Abstract
Nucleoside receptors are known to be important targets for a variety of brain diseases. However, the therapeutic modulation of their endogenous agonists by inhibitors of nucleoside metabolism represents an alternative therapeutic strategy that has gained increasing attention in recent years. Deficiency in endogenous nucleosides, in particular of adenosine, may causally be linked to a variety of neurological diseases and neuropsychiatric conditions ranging from epilepsy and chronic pain to schizophrenia. Consequently, augmentation of nucleoside function by inhibiting their metabolism appears to be a rational therapeutic strategy with distinct advantages: (i) in contrast to specific receptor modulation, the increase (or decrease) of the amount of a nucleoside will affect several signal transduction pathways simultaneously and therefore have the unique potential to modify complex neurochemical networks; (ii) by acting on the network level, inhibitors of nucleoside metabolism are highly suited to fine-tune, restore, or amplify physiological functions of nucleosides; (iii) therefore inhibitors of nucleoside metabolism have promise for the “soft and smart” therapy of neurological diseases with the added advantage of reduced systemic side effects. This review will first highlight the role of nucleoside function and dysfunction in physiological and pathophysiological situations with a particular emphasis on the anticonvulsant, neuroprotective, and antinociceptive roles of adenosine. The second part of this review will cover pharmacological approaches to use inhibitors of nucleoside metabolism, with a special emphasis on adenosine kinase, the key regulator of endogenous adenosine. Finally, novel gene-based therapeutic strategies to inhibit nucleoside metabolism and focal treatment approaches will be discussed.
Keywords: adenosine, ADK, nucleosides, focal drug delivery, neurology, psychiatry
INTRODUCTION
Half a century ago inhibitors of nucleoside metabolism received much attention as inhibitors of nucleic acid synthesis, and therefore as potent anti-neoplastic and anti-viral agents [1–3]. The past decade has brought a wealth of new understanding how nucleoside metabolism is involved in normal brain function and how dysfunctional nucleoside metabolism is implicated in the etiopathology of a wide spectrum of neurological, neurodegenerative, and neuropsychiatric disorders. Consequently, the pharmaceutical development of inhibitors of nucleoside metabolism has gained renewed interest as potential agents to treat brain disease. Augmentation of nucleoside function by inhibiting their metabolism is expected to be advantageous compared to traditional approaches that involve the direct modulation of specific receptors: A subtle change in the amount of a nucleoside will affect several signal transduction pathways simultaneously with the potential to modify complex neurochemical networks at the same time. Inhibitors of nucleoside metabolism are highly suited to fine-tune, restore, or amplify physiological functions of nucleosides with the added advantage of reduced systemic side effects. This review will focus on the nucleosides adenosine, guanosine, cytidine, uridine, and inosine, inhibitors of their metabolism, and nucleoside-based therapeutic approaches to treat brain disease. The transport of nucleosides has recently been covered elsewhere [4] and will not be discussed here.
NUCLEOSIDES IN BRAIN FUNCTION
Adenosine
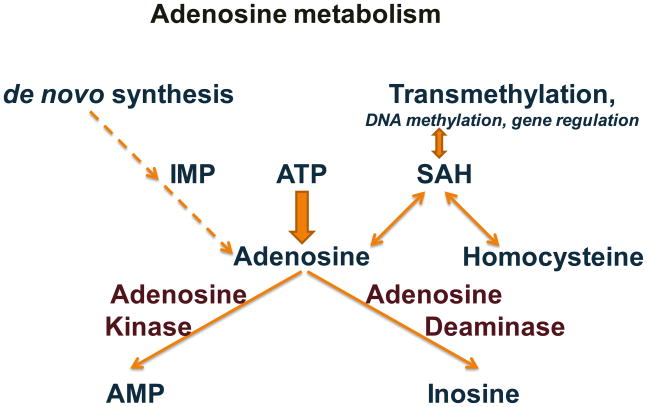
The purine ribonucleoside adenosine is a ubiquitous neuromodulator and upstream regulator of diverse brain functions [5–12]. In brain, adenosine is an endogenous distress signal [9] accumulating rapidly as an acute response to stress, e.g. under conditions of oxygen deprivation during stroke [13–15] or under conditions of excessive energy consumption during seizures [16]. Adenosine modulates many neuronal [17] and glial [10] functions in physiological and pathophysiological conditions [11, 12] such as seizure susceptibility [18], tissue damage and repair [9], and immune functions of the brain [19]. In addition, adenosine has direct impacts on other neurotransmitter systems and is an important upstream regulator to integrate and fine-tune glutamatergic and dopaminergic neurotransmission [17]. The downstream effects of adenosine are mediated by G protein-coupled adenosine receptors (ARs) A1, A2A, A2B, and A3 [20, 21], which are important targets for drug development [22, 23]. Inhibitory A1Rs and facilitatory A2ARs have in common a high affinity for adenosine, but have opposing activities based on coupling to different G proteins and a spatially distinct expression within the brain [21]. Ambient levels of adenosine (i.e. the “tone” of adenosine) within the brain is largely regulated by the astrocyte-based enzyme adenosine kinase (ADK), which is regarded as the key “upstream-regulator” of adenosinergic neuromodulation [24, 25] (Figure 1).
Figure 1. Major pathways of adenosine metabolism.
Within the central nervous system degradation of ATP constitutes the major source for synaptic adenosine, whereas the de novo synthesis plays only a minor role. In brain the major pathway for the removal of adenosine is its phosphorylation to AMP by adenosine kinase; alternatively, adenosine can be dephosphorylated by adenosine deaminase into inosine. Adenosine is a ubiquitous endproduct of transmethylation reactions. However, when adenosine levels increase the equilibrium of the s-adenosyl homocysteine (SAH) hydrolase reaction is shifted towards synthesis of SAH, resulting in the inhibition of transmethylation reactions.
The opposing activities of A1 and A2ARs indicated above imply that adenosine can exert both inhibitory and excitatory functions within the brain. A1Rs mediate inhibitory neuromodulation by coupling to inhibitory Gi or Go containing G proteins [12, 23]. As a result of this interaction adenylyl cyclase is stimulated, inwardly rectifying K+ channels are activated, presynaptic Ca2+ channels are inhibited [26], and phospholipase C is activated. Consequently, the release of predominantly excitatory neurotransmitters, such as dopamine, serotonin and acetylcholine, is inhibited, while the postsynaptic neurons are hyperpolarized. Thus, therapeutic activation of A1Rs has profound antiepileptic [27] and neuroprotective [28] functions.
In contrast to the inhibitory activity of A1Rs, excitatory functions of adenosine are largely mediated by activation of A2ARs. Thus, inhibitors of the A2AR have profound neuroprotective functions [28] and prevent apoptosis [29]. The A2AR has important functions related to the control of dopaminergic neurotransmission [17, 30–33]. Thus, the highest expression of A2ARs is found in striatal neurons, which are involved in the control of motor function [34]. A2ARs can form heteromers with other receptors, e.g. A1/A2A heteromers, or A2A/D2 heteromers and thus can modulate adenosinergic and dopaminergic neuromodulation by direct receptor interactions [31, 35]. Due to these interactions with other transmitter systems and due to its action on receptors with opposing activity (A1 versus A2), adenosine is uniquely positioned as an upstream regulator to integrate and fine tune excitatory and inhibitory functions within the central nervous system (CNS).
Ambient levels of brain adenosine appear to be largely under the control of astrocytes [36–40]. Under physiological conditions a significant part of synaptic adenosine is derived from astrocytic vesicular release of adenosine-5′-triphosphate (ATP) and its extracellular degradation to adenosine by several ectonucleotidases [41]. Extra- and intracellular levels of adenosine are rapidly equilibrated by nucleoside transporters [42–44]. Within astrocytes, adenosine is rapidly phosphorylated and removed from the adenosine pool by ADK [24, 39]. Thus, ADK controls synaptic adenosine by driving its influx into astrocytes via the nucleoside transporters [24].
Guanosine
Guanine-based purines are well recognized as intracellular modulators of G protein activity. In addition, several extracellular effects of guanosine that are not related to G proteins have recently been identified [45]. Extracellular guanosine is largely derived from astrocytic sources [46], fulfills important neurotrophic functions in the CNS [46] and stimulates astrocyte proliferation likely by increasing the endogenous extracellular adenosine concentration [47]. Current evidence suggests that guanosine stimulates glutamate uptake by astrocytes [48–50]. Consequently, guanosine reduces seizures induced by glutamatergic agents [51, 52], and is neuroprotective in response to ischemia-hypoxia [53]. On the behavioral side, guanosine impaired retention on the inhibitory avoidance task and decreased locomotor activity on the open field test [54]. Although the receptors and molecular pathways affected by guanosine are still incompletely understood, several studies suggest guanosine as potential new target for neuroprotection and neuromodulation.
Cytidine and uridine
Cytidine and uridine are not known to have direct effects on CNS function. Via pyrimidine salvage they will be transformed into cytidine-5′-triphosphate (CTP) and uridine-5′-triphosphate (UTP) and can contribute to brain phosphatidylcholine and phosphatidylethanolamine synthesis via the Kennedy pathway [55]. Based on these metabolic links cytidine and uridine metabolism might however play roles in neurodegenerative conditions.
Inosine
Inosine is a metabolite of adenosine and like adenosine, inosine fulfills important neuroprotective functions. Apart from being a degradation product of adenosine, inosine can directly be released from neurons in a N-methyl-d-aspartate receptor (NMDAR) dependent manner requiring extracellular calcium [56]. In addition, inosine can stimulate neurons to extend axons in culture and, in vivo, augments the ability of undamaged neurons to form axon collaterals after brain damage. Using a unilateral injury model limited to the sensorimotor cortex, it was recently shown that inosine increased the number of corticospinal tract axons that project from the unaffected hemisphere and enhanced the ability of these neurons to form connections on the denervated side of the spinal cord, which led to improved performance of the impaired limb [57]. This effect was attributed to inosine-induced alteration of gene expression in neurons contralateral to a stroke. Apart from direct effects of inosine within the CNS it was recently shown that inosine can contribute to beneficial effects in multiple sclerosis that have been attributed to a rise in serum urate levels [58].
NUCLEOSIDES IN BRAIN PATHOLOGY
The complex nature and ubiquitous distribution of nucleosides within the CNS implies that any dysfunction of nucleoside levels or distribution may translate into a wide spectrum of neurological diseases and psychiatric conditions. Research from recent years has provided novel evidence that dysregulation of endogenous nucleosides, in particular of adenosine, might play key roles in the pathogenesis of a wide range of neurological and neuropsychiatric conditions. The following sections will elucidate in selected examples – using adenosine as a prototype nucleoside – that any pathological dysregulation of nucleoside metabolism is expected to contribute to a vast array of neurological and neuropsychiatric disorders. Therefore, inhibitors of nucleoside metabolism hold great potential for the treatment of a vast spectrum of conditions (Table 1).
TABLE 1. THERAPEUTIC POTENTIAL OF ADENOSINERGIC DRUGS.
Selected examples are given how therapeutic modulation of the adenosine system might be therapeutically effective in a variety of conditions. See main text for disease specific discussions.
| ADK-inhibitors | A1R agonists | A2AR antagonists | |
|---|---|---|---|
| Epilepsy | Potent anticonvulsants | Potent anticonvulsants | Potential benefit for seizures DPMX: prevention of astrogliosis |
| Cerebral Stroke | Potential neuroprotectant? | Beneficial | Beneficial |
| Sleep Disorders | Potential use as sleep inducers? | Promotion of wakefulness (caffeine) | |
| Chronic Pain | Analgesia | Analgesia | SCH 58261: antinociception |
| Alzheimer’s Disease | Nootropic function? | Neuroprotection | Neuroprotection |
| Parkinson’s Disease | Neuroprotection? | Neuroprotection | KW-6002: motor stimulation |
| Amyotrophic Lateral Sclerosis | Neuroprotection? | Neuroprotection? | Neuroprotection? |
| Schizophrenia | ADK-inhibitors and AR agonists/antagonists have not directly been tested, however adenosine regulating agents might be beneficial: Allopurinol: effective on positive symptoms Dipyramidole: beneficial effects in patients |
Receptor agonists might be useful to reduce hyperdopaminergic functions. | |
Epilepsy
Adenosine is widely considered to be an endogenous anticonvulsant of the brain [16, 18, 59–61]. Recent findings indicate that dysfunction of astrocytes [37, 38, 40, 62–66] and in particular dysfunction of the adenosine system [24, 67] can cause seizures [18], or augment the spread of kainic acid- or traumatic brain injury- induced seizures and associated neuronal cell loss [67, 68]. Apart from other histopathological changes [69–73] including mossy fiber sprouting, dispersion of granule cells, and pyramidal cell loss, astrogliosis [74, 75] is one hallmark of the epileptic brain. In contrast, the loss of astrocytes – which provide a major source for adenosine – has been described in epileptic brain specimens obtained from patients with Rasmussen’s encephalitis [76].
Astrogliosis depends on the proliferation and hypertrophy of astrocytes, which is regulated in part by the ratio of A1R and A2AR expression on astrocytes [10]. Thus, activation of the A2AR potentiates synaptic actions of brain-derived neurotrophic factor (BDNF) in the hippocampus [77], stimulates glutamate outflow and leads to excessive glial activation [78]. Experimentally, astrogliosis could be increased in rat cortex by micro-infusion of the A2AR agonist 5′-(N-cyclopropyl)-carboxamidoadenosine (CPCA); this effect could be abolished by co-infusion of the adenosine A2AR antagonist 1,3-dipropyl-7-methylxanthine (DPMX) [79]. In line with these findings, A2AR blockade abolished reactive gliosis in rat striatal primary astrocytes that was induced by basic fibroblast growth factor [80]. These findings suggest that A2AR stimulation promotes astrogliosis. Since upregulation of A2ARs [28] as well as acute increases in adenosine [81] have all been described as a consequence of brain injury, hypoxia, or inflammation, it is tempting to speculate that astrogliosis might be triggered via an adenosine-based mechanism. Likewise, stimulation of A1Rs, which are downregulated during epileptogenesis [82–84], was demonstrated to lead to a reduction of astrocyte proliferation [47].
Due to astrogliosis, changes in the homeostasis of the astrocyte-based adenosine cycle are to be expected in epileptic brain. Thus, as a consequence of astrogliosis, the astrocyte-based adenosine-removing enzyme ADK is upregulated, leading to a reduction in ambient adenosine [24]. Interestingly, upregulation of ADK coincides – both spatially as well as temporally – with the emergence of spontaneous chronic seizures [24]. The hypothesis that upregulated ADK can be a direct cause for seizures was validated by creating transgenic mice with brain-wide overexpression of ADK (Adk-tg mice); as a consequence of this genetic manipulation Adk-tg mice display reduced seizure thresholds and spontaneous seizures [85]. These findings provide several rationales for therapeutic interventions in epilepsy: (i) Reconstitution of the physiological adenosine tone by increasing adenosine – ideally restricted to an astrogliotic scar; (ii) inhibition of ADK; (iii) prevention of astrogliosis by adenosine receptor modulation.
Cerebral Stroke
Adenosine, via activation of A1Rs is considered to be an important neuroprotectant against ischemic damage in the adult brain, although there is still controversy regarding the underlying mechanisms [15, 86–89]. Surprisingly, A1R activation does not protect the developing brain and impairs cerebral maturation [90] and adenosine receptor antagonists reduce ischemic brain injury in neonatal mice [91]. Thus, transient high neuronal expression of ADK in the early postnatal development of the mouse brain [39] might be a physiological mechanism to keep adenosine levels low.
In adult brain a rapid surge of adenosine following cerebral ischemia [15, 92] as an endogenous neuroprotective mechanism might initially lead to a cerebroprotective state known as ischaemic tolerance [93], a phenomenon, in which astrocytes appear to play a prominent role [94]. In line with this notion, the astrocytic enzyme ADK was downregulated within 3 hours following mild or severe stroke in the mouse [13]. Downregulation of ADK was associated with an increase in ambient adenosine [13]. Thus, activity levels of ADK in astrocytes might control the brain’s susceptibility to ischemic cell death. In support of this hypothesis Adk-tg mice overexpressing ADK in brain are hypersensitive to middle cerebral artery occlusion (MCAO) induced cell death [95]. If increased ADK in brain renders the brain more vulnerable, then focal augmentation of adenosine in the brain should be protective. This was demonstrated recently by transplantation of ADK-deficient neural or glial progenitor cells into the striatum of mice one week prior to MCAO; adenosine-releasing brain implants led to a striking reduction of the injured brain volume [96]. Thus, susceptibility to ischemia-induced brain injury seems to be tightly balanced by the tone of ambient adenosine. Consequently, as a rationale for therapeutic cerebroprotection, augmentation of the adenosine system e.g. by focal cell implants or inhibition of ADK appears to have promising potential.
