Abstract
A male advantage over females for spatial tasks has been well documented in both humans and rodents, but it remains unclear how the activational effects of testosterone influence spatial ability in males. In a series of experiments, we tested how injections of testosterone influenced the spatial working and reference memory of castrated male rats. In the eight-arm radial maze, testosterone injections (0.500 mg/rat) reduced the number of working memory errors during the early blocks of testing but had no effect on the number of reference memory errors relative to the castrated control group. In a reference memory version of the Morris water maze, injections of a wide range of testosterone doses (0.0625-1.000 mg/rat) reduced path lengths to the hidden platform, indicative of improved spatial learning. This improved learning was independent of testosterone dose, with all treatment groups showing better performance than the castrated control males. Furthermore, this effect was only observed when rats were given testosterone injections starting seven days prior to water maze testing and not when injections were given only on the testing days. We also observed that certain doses of testosterone (0.250 and 1.000 mg/rat) increased perseverative behavior in a reversal-learning task. Finally, testosterone did not have a clear effect on spatial working memory in the Morris water maze, although intermediate doses seemed to optimize performance. Overall, the results indicate that testosterone can have positive activational effects on spatial learning and memory, but the duration of testosterone replacement and the nature of the spatial task modify these effects.
Keywords: testosterone, androgen, spatial memory, working memory, reference memory, water maze, radial arm maze, dose-response, rats
Introduction
Spatial ability is broadly defined as the ability to perceive, encode, store, retrieve, transform and integrate spatial information drawn from two- or three-dimensional space (Ecuyer-Dab and Robert, 2004). Men outperform women on a variety of spatial tasks including mental rotation of objects (Kaufman, 2007; Parsons et al., 2004) , route learning (Holding and Holding, 1988; Postma et al., 2004), and maze navigation (Astur et al., 1998; Moffat et al., 1998). A male advantage for a variety of spatial learning and memory tasks has also been documented for meadow voles (Gaulin and FitzGerald, 1986), deer mice (Galea et al., 1994), and various strains of laboratory mice and rats (Jonasson, 2005), which suggests that this sex difference may be a common phenomenon among mammals possibly shaped at the ultimate level by various selective pressures (Ecuyer-Dab and Robert, 2004; Gaulin and FitzGerald, 1986). Among rats, specifically, males have been shown to have better working and reference memory based on performance in the Morris water maze (Harris et al., 2008; Markowska, 1999; Roof and Stein, 1999) and 17-arm radial maze (Gibbs and Johnson, 2008; Seymoure et al., 1996). Working memory is defined as a form of short-term memory that involves storage of information from a particular task only for as long as it is useful to complete the task, and reference memory is defined as the long-term storage of memories that are used from one task to the next (Olton and Papas, 1979).
At the proximate level, there is considerable evidence that sex steroids (estrogens and androgens) play at least some role in causing sex differences in spatial ability. Evidence from experiments with humans and rats indicates that testosterone has organizational effects upon the brain early in development that enhance spatial learning and memory (Hines et al., 2003; Isgor and Sengelaub, 1998; Mueller et al., 2008; Roof and Havens, 1992; Williams et al., 1990). Whether androgens have activational effects that enhance spatial ability in adults remains less clear. Recent reviews have concluded that there is no consistent evidence that elevated circulating testosterone levels improve spatial ability in men (Puts et al., 2010; Ulubaev et al., 2009). In spite of inconsistency, however, there is some evidence that testosterone can improve mental rotation ability (Christiansen and Knussmann, 1987; Hooven et al., 2004; Silverman et al., 1999), route-learning (Choi and Silverman, 2002), and performance of a block design task (Thilers et al., 2006) among younger men. A causal link between age-related declines in testosterone and memory has been demonstrated by some longitudinal and cross-sectional studies (Moffat et al., 2002), but other studies have found no relationship between testosterone and spatial ability among older men (Martin et al., 2007; Matousek and Sherwin, 2010). Clinical studies testing the effects of exogenous testosterone replacement therapy on the cognitive abilities of healthy older men have also produced mixed results, with some studies showing improved spatial ability in men receiving testosterone (Cherrier et al., 2001; Cherrier et al., 2005; Janowsky et al., 1994), while other studies have shown no effect of testosterone on cognition (Emmelot-Vonk et al., 2008; Vaughan et al., 2007; Wolf et al., 2000). Additionally, testosterone supplementation given to men with Alzheimer’s disease has been shown to be effective in improving spatial memory (Cherrier et al., 2005; Tan and Pu, 2003). Thus, although there is considerable evidence that testosterone supplementation may restore cognitive function (specifically spatial ability) in men with age-related memory loss, contradictory results render the benefits of such intervention questionable.
The contradictory results obtained with human studies highlight the need for the use of rodent models to experimentally test the effect of testosterone upon spatial ability. Unfortunately, the results of rodent studies are also inconsistent, but some general trends have begun to emerge. In various versions of the radial arm maze and T-maze, castration has been shown to impair spatial working memory (Daniel et al., 2003; Gibbs and Johnson, 2008; Hasegawa and Mochizuki, 2009; Kritzer et al., 2001; Spritzer et al., 2008). Surprisingly, Gibbs and Johnson (2008) found that testosterone replacement using silastic implants in castrated male rats did not restore spatial working memory performance on the radial arm maze. In contrast, testosterone implants did restore males’ performance on a T-maze alternation task and a water radial arm maze (Bimonte-Nelson et al., 2003; Kritzer et al., 2001). Therefore, most current evidence indicates that testosterone enhances spatial working memory among male rats. The effects of testosterone on spatial reference memory remain less clear. Experiments using the radial-arm maze suggest that testosterone impairs spatial reference memory (Gibbs and Johnson, 2008; Spritzer et al., 2008), whereas studies employing the Morris water maze to test spatial reference memory have shown improvement of (Khalil et al., 2005) impairment of (Goudsmit et al., 1990; Naghdi et al., 2001) or no effect of testosterone on performance (Hodosky et al., 2010; Naghdi et al., 2005b; Sandstrom et al., 2006; Spritzer et al., 2008). Some of this variation in results is likely due to differences in the doses of testosterone and the method of testosterone replacement. For example, some studies showing that testosterone impairs spatial reference memory involved injections of a high dose of testosterone directly into the CA1 region of the hippocampus (Moradpour et al., 2006; Naghdi et al., 2001; Naghdi et al., 2005b). It is also important to note that most past studies in this area have failed to assess circulating testosterone levels following an experimental manipulation, making it difficult to compare results among studies and to determine whether subjects experienced physiological or supra-physiological levels of testosterone.
The general goal of the current study was to further clarify the activational effects of testosterone on the spatial ability of males using rats as a model system. More specifically, we conducted a series of experiments designed to determine: 1) whether the effects of testosterone replacement depend on the type of maze task (i.e., radial arm maze vs. Morris water maze), 2) the dose response relationship between testosterone and spatial memory using doses within the physiological range for a male rat, 3) whether duration of exposure influences the effects of testosterone on spatial memory, and 4) whether the effects of testosterone differ between spatial working and reference memory. To achieve these goals, we used a working-reference memory version of the radial arm maze and both reference and working memory versions of the Morris water maze. For the reference memory version of the water maze, we employed a broad dose range and varied the duration of testosterone exposure prior to behavioral testing. We also assessed circulating testosterone levels following hormone replacement for all of our subjects.
Methods
Subjects
Adult male rats (approximately 60 days old) were obtained from Charles River Laboratories (St. Constant, Quebec, Canada). For Experiment 1, Sprague-Dawley rats were used to allow comparison with a previous study that tested this strain using the radial arm maze (Spritzer et al., 2008), while for Experiments 2 and 3 Long-Evans rats were used to facilitate tracking in the water maze. For all experiments, rats were individually housed in opaque polypropylene cages (21 × 42 × 21 cm) with Tek-Fresh Bedding (Harlan Laboratories, Indianapolis, IN, USA). Animals had free access to water and a soy-protein-free rodent diet (Harlan Teklad Diet 2020X), except during food restriction for rats tested on the radial arm maze. The housing room was temperature controlled (21 ± 1 °C) with a 12:12 h light/dark cycle (lights on at 0700 h). All animal procedures were approved by the Middlebury College Animal Care and Use Committee and were carried out in accordance with ethical guidelines set by the National Institutes of Health.
All subjects were bilaterally castrated 7-14 days after they arrived in the animal facility. Surgeries were performed with aseptic technique and under isofluorane anesthesia (3.5-4.0% in oxygen during induction, 2.0-2.5% in oxygen during maintenance). The analgesic Ketofen was administered before surgery (5 mg/kg body mass, s. c.), and the topical analgesic Fougera (2.5% lidocaine, 2.5% prilocaine) was applied to the incision site immediately after surgery. Each testis was excised through a small incision at the posterior end of the scrotum and ligated with chromic gut suture material (Ethicon, Somerville, NJ, USA). The muscular sheath was closed with chromic gut sutures, and the skin layer was closed with ethilon sutures (Ethicon). For each experiment, the rats were castrated over two days with half the rats castrated each day (Table 1). Seven days after surgery, the average mass of the subjects was 350.4 ± 2.7 g.
Table 1.
