Abstract
Effects of estrogen therapy on cognitive performance appear to diminish with age and time following the loss of ovarian function. We hypothesize that this is due to a reduction in basal forebrain cholinergic function and that treatment with a cholinergic enhancer can reverse the effect. This study tested whether combining the cholinesterase inhibitor donepezil with estradiol treatment can enhance/restore estradiol effects on cognitive performance in young ovariectomized rats with selective lesions of septal cholinergic neurons. 192IgG-saporin was injected directly into the medial septum to produce selective cholinergic lesions. Rats were then treated with donepezil (Don, daily injections of 3 mg/Kg/day, i.p.) or vehicle, and then with 17β-estradiol (E2, administered by silastic capsule implanted s.c.) or an empty capsule. Rats were trained on a delayed matching-to-position (DMP) T-maze task which previous studies have shown is sensitive to ovariectomy and estrogen replacement. Results show that neither estradiol nor donepezil alone significantly enhanced acquisition of the DMP task in rats with cholinergic lesions. Combination therapy was effective, however, depending on the severity of the lesion. Don+E2 significantly enhanced acquisition of the task in rats with partial lesions (<50% loss of cholinergic neurons), but not in rats with severe lesions. This effect was due largely to a reduction in perseverative behavior. Don+E2 also improved working memory in rats with partial lesions, as evidenced by significantly better performance than controls during increased intertrial delays. These findings suggest that even partial loss of septal cholinergic neurons can reduce effects of estrogen therapy on cognitive performance, and demonstrate that combining a cholinesterase inhibitor with estrogen therapy can help to restore beneficial effects on performance. We propose that combination therapy may have similar beneficial effects in women, particularly in older women who have not used estrogen therapy for many years and are beginning to show signs of cognitive impairment or early Alzheimer’s disease.
Keywords: spatial learning, hormone therapy, cholinesterase inhibitor, surgical menopause, cholinergic lesion, 192IgG-saporin
INTRODUCTION
Animal studies show that estrogens have many beneficial effects on the brain, including reducing neuronal loss following cardiac arrest and stroke (Suzuki et al., 2006), increasing neuronal connectivity (Spencer et al., 2008), improving cognitive performance (Daniel, 2006; Kim and Casadesus, 2010; Luine, 2008), and preventing or slowing age-related cognitive decline (Frick, 2009; Gibbs, 2006). However, data show that the ability to produce these effects diminishes with age and time following the loss of ovarian function (Daniel et al., 2006; Gibbs et al., 2009; Markowska and Savonenko, 2002; Smith et al., 2010; Talboom et al., 2008). Clinical observational studies as well as recent prospective randomized trials suggest that similar effects occur in humans (Henderson, 2009; Rocca et al., 2010; Sherwin, 2009). This has given rise to the critical period hypothesis, which states that there is a window of opportunity following menopause during which estrogen therapy must be initiated to produce beneficial effects on brain function and cognition.
Evidence from animal studies suggests that this window of opportunity is defined, at least in part, by the functional status of cholinergic projections to the hippocampus and cortex (Gibbs, 2010). Forebrain cholinergic projection neurons are divided into subregions known as the medial septum, diagonal band of Broca, substantial innominata, and nucleus basalis magnocellularis. Cholinergic inputs to the hippocampus originate primarily from neurons located in the medial septum and to some extent the vertical limb of the diagonal band of Broca, whereas cholinergic inputs to the neocortex originate primarily from cells located in the nucleus basalis magnocellularis (Mesulam, 1996; Woolf, 1991). Many studies show that cholinergic function in these regions declines with age (Baskerville et al., 2006; Fischer et al., 1992) as well as with specific neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease (Lanari et al., 2006; Linstow and Platt, 1999; Smith et al., 1999). These neurons also are adversely affected by loss of ovarian function (reviewed in Gibbs, 2010). According to the cholinergic hypothesis, the loss of beneficial effect of estrogen therapy occurs when basal forebrain cholinergic function decreases below a critical threshold (Gibbs, 2010). This is consistent with reports that selective destruction of cholinergic projections to the hippocampus reduces or abolishes effects of estradiol on synaptic plasticity and function (Lam and Leranth, 2003; Rudick et al., 2003). Similar lesions, or administration of a muscarinic receptor blocker, likewise prevents estradiol-mediated effects on spatial learning tasks (Daniel et al., 2005; Gibbs, 2007).
If the cholinergic hypothesis is correct, then enhancing cholinergic function should restore beneficial effects of estrogens on cognitive performance in rats with cholinergic lesions as well as in aged rats that have experienced long-term loss of ovarian function. Recently we showed that daily injections of donepezil, a cholinesterase inhibitor commonly used in treating Alzheimer’s disease, was able to restore effects of estradiol on acquisition of a delayed matching-to-position (DMP) T-maze task by aged rats that had been ovariectomized as young adults (Gibbs et al., 2009). In the present study, we evaluated the ability of donepezil to enhance estradiol effects on DMP acquisition in young ovariectomized rats with selective lesions of septal cholinergic neurons. Our goals were to test whether treatment with donepezil could restore beneficial effects of estradiol on DMP acquisition in rats with septal cholinergic lesions, and to determine whether the effectiveness of combining donepezil with estradiol was dependent on the degree of cholinergic cell loss.
MATERIALS AND METHODS
Animals
Eighty-one female Sprague-Dawley rats were ovariectomized at three months of age by Hilltop Laboratories, Inc. and then delivered to the University of Pittsburgh. Rats were maintained on a 12 hour:12 hour light/dark schedule with lights on at 0700 with free access to food and water. All procedures were consistent with PHS guidelines on the care and use of laboratory animals, and with the approval of the University’s Institutional Animal Care and Use Committee.
Cholinergic Lesions
Surgeries were performed 2–3 weeks after arrival. Rats were anesthetized with a mixture of ketamine (50 mg/mL) and xylazine (10 mg/mL) (0.25 cc/250 g.b.w. i.p.) and placed into a standard stereotaxic apparatus. Coordinates were derived based on the stereotaxic atlas of Paxinos & Watson (1986). The skull was exposed and a hole drilled at midline 0.2 mm anterior to Bregma. A 28 ga. stainless steel cannula was lowered into the medial septal nucleus (−5.6 mm from dura) and 1.0 μL 192IgG-saporin (SAP; Advanced Targeting Systems, Inc.) was injected at a rate of 0.2 μL/min. Two lots of SAP were used. Lot 41-105 was used at a dose of 0.25 μg/μL. Lot 64-124 was used at doses of 0.096 and 0.192 μg/μL. Each lot of was tested ahead of time to determine doses that would produce selective loss of cholinergic neurons without damaging nearby GABAergic neurons. Following surgery, rats received ketofen (1.0 mg/Kg i.p.) twice each day for three days to reduce discomfort.
Drug Treatments
After at least one week of recovery, rats began receiving daily injections of donepezil-HCl (Don, 3mg/kg; Ivy Fine Chemicals, Inc.) or sterile saline delivered i.p. Donepezil is a piperidine-based highly potent mixed, non-competitive reversible inhibitor of acetylcholinesterase, with an in vitro IC50 of approximately 6.7nM and an in vivo ID50 of approximately 2.6mg (6.8uMol)/kg brain tissue (Sugimoto et al., 2002). Injections were administered in the late afternoon. One week after the beginning of treatment, half of each group (Don or sterile saline) received a silastic capsule (6 mm length, 1.98mm I.D., 3.18 mm O.D) packed with 3mm of powdered 17β-estradiol (Sigma-Aldrich, Inc.), implanted s.c. The remaining rats received an empty capsule as a control.
