Abstract
Our previous studies have found that activation of Wnt/β-Catenin signaling resulted in mouse prostatic intraepithelial neoplasia (mPIN). In the large probasin promoter directed SV40-Large T-antigen (LPB-Tag) expressing mouse prostate, mPIN forms with rare areas of adenocarcinoma. Combining expression of both Wnt-signaling and Tag expression in the mouse prostate, we have studied the role of Wnt/β-Catenin signaling in the progression from mPIN to adenocarcinoma. Our results show that the prostates of mice expressing Tag alone or nuclear β-Catenin alone developed mPIN while the activation of both Tag and the Wnt/β-Catenin pathway resulted in invasive prostate adenocarcinoma. Also, Foxa2, a forkhead transcription factor, was induced by active Wnt/β-Catenin signaling; and the expression of Foxa2 was associated with the invasive phenotype in the primary prostate cancer. In the LPB-Tag/dominant active (D.A.) β-Catenin prostates, MMP7, a Wnt/β-Catenin target gene, was up-regulated. Furthermore, we also assessed AR and AR signaling pathway in these LPB-Tag/D.A. β-Catenin mice. Although β-Catenin is a well known AR co-activator in vitro, our study provides strong in vivo evidences indicating that both AR protein and the AR pathway were down-regulated in the prostate of LPB-Tag/D.A. β-Catenin mice. Histological analysis shows that prostate sections derived from the LPB-Tag/D.A. β-Catenin mice display neuroendocrine differentiation (NED) but NE cancer does not develop. Together, our findings indicate that Wnt/β-Catenin signaling plays an important role in the progression of mPIN to prostate adenocarcinoma.
Keywords: Prostate, Wnt/β-Catenin, AR, Foxa2, T-antigen
Introduction
Several genes and signaling pathways have been implicated in prostate cancer (PCa) initiation and progression, such as p53, cMyc, Nkx3.1, Pten, androgen receptor (AR), and Wnt/β-Catenin (Kasper, 2005). Mouse models established by manipulating these genes have been used to study molecular events important for PCa initiation and progression. The SV40 early region (expresses the Large T and small t-antigen) has been widely used to establish mouse models for human cancers. Studies have shown that the SV40 large T-antigen interacts with p53 and Rb to inactivate these genes (Levine and Momand, 1990), thus altering cell cycle progression to contribute to the rapid cell proliferation. A recent study showed that transgenic mouse models of breast, prostate, and lung cancer that express the SV40 early region have a gene signature highly predictive for cancer prognosis (Deeb et al., 2007), re-enforcing the importance of these mouse models in cancer research. The large probasin (LPB) promoter drives prostate specific expression of the SV40 large T-antigen (Tag) in transgenic mice. The LPB-Tag lines use a deletion construct of the SV40 early region that removes expression of the small t-antigen. These lines develop high grade prostatic intraepithelial neoplasia (HGPIN) (Kasper et al., 1998). Among the six LPB-Tag founder lines that developed HGPIN in the prostate (not including the 12T-10 line that developed small cell carcinoma), the 12T-7 line is the best characterized (Kasper et al., 1998; Masumori et al., 2001). As a result of a copy number difference on multiple chromosomes, the 12T-7 line diverged into 12T-7 fast (f) and 12T-7 slow (s). The 12T-7f line of mice received additional copies of the Tag gene from the founder than the 12T-7s line. As a result, HGPIN develops faster in the 12T-7f line than in 12T-7s, and death occurs at 17–20 weeks for 12T-7f, and 20–22 weeks for 12T-7s.
Wnt/β-Catenin signaling has been implicated in both normal prostate development and in PCa progression (Yu et al., 2009). Wnts are a family of secreted glycoproteins consisting of 19 members in mammals (Miller, 2002). In the canonical Wnt pathway, nuclear β-Catenin mediates Wnt-signaling. Wnt-signaling prevents β-Catenin degradation and results in cytoplasmic/nuclear accumulation of β-Catenin (Miller, 2002). Once β-Catenin enters the nucleus, it acts as a transcription co-activator and activates TCF target genes such as c-Myc, cyclin D1, MMP7, uPA and AR target genes (Miller, 2002; Terry et al., 2006). Studies have reported that increased nuclear β-Catenin is associated with advanced stage PCa (Chesire et al., 2002; de la et al., 2003; Yardy and Brewster, 2005). As a correlate, previous studies have shown that WIF1 (a Wnt inhibitor) is often down regulated in PCa (Wissmann et al., 2003). While mutations in exon 3 of the β-Catenin gene that result in the constitutive activation of the Wnt pathway are reported in only 5% of primary PCa (Chesire et al., 2000), several studies indicate Wnt/β-Catenin signaling can be activated in PCa via a number of additional mechanisms, such as cross-talk with the PTEN/Akt, COX-2/PGE2, PDGF, and NF-κB pathways (Castellone et al., 2005; Lamberti et al., 2001; Persad et al., 2001). Reactive stroma is associated with PCa in the tumor microenvironment, and there is increasing evidence indicating that Wnt/β-Catenin can also be activated by growth factors and inflammatory factors secreted by fibroblasts and macrophages from the tumor microenvironment (Huang and Du, 2008). In summary, a variety of mechanisms activate Wnt-signaling, thus contributing to β-Catenin driven PCa progression in vivo.
We have reported that the activation of Wnt/β-Catenin in the prostate results in HGPIN (Yu et al., 2009). To study the role of Wnt/β-Catenin signaling in PCa progression, a non-degradable β-Catenin gene was expressed in mouse prostate in the presence of SV40 large T-antigen. The results showed that the prostate-specific activation of both LPB-Tag and Wnt/β-Catenin pathways resulted in the development of invasive adenocarcinoma, while the prostates of mice expressing LPB-Tag alone or β-Catenin alone developed HGPIN, which is consistent with our previous report (Yu et al., 2009). In the LPB-Tag/D.A. β-Catenin mouse prostates, MMP7 expression levels were elevated; and Foxa2, a forkhead transcription factor that is induced by active Wnt/β-Catenin signaling (Yu et al., 2009), is expressed in the invasive PCa cells. The association of Foxa2 and active Wnt-signaling with PCa invasion suggests that activation of these pathways endows PCa cells with invasive ability, a hallmark of adenocarcinoma formation in the large T-antigen and the Wnt/β-Catenin bigenic mice. Furthermore, we also assessed androgen receptor (AR) expression levels and AR signaling in our bigenic mice. Although β-Catenin is a well known AR co-activator in vitro, our present study provides strong in vivo evidence indicating that both AR protein levels and the AR pathway are in fact, down-regulated in the prostates of LPB-Tag/D.A. β-Catenin mice. Finally, there was histological evidence of neuroendocrine differentiation in the LPB-Tag/D.A. β-Catenin mouse prostates. Although human prostate neuroendocrine cancer is rare, NED is common in advanced prostate adenocarcinoma, and the presence of NED correlates with poor prognosis. Here we show that the activation of Wnt/β-Catenin can account for the increased NED reported in advanced prostate cancer. Together, our findings indicate that Wnt/β-Catenin signaling plays an important role in the progression of PIN to prostate adenocarcinoma and the appearance of NED in advanced stage disease.
