Abstract
This study examined whether the sensory neuropeptide calcitonin gene-related peptide (CGRP) inhibits release of chemokines by dermal microvascular endothelial cells. Dermal blood vessels are associated with nerves containing CGRP, suggesting that CGRP-containing nerves may regulate cutaneous inflammation through effects on vessels. We examined CGRP effects on stimulated chemokine production by a human dermal microvascular endothelial cell line (HMEC-1) and primary human dermal microvascular endothelial cells (pHDMECs). HMEC-1 cells and pHDMECs expressed mRNA for components of the CGRP and adrenomedullin receptors and CGRP inhibited LPS-induced production of the chemokines CXCL8, CCL2, and CXCL1 by both HMEC-1 cells and pHDMECs. The receptor activity-modifying protein (RAMP)1/calcitonin receptor-like receptor (CL)-specific antagonists CGRP8-37 and BIBN4096BS, blocked this effect of CGRP in a dose-dependent manner. CGRP prevented LPS-induced IκBα degradation and NF-κB binding to the promoters of CXCL1, CXCL8 and CCL2 in HMEC-1 cells and Bay 11-7085, an inhibitor of NF-κB activation, suppressed LPS-induced production of CXCL1, CXCL8 and CCL2. Thus, the NF-κB pathway appears to be involved in CGRP-mediated suppression of chemokine production. Accordingly, CGRP treatment of LPS-stimulated HMEC-1 cells inhibited their ability to chemoattract human neutrophils and mononuclear cells. Elucidation of this pathway may suggest new avenues for therapeutic manipulation of cutaneous inflammation.
Keywords: CGRP, endothelial cell, chemotaxis, NF-κB, neutrophil, mononuclear cell
1. Introduction
Dermal microvascular endothelial cells contribute to cutaneous inflammation through many mechanisms. Amongst these is the capacity to release chemokines that play a role in recruiting inflammatory cells (Goebeler et al., 1997; Bender et al., 2008). Hence, as endothelial-derived chemokines play a crucial role in inflammation and disease, regulation of their expression presents an approach to the management of inflammatory skin disease.
Calcitonin gene-related peptide (CGRP) is a 37-amino acid neuropeptide generated by tissue-specific alternative processing of the calcitonin gene (Amara et al., 1982; Rosenfeld et al., 1983). It is widely distributed in organs of the immune system as well as the central and peripheral nervous systems and is generally co-expressed with either somatostatin or substance P in sensory neurons (Brain and Williams, 1988; Zaidi et al., 1990; Brain, 1997). CGRP attenuates various immune responses including inhibition of the production of Th1-type cytokines, attenuation of interleukin (IL)-1β induced reactive oxygen species in alveolar epithelial type II cells (Li et al., 2006), inhibition of mitogen-stimulated T lymphocyte and thymocyte proliferation (Umeda et al., 1988; Bulloch et al., 1991; Bulloch et al., 1998), and suppression of the production of IL-2 and other cytokines by CD4+ Th1 cell clones (Wang et al., 1992). CGRP can also function as a mediator of inflammation; it is a potent endogenous vasodilator and is involved in the accumulation of inflammatory cells in areas of inflammation (Li and Wang, 2006; Hartung and Toyka, 1989; Merhi et al., 1998; Benrath et al., 1995). In accord with these observations, CGRP enhances adhesion of neutrophils to endothelium in vitro (Zimmerman et al., 1992). However, in other experiments CGRP was found to inhibit inflammation not associated with adaptive immunity. For example, CGRP inhibits edema-promoting actions of inflammatory mediators (histamine, leukotrine B4, 5-hydroxytryptamine) in vivo in the hamster cheek pouch, human skin, and rat paw (Raud et al., 1991). Additionally, systemic treatment of mice with CGRP reduced blood neutrophilia induced by systemic administration of LPS and also protected against a lethal dose of LPS (Gomes et al., 2005).
With regard to adaptive immunity, CGRP-containing epidermal nerves are intimately associated with Langerhans cells (LC) in the skin and CGRP inhibits antigen presentation by LC in vitro (Hosoi et al., 1993). After intradermal administration, CGRP inhibits the induction of contact hypersensitivity at the injected site in mice (Asahina et al., 1995; Niizeki et al., 1997).
Given these findings and the knowledge that dermal blood vessels are associated with CGRP-containing nerves (Garcia-Caballero et al., 1989), we wished to examine whether CGRP regulates chemokine expression by dermal microvascular endothelial cells. In the present study, we investigated the effect of CGRP on the expression of the chemokines CXCL8 (interleukin-8), CCL2 (monocyte chemotactic protein-1), and CXCL1 (growth related oncogene-alpha) by activated HMEC-1 cells [a transformed human dermal microvascular endothelial cell (HDMEC) line] and pHDMECs and explored possible mechanisms of this effect. We have also shown directly that CGRP inhibits the ability of stimulated HMEC-1 cells or supernatants conditioned by these cells to chemoattract neutrophils or mononuclear cells. We have chosen these chemokines for study as previous work has demonstrated that stimulated HDMECs produce them (Bender et al., 2008; Seiffert et al., 2006) and they are known to play significant roles in cutaneous inflammation, wound healing and other cutaneous pathologies (Zaja-Milatovic and Richmond, 2008; Britschgi and Pichler, 2002; Dearman et al., 2004; Yamamoto, 2003).
This study has identified a novel mechanism by which CGRP may participate in the attenuation of inflammatory responses. There has been much attention to a putative role for stress-induced neurologic mediators playing a role in stress-induced exacerbation of inflammatory skin disorders (Pavlovic et al., 2008; Amano et al., 2008; Seiffert et al., 2006) and a possible role for stress reduction and avoidance behavior in ameliorating such disorders (Farber and Nall, 1993; Arndt et al., 2008). These findings may suggest a mechanism by which the nervous system can limit inflammation. They also may suggest new therapeutic approaches to the treatment of inflammatory skin disease.
2. Materials and Methods
2.1. Reagents and peptides
αCGRP and CGRP8-37 were purchased from Peninsula Laboratories (Bachem America, Torrance, CA). The CGRP antagonist BIBN4096BS was a kind gift from Dr. Henri Doods (Biological Research, Boehringer Ingelheim Pharma KG, Ingelheim, Germany); LPS (E. coli 0111:B4), 8-bromoadenosine-3′, 5′-cyclic monophosphorothioate (Rp-8-Br-cAMPS), bisindolylmaleimide VIII acetate (Ro 31-7549) and Bay 11-7085 were purchased from Sigma-Aldrich, St. Louis, MO, USA; human CXCL8, CXCL1 and CCL2 ELISA kits, and Parameter™ cAMP assay kits were purchased from R&D Systems, Minneapolis, MN, USA. Rabbit polyclonal anti-mouse IκBα was purchased from Cell Signaling Technology; anti-p50 and anti-p65 rabbit polyclonal antisera were purchased from Santa Cruz Biotechnology, Santa Cruz, CA, USA.
2.2. Media and cells
HMEC-1 cells were the kind gift of T.J. Lawley (Emory University, Atlanta, GA, USA). HMEC-1 cells were cultured in endothelial cell basal medium (Lonza, Walkersville, VA, USA) containing 10% fetal bovine serum (FBS, Gemini Bio-Products, West Sacramento, CA, USA), 10 ng/ml epidermal growth factor (BD Biosciences, San Jose, CA, USA), 1 μg/ml hydrocortisone (Sigma-Aldrich), and 100 U/ml penicillin/100 mg/ml streptomycin (Mediatech, Manassas, VA, USA). For all experiments using HMEC-1 cells other than examination of mRNA expression for components of CGRP and AM receptors, the cells were kept in endothelial cell basal medium supplemented only with 2% FBS and penicillin/streptomycin (depleted medium) without epidermal growth factor or hydrocortisone overnight and during the experimental procedures. Primary cultures of neonatal foreskin-derived HDMEC were obtained commercially (Lonza). pHDMECs were cultured in endothelial cell EBM-2 basal medium (Lonza) supplemented with 5% FBS, hydrocortisone, hFGF-B, VEGF, R3-IGF-1, ascorbic acid, HEGF, gentamicin, and amphotericin B (EGM-2 MV Bulletkit, Lonza). For all experiments other than examination of mRNA expression for components of CGRP and AM receptors, pHDMECs were kept in depleted medium (EBM-2 basal medium with 5% FBS) for 6 hrs prior to and during the experimental procedures.
The decision to use the transformed human dermal microvascular EC line HMEC-1 (immortalized by simian virus 40 transformation) as a surrogate for HDMEC was based on its relatively flexible growth requirements, homogeneity (Ades et al., 1992; Pruckler et al., 1993) and its retention of the properties of HDMEC including cell adhesion molecule expression and cytokine production (Xu et al., 1994), providing us with a reliable source of endothelial cells.