Sleep Disorders
Adenosine is considered to be an endogenous sleep inducer based on a variety of pharmacological and behavioral data. Its biological function appears to be the restoration of brain energy metabolism during sleep [97, 98]. Administration of adenosine or its analogues can induce sleep when administered to experimental animals [99]. In behavioral studies sleep deprivation leads to increases in extracellular adenosine levels in the basal forebrain, but to decreases during sleep recovery [99, 100]. Sleep induction seems to be dependent on the activation of both A1 and A2ARs, however their relative contribution to sleep induction remains unclear [99, 101]. Thus, the electroencephalographic effects of sleep deprivation could be mimicked by A1R stimulation [102]. Adenosine stimulated calcium release in cholinergic but not non-cholinergic neurons via the A1R [103]. Microdialysis perfusion of A1R antisense oligonucleotides into the basal forebrain resulted in a significant reduction in non-rapid eye movement (NREM) sleep and electroencephalogram delta power with an increase in wakefulness within the spontaneous levels of sleep-wakefulness [104]. These and the above data support the hypothesis that adenosine, acting via the A1R in the basal forebrain is a key component in the homeostatic regulation of sleep. In contrast to adenosine, the non-selective A1R and A2AR antagonist caffeine promotes wakefulness [105, 106].
More recent data demonstrate that the A2AR might play a dominant role in sleep regulation. Thus, it was recently demonstrated that caffeine promoted wakefulness in wildtype (WT) and A1R knockout (KO) mice, but not in A2AR KO mice, indicating that the arousal effect of caffeine was caused by blockade of the A2AR [107]. Likewise, caffeine was shown to reduce the hypnotic effects of alcohol via the A2AR [108]. Together, these data imply a predominance of the A2AR for sleep regulation, although the subtype of adenosine receptor responsible for sleep regulation is still a matter of debate [99, 109].
In regard to the ongoing debate on the contribution of different adenosine receptor subtypes to sleep regulation, both A1R and A2AR KO mice demonstrated clear circadian profiles of sleep-stage distribution and identical amounts of NREM and rapid eye movement (REM) sleep compared to WT mice under basal conditions [107, 110]. A major difference however is that A2AR KO mice did not show NREM sleep rebound after sleep deprivation; in contrast they exhibited a robust REM sleep rebound [109]. In contrast, A1R KO displayed a phenotype similar to WT in having a robust rebound of both NREM and REM sleep after sleep deprivation [110]. These results indicate that the A2AR, but not the A1R, is essential for the homeostatic regulation of NREM sleep and imply that any dysregulation within the adenosine system affects sleep properties.
Chronic Pain
Pro- and antinociceptive activity can both be mediated by adenosine depending on the site of application or the receptor(s) involved [8]. Pain pathways involving the A1R, which dominates in mediating the antinociceptive effects of adenosine, have received most attention [8]. Thus, A1R agonists – acting at spinal sites – are effective antinociceptive agents in conditions of neuropathic and inflammatory pain, therapeutic effects mediated probably by prejunctional inhibition of transmitter release in pain pathways [111]. In line with these findings, A1 receptor knockout mice are characterized by hyperalgesia and increased susceptibility to chronic pain [112, 113]. These findings have been translated into clinical trials, in which adenosine, infused into the intrathecal space, ameliorated hypersensitivity due to central sensitization following peripheral capsaicin injection [114]. Likewise, intravenous adenosine led to a remarkable reduction of neuropathic pain in a double blind placebo controlled crossover phase I trial [115]. More recently, antagonism of A2A receptors has been considered for the management of chronic pain [33]. In particular, spinal effects of A2A receptors are likely based on combined immunohistochemical and electrophysiological studies demonstrating responses to the A2A selective agonist 3-[4-[2-[[6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-oxolan-2-yl]purin-2-yl]amino]ethyl]phenyl] propanoic acid (CGS 21680) in spinal cord, leading to the proposal that A2A receptors are located on presynaptic inhibitory terminals of descending fibers from higher centers [116]. Consistent with these findings, the A2A selective antagonist 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine (SCH 58261) produced antinociception in two acute thermal pain tests [117]. In addition, A2A receptor knockout mice are characterized by significantly reduced NMDAR binding in all regions of the spinal cord, which may be due to altered glutamate signaling in C-fibers [118]. Following intraplanar formalin injection A2A receptor knockout mice have reduced biting and flinching responses in the first acute response phase (0–15 min) and reduced flinching only in the second phase (15–60 min) of the test [118]. The potential for A2A receptor antagonists in inflammatory pain is supported by findings that SCH 58261 blocks both phases of the formalin response in wild-type mice [118]. Interactions between A2A receptors and the opioid system is suggested by findings that A2A receptor knockout mice show altered expression of the opioid peptide precursor pro-opiomelanocortin [119]. In summary, the available evidence suggests that A1 receptors exert antinociceptive effects at peripheral and spinal sites, while A2A receptors play an opposing role. Thus, a combination of A1 receptor agonism with A2A receptor antagonism might be an effective strategy for pain control.
Alzheimer’s Disease
Alzheimer’s disease (AD) affects 20–30 million people worldwide and is characterized by progressive memory deficits, cognitive impairment and personality changes. The main cause of AD is generally attributed to the increased production and extracellular accumulation of amyloid-beta (Aβ), in association with intracellular neurofibrillary tangle (NFT) formation [120–122]. In addition, inflammatory processes appear to be crucial in the pathophysiology of AD [123] and activated astrocytes were found to be closely associated with amyloid plaques in tissue from individuals with AD [124]. Astrocytes are thought to be activated by human Aβ indicating a correlation between this protein and astrocyte dysfunction [125]. Conversely, activated glial cells appear to contribute to the development of amyloid plaques [126]. Clinically, hippocampal sclerosis is a common finding in AD autopsies [127] and the glial activation marker S100β was elevated in AD [128, 129]. Although it is generally accepted that Aβ-deposition is a potent glial activator, astrocyte and microglial activation could be an early event in the disease, occurring even in the absence of Aβ-deposition [130, 131]. Thus, in a clinical study glial activation was detected at very early stages of AD [132] and in a recent population-based study increasing gliosis has been found before the development of AD-lesions [133]. Likewise, in amyloid precursor protein mutant mice focal glial activation preceded Aβ deposition [134]. Remarkably, several transgenic AD-models show cognitive deficits well before Aβ deposition [135]. Together, these findings suggest that glial dysfunction is involved in the pathophysiology of AD.
Studies from our lab concerning the role of ADK in epilepsy have demonstrated, that – as a homotypic chronic response of the brain to injury (e.g. status epilepticus) – astrogliosis is always accompanied by upregulation of ADK, which in turn leads to a focal adenosine deficiency that triggers seizures [85, 136–141].
The neuromodulator adenosine fulfils a unique role in the brain affecting glutamatergic neurotransmission and dopaminergic signalling via activation of adenosine A1 and A2A receptors, respectively. The adenosine system is thus ideally positioned to integrate glutamatergic and dopaminergic neurotransmission, which in turn could affect behavior and cognition. To test this hypothesis, transgenic mice with an overexpression of ADK in brain (Adk-tg), and therefore reduced brain adenosine levels, were evaluated in a panel of behavioral and psychopharmacological assays to assess possible glutamatergic and dopaminergic dysfunction [142]. In comparison to non-transgenic control mice, Adk-tg mice were characterized by severe learning deficits in the Morris water maze task and in Pavlovian conditioning. These results confirmed that ADK overexpression could lead to functional concomitant alternations in dopaminergic and glutamatergic functions, which in turn affect cognition.
It is tempting to hypothesize that astrogliosis in AD leads to upregulation of ADK and adenosine deficiency: this has twofold consequences for the pathophysiology of AD: (i) A reduction in the tone of ambient adenosine (by overexpressed ADK) implies the loss of a major endogenous neuroprotectant [28]; thus upregulation or overexpression of ADK is associated with increased stroke- or seizure-induced neuronal cell death [95, 136]; consequently, upregulation of ADK in AD is likely to contribute to increased neuronal cell death in AD. (ii) Transgenic overexpression of ADK in mice causes severe cognitive impairment [142]; thus upregulated ADK in AD is expected to contribute to cognitive impairment. Adenosine-augmentation therapies are thus expected to directly improve cognitive functionality in AD and at the same time promote neuronal survival. This dual therapeutic approach will combine two endogenous properties of adenosine: neuroprotection and cognitive enhancement. Future directions thus may include the development of adenosine-augmenting therapies for AD.
Parkinson’s Disease
The adenosine A2A receptor has emerged as a leading non-dopaminergic therapeutic target in Parkinson’s disease (PD). Two lines of evidence suggest that pharmacological inhibition of A2ARs will be of benefit for patients with PD: (1) A2ARs and dopamine D2 receptors (D2Rs) are colocalized in striatopallidal neurons and display antagonistic interactions at the molecular, neurochemical and behavioral level. These interactions have been used to explain the motor stimulant effects of A2AR blockade [143–145]. Based on these considerations A2AR antagonists such as KW-6002 (istradefylline) and SCH 420814 are currently investigated in clinical trials. Phase II and III studies have confirmed that selective A2AR antagonists can stimulate motor activity by potentiating the L-dopa effect in advanced PD patients [146, 147]. (2) A2AR antagonists may also have a more direct effect to attenuate dopaminergic neurodegeneration. Several large-cohort prospective studies have confirmed a similar inverse relationship between the consumption of caffeine and the risk of developing PD [148], [149], [150]. These findings are corroborated by animal models of PD that demonstrated potentially protective effects of caffeine. Thus, it was demonstrated that pharmacological blockade or genetic disruption of the A2AR attenuates dopaminergic neurodegeneration[151–153]. Together, these findings suggest, that any (pathological) imbalance in adenosinergic neuromodulation might impact the pathophysiology of PD.
Amyotrophic Lateral Sclerosis (ALS)
Most current knowledge on ALS pathogenesis is based on the landmark discovery that 20% of all familiar and 1% of all sporadic ALS cases are linked to dominant mutations of the ubiquitously expressed enzyme Cu/Zn superoxide dismutase (SOD1) [154]. Importantly, ALS could be replicated in transgenic rodents that carry single-amino acid mutations of human SOD1; for a review see [155]. These findings suggested that mutant SOD1 might become toxic to motor neurons because of a gain of function. Whereas the primary toxic nature of mutant SOD1 is still unresolved, a seminal study demonstrated that non-cell-autonomous processes that involved the interaction with neighboring non-neuronal cells, in particular microglia and astrocytes, contributed to the death of motor neurons [156]. In line with these findings massive activation of microglia and astrocytes was found in human cases as well as in transgenic animal models [157–159]. A wealth of information now demonstrates that astrogliosis is a pathological hallmark of ALS that might contribute significantly to the etiology of ALS-pathogenesis and has extensively been covered in recent literature [159–162]. Thus, reactive astrocytosis has been identified as a uniform pathological feature in rodent models of ALS [163–165] as well as in human cases of ALS [166–169]. A recent study using genetic ablation of dividing GFAP+ astrocytes suggests that Olig2+ and NG2+ glial progenitor cells likely play a prominent role in the astroglial pathology of ALS [170].
As outlined above in “Epilepsy” and “Alzheimer’s Disease” an astroglial contribution to ALS pathology might lead to adenosine deficiency caused by a hypothesized astrogliotic overexpression of ADK. Thus, a deficiency in the neuroprotective adenosine might contribute to the loss of motor neurons in ALS and might explain the comorbidity of frontal dementia.
Schizophrenia (SZ)
The dopamine hypothesis of SZ is largely based on complementary effects of dopamine receptor (DR) antagonists and agonists to suppress and to promote psychotic symptoms, respectively. Thus, the efficacy of many antipsychotics correlates with their ability to block dopamine D2Rs [171, 172], dopamine-releasing drugs such as amphetamine can exacerbate psychotic symptoms in schizophrenia, and its prolonged abuse can produce psychotic symptoms in healthy subjects [173]. In addition, elevated basal occupation of D2Rs by dopamine [174], increased dopamine turnover [175], and enhanced amphetamine-induced dopamine-release [173] have been shown in SZ patients using positron emission tomography (PET) imaging. The latter two findings in particular are compatible with a deficiency in adenosine. An adenosine deficit can (i) enhance dopamine activity by reducing the inhibitory effect of adenosine A1Rs on dopamine release [176], and within the striatum it can (ii) potentiate amphetamine-induced locomotion [177] and dopamine release in the nucleus accumbens as suggested by the effects of A1R antagonists [178]. Interactions between A2ARs and D2Rs [179, 180] allow further opportunity for mutual modulation between the adenosine and dopamine systems [181]. Thus, increased basal D2R occupancy in SZ patients [174] could reduce the A2AR’s effect on D2Rs, and thereby increase the affinity of the D2R for dopamine [179, 180, 182]. These mechanisms could provide the rationale for a typical neuroleptic-like profile of adenosine receptor agonists.
According to the glutamate hypothesis of SZ, reduced NMDAR function may contribute to cognitive and negative symptoms of schizophrenia [183, 184]. Blockade of NMDARs is known to impair the induction of long term potentiation (LTP) and learning in animals [185–187]. Moreover, NMDAR blockade can give rise to behavioral dysfunction such as impulsivity [188] and psychotic-like behavior; e.g., phencyclidine and ketamine (two non-competitive NMDAR antagonists classified as dissociative anesthetics) are well known psychomimetics [183]. In contrast, co-agonists of the NMDAR, such as D-serine and glycine, improve cognition and negative symptoms in SZ [184]. Similarly, disruption of glycine transporter 1 has also been found to possess promnesic and antipsychotic potentials [189–192]. Adenosine is an endogenous modulator of glutamatergic activity. It inhibits glutamate release and the post-synaptic action of excitatory neurotransmitters upon neuronal hyperpolarization via A1Rs [81]. In animal models of SZ, A1 and A2AR agonists have repeatedly been shown to be effective against the behavioral as well as neurophysiological (EEG and prepulse inhibition) effects induced by NMDAR antagonists [193, 194], thus lending support for their potential antipsychotic efficacy in humans.
Several lines of evidence support dysfunction of the adenosine system in patients with schizophrenia. Thus, increased density of A2ARs was found in the striatum of patients in a post mortem study [195]. Upregulation of striatal A2ARs could be a compensatory response triggered by reduced adenosinergic activity, which in turn could produce a hyperdopaminergic state [196, 197]. Interestingly, the Golf protein that is coupled to A2ARs [198] is a candidate gene for schizophrenia [199, 200]. Although adenosine-based drugs have not yet been tested in schizophrenia, the purine metabolic drug allopurinol was studied as add-on therapy for schizophrenia [201–203]. Adjunctive allopurinol was effective on positive and general symptoms. Similarly, the adenosine transport inhibitor dipyramidole (by raising adenosine) was beneficial in patients with schizophrenia [204]. Thus, preliminary clinical data support the adenosine hypothesis of schizophrenia.
INHIBITORS OF NUCLEOSIDE METABOLISM
The preceding sections illustrate that any imbalance in the level or distribution of nucleosides is expected to be associated with a wide spectrum of neurological and/or neuropsychiatric disturbances. Therefore inhibitors of nucleoside metabolism hold great promise for the treatment of a wide spectrum of conditions. In the following the major enzymes and inhibitors of nucleoside metabolism and their specific therapeutic relevance will be reviewed. Inhibitors of nucleotide metabolism (e.g. ecto-ATPases) will not be covered here.
5′nucleotidase (5′NT)
5′-nucleotidase (EC 3.1.3.5) degrades guanosine-5′-monophosphate (GMP), inosine-5′-monophosphate (IMP) and adenosine-5′-monophosphate (AMP) to guanosine, inosine, and adenosine, respectively. In addition to the regulation of endogenous nucleosides the enzymes regulate the activation of nucleoside analogues, a class of anticancer and antiviral agents that rely on nucleoside kinases for phosphorylation to their active forms. Both clinical and in vitro studies suggest that an increase in nucleotidase activity can inhibit nucleoside analogue activation and result in drug resistance [205]. The enzyme can either be located in the plasma membrane (ecto-5′-nucleotidase) or in the cytoplasm (cytosolic 5′-nucleotidase). Cytosolic 5′-nucleotidase forms a substrate cycle with ADK to the effect that small activity changes in either of those enzymes can result in large changes in ambient adenosine [206, 207]. In particular, cytosolic 5′-nucleotidase is an important contributor to the generation of adenosine and has been implicated in coupling adenosine to the energy state of a cell [208].