Results
Wnt/β-Catenin signaling promotes tumor progression in the prostates of LPB-Tag mice
Previously, our laboratory showed that the use of a large fragment of the PB promoter to drive the prostate-specific expression of the large T antigen resulted in reproducible pathological alterations and PIN (Kasper et al., 1998). To study whether expression of stabilized β-Catenin (active Wnt/β-Catenin signaling) promotes tumor progression, we developed 12T-7s/Catnblox(ex3)/PBCre4 (designated as LPB-Tag/D.A. β-Catenin) mice where Wnt/β-Catenin signaling was activated in mouse prostate in the presence of large T-antigen. The D.A. β-Catenin transgenic mouse contains a deletion of exon 3 in β-Catenin through the expression of probasin driven Cre, resulting in the blockage of β-Catenin degradation and subsequent accumulation of β-Catenin in the cytoplasm/nucleus in the prostate. Therefore, the LPB-Tag/D.A. β-Catenin mice have a compound, prostate-specific activation of both large T-antigen and nuclear β-Catenin.
All the mice used in this study were sacrificed at 18–20 weeks of age. There are at least 7 mice for each genotype. H&E staining of prostate tissue from both LPB-Tag mice and D.A. β-Catenin mice showed epithelial cell expansion and the presence of focally filled prostatic lumens, but prostate epithelial cells in these mice are still confined within glands, indicating the presence of PIN and HGPIN, but not adenocarcinoma in the LPB-Tag or D.A. β-Catenin mouse prostates (Fig. 1D-I). In the LPB-Tag/D.A. β-Catenin mice, the VP (Fig. 1L) displayed a more severe phenotype than the DLP (Fig. 1K) or AP (Fig. 1J). The VP, derived from LPB-Tag/D.A. β-Catenin mice showed epithelial cell invasion into surrounding stroma (Fig. 1L), which was not seen in age matched LPB-Tag (Fig. 1D-F) or D.A. β-Catenin mice (Fig. 1G-I). The invasive cells in LPB-Tag/D.A. β-Catenin mouse prostates were epithelial in origin as they expressed β-Catenin, Foxa2, and pan-cytokeratin (Fig. 2A-D). It is noteworthy that the invading edge and cells scattered among surrounding stroma in LPB-Tag/D.A β-Catenin mouse prostate were positive for Foxa2 (Fig. 2D and 2E), which was induced by active Wnt/β-Catenin signaling, suggesting that Foxa2 plays a role in the focal invasion of PCa. Together, the histology showed that LPB-Tag/D.A. β-Catenin mouse prostates developed an invasive phenotype, a hallmark of adenocarcinoma.
Figure 1. Compound activation of SV40 large T-antigen and β-Catenin causes prostate adenocarcinoma.
H&E stainings were performed on prostate sections derived from 20 weeks old LPB-Tag/D.A. β-Catenin mice and age matched wild type (WT), LPB-Tag, and D.A. β-Catenin mice. AP: anterior prostate; DLP: dorsolateral prostate; VP: ventral prostate. A-C are prostate specimens derived from wild type (WT) mouse; D-F are derived from LPB-Tag mouse; G-I are derived from D.A. β-Catenin mouse; J-L are derived from LPB-Tag/D.A. β-Catenin mouse. The histology showed that the LPB-Tag mice or D.A. β-Catenin mice developed PIN to HGPIN, but not prostate carcinoma since the growing epithelial cells were still confined within the gland (D-I); whereas, the LPB-Tag/D.A. β-Catenin mice developed HGPIN (J) and prostate carcinoma (K and L). Scale bar represents 100 µm.
Figure 2. Activation of Wnt/β-Catenin signaling promotes PCa progression and the expression of Foxa2 is associated with the invasive phenotype.
A-D: H&E staining and immunostainings performed on serial sections derived from LPB-Tag/D.A. β-Catenin mouse prostate. A: H&E staining shows the presence of prostate adenocarcinoma. B-D: immunostainings against wide spectrum cytokeratin (panCK), β-Catenin (beta-Catenin) and Foxa2. Cells scattered among the surrounding stromas are positive for pan-cytokeratin, β-Catenin, and Foxa2, confirming an epithelial origin. E: Foxa2 staining performed on section derived from a LPB-Tag/D.A. β-Catenin mouse prostate showing the histology of adenocarcinoma featured by the appearance of Foxa2 positive, invasive prostate epithelial cells in surrounding stroma. Arrows indicate Foxa2 positive cells at the invading edge or cells scattered among stromas.
Activation of Wnt/β-Catenin down-regulates AR and AR signaling pathway
β-Catenin is reported as a co-activator of AR (Terry et al., 2006). To study the cross-talk of active Wnt/β-Catenin signaling with the AR pathway, the expression AR and T-antigen in the LPB-Tag/D.A. β-Catenin mouse and control mouse prostates was assessed by immunostaining and by western blotting analysis. In agreement with our previous report (Yu et al., 2009), AR protein levels were slightly decreased in D.A. β-Catenin mouse prostates (Fig. 3H) when compared with wild type (Fig. 3B), or with the LPB-Tag mouse prostates (Fig. 3E). However, a further decrease in AR protein levels was observed in prostates derived from LPB-Tag/D.A. β-Catenin mice (Fig. 3K, 3N & Fig. 4B) while AR mRNA levels increased (Fig 4C). Western blotting analysis (Figs. 4A) also shows that the endogenous β-catenin is degraded while the exon3 deletion in β-catenin results in accumulation of the non-degradable mutated β-catenin. In addition, large T-antigen protein levels were reduced in LPB-Tag/D.A. β-catenin prostate tissue (Figs. 3M and Fig. 4D), and displayed a mutually exclusive expression pattern with Foxa2 (compare * labeled area in Fig. 3L with that in Fig. 3M, and Fig. 3O-R). Since the large T-antigen transgene expression is driven by the androgen-responsive probasin promoter, decreased large T-antigen expression potentially results from the decreased levels of AR protein. Thus, both AR levels and the AR signaling pathway are down-regulated in the prostates of LPB-Tag/D.A. β-Catenin mice.