DMP Training
One week after receiving the silastic capsule, rats were food restricted to 85% body weight and then trained on a delayed matching to position (DMP) T-maze task as described previously (Gibbs and Johnson, 2007; Gibbs et al., 2009). Briefly, rats were trained to traverse the maze and enter the goal arms by using a series of 6 forced “choice” trials per day for at least 4 days, each of which was rewarded with 4 food pellets (45 mg, formula 5TUM, Test Diets, Inc.). Right and left arms were alternated randomly in order to avoid the introduction of a side bias. DMP training was then performed in trial pairs. Each rat received 8 trial pairs per day. The first trial of each pair consisted of a “forced” choice in which one goal arm was blocked, requiring the rat to enter the open arm to receive a food reward (2 pellets). The rat was then immediately returned to the start box. All arms of the maze were wiped with 50% ethanol, and both goal arms were opened for the second trial. A choice was defined as an animal placing both front legs and at least one rear leg into a goal arm. Returning to the same arm as the previous “forced” trial resulted in food reward (4 pellets). Entering the opposite arm resulted in no reward and confinement to the arm for 10 seconds. During each day of training, four arms on the right and four arms on the left were selected in random order for the “forced” trials. After each trial pair, the rat was returned to its cage for 5–10 min. Each rat continued to receive 8 trial pairs per day until it reached a criterion of 15/16 correct choices over two consecutive days, or until it received 30 days of training.
Post-criterion testing
All rats that reached criterion also received post-criterion testing. One day after reaching criterion, animals received a probe trial during which the T-maze was rotated 180 degrees (relative to extramaze cues) between the forced and open trials. This probe trial was done to determine whether rats were utilizing a place strategy (i.e., were utilizing extramaze cues) or a response strategy (i.e., were utilizing internal or kinetic cues) to perform the task. An animal using extramaze cues is expected to turn in the opposite direction (i.e., enter the opposite physical arm now located in the same position of the room as the goal arm during the “forced” trial), while an animal using internal or kinetic cues would be expected to turn in the same direction (i.e., enter the same physical arm as during the “forced” trial) even though the arm occupies a different position in the room. Selecting the arm located in the same position of the room as during the “forced” trial was assigned a score of 0, while selecting the opposite arm was assigned a score of 1. One day after the probe trial, animals received four days of additional DMP training to assess delay-dependent effects on performance. On these days animals received 8 trial pairs/day and the delay between the “forced” and “open” trial was increased each day (day 1=minimal delay as during training, day 2=30 seconds, day 3=60 seconds, day 4=90 seconds).
Tissue Collection
After DMP training, rats were anesthetized with an overdose of ketamine and xylazine and then decapitated. Blood was collected for the determination of serum estradiol levels. Brains were removed and hippocampi were dissected and frozen at −80°C for analysis of choline acetyltransferase (ChAT) activity. Tissues containing the medial septum (MS) and diagonal band of Broca (DBB) were fixed by immersion in 4% paraformaldehyde in 50 mM phosphate buffered saline (PBS, pH 7.2) at 4°C overnight. These tissues were then transferred to 20% sucrose in PBS at 4°C for several days. Sections through the MS and DBB were cut and stained for ChAT- and for parvalbumin- like immunoreactivity as described below.
Estradiol assay
Serum levels of 17β-estradiol (E2) were determined using a sensitive LC-MS/MS assay recently developed in our department. Samples were spiked with internal standard (2,4,16,16,17-d3-17β-estradiol) and then extracted with n-butyl chloride. After centrifugation and evaporation, the residue was derivatized in 0.1 mL buffered dansyl chloride solution (pH 10.5). E2 was eluted from a Waters Acquity UPLC BEH C18, 1.7 um, 2.1 X 150 mm reversed-phase column, with an acetonitrile:water (0.1% formic acid) gradient. Detection and quantitation were achieved in the positive mode with a Thermo Fisher TSQ Quantum Ultra mass spectrometer interfaced via an electrospray ionization (ESI) probe with the Waters UPLC Acquity solvent delivery system. Transitions used for analysis were 506 → 171 for E2, and 511 → 171 for the deuterated internal standard. Area under the peak was quantified and used to determine absolute levels of E2/mL of sample by comparison with a series of standards. The limit of detectability for this assay is 2.5 pg/mL. Intra-assay statistics show errors below 8.1% and relative standard deviations below 10.4%. Inter-assay statistics show errors below 5.0% with relative standard deviations below 7.4%.
ChAT Assay
ChAT activity in hippocampal tissues was analyzed as previously described (Gibbs and Johnson, 2007). Tissues were dissociated by sonication in a medium containing EDTA (10 mM) and Triton X-100 (0.5%) and diluted to a concentration of 10 mg tissue/mL. An aliquot of each sample was used for the determination of total protein. Three 5 ul aliquots of each sample were incubated for 30 min at 37°C in a medium containing 3[H] acetyl-CoA (50,000–60,000 d.p.m./tube, final concentration 0.25mM acetyl-CoA), choline chloride (10.0mM), physostigmine sulfate (0.2mM), NaCl (300mM), sodium phosphate buffer (pH 7.4, 50mM), and EDTA (10 mM). The reaction was terminated with 4ml sodium phosphate buffer (10 mM) followed by the addition of 1.6ml of acetonitrile containing 5mg/ml tetrephenylboron. The amount of [3H] acetylcholine produced was determined by adding 8ml of EconoFluor scintillation cocktail (Packard Instruments, Meriden, CT) and counting total cpm in the organic phase using an LKB beta-counter. Background was determined using identical tubes to which no sample was added. For each sample, triplicates were averaged and the difference between total cpm and background cpm was calculated. This was used to estimate the total amount of acetylcholine produced per sample. ChAT activity was then calculated for each sample as pmol acetylcholine manufactured/h/ug protein.
Histology
Forty micron frozen sections in the coronal plane were cut through the MS and DBB and placed into PBS. Four adjacent series of sections were cut from each brain. One series was stained for ChAT, and an adjacent series was stained for parvalbumin (Parv) as previously described (Gibbs, 2002; Johnson et al., 2002). Briefly, sections were incubated with primary antibodies (goat anti-ChAT, 1:3500, AP144P, Chemicon, Inc.; rabbit anti-Parv,1:12,000, F3171, Sigma-Aldrich, Inc.) diluted with PBS containing 5% normal horse serum and 0.05% Triton X-100. After three days at 4°C, sections were rinsed with PBS, and then placed in secondary antibody (biotinylated donkey-anti-goat, or biotinylated donkey-anti-rabbit, 1:200, Vector Laboratories, Inc.) for 1 h at room temperature. Sections were then rinsed with PBS and placed in a solution containing avidin-HRP complex (Vectastain Elite Kit, Vector Laboratories, Inc.) for 1 h at room temperature. Sections were then rinsed with Tris acetate solution (50 mM, pH 7.6) and transferred to a Tris acetate solution containing 3,3′-diaminobenzidine (0.5 mg/mL), H2O2 (0.01%), and NiCl2 (0.032%) for 10–15 min. Sections were then rinsed with PBS, mounted onto glass slides, dehydrated, coverslipped, and examined with a Leitz photomicroscope.