Timelines for the Experiments 1-3 (including 2A and 2B) with the days shown during which each procedure was conducted.
| Exp. | GDX | Handle | Habituate and shape |
Testosterone injections |
Acquisition* | Probe trials |
Reversal trials |
Blood draw |
|---|---|---|---|---|---|---|---|---|
| 1 | 1-2 | 14-17 | 18-23 | 24-53 | 24-53 | NA | NA | 54 |
| 2A | 1-2 | 9-12 | NA | 13-19 | 13-18 | 19 | NA | 19 |
| 2B | 1-2 | 9-12 | NA | 13-27 | 20-25 | 26 | 27 | 27 |
| 3 | 1-2 | 9-12 | NA | 13-25 | 20-25 | NA | NA | 25 |
NA (not applicable) refers to procedures that were not used for a particular experiment. GDX (gonadectomy) refers to the days when castration surgeries were performed.
Acquisition refers to training days on the radial arm maze (Experiment 1) or acquisition trials in the water maze (Experiments 2 and 3).
Apparatus
For Experiment 1, testing was conducted on an eight-arm radial arm maze elevated 53 cm above the floor, with arms (57 cm × 10 cm) projecting at equal angles from a central platform (34 cm diameter). During testing, 45 mg dustless reward pellets (Bio-Serv, Frenchtown, New Jersey) were placed in small plastic cups affixed 1 cm from the end of each arm, which prevented rats from seeing the pellets. The maze remained in the same location in a dimly lit room for the duration of behavioral testing. Each wall of the room was visually distinct from the others to create clear extra-maze cues. Large, high contrast cues were mounted on two of the white walls (a rectangular poster and a large X), one wall had no cues, and the remaining wall consisted of a large black curtain used to divide the testing room. To minimize intra-maze cues, the maze was rotated random 90° increments before beginning testing each day while remaining in the same relative position in the room. Each rat was placed in the same direction in the center of the maze for all trials, and after placing each rat the experimenter sat nearby in the same location for all trials.
For Experiments 2 and 3, the Morris water maze used was a circular white steel pool (180 cm diameter; 60 cm high). The pool was filled to approximately 40 cm with water at equilibrium with room temperature (20 ± 2°C), which was made opaque with non-toxic white powdered paint (Sargent Art, Hazelton, PA, USA). The pool was divided virtually into four quadrants with four equidistant release points around the edge. The goal platform (10 cm diameter) was submerged 2.5 cm beneath the water surface in the center of one of the quadrants (50 cm from the pool edge). Each trial was recorded by a digital USB camera mounted on the ceiling directly above the maze and interfaced with a laptop computer running a video tracking program (ANY-maze™ Video Tracking System, 4.62a beta; Stoelting Co., Wood Dale, IL, USA). For all trials, distal cues around the maze remained constant throughout testing; these included two large paper patterns affixed to two walls, a black curtain used to obscure observers, two electrical outlets, and asymmetrical overhead lighting.
Experiment 1
Experiment 1 tested the effects of testosterone on spatial memory using a working-reference memory version of the radial arm maze (Olton and Papas, 1979). This experiment involved 20 adult male rats divided into two groups (N = 10/group), which received s.c. injections of either 0.500 mg of testosterone propionate dissolved in 0.1 ml of sesame oil (0.500 mg T group) or 0.1 ml sesame oil (Control group). After recovery from surgery (12-13 days), rats were handled 4-5 min per day for four days to habituate them to the researchers. Subjects were injected with testosterone or oil daily (0800-0900 h) throughout maze testing (30 days total) beginning on the first day of maze testing. Because injections did not occur during the maze habituation and shaping period, the first day of injections was 22-23 days after castrations (Table 1).
Behavioral testing consisted of three habituation days, three shaping days, and 30 training days. Beginning on the first day of maze habituation and continuing through all the training days, animals were maintained at 90% of their free-feeding body mass using food restriction. To determine the target mass, all rats were weighed each day and two free-feeding rats were used as a reference to determine the expected daily growth rate. The amount of food that rats received was weighed each day and adjusted to maintain each rat as close as possible its target body mass. All animals received ten reward pellets in their home cages daily during the habituation and shaping phases of testing. For each rat, four arms of the maze were consistently baited throughout the experiment. Maze habituation began immediately after the four-day handling period. On the first day of habituation, rats were placed in the center of the maze and allowed to explore the apparatus for 5 min. During the second and third days of habituation, time on the maze was extended to 10 min. During shaping, several reward pellets were positioned sequentially along assigned arms and the rats were allowed 10 min to explore the maze. Finally, during training days, a single pellet was placed in the plastic cup at the end of each assigned arm. Training trials were conducted each afternoon (1200-1700 h), and the order in which rats were tested was randomized each day. Training trials ended when either the rat retrieved all four of the pellets or 10 min had elapsed. Two rats, both from the 0.500 mg T group, failed to complete the task in the allotted 10 min during all of the first 15 days of testing and were dropped from the experiment. For an additional six trials involving four rats (two from each group), the subjects remained in the center of the maze and did not move for the entire 10 min. For these trials, each rat was assigned the average of the number of errors made on the trial before and after the incomplete trial.
Rats could make two different types of errors during training trials: reference memory errors (RME), defined as entries into non-baited arms, and working memory errors (WME), defined as repeat entries into an arm within a single trial. We also scored working-reference errors (WRE), which were a subset of WME’s defined as within-trial re-entry into arms that have never been baited. Latency to make the first arm choice was used as a measure of motivation to perform the task.
Experiment 2
Experiment 2 consisted of two sub-experiments (2A and 2B) designed to test the effects of testosterone on male performance in the standard reference memory version of the Morris water maze (Morris, 1984). Experiment 2A involved 48 adult male rats (N = 8/group), and Experiment 2B involved 72 adult male rats (N = 12/group). All rats were given 7-8 days of recovery followed by 4 days of handling for 4-5 min per day; therefore, rats started testosterone injections 11-12 days after castrations (Table 1). The main difference between the two sub-experiments was the duration of testosterone injections: for Experiment 2A rats were injected only during the seven days of testing, whereas for Experiment 2B injections began seven days prior to the start of maze testing and continued throughout testing (15 days of injections total). For both experiments, the rats were divided into six groups, with each group receiving daily (0900-1000 h) s.c. injections of a specific dose of testosterone propionate dissolved in 0.1 ml of sesame oil: Control, 0.0625 mg T, 0.125 mg T, 0.250 mg T, 0.500 mg T, and 1.000 mg T.
Both experiments included six consecutive days of acquisition trials followed by one day of probe trials, and Experiment 2B included an additional single day of reversal-learning trials on the day after the probe trials. Trials were conducted each afternoon (1300-1700 h), and the order in which rats were tested was the same each day and was counter-balanced for treatment group. For acquisition trials, rats were subjected to blocks of four trials each day, in which they were released from each of the four release sites around the pool in a random order. The platform remained in the same quadrant for all trials and for all rats. Rats were released near the edge of the pool facing the edge, and the researcher went out of view behind the black curtain immediately after each release. The rats were allowed a maximum of 90 s to find the platform and then allowed to remain on the platform for 15 s. If at the end of 90 s the rat was unsuccessful, it was placed on the platform and allowed to remain there for 15 s. Between acquisition trials, rats were towel-dried and placed in a clean holding cage for a 45 s inter-trial interval. The time needed to reach the platform (escape latency) and path length to the platform were analyzed as indices of spatial learning. Percentage of time spent thigmotactic (within 20 cm of the pool edge) was also analyzed, with high levels of thigmotaxis indicative of an inability to initiate spatial learning in the water maze (Devan et al., 1999) or possibly a stronger stress response (Beiko et al., 2004; Perrot-Sinal et al., 1996). A single probe trial was conducted on the day after the final day of acquisition trials. For the probe trial, the platform was removed and the amount of time spent swimming in each quadrant was recorded for 90 s. Percentage of time spent in the quadrant where the platform had been located during the acquisition trials was used as an index of spatial memory retention. For Experiment 2B only, reversal-learning trials were conducted on the day after the probe trials. For reversal-learning trials, the platform was placed in the quadrant opposite of where it had been during the acquisition trials, and rats were released from all four release points in a random order. Time spent in the quadrant where the platform had been located during acquisition trials was used as an index of perseverance.
Experiment 3
Experiment 3 tested the effects of testosterone upon spatial working memory using a working memory version of the Morris water maze (Hodges et al., 1995; Vorhees and Williams, 2006). This experiment involved 48 adult male rat divided into four groups (N = 12/group), with each group assigned a dose of testosterone propionate dissolved in 0.1 mL of sesame oil: Control, 0.125 mg T, 0.250 mg T, and 0.500 mg T. As for Experiment 2, all rats were given 7-8 days of recovery followed by 4 days of handling for 4-5 min per day; therefore, rats started testosterone injections 11-12 days after castrations (Table 1). Each rat received daily (0900-1000 h) s.c. injections for 13 days beginning seven days prior to the start of maze testing and continuing throughout testing.
Water maze testing was conducted each afternoon (1300-1700 h), and the order in which rats were tested was the same each day and was counter-balanced for treatment group. The testing protocol was similar to that described for the acquisition trials in Experiment 2, with 6 days of trials and 4 trials each day. The goal platform remained in the same location during each day of testing, but unlike Experiment 2 the goal platform was moved to a new quadrant each day. The location of the platform was determined in a semi-random manner such that the same location was not used on consecutive days and no quadrant was used more than twice. Also unlike Experiment 2, the inter-trial interval was only 30 s to allow testing of working memory (Hodges et al., 1995). Changes in escape latency and path length within each day of testing were considered indicative of spatial working memory.