Within a pathological context increased activity of ecto-5′-nucleotidase has been associated with pilocarpine-induced epilepsy in rats, possible an endogenous protective mechanism in an attempt to raise levels of the endogenous anticonvulsant adenosine [209]. In addition, the enzyme is implicated in regulating vascular inflammatory responses that play a major role in many CNS pathologies [210].
Nucleotide and nucleoside analogues have been developed as inhibitors of cytosolic 5′-nucleotidase I from heart and brain, mostly as a biochemical tool to discriminate between functions of different 5′-nucleotidases [211, 212]. Similar analogues have also been used to study mechanisms how cells acquire nucleoside analogue resistance [205, 213]. Treatment with 0.001 mM adenosine 5′-(alpha, beta-methylene)diphosphate, a competitive ecto-5′-NT/CD73 inhibitor, caused a significant reduction of 30% in glioma cell proliferation [214]. Apart from a potential use as anti-neoplastic agents, inhibitors of 5′-nucleotidase activity are likely of limited therapeutic value as they would lead to a reduction of neuromodulatory nucleosides. In contrast, however, statins were found to increase 5′-nucleotidase activity. Thus, rosuvastatin increased extracellular adenosine formation in humans in vivo and was shown to provide protection against ischemia-reperfusion injury in humans [215].
Purine nucleoside phosphorylase (PNP)
Purine nucleoside phosphorylase (EC: 2.4.2.1) can catalyze guanosine to guanine, inosine to hypoxanthine, and adenosine to adenine conversions. Genetic deficiency in PNP causes immunodeficiency similar to immunodeficiency caused by adenosine deaminase deficiency [216]. The therapeutic potential for PNP inhibitors seems to be limited to the realm of oncology. Forodesine is a highly potent, orally active, rationally designed PNP inhibitor, that is active in preclinical studies with malignant cells and clinical utility against T-cell acute lymphoblastic leukemia and cutaneous T-cell lymphoma [217]. Therapeutic potential for CNS conditions is currently not known.
Adenosine kinase (ADK)
Adenosine kinase (EC 2.7.1.20) phosphorylates adenosine into AMP and is part of a highly active substrate cycle between adenosine and AMP [206, 207]. In adult brain, ADK is mostly expressed in astrocytes [39]. Two alternatively spliced isoforms exist, a short isoform that is specific for the cytoplasm and a long isoform that is specific for the nucleus [218]. In adult rodent brain ADK is the key regulator for ambient levels of adenosine that are part of an astrocyte-based adenosine cycle [137, 219, 220]. It was demonstrated more than 15 years ago that inhibition of ADK, but not of adenosine deaminase depressed neuronal activity in hippocampal slices [221]. Increased expression of ADK is linked to astrogliosis in several CNS pathologies leading to a reduction in ambient levels of adenosine [136, 137, 222]. In epilepsy, it was demonstrated that increased levels of ADK, rather than any other epileptogenetic event is sufficient to trigger spontaneous recurrent seizures [136, 222]. Likewise, increased levels of ADK render the brain more susceptible to neuronal injury [95, 223]. Due to its key role in regulating the brain’s endogenous anticonvulsant and neuroprotectant adenosine, inhibitors of ADK hold great promise for the treatment of neurological conditions that are based on adenosine deficiency, or that can be treated by adenosine augmentation. Therefore, ADK inhibitors have been developed with promising activities including seizure suppression, antinociception, anti-inflammatory action, and neuroprotection [224–242]. Importantly, ADK inhibitors have the potential to potentiate an event-specific endogenous surge in adenosine (by blocking its degradation) and thereby avoiding unspecific side effects that are common with adenosine A1R agonists [224–226]. The wide potential utility of ADK inhibitors is illustrated by the following examples: Following systemic administration the nucleoside ADK inhibitors 5′-amino-5′-deoxy-adenosine, 5′-deoxy-5-iodotubercidin, 5-iodotubercidin alleviated acute thermal nociception as measured by the hot-plate test in mice [227]. In addition to antinociception, ADK inhibitors are highly effective anticonvulsants [228, 229] with proven efficacy in animal models of pharmacoresistant epilepsy [141, 230].
Adenosine deaminase (ADA)
Adenosine deaminase (EC 3.5.4.4) deaminates adenosine into inosine and constitutes a major catabolic pathway for adenosine. In contrast to ADK that plays a major role in the adult CNS, ADA is expressed at high levels in placenta and seems to play a crucial role for fetal and perinatal development [231–233]. During early brain development transient ADA expression was found in specific subsets of neurons [234]. In adulthood, the highest expression levels of ADA are found in tongue and in cells lining the intestinal tract [235, 236]. In adult brain ADA is associated with neurons and exhibits an uneven expression pattern [237]. Whereas many brain areas such as hippocampus are characterized by very low levels of ADA, higher ADA levels are only found in specialized nuclei such as the posterior hypothalamic magnocellular nuclei [237]. It is important to note that ADA activity decreases during brain maturation in several brain areas such as superior colliculus, cortex, hippocampus, cerebellum, olfactory bulbs, and olfactory nucleus indicating further that ADA plays important roles during brain development rather than in mature brain [238]. In contrast, ADK expression in astrocytes increases during brain maturation [39] indicating that the brain undergoes a developmental switch from ADA to ADK as the key regulators of ambient brain adenosine. Thus, in slices from adult brain, pharmacological inhibition of ADK, but not of ADA leads to adenosine-induced inhibition of neuronal activity [221].
Inhibitors of ADA include erythro-9-(2-hydroxy-3-nonyl) adenine (EHNA, specific for isozyme ADA1, no activity on ADA2), 2-deoxycoformycin (pentostatin; (8R)-3-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydro-2-furanyl]-3,6,7,8-tetrahydroimidazo[4,5-d][1,3]diazepin-8-ol) and the non-nucleoside inhibitor 1-((1R,2S)-2-hydroxy-1-(2-(1-naphthyl)ethyl)propyl)1H-imidazole-4-carboxamine (FR234938). Pentostatin has been (Federal Drug Administration (FDA) approved for the treatment of hematological malignancies [239–241], whereas the non-nucleoside inhibitor FR234938 has good potential as a new type of anti-rheumatic and anti-inflammatory drug, by modulating host-defense concentrations of adenosine [242]. While most attention to ADA inhibitors is directed at anti-neoplastic and anti-inflammatory activity, few studies have explored the potential use of ADA inhibitors for CNS disorders. Thus, it was shown that the novel putative anticonvulsant drug 1-[2,6-difluorophenyl)-methyl]-1H-1,2,3-triazolo[4,5-c]) pyridine-4-amine monohydrochloride (BW534U87) effectively reduced seizures induced in rodents by threshold maximal and supramaximal electroshock, electrical kindling, pentylenetetrazole (PTZ) infusion and by vestibular stimulation in the genetically seizure-prone epilepsy-like (EL) mouse, likely via inhibition of ADA [243, 244]. Protection against focal ischemic brain damage in rats was evident when deoxycoformycin (500 micrograms/kg), was administered prior to the onset of ischemia, whereas delayed dosage was without any effect [245].
Guanase
Guanine deaminase (guanase; EC 3.5.4.3) catalyzes the deamination of guanine to xanthine. Like ADA, guanase plays a prominent role for purine catabolism in the small intestine [235] and is expressed in liver, kidney and brain [246]. In the CNS extracellular guanine is known to exert trophic effects that are likely regulated by conversion of extracellular guanosine into guanine by a membrane located purine nucleoside phosphorylase (ecto-PNP) and by conversion of extracellular guanine to xanthine by guanase [46]. In addition, guanase appears to be essential for ammoniagenesis in brain [247]
Azepinomycin is a natural guanase inhibitor with antitumor and antibiotic activities [248, 249], however guanase inhibitors are currently not used for any CNS indications.
Xanthine oxidase (XO)
Xanthine oxidase (EC 1.17.3.2) degrades hypoxanthine to xanthine and xanthine to uric acid and is part of the major catabolic pathway for purines. The enzyme also produces free radicals, which contributes to neurotoxicity; XO-induced free radical production is therefore thought to contribute to the pathophysiology of stroke, traumatic brain injury, as well as to the development of neurodegenerative diseases [250–252]. Inhibition of XO is therefore a rational neuroprotective strategy. The most widely used inhibitors of XO are allopurinol and oxypurinol, which are used primarily in the treatment of hyperuricemia and gout. XO inhibitors lower uric acid but also attenuate expression of inflammatory adhesion molecules in murine models, reduce oxidative stress in the vasculature, and improve endothelial function. Two distinct mechanisms may account for the therapeutic activity of XO-inhibitors: (i) suppression of free radical formation and (ii) inhibition of purine degradation, which ultimately leads to an increase in adenosine. A recent study demonstrates that allopurinol exerts potent anti-nociceptive activity via augmentation of adenosine [253]. In this study allopurinol presented dose-dependent anti-nociceptive effects in four different chemical or thermal pain models. The observed anti-nociceptive effects were not affected by the opioid antagonist naloxone. However, the non-selective adenosine-receptor antagonist caffeine and the selective A1R antagonist DPCPX, but not the selective A2AR antagonist SCH58261, completely prevented allopurinol-induced anti-nociception. Allopurinol, at doses up to 200 mg/kg did not produce any obvious motor deficits. In addition, allopurinol raised cerebrospinal fluid levels of purines, including the nucleosides adenosine and guanosine. Together, these findings suggest that allopurinol exerts beneficial therapeutic effect by leading to a rise in the endogenous anti-nociceptive nucleoside adenosine [253]. Furthermore, adenosine-based antinociception might further be enhanced by reduction of pro-nociceptive reactive oxygen species via XO-inhibition [254]. Allopurinol has clinically been used. In a double-blind adjunctive allopurinol trial significant symptom improvement was noted in patients with schizophrenia, however the underlying mechanism responsible for the therapeutic benefit was not further investigated [255]. In stroke patients allopurinol was shown to have beneficial effects on inflammatory [256] and vascular [257] parameters. Due to its relative safety, allopurinol may hold promise for future adenosine augmentation therapies.
In contrast to the beneficial actions of XO-inhibitors, recent findings demonstrate that the obligatory end-product of purine catabolism, uric acid has neuroprotective functions in Parkinson’s Disease [258]. Based on these new findings XO-inhibitors might reduce uric acid-based neuroprotection.
Hypoxanthine-guanine phosphoribosyltransferase (HGPRT)
Hypoxanthine-guanine phosphoribosyltransferase (EC 2.4.2.8) is a purine salvage pathway enzyme, which can catalyze the guanine and hypoxanthine conversion to GMP and IMP, respectively. The gene for HGPRT is considered to be a housekeeping gene and commonly used as a reference standard [259, 260]. Deficiency of HGPRT in humans is associated with Lesch-Nyhan syndrome and severe neurological deficits that include self-mutilation [261, 262]. It has been suggested that Lesch-Nyhan syndrome is primarily due to deficiency of dopamine in basal ganglia. This neurochemical lesion may result from a deficiency of purine nucleotides, which impairs arborization of nigrostriatal neurons during perinatal development [263]. Enzyme inhibitors include 6-thioguanine and 6-mercaptoguanine, however their therapeutic use is largely restricted to inhibit the growth of parasites. Due to the general toxicity of HGPRT inhibition, HGPRT inhibitors are of little value for any CNS applications.
Uridine phosphorylase (UP)
Uridine phosphorylase (EC 2.4.2.3) catalyzes the uridine – uracil conversion. Recent evidence suggests that uridine protects neurons against ischemic insult-induced neuronal death, possibly through the action of UP [264]. In addition, the enzyme seems to underlie circadian regulation. Thus, the circadian rhythm observed in the plasma concentration of uridine was found to be inverse of that for uridine phosphorylase activity [265]. It was further suggested that hepatic uridine phosphorylase plays an important role in the regulation of uridine levels in blood which, in turn, may be involved in the humoral control of sleep by uridine [265]. Enzyme inhibitors fall largely into the class of alkylated or benzylated uridine derivatives. Those drugs, such as benzylacyclouridine are largely of interest for their antineoplastic activity [266–268], however benefit for CNS applications is unknown.
Other nucleoside metabolizing enzymes
Several other nucleoside metabolizing enzyme exist, however their relevance for CNS pathology and therapy are not yet defined. Among those enzymes not discussed here are: S-adenosyl-homocysteine hydrolase, guanosine kinase, uridine kinase, uridine-cytidine kinase, cytidine kinase, cytidine deaminase, and enzymes of inosine metabolism.
THERAPEUTIC RATIONALE
The examples above demonstrate that relatively few inhibitors of nucleoside metabolism are of interest for applications within the CNS. Among those drugs, ADA inhibitors, ADK inhibitors, and allopurinol are of most interest, all acting by augmenting adenosinergic signalling with its proven benefit in a wide spectrum of neurological and neuropsychiatric conditions. The following discussion therefore centers on the therapeutic benefit of adenosine augmentation therapies (AATs).
Effects on multiple neurochemical networks
Adenosine is an important upstream regulator of neuronal function [269–273]. By activation of presynaptic A1Rs it mediates presynaptic inhibition [274, 275]. In addition, adenosine promotes hyperpolarization of the postsynaptic neuron [276–278]. Furthermore, adenosine interacts on multiple levels with complex neuronal networks and directly modulates the activity of all major neurotransmitters [5, 269]. Attenuation of the glutamate response can explain the potent antinociceptive, neuroprotective, and antiepileptic effects of AATs [253, 279–281]. In addition, the ambient adenosine concentration determines the stability of GABAA receptors [272, 282]. Adenosine controls dopamine signalling largely via heteromerization of adenosine A2A and dopamine D2 receptors; therefore A2AR antagonists are of high therapeutic interest for Parkinson’s disease [31, 32, 146, 283, 284]. Adenosine A2A receptors interact with trkB receptors and thereby influence BDNF signalling [77, 285]. These examples illustrate that any pathological imbalance in adenosine levels is likely to affect several different downstream pathways simultaneously, contributing to the complex phenotypes of most neurological and neuropsychiatric disorders. On the other hand, AAT is uniquely suited to affect several signalling pathways simultaneously and thereby to offer the potential for soft and smart therapeutic applicability. This therapeutic concept is in contrast to industrial drug development efforts that seek to find highly selective compounds to affect only one specific pathway, e.g. by blocking or activating a specific receptor subtype. However, those approaches are not able to affect the complex pathophysiology of a given disease; they rather provide symptomatic relief for a specific endophenotype of a disorder, and are associated with side effects. As an example pharmacological blockade of NMDA receptors is not an option to treat epilepsy, as NMDA receptor blockade is associated with psychosis. However, AAT may provide an indirect and more elegant way to reduce NMDA receptor function, and to prevent seizures, however, without inducing psychosis.
Amplification of physiological functions of nucleosides
The section above illustrates that AAT allows modulating several downstream pathways simultaneously, which is considered to be a major advance compared to more standard pharmacotherapeutic approaches. The potential efficacy of AAT is further enhanced by its ability to potentiate an endogenous protective response of the brain in an event and site-specific manner. This has best been illustrated with ADK inhibitors [224–228, 286–294]. After any type of brain injury, the brain reacts with a surge of endogenous adenosine as an acute protective response of the brain [24]. If this surge of adenosine can be amplified by reducing adenosine clearance, the endogenous protective response of the brain should be potentiated. In a seminal study by Britton et al., KA-evoked striatal adenosine release was augmented in animals receiving systemic treatment with the ADK inhibitor 5′deoxy-5-iodotubercidin (5′d-5IT) (cumulative dose of 7.5 μmol/kg, i.p.) compared with i.p. vehicle controls. In contrast, 5′d-5IT administration had no significant effect on basal or contralateral (artificial CSF-perfused) striatal adenosine levels. This experiment demonstrates that inhibition of adenosine clearance can potentiate the beneficial effects of adenosine in a site- and event-specific manner. However, blocking the clearance of an endogenous adenosine surge can have also detrimental effects as has recently been suggested in a model of sudden unexpected death in epilepsy, in which a combined surge in KA-triggered adenosine release with defective adenosine clearance, led to prolonged apnea in mice with lethal outcome [295].