Figure 3. Activation of Wnt/β-Catenin reduces the expression of AR and AR regulated gene.
Immunostainings against β-Catenin, AR, Fox2, and large T antigen (Tag) were performed on prostate specimens derived from wile type (WT, A-C), LPB-Tag (D-F), D.A. β-Catenin (G-I), and LPB-Tag/D.A. β-Catenin (J-N) mice. The WT prostate and the LPB-Tag prostate displayed membrane β-Catenin staining (A and D); the D.A. β-Catenin prostate showed cytoplasmic/nuclear accumulation of β-Catenin (G); the LPB-Tag prostate highly expressed Tag (F); the LPB-Tag/D.A. β-Catenin mice showed cytoplasmic/nuclear β-Catenin (J), and expressed Tag at some areas (arrow in panel M). * in panel L and panel M indicate the cells that are expressing Foxa2, but losing T-antigen. N is higher magnification picture of the boxed area from panel K to show that some cells lost AR and some have reduced level of AR expression. O-R, Dual immunofluorescence staining of Foxa2 (in green) and large T-antigen (in red) was performed on prostate sections from a LPB-Tag/D.A. β-Catenin mouse. O: low magnification; P-R: high magnification of the boxed areas from panel O. DAPI was used for counterstaining. The expression of Foxa2 was not co-localized with large T-antigen; instead, the expression pattern of Foxa2 and large T-antigen was exclusive with each other. Scale bar represents 25 µm.
Figure 4. Quantitative RT-PCR and western blot to analyze AR and T-antigen level.
A, western blot of AR. B, quantitation of the western blotting data. Protein lysates were prepared from prostates of the following mice: wild type (WT), LPB-Tag (Tag), D.A. β-Catenin (b-Cat), and LPB-Tag/D.A. β-Catenin (Tag/b-Cat). The exon 3 deleted β-Catenin was indicated by D.A. β-Catenin. With the accumulation of non-degradable β-Catenin, endogenous β-Catenin (endo. b-Catenin) levels were decreased in both D.A. β-Catenin and LPB-Tag/D.A. β-Catenin mouse prostates. AR levels were only slightly decreased in D.A. β-Catenin mouse prostates, but significantly decreased in LPB-Tag/D.A. β-Catenin mouse prostates. C, qRT-PCR to assess AR mRNA level. In contrast to the western blotting results that indicate AR protein was reduced in D.A. β-Catenin and LPB-Tag/D.A. β-Catenin mouse prostates, AR mRNA level was increased in these mouse prostates. D, western blot of T-antigen. T-antigen was not expressed in prostate of wild type (WT) or D.A. β-Catenin (b-Cat) mouse; it was highly expressed in prostate of LPB-Tag mouse (Tag), but decreased in prostate of LPB-Tag/D.A. β-Catenin mouse (Tag/b-Cat).
MMP7 is elevated in mouse prostates following activation of nuclear β-Catenin
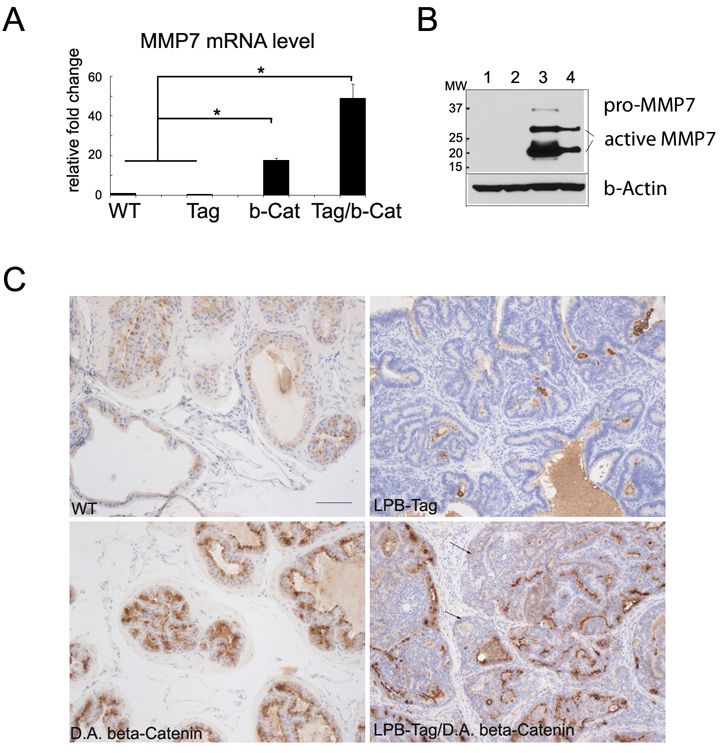
LPB-Tag/D.A. β-Catenin mice developed invasion, a hallmark of adenocarcinoma. To determine the mechanism(s) to explain the more aggressive phenotype that we observed in the LPB-Tag/D.A. β-Catenin mice, we examined the expression of several known β-Catenin target genes, including uPA, MMP7, and MMP9. Comparisons of D.A. β-Catenin mice, LPB-Tag mice, or LPB-Tag/D.A. β-Catenin mice with wild-type mice revealed that the expression of uPA and MMP9 was not significantly different (data not shown). However, qRT-PCR revealed that prostatic MMP7 levels were significantly higher in D.A. β-Catenin mice when compared with wild type control or with LPB-Tag mice (p<0.01) (Fig. 5A). In addition, MMP7 levels were further increased in LPB-Tag/D.A. β-Catenin mice when compared to wild-type control or with LPB-Tag mice (p<0.01) (Fig. 5A). The elevation of MMP7 level in mouse prostates that have nuclear β-Catenin was also confirmed by western blot and immunostaining (Fig. 5B &5C). The results show that MMP7 protein levels are elevated in both in D.A. β-Catenin mouse and LPB-Tag/D.A. β-Catenin mouse when compared with wild type or with LPB-Tag mice. D.A. β-Catenin mouse prostate has the highest level of MMP7 protein. However, the expression of MMP7 in LPB-Tag/D.A. β-Catenin prostates is not spatially associated with the invasive front of tumors (Fig. 5C).