Testosterone assays
Blood samples were collected from all rats to assay circulating testosterone levels. For Experiment 1, blood was collected one day after the last day of testing (i.e., 24 h after the last testosterone injections), whereas for Experiments 2 and 3 blood was collected immediately after water maze testing was completed on the final day of testing (i.e., 6-7 h after the last testosterone injections). For Experiments 1 and 3, rats were given a lethal dose of sodium pentobarbital (approximately 150 mg/kg, i. p.) and blood was collected via heart puncture. For Experiment 2, blood was collected via tail clip while the rat was in a plastic restrainer. Blood was refrigerated overnight at 4 °C before being centrifuged (15 min, 10,000 RPM), and the serum was extracted and stored at −20 °C.
Total serum testosterone was assayed using coated-tube radioimunnoassay (RIA) kits with all samples run in duplicate. For Experiments 1 and 2, we used kits supplied by Diagnostic Systems Laboratories (DSL, Webster, TX, USA), which had a lower limit of detection of 0.08 ng/ml. The testosterone antibody had some cross-reactivity with dihydrotestosterone (5.8%), 11-oxotestosterone (4.2%), and androstenedione (2.3%), and other androgens (< 1.0%), but had no detectable cross-reactivity with progesterone, estrogens, or glucocorticoids. The inter-assay coefficient of variation for this kit was 8.7%. Unfortunately, prior to completing Experiment 3, the DSL kits were discontinued by the supplier, and we therefore switched to a different supplier (Siemens Healthcare Diagnostics, Los Angeles, CA, USA). The Siemens kits had a lower limit of detection of 0.04 ng/ml, and the testosterone antibody had some cross-reactivity with dihydrotestosterone (3.3%), other androgens (< 1.0%), and very low cross-reactivity with progesterone, estrogens, or glucocorticoids (< 0.1%). Relatively lower circulating testosterone levels were found for Experiment 3 than for the other experiments (Table 2), indicating possible differences between the RIA kits. To test for this, 10 samples from Experiment 2 were run with both kits. This resulted in a fairly low inter-assay coefficient of variation (7.7%), suggesting that differences between the kits were minimal and that the lower testosterone concentrations obtained for Experiment 3 may have been due to other factors (e.g., differences in blood collection methods). The intra-assay coefficient of variation for all experiments combined was 9.2%.
Table 2.
Testosterone concentrations (mean ± SEM) in serum collected at 24 h after the last injection for Experiment 1 and 6-7 h after the last injection for Experiments 2 and 3.
| Experiment | Treatment | N | Serum testosterone (ng/ml) |
|---|---|---|---|
| 1 | Control | 10 | 0.00 ± 0.00a |
| 0.500 mg T | 8 | 4.59 ± 0.34* | |
| 2A | Control | 8 | 0.23 ± 0.13a |
| 0.0625 mg T | 8 | 1.87 ± 0.17 | |
| 0.125 mg T | 8 | 2.83 ± 0.27 | |
| 0.250 mg T | 8 | 5.73 ± 0.34* | |
| 0.500 mg T | 8 | 15.17 ± 0.34* | |
| 1.000 mg T | 8 | 26.53 ± 2.14* | |
| 2A | Control | 12 | 0.02 ± 0.02a |
| 0.0625 mg T | 12 | 1.50 ± 0.14 | |
| 0.125 mg T | 12 | 3.05 ± 0.48 | |
| 0.250 mg T | 12 | 5.58 ± 0.39* | |
| 0.500 mg T | 12 | 12.43 ± 1.86* | |
| 1.000 mg T | 12 | 27.09 ± 2.71* | |
| 3 | Control | 12 | 0.02 ± 0.07 a |
| 0.125 mg T | 12 | 1.98 ± 0.20* | |
| 0.250 mg T | 12 | 3.66 ± 0.27* | |
| 0.500 mg T | 12 | 7.79 ± 0.47* |
Significantly different from control levels (P < 0.05).
All samples below the detection limit of the assay (0.08 ng/ml) were assigned a value of 0 ng/ml.
Statistical analysis
For Experiment 1, the radial arm maze data were divided into six five-day blocks for analyses. Each of the different types of memory errors (WME, RME, and WRE) and the latency to make the first arm choice were analyzed using repeated measures analysis of variance (ANOVA). The five-day blocks were the within-subjects factor and testosterone treatment was the between-subjects factor. Differences in serum testosterone concentrations among the treatment groups were analyzed using a t-test (Experiment 1) or one-way ANOVA (Experiments 2 and 3). For Experiment 2, measures of performance during acquisition trials (i.e., path length, escape latency, and percent time thigmotactic) were averaged within each day for each animal. These data were then analyzed using repeated measures ANOVA’s, with day as the within-subjects factor and testosterone treatment as the between-subjects factor. For the probe trials, the percentage of time spent in the target quadrant by each treatment group was compared using a one-way ANOVA, and Fisher’s least significant difference (LSD) test was used for post hoc comparisons. The percentage of time spent in the target quadrant was also compared to that expected for random swim paths (25%) using one-sample t-tests for each group of rats. For the reversal-learning trials, path length, escape latency, and time spent in the old target quadrant were each analyzed in two ways. First, repeated measures ANOVA’s were used, with trial as the within-subjects factor and testosterone treatment as the between-subjects factor. Second, the data were averaged across trials for each subject and differences between groups were analyzed using one-way ANOVA’s. Finally for Experiment 3, measures of performance during working memory trials (i.e., path length, escape latency, and percent time thigmotactic) were analyzed using repeated measures ANOVA’s, with day and trial as the within-subjects factors and testosterone treatment as the between-subjects factor. Data within days were subsequently analyzed with repeated measures ANOVA with trial as the within-subjects factor and treatment as the between-subjects factor. When significant day × treatment interactions were observed, the data were analyzed within each day using either t-tests (Experiment 1) or ANOVAs (Experiment 2 and 3), and Fisher’s LSD was used for post-hoc comparisons when an ANOVA for a particular day was significant. SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used for all analyses, and P < 0.05 was considered statistically significant.
Results
Experiment 1: Effects of testosterone on performance on the radial arm maze
The rats injected with testosterone (0.500 mg T) made few WME’s throughout testing, whereas the control rats made more WME’s during the early days of testing than during the later days (Fig. 1A). The day × treatment interaction was statistically significant (F(5,80) = 3.07, P = 0.014). There was also a significant main effect of day indicating that rats were learning the task (F(5,80) = 6.75, P < 0.0005), but there was not a significant main effect of treatment (P = 0.19). Comparisons within the five-day blocks showed that the Control group committed significantly more WME’s than did the 0.500 mg T group on days 1-5 (t(16) = 2.31, P = 0.035). A similar difference occurred on days 11-15, although this was not statistically significant (t(16) = 1.97, P = 0.066), and there were no significant differences for any of the other 5-day blocks (all P > 0.15). Similar trends were observed for WRE’s (Fig. 1B), with a significant day × treatment interaction (F(5,80) = 3.20, P = 0.011), significant main effect of day (F(5,80) = 5.07, P < 0.0005), but the main effect of treatment was not significant (F(1,16) = 4.19, P = 0.058). Comparisons within the five-day blocks showed that the Control group committed significantly more WRE’s than did the 0.500 mg T group on days 1-5 (t(16) = 2.38, P = 0.033) and days 6-10 (t(16) = 2.42, P = 0.027), and there were no significant differences between groups for any of the other five-day blocks (all P > 0.45). There were no significant effects of day, treatment, or the day × treatment interaction for RME’s (all P > 0.40), indicating that rats from both groups made similar numbers of RME’s and there were no clear improvements in reference memory during the course of testing (Fig. 1C).
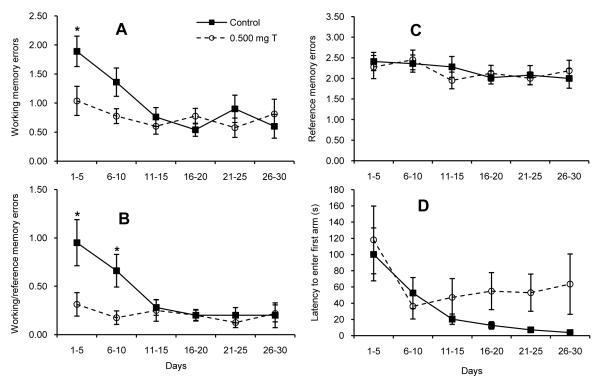
Fig. 1.
Number of (A) WME’s, (B) WRE’s, and (C) RME’s performed by castrated male rats injected daily with sesame oil (Control; N = 10) or 0.500 mg testosterone propionate (0.500 mg T; N = 8) during 5-day blocks of trials over 30 days of testing on the radial arm maze. Control males committed significantly more WME’s and WRE’s during the early blocks of trials (* P < 0.05). There was no significant difference between groups for RME’s.
The difference between groups was also not significant difference between groups in (D) the latency to make the first arm choice. All data are shown as means ± SEM (N = 8/group).
All rats showed a significant reduction in the latency to make the first arm choice over the course of testing, suggesting increased motivation to complete the task as the days progressed (Fig. 1D; day effect: F(5,80) = 9.15, P < 0.0005). Rats in the 0.500 mg T group tended to take longer to make their first arm entry than did the control rats during the later days of testing, which suggests that the 0.500 mg T group may have been less motivated to perform on the maze. However, the day × treatment interaction was not significant (P = 0.13) and the main effect of treatment was not significant (P = 0.26).