Reduced side effects
The second generation of non-nucleoside ADK inhibitors, e.g. ABT-702 (5-(3-bromophenyl)-7-(6-morpholin-4-ylpyridin-3-yl)pyrido[2,3-d]pyrimidin-4–ylamine) was shown to exhibit potent antinociceptive and anti-inflammatory activity with a favorable therapeutic window [289, 292, 293] in particular, when compared to adenosine receptor agonists [288]. Based on ABT-702 novel 4-amino-5-aryl-6-arylethynylpyrimidines have recently been developed [227].
THERAPEUTIC FUTURE FOR ADK INHIBITORS
Despite an excellent therapeutic rationale and demonstrated efficacy in chronic pain and epilepsy, the systemic use of ADK inhibitors interferes with methionine metabolism in liver [296, 297] and brain hemorrhage after systemic administration of an ADK inhibitor has been reported in dogs [228]. Although effective in pharmacoresistant epilepsy, the ADK inhibitor iodotubercidin still caused significant sedative side effects [141]. The bulk of pharmaceutical studies on ADK-inhibitor development was published between 1998 and 2005 with a peak in 2001. These findings suggest that systemic ADK inhibitors are no longer considered to be promising drugs for pharmaceutical drug development. Although therapeutically very effective in augmenting adenosine signalling, any systemic adenosine augmentation will affect multiple organ systems and will lead to unspecific augmentation of a multitude of signalling pathways that are regulated by adenosine. One therapeutically viable strategy to circumvent this conceptual problem constitutes the development of focal AATs. The underlying rationale for the use of focal AATs is first to define a local area of adenosine dysfunction as contributor to disease pathology, and second to augment adenosine selectively within this pathological area. Partial epilepsy is an ideal target for those approaches. In particular, mesial temporal lobe epilepsy is characterized by astrogliosis and focal adenosine deficiency caused by pathological overexpression of ADK. Therefore reconstitution of normal (rather than providing excessive levels of adenosine) within an epileptogenic focus is considered to be a rational and effective approach for seizure control.
NOVEL THERAPEUTIC AVENUES
Based on the neurochemical rationale outlined above, we have developed a wide spectrum of focal AAT approaches for the treatment of seizures in epilepsy [298–300].
Polymer-based adenosine delivery
The biomaterial silk constitutes an ideal system for the controlled and sustained focal delivery of small molecule drugs. Silk is biocompatible and biodegradable, can be used for both drug- and cell-encapsulation and has been used for a wide spectrum of therapeutic drug-delivery approaches [301–303]. We recently generated and tested silk-based brain implants for the focal delivery of adenosine to the epileptic brain. Silk polymers were generated to deliver target doses of 40, 200, or 1000 ng adenosine per day [304]. Implantation of those polymers into the infrahippocampal fissure of rats prior to the onset of electrical kindling suppressed the development of kindled seizures in a dose-dependent way [304]. Polymers engineered to release a target dose of 1000 ng over a time span of 10 days completely suppressed stage 5 (highest possible seizure grade) seizures in fully kindled epileptic rats. Duration of suppression correlated with the release profile of adenosine. As soon as adenosine release from the polymers was expired, seizure activity resumed, indicating that seizure suppression was due to implant-based adenosine release [305]. In addition, when implanted before kindling initiation, the same polymers almost completely prevented the expression of any seizures. After expiration of adenosine release from the polymers kindling was resumed and the subsequent curve of kindling development paralleled kindling development in control animals. These data strongly indicate that the focal delivery of adenosine to the brain might not only suppress seizures but also the process leading to epilepsy, i.e. epileptogenesis [305].
Stem cell-based adenosine delivery
To provide a source for the sustained release of adenosine we engineered mouse embryonic stem cells (mESCs) to release adenosine based on a biallelic genetic disruption of the Adk-gene [306]. The cells were subjected to an in vitro differentiation paradigm [307] to induce the formation of adenosine-releasing neural precursor (NP) cells [306]. These cells were transplanted into the infrahippocampal fissure of rats prior to the onset of kindling. Recipients of adenosine-releasing mESC-derived NPs were characterized by robust suppression of kindling development compared to sham or wild-type cell-treated control animals [308]. In a similar approach, the same cells were transplanted into mice 24 hours after the intraamygdaloid injection of KA (= trigger for epileptogenesis). Three weeks later, recipients of wild-type cells or of a sham procedure were characterized by focal CA3-selective astrogliosis that included overexpression of ADK and the expression of about 4 spontaneous electrographic seizures per hour. In contrast, recipients of adenosine-releasing cells were characterized by a significant reduction in astrogliosis, by almost normal expression levels of ADK and by complete lack of seizures [136]. Together, these experiments demonstrate antiepileptogenic activity of focal stem cell-based adenosine delivery. In both experiments [136, 308], but also in recipients of human ESCs [300], 4 weeks after transplantation dense grafts were found within the infrahippocampal fissure, likely forming a reservoir for paracrine adenosine release, whereas some cells migrated into the ipsilateral CA1 and assumed a neuronal morphology. To develop human stem cells for future clinical use in cell-based AAT approaches, we developed a lentiviral expression system to express a micro-RNA (miRNA) directed against ADK. Human mesenchymal stem cells (hMSCs) can easily be derived from the bone marrow of a patient with epilepsy and used for autologous cell grafting approaches without the need for immunosuppression. ADK knockdown and adenosine release was induced in hMSCs after lentiviral transduction with the anti ADK miRNA construct, whereas a scrambled control sequence had no effect [309]. Infrahippocampal grafts of adenosine-releasing hMSCs in mice attenuated acute KA-induced neuronal injury and attenuated seizure development after KA-injection [309, 310]. These experiments suggest that stem cells derived from a patient might be engineered for therapeutic adenosine delivery.
Gene therapy
Since pathogenetic overexpression of ADK is directly related to seizure generation, the enzyme constitutes an ideal target for future gene therapy approaches. Whereas in conventional gene therapy a therapeutic gene is added, in this case the therapeutic target would be to construct a gene therapy vector capable of downregulating ADK, ideally within an astrogliotic area of increased ADK expression. Limitation of such an approach to the focus of seizure generation might provide a rational long-term strategy for sustain seizure suppression or prevention.
CONCLUSIONS AND FUTURE PERSPECTIVES
Nucleosides are ubiquitous regulators of a wide spectrum of physiological functions that are of importance for every organ system. While inhibitors of nucleoside metabolism are primarily of therapeutic interest for antimicrobial or anti-neoplastic therapies, surprisingly few of those inhibitors have been developed for CNS applications. Even though highly effective in a variety of paradigms (e.g. epilepsy, inflammation, or chronic pain) therapeutic use of systemic nucleoside inhibitors appears to be of limited use due to lack of regional specificity and due to wide-spread side effects. Eventually, focal augmentation of nucleoside function might represent a more promising option. Focal augmentation of nucleosides is ideally suited to limit their therapeutic action to only those regions where nucleoside augmentation appears to be rationally justified.
The bulk of research has focused on adenosine, however the emerging roles of guanosine and inosine as neuromodulators need to be further explored and respective therapeutic tools need to be developed. Eventually, the further development of focal nucleoside augmentation approaches, e.g. by polymeric brain implants, or the development of stem cell or gene therapies may provide the basis for a soft and intelligent management of a wide range of neurological and neuropsychiatric disorders.
Acknowledgments
The work of the author is funded by grants R01MH083973, R01NS058780, R01NS061844, R21NS057538, and R21NS058780 from the National Institutes of Health (NIH).
ABBREVIATIONS
- Aβ
Amyloid-beta
- AAT
Adenosine Augmentation Therapy
- AD
Alzheimer’s Disease
- ADA
Adenosine Deaminase
- ADK
Adenosine Kinase
- ALS
Amyotrophic Lateral Sclerosis
- AMP
Adenosine-5′-monophosphate
- AR
Adenosine Receptor
- ATP
Adenosine-5′-triphosphate
- BDNF
Brain Derived Neurotrophic Factor
- CNS
Central Nervous System
- CPCA
5-(N-cyclopropyl)-carboxamido-adenosine
- CTP
Cytidine-5′-triphosphate
- DPMX
1,3-dipropyl-7-methyl-xanthine
- DR
Dopamine Receptor
- ESC
Embryonic Stem Cell
- GABA
Gamma Amino Butyric Acid
- GMP
Guanidine-5′-monophosphate
- HGPRT
Hypoxantine Guanine Phosphoribosyltransferase
- IMP
Inosine-5′-monophosphate
- KA
Kainic Acid
- KO
Knockout
- KW-6002
Istradefylline
- LTP
Long Term Potentiation
- MCAO
Middle Cerebral Artery Occlusion
- miRNA
micro-RNA
- MSC
Mesenchymal Stem Cell
- NFT
Neurofibrillary Tangle
- NMDAR
N-methyl-D-aspartate Receptor
- NP
Neural Precursor
- NREM
Non-Rapid Eye Movement
- PET
Positron Emission Tomography
- PD
Parkinson’s Disease
- PNP
Purine Nucleoside Phosphorylase
- PTZ
Pentylene Tetrazole
- REM
Rapid Eye Movement
- SOD-1
Cu/Zn Superoxide Dismutase 1
- SZ
Schizophrenia
- UP
Uridine Phosphorylase
- UTP
Uridine-5′-Triphosphate
- WT
Wild Type
- XO
Xanthine Oxidase
References
- 1.Inagaki A, Nakamura T, Wakisaka G. Studies on the mechanism of action of 1-beta-D-arabinofuranosylcytosine as an inhibitor of DNA synthesis in human leukemic leukocytes. Cancer Res. 1969;29(12):2169–2176. [PubMed] [Google Scholar]
- 2.Jarman M, Kuszmann J, Stock JA. Aminoacyl nucleosides derived from the tumour inhibitor, 1-aminocyclopentanecarboxylic acid. Biochem Pharmacol. 1969;18(10):2473–2484. doi: 10.1016/0006-2952(69)90363-3. [DOI] [PubMed] [Google Scholar]
- 3.Brink JJ, Lepage GA. 9-Beta-D-Arabinofuranosyladenine as an Inhibitor of Metabolism in Normal and Neoplastic Cells. Can J Biochem Physiol. 1965;43:1–15. doi: 10.1139/o65-001. [DOI] [PubMed] [Google Scholar]
- 4.King AE, Ackley MA, Cass CE, Young JD, Baldwin SA. Nucleoside transporters: from scavengers to novel therapeutic targets. Trends Pharmacol Sci. 2006;27(8):416–425. doi: 10.1016/j.tips.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Sebastiao AM, Ribeiro JA. Fine-tuning neuromodulation by adenosine. Trends Pharmacol Sci. 2000;21(9):341–346. doi: 10.1016/s0165-6147(00)01517-0. [DOI] [PubMed] [Google Scholar]
- 6.Boison D. Adenosine as a modulator of brain activity. Drug News Persp. 2007;20(10):607–611. doi: 10.1358/dnp.2007.20.10.1181353. [DOI] [PubMed] [Google Scholar]
- 7.Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawynok J. Adenosine and ATP receptors. Handb Exp Pharmacol. 2007;(177):309–328. doi: 10.1007/978-3-540-33823-9_11. [DOI] [PubMed] [Google Scholar]
- 9.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14(7):1315–1323. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 10.Dare E, Schulte G, Karovic O, Hammarberg C, Fredholm BB. Modulation of glial cell functions by adenosine receptors. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: Insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- 12.Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- 13.Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2007 doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- 14.Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem. 2007;101(5):1400–1413. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson T, Damian K, Lynas RE, Frenguelli BG. Sustained elevation of extracellular adenosine and activation of A(1) receptors underlie the post-ischaemic inhibition of neuronal function in rat hippocampus in vitro. Journal of Neurochemistry. 2006;97(5):1357–1368. doi: 10.1111/j.1471-4159.2006.03823.x. [DOI] [PubMed] [Google Scholar]
- 16.During MJ, Spencer DD. Adenosine: a potential mediator of seizure arrest and postictal refractoriness. Ann Neurol. 1992;32(5):618–624. doi: 10.1002/ana.410320504. [DOI] [PubMed] [Google Scholar]
- 17.Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferre S. Adenosine A(2A) receptors and basal ganglia physiology. Prog Neurobiol. 2007 doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boison D. Adenosine and epilepsy: from therapeutic rationale to new therapeutic strategies. Neuroscientist. 2005;11(1):25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- 19.Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005;26(10):511–516. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredholm BB, Hokfelt T, Milligan G. G-protein-coupled receptors: an update. Acta Physiol (Oxf) 2007;190(1):3–7. doi: 10.1111/j.1365-201X.2007.01689.x. [DOI] [PubMed] [Google Scholar]
- 21.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 22.Fredholm BB. Adenosine receptors as targets for drug development. Drug News Perspect. 2003;16(5):283–289. doi: 10.1358/dnp.2003.16.5.829316. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. Trends Pharmacol Sci. 2006;27(12):652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Wall MJ, Atterbury A, Dale N. Control of basal extracellular adenosine concentration in rat cerebellum. Journal of Physiology-London. 2007;582(1):137–151. doi: 10.1113/jphysiol.2007.132050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gundlfinger A, Bischofberger J, Johenning FW, Torvinen M, Schmitz D, Breustedt J. Adenosine modulates transmission at the hippocampal mossy fibre synapse via direct inhibition of presynaptic calcium channels. Journal of Physiology-London. 2007;582(1):263–277. doi: 10.1113/jphysiol.2007.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boison D. Adenosine-based cell therapy approaches for pharmacoresistant epilepsies. Neurodegener Dis. 2007;4(1):28–33. doi: 10.1159/000100356. [DOI] [PubMed] [Google Scholar]
- 28.Cunha RA. Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signaling. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva CG, Porciuncula LO, Canas PM, Oliveira CR, Cunha RA. Blockade of adenosine A(2A) receptors prevents staurosporine-induced apoptosis of rat hippocampal neurons. Neurobiol Dis. 2007 doi: 10.1016/j.nbd.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, Tinner-Staines B, Staines W, Rosin D, Terasmaa A, Popoli P, Leo G, Vergoni V, Lluis C, Ciruela F, Franco R, Ferre S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61(11 Suppl 6):S19–23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- 31.Fuxe K, Ferre S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 32.Fuxe K, Marcellino D, Genedani S, Agnati L. Adenosine A(2A) receptors, dopamine D(2) receptors and their interactions in Parkinson’s disease. Mov Disord. 2007 doi: 10.1002/mds.21440. [DOI] [PubMed] [Google Scholar]