Analysis of DMP Performance
Days to criterion (DTC) was analyzed either by ANOVA or by the nonparametric Wilcoxin test (when rats did not reach criterion) using Treatment as the between factor. Performance during acquisition was blocked into ten 3-day blocks of training. Once an animal reached criterion, a value of 0.9375 (15/16) was recorded for performance on subsequent days. The blocked data were then analyzed by ANOVA with repeated measures on ‘block’. The effects of rotating the maze 180° during the probe trial were analyzed by contingency table and Chi-square test. Performance during increased intertrial delays was analyzed by ANOVA with repeated measures on ‘Delay’.
We have observed that after several days of DMP training, some rats adopt a persistent turn whereby they consistently enter either the right or left arm of the maze. This behavior increases significantly with age (Gibbs et al., 2009) and following septal cholinergic lesions (Gibbs and Johnson, 2007). We quantified this as described previously by counting the total number of days during training that an animal chose the same arm of the maze 15 out of 16 times over a two-day period. Any animal that met this criterion was defined as having adopted a persistent turn. Chi-Square analysis was used to compare the number of animals that adopted a persistent turn between groups, and ANOVA was used to compare the number of days that this pattern persisted among the treatment groups. To evaluate the contribution that the number of days engaged in a persistent turn made to effects on DTC, the duration of the persistent turn was subtracted from DTC for each animal and the results analyzed using ANOVA.
Analysis of ChAT-IR Cells
Our studies show that sections through the anterior septum of non-lesioned rats contain approximately 100–120 ChAT-IR cells/section (unpublished observations) with approximately 700–800 ChAT-IR cells within a 500 μm slab extending 0.2 – 0.7 mm anterior to Bregma (Aggarwal and Gibbs, 2000) (corresponding to plates 15–17 of Paxinos & Watson (1986)). In this study, ChAT-IR staining was examined in matched sections through the MS of each rat and the degree of ChAT-IR cell loss was estimated based on cell counts and using both published and unpublished numbers as a reference. Rats were then assigned to one of four categories: >75% loss of cells, 50%–75% loss of cells, 25%–50% loss of cells, <25% loss of cells. This analysis was performed by a trained observer blind to treatment group. These categories were then analyzed for association with hippocampal ChAT activity and with DMP performance by ANOVA.
RESULTS
Serum levels of E2
Levels of E2 in non-E-treated rats were below the level of detection. Mean serum levels of E2 in E-treated rats were 28.6 ± 1.9 pg/mL and 29.9 ± 7.5 pg/mL for E- and Don+E-treated rats. These values did not differ significantly from each other.
Degree and specificity of the cholinergic lesions
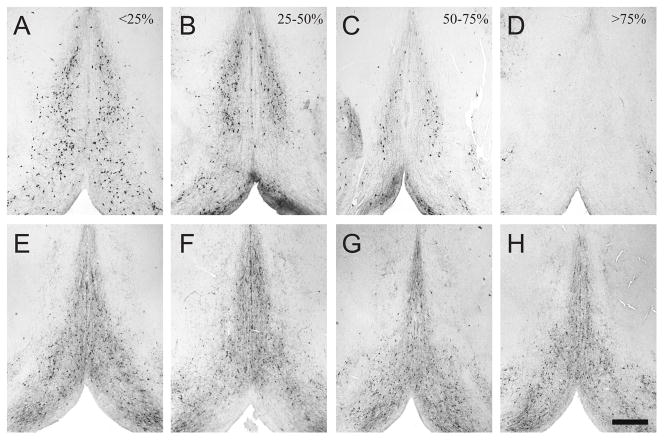
Photomicrographs of ChAT and Parv staining in sections from rats with good lesions and poor lesions are shown in Figure 1. Note the severe depletion of ChAT-IR cells in rats with very good lesions as well as the different degrees of cell loss among different rats. Effects on Parv staining in adjacent sections appeared minimal.
Figure 1.
Panels A–D show choline acetyltransferase (ChAT) immunoreactivity in the medial septum and vertical limb of the diagonal band of Broca of rats with different degrees of cholinergic cell loss. Panels E–H show parvalbumin (Parv) immunoreactivity in sections adjacent to those in the panels above. In all panels, dorsal is towards the top and midline extends top to bottom through the middle of the panel. The estimated percent loss of ChAT-IR cells is indicated in the upper right corner of panels A–D. Note that Parv-immunoreactive staining is relatively intact, even in rats with severe loss of ChAT-immunoreactive cells. Scale bar = 0.5 mm.
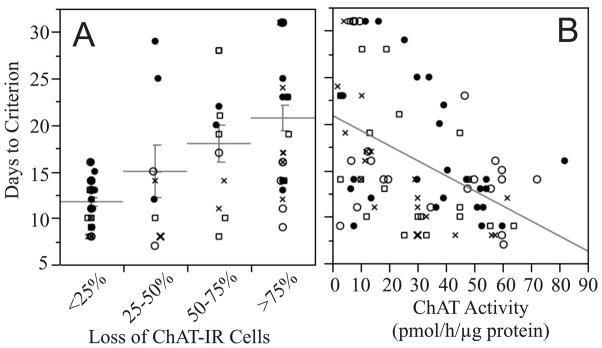
Severity of the lesions also was evaluated by measuring ChAT activity in the hippocampus. This was done to have two independent measures of lesion severity, and to evaluate the sensitivity of using hippocampal ChAT activity as an indicator of partial cholinergic denervation. Results of ANOVA indicate a highly significant association between ChAT activity and loss of ChAT-IR cells (F[3,77]=46.4, p<0.0001; Figure 2), indicating that these measures are in good agreement as indicators of lesion severity.
Figure 2.
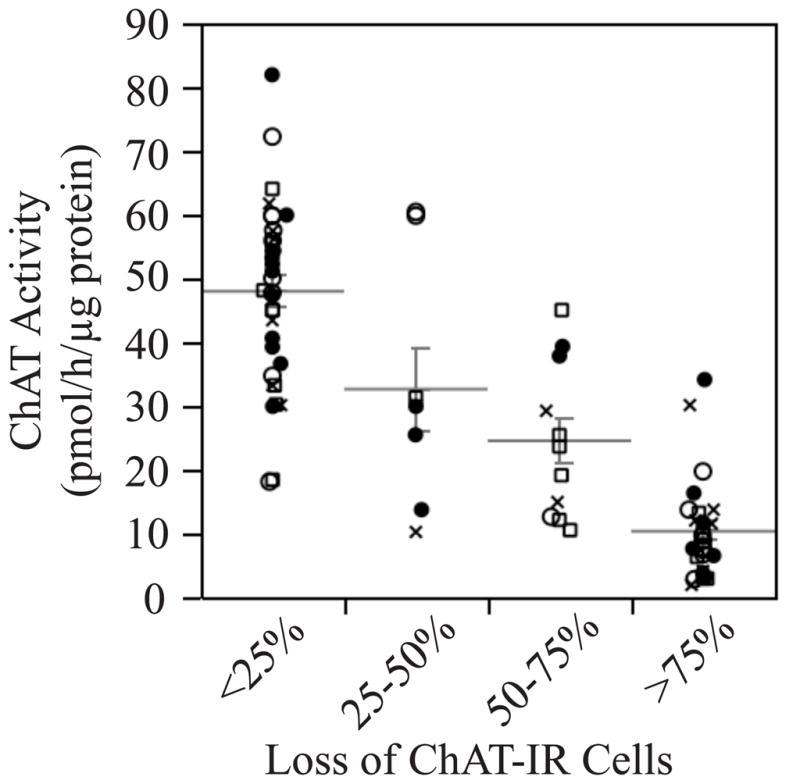
Plot showing a strong association between hippocampal choline acetyltransferase (ChAT) activity and loss of ChAT-immunoreactive cells in the medial septum. ●Controls; ○Don; □E2; ✕ Don+E2.