All of the control rats had serum testosterone concentrations below the detection limit for the assay (0.08 ng/ml). The serum testosterone concentration for the 0.500 mg T group was significantly greater than 0 (Table 2; one-sample t-test: t(7) = 13.34, P < 0.0005).
Experiment 2A: Effects of acute testosterone exposure on performance of reference memory version of the Morris water maze
All rats showed significant decreases in escape latency and path length over the six days of acquisition trials on the Morris water maze (Figs 2A and 2B; escape latency: F(5, 210) = 138.5, P < 0.0005; path length: F(5, 210) = 197.2, P < 0.0005), which indicates that rats learned the location of the platform. However, the day × treatment interaction was not significant (both P < 0.65) and the main effect of treatment was not significant (both P < 0.17), indicating that testosterone did not influence learning. Similarly, there was a significant reduction in the percentage of time spent thigmotactic over the testing days (F(5, 210 = 99.48, P < 0.0005), but the day × treatment interaction (P = 0.99) and the treatment effect (P = 0.10) were not significant.
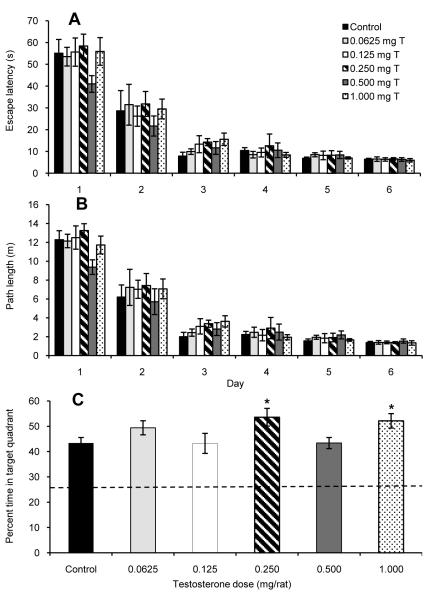
Fig. 2.
Performance on a reference memory version of the Morris water maze by castrated male rats injected daily for seven days with sesame oil (Control) or various doses of testosterone propionate beginning on the first day of maze testing. During six days of acquisition trials, all groups showed a significant decrease in both (A) escape latency and (B) path length (both P < 0.0005), but testosterone injections had no effect on either of these measurements of learning. (C) During probe trials, all treatment groups spent more time swimming in the target quadrant than would be expected by a random swim path (represented by dashed line), and the 0.250 mg T and 1.000 mg T groups each showed significantly better retention than the Control group (*Fisher’s LSD: P < 0.05). All data are shown as means ± SEM (N = 8/group).
During the probe trails, all groups showed a significant bias toward swimming in the quadrant where the platform had been during the acquisition trials (Fig. 2C; all P < 0.005). Interestingly, there were also significant differences between groups in the amount of time spent in the target quadrant (F(5, 42) = 2.57, P = 0.041). Post hoc analyses showed that both the 0.250 mg T group (P = 0.020) and the 1.000 mg T group (P = 0.044) spent a significantly greater percentage of time in the target quadrant than did the Control group. This suggests that these doses of testosterone improved memory retention.
Three of the rats in the Control group had detectable serum testosterone, although at low concentrations (0.43-0.95 ng/ml). Serum testosterone concentrations differed significantly among the groups (Table 2; F(5, 42) = 49.62, P < 0.0005). The 0.250, 0.500, and the 1.000 mg T groups each had serum testosterone concentrations significantly higher than the control group (all P < 0.01).
Experiment 2B: Effects of prolonged testosterone exposure on performance of reference memory version of the Morris water maze
Unlike Experiment 2A, rats in Experiment 2B received seven days of hormone injections prior to starting testing in the water maze. Similar to Experiment 2A, all rats showed significant decreases in escape latency and path length over the six days of acquisition trials on the Morris water maze (Figs 3A and 3B; escape latency: F(5, 325) = 226.5, P < 0.0005; path length: F(5, 325) = 219.2, P < 0.0005). Testosterone injections did not have significant main effects on escape latency (P = 0.096) or path length (P = 0.068), but the Control group did tend to perform worse than most of the other groups (Fig. 3A and 3B). The day × treatment interaction was not significant for escape latency (P = 0.23), but it just reached significance for path length (F(25, 325) = 1.53, P = 0.049). Analyses of path lengths within each day revealed a significant effect of treatment on day 2 (F(5, 65) = 2.45, P = 0.043) and day 5 (F(5, 65) = 2.99, P = 0.017). Post hoc analyses for day 2 showed that all of the testosterone-injected groups had significantly shorter path lengths than the Control group (all P < 0.05) and there were no significant differences between any of the other groups. The differences in path length on day 5 seem less interesting because by this day all the rats were performing very efficiently on the maze (Fig. 3B). Post hoc tests for day 5 showed that the 1.000 mg T group had significantly shorter path lengths than did the 0.125 and 0.250 mg T groups (both P < 0.02) and there was also a significant difference between the 0.0625 mg T and the 0.250 mg T group (P = 0.012). All groups showed a significant reduction in the percentage of time spent thigmotaictic over the testing days (F(5,325) = 188.2, P < 0.0005), but the day × treatment interaction (P = 0.35) and the treatment effect (P = 0.53) were not significant.
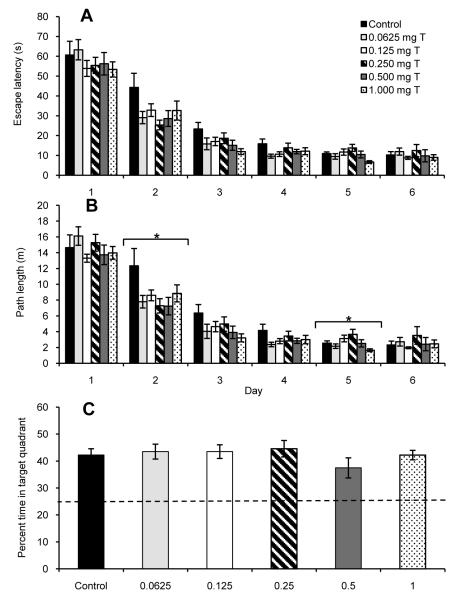
Fig. 3.
Performance on a reference memory version of the Morris water maze by castrated male rats injected daily for fifteen days with sesame oil (Control) or various doses of testosterone propionate beginning on the seven days before the first day of maze testing. During six days of acquisition trials, all groups showed a significant decrease in both (A) escape latency and (B) path length (P < 0.0005). For path length, but not escape latency, there was a significant day × treatment interaction (P < 0.05), and within day analyses revealed significant treatment effects for days 2 and 5 (*P < 0.05). (C) During probe trials, all treatment groups spent more time swimming in the target quadrant than would be expected by a random swim path (represented by dashed line), but there were no significant differences between groups. All data are shown as means ± SEM (N = 11-12/group).
During the probe trials, all groups showed a significant bias toward swimming in the quadrant where the platform had been during the acquisition trials (Fig. 3C; all P < 0.005). Treatment did not have a significant effect on the percentage of time that rats spent in the target quadrant (P = 0.56). Note that as for Experiment 2A, the 0.500 mg T group spent the least amount of time in the target quadrant (Figs. 2C and 3C).
For the reversal-learning trials, the data were initially analyzed across the four trials to determine whether rats learned the new position of the platform. The trial × treatment interaction was not significant for any of the variables analyzed (all P > 0.14). All rats showed significant decreases in escape latency and path length over the four reversal-learning trials (Figs 4A and 4B; escape latency: F(3, 195) = 76.52, P < 0.0005; path length: F(3, 195) = 67.57, P < 0.0005). There was a significant effect of treatment for path length (Fig. 4B; F(1,65) = 2.69, P = 0.029), but not for escape latency (Fig. 4A; P = 0.052). For the amount of time spent in the old target quadrant (Fig. 4C), there was a significant decrease over the trails (F(3,192) = 81.39, P < 0.0005), but no significant effect of treatment (P = 0.14) or a trial × treatment interaction (P = 0.14). Analyzing the data with the four trials averaged for each subject revealed clearer differences between the groups. There were significant differences between the groups in the amount of time spent swimming in the old target quadrant (Fig. 4D; F(5,70) = 2.37, P = 0.049). Post hoc comparisons showed that the 0.250 mg T group spent significantly more time in the old quadrant than did the 0.0625, 0.125, and 0.500 mg T groups (all P < 0.05). Furthermore, the 1.000 mg T group spent significantly more time in the old target quadrant than did the 0.125 mg T group (P = 0.032). Escape latency and path length to the new platform location also differed significantly among the groups (escape latency: F(5,70) = 2.39, P =0.047; path length: F(5,70) = 2.72, P = 0.027), and post hoc comparisons revealed a pattern similar to that found for the amount of time spent in the old quadrant. This suggests that the 0.250 mg T group, in particular, was slower than some of the other groups in locating the platform due to perseverative behavior.
Fig. 4.
Performance during four reversal-learning trials in the Morris water maze by castrated male rats injected daily for fifteen days with sesame oil (Control) or various doses of testosterone propionate beginning on the seven days before the first day of maze testing. Reversal-learning trials were conducted on a single day after six days of acquisition trials and one day of probe trials. The platform was located in the quadrant opposite of that used for acquisition trials. All groups showed a significant decrease in (A) escape latency, (B) path length, and (C) time spent in the old target quadrant (P < 0.0005). Averaging the data across trials (D) revealed that the 0.250 mg T group spent significantly more time in the old target quadrant than did most of the other groups (letters indicate groups that differ from each other, P < 0.05). All data are shown as means ± SEM (N = 11-12/group).