- 33.Ferre S, Diamond I, Goldberg SR, Yao L, Hourani SM, Huang ZL, Urade Y, Kitchen I. Adenosine A(2A) receptors in ventral striatum, hypothalamus and nociceptive circuitry Implications for drug addiction, sleep and pain. Prog Neurobiol. 2007 doi: 10.1016/j.pneurobio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 35.Ciruela F, Casado V, Rodrigues RJ, Lujan R, Burgueno J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, Cortes A, Canela EI, Lopez-Gimenez JF, Milligan G, Lluis C, Cunha RA, Ferre S, Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J Neurosci. 2006;26(7):2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55(1):36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- 37.Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007;27(24):6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13(2):54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Studer FE, Fedele DE, Marowsky A, Schwerdel C, Wernli K, Vogt K, Fritschy JM, Boison D. Shift of adenosine kinase expression from neurons to astrocytes during postnatal development suggests dual functionality of the enzyme. Neuroscience. 2006;142:125–137. doi: 10.1016/j.neuroscience.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiological Reviews. 2006;86(3):1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 41.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310(5745):113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 42.Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5(1):63–84. doi: 10.2174/1389200043489162. [DOI] [PubMed] [Google Scholar]
- 43.Gray JH, Owen RP, Giacomini KM. The concentrative nucleoside transporter family, SLC28. Pflugers Arch. 2004;447(5):728–734. doi: 10.1007/s00424-003-1107-y. [DOI] [PubMed] [Google Scholar]
- 44.Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447(5):735–743. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt AP, Lara DR, Souza DO. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol Ther. 2007;116(3):401–416. doi: 10.1016/j.pharmthera.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Rathbone M, Pilutti L, Caciagli F, Jiang S. Neurotrophic effects of extracellular guanosine. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):666–672. doi: 10.1080/15257770802143913. [DOI] [PubMed] [Google Scholar]
- 47.Rathbone MP, Middlemiss PJ, DeLuca B, Jovetich M. Extracellular guanosine increases astrocyte cAMP: inhibition by adenosine A2 antagonists. Neuroreport. 1991;2(11):661–664. doi: 10.1097/00001756-199111000-00007. [DOI] [PubMed] [Google Scholar]
- 48.Frizzo ME, Antunes Soares FA, Dall’Onder LP, Lara DR, Swanson RA, Souza DO. Extracellular conversion of guanine-based purines to guanosine specifically enhances astrocyte glutamate uptake. Brain Res. 2003;972(1–2):84–89. doi: 10.1016/s0006-8993(03)02506-x. [DOI] [PubMed] [Google Scholar]
- 49.Frizzo ME, Lara DR, Dahm KC, Prokopiuk AS, Swanson RA, Souza DO. Activation of glutamate uptake by guanosine in primary astrocyte cultures. Neuroreport. 2001;12(4):879–881. doi: 10.1097/00001756-200103260-00051. [DOI] [PubMed] [Google Scholar]
- 50.Deutsch SI, Rosse RB, Long KD, Gaskins BL, Mastropaolo J. Guanosine possesses specific modulatory effects on NMDA receptor-mediated neurotransmission in intact mice. Eur Neuropsychopharmacol. 2008;18(4):299–302. doi: 10.1016/j.euroneuro.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Tavares RG, Schmidt AP, Tasca CI, Souza DO. Quinolinic acid-induced seizures stimulate glutamate uptake into synaptic vesicles from rat brain: effects prevented by guanine-based purines. Neurochem Res. 2008;33(1):97–102. doi: 10.1007/s11064-007-9421-y. [DOI] [PubMed] [Google Scholar]
- 52.Torres FV, da Silva Filho M, Antunes C, Kalinine E, Antoniolli E, Portela LV, Souza DO, Tort AB. Electrophysiological effects of guanosine and MK-801 in a quinolinic acid-induced seizure model. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 53.Moretto MB, Boff B, Lavinsky D, Netto CA, Rocha JB, Souza DO, Wofchuk ST. Importance of schedule of administration in the therapeutic efficacy of guanosine: early intervention after injury enhances glutamate uptake in model of hypoxia-ischemia. J Mol Neurosci. 2009;38(2):216–219. doi: 10.1007/s12031-008-9154-7. [DOI] [PubMed] [Google Scholar]
- 54.Vinade ER, Schmidt AP, Frizzo ME, Portela LV, Soares FA, Schwalm FD, Elisabetsky E, Izquierdo I, Souza DO. Effects of chronic administered guanosine on behavioral parameters and brain glutamate uptake in rats. J Neurosci Res. 2005;79(1–2):248–253. doi: 10.1002/jnr.20327. [DOI] [PubMed] [Google Scholar]
- 55.Cansev M. Uridine and cytidine in the brain: their transport and utilization. Brain Res Rev. 2006;52(2):389–397. doi: 10.1016/j.brainresrev.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Zamzow CR, Bose R, Parkinson FE. N-methyl-D-aspartate-evoked adenosine and inosine release from neurons requires extracellular calcium. Can J Physiol Pharmacol. 2009;87(10):850–858. doi: 10.1139/Y09-075. [DOI] [PubMed] [Google Scholar]
- 57.Zai L, Ferrari C, Subbaiah S, Havton LA, Coppola G, Strittmatter S, Irwin N, Geschwind D, Benowitz LI. Inosine alters gene expression and axonal projections in neurons contralateral to a cortical infarct and improves skilled use of the impaired limb. J Neurosci. 2009;29(25):8187–8197. doi: 10.1523/JNEUROSCI.0414-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Markowitz CE, Spitsin S, Zimmerman V, Jacobs D, Udupa JK, Hooper DC, Koprowski H. The treatment of multiple sclerosis with inosine. J Altern Complement Med. 2009;15(6):619–625. doi: 10.1089/acm.2008.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dunwiddie TV. Endogenously released adenosine regulates excitability in the in vitro hippocampus. Epilepsia. 1980;21(5):541–548. doi: 10.1111/j.1528-1157.1980.tb04305.x. [DOI] [PubMed] [Google Scholar]
- 60.Dragunow M, Goddard GV, Laverty R. Is adenosine an endogenous anticonvulsant? Epilepsia. 1985;26(5):480–487. doi: 10.1111/j.1528-1157.1985.tb05684.x. [DOI] [PubMed] [Google Scholar]
- 61.Lee KS, Schubert P, Heinemann U. The anticonvulsive action of adenosine: a postsynaptic, dendritic action by a possible endogenous anticonvulsant. Brain Res. 1984;321(1):160–164. doi: 10.1016/0006-8993(84)90694-2. [DOI] [PubMed] [Google Scholar]
- 62.Tian GF, Azmi H, Takano T, Xu QW, Peng WG, Lin J, Oberheim N, Lou NH, Wang XH, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nature Medicine. 2005;11(9):973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duffy S, MacVicar BA. Modulation of neuronal excitability by astrocytes. Adv Neurol. 1999;79:573–581. [PubMed] [Google Scholar]
- 64.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 65.Tashiro A, Goldberg J, Yuste R. Calcium oscillations in neocortical astrocytes under epileptiform conditions. J Neurobiol. 2002;50(1):45–55. doi: 10.1002/neu.10019. [DOI] [PubMed] [Google Scholar]
- 66.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22(5):208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 67.Fedele DE, Li T, Lan JQ, Fredholm BB, Boison D. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol. 2006;200(1):184–190. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- 68.Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RS, Homanics GE, Dixon CE, Schnermann J, Jackson EK. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:565–575. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- 69.Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239(4844):1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- 70.Represa A, Jorquera I, Le Gal La Salle G, Ben-Ari Y. Epilepsy induced collateral sprouting of hippocampal mossy fibers: does it induce the development of ectopic synapses with granule cell dendrites? Hippocampus. 1993;3(3):257–268. doi: 10.1002/hipo.450030303. [DOI] [PubMed] [Google Scholar]
- 71.Sloviter RS, Zappone CA, Harvey BD, Frotscher M. Kainic acid-induced recurrent mossy fiber innervation of dentate gyrus inhibitory interneurons: possible anatomical substrate of granule cell hyper-inhibition in chronically epileptic rats. J Comp Neurol. 2006;494(6):944–960. doi: 10.1002/cne.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kharatishvili I, Nissinen JP, McIntosh TK, Pitkanen A. A model of posttraumatic epilepsy induced by lateral fluid-percussion brain injury in rats. Neuroscience. 2006;140(2):685–697. doi: 10.1016/j.neuroscience.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 73.Pitkanen A, Kharatishvili I, Karhunen H, Lukasiuk K, Immonen R, Nairismagi J, Grohn O, Nissinen J. Epileptogenesis in experimental models. Epilepsia. 2007;48(Suppl 2):13–20. doi: 10.1111/j.1528-1167.2007.01063.x. [DOI] [PubMed] [Google Scholar]
- 74.Thom M, Sisodiya SM, Beckett A, Martinian L, Lin WR, Harkness W, Mitchell TN, Craig J, Duncan J, Scaravilli F. Cytoarchitectural abnormalities in hippocampal sclerosis. J Neuropathol Exp Neurol. 2002;61(6):510–519. doi: 10.1093/jnen/61.6.510. [DOI] [PubMed] [Google Scholar]
- 75.Khurgel M, Ivy GO. Astrocytes in kindling: relevance to epileptogenesis. Epilepsy Res. 1996;26(1):163–175. doi: 10.1016/s0920-1211(96)00051-4. [DOI] [PubMed] [Google Scholar]
- 76.Bauer J, Eiger CE, Hans VH, Schramm J, Urbach H, Lassmann H, Bien CG. Astrocytes are a specific immunological target in Rasmussen’s encephalitis. Annals of Neurology. 2007;62(1):67–80. doi: 10.1002/ana.21148. [DOI] [PubMed] [Google Scholar]
- 77.Diogenes MJ, Assaife-Lopes N, Pinto-Duarte A, Ribeiro JA, Sebastiao AM. Influence of age on BDNF modulation of hippocampal synaptic transmission: Interplay with adenosine A(2A) receptors. Hippocampus. 2007;17(7):577–585. doi: 10.1002/hipo.20294. [DOI] [PubMed] [Google Scholar]
- 78.Popoli P, Blum D, Martire A, Ledent C, Ceruti S, Abbracchio MP. Functions, dysfunctions and possible therapeutic relevance of adenosine A2A receptors in Huntington’s disease. Prog Neurobiol. 2007;81(5–6):331–348. doi: 10.1016/j.pneurobio.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 79.Hindley S, Herman MA, Rathbone MP. Stimulation of reactive astrogliosis in vivo by extracellular adenosine diphosphate or an adenosine A2 receptor agonist. J Neurosci Res. 1994;38(4):399–406. doi: 10.1002/jnr.490380405. [DOI] [PubMed] [Google Scholar]
- 80.Brambilla R, Cottini L, Fumagalli M, Ceruti S, Abbracchio MP. Blockade of A2A adenosine receptors prevents basic fibroblast growth factor-induced reactive astrogliosis in rat striatal primary astrocytes. Glia. 2003;43(2):190–194. doi: 10.1002/glia.10243. [DOI] [PubMed] [Google Scholar]
- 81.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 82.Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonca A, Cunha RA. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur J Neurosci. 2003;18(4):820–828. doi: 10.1046/j.1460-9568.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- 83.Rebola N, Porciuncula LO, Lopes LV, Oliveira CR, Soares-da-Silva P, Cunha RA. Long-term effect of convulsive behavior on the density of adenosine A1 and A 2A receptors in the rat cerebral cortex. Epilepsia. 2005;46(Suppl 5):159–165. doi: 10.1111/j.1528-1167.2005.01026.x. [DOI] [PubMed] [Google Scholar]
- 84.Ekonomou A, Sperk G, Kostopoulos G, Angelatou F. Reduction of A1 adenosine receptors in rat hippocampus after kainic acid-induced limbic seizures. Neurosci Lett. 2000;284(1–2):49–52. doi: 10.1016/s0304-3940(00)00954-x. [DOI] [PubMed] [Google Scholar]
- 85.Fedele DE, Gouder N, Güttinger M, Gabernet L, Scheurer L, Rulicke T, Crestani F, Boison D. Astrogliosis in epilepsy leads to overexpression of adenosine kinase resulting in seizure aggravation. Brain. 2005;128:2383–2395. doi: 10.1093/brain/awh555. [DOI] [PubMed] [Google Scholar]
- 86.Arrigoni E, Crocker AJ, Saper CB, Greene RW, Scammell TE. Deletion of presynaptic adenosine A1 receptors impairs the recovery of synaptic transmission after hypoxia. Neuroscience. 2005;132(3):575–580. doi: 10.1016/j.neuroscience.2004.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pugliese AM, Coppi E, Spalluto G, Corradetti R, Pedata F. A3 adenosine receptor antagonists delay irreversible synaptic failure caused by oxygen and glucose deprivation in the rat CA1 hippocampus in vitro. Br J Pharmacol. 2006;147(5):524–532. doi: 10.1038/sj.bjp.0706646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tomaselli B, Podhraski V, Heftberger V, Bock G, Baier-Bitterlich G. Purine nucleoside-mediated protection of chemical hypoxia-induced neuronal injuries involves p42/44 MAPK activation. Neurochem Int. 2005;46(7):513–521. doi: 10.1016/j.neuint.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 89.Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10(10):1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]
- 90.Aden U, Leverin AL, Hagberg H, Fredholm BB. Adenosine A(1) receptor agonism in the immature rat brain and heart. Eur J Pharmacol. 2001;426(3):185–192. doi: 10.1016/s0014-2999(01)01220-1. [DOI] [PubMed] [Google Scholar]
- 91.Back SA, Craig A, Luo NL, Ren J, Akundi RS, Ribeiro I, Rivkees SA. Protective effects of caffeine on chronic hypoxia-induced perinatal white matter injury. Annals of Neurology. 2006;60(6):696–705. doi: 10.1002/ana.21008. [DOI] [PubMed] [Google Scholar]
- 92.Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. Journal of Neurochemistry. 2007;101(5):1400–1413. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7(6):437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 94.Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: focus on ischemic preconditioning. Glia. 2005;50(4):307–320. doi: 10.1002/glia.20204. [DOI] [PubMed] [Google Scholar]
- 95.Pignataro G, Simon RP, Boison D. Transgenic overexpression of adenosine kinase aggravates cell death in ischemia. J Cereb Blood Flow Metab. 2007;27(1):1–5. doi: 10.1038/sj.jcbfm.9600334. [DOI] [PubMed] [Google Scholar]
- 96.Pignataro G, Studer FE, Wilz A, Simon RP, Boison D. Neuroprotection in ischemic mouse brain induced by stem cell-derived brain implants. J Cereb Blood Flow Metab. 2007;27(5):919–927. doi: 10.1038/sj.jcbfm.9600422. [DOI] [PubMed] [Google Scholar]
- 97.Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45(4):347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- 98.Benington JH. Sleep homeostasis and the function of sleep. Sleep. 2000;23(7):959–966. [PubMed] [Google Scholar]
- 99.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73(6):379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 100.Porkka-Heiskanen T, Kalinchuk A, Alanko L, Urrila A, Stenberg D. Adenosine, energy metabolism, and sleep. Scientific World Journal. 2003;3(8):790–798. doi: 10.1100/tsw.2003.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang ZL, Urade Y, Hayaishi O. Prostaglandins and adenosine in the regulation of sleep and wakefulness. Current Opinion in Pharmacology. 2007;7(1):33–38. doi: 10.1016/j.coph.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 102.Benington JH, Kodali SK, Heller HC. Stimulation of A1 adenosine receptors mimics the electroencephalographic effects of sleep deprivation. Brain Res. 1995;692(1–2):79–85. doi: 10.1016/0006-8993(95)00590-m. [DOI] [PubMed] [Google Scholar]
- 103.Basheer R, Arrigoni E, Thatte HS, Greene RW, Ambudkar IS, McCarley RW. Adenosine induces inositol 1,4,5-trisphosphate receptor-mediated mobilization of intracellular calcium stores in basal forebrain cholinergic neurons. J Neurosci. 2002;22(17):7680–7686. doi: 10.1523/JNEUROSCI.22-17-07680.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thakkar MM, Winston S, McCarley RW. A1 receptor and adenosinergic homeostatic regulation of sleep-wakefulness: effects of antisense to the A1 receptor in the cholinergic basal forebrain. J Neurosci. 2003;23(10):4278–4287. doi: 10.1523/JNEUROSCI.23-10-04278.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fredholm BB. Astra Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76(2):93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 106.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51(1):83–133. [PubMed] [Google Scholar]
- 107.Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8(7):858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 108.El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology. 2003;45(7):977–985. doi: 10.1016/s0028-3908(03)00254-5. [DOI] [PubMed] [Google Scholar]
- 109.Urade Y, Eguchi N, Qu WM, Sakata M, Huang ZL, Chen JF, Schwarzschild MA, Fink JS, Hayaishi O. Sleep regulation in adenosine A2A receptor-deficient mice. Neurology. 2003;61(11 Suppl 6):S94–96. doi: 10.1212/01.wnl.0000095222.41066.5e. [DOI] [PubMed] [Google Scholar]