Figure 5. expression of nuclear β-Catenin increases MMP7 level.
A, MMP7 mRNA level was assessed by qRT-PCR. MMP7 was elevated in prostates from D.A. β-Catenin mice (b-Catenin) or from LPB-Tag/D.A. β-Catenin (Tag/b-Catenin) mice when compared with wild type (WT) or LPB-Tag (Tag) mice. * p<0.01. B, western blotting to assess the protein levels of MMP7. Protein lysates were prepared from 1: wild type; 2: LPB-Tag; 3: D.A. β-Catenin; and 4: LPB-Tag/D.A. β-Catenin mouse prostates. Active MMP7 protein levels are significantly increased in both D.A. β-Catenin mouse and LPB-Tag/D.A. β-Catenin mouse. D.A. β-Catenin mouse prostate has the highest level of active MMP7. C, immunostaining of MMP7. MMP7 is lightly expressed in wild type mouse prostate, hardly detected in LPB-Tag mouse prostate, highly expressed in D.A. β-Catenin mouse and LPB-Tag/D.A. β-Catenin mouse prostate. The expression of MMP7 in LPB-Tag/D.A. β-Catenin mouse prostate is not associated with invading edges (arrows indicate cells at invasive front). Scale bar represents 50 µm.
The LPB-Tag/D.A. β-Catenin mouse prostates display focal areas of neuroendocrine differentiation
Studies have shown that neuroendocrine (NE) cells in the wild type prostate and PCa have little or no AR expression (Sciarra et al., 2003); however, AR and PSA expression is still detected in adenocarcinoma that begins to express NE factors and undergoes neuroendocrine differentiation (NED). Additionally, we have reported that Foxa2 expression is associated with prostate NE tumor and NE cells found in the normal adult prostate (Gupta et al., 2008; Mirosevich et al., 2005; Mirosevich et al., 2006; Qi et al., 2010). The induction of Foxa2 and loss of AR in the LPB-Tag/D.A. β-Catenin mice prompted us to examine the expression of the NE markers synaptophysin (Syn) and chromogranin A (Chr. A) in these samples. Our analysis showed that Syn was not expressed by luminal epithelial cells in wild type prostates (Fig. 6C), LPB-Tag prostates (Fig. 6F), minimally expressed in the D.A. β-Catenin prostate (Fig. 6I), but was detected in prostate specimens derived from LPB-Tag/D.A. β-Catenin mice (Fig. 6L). Chr. A, another NE marker, was elevated in LPB-Tag/D.A. β-Catenin mice when compared with wild type, LPB-Tag, or D.A. β-Catenin mice (Figs. 7C&7D). Foxa2, which has been associated with prostate NE tumor (Mirosevich et al., 2006; Yu et al., 2005), was not detected in wild type or LPB-Tag mice, but was expressed in both D.A. β-Catenin and LPB-Tag/D.A. β-Catenin mice (Figs. 6H and 6K). These data indicate that prostate specimens derived from the LPB-Tag/D.A. β-Catenin prostates show the presence of prostate adenocarcinoma that undergoes NED, and that Foxa2 expression is associated with cells that are both positive and negative for Syn staining (compare Figs. 6H&I with Figs. 6K&L).
Figure 6. Compound activation of SV40 large T-antigen and β-Catenin causes prostate carcinoma with NED features.
H&E staining and immunostaining against Foxa2 and synaptophysin (Syn.) were performed on prostate specimens derived from wile type (A-C), LPB-Tag (D-F), D.A. β-Catenin (G-I), and LPB-Tag/D.A. β-Catenin (J-L) mice. Foxa2, which has been associated with neuroendocrine differentiation in prostate, was expressed in prostate derived from both D.A. β-Catenin mouse (H) and LPB-Tag/D.A. β-Catenin mouse (K), but was not expressed in prostate luminal epithelial cells derived from WT mouse (B), or from LPB-Tag mouse (E). Syn. was not expressed in prostate from wild type mouse (C) or from LPB-Tag mouse (F), hardly detected in prostate derived from D.A. β-Catenin mouse (I), but was expressed in prostate tissue derived from LPB-Tag/D.A. β-Catenin mouse (L). Arrows in panel J-L indicate cells undergoing NED (positive for Syn. and Foxa2, and show hyperchromatic nuclei, granular chromatin, and high nuclear/cytoplasmic ratio). Scale bar represents 50 µm.
Figure 7. Quantitative RT-PCR and western blot to analyze the levels of NE markers.
A, western blotting of cell lysates from NeoTag1 cells for NSE and Foxa2. Mutant β-Catenin gene (D.A. β-Catenin) was introduced into NeoTag1 cells by viral infection. NE markers were examined in the β-Catenin over-expressing cells. NSE and Foxa2 were induced in NeoTag1/β-Catenin cells. P.C.: positive control. B, qRT-PCR for Chromogranin A (Chr. A). Chr A was increased in NeoTag1/β-Catenin cells compared with empty vector control. C, qRT-PCR of Chromogranin A (Chr. A). RNA was extracted from prostates of wild type (WT), LPB-Tag (Tag), D.A. β-Catenin (b-Cat), and LPB-Tag/D.A. β-Catenin (Tag/b-Cat.) mice. Chr. A was significantly increased in LPB-Tag/D.A. β-Catenin mouse prostates. * p<0.01. D, western blotting to assess the protein levels of chromogranin A (Chr. A). Protein lysates were prepared from 1: wild type; 2: LPB-Tag; 3: D.A. β-Catenin; and 4: LPB-Tag/D.A. β-Catenin mouse prostates.
β-Catenin stabilization correlates with increases in Foxa2 expression and increases in markers of NED
To confirm our in vivo finding that activation of Wnt/β-Catenin signaling promoted the development of NED in T-antigen expressing prostate cells, we stably integrated a mutant, non-degradable β-Catenin gene into NeoTag1 cells. The NeoTag1 prostate epithelial cell line was derived from 12T-7f mouse line, which is a well characterized mouse model of HGPIN (Wang et al., 2006). Thus, the NeoTag1 murine prostate cell line has similar characteristics as the LPB-Tag transgenic line from which it originated (Wang et al., 2006). Ectopic expression of this mutant β-Catenin construct results in cytoplasmic/nuclear accumulation of non-degradable β-Catenin, effectively acting as a dominant active Wnt signal (Barth et al., 1999). Expression of dominant active β-Catenin resulted in the increased expression of the two common NE markers (Sciarra et al., 2003) chromogranin A (Chr.A) and Neuron-specific enolase (NSE) (Figs. 7A&B). These results indicate that stabilization of β-Catenin induces NED in NeoTag1 cells.