Only one of the rats in the Control group had detectable testosterone in its serum (0.26 ng/ml). Serum testosterone concentrations differed significantly among the groups (Table 2; F(5, 64) = 57.50, P < 0.0005). The 0.250, 0.500, and the 1.000 mg T groups each had serum testosterone concentrations significantly higher than did the Control group (all P < 0.005).
Experiment 3: Effects of prolonged testosterone exposure on performance of working memory version of the Morris water maze
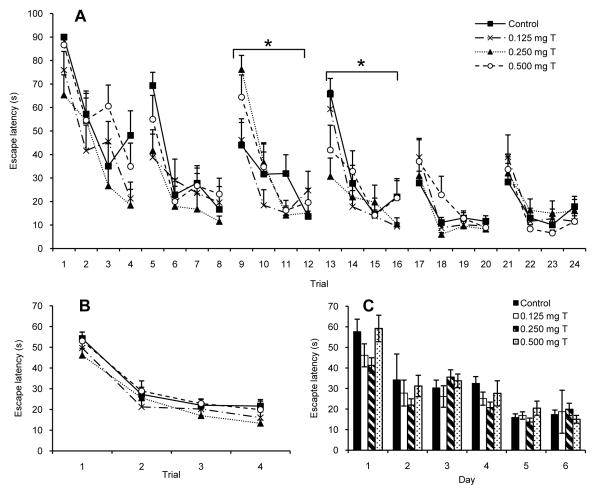
Testosterone did not have consistent effects on rats’ performance on the working memory version of the Morris water maze, although some subtle effects were noted (Fig. 5). Rats showed a significant decrease in escape latency across the six days of testing (F(5,220) = 44.26, P < 0.0005) and within each day there was a significant decrease in escape latency over the four trials (Fig. 5B; F(3, 44) = 110.85, P < 0.0005). There was also a significant day × trial interaction (F(15, 660) = 2.73, P < 0.0005) and a significant day × trial × treatment interaction (F(45, 660) = 1.90, P < 0.0005). Although there was a tendency for the 0.250 mg T group to perform better in the maze than any of the other groups on most days (Fig. 5C), none of the other main effects or interaction effects were statistically significant (all P > 0.10). Analyses within days showed a significant trial × treatment interaction only for days 3 and 4 (Fig. 5A; both P < 0.05). On day 3 it is noteworthy that the Control group was slower to improve on the task than were any of the testosterone-injected groups. On day 4 the Control group and the 0.500 mg T group performed poorer than did the other groups, especially on the fourth trial. Analyses of path length produced similar results to those obtained for escape latency, with the same effects having statistical significance (data not shown). One noteworthy exception was that for path length, the trial × treatment effect did not reach statistical significance on day 4 (P = 0.13). As for Experiment 2, all groups showed a significant reduction in the percentage of time spent thigmotactic over the testing days (F(5,220) = 109.6, P < 0.0005), but the day × treatment interaction (P = 0.77) and the treatment effect (P = 0.34) were not significant.
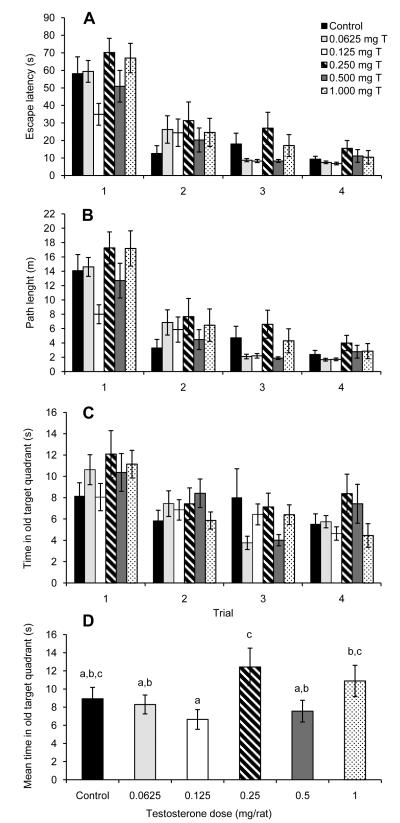
Fig. 5.
Escape latencies for a working memory version of the Morris water maze by castrated male rats injected daily for fifteen days with sesame oil (Control) or various doses of testosterone propionate beginning on the seven days before the first day of maze testing. Data are presented for (A) all trials, (B) averaged within trials, and (C) averaged within days. During six days of trials, all groups showed a significant decrease in escape latencies (P < 0.0005). There was also a significant day × trial × treatment interaction (P < 0.0005). Analyses within days showed a significant trial × treatment interaction for days 3 and 4 (*P < 0.05). All data are shown as means ± SEM (N = 12/group).
Only one rat in the Control group had detectable testosterone in its serum (0.24 ng/ml). Serum testosterone concentrations differed significantly among the groups (Table 2; F(3,44) = 127.8, P < 0.0005). Each of the testosterone-injected groups had serum testosterone concentrations that were significantly higher than the Control group (all P < 0.0005).
Discussion
Overall, the results of this study revealed some activational effects of testosterone on spatial ability in male rats, which were influenced by the nature of the task (water maze vs. radial arm maze), the duration of testosterone replacement, and the type of spatial memory being measured (reference memory vs. working memory). More specifically, testosterone replacement improved acquisition of the working memory component of the radial arm maze but had no effect on the reference memory component of the task. In contrast, in the water maze testosterone improved reference memory but its effects were less obvious for working memory. The enhancing effects of testosterone on learning in the reference memory version of the water maze were observed when rats were given testosterone injections for seven days prior to starting testing, whereas no effects were observed when rats were only injected on the days of testing in the water maze. Although we used a broad range of testosterone doses for rats tested in the reference memory version of the water maze, all of the doses improved spatial learning (i.e., shorter path lengths) to an equal degree. Finally, an unexpected finding was that certain doses of testosterone increased perseverative behavior in the water maze during reversal-learning trials.
Testosterone and working memory
In Experiment 1, testosterone replacement (0.5 mg/rat) resulted in a significant decrease in the number of WME’s committed during the initial days of testing on the radial arm maze. This result supports a number of previous studies with rats showing that testosterone improves spatial working memory on appetitively motivated tasks. In an experiment following an identical testing protocol, Spritzer et al. (2008) observed that castration caused a significant increase in the number of WME’s relative to intact control rats. Two other experiments using the eight-arm radial maze have demonstrated a similar reduction in working memory due to castration (Daniel et al., 2003; Hasegawa and Mochizuki, 2009). Gibbs (2005) observed that testosterone implants resulting in a high physiological level of serum testosterone (about 14 ng/ml) improved working memory on a T-maze task. Bimonte-Nelson et al. (2003) found that testosterone implants given to aged male rats resulting in average physiological levels of serum testosterone (about 4 ng/ml) restored working memory in a water radial arm maze. In our study, serum testosterone levels in the experimental males were 4.6 ng/ml, but blood samples were collected 24 h after the last injection and, therefore, testosterone levels were likely higher at the time of behavioral testing. Given that the physiological range of circulating testosterone for an intact rat is 1-14 ng/ml (Bartke et al., 1973; Keating and Tcholakian, 1979), the current results in combination with past work indicate that restoring testosterone to the physiological range in castrated male rats also restores their spatial working memory. In contrast, one study involving the 12-arm radial maze showed that testosterone implants resulting in normal physiological levels of serum testosterone (3.6 ng/ml) did not restore working memory among castrated males (Gibbs and Johnson, 2008). This difference may be because Gibbs and Johnson (2008) used a procedure that prevented the rats from using a simple motor response (i.e., response strategy) to solve the task. It has been previously noted that estradiol biases female rats to prefer place strategy (i.e., learn the spatial position of the reward) and against a response strategy (Korol and Kolo, 2002). Perhaps testosterone has the opposite effect in male rats, biasing them toward a response strategy. In tasks that involved the use of both place and response strategies, Schmidt et al. (2009) found that males were better than females at switching between strategies However, sex differences in strategy preference would not fully explain the results of Gibbs and Johnson (2008) because they did find that castrated males performed more poorly than intact males. Thus, although a growing number of studies indicate that testosterone improves spatial working memory in male rats, some unexplained contradictions remain.
While most previous studies have used appetitively motivated tasks to test the effects of testosterone on working memory in rats, Experiment 3 employed an aversively motivated task (Morris water maze). Testosterone did not have a clear effect on males’ performance in this task, but some trends were noted. In particular, the intermediate dose of testosterone (0.250 mg T group) seemed to result in better performance on the task compared to the Control group and the 0.500 mg T group. This supports some previous studies with humans indicating that there may be a negative parabolic (inverted U) relationship between testosterone and spatial memory (Cherrier et al., 2007; Gouchie and Kimura, 1991; Moffat and Hampson, 1996). For the human studies, however, the curvilinear relationship is due to a positive relationship between testosterone and spatial ability in women and a negative relationship in men, making it unclear whether such a relationship might be expected within an all male population.