2.3. RNA isolation and RT-PCR
Total RNA was extracted from HMEC-1 cells and pHDMECs using a total RNA extraction kit as per the manufacturer’s instructions (Qiagen, Valencia, CA, USA). A genomic DNA eliminator spin column was used to remove DNA from the samples. Total RNA treated with RNase-free DNase (Qiagen) (500 ng per 20 μl reaction) was reverse-transcribed into cDNA using Superscript™ II reverse transcriptase (Invitrogen, Carisbad, CA), following the manufacturer’s instructions. Specific primers were constructed for the human calcitonin receptor-like receptor (CL), CGRP-receptor component protein (CRCP), and receptor activity modifying proteins (RAMPs) 1 through 3 on the basis of sequences published in GenBank: CL 5′-TCAAGAGCCTAAGTTGCCAAA-3′ (sense) and 5′-AATCAGCACAAATT CAATGCC-3′ (anti-sense); CRCP, 5′-AACTGATCTGAAAGAGCAGCG-3′ (sense) and 5′-TCTTCTTCTGCTCAGCCTCTG-3′ (anti-sense); human RAMP1, 5′-GAGACGCTGTGGTGTGACTG-3′ (sense) and 5′-TCGGCTACTCTGGACTCCTG-3′ (anti-sense) (Swerlick and Lawley, 1993); RAMP2, 5′-GGGGGACGGTGAAGAACTAT-3′ (sense) and 5′-GTTGGCAAAGTGGATCTGGT-3′ (anti-sense) (Gupta et al., 2006) and RAMP3, 5′-TCGTGGGCTGCTACTGG-3′ (sense) and 5′-CTCACAGCAGCGTGTCG-3′ (anti-sense) (Nikitenko et al., 2001). PCR was performed by transferring 2 μl of cDNA to a PCR mixture containing 200 nM of each specific primer and platinum PCR Supermix (Invitrogen, Carlsbad, CA, USA) for a total volume of 25 μl, using a thermal cycler (Gene AMP PCR System 9700; Perkin Elmer, Waltham, MA, USA). PCR products were resolved on a 1.5% agarose gel and visualized by ethidium bromide.
2.4. DNA sequencing
PCR products were gel isolated and DNA was extracted using a QIAquick Gel Extraction kit as per the manufacturer’s instructions (Qiagen). DNA was sequenced by the Cornell University Life Sciences Core Laboratories Center.
2.5. Chemokine determinations
To measure CXCL8, CCL2 and CXCL1 secretion by HMEC-1 cells and pHDMECs, cells were plated at a concentration of 0.25×106 cells/well in twelve-well plates in triplicate in 2 ml of depleted medium. After overnight incubation for HMEC-1 cells or 6-h incubation for pHDMECs, cells were treated with or without 1 μg/ml LPS in the presence or absence of CGRP with CGRP added 1 h prior to LPS. Supernatants were harvested at the indicated times. CXCL8, CCL2 and CXCL1 production was quantified by sandwich ELISA following the manufacturer’s instructions. In experiments examining the effects of inhibitors of the CGRP1 receptor, the inhibitor was added to cells 5 min prior to addition of CGRP and, in these experiments, LPS was added immediately following addition of CGRP.
2.6. RNA extraction and Northern blot analysis
HMEC-1 cells were cultured at a concentration of 1.5×106 cells per 100 mm tissue culture dish in 10 ml of depleted medium and stimulated with or without LPS (1 μg/ml) in the presence or absence of CGRP (10 nM) for up to 12 h. CGRP was added 1 hr prior to addition of LPS. Cells were collected and total cellular RNA extracted by TRIzol (Invitrogen) according to the manufacturer’s protocol. Briefly, HMEC-1 cells were lysed in TRIzol, followed by extraction with chloroform and precipitation with isopropanol. The RNA was resuspended in diethylpyrocarbonate-treated water and quantitated spectrophotometrically at 260/280 nm. Fifteen μg of total RNA from each sample was electrophoresed on a 1.5% agarose-formadehyde gel, transferred in 20 × saline sodium citrate (SSC) onto a Hybond-N+ membrane (Amersham, GE Healthcare, Piscataway, NJ, USA) and cross-linked to the membrane using UV light.
Probes for human CXCL8, CCL2 and CXCL1 were generated by RT-PCR using specific primers for CCL2, 5′-ATGAAAGTCTCTGCCGCC-3′ (sense) and 5′-TTGCTTGTCCA GGTGGTC-3′ (anti-sense); CXCL1, 5′-ATGGCCCGCGCTGCTCTCTCC-3′ (sense) and reverse 5′-GTTGGATTTGTCACTGTTCAG-3′ (anti-sense) and CXCL8, 5′-TTG GCAGCCTTCCTGATTTC-3′ (sense) and reverse 5′-AACTTCTCCACAACCCTCTG CA-3′ (anti-sense). Oligonucleotides were end-labeled with 32P-dCTP (Perkin-Elmer) by using Klenow kinase (Invitrogen). The RNA-containing membranes were pre-hybridized for 24 h at 42°C and hybridized for 24 h at 42°C with labeled probes [106 cpm] in Hybrisol®-1 buffer (Millipore, Billerica, MA). The membranes were washed twice in 2 × SSC containing 0.1% SDS (20 min; 25°C) and once with 0.1% SSC containing 0.1% SDS (10 min; 55°C). The membranes were then exposed to X-ray film (Kodak, Rochester, NY, USA). The intensity of the transcript was digitized and quantified using a phosphor imaging system (Typhoon Trio+, GE Healthcare) and then normalized to the intensity of β-actin mRNA with results expressed as fold-increase compared with the level obtained with medium alone.
2.7. Detection of IκBα
IκBα was detected by Western blotting with antibodies specific to IκBα. Five hundred thousand HMEC-1 cells per well in a six-well plate were cultured in depleted EBM medium for 24 h, they were then stimulated with or without LPS in the absence or presence of 10 nM of CGRP, for indicated time. The cells in each well were lysed by adding 250 μl of cell lysis buffer (20 mM Tris-HCL, pH 7.5; 150 mM NaCl; 1 mM Na2EDTA; 1 mM EGTA; 1% Triton; 2.5 mM sodium pyrophosphate; 1 mM glycerophosphate; 1 mM NaVO4; 1 μg/ml leupeptin, 1 mM phenylmethylsulfonylfluoride). Cells were then sonicated for 15 seconds and microcentrifuged for 5 min. The protein concentration in each sample was quantitative by the bicinchoninic acid assay. Twenty-five micrograms of cell lysates were separated on a 7.5% SDS-PAGE gel and then electrotransferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline buffer with 0.1% Tween-20 for 1 h and then incubated with 1:1,000 diluted of rabbit polyclonal anti-mouse IκBα antibodies (Cell Signaling Technology, Boston, MA, USA) overnight at 4°C. The membrane was washed three times and incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000 dilution) for 1 h, and proteins were detected by a chemiluminescence detection system (Amersham, GE Healthcare).
2.8. Preparation of nuclear extracts
HMEC-1 cells were plated at a density of 1.5×106 cells/plate in 25 cm2 tissue culture dishes and cultured in depleted EBM medium for 24 h. Cells were then cultured for 1 h in the presence or absence of CGRP followed by stimulation with or without LPS in the continued presence or absence of CGRP for 4 h. Cells were then washed twice with ice-cold PBS and pelleted. Cell pellets were homogenized with 400 μl of cell lysis buffer (10 mM HEPES, PH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 0.5mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 5 mM NaF, 1 mM Na3VO4, and 1 mM NaN3). After a 10 minute ice bath, Nonidet P-40 was added to a final concentration of 0.5% and nuclei were isolated by centrifugation at 10,000 × g for 1 min. Pelleted nuclei were lysed by incubation for 30 min on ice in 50 μl of nuclear lysis buffer (20 mM HEPES, pH 7.9, 420 mM NaCl, 0.2 mM EDTA, 0.2 mM EGTA, 1.5 mM MgCl2. 40 mM KCl, 25% glycerol, 1 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl and 1 μg/ml lepeptin) with agitation. Supernatants containing nuclear protein were harvested by centrifugation for 15 min at 12,000 × g at 4°C and the protein concentration was determined. Aliquots were stored at −80°C
2.9. Electrophoretic mobility shift assay (EMSA)
Double-stranded oligonucleotides corresponding to the NF-κB sites of murine CXCL8 (AAATC GTGGAATTTCCTCTGACAT), CCL2 (CTCATGGAAGATCCCTCCTCCT) and CXCL1 (GAACTCCGGGAATTTCCCTGGC CC) promoters were end-labeled with 32P-ATP to a specific activity of 0.3–1.0 × 106 cpm/ng. Five μg of nuclear extracts from each test were incubated with the labeled oligonucleotide probe (2–4 × 104 cpm) in 15 μl of reaction mixture containing 20 mM HEPES (pH 7.9), 1 mM EDTA, 60 mM KCL, 12% glycerol, 1 mM dithiothreitol, 2 μg poly(dI-dC) at room temperature for 20 min. The samples were loaded onto 4.8% nondenaturing polyacrylamide gel and electrophoresed in TBE buffer (45 mM Tris-HCl, PH 8.4, 1 mM EDTA, 45 mM boric acid) at 4°C, followed by drying of the gel and autoradiography. In competition and antibody supershift experiments, nuclear extracts were incubated for 15 min at room temperature with 1 μg of anti-rabbit polyclonal anti-p50 and 1 μg of anti-rabbit polyclonal anti-p65 (Santa Cruz Biotechnology) before the addition of the labeled probe.
2.10. Neutrophil and mononuclear cell isolation
Blood was drawn from healthy donors using a protocol approved by the Weill Cornell Medical College Institutional Review Board. Neutrophils were isolated from heparinized human blood using Percoll Plus (GE Healthcare, Piscataway, NJ). Fifteen ml of a 1.088 density was prepared by mixing 9.5 ml of Percoll Plus with 1.5 ml of 10X Hanks balanced salt solution and 4 ml of H2O in a 50 ml conical centrifuge tube. Thirty ml of blood diluted 1:3 with PBS was overlayed onto the 15 ml of Percoll Plus in each of several tubes. Tubes were then centrifuged at 400 × g for 30 min at 20°C. Neutrophils were collected from the layer directly above the red blood cells. Red blood cells in the neutrophil preparation were lysed by hypotonic lysis buffer followed by washing 3 times with PBS containing 10 mM Hepes and 0.1% bovine serum albumin. Mononuclear cells were isolated using Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ). Fifteen ml of Ficoll-Paque Plus was overlayed with 30 ml of diluted blood in each of several centrifuge tubes as above and centrifuged at 400 × g for 30 min. The white cell layer was then collected and red blood cells lysed as above.