Data were then sorted into good lesions and poor lesions. Two methods were used. In one method, good lesions were defined as >50% loss of ChAT-IR cells in the medial septum. In another, good lesions were defined as rats with hippocampal ChAT activity below the overall mean of 30 pmol/h/μg protein. The data are summarized in (Table 1). Note that ChAT activity in the hippocampus of young Ovx rats without cholinergic lesions is typically in the range of 46–75 pmol/h/μg protein (Gibbs, 2000; Rudick et al., 2003), which is consistent with the present findings. In contrast, the mean ChAT activity in the hippocampus of middle-aged and aged Ovx rats is approximately 25–30 pmol/h/μg protein (Gibbs et al., 2009), which is similar to the cut-off used to separate poor vs. good lesions. As expected, ChAT activity was significantly greater in the hippocampus of rats with poor lesions vs. good lesions (F[1,73]=98.6, p<0.0001 for Method 1; F[1,73]=235.8, p<0.0001 for Method 2). This makes sense since most of the ChAT activity in the hippocampus is associated with projections from septal cholinergic neurons. The effect of treatment on ChAT activity was assessed by ANOVA in order to assess any effects of treatment on lesion severity. There was no significant effect of treatment and no significant interaction between treatment and lesion severity on ChAT activity.
Table 1.
Summary of Hippocampal choline acetyltransferase (ChAT) Activity by Treatment Group for Good and Poor Lesions
| Good Lesions Defined by loss of ChAT-IR Cells | Poor Lesions Defined by loss of ChAT-IR Cells | |||
|---|---|---|---|---|
| Tx | N | ChAT Activity pmol ACh/h/μg protein | N | ChAT Activity pmol ACh/h/μg protein |
| Controls | 11 | 20.1 ± 4.4 | 13 | 44.6 ± 3.7 |
| Don | 9 | 9.9 ± 1.6 | 11 | 52.3 ± 4.5 |
| E2 | 11 | 15.6 ± 3.7 | 9 | 40.1 ± 6.1 |
| Don+E2 | 9 | 13.3 ± 2.8 | 8 | 36.9 ± 7.4 |
| Good Lesions Defined by ChAT Activity | Poor Lesions Defined by ChAT Activity | |||
| Controls | 10 | 14.8 ± 3.3 | 14 | 48.7 ± 3.4 |
| Don | 10 | 10.7 ± 1.7 | 10 | 55.8 ± 3.1 |
| E2 | 11 | 13.1 ± 2.3 | 9 | 44.2 ± 3.8 |
| Don+E2 | 9 | 12.0 ± 2.6 | 8 | 42.8 ± 4.9 |
Effects of cholinergic lesions on DMP acquisition
The degree of cholinergic cell loss was analyzed for association with days to criterion (DTC). A highly significant association was observed by ANOVA (F[3,77]=13.1, p<0.0001), with DTC increasing with the severity of the lesion (Figure 3A). A highly significant correlation between DTC and hippocampal ChAT activity also was observed (F[1,79]=24.2, p<0.0001, adjusted RSquare = 0.22; Figure 3B).
Figure 3.
(A) Plots showing association between Days to Criterion and loss of choline acetyltransferase-immunoreactive (ChAT-IR) cells in the septum (A) or hippocampal ChAT activity (B). Note that rats take longer to reach criterion as the severity of ChAT-IR cell loss increases and hippocampal ChAT activity decreases. The relationship between loss of ChAT-IR Cells and DTC in panel A is highly significant (p<0.0001). Likewise, the relationship between hippocampal ChAT activity and DTC in panel B is highly significant (least squares regression line has adjusted RSquare=0.22, p<0.0001). ●Controls; ○Don; □E2; ✕ Don+E2.
Rats with >50% loss of ChAT-IR cells also were more likely to adopt a persistent turn during DMP training (87.5%) than rats with <50% loss of ChAT-IR cells (56.1%) (χ2(1)= 9.8, p<0.002), and to engage in a persistent turn for a greater number of days (10.2 ± 1.0 days vs. 2.7 ± 1.0 days; F[1,79]=28.1, p<0.0001). Plotting the number of days engaged in a persistent turn vs. lesion severity revealed that, like DTC, this measure increased in accordance with the severity of the lesions (supplemental Figures 1A & 1B). This was true regardless of whether lesion severity was based on cell counts (F[3,77]=10.6, p<0.0001) or on ChAT activity (F[1,79]=16.0, p<0.0001). Subtracting the number of days that rats engaged in a persistent turn from DTC eliminated the significant effect of lesion severity (supplemental Figures 1C & 1D), demonstrating that the increase in persistent turn accounted in large part for the effect on DTC.
Effects of Treatment on DMP acquisition
Initial analyses revealed no significant effects of treatment on DTC (χ2(3)= 3.2, p=0.36). Seven rats failed to reach criterion on the task (2 controls, 3 Don, 1 E2, 1 Don + E2). All of these rats had >50% loss of ChAT-IR cells. Therefore, we next tested whether treatments produced different effects in rats with good vs. poor lesions. Similar results were obtained when good lesions were defined based on ChAT activity or on ChAT-IR cell loss. Since ChAT activity is the more objective measure, only these results are summarized.
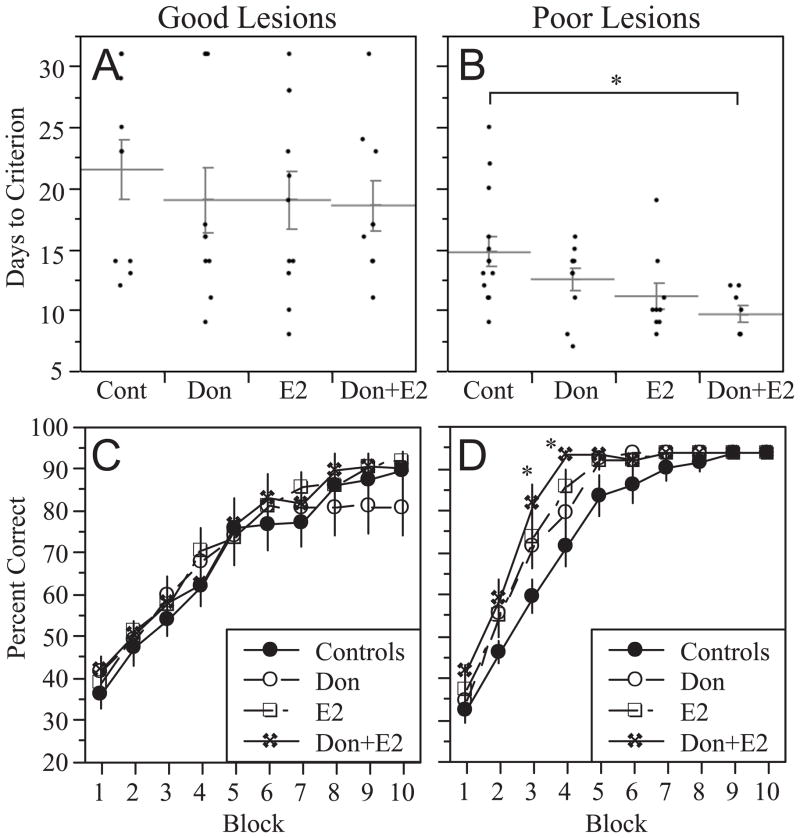
In rats with good lesions, there were no significant effects of treatment on days to criterion (χ2(3)= 0.60, p=0.90; Figure 4A) and no significant effects on the learning curves (Figure 4C). ANOVA with repeated measures on Block showed no effect of Treatment (F[3,36]=0.24, p=0.87), a significant effect of Block (F[9,324]=102.0, p<0.0001), and no Treatment x Block interaction (F[27,324]=0.8, p=0.78) for rats with good lesions. Hence, treatment was not effective in rats with good lesions. In contrast, rats with poor lesions showed a significant effect of treatment on days to criterion (F[3,37]=4.0, p<0.05; Figure 4B). Comparison of group means revealed that rats treated with Don+E2 took significantly fewer days to reach criterion than controls (9.6 ± 0.7 days 14.7 ± 1.2 days; p<0.05). Neither donepezil nor E2 alone had a significant effect. The learning curves likewise showed that rats with poor lesions that were treated with Don+E2 learned the task significantly faster than controls (Figure 4D). ANOVA with repeated measures on Block revealed a significant effect of Treatment (F[3,37]=4.5, p<0.01), a significant effect of Block (F[9,333]=204.6, p<0.0001), and a significant Treatment x Block interaction (F[27,333]=1.8, p<0.01). Post-hoc analyses showed that rats with poor lesions that were treated with Don+E2 performed significantly better than controls on Blocks 3 and 4.