It is noteworthy that the dose that improved working memory in the radial arm maze (0.500 mg/rat) did not improve working memory in the water maze, indicating that the effects of testosterone on working memory may be task dependent. The motivation of the rats to perform the two tasks may have differed. This explanation seems unlikely, however, because there were no significant differences in swimming speed between the groups in the water maze (data not shown), and the latency to enter the first arm of the radial arm maze did not differ significantly between groups. Furthermore, a study using an aversively motivated water radial arm maze showed that testosterone improved spatial working memory (Bimonte-Nelson et al., 2003). Another explanation is that the two tasks used may differ in their ability to accurately assess spatial working memory. Re-entry into a previously visited arm on the radial arm maze is arguably a purer index of working memory than is a change in a rat’s performance within days on the water maze. A rat’s performance in the working memory version of the water maze is dictated, to some degree, by the random chance that its search path during the first trial on each day of testing intersects with the platform, and this element of randomness could have obscured differences between the groups. Another distinction between the tasks is that the water maze includes an inter-trial interval and the radial arm maze does not, and the inter-trial interval may reduce the degree to which working memory is actually measured in the water maze. We chose a 30 sec inter-trial interval to model our protocol after a previous study showing lesion-induced decrements in working memory among male rats (Hodges et al., 1995). Other protocols have involved much longer inter-trial intervals (8-75 min) to reportedly assess working memory in rats (Hoane et al., 2008; Sarihi et al., 2000). Some more recent protocols have suggested that shorter inter-trial intervals (10-15 sec) should be used to accurately assess working memory (Aultman and Moghaddam, 2001; Feldman et al., 2010; Vorhees and Williams, 2006). Shorter inter-trial intervals (10-20 sec) may provide more meaningful comparisons with the radial arm maze and with human data (Cowan, 2008).
The differences in the results of Experiments 1 and 3 may have been due to other differences in the design of the two experiments. Testosterone injections started 22-23 days after castrations for Experiment 1 and 11-12 days after castrations for Experiment 3. A prolonged period without circulating testosterone could have caused increased down-regulation of androgen and/or estrogen receptors among the rats in Experiment 1 and caused clearer differences in working memory. However, a single high dose of testosterone (2.0 mg/rat) was sufficient to restore androgen receptor expression two hours later within the hippocampus of male rats that had been castrated 14 days earlier (Xiao and Jordan, 2002), suggesting that our testosterone injections may have rapidly restored androgen receptors levels. An additional difference between experiments was that the rats in Experiment 3 received testosterone injections for one full week prior beginning maze testing, while the rats in Experiment 1 did not begin testosterone injections until the first day of maze testing. The results of Experiments 2A and 2B indicate that prolonged exposure to testosterone injections was necessary to observe treatment effects for reference memory, so duration of exposure should be considered in future studies as a variable that may also influence working memory. Another experimental confound was the fact that Sprague-Dawley rats were used for Experiment 1, while Long-Evans rats were used for Experiment 3. Some studies have shown that Long-Evans rats perform better than do Sprague-Dawley rats in both reference memory and working memory versions of the water maze (Harker, 2002; Tonkiss et al., 1993). However, one recent study using a water maze protocol very similar to ours found no strain differences in performance (Epp et al., In Press). Furthermore, in both Experiments 1 and 3, the rats demonstrated a clear learning curve, making it unlikely that strain differences would have obscured any treatment effects.
In both Experiments 1 and 3, differences between groups were only noted during the early stages of testing, and by the final testing days performance seemed to be optimized with all groups performing at similar levels. This was also true for the effects of testosterone on reference memory in Experiment 2b. This indicates that low circulating testosterone reduces the speed with which an individual can learn a spatial working memory task rather than entirely eliminating the ability to learn the task. Some past studies with rats have observed the largest sex differences during the early stages of maze training (Markowska, 1999; Schmidt et al., 2009), suggesting that the activational effects of testosterone may be the underlying cause of sex differences in spatial ability. Estradiol has also been shown to reduce working memory errors mainly during the early stages of testing in the radial arm maze for both male rats (Luine and Rodriguez, 1994) and female rats (Daniel et al., 1999; Luine et al., 1998). Furthermore, the positive effects of testosterone on learning speed are supported by some previous studies with humans (Beer et al., 2006; Hooven et al., 2004). These results suggest that the effects of sex steroids may generally occur during the initial acquisition of a working memory task.
Testosterone and reference memory
In Experiment 1, we observed no effects of testosterone on the number of RME’s that rats performed on the radial arm maze. One past study showed that castration caused an increase in total number of RME’s on the radial arm maze over 30 days of testing, but similar to the current results no significant effects were observed within five-day blocks (Spritzer et al., 2008). Gibbs and Johnson (2008) also found no difference in reference memory between castrated and intact males, but they did notice that testosterone implants caused increased RME’s relative to castrated and intact males. Therefore, there is some past evidence that testosterone can impair spatial reference memory, but we did not replicate that finding. In Experiment 1, both groups performed about 2.5 errors per day during all of the blocks of testing in the radial arm maze. This suggests that the task was either too easy or too difficult to produce a clear learning curve, and this may have obscured any effects of testosterone on reference memory. Past studies showing differences between groups have also failed to demonstrate a clear learning curve for reference memory on the radial arm maze (Gibbs and Johnson, 2008; Hasegawa and Mochizuki, 2009; Spritzer et al., 2008), suggesting that this may not be a useful task for assessing spatial reference memory in male rats.
Unlike in the radial arm maze, rats showed a clear learning curve in the reference memory version of the Morris water maze. In Experiment 2A, we observed no effect of a wide range of testosterone doses upon acquisition trials in the Morris water maze (i.e., path lengths and escape latencies), and this result supports multiple past studies with male rats (Hodosky et al., 2010; Isgor and Sengelaub, 1998; Naghdi et al., 2005a, 2005b; Sandstrom et al., 2006; Spritzer et al., 2008). In Experiment 2B, rats were given testosterone injections for one week prior to beginning testing in the water maze, and this resulted in more rapid acquisition of the task by testosterone-injected rats compared to castrated controls. This suggests that prolonged exposure to testosterone may be needed to improve spatial reference memory. This contradicts a number of past studies, but in two studies intact males exhibited shorter path lengths than did castrated males on most days of testing even though the differences were not statistically significant (Sandstrom et al., 2006; Spritzer et al., 2008). Clearly, the effects of testosterone upon reference memory are subtle, and treatment effects may be obscured when intact males are used due to their natural variability in circulating testosterone levels. In contrast to the results from our Experiment 2A, one previous study in which rats were injected with testosterone only on the days of testing showed a testosterone-induced enhancement of reference memory in the Morris water maze (Khalil et al., 2005). This difference may have been due to the supra-physiological dose of testosterone that was used (2.5 mg/rat), but another study using very high doses of testosterone (20-120 mg/kg) failed to find any effects of testosterone on performance in the Morris water maze (Naghdi et al., 2005b). Clearly more experiments are needed in the high dose range to clarify these differences, but the results of our study and some others indicate that testosterone can cause a small enhancement of spatial reference memory relative to hypogonadal individuals.
Although a wide range of testosterone doses was used in Experiment 2B (0.0625-1.000 mg/rat), the effects of testosterone on path lengths during testing day 2 were independent of dose. This suggests that there may be a low threshold for the ability of testosterone to restore spatial reference memory in hypogonadal males. This may explain why some studies with eugonadal men have failed to demonstrate a correlation between circulating testosterone levels and spatial ability (Fonda et al., 2005; Matousek and Sherwin, 2010; Puts et al., 2010). Similar to our results, some studies of aged men with relatively low testosterone levels have shown that testosterone replacement can improve spatial ability (Cherrier et al., 2001; Cherrier et al., 2005; Janowsky et al., 1994). The results of Experiment 2B do not indicate that there is an optimal dose of testosterone for spatial reference memory. This differs from our findings for spatial working memory in the water maze (Experiment 3) in which an apparent curvilinear relationship between testosterone and performance was noted. This highlights the fact that the effects of testosterone on spatial working and reference memory seem to differ.
Small, but statistically significant, effects of testosterone on spatial memory retention were observed during the probe trials in Experiment 2A. Specifically, the 0.250 mg T and 1.000 mg T groups had better memory retention than did the Control group. No significant differences in memory retention were observed in Experiment 2B, although a relatively low level of retention in the 0.500 mg T group was observed in both experiments. These results indicate that testosterone may have a small dose-dependent effect on retention of spatial reference memories. Two previous studies observed no differences in memory retention between intact and castrated male rats (Sandstrom et al., 2006; Spritzer et al., 2008), but this may be because the variability in testosterone levels among the intact males obscured any dose-dependent effects. Spritzer et al. (2008) did observe a trend for intact males with higher testosterone levels to spend more time in the target quadrant during probe trials. However, Goudsmit et al. (1990) found that testosterone implants given to intact male rats impaired memory retention relative to intact controls. This discrepancy suggests that the chronic exposure to elevated testosterone caused by implants may have different effects than acute daily injections of testosterone. We chose to use injections due to the tighter control over daily testosterone levels and to better mimic the daily surges in testosterone that intact male rats normally experience (Bartke et al., 1973; Keating and Tcholakian, 1979).