2.11. Chemotaxis assays
Human neutrophil and mononuclear migration in response to LPS-stimulated HMEC-1 cells and supernatants conditioned by LPS-stimulated HMEC-1 cells was evaluated using 24-well Transwell plates (Corning Life Sciences, Lowell, MA). In brief, sets of 3 wells (lower chambers), each containing 1.25 × 105 HMEC-1 cells were stimulated with 1 μg/ml of LPS in the presence or absence of CGRP (10 nM or 100 nM), CGRP alone or medium alone for 24 h. Then, 200 μl of depleted EBM containing 2 × 105 neutrophil or mononuclear cells was placed in the upper chambers of Transwell inserts (6.5 mm diameter, 5.0 μM pore-size polycarbonate membrane). Transwell inserts were then placed into the plate wells and plates then incubated at 37°C in 5% CO2 for 90 min. The inserts were removed and cells that migrated through the upper chamber’s filter to the lower chamber were determined by quantifying cells in the medium by light microscopy.
In other experiments, supernatants conditioned by HMEC-1 cells treated with LPS, LPS plus CGRP, CGRP alone or medium alone in an identical manner for 24 h were transferred to wells of the Transwell plates and examined for chemotatic ability as above. As controls, a parallel experiment was set-up with fresh depleted EBM added to the wells of Transwell plates followed by addition of 2 × 105 neutrophils or mononuclear cells in 200 μl of condition medium conditioned by LPS in the presence or absence of CGRP, CGRP alone or medium alone to Transwell inserts (in triplicate) followed by placement in wells. After incubation for 90 min, the inserts were removed and cells that migrated through the upper chamber’s filter to the lower chamber were quantified by light microscopy.
2.12. Statistical analyses
For experiments examining the effects of time of exposure and concentration of LPS on CGRP-inhibition of chemokine release (Figs. 1A and 1B), multiple linear regression was used to examine the interaction between LPS and CGRP. Student t-test was then used to compare the biomarker levels between cells treated with and without CGRP at various timepoints or concentrations of LPS. p-values were adjusted for multiple comparisons by controlling the false discovery rate (FDR). For all other experiments, ANOVA was carried out to examine the difference in average biomarker levels across different groups. Multiple comparisons of means were carried out by using simultaneous tests for general linear hypotheses (Hothorn et al., 2008). p-values were adjusted for multiple comparisons by controlling the FDR.
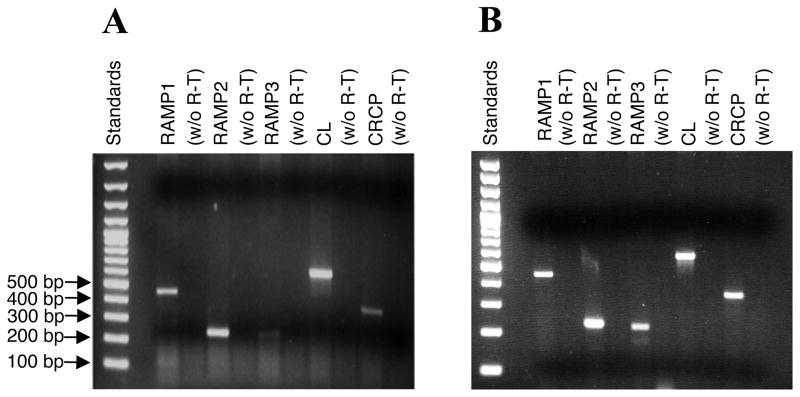
Fig. 1.
HMEC-1 cells (A) and pHMDECs (B) express mRNA for components of the AM and CGRP receptors (RAMP1/CL, RAMP2/CL and RAMP3/CL). Total RNA was extracted from unstimulated HMEC-1 cells and subjected to RT-PCR with primers specific for RAMP1, RAMP2, RAMP3, CL and CRCP. The expected sizes for the amplified fragments are 450 bp for RAMP1; 227 bp for RAMP2; 212 bp for RAMP3; 560 bp for CL and 344 bp for CRCP.
3. Results
3.1. HMEC-1 cells and pHDMECs express mRNA for components of both CGRP and AM receptors
RT- PCR was employed to identify the components of the CGRP receptor expressed in HMEC-1 cells. We found that HMEC-1 cells (Fig. 1A) and pHDMECs (Fig. 1B) expressed mRNA for the calcitonin receptor-like receptor (CL) and for the G-protein coupling protein CRCP. HMEC-1 cells also expressed the accessory protein components RAMP1, RAMP2 and RAMP3, with both RT-PCR and real-time RT-PCR (not shown) demonstrating lower expression of RAMP3 relative to the other two RAMPs. The identity of all PCR products was verified via cDNA sequencing. HMEC-1 cells expressed the components of both functional CGRP and adrenomedulin (AM) receptors.
3.2. CGRP inhibited LPS-induced CXCL8, CCL2 and CXCL1 production by HMEC-1 cells and pHDMECs
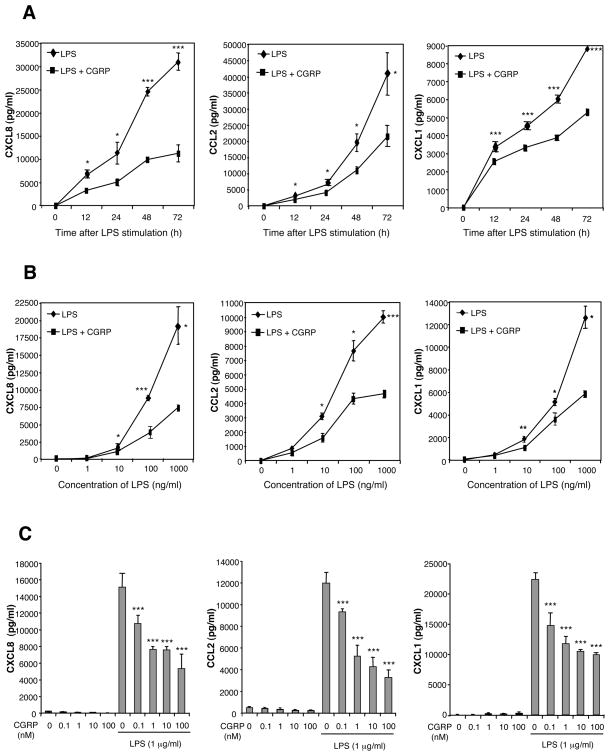
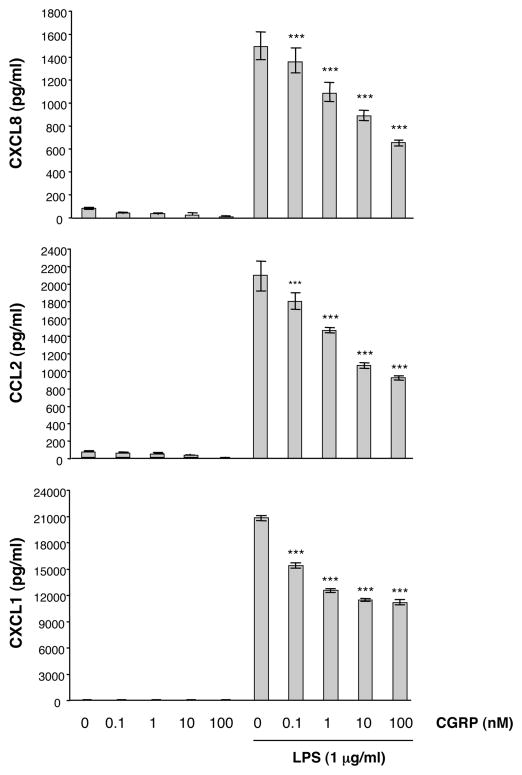
We investigated the effect of CGRP on CXCL8, CCL2 and CXCL1 production in LPS-stimulated HMEC-1 cells and pHDMECs (Fig. 2). HMEC-1 cells were stimulated with different concentrations of LPS in the presence or absence of graded doses of CGRP. The concentrations of CXCL8, CCL2 and CXCL1 in culture supernatants were assayed by ELISA at 12, 24, 48, and 72 h. Trypan blue staining confirmed that chemokine inhibition was not the result of cell death, as CGRP did not affect the viability of stimulated HMEC-1 cells or pHDMECs after 72 h in culture (data not shown). We found that LPS induced the production of CXCL8, CCL2 and CXCL1 within 12 h, and that chemokine production was significantly inhibited by CGRP as early as 12 h (Fig. 2A). This inhibition was maintained throughout a 72 h incubation period, indicating that CGRP attenuated rather than delayed chemokine production (Fig. 2A); CGRP inhibited CXCL8, CCL2 and CXCL1 production at all concentrations of LPS tested (0.01–1000 ng/ml) (Fig. 2B); dose-dependent inhibition of CXCL8, CCL2 and CXCL1 production by HMEC-1 was observed over the concentration range 0.1–100 nM of CGRP (Fig. 2C). Furthermore, CGRP also inhibited CXCL8, CCL2 and CXCL1 production by pHDMECs cells dose-dependently over the CGRP concentration range 0.1–100 nM (Fig. 3).
Fig. 2.
CGRP inhibits LPS-induced CXCL8, CCL2, and CXCL1 production by HMEC-1 cells. (A) Time-dependent chemokine inhibition by CGRP. HMEC-1 cells (0.25 ×106 cells/ml) were exposed to LPS in the presence or absence of CGRP, and supernatants were collected at different time points and assayed for CXCL8, CCL2, and CXCL1 content. (B) CGRP inhibited chemokine production over a range of LPS concentrations (0.001–1 μg/ml). HMEC-1 cells were stimulated with increasing concentrations of LPS in the presence or absence of CGRP (10 nM). Supernatants were collected 72 h after LPS stimulation and assayed for chemokine content. (C) Dose-dependent chemokine inhibition by CGRP. HMEC-1 cells were cultured with various concentrations of CGRP (0.1–100 nM) and stimulated with LPS for 72 h. Chemokine contents in culture supernatants were determined. Each result is the mean ± SD of 3 separate replicates (each with duplicate wells) performed at the same time. Each experiment was performed at least 3 times with the same result except the time-dependent determination of CXCL1 in which the result illustrated was seen in 4 of 6 experiments. (*p<0.05, **p<0.01, ***p<0.001)
Fig. 3.