- 110.Stenberg D, Litonius E, Halldner L, Johansson B, Fredholm BB, Porkka-Heiskanen T. Sleep and its homeostatic regulation in mice lacking the adenosine A1 receptor. J Sleep Res. 2003;12(4):283–290. doi: 10.1046/j.0962-1105.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 111.Sawynok J, Liu XJ. Adenosine in the spinal cord and periphery: release and regulation of pain. Prog Neurobiol. 2003;69(5):313–340. doi: 10.1016/s0301-0082(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 112.Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu X-J, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci USA. 2001;98(16):9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu WP, Hao JX, Halldner L, Lovdahl C, DeLander GE, Wiesenfeld-Hallin Z, Fredholm BB, Xu XJ. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain. 2005;113(3):395–404. doi: 10.1016/j.pain.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 114.Eisenach JC, Hood DD, Curry R. Preliminary efficacy assessment of intrathecal injection of an American formulation of adenosine in humans. Anesthesiology. 2002;96(1):29–34. doi: 10.1097/00000542-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 115.Lynch ME, Clark AJ, Sawynok J. Intravenous adenosine alleviates neuropathic pain: a double blind placebo controlled crossover trial using an enriched enrolment design. Pain. 2003;103(1–2):111–117. doi: 10.1016/s0304-3959(02)00419-0. [DOI] [PubMed] [Google Scholar]
- 116.Brooke RE, Deuchars J, Deuchars SA. Input-specific modulation of neurotransmitter release in the lateral horn of the spinal cord via adenosine receptors. J Neurosci. 2004;24(1):127–137. doi: 10.1523/JNEUROSCI.4591-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Godfrey L, Yan L, Clarke GD, Ledent C, Kitchen I, Hourani SM. Modulation of paracetamol antinociception by caffeine and by selective adenosine A2 receptor antagonists in mice. Eur J Pharmacol. 2006;531(1–3):80–86. doi: 10.1016/j.ejphar.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 118.Hussey MJ, Clarke GD, Ledent C, Hourani SM, Kitchen I. Reduced response to the formalin test and lowered spinal NMDA glutamate receptor binding in adenosine A2A receptor knockout mice. Pain. 2007;129(3):287–294. doi: 10.1016/j.pain.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 119.Jegou S, El Yacoubi M, Mounien L, Ledent C, Parmentier M, Costentin J, Vaugeois JM, Vaudry H. Adenosine A2A receptor gene disruption provokes marked changes in melanocortin content and pro-opiomelanocortin gene expression. J Neuroendocrinol. 2003;15(12):1171–1177. doi: 10.1111/j.1365-2826.2003.01116.x. [DOI] [PubMed] [Google Scholar]
- 120.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8(2):101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 121.Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426(6968):900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- 122.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 123.Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184(1–2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 124.Wisniewski HM, Wegiel J. Spatial relationships between astrocytes and classical plaque components. Neurobiol Aging. 1991;12(5):593–600. doi: 10.1016/0197-4580(91)90091-w. [DOI] [PubMed] [Google Scholar]
- 125.DeWitt DA, Perry G, Cohen M, Doller C, Silver J. Astrocytes regulate microglial phagocytosis of senile plaque cores of Alzheimer’s disease. Exp Neurol. 1998;149(2):329–340. doi: 10.1006/exnr.1997.6738. [DOI] [PubMed] [Google Scholar]
- 126.Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging. 2004;25(5):663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 127.Attems J, Jellinger KA. Hippocampal sclerosis in Alzheimer disease and other dementias. Neurology. 2006;66(5):775. doi: 10.1212/01.wnl.0000200959.50898.26. [DOI] [PubMed] [Google Scholar]
- 128.Sheng JG, Mrak RE, Griffin WS. S100 beta protein expression in Alzheimer disease: potential role in the pathogenesis of neuritic plaques. J Neurosci Res. 1994;39(4):398–404. doi: 10.1002/jnr.490390406. [DOI] [PubMed] [Google Scholar]
- 129.Van Eldik LJ, Griffin WS. S100 beta expression in Alzheimer’s disease: relation to neuropathology in brain regions. Biochim Biophys Acta. 1994;1223(3):398–403. doi: 10.1016/0167-4889(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 130.Moreira PI, Santos MS, Oliveira CR, Shenk JC, Nunomura A, Smith MA, Zhu X, Perry G. Alzheimer disease and the role of free radicals in the pathogenesis of the disease. CNS Neurol Disord Drug Targets. 2008;7(1):3–10. doi: 10.2174/187152708783885156. [DOI] [PubMed] [Google Scholar]
- 131.Nunomura A, Perry G, Pappolla MA, Friedland RP, Hirai K, Chiba S, Smith MA. Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. J Neuropathol Exp Neurol. 2000;59(11):1011–1017. doi: 10.1093/jnen/59.11.1011. [DOI] [PubMed] [Google Scholar]
- 132.Cagnin A, Brooks DJ, Kennedy AM, Gunn RN, Myers R, Turkheimer FE, Jones T, Banati RB. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358(9280):461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 133.Wharton SB, O’Callaghan JP, Savva GM, Nicoll JA, Matthews F, Simpson JE, Forster G, Shaw PJ, Brayne C, Ince PG. Population variation in glial fibrillary acidic protein levels in brain ageing: relationship to Alzheimer-type pathology and dementia. Dement Geriatr Cogn Disord. 2009;27(5):465–473. doi: 10.1159/000217729. [DOI] [PubMed] [Google Scholar]
- 134.Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, Van Leuven F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Haute CV, Checler F, Godaux E, Cordell B, Van Leuven F. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274(10):6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- 136.Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118(2):571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boison D. The adenosine kinase hypothesis of epileptogenesis. Progress in Neurobiology. 2008;84(3):249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Boison D. Astrogliosis and adenosine kinase: a glial basis of epilepsy. Future Neurology. 2008;3(3):221–224. [Google Scholar]
- 139.Li T, Lan JQ, Fredholm B, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology. 2007;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boison D. Adenosine, astrogliosis and seizures: a new perspective of epileptogenesis. Neuron Glia Biology. 2007;2:S9–S9. [Google Scholar]
- 141.Gouder N, Scheurer L, Fritschy J-M, Boison D. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci. 2004;24(3):692–701. doi: 10.1523/JNEUROSCI.4781-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yee BK, Singer P, Chen JF, Feldon J, Boison D. Transgenic overexpression of adenosine kinase in brain leads to multiple learning impairments and altered sensitivity to psychomimetic drugs. Eur J Neurosci. 2007;26:3237–3252. doi: 10.1111/j.1460-9568.2007.05897.x. [DOI] [PubMed] [Google Scholar]
- 143.Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A. 1991;88(16):7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Richardson PJ, Gubitz AK, Freeman TC, Dixon AK. Adenosine receptor antagonists and Parkinson’s disease: actions of the A2A receptor in the striatum. Adv Neurol. 1999;80:111–119. [PubMed] [Google Scholar]
- 145.Schwarzschild MA, Agnati L, Fuxe K, Chen JF, Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29(11):647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 146.Jenner P, Mori A, Hauser R, Morelli M, Fredholm BB, Chen JF. Adenosine, adenosine A(2A) antagonists, and Parkinson’s disease. Parkinsonism Relat Disord. 2009 doi: 10.1016/j.parkreldis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 147.Schwarzschild MA. Adenosine A2A antagonists as neurotherapeutics: crossing the bridge. Prog Neurobiol. 2007;83(5):261–262. doi: 10.1016/j.pneurobio.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR. Association of coffee and caffeine intake with the risk of Parkinson disease. Jama. 2000;283(20):2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 149.Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Ann Neurol. 2001;50(1):56–63. doi: 10.1002/ana.1052. [DOI] [PubMed] [Google Scholar]
- 150.Saaksjarvi K, Knekt P, Rissanen H, Laaksonen MA, Reunanen A, Mannisto S. Prospective study of coffee consumption and risk of Parkinson’s disease. Eur J Clin Nutr. 2008;62(7):908–915. doi: 10.1038/sj.ejcn.1602788. [DOI] [PubMed] [Google Scholar]
- 151.Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N, Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci. 2001;21(10):RC143. doi: 10.1523/JNEUROSCI.21-10-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ikeda K, Kurokawa M, Aoyama S, Kuwana Y. Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. J Neurochem. 2002;80(2):262–270. doi: 10.1046/j.0022-3042.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- 153.Kelly S, Zhang ZJ, Zhao H, Xu L, Giffard RG, Sapolsky RM, Yenari MA, Steinberg GK. Gene transfer of HSP72 protects cornu ammonis 1 region of the hippocampus neurons from global ischemia: influence of Bcl-2. Ann Neurol. 2002;52(2):160–167. doi: 10.1002/ana.10264. [DOI] [PubMed] [Google Scholar]
- 154.Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 155.Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85(1):94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 156.Clement AM, Nguyen MD, Roberts EA, Garcia ML, Boillee S, Rule M, McMahon AP, Doucette W, Siwek D, Ferrante RJ, Brown RH, Jr, Julien JP, Goldstein LS, Cleveland DW. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302(5642):113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 157.Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52(1):39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 158.Boillee S, Yamanaka K, Lobsiger CS, Copeland NG, Jenkins NA, Kassiotis G, Kollias G, Cleveland DW. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312(5778):1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 159.Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11(3):251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Staats KA, Van Den Bosch L. Astrocytes in amyotrophic lateral sclerosis: direct effects on motor neuron survival. J Biol Phys. 2009 doi: 10.1007/s10867-009-9141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rossi D, Volterra A. Astrocytic dysfunction: Insights on the role in neurodegeneration. Brain Res Bull. 2009 doi: 10.1016/j.brainresbull.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 162.Neusch C, Bahr M, Schneider-Gold C. Glia cells in amyotrophic lateral sclerosis: new clues to understanding an old disease? Muscle Nerve. 2007;35(6):712–724. doi: 10.1002/mus.20768. [DOI] [PubMed] [Google Scholar]
- 163.Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57(7):1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- 164.Feeney SJ, McKelvie PA, Austin L, Jean-Francois MJ, Kapsa R, Tombs SM, Byrne E. Presymptomatic motor neuron loss and reactive astrocytosis in the SOD1 mouse model of amyotrophic lateral sclerosis. Muscle Nerve. 2001;24(11):1510–1519. doi: 10.1002/mus.1176. [DOI] [PubMed] [Google Scholar]
- 165.Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23(3):249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 166.Kushner PD, Stephenson DT, Wright S. Reactive astrogliosis is widespread in the subcortical white matter of amyotrophic lateral sclerosis brain. J Neuropathol Exp Neurol. 1991;50(3):263–277. doi: 10.1097/00005072-199105000-00008. [DOI] [PubMed] [Google Scholar]
- 167.Nagy D, Kato T, Kushner PD. Reactive astrocytes are widespread in the cortical gray matter of amyotrophic lateral sclerosis. J Neurosci Res. 1994;38(3):336–347. doi: 10.1002/jnr.490380312. [DOI] [PubMed] [Google Scholar]
- 168.Schiffer D, Cordera S, Cavalla P, Migheli A. Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. J Neurol Sci. 1996;139(Suppl):27–33. doi: 10.1016/0022-510x(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 169.Schiffer D, Fiano V. Astrogliosis in ALS: possible interpretations according to pathogenetic hypotheses. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(1):22–25. doi: 10.1080/14660820310016822. [DOI] [PubMed] [Google Scholar]
- 170.Lepore AC, Dejea C, Carmen J, Rauck B, Kerr DA, Sofroniew MV, Maragakis NJ. Selective ablation of proliferating astrocytes does not affect disease outcome in either acute or chronic models of motor neuron degeneration. Exp Neurol. 2008;211(2):423–432. doi: 10.1016/j.expneurol.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10(4):515–531. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- 172.Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, Yanai K, Weinshenker D, Sumiyoshi T. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60(4):319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- 173.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 174.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, Weiss R, Cooper TB, Mann JJ, Van Heertum RL, Gorman JM, Laruelle M. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lindstrom LH, Gefvert O, Hagberg G, Lundberg T, Bergstrom M, Hartvig P, Langstrom B. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by L-(beta-11C) DOPA and PET. Biol Psychiatry. 1999;46(5):681–688. doi: 10.1016/s0006-3223(99)00109-2. [DOI] [PubMed] [Google Scholar]
- 176.Golembiowska K, Zylewska A. Agonists of A1 and A2A adenosine receptors attenuate methamphetamine-induced overflow of dopamine in rat striatum. Brain Res. 1998;806(2):202–209. doi: 10.1016/s0006-8993(98)00743-4. [DOI] [PubMed] [Google Scholar]
- 177.Popoli P, Pezzola A, de Carolis AS. Modulation of striatal adenosine A1 and A2 receptors induces rotational behaviour in response to dopaminergic stimulation in intact rats. Eur J Pharmacol. 1994;257(1–2):21–25. doi: 10.1016/0014-2999(94)90689-0. [DOI] [PubMed] [Google Scholar]
- 178.Solinas M, Ferre S, You ZB, Karcz-Kubicha M, Popoli P, Goldberg SR. Caffeine induces dopamine and glutamate release in the shell of the nucleus accumbens. J Neurosci. 2002;22(15):6321–6324. doi: 10.1523/JNEUROSCI.22-15-06321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ferre S, Herrera-Marschitz M, Grabowska-Anden M, Casas M, Ungerstedt U, Anden NE. Postsynaptic dopamine/adenosine interaction: II. Postsynaptic dopamine agonism and adenosine antagonism of methylxanthines in short-term reserpinized mice. Eur J Pharmacol. 1991;192(1):31–37. doi: 10.1016/0014-2999(91)90065-x. [DOI] [PubMed] [Google Scholar]
- 180.Ferre S, Herrera-Marschitz M, Grabowska-Anden M, Ungerstedt U, Casas M, Anden NE. Postsynaptic dopamine/adenosine interaction: I. Adenosine analogues inhibit dopamine D2-mediated behaviour in short-term reserpinized mice. Eur J Pharmacol. 1991;192(1):25–30. doi: 10.1016/0014-2999(91)90064-w. [DOI] [PubMed] [Google Scholar]
- 181.Ferre S, Popoli P, Gimenez-Llort L, Rimondini R, Muller CE, Stromberg I, Ogren SO, Fuxe K. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2001;7(3):235–241. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- 182.Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A. 1991;88(16):7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Farber NB. The NMDA receptor hypofunction model of psychosis. Ann N Y Acad Sci. 2003;1003:119–130. doi: 10.1196/annals.1300.008. [DOI] [PubMed] [Google Scholar]
- 184.Coyle JT, Tsai G. NMDA receptor function, neuroplasticity, and the pathophysiology of schizophrenia. Int Rev Neurobiol. 2004;59:491–515. doi: 10.1016/S0074-7742(04)59019-0. [DOI] [PubMed] [Google Scholar]
- 185.Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9(9):3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 187.Davis S, Butcher SP, Morris RG. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J Neurosci. 1992;12(1):21–34. doi: 10.1523/JNEUROSCI.12-01-00021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tonkiss J, Morris RG, Rawlins JN. Intra-ventricular infusion of the NMDA antagonist AP5 impairs performance on a non-spatial operant DRL task in the rat. Exp Brain Res. 1988;73(1):181–188. doi: 10.1007/BF00279671. [DOI] [PubMed] [Google Scholar]