In addition to ChrA and NSE expression, our previous studies have shown that Foxa2 is associated with NE cancer in mouse models and in human NE PCa (Mirosevich et al., 2006; Yu et al., 2005). We have also reported that activation of Wnt-signaling results in the expression of Foxa2 in the mouse prostate (Yu et al., 2009). Therefore, Foxa2 expression was also examined in these NeoTag1/β-Catenin cells by western-blotting analysis. Foxa2 was induced by over-expression of the non-degradable, dominant active β-Catenin (Fig. 7A). Taken together, these data indicate that active Wnt/β-Catenin signaling can induce the expression of Foxa2, and increased expression of NED markers in NeoTag1 cells.
Discussion
PCa begins with abnormal growth of prostate epithelial cells. However, the rapid cell proliferation alone is not sufficient to cause cancer transformation as evidenced by several mouse models that display active cell proliferation but develop PIN instead of PCa (Bhatia-Gaur et al., 1999; Song et al., 2002; Zhang et al., 1997). Over-expression of growth factors or oncogenes, or inactivation of tumor suppressor genes in these transgenic mice alters cell cycle thus enabling prostatic cells to proliferate rapidly and form PIN lesions, but is not sufficient to form PCa. These pre-cancerous cells generally need to acquire a second mutation or “hit” to acquire the ability to invade into the surrounding stromal tissue and form malignant PCa (Kasper, 2005), as observed in the Tag-Hepsin (Klezovitch et al., 2004) or the PTEN+/−/Nkx3.1−/− (Kim et al., 2002) mouse models. Hepsin is a cell surface serine protease; over-expression of hepsin promotes T-antigen expressing cell invasion at the primary site and metastasis to distal organs. This model supports the involvement of proteases in PCa progression. It is now widely accepted that the process of invading through the basement membrane is a hallmark characteristic of prostate adenocarcinoma, which is accomplished by the destruction of the extracellular matrix, including the basement membrane and connective tissue by proteases (Bonfil et al., 2007). Increased expression of MMPs and other proteases such as uPA and hepsin has been shown to facilitate tumor cell invasion through the basement membrane to gain access to the vascular bed (Bonfil et al., 2007). It is also important to note that proteolytic activity can also facilitate angiogenesis, thus providing nutrient supply to tumors, as well as a conduit for the promotion of tumor cell dissemination.
Activation of Wnt/β-Catenin signaling has been shown to facilitate the invasive phenotype by endowing cell mobility and inducing the expression of proteases such as MMPs and uPA, thus implicating this pathway in tumor invasion (Brabletz et al., 2005; Polakis, 2000). In this study, we activated Wnt/β-Catenin in the presence of large T-antigen in mouse prostate. We found compound activation of Wnt/β-Catenin and T-antigen resulted in invasive PCa; whereas, activation Wnt/β-Catenin alone or expression of Large T-antigen alone caused PIN. Our data indicate that activation of Wnt/β-Catenin signaling in the large T-antigen expressing prostate epithelial cells results in progression from HGPIN to invasive adenocarcinoma.
To identify possible mechanism(s) explaining the more aggressive phenotype seen in the Tag/β-Catenin mouse prostate, we analyzed the expression of several well known β-catenin target genes (MMP7, MMP9, and uPA) (Crawford et al., 2001). Expression of MMP7 was found to be elevated in the D.A. β-Catenin mouse and LPB-Tag/D.A. β-Catenin mouse prostates (Fig. 5) but not MMP9 or uPA. The MMPs are a family of proteases that catalyze the degradation of extracellular matrix proteins (Fingleton, 2006; Wilson and Matrisian, 1996) and also process a number of proteins on the cell surface to generate bioactive molecules (Fingleton, 2006). The MMPs are implicated in several cellular processes including proliferation, migration, differentiation, and angiogenesis- all of which play important roles during cancer progression (Fingleton, 2006). The MMP7 is a membrane type MMP (MT4-MMP), which is cell membrane-associated instead of being secreted into extracellular matrix. Unlike most other MMPs that are produced by stromal cells, MMP7 is exclusively produced by epithelial cells, and has been associated with cancer initiation and progression (Wilson and Matrisian, 1996). Although the expression of MMP7 is not associated with invasion phenotype observed in the LPB-Tag/D.A. β-Catenin prostates, the induction of MMP7 by stabilized β-Catenin may still contribute to the carcinoma development in these prostates. MMP7 has been shown to be important for processing of growth factor molecules (Fingleton, 2006). Therefore, MMP7 expression distal to the site of frank invasion may suggest a function for MMP7 in the regulated processing of paracrine growth factors. We also observed that the amount of pro-MMP7 (or inactive MMP7) was decreased in the LPB-Tag/D.A. β-Catenin mouse prostate. This could indicate that there is less inactive MMP7 in the LPB-Tag/D.A. β-Catenin mouse prostate compared to the D.A. β-Catenin mouse prostate. However, more mechanistic studies would be required to confirm this.
Our studies show that the prostate-specific activation of both LPB-Tag and Wnt/β-Catenin pathways results in the development of invasive adenocarcinoma (Fig. 1 – Fig. 3), with elements of limited NED, while the prostates of mice expressing LPB-Tag alone or β-Catenin alone developed HGPIN (Kasper et al., 1998; Yu et al., 2009). It is noteworthy that our data shows Foxa2 is expressed at the tumor invading edge and in cells scattered among the surrounding stroma (Fig. 3). These data indicate that the active Wnt/β-Catenin signaling and the expression of Foxa2 is associated with PCa tumor invasion at the primary site. A similar phenotype has been described in colorectal cancer, where loss of APC functions and/or oncogenic β-Catenin mutations contribute to a majority of colorectal cancer cases. Studies have found that in colorectal cancer, nuclear β-Catenin displayed a heterogeneous pattern that membrane expression of β-Catenin is associated with well-differentiated central area of colorectal tumors, whereas nuclear β-Catenin and its down-stream target genes MMP7, uPA and uPAR are frequently detected in invasive cells, suggesting that growth factors and Wnts from extracellular matrix (tumor microenvironment) may account for the enhanced Wnt/β-Catenin signaling in these epithelial cells (Brabletz et al., 2001; Huang and Du, 2008). The heterogeneous distribution of cells with nuclear β-Catenin in the invasive front of tumor mass may confer these cells with malignant capabilities. Similarly, we found that in the prostates of the LPB-Tag/D.A. β-Catenin mouse model, the invasive front and cells scattered in the adjacent stroma were positive for Foxa2, an indicator of active β-Catenin signaling in prostate, suggesting that activation of Wnt/β-Catenin signaling and the expression of Foxa2 in these cells may endow them invasive ability.