Perseverative behavior
One challenge in interpreting the results of the probe trials in the water maze is that a high percentage of time in the target quadrant may indicate either good memory retention or high perseverance (i.e., an inability to flexibly learn a new task). To tease apart these interpretations, a reversal-learning task was performed in Experiment 2b. Interestingly, we observed that the 0.250 mg T and the 1.000 mg T groups showed the highest levels of perseverance as indicated by the time spent in the old target quadrant. This dose-dependent pattern corresponds surprisingly well with the dose-dependent effects of testosterone observed for the probe trials in Experiment 2a. Thus, rather than improving spatial memory, testosterone may affect male performance on spatial tasks by increasing perseverative behavior. A positive relationship between serum testosterone levels and perseverance in the Morris water maze was observed in one previous study (Spritzer et al., 2008). Furthermore, testosterone injections have been shown to increase perseverative behavior among rats in some non-spatial tasks (Thompson and Wright, 1979; van Hest et al., 1989). In the case of the water maze, a rat must first remember where the platform is located before it can engage in perseverative behaviors; therefore, certain doses of testosterone may increase both reference memory and perseverance.
Physiological mechanisms
Our finding that testosterone injections improved some aspects of learning and memory in both the radial arm maze and water maze suggest that testosterone is influencing functioning of the hippocampus and/or prefrontal cortex, as these are brain regions that play critical roles in the processing and storage of spatial memories (Eichenbaum et al., 1990; Floresco et al., 1997; Olton and Papas, 1979; Seamans et al., 1995). The major metabolites of testosterone in the brain are estradiol and dihydrotestosterone (DHT), which bind to estrogen and androgen receptors, respectively (Edinger and Frye, 2004). Hippocampal and cortical regions of male rats possess both estrogen and androgen receptors (Gerlach and McEwen, 1988; Kerr et al., 1995; Kritzer, 2004; Li et al., 1997), which suggests that androgens and estrogens may have direct effects on these brain regions. In support of a role for androgen receptors, male rats with dysfunctional androgen receptors exhibit poorer acquisition on the reference memory version of the Morris water maze (Jones and Watson, 2005). Furthermore, DHT, but not estradiol, was shown to enhance synaptic density and neurogenesis within the hippocampus of male rats (Leranth et al., 2003; Spritzer and Galea, 2007). One study with castrated male mice showed that DHT restored memory retention in a water maze task (Benice and Raber, 2009). Relatively few studies have tested the effects of estradiol on the spatial ability of male rodents, but estradiol implants were shown to improve spatial working memory in castrated male rats (Gibbs, 2005; Luine and Rodriguez, 1994). Similarly, intrahippocampal injections of estradiol enhanced the performance of male rats on a reference memory version of the Morris water maze (Packard et al., 1996). Finally, one recent study showed male rats implanted with 3α-diol, a metabolite of DHT that binds estrogen receptors, had reduced escape latencies in a reference memory version of the Morris water maze (Osborne et al., 2009). Thus, current evidence suggests that the spatial memory enhancing effects of testosterone observed in the current study may involve either androgen or estrogen receptors within the brain.
Rather than testosterone metabolites having memory-enhancing effects, an alternative explanation for our results is that castration impairs memory by indirectly causing abnormal levels of other hormones. For example, castrated male rats show elevated release of corticosterone in response to restraint stress (Viau and Meaney, 1996). This seems to be because testosterone inhibits arginine vasopressin synthesis in the hypothalamus, which reduces basal adrenocorticotropic hormone (ACTH) levels, which in turn causes reduced corticosterone levels (Viau, 2002). Numerous studies have shown that a variety of chronic stressors impair spatial working and reference memory (Conrad et al., 1996; Hölscher, 1999; Nishimura et al., 1999). The effect of stress on spatial memory seems to be mediated by corticosterone binding to receptors in the hippocampus (de Quervain et al., 1998; Oitzl et al., 1998). Thus, testosterone injections may have prevented stress-induced impairment of spatial learning and memory in our experiments. This hypothesis is supported by findings that the sex difference in spatial memory was eliminated when rats were given preliminary trials in the water maze to reduce stress (Beiko et al., 2004; Perrot-Sinal et al., 1996). Furthermore, eliminating the hormonal stress response via adrenalectomy also eliminated sex differences in water maze performance (Beiko et al., 2004). However, in these previous studies sex differences in learning the location of the platform were associated with high levels of thigmotaxis among the females. We did not observe effects of treatment upon thigmotaxis for any of our experiments, suggesting that testosterone may not have a strong effect upon male stress levels in the water maze.
Another indirect effect is that castration may cause dysregulation of the hypothalamic- pituitary-gonadal axis, which could impair spatial ability. Testosterone normally has negative feedback effects on the brain that keep levels of gonadotropins relatively low; therefore, castration results in a chronic up-regulation of luteinizing hormone (LH) and follicle stimulating hormone (Brar et al., 1985). Some recent studies have shown that elevated LH impairs spatial memory in female rats (Berry et al., 2008; Ziegler and Thornton, In Press), and therefore elevated LH may have similar effects in castrated males. Interestingly, elevated LH has also been associated with increased risk of age-related dementia (Bowen et al., 2000).
Conclusion
In this study, testosterone replacement was shown to enhance components of spatial working and reference memory. Effects on spatial working memory were clearer for the radial arm maze than for the water maze, and this may be because the radial arm maze provides a more accurate index of working memory. Prolonged exposure to testosterone was necessary to observe effects on acquisition of the reference memory version of the water maze, indicating that acute changes in testosterone are less likely to influence males’ spatial abilities. It is also noteworthy that castration did not eliminate rats’ ability to learn the tasks, but mainly slowed the rate at which they learned. In the reference memory version of the water maze, we found that testosterone dose did not influence acquisition of the spatial task but dose did influence level of perseverance during reversal learning. The mechanism by which testosterone influences spatial learning and memory also remains unclear, but may involve the direct effects of testosterone metabolites on the brain or indirect effects of hormones that are dysregulated by castration. Continued experiments using rodent models will further clarify lingering contradictions regarding which specific aspects of spatial ability are influenced by the activational effects of testosterone.
Acknowledgements
We thank Josh Chan, Molly Curtis, Elliott Fox, Greg Larsen, Graeme Rosenberg, Julia Stern, QiaQia Wu, and Jane Yoon for assistance with data collection. We also thank Vicki Major, Sarah Froebel, and the rest of the Middlebury College animal facility staff for providing excellent care for our animals. This project was funded by Middlebury College, Sigma Xi, and the Vermont Genetics Network (P20 RR16462) from the INBRE Program of the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). The contents of this project are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Astur RS, Ortiz ML, Sutherland RJ. A characterization of performance by men and women in a virtual Morris water maze: A large and reliable sex difference. Behav. Brain Res. 1998;93:185–190. doi: 10.1016/s0166-4328(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Aultman JM, Moghaddam B. Distinct contributions of glutamate and dopamine receptors to temporal aspects of rodent working memory using a clinically relevant task. Psychopharmacology. 2001;153:353–364. doi: 10.1007/s002130000590. [DOI] [PubMed] [Google Scholar]
- Bartke A, Steele RE, Musto N, Caldwell BV. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973;92:1223–1228. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- Beer TM, Bland LB, Bussiere JR, Neiss MB, Wersinger EM, Garzotto M, Ryan CW, Janowsky JS. Testosterone loss and estradiol administration modify memory in men. J. Urol. 2006;175:130–135. doi: 10.1016/S0022-5347(05)00049-2. [DOI] [PubMed] [Google Scholar]
- Beiko J, Lander R, Hampson E, Boon F, Cain DP. Contribution of sex differences in the acute stress response to sex differences in water maze performance. Behav. Brain Res. 2004;151:239–253. doi: 10.1016/j.bbr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- Benice TS, Raber J. Dihydrotestosterone modulates spatial working-memory performance in male mice. J. Neurochem. 2009;110:902–911. doi: 10.1111/j.1471-4159.2009.06183.x. [DOI] [PubMed] [Google Scholar]
- Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases amyloid-β levels in female rats. Horm. Behav. 2008;54:143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Nelson ME, Eckman CB, Barber J, Scott TY, Granholm ACE. Testosterone, but not nonaromatizable dihydrotestosterone, improves working memory and alters nerve growth factor levels in aged male rats. Exp. Neurol. 2003;181:301–312. doi: 10.1016/s0014-4886(03)00061-x. [DOI] [PubMed] [Google Scholar]