Figure 4.
Plots summarizing the effects of treatment on delayed matching-to-position (DMP) acquisition for rats with good lesions (A & C) and poor lesions (B & D). Panels A & B illustrate effects on Days to Criterion whereas panels C & D show the learning curves. No significant effects of treatment in rats were observed in rats with good lesions; however, rats with poor lesions that were treated with Don+E2 took significantly fewer days to reach criterion than corresponding controls (panel B). Learning curves show that rats with poor lesions that were treated with Don+E2 performed significantly better than controls on blocks 3 and 4 of training (panel D). *p<0.05 relative to controls.
Effects on Persistent Turn
Next we tested whether treatments affected the predisposition of rats to adopt and maintain a persistent turn while performing the DMP task and whether this might account for the effects of Don+E2 on DMP acquisition. No significant effects were observed in rats with good lesions. Among rats with poor lesions, treatments significantly affected the percentage of rats that adopted a persistent turn during training (χ2(3)= 8.7, p<0.05). This percentage was greatest for the controls (85.7%) and for rats treated with Don alone (70.0%), and was significantly less for rats treated with E2 (33.3%) and Don+E2 (37.5%). Treatments also significantly affected the number of days that rats engaged in a persistent turn (F[3,37]=4.7, p<0.01) with controls exhibiting the greatest number of days (6.6 ± 1.4) followed by rats treated with Don (3.0 ± 1.0), Don+E2 (2.0 ± 1.0) and E2 (1.2 ± 0.8). Both E2 and Don+E2-treated groups differed significantly from controls.
Subtracting the number of days engaged in a persistent turn from DTC eliminated the significant effect of Treatment on performance in rats with poor lesions (F[3,37]=1.2, p=0.34; supplemental Figure 2). This suggests that the effect of Don+E2 on DTC in rats with poor lesions was due largely to an effect on persistent turn.
Post-Criterion Testing
Probe Trial
All rats that reached criterion received a probe trial in which the maze was rotated 180° between the forced and open choice. For rats with poor lesions, rotating the maze decreased performance to chance and there was no differential effect of treatment on arm choice during the open trial (χ2(3)= 3.0, p=0.39)(Figure 5). This suggests that rats with poor lesions relied on extramaze visual cues to some degree to perform the task. For rats with good lesions, there was a significant effect of treatment on arm choice during the open trial (χ2(3)= 8.5, p<0.05). Controls and Don-treated rats performed near chance levels, similar to rats with poor lesions; however, rats that received E2 alone were more likely to select the arm located in the same position of the room as the goal arm during the forced trial (8/10 rats). Rats that received Don+E2 were more likely to select the same physical arm of the maze that was entered during the forced trial (7/8 rats), even though it was now located in a different position of the room. This suggests that in rats with good lesions that were able to reach criterion, treatment with Don+E2 decreased the predisposition to rely on extramaze visual cues relative to rats treated with E2 alone.
Figure 5.
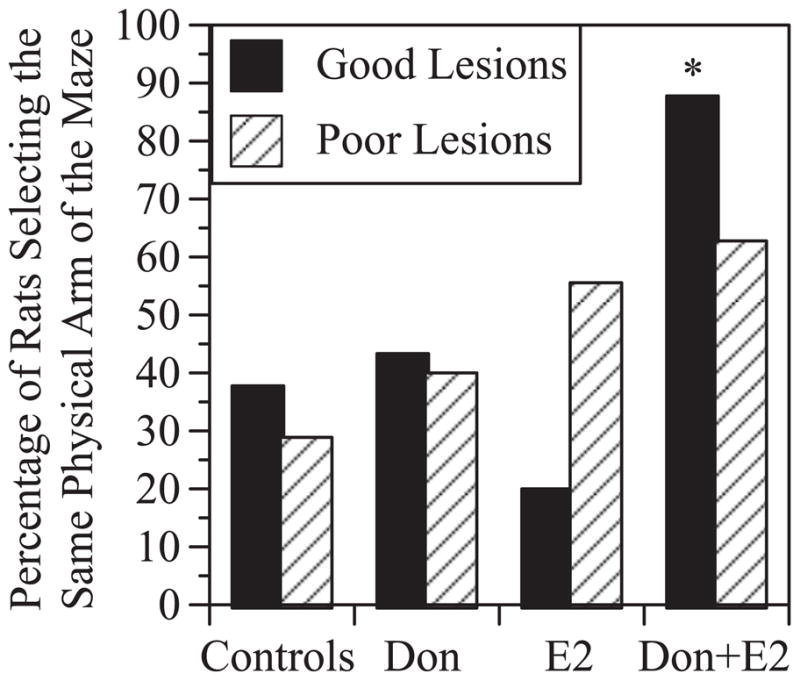
Effects of rotating the maze during the probe trial administered after rats reached criterion. The Y-axis indicates the percentage of rats that chose the same physical arm of the maze during the open trial, despite it’s altered position relative to extramaze cues. Results indicate that rotating the maze disrupted performance. No significant treatment effects were observed in rats with poor lesions. Among good lesions, rats treated with Don+E2 were more likely to choose the same physical arm of the maze despite it’s altered position than rats treated with E2 alone. *p<0.05 relative to rats with good lesions treated with E2 alone.