- 189.Mohler H, Rudolph U, Boison D, Singer P, Feldon J, Yee BK. Regulation of cognition and symptoms of psychosis: Focus on GABAA receptors and glycine transporter 1. Pharmacology, Biochemistry and Behavior. 2008 doi: 10.1016/j.pbb.2008.03.003. in press. [DOI] [PubMed] [Google Scholar]
- 190.Singer P, Boison D, Mohler H, Feldon J, Yee BK. Enhanced recognition memory following glycine transporter 1 deletion in forebrain neurons. Behav Neurosci. 2007;121(5):815–825. doi: 10.1037/0735-7044.121.5.815. [DOI] [PubMed] [Google Scholar]
- 191.Yee BK, Balic E, Singer P, Schwerdel C, Grampp T, Gabernet L, Knuesel I, Benke D, Feldon J, Mohler H, Boison D. Disruption of glycine transporter 1 restricted to forebrain neurons is associated with a pro-cognitive and anti-psychotic phenotypic profile. J Neurosci. 2006;26(12):3169–3181. doi: 10.1523/JNEUROSCI.5120-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Singer P, Feldon J, Yee BK. Interference of glycine transporter 1: Modulation of cognitive functions via activation of glycine-B site of the NMDA receptor. CNS Agents in Medicinal Chemistry. 2007;7:259–268. [Google Scholar]
- 193.Kafka SH, Corbett R. Selective adenosine A2A receptor/dopamine D2 receptor interactions in animal models of schizophrenia. Eur J Pharmacol. 1996;295(2–3):147–154. doi: 10.1016/0014-2999(95)00668-0. [DOI] [PubMed] [Google Scholar]
- 194.Popoli P, Reggio R, Pezzola A, Fuxe K, Ferre S. Adenosine A1 and A2A receptor antagonists stimulate motor activity: evidence for an increased effectiveness in aged rats. Neurosci Lett. 1998;251(3):201–204. doi: 10.1016/s0304-3940(98)00533-3. [DOI] [PubMed] [Google Scholar]
- 195.Kurumaji A, Toru M. An increase in [3H] CGS21680 binding in the striatum of postmortem brains of chronic schizophrenics. Brain Res. 1998;808(2):320–323. doi: 10.1016/s0006-8993(98)00840-3. [DOI] [PubMed] [Google Scholar]
- 196.Lara DR, Dall’Igna OP, Ghisolfi ES, Brunstein MG. Involvement of adenosine in the neurobiology of schizophrenia and its therapeutic implications. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2006;30(4):617–629. doi: 10.1016/j.pnpbp.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 197.Lara DR, Souza DO. Schizophrenia: a purinergic hypothesis. Med Hypotheses. 2000;54(2):157–166. doi: 10.1054/mehy.1999.0003. [DOI] [PubMed] [Google Scholar]
- 198.Kull B, Svenningsson P, Fredholm BB. Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol Pharmacol. 2000;58(4):771–777. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- 199.Schwab SG, Hallmayer J, Albus M, Lerer B, Hanses C, Kanyas K, Segman R, Borrman M, Dreikorn B, Lichtermann D, Rietschel M, Trixler M, Maier W, Wildenauer DB. Further evidence for a susceptibility locus on chromosome 10p14-p11 in 72 families with schizophrenia by nonparametric linkage analysis. Am J Med Genet. 1998;81(4):302–307. [PubMed] [Google Scholar]
- 200.Schwab SG, Hallmayer J, Lerer B, Albus M, Borrmann M, Honig S, Strauss M, Segman R, Lichtermann D, Knapp M, Trixler M, Maier W, Wildenauer DB. Support for a chromosome 18p locus conferring susceptibility to functional psychoses in families with schizophrenia, by association and linkage analysis. Am J Hum Genet. 1998;63(4):1139–1152. doi: 10.1086/302046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lara DR, Brunstein MG, Ghisolfi ES, Lobato MI, Belmonte-de-Abreu P, Souza DO. Allopurinol augmentation for poorly responsive schizophrenia. Int Clin Psychopharmacol. 2001;16(4):235–237. doi: 10.1097/00004850-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 202.Brunstein MG, Ghisolfi ES, Ramos FL, Lara DR. Clinical trial for allopurinol adjuvant therapy for poorly responsive schizophrenia. Schizophr Res. 2004:S142. [Google Scholar]
- 203.Akhondzadeh S, Safarcherati A, Amini H. Beneficial antipsychotic effects of allopurinol as add-on therapy for schizophrenia: a double blind, randomized and placebo controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):253–259. doi: 10.1016/j.pnpbp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 204.Akhondzadeh S, Shasavand E, Jamilian H, Shabestari O, Kamalipour A. Dipyridamole in the treatment of schizophrenia: adenosine-dopamine receptor interactions. J Clin Pharm Ther. 2000;25(2):131–137. doi: 10.1046/j.1365-2710.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- 205.Hunsucker SA, Mitchell BS, Spychala J. The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol Ther. 2005;107(1):1–30. doi: 10.1016/j.pharmthera.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 206.Arch JR, Newsholme EA. Activities and some properties of 5′-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bontemps F, Van den Berghe G, Hers HG. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983;80(10):2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Newby AC, Worku Y, Holmquist CA. Adenosine formation. Evidence for a direct biochemical link with energy metabolism. Adv Myocardiol. 1985;6:273–284. [PubMed] [Google Scholar]
- 209.Vianna EP, Ferreira AT, Dona F, Cavalheiro EA, da Silva Fernandes MJ. Modulation of seizures and synaptic plasticity by adenosinergic receptors in an experimental model of temporal lobe epilepsy induced by pilocarpine in rats. Epilepsia. 2005;46(Suppl 5):166–173. doi: 10.1111/j.1528-1167.2005.01027.x. [DOI] [PubMed] [Google Scholar]
- 210.Koszalka P, Ozuyaman B, Huo Y, Zernecke A, Flogel U, Braun N, Buchheiser A, Decking UK, Smith ML, Sevigny J, Gear A, Weber AA, Molojavyi A, Ding Z, Weber C, Ley K, Zimmermann H, Godecke A, Schrader J. Targeted disruption of cd73/ecto-5′-nucleotidase alters thromboregulation and augments vascular inflammatory response. Circ Res. 2004;95(8):814–821. doi: 10.1161/01.RES.0000144796.82787.6f. [DOI] [PubMed] [Google Scholar]
- 211.Garvey EP, Lowen GT, Almond MR. Nucleotide and nucleoside analogues as inhibitors of cytosolic 5′-nucleotidase I from heart. Biochemistry. 1998;37(25):9043–9051. doi: 10.1021/bi980209d. [DOI] [PubMed] [Google Scholar]
- 212.Borowiec A, Lechward K, Tkacz-Stachowska K, Skladanowski AC. Adenosine as a metabolic regulator of tissue function: production of adenosine by cytoplasmic 5′-nucleotidases. Acta Biochim Pol. 2006;53(2):269–278. [PubMed] [Google Scholar]
- 213.Hunsucker SA, Spychala J, Mitchell BS. Human cytosolic 5′-nucleotidase I: characterization and role in nucleoside analog resistance. J Biol Chem. 2001;276(13):10498–10504. doi: 10.1074/jbc.M011218200. [DOI] [PubMed] [Google Scholar]
- 214.Bavaresco L, Bernardi A, Braganhol E, Cappellari AR, Rockenbach L, Farias PF, Wink MR, Delgado-Canedo A, Battastini AM. The role of ecto-5′-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem. 2008;319(1–2):61–68. doi: 10.1007/s11010-008-9877-3. [DOI] [PubMed] [Google Scholar]
- 215.Meijer P, Oyen WJ, Dekker D, van den Broek PH, Wouters CW, Boerman OC, Scheffer GJ, Smits P, Rongen GA. Rosuvastatin increases extracellular adenosine formation in humans in vivo: a new perspective on cardiovascular protection. Arterioscler Thromb Vasc Biol. 2009;29(6):963–968. doi: 10.1161/ATVBAHA.108.179622. [DOI] [PubMed] [Google Scholar]
- 216.Hershfield MS, Mitchell BS. Immunodeficiency diseases caused by adenosine deaminase deficiency and purine nucleoside phosphorylase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease. Vol. 1. McGraw-Hill Inc; New York: 1995. pp. 1725–1768. [Google Scholar]
- 217.Furman RR, Hoelzer D. Purine nucleoside phosphorylase inhibition as a novel therapeutic approach for B-cell lymphoid malignancies. Semin Oncol. 2007;34(6 Suppl 5):S29–34. doi: 10.1053/j.seminoncol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 218.Cui XA, Singh B, Park J, Gupta RS. Subcellular localization of adenosine kinase in mammalian cells: The long isoform of AdK is localized in the nucleus. Biochem Biophys Res Commun. 2009;388(1):46–50. doi: 10.1016/j.bbrc.2009.07.106. [DOI] [PubMed] [Google Scholar]
- 219.Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, Frenguelli B. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56(2):429–437. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Boison D. Adenosine dysfunction and adenosine kinase in epileptogenesis. The Open Neuroscience Journal. 2010 doi: 10.2174/1874082001004020093. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Pak MA, Haas HL, Decking UKM, Schrader J. Inhibition of adenosine kinase increases endogenous adenosine and depresses neuronal activity in hippocampal slices. Neuropharmacol. 1994;33:1049–1053. doi: 10.1016/0028-3908(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 222.Li T, Lan JQ, Boison D. Uncoupling of astrogliosis from epileptogenesis in adenosine kinase (ADK) transgenic mice. Neuron Glia Biology. 2008;4(2):91–99. doi: 10.1017/S1740925X09990135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li T, Lan JQ, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology. 2007;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Britton DR, Mikusa J, Lee CH, Jarvis MF, Williams M, Kowaluk EA. Site and event specific increase of striatal adenosine release by adenosine kinase inhibition in rats. Neurosci Lett. 1999;266(2):93–96. doi: 10.1016/s0304-3940(99)00280-3. [DOI] [PubMed] [Google Scholar]
- 225.Erion MD, Wiesner JB, Rosengren S, Ugarkar BG, Boyer SH, Tsuchiya M. Therapeutic potential of adenosine kinase inhibitors as analgesic agents. Drug Dev Res. 2000;50:S14–06. [Google Scholar]
- 226.Kowaluk EA, Jarvis MF. Therapeutic potential of adenosine kinase inhibitors. Expert Opin Investig Drugs. 2000;9(3):551–564. doi: 10.1517/13543784.9.3.551. [DOI] [PubMed] [Google Scholar]
- 227.Matulenko MA, Paight ES, Frey RR, Gomtsyan A, DiDomenico S, Jr, Jiang M, Lee CH, Stewart AO, Yu H, Kohlhaas KL, Alexander KM, McGaraughty S, Mikusa J, Marsh KC, Muchmore SW, Jakob CL, Kowaluk EA, Jarvis MF, Bhagwat SS. 4-amino-5-aryl-6-arylethynylpyrimidines: structure-activity relationships of non-nucleoside adenosine kinase inhibitors. Bioorg Med Chem. 2007;15(4):1586–1605. doi: 10.1016/j.bmc.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 228.McGaraughty S, Cowart M, Jarvis MF, Berman RF. Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Curr Top Med Chem. 2005;5(1):43–58. doi: 10.2174/1568026053386845. [DOI] [PubMed] [Google Scholar]
- 229.McGaraughty S, Cowart M, Jarvis MF. Recent developments in the discovery of novel adenosine kinase inhibitors: mechanism of action and therapeutic potential. CNS Drug Rev. 2001;7(4):415–432. doi: 10.1111/j.1527-3458.2001.tb00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gouder N, Fritschy JM, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia. 2003;44(7):877–885. doi: 10.1046/j.1528-1157.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- 231.Blackburn MR, Wakamiya M, Caskey CT, Kellems RE. Tissue-specific rescue suggests that placental adenosine deaminase is important for fetal development in mice. J Biol Chem. 1995;270(41):23891–23894. doi: 10.1074/jbc.270.41.23891. [DOI] [PubMed] [Google Scholar]
- 232.Gao X, Blackburn MR, Knudsen TB. Activation of apoptosis in early mouse embryos by 2′-deoxyadenosine exposure. Teratology. 1994;49(1):1–12. doi: 10.1002/tera.1420490103. [DOI] [PubMed] [Google Scholar]
- 233.Knudsen TB, Winters RS, Otey SK, Blackburn MR, Airhart MJ, Church JK, Skalko RG. Effects of (R)-deoxycoformycin (pentostatin) on intrauterine nucleoside catabolism and embryo viability in the pregnant mouse. Teratology. 1992;45(1):91–103. doi: 10.1002/tera.1420450109. [DOI] [PubMed] [Google Scholar]
- 234.Senba E, Daddona PE, Nagy JI. Transient expression of adenosine deaminase in facial and hypoglossal motoneurons of the rat during development. J Comp Neurol. 1987;255(2):217–230. doi: 10.1002/cne.902550206. [DOI] [PubMed] [Google Scholar]
- 235.Mohamedali KA, Guicherit OM, Kellems RE, Rudolph FB. The highest levels of purine catabolic enzymes in mice are present in the proximal small intestine. J Biol Chem. 1993;268(31):23728–23733. [PubMed] [Google Scholar]
- 236.Chinsky JM, Ramamurthy V, Fanslow WC, Ingolia DE, Blackburn MR, Shaffer KT, Higley HR, Trentin JJ, Rudolph FB, Knudsen TB, et al. Developmental expression of adenosine deaminase in the upper alimentary tract of mice. Differentiation. 1990;42(3):172–183. doi: 10.1111/j.1432-0436.1990.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 237.Geiger JD, Nagy JI. Distribution of adenosine deaminase activity in rat brain and spinal cord. J Neurosci. 1986;6(9):2707–2714. doi: 10.1523/JNEUROSCI.06-09-02707.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Geiger JD, Nagy JI. Ontogenesis of adenosine deaminase activity in rat brain. J Neurochem. 1987;48(1):147–153. doi: 10.1111/j.1471-4159.1987.tb13139.x. [DOI] [PubMed] [Google Scholar]
- 239.Robak T, Korycka A, Lech-Maranda E, Robak P. Current status of older and new purine nucleoside analogues in the treatment of lymphoproliferative diseases. Molecules. 2009;14(3):1183–1226. doi: 10.3390/molecules14031183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Robak T, Jamroziak K, Robak P. Current and emerging treatments for chronic lymphocytic leukaemia. Drugs. 2009;69(17):2415–2449. doi: 10.2165/11319270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 241.Lamanna N, Kay NE. Pentostatin treatment combinations in chronic lymphocytic leukemia. Clin Adv Hematol Oncol. 2009;7(6):386–392. [PubMed] [Google Scholar]
- 242.Kuno M, Seki N, Tsujimoto S, Nakanishi I, Kinoshita T, Nakamura K, Terasaka T, Nishio N, Sato A, Fujii T. Anti-inflammatory activity of non-nucleoside adenosine deaminase inhibitor FR234938. Eur J Pharmacol. 2006;534(1–3):241–249. doi: 10.1016/j.ejphar.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 243.Southam E, Stratton SC, Sargent RS, Brackenborough KT, Duffy C, Hagan RM, Pratt GD, Jones SA, Morgan PF. Broad spectrum anticonvulsant activity of BW534U87: possible role of an adenosine-dependent mechanism. Pharmacol Biochem Behav. 2002;74(1):111–118. doi: 10.1016/s0091-3057(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 244.Dupere JR, Dale TJ, Starkey SJ, Xie X. The anticonvulsant BW534U87 depresses epileptiform activity in rat hippocampal slices by an adenosine-dependent mechanism and through inhibition of voltage-gated Na+ channels. Br J Pharmacol. 1999;128(5):1011–1020. doi: 10.1038/sj.bjp.0702881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Lin Y, Phillis JW. Deoxycoformycin and oxypurinol: protection against focal ischemic brain injury in the rat. Brain Res. 1992;571(2):272–280. doi: 10.1016/0006-8993(92)90665-v. [DOI] [PubMed] [Google Scholar]
- 246.Sannomiya K, Honda H, Kubo K, Ii K, Yuan Y, Aoyagi E, Muguruma N, Shimizu I, Ito S. A histochemical and immunohistochemical investigation of guanase and nedasin in rat and human tissues. J Med Invest. 2006;53(3–4):246–254. doi: 10.2152/jmi.53.246. [DOI] [PubMed] [Google Scholar]
- 247.Miyamoto S, Ogawa H, Shiraki H, Nakagawa H. Guanine deaminase from rat brain. Purification, characteristics, and contribution to ammoniagenesis in the brain. J Biochem. 1982;91(1):167–176. doi: 10.1093/oxfordjournals.jbchem.a133673. [DOI] [PubMed] [Google Scholar]
- 248.Rajappan VP, Hosmane RS. Analogues of azepinomycin as inhibitors of guanase. Nucleosides Nucleotides. 1998;17(7):1141–1151. doi: 10.1080/07328319808004227. [DOI] [PubMed] [Google Scholar]
- 249.Ujjinamatada RK, Bhan A, Hosmane RS. Design of inhibitors against guanase: synthesis and biochemical evaluation of analogues of azepinomycin. Bioorg Med Chem Lett. 2006;16(21):5551–5554. doi: 10.1016/j.bmcl.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 250.Stover JF, Lowitzsch K, Kempski OS. Cerebrospinal fluid hypoxanthine, xanthine and uric acid levels may reflect glutamate-mediated excitotoxicity in different neurological diseases. Neuroscience Letters. 1997;238(1–2):25–28. doi: 10.1016/s0304-3940(97)00840-9. [DOI] [PubMed] [Google Scholar]