Studies have found that β-Catenin is an AR co-activator (Terry et al., 2006). We have also found that when co-transfected with β-Catenin, AR exhibits increased activity on the ARR2PB promoter in the presence of DHT (data not shown,) confirming that β-Catenin acts as an AR co-activator in transient transfection. Additionally, we have reported that activation of Wnt/β-Catenin signaling in the mouse prostate results in an initial early increase in AR activity concomitant with the early development of hyperplasia. However, it is important to point out that with the subsequent development of PIN and HGPIN in this model, epithelial cell AR levels are reduced. Consistent with the altered AR protein levels, an increase in androgen regulated target genes was observed in early stage prostatic hyperplasia and down-regulated in HGPIN (Yu et al., 2009). Here we report that in the LPB-Tag/D.A. β-Catenin mice, there is a decrease in the androgen receptor levels and in the androgen regulated probasin promoter as seen by decreased Tag levels (Fig. 3 and Fig. 4). While large T-antigen expression is decreased, Foxa2 is now expressed in LPB-Tag/D.A. β-Catenin mouse prostate. The expression of Foxa2 indicates that Wnt/β-Catenin signaling is active in these cells. The mutually exclusive expression pattern of large T-antigen and Foxa2, as detected by IHC, indicates that Tag (directed by AR signaling) is reduced in the cells that have activated Wnt/β-Catenin signaling. Interestingly, the level of AR mRNA increases in LPB-Tag/D.A. β-Catenin prostate but the level of the AR protein decreases (Fig 4). This suggests that activation of the Wnt-signaling pathway increases protein degradation pathway(s). For example, we have reported that increased levels of the non-degradable exon 3 deleted β-Catenin results in increased levels of F-box β-TrCP ubiquitin ligase, an enzyme that causes the degradation of endogenous β-Catenin and functions as a feedback inhibitor to Wnt signaling (Yu et al., 2009). In conclusion, continuous Wnt/β-Catenin signaling appears to down-regulate AR and AR target genes.
Although prostatic small cell carcinoma (NE cancer) is rare, NED of prostatic adenocarcinoma is commonly reported and associated with a poor patient outcome (Sciarra et al., 2003; Wang and Epstein, 2008; Yao et al., 2006). Previous studies have shown that the expression of Foxa2 is associated with NE tumors (Gupta et al., 2008; Mirosevich et al., 2005; Mirosevich et al., 2006; Qi et al., 2010). In addition, several studies have also shown that NE cells in the wild type prostate and PCa have little or no AR expression, and that loss of AR expression is correlated with NED (Sciarra et al., 2003). A reduction in AR induced by castration of TRAMP mice results in an increase in the appearance of small cell carcinoma (NE cancer), regardless of the mouse strain used (Huss et al., 2007). Thus, the loss of AR activity would foster NED, consistent with the fact that small cell carcinomas show no or reduced levels of AR expression. The suppression of AR and AR signaling and the induction of Foxa2 by the activation of Wnt/β-Catenin in prostatic PIN provide connections between Wnt/β-Catenin pathway and NED of tumors. In our study, we found that the LPB-Tag/D.A. β-Catenin mouse prostates developed adenocarcinoma with some focal areas showing NE features, suggesting that activation of Wnt/β-Catenin in the presence of T-antigen causes prostate NED. This finding was confirmed by in vitro study when a non-degradable β-Catenin was expressed in T-antigen expressing NeoTag1 cells. Our results showed that expression of the mutant β-Catenin induced NED in these cells as evidenced by the induction of Foxa2 and two other well established NE markers- NSE and Chromogranin A. More evidence supporting the involvement of Wnt/β-Catenin in prostatic NED appears in an in vitro study showing that activation of Wnt/β-Catenin in LNCaP cells resulted in NED as reflected by the expression of two NE markers, NSE and ChrA (Yang et al., 2005). Taken together, these data strongly support the implication of Wnt/β-Catenin signaling in NED of prostate adenocarcinoma.
LPB-Tag/D.A. β-Catenin mouse prostates developed HGPIN and prostatic adenocarcinoma, whereas when Tag or nuclear β-Catenin alone is targeted to the prostate, the mice developed only PIN to HGPIN. Although we have not seen the development of adenocarcinoma when nuclear β-Catenin is targeted to the prostate, another report describes both HGPIN and adenocarcinoma appears when APC is specifically deleted in the mouse prostate (Bruxvoort et al., 2007), indicating that loss of APC has broader effects than the expression of nuclear β-Catenin. In summary, our study found that activation of Wnt/β-Catenin signaling in T-antigen expressing prostatic cells promoted the appearance of prostate adenocarcinoma, demonstrating that the role of Wnt-signaling pathway in tumor progression.
Materials and methods
Cell line, Plasmids, and retroviral infection
NeoTag1 cells used in this study were cultured as describe previously (Wang et al., 2006). The NeoTag1 cell is a prostate epithelial cell line established from ARR2PB-Neo/12T-7 Tag transgenic mouse prostate. The large T-antigen immortalized the epithelial cells, and since the ARR2PBneo expression is driven by the androgen responsive probasin promoter, the NeoTag1 cells are androgen receptor positive with G418 selection. β-Catenin retroviral expression plasmids and control GFP plasmids (empty vector, EV) were kindly provided by Dr. Angela Barth (Barth et al., 1999). The β-Catenin gene in this retroviral vector has several point mutations on the putative GSK3β phosphorylation sites (Ser-33, Ser-37, Thr-41, and Ser-45) that prevent β-Catenin degradation. For retroviral infection, the β-Catenin or control GFP retroviral vector plasmids were transfected into Phoenix packaging cells. 24hours later, culture media were collected and used for infecting NeoTag1 cells. The infection procedure was repeated for 3 times. β-Catenin or GFP expressing cells were obtained by fluorescence-activated cell sorting.