Effects of Delays
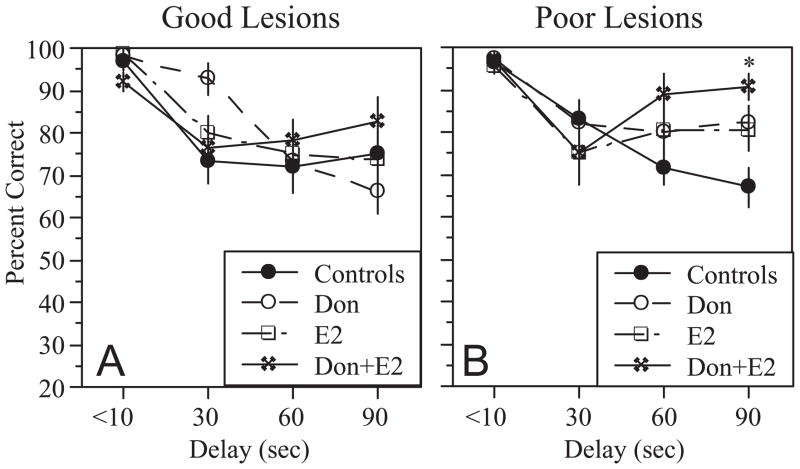
Following the probe trial, rats underwent testing with increasing delays between the forced and open trials. Increasing the delay impaired performance in rats with good and poor lesions (Figure 6). Among rats with good lesions there was a significant effect of Delay (F[3,87]=23.2, p<0.0001) as well as a significant Treatment x Delay interaction (F[9,87]=2.1, p<0.05). Post-hoc analysis, however, did not reveal any significant effects of treatment at individual delays (Figure 6A). Among rats with poor lesions, ANOVA revealed a significant effect of Delay (F[3,111]=18.9, p<0.0001) and a significant Treatment x Delay interaction (F[9,111]=2.8, p<0.01). In this case, post-hoc analysis showed that rats treated with Don+E2 performed significantly better than controls at the 90 second delay (Figure 6B), indicating an effect on delay-dependent performance.
Figure 6.
Effects of increasing the intertrial delay on performance in rats with good lesions (A) and poor lesions (B). Only rats that reached criterion were tested. Note that increasing the intertrial delay reduces performance accuracy in rats with good and poor lesions. No significant effect of treatment was observed in rats with good lesions; however, rats with poor lesions that were treated with Don+E2 performed significantly better that controls at the 90 second delay. *p<0.05 relative to controls.
DISCUSSION
Septal cholinergic lesions impair DMP acquisition
Our data confirm previous reports showing that septal cholinergic lesions significantly reduce the rate of DMP acquisition, as demonstrated by an increase in DTC (Gibbs and Johnson, 2007; Johnson et al., 2002). Additionally, we now show that even partial loss of septal cholinergic neurons affects days to criterion, and the effect increases with the degree of cell loss. This effect was due primarily to an increase in the predisposition to adopt and maintain a persistent turn during training. The effect was not due to a side bias or motor asymmetry since the behavior developed only after several days of training. It is more likely that the behavior reflects perseveration (Cutuli et al., 2009) or an increased preference to adopt an egocentric vs. allocentric learning strategy (Fitz et al., 2008). In addition, the effect on acquisition does not appear to be due to the loss of non-cholinergic neurons, since staining for GABAergic (Parv-containing) cells in the MS was not affected. Cholinergic neurons in the MS project primarily to the hippocampus. In contrast, cholinergic input to the frontal cortex originates primarily from neurons located in the nucleus basalis magnocellularis. Prior studies from our laboratory show that selectively destroying cholinergic neurons in the MS does not significantly affect ChAT activity in the frontal cortex (Gibbs and Johnson, 2007). Our data demonstrate a good correlation between loss of ChAT-IR cells in the septum and decreased ChAT activity in the hippocampus. In addition, the relationship between DTC and hippocampal ChAT activity was highly significant. We conclude, therefore, that the effects of the lesions on DMP acquisition were due primarily to cholinergic denervation of the hippocampus.
Combining Don + E2 enhanced DMP acquisition in rats with cholinergic lesions
The primary goal of this study was to test whether treatment with donepezil could restore beneficial effects of E2 on DMP acquisition in rats with septal cholinergic lesions. Results show that combining donepezil with E2 treatment was able to enhance DMP acquisition in rats with partial loss of septal cholinergic neurons. This was demonstrated by a significant reduction in DTC, as well as by a significant effect on the learning curves, for rats with poor lesions. Neither donepezil nor E2 alone produced significant effects. Inspection of the data suggests that the combination was not synergistic but may be additive, with each agent producing some improvement which when combined achieved significance. Treatments also significantly affected the number of days that rats engaged in a persistent turn, and much of the effect on DTC appeared to be due to a decrease in this behavior. Subtracting the number of days that rats engaged in a persistent turn eliminated the effect of Don+E2 on DTC. Rats with poor lesions that were treated with Don+E2 also performed better than controls when the delay between the forced choice and the open choice was increased. This suggests that some of the benefit on DMP acquisition also may be due to an improvement in spatial working memory. Collectively, these data demonstrate that donepezil and estradiol can be used effectively in combination to improve cognitive performance in rats with partial loss of hippocampal cholinergic afferents.
The effectiveness of combination therapy is limited by the degree of cholinergic cell loss
A second goal of this study was to determine whether the effectiveness of combining donepezil with E2 was dependent on the degree of cholinergic cell loss. None of the treatments had any significant effect on DMP acquisition in rats with good lesions. Good lesions were defined as >50% loss of septal ChAT-IR cells, or hippocampal ChAT activity less than half of the overall mean. Studies show that E2 alone significantly enhances DMP acquisition in ovariectomized rats without cholinergic lesions (Gibbs, 1999; Gibbs, 2002; Gibbs, 2007; Gibbs et al., 2004; Hammond et al., 2009). The fact that E2 failed to increase DMP acquisition in rats with good lesions is consistent with prior reports (Gibbs, 2002; Gibbs, 2007). It also is consistent with studies showing that inhibition of muscarinic cholinergic receptors prevents E2 enhancement of working memory (Daniel and Dohanich, 2001; Daniel et al., 2005), and with studies showing that cholinergic lesions reduce E2-mediated disinhibition of hippocampal CA1 synapses (Rudick et al., 2003), and prevent E2-mediated effects on the density of dendritic spines on CA1 neurons (Lam and Leranth, 2003). These findings demonstrate that cholinergic inputs to the hippocampus are required for E2-mediated effects on hippocampal function as well as for effects on specific cognitive tasks.
The fact the Don+E2 was ineffective at enhancing DMP acquisition in rats with good lesions suggests that combination therapy is ineffective when reductions in basal forebrain cholinergic function exceed a certain threshold. Cholinesterase inhibitors work by inhibiting the breakdown of acetylcholine at the synapse, thereby enhancing the effects of endogenous cholinergic afferents. Hence, sufficient cholinergic afferents must be present and functional in order for cholinesterase inhibitors to be effective. This could explain why combining donepezil with E2 was effective in rats with poor lesions, but not in rats with good lesions.
Comparisons with prior studies and Implications for therapy
The findings have several important implications for estrogen therapy in older women. The fact that E2 alone was ineffective in rats with poor lesions as well as good lesions suggests that even partial loss of cholinergic function is sufficient to reduce the beneficial effects of E2 on performance. The current data show that combining a cholinesterase inhibitor with E2 can reverse this effect provided that loss of cholinergic function is not too severe. These data are consistent with a prior study showing beneficial effects of combining donepezil with E2 in aged ovariectomized rats (Gibbs et al., 2009). As in the current study, the combination of Don+E2 enhanced the rate of DMP acquisition in aged rats by significantly decreasing the predisposition to engage in a persistent turn, whereas treatment with donepezil or E2 alone had no effect. The findings also are consistent with a report by Schneider et al. (Schneider et al., 1996) that postmenopausal women with AD and who also received estrogen therapy responded better to tacrine (a cholinesterase inhibitor formerly used to treat AD) than women not on estrogen therapy. These findings support the idea that combining a cholinesterase inhibitor with estrogen therapy may be beneficial in individuals experiencing cognitive decline associated with partial loss of basal forebrain cholinergic function.
