [Google Scholar]
- Tucker P, Ruwe WD, Masters B, Parker DE, Hossain A, Trautman RP, Wyatt DB. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol Psychiatry. 2004;56:121–128. doi: 10.1016/j.biopsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Cox NK, Wilcock GK, Perks P, Hunt M, Anderson S, Lightman SL, Shanks NM. Chronic stress in elderly carers of dementia patients and antibody response to influenza vaccination [see comments] Lancet. 1999;353:627–631. doi: 10.1016/S0140-6736(98)06098-X. [DOI] [PubMed] [Google Scholar]
- von Korff M, Wagner EH, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidem. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, Irwin MR, Clair J, Smith JG, Stanley H, Marchese RD, Harbecke R, Williams HM, Chan IS, Arbeit RD, Gershon AA, Schodel F, Morrison VA, Kauffman CA, Straus SE, Schmader KE, Davis LE, Levin MJ Investigators of the SPS. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]