- 251.Ono T, Tsuruta R, Fujita M, Aki HS, Kutsuna S, Kawamura Y, Wakatsuki J, Aoki T, Kobayashi C, Kasaoka S, Maruyama I, Yuasa M, Maekawa T. Xanthine oxidase is one of the major sources of superoxide anion radicals in blood after reperfusion in rats with forebrain ischemia/reperfusion. Brain Res. 2009;1305:158–167. doi: 10.1016/j.brainres.2009.09.061. [DOI] [PubMed] [Google Scholar]
- 252.Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27(5):1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Schmidt AP, Bohmer AE, Antunes C, Schallenberger C, Porciuncula LO, Elisabetsky E, Lara DR, Souza DO. Anti-nociceptive properties of the xanthine oxidase inhibitor allopurinol in mice: role of A1 adenosine receptors. Br J Pharmacol. 2009;156(1):163–172. doi: 10.1111/j.1476-5381.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Connor M. Allopurinol for pain relief: more than just crystal clearance? Br J Pharmacol. 2009;156(1):4–6. doi: 10.1111/j.1476-5381.2008.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Dickerson FB, Stallings CR, Origoni AE, Sullens A, Khushalani S, Sandson N, Yolken RH. A double-blind trial of adjunctive allopurinol for schizophrenia. Schizophr Res. 2009;109(1–3):66–69. doi: 10.1016/j.schres.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 256.Muir SW, Harrow C, Dawson J, Lees KR, Weir CJ, Sattar N, Walters MR. Allopurinol use yields potentially beneficial effects on inflammatory indices in those with recent ischemic stroke: a randomized, double-blind, placebo-controlled trial. Stroke. 2008;39(12):3303–3307. doi: 10.1161/STROKEAHA.108.519793. [DOI] [PubMed] [Google Scholar]
- 257.Khan F, George J, Wong K, McSwiggan S, Struthers AD, Belch JJ. Allopurinol treatment reduces arterial wave reflection in stroke survivors. Cardiovasc Ther. 2008;26(4):247–252. doi: 10.1111/j.1755-5922.2008.00057.x. [DOI] [PubMed] [Google Scholar]
- 258.Guerreiro S, Ponceau A, Toulorge D, Martin E, Alvarez-Fischer D, Hirsch EC, Michel PP. Protection of midbrain dopaminergic neurons by the end-product of purine metabolism uric acid: potentiation by low-level depolarization. J Neurochem. 2009;109(4):1118–1128. doi: 10.1111/j.1471-4159.2009.06040.x. [DOI] [PubMed] [Google Scholar]
- 259.Lee KS, Alvarenga TA, Guindalini C, Andersen ML, Castro RM, Tufik S. Validation of commonly used reference genes for sleep-related gene expression studies. BMC Mol Biol. 2009;10:45. doi: 10.1186/1471-2199-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Meldgaard M, Fenger C, Lambertsen KL, Pedersen MD, Ladeby R, Finsen B. Validation of two reference genes for mRNA level studies of murine disease models in neurobiology. J Neurosci Methods. 2006;156(1–2):101–110. doi: 10.1016/j.jneumeth.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 261.Peco-Antic A, Smoljanic Z, Dimitrijevic N, Kostic M, Marsenic O, Djordjevic M, Kruscic D. The Lesch-Nyhan syndrome. Srp Arh Celok Lek. 2001;129(9–10):260–263. [PubMed] [Google Scholar]
- 262.Pellicer F, Buendia-Roldan I, Pallares-Trujillo VC. Self-mutilation in the Lesch-Nyhan syndrome: a corporal consciousness problem?--a new hypothesis. Med Hypotheses. 1998;50(1):43–47. doi: 10.1016/s0306-9877(98)90176-1. [DOI] [PubMed] [Google Scholar]
- 263.Baumeister AA, Frye GD. The biochemical basis of the behavioral disorder in the Lesch-Nyhan syndrome. Neurosci Biobehav Rev. 1985;9(2):169–178. doi: 10.1016/0149-7634(85)90043-0. [DOI] [PubMed] [Google Scholar]
- 264.Choi JW, Shin CY, Choi MS, Yoon SY, Ryu JH, Lee JC, Kim WK, El Kouni MH, Ko KH. Uridine protects cortical neurons from glucose deprivation-induced death: possible role of uridine phosphorylase. J Neurotrauma. 2008;25(6):695–707. doi: 10.1089/neu.2007.0409. [DOI] [PubMed] [Google Scholar]
- 265.el Kouni MH, Naguib FN, Park KS, Cha S, Darnowski JW, Soong SJ. Circadian rhythm of hepatic uridine phosphorylase activity and plasma concentration of uridine in mice. Biochem Pharmacol. 1990;40(11):2479–2485. doi: 10.1016/0006-2952(90)90089-4. [DOI] [PubMed] [Google Scholar]
- 266.Roosild TP, Castronovo S, Fabbiani M, Pizzorno G. Implications of the structure of human uridine phosphorylase 1 on the development of novel inhibitors for improving the therapeutic window of fluoropyrimidine chemotherapy. BMC Struct Biol. 2009;9:14. doi: 10.1186/1472-6807-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Liu M, Cao D, Russell R, Handschumacher RE, Pizzorno G. Expression, characterization, and detection of human uridine phosphorylase and identification of variant uridine phosphorolytic activity in selected human tumors. Cancer Res. 1998;58(23):5418–5424. [PubMed] [Google Scholar]
- 268.Pizzorno G, Yee L, Burtness BA, Marsh JC, Darnowski JW, Chu MY, Chu SH, Chu E, Leffert JJ, Handschumacher RE, Calabresi P. Phase I clinical and pharmacological studies of benzylacyclouridine, a uridine phosphorylase inhibitor. Clin Cancer Res. 1998;4(5):1165–1175. [PubMed] [Google Scholar]
- 269.Sebastiao AM, Ribeiro JA. Tuning and fine-tuning of synapses with adenosine. Curr Neuropharmacol. 2009;7(3):180–194. doi: 10.2174/157015909789152128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Boison D, Chen JF, Fredholm BB. Adenosine signalling and function in glial cells. Cell Death Differ. 2009 September 18; doi: 10.1038/cdd.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Boison D. Adenosine-based modulation of brain activity. Curr Neuropharmacol. 2009;7(3):158–159. doi: 10.2174/157015909789152173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Roseti C, Martinello K, Fucile S, Piccari V, Mascia A, Di Gennaro G, Quarato PP, Manfredi M, Esposito V, Cantore G, Arcella A, Simonato M, Fredholm BB, Limatola C, Miledi R, Eusebi F. Adenosine receptor antagonists alter the stability of human epileptic GABAA receptors. Proc Natl Acad Sci U S A. 2008;105(39):15118–15123. doi: 10.1073/pnas.0807277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cunha RA. Different cellular sources and different roles of adenosine: A(1) receptor-mediated inhibition through astrocytic-driven volume transmission and synapse-restricted A(2A) receptor-mediated facilitation of plasticity. Neurochemistry International. 2008;52(1–2):65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 274.Coelho JE, de Mendonca A, Ribeiro JA. Presynaptic inhibitory receptors mediate the depression of synaptic transmission upon hypoxia in rat hippocampal slices. Brain Res. 2000;869(1–2):158–165. doi: 10.1016/s0006-8993(00)02381-7. [DOI] [PubMed] [Google Scholar]
- 275.Masino SA, Mesches MH, Bickford PC, Dunwiddie TV. Acute peroxide treatment of rat hippocampal slices induces adenosine- mediated inhibition of excitatory transmission in area CA1. Neurosci Lett. 1999;274(2):91–94. doi: 10.1016/s0304-3940(99)00693-x. [DOI] [PubMed] [Google Scholar]
- 276.Li H, Henry JL. Adenosine action on interneurons and synaptic transmission onto interneurons in rat hippocampus in vitro. Eur J Pharmacol. 2000;407(3):237–244. doi: 10.1016/s0014-2999(00)00661-0. [DOI] [PubMed] [Google Scholar]
- 277.Dunwiddie TV, Miller KK. Effects of adenosine and cadmium on presynaptic fiber spikes in the CA1 region of rat hippocampus in vitro. Neuropharmacology. 1993;32(10):1061–1068. doi: 10.1016/0028-3908(93)90071-a. [DOI] [PubMed] [Google Scholar]
- 278.Alzheimer C, Sutor B, ten Bruggencate G. Disinhibition of hippocampal CA3 neurons induced by suppression of an adenosine A1 receptor-mediated inhibitory tonus: pre- and postsynaptic components. Neuroscience. 1993;57(3):565–575. doi: 10.1016/0306-4522(93)90006-2. [DOI] [PubMed] [Google Scholar]
- 279.Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb Exp Pharmacol. 2009;(193):535–587. doi: 10.1007/978-3-540-89615-9_17. [DOI] [PubMed] [Google Scholar]
- 280.Li W, Dai S, An J, Li P, Chen X, Xiong R, Liu P, Wang H, Zhao Y, Zhu M, Liu X, Zhu P, Chen JF, Zhou Y. Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience. 2008;151(4):1198–1207. doi: 10.1016/j.neuroscience.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 281.Togha M, Akhondzadeh S, Motamedi M, Ahmadi B, Razeghi S. Allopurinol as adjunctive therapy in intractable epilepsy: a double-blind and placebo-controlled trial. Arch Med Res. 2007;38(3):313–316. doi: 10.1016/j.arcmed.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 282.Roseti C, Palma E, Martinello K, Fucile S, Morace R, Esposito V, Cantore G, Arcella A, Giangaspero F, Aronica E, Mascia A, Di Gennaro G, Quarato PP, Manfredi M, Cristalli G, Lambertucci C, Marucci G, Volpini R, Limatola C, Eusebi F. Blockage of A2A and A3 adenosine receptors decreases the desensitization of human GABA(A) receptors microtransplanted to Xenopus oocytes. Proc Natl Acad Sci U S A. 2009;106(37):15927–15931. doi: 10.1073/pnas.0907324106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yu L, Shen HY, Coelho JE, Araujo IM, Huang QY, Day YJ, Rebola N, Canas PM, Rapp EK, Ferrara J, Taylor D, Muller CE, Linden J, Cunha RA, Chen JF. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol. 2008;63(3):338–346. doi: 10.1002/ana.21313. [DOI] [PubMed] [Google Scholar]
- 284.Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, Port RG, Sink KS, Bunce JG, Chrobak JJ, Salamone JD. Nucleus accumbens adenosine A(2A) receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. Journal of Neuroscience. 2008;28(36):9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Diogenes MJ, Fernandes CC, Sebastiao AM, Ribeiro JA. Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices. J Neurosci. 2004;24(12):2905–2913. doi: 10.1523/JNEUROSCI.4454-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Boyer SH, Ugarkar BG, Solbach J, Kopcho J, Matelich MC, Ollis K, Gomez-Galeno JE, Mendonca R, Tsuchiya M, Nagahisa A, Nakane M, Wiesner JB, Erion MD. Adenosine kinase inhibitors. 5. Synthesis, enzyme inhibition, and analgesic activity of diaryl-erythro-furanosyltubercidin analogues. J Med Chem. 2005;48(20):6430–6441. doi: 10.1021/jm0503650. [DOI] [PubMed] [Google Scholar]
- 287.Gomtsyan A, Didomenico S, Lee CH, Stewart AO, Bhagwat SS, Kowaluk EA, Jarvis MF. Synthesis and biological evaluation of pteridine and pyrazolopyrimidine based adenosine kinase inhibitors. Bioorg Med Chem Lett. 2004;14(16):4165–4168. doi: 10.1016/j.bmcl.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 288.Jarvis MF, Mikusa J, Chu KL, Wismer CT, Honore P, Kowaluk EA, McGaraughty S. Comparison of the ability of adenosine kinase inhibitors and adenosine receptor agonists to attenuate thermal hyperalgesia and reduce motor performance in rats. Pharmacol Biochem Behav. 2002;73(3):573–581. doi: 10.1016/s0091-3057(02)00840-7. [DOI] [PubMed] [Google Scholar]
- 289.Suzuki R, Stanfa LC, Kowaluk EA, Williams M, Jarvis MF, Dickenson AH. The effect of ABT-702, a novel adenosine kinase inhibitor, on the responses of spinal neurones following carrageenan inflammation and peripheral nerve injury. Br J Pharmacol. 2001;132(7):1615–1623. doi: 10.1038/sj.bjp.0703972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Ugarkar BG, DaRe JM, Kopcho JJ, Browne CE, 3rd, Schanzer JM, Wiesner JB, Erion MD. Adenosine kinase inhibitors. 1. Synthesis, enzyme inhibition, and antiseizure activity of 5-iodotubercidin analogues. J Med Chem. 2000;43(15):2883–2893. doi: 10.1021/jm000024g. [DOI] [PubMed] [Google Scholar]
- 291.Ugarkar BG, Castellino AJ, DaRe JM, Kopcho JJ, Wiesner JB, Schanzer JM, Erion MD. Adenosine kinase inhibitors. 2. Synthesis, enzyme inhibition, and antiseizure activity of diaryltubercidin analogues. J Med Chem. 2000;43(15):2894–2905. doi: 10.1021/jm0000259. [DOI] [PubMed] [Google Scholar]
- 292.Kowaluk EA, Mikusa J, Wismer CT, Zhu CZ, Schweitzer E, Lynch JJ, Lee CH, Jiang M, Bhagwat SS, Gomtsyan A, McKie J, Cox BF, Polakowski J, Reinhart G, Williams M, Jarvis MF. ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholino-pyridin-3-yl)pyrido[2,3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties. II. In vivo characterization in the rat. J Pharmacol Exp Ther. 2000;295(3):1165–1174. [PubMed] [Google Scholar]
- 293.Jarvis MF, Yu H, Kohlhaas K, Alexander K, Lee CH, Jiang M, Bhagwat SS, Williams M, Kowaluk EA. ABT-702 (4-amino-5-(3-bromophenyl)-7-(6-morpholinopyridin-3-yl)pyrido[2, 3-d]pyrimidine), a novel orally effective adenosine kinase inhibitor with analgesic and anti-inflammatory properties: I. In vitro characterization and acute antinociceptive effects in the mouse. J Pharmacol Exp Ther. 2000;295(3):1156–1164. [PubMed] [Google Scholar]
- 294.Wiesner JB, Ugarkar BG, Castellino AJ, Barankiewicz J, Dumas DP, Gruber HE, Foster AC, Erion MD. Adenosine kinase inhibitors as a novel approach to anticonvulsant therapy. J Pharmacol Exp Ther. 1999;289(3):1669–1677. [PubMed] [Google Scholar]
- 295.Shen HY, Chen JF. Adenosine A2A receptors in psychopharmacology: Modulators of bahavior, mood, and cognition. Curr Neuropharmacol. 2009;7(3):195–206. doi: 10.2174/157015909789152191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Boison D, Scheurer L, Zumsteg V, Rülicke T, Litynski P, Fowler B, Brandner S, Mohler H. Neonatal hepatic steatosis by disruption of the adenosine kinase gene. Proc Natl Acad Sci USA. 2002;99(10):6985–6990. doi: 10.1073/pnas.092642899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mato JM, Martinez-Chantar ML, Lu SC. Methionine metabolism and liver disease. Annual Review of Nutrition. 2008;28:273–293. doi: 10.1146/annurev.nutr.28.061807.155438. [DOI] [PubMed] [Google Scholar]
- 298.Boison D, Stewart K-A. Therapeutic epilepsy research: from pharmacological rationale to focal adenosine augmentation. Biochem Pharmacol. 2009;78(12):1428–1437. doi: 10.1016/j.bcp.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Boison D. Adenosine augmentation therapies (AATs) for epilepsy: prospect of cell and gene therapies. Epilepsy Res. 2009;85(2):131–141. doi: 10.1016/j.eplepsyres.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Boison D. Engineered adenosine-releasing cells for epilepsy therapy: human mesenchymal stem cells and human embryonic stem cells. Neurotherapeutics. 2009;6(2):278–283. doi: 10.1016/j.nurt.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, Kirker-Head C, Kaplan DL. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29(24–25):3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Wang XQ, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29(8):1054–1064. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wang X, Zhang X, Castellot J, Herman I, Iafrati M, Kaplan DL. Controlled release from multilayer silk biomaterial coatings to modulate vascular cell responses. Biomaterials. 2008;29(7):894–903. doi: 10.1016/j.biomaterials.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials. 2008;29(26):3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Szybala C, Pritchard EM, Wilz A, Kaplan DL, Boison D. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Exp Neurol. 2009;219:126–135. doi: 10.1016/j.expneurol.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Fedele DE, Koch P, Brüstle O, Scheurer L, Simpson EM, Mohler H, Boison D. Engineering embryonic stem cell derived glia for adenosine delivery. Neurosci Lett. 2004;370(2–3):160–165. doi: 10.1016/j.neulet.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 307.Okabe S, Forsberg-Nilsson K, Spiro AC, Segal M, McKay RD. Development of neuronal precursor cells and functional postmitotic neurons from embryonic stem cells in vitro. Mech Dev. 1996;59(1):89–102. doi: 10.1016/0925-4773(96)00572-2. [DOI] [PubMed] [Google Scholar]
- 308.Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A, Segschneider M, Simon RP, Brustle O, Boison D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007;130(Pt 5):1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- 309.Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp Neurol. 2007;208:26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Li T, Ren G, Kaplan DL, Boison D. Human mesenchymal stem cell grafts engineered to release adenosine reduce chronic seizures in a mouse model of CA3-selective epileptogenesis. Epilepsy Res. 2009;84:238–241. doi: 10.1016/j.eplepsyres.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]