The fact that Don+E2 was ineffective in rats with good lesions, however, suggests that the benefits of combination therapy may not occur once basal forebrain cholinergic impairment becomes too severe. This could explain why several studies failed to detect any beneficial effect of estrogen therapy on cognition in women with advanced AD (Henderson et al., 2000; Mulnard et al., 2004; Mulnard et al., 2000; Wang et al., 2000). It is well known that AD is associated with a substantial loss of basal forebrain cholinergic neurons in late stages of the disease, and it is likely that decreases in basal forebrain cholinergic function occur even in early and middle stages of the disease (Lanari et al., 2006; Linstow and Platt, 1999). Based on our findings, this would significantly curtail beneficial effects of E2 on cognitive performance. Studies also show that long-term loss of ovarian function has negative effects on basal forebrain cholinergic function in rats beyond the effects of normal aging (Gibbs, 1998; Gibbs, 2003). In women, long-term hormone deprivation has been associated with significant reductions in cholinergic synaptic terminals in multiple cortical brain regions (Smith et al., 2001). This could help explain the lack of beneficial effect of estrogen therapy on cognitive performance in older women who have not used estrogen therapy for many years, such as in the very large and highly publicized Women’s Health Initiative Memory Study (Coker et al., 2010). Use of co-medications with significant anti-cholinergic activity also could significantly curtail the beneficial effects of estrogen therapy on cognitive performance and contribute to diminished effect in specific patient populations (Carriere et al., 2009; Nebes et al., 2010; Vinogradov et al., 2009). Collectively, these findings suggest that combining a cholinesterase inhibitor with E2 therapy may be most effective in recently postmenopausal women or women with early signs of mild cognitive impairment or AD, and least effective in women with advanced AD or who are on medications with significant anti-cholinergic activity.
Whether it is possible to restore beneficial effects of E2 on cognitive performance in subjects with severe basal forebrain cholinergic deficits requires further study. Only one dose of donepezil was used in this study, and other doses may have different effects. Likewise, only one behavioral task was evaluated. Results need to be expanded to other cholinesterase inhibitors and to other estrogen-sensitive cognitive tasks, including tests of working and reference memory. Direct acetylcholine receptor (AChR) agonists may be more effective, since they activate the cholinergic receptors independent of endogenous cholinergic afferents. Recent data support this idea. One study tested the effects of nicotine (0.3 & 0.7 mg/Kg/d) on performance of a visuo-spatial orientation task by adult rats (Taylor and Maloney, 2010). Nicotine improved performance in both males and females; however, gonadally intact females that received nicotine at 0.3mg/Kg/d outperformed all other groups. Ovariectomized rats treated with 0.3 mg/Kg/d nicotine + E2 also performed better than other groups. Another study reported that chronic treatment with an α4β2-selective nAChR agonist in combination with E2 produced a pronounced anti-amnestic effect in a rat model of AD-type dementia (Sapronov et al., 2006). The effect was blocked by the nAChR antagonist mecamylamine. These data show that combining an acetylcholine receptor agonist, in this case a nicotinic receptor agonist, with E2 can significantly enhance cognitive performance in normal rats and in a rat model of AD. Whether similar effects occur in women needs to be investigated.
Conclusions
Our data suggest that even partial loss of cholinergic projections to the hippocampus reduces estradiol effects on a delayed matching-to-position T-maze task, and demonstrate that beneficial effects on performance can be restored by combining donepezil with estradiol treatment. The animals used in this study were only 3 months of age; however the results are similar to those observed in aged ovariectomized rats that were treated with Don +E2 (Gibbs et al., 2009). By extrapolation, similar effects in women could be beneficial for older women who have not used hormone therapy for many years since menopause, and for women with signs of mild cognitive impairment or early AD. Further studies are necessary to see if effects generalize to other cognitive tasks and cholinesterase inhibitors, and to evaluate the efficacy of this approach in women.
Supplementary Material
Supplemental Figure 1: Plots showing that the number of days that rats engaged in a persistent turn increased significantly with the loss of choline acetyltransferase-immunoreactive (ChAT-IR) cells (A) and in association with decreased hippocampal ChAT activity (B). The relationship between loss of ChAT-IR Cells and number of days engaged in a persistent turn in panel A is highly significant (p<0.0001). Likewise, the relationship between hippocampal ChAT activity and the number of days engaged in a persistent turn in panel B is highly significant (least squares regression line has adjusted RSquare=0.16, p<0.0001). Panels C & D show that when the number of days that rats engage in a persistent turn is subtracted from DTC, the relationship between DTC and lesion severity is no longer significant (compare with Figures 3A and 3B). ●Controls; ○Don; □E2; ✕ Don+E2.
Supplemental Figure 2: Plots showing days to criterion (DTC) minus the number of days that rats engaged in a persistent turn as a function of treatment for rats with good lesions (A) and poor lesions (B). Note that when the number of days that rats engaged in a persistent turn is subtracted from DTC, the effect of treatment in rats with poor lesions is no longer observed (compare panel B with Figure 4B).
Acknowledgments
This work was supported by NIH grant 1R21AG031794.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal P, Gibbs RB. Estrogen replacement does not prevent the loss of choline acetyltransferase-positive cells in the basal forebrain following either neurochemical or mechanical lesions. Brain Research. 2000;882:75–85. doi: 10.1016/s0006-8993(00)02832-8. [DOI] [PubMed] [Google Scholar]
- Baskerville KA, Kent C, Nicolle MM, Gallagher M, McKinney M. Aging causes partial loss of basal forebrain but no loss of pontine reticular cholinergic neurons. Neuroreport. 2006;17:1819–23. doi: 10.1097/WNR.0b013e32800fef5a. [DOI] [PubMed] [Google Scholar]
- Carriere I, Fourrier-Reglat A, Dartigues JF, Rouaud O, Pasquier F, Ritchie K, Ancelin ML. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med. 2009;169:1317–24. doi: 10.1001/archinternmed.2009.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Gaussoin S, Dailey M, Shumaker SA. Postmenopausal hormone therapy and cognitive outcomes: the Women’s Health Initiative Memory Study (WHIMS) J Steroid Biochem Mol Biol. 2010;118:304–10. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutuli D, Foti F, Mandolesi L, De Bartolo P, Gelfo F, Federico F, Petrosini L. Cognitive Performances of Cholinergically Depleted Rats Following Chronic Donepezil Administration. J Alzheimers Dis. 2009;17:161–176. doi: 10.3233/JAD-2009-1040. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–95. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147:607–14. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Lee CD. Role of hippocampal M2 muscarinic receptors in the estrogen-induced enhancement of working memory. Neuroscience. 2005;132:57–64. doi: 10.1016/j.neuroscience.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Fischer W, Chen KS, Gage FH, Bjorklund A. Progressive decline in spatial learning and integrity of forebrain cholinergic neurons in rats during aging. Neurobiol Aging. 1992;13:9–23. doi: 10.1016/0197-4580(92)90003-g. [DOI] [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA. Selective lesion of septal cholinergic neurons in rats impairs acquisition of a delayed matching to position T-maze task by delaying the shift from a response to a place strategy. Brain Res Bull. 2008;77:356–60. doi: 10.1016/j.brainresbull.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. Preclinical data relating to estrogen’s effects on cognitive performance. In: Rasgon NL, editor. The Effects of Estrogen on Brain Function. The Johns Hopkins University Press; Baltimore: 2006. pp. 9–45. [Google Scholar]