CGRP inhibits LPS-induced CXCL8, CCL2, and CXCL1 production by pHDMECs. pHDMECs (0.25 ×106 cells/ml) were cultured with various concentrations of CGRP (0.1–100 nM) and stimulated with 1 μg/ml of LPS. Supernatants were collected 24 h later and chemokine concentrations were determined. Each result is the mean ± SD of three separate replicates (each with duplicate wells) performed at the same time. This experiment was performed 3 times and the result illustrated by this figure was seen 3 times. (*p<0.05, ***p<0.001)
3.3 CGRP reduced LPS-induced CXCL8, CCL2 and CXCL1 mRNA expression
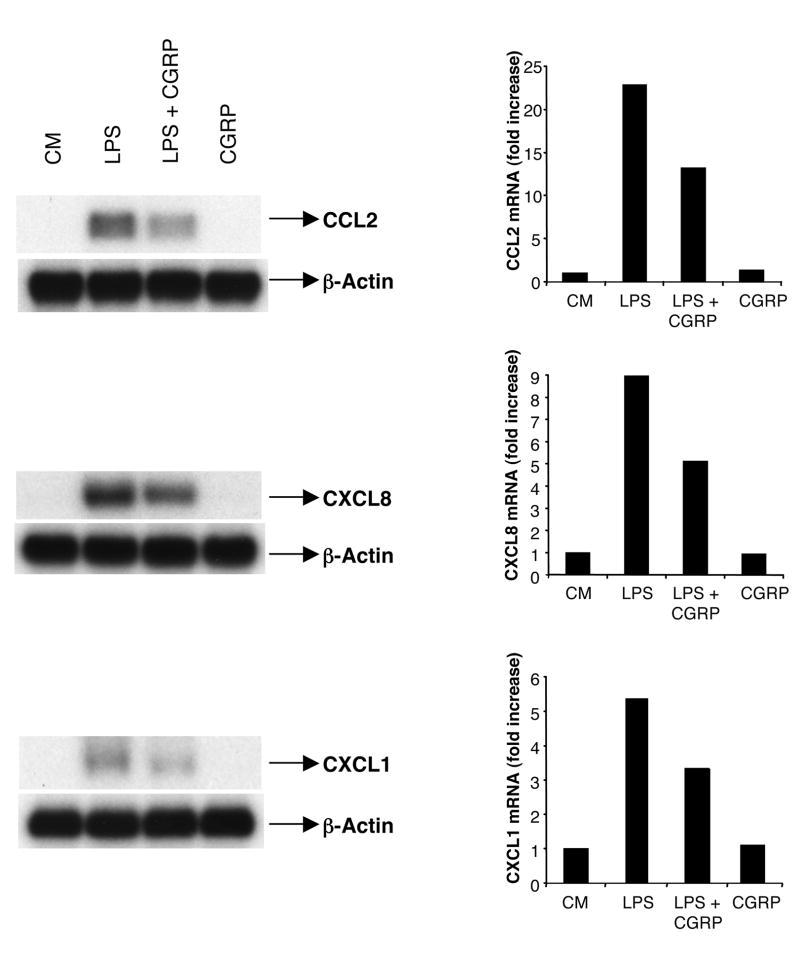
Having demonstrated that CGRP inhibits LPS-induced CXCL8, CCL2 and CXCL1 production, we explored the possibility that the inhibition occurred at a transcriptional level. HMEC-1 cells were stimulated with LPS in the presence or absence of 10 nM CGRP. Total RNA was prepared from 12 h cultures and subjected to Northern blot analysis. Although CCL2, CXCL8, and CXCL1 mRNA were not detected in non-stimulated cells, high expression of CCL2, CXCL8 and CXCL1 mRNA was present in cells cultured with LPS alone. Cells preincubated with 10 nM of CGRP had reduced mRNA expression for all three chemokines (Fig. 4). These results indicate that CGRP inhibited HMEC-1 cell production of CCL2, CXCL8 or CXCL1 at the transcriptional level.
Fig. 4.
CGRP inhibits the expression of LPS-induced chemokines at the mRNA level. HMEC-1 cells (1.5 ×106 cells) were stimulated with LPS (1 μg/ml) in the presence or absence of CGRP (10 nM) for 12 h. Total RNA was extracted and transferred onto nylon membranes. The expression of CXCL8, CCL2, and CXCL1 mRNA in HMEC-1 cells was analyzed by Northern blotting. This experiment was performed 4 times and the result illustrated by this figure was seen in all 4 experiments.
3.4. CGRP inhibited CXCL8, CCL2 and CXCL1 production via the CGRP1 receptor
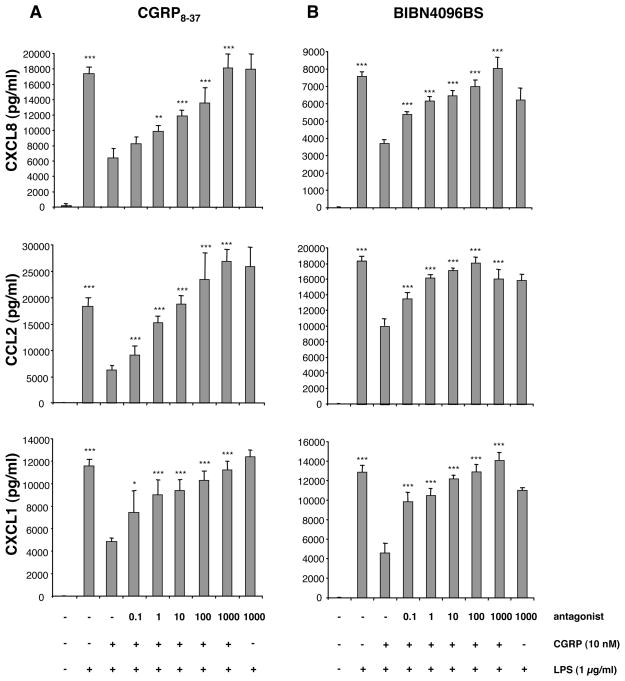
Given that RAMPS 1, 2, and 3 were all detected by RT-PCR, we endeavored to determine whether the effects of CGRP that we observed were receptor-mediated. CGRP8-37 is a competitive inhibitor of the CGRP1 receptor but has, reportedly, only weak antagonist activity at the CGRP2 receptor (Hay et al., 2003; Hinson et al., 2000). HMEC-1 cells were treated for 1 h with increasing concentrations of CGRP8-37 (0.1–1000 nM) followed immediately by addition of 10 nM CGRP and 1 μg/ml of LPS. The concentrations of chemokines in supernatants were quantified by ELISA. The inhibitory effect of CGRP on CXCL8, CCL2 and CXCL1 production was suppressed by CGRP8-37 in a dose-dependent manner (Fig. 5A), with complete loss of the CGRP effect at an antagonist concentration at 1000 nM.
Fig. 5.
CGRP8-37 and BIBN4096BS prevent CGRP-induced suppression of CXCL8, CCL2, and CXCL1 expression. (A) CGRP8-37. HMEC-1 cells were stimulated with 10 nM of CGRP in the presence or absence of various concentrations (0–1000 nM) of the CGRP antagonist CGRP8-37 followed by the addition of 1 μg/ml of LPS. Supernatants were collected 48 h later and assayed for chemokine content. (B) BIBN4096BS. This experiment was performed in the same manner except BIBN4096BS (0–1000 nM) was substituted for CGRP8-37. Each result is the mean ± SD of three separate replicates (each with duplicate wells) performed at the same time. This experiment was performed 3 times and the result illustrated by this figure was seen 3 times. (*p<0.05, **p<0.01, ***p<0.001)
To further confirm that the CGRP effect is mediated via the CGRP1 receptor, we also employed the non-peptide CGRP antagonist BIBN4096BS, which acts selectively upon the CGRP1 receptor subtype (RAMP1/CL) (Doods et al., 2000), whereas RAMP2 and RAMP3 are insensitive to its effects (Hay et al., 2003). HMEC-1 cells were treated with increasing concentrations of BIBN4096BS (0.1–1000 nM) followed by addition of CGRP to 10 nM and stimulation with 1 μg/ml of LPS. As with CGRP8-37, the effect of CGRP was inhibited by BIBN4096BS in a dose-dependent manner (Fig. 5B). These results indicate that CGRP exerts its effects primarily through the CGRP1 receptor, namely the RAMP1/CL complex.