Mouse lines and mouse breeding
12T-7 is one of the seven LPB-Tag transgenic mouse lines and widely used in prostate cancer research (Kasper et al., 1998). As a result of a copy number difference on multiple chromosomes, the 12T-7 line diverged into 12T-7 fast (f) and 12T-7 slow (s) (Masumori et al., 2001). In this study, 12T-7s line was used. To get mice with compound activation of LPB-Tag and β-Catenin, PBCre4 mice (Wu et al., 2001) (on C57BL/6 background) were bred with 12T-7s mice (Kasper et al., 1998) (on CD1 background) to obtain 12T-7s/PBCre4 mice, or with Catnblox(ex3) mice(Harada et al., 1999) (on C57BL/6 background) to obtain Catnblox(ex3)/PBCre4 mice. 12T-7s/Catnblox(ex3) /PBCre4 mice were obtained through breeding 12T-7s/PBCre4 mice with Catnblox(ex3) mice or through breeding 12T-7s mice with Catnblox(ex3)/PBCre4 mice. The 12T-7s mice were designated as LPB-Tag mice, the Catnblox(ex3)/PBCre4 as D.A. β-Catenin mice, and the 12T-7s/Catnblox(ex3)/PBCre4 as LPB-Tag/D.A. β-Catenin mice. The LPB-Tag/D.A. β-Catenin mice have a mixed C57BL/6 and CD1 background.
Immunohistochemistry, immunofluorescence staining and western blot
Immunostaining, immunofluorescence staining, H&E staining, and western-blot were conducted as described (Yu et al., 2009). Antibodies used are: AR and Foxa2 from Santa Cruz (Santa Cruz, CA), synaptophysin and β-Catenin from BD Biosciences (San Jose, CA), NSE from NeoMarkers (Fremont, CA), large T-antigen from Calbiochem (San Diego, CA), chromogranin A from ImmunoStar (Hudson, WI), MMP7 from Cell Signaling (Danvers, MA), β-Actin from Sigma (St Louis, MO), and wide spectrum cytokeratin (pan-cytokeratin) from Dako (Carpentaria, CA).
Quantitative RT-PCR (qRT-PCR)
RNA was extracted using the RNeasy kit from Qiagen (Valencia, CA). Reverse transcription was conducted using SuperScriptII from Invitrogen (Carlsbad, CA). Primer sequences are: TTTGCCCTTCCTGTGAACAGC(f) and CTTGGAGAGCCAGGTCTTGAAGTT (r) for Chromogranin A (Chr. A); GTGGACAACCTCAAGGAAATGCAG(f) and TCCACTACGATCCGAGGTAAGTCT(r) for MMP7; TGTGGAGATGAAGCTTCTGGCTGT(f) and TGGTACAATCGTTTCTGCTGGCAC(r) for AR; TGCACCACCAACTGCTTAGC (f) and GGCATGGACTGTGGTCATGAG (r) for GAPDH. Real-time PCR was performed on iCycler using iQ SYBR Green Supermix from Bio-Rad (Hercules, CA). PCR was performed as follows: 95°C for 4 mins, followed by 40 cycles of 95°C/30 secs, 58°C/30 secs, and 72°C/30 secs. All samples were normalized by GAPDH. Results were expressed as fold change of each sample versus control.
Acknowledgement
we thank Dr. Angela Barth, Stanford University, for kindly providing the mutant β-Catenin expressing vector, Tom Case and Manik Paul for technical support, Dr. Barbara Fingleton and Dr. Lynn Matrisian for advice and discussion. Grant support was provided by NIH to RJM (Grant Numbers: 2R01 CA76142-11; 2R01-DK055748-10), DOD to RJM (Grant Number: PC074022), and the Joe C. Davis Foundation. David DeGraff, PhD was supported by the American Cancer Society Great Lakes Division-Michigan Cancer Research Fund Postdoctoral Fellowship.
Frequently used abbreviation in this paper
- PCa
prostate cancer
- AR
androgen receptor
- HGPIN
high grade prostatic intraepithelial neoplasia
- Tag
large T-antigen
- NE
neuroendocrine
Footnotes
Disclosure statement: The authors have nothing to disclose. There is no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reference List
- Barth AI, Stewart DB, Nelson WJ. T cell factor-activated transcription is not sufficient to induce anchorage-independent growth of epithelial cells expressing mutant beta-catenin. Proc Natl Acad Sci U S A. 1999;96(9):4947–4952. doi: 10.1073/pnas.96.9.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, et al. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13(8):966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfil RD, Chinni S, Fridman R, Kim HR, Cher ML. Proteases, growth factors, chemokines, and the microenvironment in prostate cancer bone metastasis. Urol Oncol. 2007;25(5):407–411. doi: 10.1016/j.urolonc.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, et al. Invasion and metastasis in colorectal cancer: epithelial-mesenchymal transition, mesenchymal-epithelial transition, stem cells and beta-catenin. Cells Tissues Organs. 2005;179(1–2):56–65. doi: 10.1159/000084509. [DOI] [PubMed] [Google Scholar]
- Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98(18):10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruxvoort KJ, Charbonneau HM, Giambernardi TA, Goolsby JC, Qian CN, Zylstra CR, et al. Inactivation of apc in the mouse prostate causes prostate carcinoma. Cancer Res. 2007;67(6):2490–2496. doi: 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- Castellone MD, Teramoto H, Williams BO, Druey KM, Gutkind JS. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310(5753):1504–1510. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear beta-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21(17):2679–2694. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Detection and analysis of beta-catenin mutations in prostate cancer. Prostate. 2000;45(4):323–334. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Crawford HC, Fingleton B, Gustavson MD, Kurpios N, Wagenaar RA, Hassell JA, et al. The PEA3 subfamily of Ets transcription factors synergizes with beta-catenin-LEF-1 to activate matrilysin transcription in intestinal tumors. Molecular & Cellular Biology. 2001;21(4):1370–1383. doi: 10.1128/MCB.21.4.1370-1383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la TA, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, et al. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res. 2003;9(5):1801–1807. [PubMed] [Google Scholar]
- Deeb KK, Michalowska AM, Yoon CY, Krummey SM, Hoenerhoff MJ, Kavanaugh C, et al. Identification of an integrated SV40 T/t-antigen cancer signature in aggressive human breast, prostate, and lung carcinomas with poor prognosis. Cancer Research. 2007;67(17):8065–8080. doi: 10.1158/0008-5472.CAN-07-1515. [DOI] [PubMed] [Google Scholar]
- Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front Biosci. 2006;11:479–491. doi: 10.2741/1811. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y-Q, Browne C, Kim S, Case TC, Paul M, et al. Neuroendocrine differentiation in the 12T-10 transgenic prostate mouse model mimics endocrine differentiation of pancreatic beta cells. The Prostate. 2008;68(1):50–60. doi: 10.1002/pros.20650. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18(21):5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Du X. Crosstalk between tumor cells and microenvironment via Wnt pathway in colorectal cancer dissemination. World J Gastroenterol. 2008;14(12):1823–1827. doi: 10.3748/wjg.14.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huss WJ, Gray DR, Tavakoli K, Marmillion ME, Durham LE, Johnson MA, et al. Origin of androgen-insensitive poorly differentiated tumors in the transgenic adenocarcinoma of mouse prostate model. Neoplasia. 2007;9(11):938–950. doi: 10.1593/neo.07562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S. Survey of genetically engineered mouse models for prostate cancer: analyzing the molecular basis of prostate cancer development, progression, and metastasis. J Cell Biochem. 2005;94(2):279–297. doi: 10.1002/jcb.20339. [DOI] [PubMed] [Google Scholar]
- Kasper S, Sheppard PC, Yan Y, Pettigrew N, Borowsky AD, Prins GS, et al. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab Invest. 1998;78(6):319–334. [PubMed] [Google Scholar]
- Kim MJ, Cardiff RD, Desai N, Banach-Petrosky WA, Parsons R, Shen MM, et al. Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis. Proc Natl Acad Sci U S A. 2002;99(5):2884–2889. doi: 10.1073/pnas.042688999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klezovitch O, Chevillet J, Mirosevich J, Roberts RL, Matusik RJ, Vasioukhin V. Hepsin promotes prostate cancer progression and metastasis. Cancer Cell. 2004;6(2):185–195. doi: 10.1016/j.ccr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Lamberti C, Lin KM, Yamamoto Y, Verma U, Verma IM, Byers S, et al. Regulation of beta-catenin function by the IkappaB kinases. J Biol Chem. 2001;276(45):42276–42286. doi: 10.1074/jbc.M104227200. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Momand J. Tumor suppressor genes: the p53 and retinoblastoma sensitivity genes and gene products. Biochim Biophys Acta. 1990;1032:119–136. doi: 10.1016/0304-419x(90)90015-s. [DOI] [PubMed] [Google Scholar]
- Masumori N, Thomas TZ, Case T, Paul M, Kasper S, Chaurand P, et al. A probasin-large T antigen transgenic mouse line develops prostate adeno and neuroendocrine carcinoma with metastatic potential. Cancer Res. 2001;61:2239–2249. [PubMed] [Google Scholar]
- Miller JR. The Wnts. Genome Biol. 2002;3(1):1–15. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirosevich J, Gao N, Gupta A, Shappell SB, Jove R, Matusik RJ. Expression and role of Foxa proteins in prostate cancer. Prostate. 2006;66:1013–1029. doi: 10.1002/pros.20299. [DOI] [PubMed] [Google Scholar]
- Mirosevich J, Gao N, Matusik RJ. Expression of Foxa transcription factors in the developing and adult murine prostate. Prostate. 2005;62(4):339–352. doi: 10.1002/pros.20131. [DOI] [PubMed] [Google Scholar]
- Persad S, Troussard AA, McPhee TR, Mulholland DJ, Dedhar S. Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J Cell Biol. 2001;153(6):1161–1174. doi: 10.1083/jcb.153.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- Qi J, Nakayama K, Cardiff RD, Borowsky AD, Kaul K, Williams R, et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell. 2010;18(1):23–38. doi: 10.1016/j.ccr.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciarra A, Mariotti G, Gentile V, Voria G, Pastore A, Monti S, et al. Neuroendocrine differentiation in human prostate tissue: is it detectable and treatable? BJU Int. 2003;91(5):438–445. doi: 10.1046/j.1464-410x.2003.03066.x. [DOI] [PubMed] [Google Scholar]
- Song Z, Wu X, Powell WC, Cardiff RD, Cohen MB, Tin RT, et al. FGF8, Isoform b overexpression in prostate epithelium: A new mouse model for prostatic intraepithelial neoplasia. Cancer Res. 2002 In press. [PubMed] [Google Scholar]
- Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem. 2006;99(2):402–410. doi: 10.1002/jcb.20983. [DOI] [PubMed] [Google Scholar]
- Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32(1):65–71. doi: 10.1097/PAS.0b013e318058a96b. [DOI] [PubMed] [Google Scholar]
- Wang Y-Q, Kasper S, Yuan J, Jin RJ, Zhang J, Ishii K, et al. Androgen dependent prostatic epithelial cell selection by targeting ARR2PBNeo to the LPB-Tag transgenic model of prostate cancer. Lab Invest. 2006;86:1074–1088. doi: 10.1038/labinvest.3700463. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28(2):123–136. doi: 10.1016/1357-2725(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol. 2003;201(2):204–212. doi: 10.1002/path.1449. [DOI] [PubMed] [Google Scholar]
- Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101(1–2):61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- Yang X, Chen MW, Terry S, Vacherot F, Chopin DK, Bemis DL, et al. A human- and male-specific protocadherin that acts through the wnt signaling pathway to induce neuroendocrine transdifferentiation of prostate cancer cells. Cancer Research. 2005;65(12):5263–5271. doi: 10.1158/0008-5472.CAN-05-0162. [DOI] [PubMed] [Google Scholar]
- Yao JL, Madeb R, Bourne P, Lei J, Yang X, Tickoo S, et al. Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol. 2006;30(6):705–712. doi: 10.1097/00000478-200606000-00005. [DOI] [PubMed] [Google Scholar]
- Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8(2):119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]
- Yu X, Gupta A, Wang Y-Q, Suzuki K, Mirosevich J, Orgebin-Crist MC, et al. Foxa1 and Foxa2 interact with the androgen receptor to regulate prostate and epididymal genes differentially. Annals New York Academy of Sciences. 2005;1061:77–93. doi: 10.1196/annals.1336.009. [DOI] [PubMed] [Google Scholar]
- Yu X, Wang Y-Q, Jiang M, Bierie BB, Hayward SW, Shen MM, et al. Activated beta-catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. The Prostate. 2009;69(3):249–262. doi: 10.1002/pros.20877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen MW, Ng A, Ng PY, Lee C, Rubin M, et al. Abnormal Prostate Development in C3(1)-bcl-2 Transgenic Mice. The Prostate. 1997;32(1):16–26. doi: 10.1002/(sici)1097-0045(19970615)32:1<16::aid-pros3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]