- Gibbs RB. Impairment of basal forebrain cholinergic neurons associated with aging and long-term loss of ovarian function. Exp Neurol. 1998;151:289–302. doi: 10.1006/exnr.1998.6789. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Hormones & Behavior. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal Forebrain Cholinergic Neurons are Necessary for Estrogen to Enhance Acquisition of a Delayed Matching-To-Position T-maze Task. Horm & Behav. 2002;42:245–257. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of ageing and long-term hormone replacement on cholinergic neurones in the medial septum and nucleus basalis magnocellularis of ovariectomized rats. J Neuroendocrinol. 2003;15:477–85. doi: 10.1046/j.1365-2826.2003.01012.x. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–9. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–53. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–748. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Cholinergic lesions produce task-selective effects on delayed matching to position and configural association learning related to response pattern and strategy. Neurobiol Learn Mem. 2007;88:19–32. doi: 10.1016/j.nlm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm Behav. 2009;56:73–83. doi: 10.1016/j.yhbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–14. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson VW. The critical window hypothesis: hormone exposures and cognitive outcomes after menopause. In: Hogervorst E, et al., editors. Hormones, Cognition and Dementia: State of the Art and Emergent Therapeutic Strategies. Cambridge University Press; Cambridge, UK: 2009. pp. 131–177. [Google Scholar]
- Henderson VW, PaganiniHill A, Miller BL, Elble RJ, Reyes PF, Shoupe D, McCleary CA, Klein RA, Hake AM, Farlow MR. Estrogen for Alzheimer’s disease in women - Randomized, double-blind, placebo-controlled trial. Neurology. 2000;54:295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Zambon NJ, Gibbs RB. Selective lesion of cholinergic neurons in the medial septum by 192 IgG-saporin impairs learning in a delayed matching to position T-maze paradigm. Brain Research. 2002;943:132–141. doi: 10.1016/s0006-8993(02)02623-9. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Casadesus G. Estrogen-mediated effects on cognition and synaptic plasticity: What do estrogen receptor knockout models tell us? Biochim Biophys Acta. 2010;1800:1090–3. doi: 10.1016/j.bbagen.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam TT, Leranth C. Role of the medial septum diagonal band of Broca cholinergic neurons in oestrogen-induced spine synapse formation on hippocampal CA1 pyramidal cells of female rats. Eur J Neurosci. 2003;17:1997–2005. doi: 10.1046/j.1460-9568.2003.02637.x. [DOI] [PubMed] [Google Scholar]
- Lanari A, Amenta F, Silvestrelli G, Tomassoni D, Parnetti L. Neurotransmitter deficits in behavioural and psychological symptoms of Alzheimer’s disease. Mech Ageing Dev. 2006;127:158–65. doi: 10.1016/j.mad.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Linstow Ev, Platt B. Biochemical dysfunction and memory loss: the case of Alzheimer’s dementia. Cell Mol Life Sci. 1999;55:601–616. doi: 10.1007/s000180050318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Sex steroids and cognitive function. J Neuroendocrinol. 2008;20:866–72. doi: 10.1111/j.1365-2826.2008.01710.x. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–95. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. The systems-level organization of cholinergic innervation in the human cerebral cortex and its alterations in Alzheimer’s disease. Prog Brain Res. 1996;109:285–97. doi: 10.1016/s0079-6123(08)62112-3. [DOI] [PubMed] [Google Scholar]
- Mulnard RA, Corrada MM, Kawas CH. Estrogen replacement therapy, Alzheimer’s disease, and mild cognitive impairment. Curr Neurol Neurosci Rep. 2004;4:368–73. doi: 10.1007/s11910-004-0083-8. [DOI] [PubMed] [Google Scholar]
- Mulnard RA, Cotman CW, Kawas C, Dyck CHv, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen Replacement Therapy for Treatment of Mild to Moderate Alzheimer Disease. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Halligan EM, Houck P, Saxton JA. Cognitive Slowing Associated With Elevated Serum Anticholinergic Activity in Older Individuals is Decreased by Caffeine Use. Am J Geriatr Psychiatry. 2010 doi: 10.1097/JGP.0b013e3181e4490d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Atlas. Academic Press; London: 1986. [Google Scholar]
- Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen, and cognitive aging: the timing hypothesis. Neurodegener Dis. 2010;7:163–6. doi: 10.1159/000289229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick CN, Gibbs RB, Woolley CS. A role for the basal forebrain cholinergic system in estrogen-induced disinhibition of hippocampal pyramidal cells. J Neurosci. 2003;23:4479–90. doi: 10.1523/JNEUROSCI.23-11-04479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapronov NS, Fedotova YO, Kuznetsova NN. Antiamnestic effect of alpha7-nicotinic receptor agonist RJR-2403 in middle-aged ovariectomized rats with Alzheimer type dementia. Bull Exp Biol Med. 2006;142:700–2. doi: 10.1007/s10517-006-0455-y. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Farlow MR, Henderson VW, Pogoda JM. Effects of estrogen replacement therapy on response to tacrine in patients with Alzheimer’s disease. Neurology. 1996;46:1580–4. doi: 10.1212/wnl.46.6.1580. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5:620–7. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Smith CC, Vedderb LC, Nelsonb AR, Bredemannb TM, McMahon LL. Duration of Estrogen Deprivation, Not Chronological Age, Prevents Estrogen’s Ability to Enhance Hippocampal Synaptic Physiology. PNAS. 2010 doi: 10.1073/pnas.1009307107. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:10893–8. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Minoshima S, Kuhl DE, Zubieta JK. Effects of long-term hormone therapy on cholinergic synaptic concentrations in healthy postmenopausal women. J Clin Endocrinol Metab. 2001;86:679–84. doi: 10.1210/jcem.86.2.7222. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Ogura H, Arai Y, Limura Y, Yamanishi Y. Research and development of donepezil hydrochloride, a new type of acetylcholinesterase inhibitor. Jpn J Pharmacol. 2002;89:7–20. doi: 10.1254/jjp.89.7. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–15. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–63. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GT, Maloney S. Gender differences and the role of estrogen in cognitive enhancements with nicotine in rats. Pharmacol Biochem Behav. 2010;95:139–45. doi: 10.1016/j.pbb.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166:1055–62. doi: 10.1176/appi.ajp.2009.09010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PN, Liao SQ, Liu RS, Liu CY, Chao HT, Lu SR, Yu HY, Wang SJ, Liu HC. Effects of estrogen on cognition, mood, and cerebral blood flow in AD: a controlled study. Neurology. 2000;54:2061–6. doi: 10.1212/wnl.54.11.2061. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Plots showing that the number of days that rats engaged in a persistent turn increased significantly with the loss of choline acetyltransferase-immunoreactive (ChAT-IR) cells (A) and in association with decreased hippocampal ChAT activity (B). The relationship between loss of ChAT-IR Cells and number of days engaged in a persistent turn in panel A is highly significant (p<0.0001). Likewise, the relationship between hippocampal ChAT activity and the number of days engaged in a persistent turn in panel B is highly significant (least squares regression line has adjusted RSquare=0.16, p<0.0001). Panels C & D show that when the number of days that rats engage in a persistent turn is subtracted from DTC, the relationship between DTC and lesion severity is no longer significant (compare with Figures 3A and 3B). ●Controls; ○Don; □E2; ✕ Don+E2.
Supplemental Figure 2: Plots showing days to criterion (DTC) minus the number of days that rats engaged in a persistent turn as a function of treatment for rats with good lesions (A) and poor lesions (B). Note that when the number of days that rats engaged in a persistent turn is subtracted from DTC, the effect of treatment in rats with poor lesions is no longer observed (compare panel B with Figure 4B).