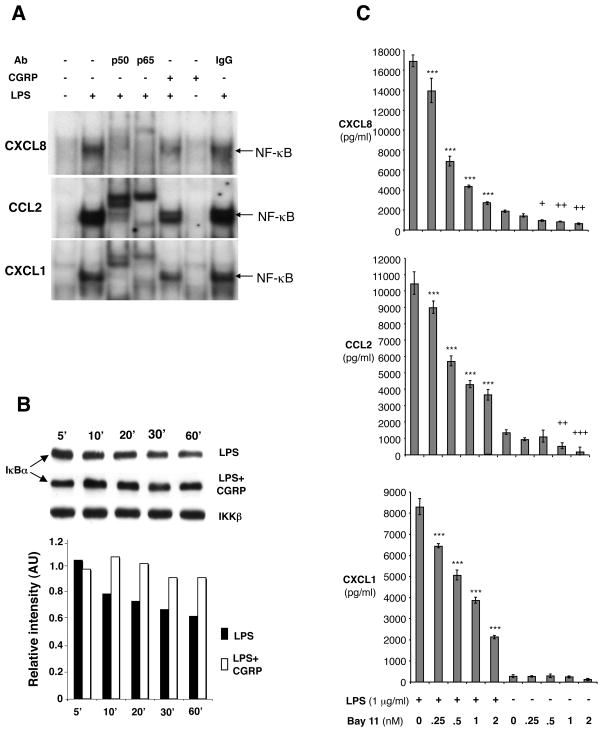
3.5. CGRP prevented LPS-induced IκBα degradation and NF-κB binding to promoters of CXCL1, CXCL8 and CCL2
To test the hypothesis that the inhibitory effect of CGRP on chemokine production by endothelial cells occurs through inhibition of NF-κB activation, we examined the effects of CGRP on LPS-stimulated NF-κB activation using an electrophoretic mobility shift assay (EMSA). HMEC-1 cells were cultured in the presence or absence of CGRP for 1 h, followed by LPS addition to certain cultures for 4 h to activate NF-κB. LPS caused a significant increase in NF-κB activation (Fig. 6A, lane 1 vs. 2). This band was accordingly shifted by incubation with antibody to p50 or p65 (Fig. 6A, lane 3 and lane 4), as shown by the marked reduction in intensity of the NF-κB band, indicating that the NF-κB-binding complex is composed primarily of p50/p65 heterodimers. Addition of CGRP before LPS stimulation reduced in intensity the NF-κB band (Fig. 6A, lane 5). A similar result was seen with pHDMECs treated the same way (data not shown). Our results suggest that CGRP inhibits LPS-induced NF-κB binding to NF-κB binding sites on promoters of CXCL1, CXCL8 and CCL2. NF-κB activation is mediated by the phosphorylation and subsequent degradation of IκB (Viatour et al., 2005). To study whether CGRP alters IκB degradation, we treated HMEC-1 cells with 1 μg/ml LPS in the presence or absence of CGRP and the cytoplasmic IκBα levels were measured by Western blot. When LPS was introduced to HMEC-1 cells, IκBα degraded as expected over the time range of 5 – 60 min (Fig. 6B). However, when HMEC-1 cells were exposed to 10 nM CGRP prior to and during the period of LPS stimulation, LPS-induced IκBα degradation was suppressed (Fig 6B). IKKβ was measured to ensure equivalent loading of proteins. To further confirm that the NF-κB pathway is indeed involved in the regulation of CXCL1, CXCL8 and CCL2, we examined the ability of an inhibitor of NF-κB activation, Bay 11-7085 (Pierce et al., 1997; Mabuchi et al., 2004) to affect LPS-induced chemokine release from HMEC-1 cells. We found that Bay 11-7085 inhibited LPS-induced CXCL1, CXCL8 and CCL2 production in a dose-dependent manner (Fig. 6C). It also significantly inhibited background release of CXCL8 and CCL2 by unstimulated cells (Fig. 6C).
Fig. 6.
CGRP prevents NF-κB binding to the promoters of CXCL1, CXCL8 and CCL2 and LPS-induced IκBα degradation in HMEC-1 cells. (A) CGRP inhibits NF-κB DNA binding to promoters. Nuclear extracts were prepared from HMEC-1 cells stimulated for 4 h with LPS (1 μg/ml) in the presence or absence of CGRP with CGRP added to cultures 1 hr before LPS. NF-κB binding was assayed by EMSA. Nuclear extracts were incubated with antisera against p50, p65 or IgG control for 15 min before adding the radiolabeled probe. Similar results were observed in 3 independent experiments. (B) CGRP prevents LPS-induced IκBα degradation. HMEC-1 cells were stimulated with LPS in the presence or absence of CGRP as in (A). Cytosolic amounts of IκBα at different time points were determined by Western blot. One representative experiment of 3 is shown. (C) Bay11-7085 blocked LPS-induced CXCL8, CCL2 and CXCL1 production by HMEC-1 cells. HMEC-1 cells were stimulated with LPS (1 μg/ml) in the presence or absence of graded concentrations of Bay11-7085. Supernatants were collected 24 h after LPS stimulation and chemokine content assayed. Each result is the mean ± SD of three separate replicates (each with duplicate wells) performed at the same time. This result is representative of 2 such experiments. (***p<0.001 vs LPS, no Bay11-7085; *p<0.05, **p<0.01, ***p<0.001 vs no LPS, no Bay11-7085).
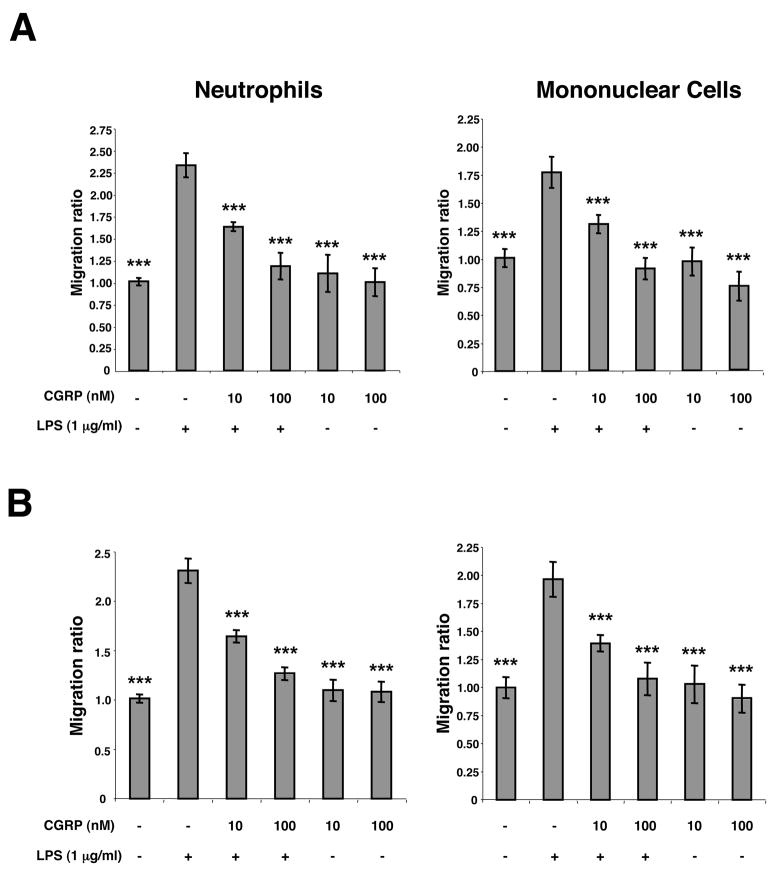
3.6. CGRP inhibits chemotaxis of neutrophils and peripheral blood mononuclear cells towards LPS stimulated HMEC-1 cells
To examine directly whether CGRP can inhibit the ability of LPS-activated endothelial cells to chemoattract neutrophils or mononuclear cells, we performed chemotaxis assays using Transwell inserts. HMEC-1 cells were plated in the wells of 24-well Falcon plates and stimulated for 24 h with LPS in the presence or absence of CGRP. Control wells were treated with medium in the presence or absence of CGRP without LPS. Then, Transwell inserts containing either human peripheral blood neutrophils or human peripheral blood mononuclear cells were placed into the wells. Ninety min later, Transwell inserts were removed and migrated cells enumerated by light microscopy in the medium. As shown in Fig. 7A, CGRP significantly inhibited the migration of both neutrophils and mononuclear cells. The addition of LPS or LPS plus CGRP to Transwell inserts instead of to Falcon wells did not result in a statistically significant change in migration of neutrophils or mononuclear cells to the lower chamber.
Fig. 7.
Exposure of HMEC-1 cells to CGRP during stimulation inhibits chemotaxis of neutrophils and monocunclear cells towards LPS-stimulated HMEC-1 cells or supernatants conditioned by LPS-stimulated HMEC-1 cells. (A) Inhibition of chemotaxis towards stimulated cells. HMEC-1 cells were treated with 1 μg/ml LPS in the presence or absence of CGRP, CGRP alone or medium alone in the lower chambers of the Transwell apparatus for 24 h. Neutrophils or mononuclear cells were then placed in the upper chambers and after 90 min cells that migrated into the lower chambers were enumerated by light microscopy. (B) Inhibition of chemotaxis towards conditioned supernatants. HMEC-1 cells were stimulated with LPS in the presence or absence of CGRP, CGRP alone or medium alone. Supernatants were harvested after 24 h and placed in the lower chambers of the Transwell apparatus. Neutrophils or mononuclear cells were then placed in the upper chambers and after 90 min cells that migrated into the lower chambers were enumerated by light microscopy. The experiment shown is representative of three experiments with similar results (***p<0.001 vs LPS, no CGRP).
To examine directly whether the effect of CGRP observed can be related to the inhibition of release of chemotactic substances, HMEC-1 cells were treated with LPS, LPS plus CGRP, CGRP alone, or medium alone for 24 h. Conditioned supernatants were removed and transferred to Falcon plates followed by placement of Transwell inserts containing neutrophils or mononuclear cells. Supernatants conditioned by LPS alone induced significant migration of cells out of the upper chamber. However, this effect was lost when supernatants were conditioned by HMEC-1 cells treated with both LPS and CGRP, as shown in Fig. 7B.
4. Discussion
Dermal blood vessels are innervated and it has long been known that CGRP-containing nerves surround them (Garcia-Caballero et al., 1989). The findings presented within this paper strongly suggest that CGRP-containing nerves play a role in the regulation of cutaneous inflammation through effects on dermal vessel endothelial cells.
CGRP mediates its activities through the 7-transmembrane calcitonin-receptor-like-receptor (CL), a G protein-coupled receptor linked to the cellular signal transduction pathway by the accessory protein, CRCP (Evans et al., 2000; Prado et al., 2002). However, various CGRP receptor subtypes have been identified, which, depending on the expression of RAMPs, differ in their affinities for CGRP and for homologous peptides such as AM (Kitamura et al., 1993; Eguchi et al., 1994; Ishizaka et al., 1994; Shimekake et al., 1995; Fernandes et al., 2009). Studies on RAMPs demonstrate that they behave essentially as chaperones that are required for terminal glycosylation and correct cell surface targeting of the CGRP receptor, essentially conferring function and ligand specificity to the orphan CL receptor (Conner et al., 2004). Receptor subtypes are characterized by their RAMP-mediated sensitivity to the CGRP antagonist, CGRP8-37, with CGRP1 (RAMP1/CL) types having high affinity and CGRP2 (RAMP2/CL and RAMP3/CL) types having low affinity (Poyner, 1995). Of the three RAMPs that have been studied, the RAMP1/CL complex demonstrates the highest affinity for CGRP, while both RAMP2 and RAMP3 complexed receptors serve as functional AM receptors with RAMP3 having limited affinity for CGRP (McLatchie et al., 1998; Kamitani et al., 1999; Nikitenko et al., 2006). Hence, it seems likely that the cellular effects observed with CGRP treatment may arise through the RAMP1/CL receptor complex rather than the other subtypes with relatively lower CGRP sensitivity.
We have demonstrated that HMEC-1 cells and the population of pHDMECs that we utilized express the CGRP orphan receptor CL, the receptor component protein CRCP, and the accessory proteins RAMP1, RAMP2 and RAMP3. The presence of RAMP1 in HMEC-1 cells is consistent with past studies which have concluded that the RAMP1/CL is pharmacologically identical to the CGRP1 receptor subtype (Prado et al., 2002) which has the highest affinity for both CGRP, the antagonist CGRP8-37, and the RAMP1-selective antagonist, BIBN4096BS. Furthermore, HMEC-1 cell expression of accessory proteins such as CRCP, an intracellular peripheral membrane protein shown to couple CL/RAMP complexes to downstream effectors (Evans et al., 2000), provides a plausible mechanism by which the CGRP receptor is linked to G proteins and downstream signaling pathways. The experiments reported herein showing that CGRP1 inhibitors block the suppressive effect of CGRP on inhibition of chemokine release demonstrate that stimulation of the CGRP1 is necessary for the effect, although they do not prove that it is sufficient.
It is also of interest that, as shown in Fig. 5, the CCL2 level released by cultured cells stimulated by LPS is higher (p<0.001) in the presence of 1000 nM CGRP8-37 than with LPS alone. This was not seen with the BIBN4096BS inhibitor nor was it seen with the other chemokines examined. This observation raises the possibility that CCL2 is so sensitive to CGRP that release of small amounts of CGRP by cells in the culture wells are inhibiting CCL2 release. In this regard, some non-nervous system cells have been reported to express CGRP. For example, human monocytes and macrophage-activated human adipocytes reportedly express CGRP (Linscheid et al., 2004; Bracci-Laudiero et al., 2005) as do rat lymphocytes (Xing et al, 1998) and human Langerhans cells within psoriatic lesions (He et al., 2000). CGRP is also expressed in the thymus of several species including humans and mice (Silva et al., 2006). However, the failure of BIBN4096BS to have this effect argues against this possibility. A detailed examination of this observation is beyond the scope of this paper but it is an interesting question.
The promoters of most CXC and CC chemokines contain various transcription factor binding sites where NF-κB may act to regulate the expression of these and other inflammatory mediators (Siebenlist et al., 1994; Freter et al., 1996; Ohmori et al., 1995; Ueda et al., 1997; Mauviel et al., 1992; Anisowicz et al., 1991; Lim and Garzino-Demo, 2000). For example, induction of chemokine expression in endothelial cells by Borrelia burgdorferi (Ebnet et al., 1997) was blocked by NF-κB inhibitors and magnesium deficiency promotes chemokine release by endothelial cells via NF-κB activation (Ferrè et al., 2010), suggesting that NF-κB activation is important for chemokine production in these cells Thus, we also investigated whether the inhibitory effect of CGRP on endothelial cell chemokine release results from effects on the NF-κB signal pathway.
As a major regulator of inflammation, NF-κB modulates the expression of various chemokines and cytokines (Bierhaus and Nawroth, 2003); hence, it is plausible that CGRP may affect the NF-κB pathway as a way of inhibiting CXCL1, CXCL8 and CCL2 production. The results described above strongly support this hypothesis. CGRP suppressed the degradation of IκBα, thus inhibiting activation of NF-κB and its subsequent binding to the promoters of CXCL1, CXCl8 and CCL2. We also employed the pharmacologic inhibitor of NF-κB activation, Bay 11-7085 [which acts by inhibiting IκB kinase (IKK)], to determine its ability to inhibit production of CXCL1, CXCL8 and CCL2 (Pierce et al., 1997; Mabuchi et al., 2004). We found that Bay 11-7085 inhibited LPS-induced CXCL1, CXCL8 and CCL2 production by HMEC-1 cells. Without P-IKKα/β, the NF-κB dimer is not released and is unable to translocate into the nucleus to induce gene expression. Thus, this pharmacologic NF-κB inhibitor strikingly mimics the effects of CGRP in this system, supporting the idea that the inhibitory effect of CGRP on chemokine production may be mediated at least partly through inhibition of the NF-κB activation pathway.
These results show that LPS-induced chemokine production by HMEC-1 cells is transcriptionally suppressed by CGRP in a dose- and time-dependent manner in the nanomolar concentration range. While the concentration of CGRP in the vicinity of a nerve ending after release is not known, we chose to study this dose range as nervous tissue contains CGRP as high as 1197 pg/mg (Xu et al., 1989). While we chose LPS-induced chemokine release as our model to study, similar suppressive effects of CGRP were observed on CXCL1, CXCL8 and CCL2 release from HMEC-1 cells stimulated with IL-1β or TNFα (data not shown).
A question that, of course, arises in a study of this type is whether it has in vivo relevance. Fortunately, others have performed experiments yielding findings that bear directly on this question. As discussed in the introduction, systemic administration of CGRP to mice was found to inhibit the accumulation of neutrophils in the peritoneal cavity after subsequent injection with LPS (Gomes et al., 2005). It also prevented death from lethal endotoxemia in mice given a fatal dose of LPS intraperitoneally and this effect was lost with co-administration of CGRP8-37 (Gomes et al., 2005). Subplantar injection of CGRP into the rat paw inhibited paw swelling induced by subsequent injection with 5-hydroxytryptamine (Raud et al., 1991). Of particular interest, topical application of CGRP inhibited ear inflammation induced by topical treatment with croton oil, arachidonic acid or tetradecanoylphorbol acetate (O’Kane et al., 2006; Clementi et al., 1994; Clementi et al., 1995). Although not proven, these observations are consistent with an inhibitory effect of CGRP on endothelial cells. The chemotaxis experiments described in the results section, however, directly support the concept that the CGRP–induced inhibition seen in these types of experiments results from a direct effect on endothelial cells inhibiting their release of chemotactic factors. Indeed, the chemotaxis experiments directly link the suppression of release of chemokines by endothelial cells with a functional (inhibition of chemotaxis of neutrophils and mononuclear cells) result.
The observation that a product of nerves surrounding dermal blood vessels (CGRP) can alter release of chemotactic factors by endothelial cells has several implications. First, as mentioned above, it provides a potential mechanism by which the nervous system can regulate inflammation in the skin. Second, it suggests the possibility that therapeutic agents might be developed that could exploit CGRP receptor signaling to inhibit chemokine release by dermal endothelial cells. Third, if release or non-release of CGRP by nerves surrounding dermal blood vessels is regulated by stress, these finding might help to explain why certain skin disorders (such as rosacea, psoriasis and atopic dermatitis) may worsen with stress. The possible role of nerve-derived substances such as subtance P and adenosine triphosphate in enhancement of cutaneous inflammation during stress has been explored experimentally (Pavlovic et al., 2008; Amano et al., 2008; Seiffert et al., 2006). Interestingly, a possible role for stress reduction and avoidance behavior in ameliorating inflammatory skin disorders such as atopic dermatitis and psoriasis has been suggested (Farber and Nall, 1993; Arndt et al., 2008). These findings may suggest a mechanism by which CGRP may play a role in stress modulation of skin inflammation. Indeed, cutaneous nerve fiber expression of CGRP was shown to be reduced 24 hours after experimental acute social stress (Kleyn et al., 2008). An important area of future research will be to determine the conditions by which neuropeptides are released by dermal nerves.
Acknowledgments
This work was funded by R01 AR42429 (RDG), an agreement with Clinique Laboratories, LLC (RDG), a grant from the National Rosacea Society (RDG), a grant from the Dana Foundation (RDG), a gift from the Jacob L. and Lillian Holtzmann Foundation (RDG), a grant from the Edith C. Blum Foundation (RDG), contributions from the Carl and Fay Simons Family Trust (RDG), a contribution from the Seth Sprague Educational and Charitable Foundation and a grant from the Lewis B. and Dorothy Cullman Foundation (RDG).
Footnotes
Conflict of interest statement
The senior author was the principal investigator in the research agreement with Clinique Laboratories, LLC that funded part of this work. Clinique Laboratories, LLC provided other funds to the Department of Dermatology and the Weill Cornell Medical College.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- Amano H, Negishi I, Akiyama H, Ishikawa O. Psychological stress can trigger atopic dermatitis in NC/Nga mice: An inhibitory effect of corticotropin-releasing factor. Neuropsychophrmacology. 2008;33:566–573. doi: 10.1038/sj.npp.1301435. [DOI] [PubMed] [Google Scholar]
- Amara SG, Jonas V, Rosenfeld MG, Ong ES, Evans RM. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982;298:240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Anisowicz A, Messineo M, Lee SW, Sager R. An NF-κB-like transcription factor mediates IL-1/TNFα induction of gro in human fibroblasts. J Immunol. 1991;147:520–527. [PubMed] [Google Scholar]
- Arndt J, Smith N, Tausk F. Stress and atopic dermatitis. Curr Allergy Asthma Rep. 2008;8:312–317. doi: 10.1007/s11882-008-0050-6. [DOI] [PubMed] [Google Scholar]
- Asahina A, Hosoi J, Beissert S, Stratigos A, Granstein RD. Inhibition of the induction of delayed-type and contact hypersensitivity by calcitonin gene-related peptide. J Immunol. 1995;154:3056–3061. [PubMed] [Google Scholar]
- Bender A, Zapolanski T, Watkins S, Khosraviani A, Seiffert K, Ding W, Wagner JA, Granstein RD. Tetracycline suppresses ATPgammaS-induced CXCL8 and CXCL1 production by the human dermal microvascular endothelial cell-1 (HMEC-1) cell line and primary human dermal microvascular endothelial cells. Exp Dermatol. 2008;17:752–760. doi: 10.1111/j.1600-0625.2008.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benrath J, Eschenfelder C, Zimmerman M, Gillardon F. Calcitonin gene-related peptide, substance P and nitric oxide are involved in cutaneous inflammation following ultraviolet irradiation. Eur J Pharmacol. 1995;293:87–96. doi: 10.1016/0926-6917(95)90022-5. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Nawroth PP. Modulation of the vascular endothelium during infection—the role of NF-kappa B activation. Contrib Microbiol. 2003;10:86–105. doi: 10.1159/000068133. [DOI] [PubMed] [Google Scholar]
- Bracci-Laudiero L, Aloe L, Caroleo MC, Buanne P, Costa N, Starace G, Lundeberg T. Endogenous NGF regulates CGRP expression in human monocytes, and affects HLA-DR and CD86 expression and IL-10 production. Blood. 2005;106:3507–3514. doi: 10.1182/blood-2004-10-4055. [DOI] [PubMed] [Google Scholar]
- Brain SD. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacol. 1997;37:133–152. doi: 10.1016/s0162-3109(97)00055-6. [DOI] [PubMed] [Google Scholar]
- Brain SD, Williams TJ. Neuropharmacology of peptides in skin. Semin Dermatol. 1988;7:278–283. [PubMed] [Google Scholar]
- Britschgi M, Pichler WJ. Acute generalized exanthematous pustulosis, a clue to neutrophil-mediated inflammatory processes orchestrated by T cells. Curr Opin Allergy Clin Immunol. 2002;2:325–331. doi: 10.1097/00130832-200208000-00006. [DOI] [PubMed] [Google Scholar]
- Bulloch K, Hausman J, Radojcic T, Short S. Calcitonin gene-related peptide in the developing and aging thymus. An immunocytochemical study. Ann NY Acad Sci. 1991;621:218–228. doi: 10.1111/j.1749-6632.1991.tb16981.x. [DOI] [PubMed] [Google Scholar]
- Bulloch K, McEwen BS, Nordberg J, Diwa A, Baird S. Selective regulation of T-cell development and function by calcitonin gene-related peptide in thymus and spleen. An example of differential regional regulation of immunity by the neuroendocrine system. Ann NY Acad Sci. 1998;840:551–562. doi: 10.1111/j.1749-6632.1998.tb09594.x. [DOI] [PubMed] [Google Scholar]
- Clementi G, Amico-Roxas M, Caruso A, Catena Cutuli VM, Prato A, Maugeri S, de Bernardis E, Scapagnini U. Effects of CGRP in different models of mouse ear inflammation. Life Sci. 1994;54:PL119–124. doi: 10.1016/0024-3205(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Clementi G, Caruso A, Cutuli VM, Prato A, de Bernardis E, Fiore CE, Amico-Roxas M. Anti-inflammatory activity of amylin and CGRP in different experimental models of inflammation. Life Sci. 1995;57:PL193–197. doi: 10.1016/0024-3205(95)02100-w. [DOI] [PubMed] [Google Scholar]
- Conner AC, Simms J, Hay DL, Mahmoud K, Howitt SG, Wheatley M, Poyner DR. Heterodimers and family-B GPCRs: RAMPs, CGRP and adrenomedullin. Biochem Soc Trans. 2004;32:843–846. doi: 10.1042/BST0320843. [DOI] [PubMed] [Google Scholar]
- Dearman RJ, Bhushan M, Cumberbatch M, Kimber I, Griffiths CE. Measurement of cytokine expression and Langerhans cell migration in human skin following suction blister formation. Exp Dermatol. 2004;13:452–460. doi: 10.1111/j.0906-6705.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- Doods H, Hallermayer G, Wu D, Entzeroth M, Rudolf K, Engel W, Eberlein W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br J Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Brown KD, Siebenlist UK, Simon MM, Shaw S. Borrelia burgdorferi activates nuclear factor-kappa B and is a potent inducer of chemokine and adhesion molecule gene expression in endothelial cells and fibroblasts. J Immunol. 1997;158:3285–3292. [PubMed] [Google Scholar]
- Eguchi S, Hirata Y, Kano H, Sato K, Watanabe Y, Watanabe TX, Nakajima K, Sakakibara S, Marumo F. Specific receptors for adrenomedullin in cultured rat vascular smooth muscle cells. FEBS Lett. 1994;340:226–230. doi: 10.1016/0014-5793(94)80143-6. [DOI] [PubMed] [Google Scholar]
- Evans BN, Rosenblatt MI, Mnayer LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem. 2000;275:31438–31443. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- Farber EM, Nall L. Psoriasis: a stress-related disease. Cutis. 1993;51:322–326. [PubMed] [Google Scholar]
- Fernandes ES, Schmidhuber SM, Brain SD. Sensory-nerve-derived neuropeptides: possible therapeutic targets. Handb Exp Pharmacol. 2009;194:393–416. doi: 10.1007/978-3-540-79090-7_11. [DOI] [PubMed] [Google Scholar]
- Ferrè S, Baldoli E, Leidi M, Maier JA. Magnesium deficiency promotes a pro-atherogenic phenotype in cultured human endothelial cells via activation of NFκB. Biochim Biophys Acta. 2010;1802:952–958. doi: 10.1016/j.bbadis.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Freter RR, Alberta JA, Hwang GY, Wrentmore AL, Stiles CD. Platelet-derived growth factor induction of the immediate-early gene MCP-1 is mediated by NF-κB and a 90-kDa phosphoprotein coactivator. J Biol Chem. 1996;271:17417–17424. doi: 10.1074/jbc.271.29.17417. [DOI] [PubMed] [Google Scholar]
- Garcia-Caballero T, Gallego R, Roson E, Fraga M, Beiras A. Calcitonin gene-related peptide (CGRP) immunoreactivity in the neuroendocrine Merkel cells and nerve fibres of pig and human skin. Histochemistry. 1989;92:127–132. doi: 10.1007/BF00490231. [DOI] [PubMed] [Google Scholar]
- Goebeler M, Yoshimura T, Toksoy A, Ritter U, Brocker EB, Gillitzer R. The chemokine repertoire of human dermal microvascular endothelial cells and its regulation by inflammatory cytokines. J Invest Dermatol. 1997;108:445–451. doi: 10.1111/1523-1747.ep12289711. [DOI] [PubMed] [Google Scholar]
- Gomes RN, Castro-Faria-Neto HC, Bozza PT, Soares MB, Shoemaker CB, David JR, Bozza MT. Calcitonin gene-related peptide inhibits local acute inflammation and protects mice against lethal endotoxemia. Shock. 2005;24:590–594. doi: 10.1097/01.shk.0000183395.29014.7c. [DOI] [PubMed] [Google Scholar]
- Gupta S, Mehrotra S, Avezaat CJ, Villalon CM, Saxena PR, Maassenvandenbrink A. Characterisation of CGRP receptors in the human isolated middle meningeal artery. Life Sci. 2006;79:265–271. doi: 10.1016/j.lfs.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Toyka KV. Substance P, the immune system and inflammation. Int Rev Immunol. 1989;4:229–249. doi: 10.3109/08830188909054420. [DOI] [PubMed] [Google Scholar]
- Hay DL, Howitt SG, Conner AC, Schindler M, Smith DM, Poyner DR. CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors: a comparison of effects of adrenomedullin22-52, CGRP8-37 and BIBN4096BS. Br J Pharmacol. 2003;140:477–486. doi: 10.1038/sj.bjp.0705472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Ding G, Wang X, Zhu T, Fan S. Calcitonin gene-related peptide in Langerhans cells in psoriatic plaque lesions. Chin Med J (Engl) 2000;113:747–51. [PubMed] [Google Scholar]
- Hinson JP, Kapas S, Smith DM. Adrenomedullin, a multifunctional regulatory peptide. Endocr Rev. 2000;21:138–167. doi: 10.1210/edrv.21.2.0396. [DOI] [PubMed] [Google Scholar]
- Hosoi J, Murphy GF, Egan CL, Lerner EA, Grabbe S, Asahina A, Granstein RD. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993;363:159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz FB, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- Ishizaka Y, Tanaka M, Kitamura K, Kangawa K, Minamino N, Matsuo H, Eto T. Adrenomedullin stimulates cyclic AMP formation in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1994;200:642–646. doi: 10.1006/bbrc.1994.1496. [DOI] [PubMed] [Google Scholar]
- Kamitani S, Asakawa M, Shimekake Y, Kuwasako K, Nakahara K, Sakata T. The RAMP2/CRLR complex is a functional adrenomedullin receptor in human endothelial and vascular smooth muscle cells. FEBS Lett. 1999;448:111–114. doi: 10.1016/s0014-5793(99)00358-0. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T. Adrenomedullin, a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Kleyn CE, Schneider L, Saraceno R, Mantovani C, Richards HL, Fortune DG, Cumberbatch M, Dearman RJ, Terenghi G, Kimber I, Griffiths CE. The effects of acute social stress on epidermal Langerhans’ cell frequency and expression of cutaneous neuropeptides. J Invest Dermatol. 2008;128:1273–1279. doi: 10.1038/sj.jid.5701144. [DOI] [PubMed] [Google Scholar]
- Li W, Wang T, Ma C, Xiong T, Zhu Y, Wang X. Calcitonin gene-related peptide inhibits interleukin-1beta-induced endogenous monocyte chemoattractant protein-1 secretion in type II alveolar epithelial cells. Am J Physiol Cell Physiol. 2006;291:C456–465. doi: 10.1152/ajpcell.00538.2005. [DOI] [PubMed] [Google Scholar]
- Li WJ, Wang TK. Calcitonin gene-related peptide inhibits interleukin-1beta-induced interleukin-8 secretion in human type II alveolar epithelial cells. Acta Pharmacol Sin. 2006;27:1340–1345. doi: 10.1111/j.1745-7254.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- Lim SP, Garzino-Demo A. The human immunodeficiency virus type 1 Tat protein up-regulates the promoter activity of the beta-chemokine monocyte chemoattractant protein 1 in the human astrocytoma cell line U-87 MG: role of SP-1, AP-1, and NF-kappaB consensus sites. J Virol. 2000;74:1632–1640. doi: 10.1128/jvi.74.4.1632-1640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linscheid P, Seboek D, Schaer DJ, Zulewski H, Keller U, Müller B. Expression and secretion of procalcitonin and calcitonin gene-related peptide by adherent monocytes and by macrophage-activated adipocytes. Crit Care Med. 2004;32:1715–21. doi: 10.1097/01.ccm.0000134404.63292.71. [DOI] [PubMed] [Google Scholar]
- Mabuchi S, Ohmichi M, Nishio Y, Hayasaka T, Kimura A, Ohta T, Saito M, Kawagoe J, Takahashi K, Yada-Hashimoto N, Sakata M, Motoyama T, Kurachi H, Tasaka K, Murata Y. Inhibition of NFkappaB increases the efficacy of cisplatin in in vitro and in vivo ovarian cancer models. J Biol Chem. 2004;279:23477–23485. doi: 10.1074/jbc.M313709200. [DOI] [PubMed] [Google Scholar]
- Mauviel A, Reitamo S, Remitz A, Lapière JC, Ceska M, Baggiolini M, Walz A, Evans CH, Uitto J. Leukoregulin, a T cell-derived cytokine, induces IL-8 gene expression and secretion in human skin fibroblasts. Demonstration and secretion in human skin fibroblasts. Demonstration of enhanced NF-kappa B-driven promoter activity. J Immunol. 1992;149:2969–2976. [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Merhi M, Dusting GJ, Khalil Z. CGRP and nitric oxide of neuronal origin and their involvement in neurogenic vasodilatation in rat skin microvasculature. Br J Pharmacol. 1998;123:863–868. doi: 10.1038/sj.bjp.0701696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizeki H, Alard P, Streilein JW. Calcitonin gene-related peptide is necessary for ultraviolet B-impaired induction of contact hypersensitivity. J Immunol. 1997;159:5183–5186. [PubMed] [Google Scholar]
- Nikitenko LL, Blucher N, Fox SB, Bicknell R, Smith DM, Rees MC. Adrenomedullin and CGRP interact with endogenous calcitonin-receptor-like receptor in endothelial cells and induce its desensitization by different mechanisms. J Cell Sci. 2006;119:910–922. doi: 10.1242/jcs.02783. [DOI] [PubMed] [Google Scholar]
- Nikitenko LL, Brown NS, Smith DM, MacKenzie IZ, Bicknell R, Rees MC. Differential and cell-specific expression of calcitonin receptor-like receptor and receptor activity modifying proteins in the human uterus. Mol Hum Reprod. 2001;7:655–664. doi: 10.1093/molehr/7.7.655. [DOI] [PubMed] [Google Scholar]
- Ohmori Y, Fukumoto S, Hamilton TA. Two structurally distinct kappa B sequence motifs cooperatively control LPS-induced KC gene transcription in mouse macrophages. J Immunol. 1995;155:3593–3600. [PubMed] [Google Scholar]
- O’Kane M, Murphy EP, Kirby B. The role of corticotropin-releasing hormone in immune-mediated cutaneous inflammatory disease. Exp Dermatol. 2006;15:143–153. doi: 10.1111/j.1600-0625.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- Pavlovic S, Daniltchenko M, Tobin DJ, Hagen E, Hunt SP, Klapp BF, Arck PC, Peters EM. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J Invest Dermatol. 2008;128:434–446. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- Poyner D. Pharmacology of receptors for calcitonin gene-related peptide and amylin. Trends Pharmacol Sci. 1995;16:424–428. doi: 10.1016/s0165-6147(00)89093-8. [DOI] [PubMed] [Google Scholar]
- Prado MA, Evans-Bain B, Dickerson IM. Receptor component protein (RCP): a member of a multi-protein complex required for G-protein-coupled signal transduction. Biochem Soc Trans. 2002;30:460–464. doi: 10.1042/bst0300460. [DOI] [PubMed] [Google Scholar]
- Pruckler JM, Lawley TJ, Ades EW. Use of a human microvascular endothelial cell line as a model system to evaluate cholesterol uptake. Pathobiol. 1993;61:283–287. doi: 10.1159/000163806. [DOI] [PubMed] [Google Scholar]
- Raud J, Lundeberg T, Brodda-Jansen G, Theodorsson E, Hedqvist P. Potent anti-inflammatory action of calcitonin gene-related peptide. Biochem Biophys Res Commun. 1991;180:1429–1435. doi: 10.1016/s0006-291x(05)81356-7. [DOI] [PubMed] [Google Scholar]
- Rosenfeld MG, Mermod JJ, Amara SG, Swanson LW, Sawchenko PE, Rivier J, Vale WW, Evans RM. Production of a novel neuropeptide encoded by the calcitonin gene via tissue-specific RNA processing. Nature. 1983;304:129–135. doi: 10.1038/304129a0. [DOI] [PubMed] [Google Scholar]
- Seiffert K, Ding W, Wagner JA, Granstein RD. ATPgammaS enhances the production of inflammatory mediators by a human dermal endothelial cell line via purinergic receptor signaling. J Invest Dermatol. 2006;126:1017–1027. doi: 10.1038/sj.jid.5700135. [DOI] [PubMed] [Google Scholar]
- Shimekake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, Eto T, Kangawa K, Matsuo H. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. J Biol Chem. 1995;270:4412–4417. doi: 10.1074/jbc.270.9.4412. [DOI] [PubMed] [Google Scholar]
- Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Ann Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- Silva AB, Aw D, Palmer DB. Evolutionary conservation of neuropeptide expression in the thymus of different species. Immunology. 2006;118:131–140. doi: 10.1111/j.1365-2567.2006.02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerlick RA, Lawley TJ. Role of microvascular endothelial cells in inflammation. J Invest Dermatol. 1993;100:111S–115S. doi: 10.1111/1523-1747.ep12356595. [DOI] [PubMed] [Google Scholar]
- Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem. 1997;272:31092–31099. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- Umeda Y, Takamiya M, Yoshizaki H, Arisawa M. Inhibition of mitogen-stimulated T lymphocyte proliferation by calcitonin gene-related peptide. Biochem Biophys Res Commun. 1988;154:227–235. doi: 10.1016/0006-291x(88)90674-2. [DOI] [PubMed] [Google Scholar]
- Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30:43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Wang F, Millet I, Bottomly K, Vignery A. Calcitonin gene-related peptide inhibits interleukin 2 production by murine T lymphocytes. J Biol Chem. 1992;267:21052–21057. [PubMed] [Google Scholar]
- Xing LY, Xing YT, Tang YM, Guo JX, Wang X. Expression of calcitonin gene-related peptide (CGRP) mRNA in rat lymphocytes. Sheng Li Xue Bao. 1998;50:423–30. [PubMed] [Google Scholar]
- Xu D, Wang XA, Wang JP, Yuan QX, Fiscus RR, Chang JK, Tang JA. Calcitonin gene-related peptide (CGRP) in normotensive and spontaneously hypertensive rats. Peptides. 1989;10:309–312. doi: 10.1016/0196-9781(89)90035-1. [DOI] [PubMed] [Google Scholar]
- Xu Y, Swerlick RA, Sepp N, Bosse B, Ades EW, Lawley TJ. Characterization of expression and modulation of cell adhesion molecules on an immortalized human dermal microvascular endothelial cell line (HMEC-1) J Invest Dermatol. 1994;102:833–837. doi: 10.1111/1523-1747.ep12382086. [DOI] [PubMed] [Google Scholar]
- Yamamoto T. Potential roles of CCL2/monocyte chemoattractant protein-1 in the pathogenesis of cutaneous sclerosis. Clin Exp Rheumatol. 2003;21:369–375. [PubMed] [Google Scholar]
- Zaidi M, Moonga BS, Bevis PJ, Bascal ZA, Breimer LH. The calcitonin gene peptides: biology and clinical relevance. Crit Rev Clin Lab Sci. 1990;28:109–174. doi: 10.3109/10408369009105900. [DOI] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 2008;23:1399–1407. doi: 10.14670/hh-23.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman BJ, Anderson DC, Granger DN. Neuropeptides promote neutrophil adherence to endothelial cell monolayers. Am J Physiol. 1992;263:G678–682. doi: 10.1152/ajpgi.1992.263.5.G678. [DOI] [PubMed] [Google Scholar]