- Bowen RL, Isley JP, Atkinson RL. An Association of elevated serum gonadotropin concentration and Alzheimer disease? J. Neuroendocrinol. 2000;12:351–354. doi: 10.1046/j.1365-2826.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- Brar AK, McNeilly AS, Fink G. Effects of hyperprolactinaemia and testosterone on the release of LH-releasing hormone and the gonadotrophins in intact and castrated rats. J. Endocrinol. 1985;104:35–43. doi: 10.1677/joe.0.1040035. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Plymate S, Baker L, Matsumoto AM, Peskind E, Raskind MA, Brodkin K, Bremner W, Petrova A, LaTendresse S, Craft S. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neuology. 2001;57:80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Johnson M, Craft S, Peskind ER, Raskind MA. Characterization of verbal and spatial memory changes from moderate to supraphysiological increases in serum testosterone in healthy older men. Psychoneuroendocrinology. 2007;32:72–79. doi: 10.1016/j.psyneuen.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- Choi J, Silverman I. The relationship between testosterone and route-learning strategies in humans. Brain Cogn. 2002;50:116–120. doi: 10.1016/s0278-2626(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Christiansen K, Knussmann R. Sex hormones and cognitive functioning in men. Neuropsychobiology. 1987;18:27–36. doi: 10.1159/000118389. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is block by tianeptine pretreatment. Behav. Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- Cowan N. What are the differences between long-term, short-term, and working memory? Prog. Brain Res. 2008;169:323–338. doi: 10.1016/S0079-6123(07)00020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormone and environment on radial maze and water maze performance of female rats. Physiol. Behav. 1999;66:11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology (Berl. ) 2003;170:294–300. doi: 10.1007/s00213-003-1537-4. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ-, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–789. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Devan BD, McDonald RJ, White NM. Effects of medial and lateral caudate-putamen lesion on place- and cue-guided behaviors in the water maze: relation to thigmotaxis. Behav. Brain Res. 1999;100:5–14. doi: 10.1016/s0166-4328(98)00107-7. [DOI] [PubMed] [Google Scholar]
- Ecuyer-Dab I, Robert M. Have sex differences in spatial ability evolved from male competition for mating and female concern for survival? Cognition. 2004;91:221–257. doi: 10.1016/j.cognition.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA. Testosterone’s analgesic, anxiolytic, and cognitive enhancing effects may be due in part to actions of its 5a-reduced metabolites in the hippocampus. Behav. Neurosci. 2004;118:1352–1364. doi: 10.1037/0735-7044.118.6.1352. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Stewart C, Morris RGM. Hippocampal representation in place learning. Journal of Neuroscience. 1990;10:3531–3542. doi: 10.1523/JNEUROSCI.10-11-03531.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJJ, Pour HRN, Aleman A, Lock TMTW, Bosch JLHR, Grobbee DE, van der Schouw YT. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men. J. Am. Med. Assoc. 2008;299:39–52. doi: 10.1001/jama.2007.51. [DOI] [PubMed] [Google Scholar]
- Epp JR, Scott NA, Galea LAM. Strain differences in neurogenesis and activation of new neurons in the dentate gyrus in response to spatial learning. Neuroscience. doi: 10.1016/j.neuroscience.2010.10.025. In Press. [DOI] [PubMed] [Google Scholar]
- Feldman LA, Shapiro ML, Nalbantoglu J. A rapidly acquired and persistent spatial memory task that induces immediate early gene expression. Behav. Brain Func. 2010;6:35. doi: 10.1186/1744-9081-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles of hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J. Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonda SJ, Bertrand R, O’Donnell A, Longcope C, McKinlay JB. Age, hormones, and cognitive functioning among middle-aged and elderly men: cross-sectional evidence from the Massachusetts male aging study. J. Gerontol. 2005;60A:385–390. doi: 10.1093/gerona/60.3.385. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Kavaliers M, Ossenkopp K-, Innes D, Hargreaves EL. Sexually dimorphic spatial learning varies seasonally in two populations of deer mice. Brain Res. 1994;635:18–26. doi: 10.1016/0006-8993(94)91419-2. [DOI] [PubMed] [Google Scholar]
- Gaulin SJC, FitzGerald RW. Sex differences in spatial ability: An evolutionary hypothesis and test. Am. Nat. 1986;127:74–88. [Google Scholar]
- Gerlach LR, McEwen BS. Autoradiographic localization of estradiol-binding neurons in the rat hippocampal formation and entorhinal cortex. Brain Res. 1988;467:245–251. doi: 10.1016/0165-3806(88)90028-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm. Behav. 2005;48:268–277. doi: 10.1016/j.yhbeh.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology. 2008;149:3176–3183. doi: 10.1210/en.2007-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16:323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Goudsmit E, Vandepoll NE, Swaab DF. Testosterone fails to reverse spatial memory decline in aged rats and impairs retention in your and middle-aged animals. Behav. Neural Biol. 1990;53:6–20. doi: 10.1016/0163-1047(90)90729-p. [DOI] [PubMed] [Google Scholar]
- Harker KT. Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fishcer 344) and albinism (Wistar, Sprague-Dawley) but no domestication (wild rat vs. Long-Evans, Fischer-Norway) Behav. Brain Res. 2002;234:467–477. doi: 10.1016/s0166-4328(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Harris AP, D’Eath RB, Healy SD. Sex differences, or not, in spatial cognition in albino rats: acute stress is the key. Anim. Behav. 2008;76:1579–1589. [Google Scholar]
- Hasegawa N, Mochizuki M. Improved effect of pycnogenol on impaired spatial memory function in partial androgen deficiency rat model. Phytother. Res. 2009;23:840–843. doi: 10.1002/ptr.2702. [DOI] [PubMed] [Google Scholar]
- Hines M, Fane BA, Pasterski VL, Mathews GA, Conway GS, Brook C. Spatial abilities following prenatal androgen abnormality: targeting and mental rotations performance in individuals with congenital adrenal hyperplasia. Psychoneuroendocrinology. 2003;28:1010–1026. doi: 10.1016/s0306-4530(02)00121-x. [DOI] [PubMed] [Google Scholar]
- Hoane MR, Pierce JL, Holland MA, Anderson GD. Nicotinamide treatment induces behavioral recovery when administered up to 4 hours following cortical contusion injury in the rat. Neuroscience. 2008;154:861–868. doi: 10.1016/j.neuroscience.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges H, Sowinski P, Sinden JD, Netto CA, Fletcher A. The selective 5-HT3 receptor antagonist, WAY100289, enhances spatial memory in rats with ibotenate lesions of the forebrain cholinergic projection system. Psychopharmacology. 1995;117:318–332. doi: 10.1007/BF02246107. [DOI] [PubMed] [Google Scholar]
- Hodosky J, Pales J, Ostatnikova D, Celec P. The effects of exogenous testosterone on spatial memory in rats. Cent. Eur. J. Biol. 2010;5:466–471. [Google Scholar]
- Holding CS, Holding DH. Acquisition of route network knowledge by males and females. J. Gen. Psychol. 1988;116:29–41. [Google Scholar]
- Hölscher C. Stress impairs performance in spatial water maze learning tasks. Behav. Brain Res. 1999;100:225–235. doi: 10.1016/s0166-4328(98)00134-x. [DOI] [PubMed] [Google Scholar]
- Hooven CK, Chabris CF, Ellison PT, Kosslyn SM. The relationship of male testosterone to components of mental rotation. Neuropsychologia. 2004;42:782–790. doi: 10.1016/j.neuropsychologia.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Isgor C, Sengelaub DR. Prenatal gonadal steroids affect adult spatial behavior, CA1 and CA3 cell morphology in rats. Horm. Behav. 1998;34:183–198. doi: 10.1006/hbeh.1998.1477. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwll ES. Testosterone influences spatial cognition in older men. Behav. Neurosci. 1994;2:325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci. Biobehav. Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Spatial memory performance in androgen insensitive male rats. Physiol. Behav. 2005;85:135–141. doi: 10.1016/j.physbeh.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Kaufman SB. Sex differences in mental rotation and spatial visualization ability: Can they be accounted for by differences in working memory capacity? Intelligence. 2007;35:211–223. [Google Scholar]
- Keating RJ, Tcholakian RK. In Vivo patterns of circulating steroids in adult male rats. I. Variations in testosterone during 24- and 48- hour standard and reverse light/dark cycles. Endocrinology. 1979;104:184–188. doi: 10.1210/endo-104-1-184. [DOI] [PubMed] [Google Scholar]
- Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution of hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- Khalil R, King MA, Soliman MRI. Testosterone reverses ethanol-induced deficit in spatial reference memory in castrated rats. Pharmacology. 2005;75:87–92. doi: 10.1159/000087188. [DOI] [PubMed] [Google Scholar]
- Korol KL, Kolo LI. Estrogen-induced changes in place and response learning in yough adult female rats. Behav. Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kritzer M. The distribution of immunoreactivity for intracellular androgen receptors in the cerebral cortex of hormonally intact adult male and female rats: localization in pyramidal neurons making corticocortical connections. Cereb. Cortex. 2004;14:268–280. doi: 10.1093/cercor/bhg127. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirilis T. Gonadectomy impairs T-maze acquisition in adult male rats. Horm. Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J. Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Schwartz PE, Rissman EF. Distribution of estrogen receptor- β-Like immunoreactivity in rat forebrain. Neuroendocrinology. 1997;66:63–67. doi: 10.1159/000127221. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behav. Neural. Biol. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm. Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrus cycle. J. Neurosci. 1999;15:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Wittert G, Burns NR, Haren MT, Sugarman R. Testosterone and cognitive function in ageing men: Data from the Florey Adelaide male ageing study (FAMAS) Maturitas. 2007;57:182–194. doi: 10.1016/j.maturitas.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Matousek RH, Sherwin BB. Sex steroid hormone and cognitive functioning in healthy, older men. Horm. Behav. 2010;57:352–359. doi: 10.1016/j.yhbeh.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Hatzipantelis M. Navigation in a “Virtual” maze: sex differences and correlation with psychometric measure of spatial ability in humans. Evol. Hum. Behav. 1998;19:73–87. [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J. Clin. Endocrinol. Metab. 2002;87:5001–5007. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference. Psychoneuroendocrinology. 1996;21:323–337. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Moradpour F, Naghdi N, Fathollahi Y. Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behav. Brain Res. 2006;175:223–232. doi: 10.1016/j.bbr.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Temple V, Oh E, VanRyzin C, Williams A, Cornwell B, Grillon C, Pine DS, Ernst M, Merke DP. Early androgen exposure modulates spatial cognition in congenital adrenal hyperplasia (CAH) Psychoneuroendocrinology. 2008;33:973–980. doi: 10.1016/j.psyneuen.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghdi N, Majlessi N, Bozorgmehr T. The effect of intrahippocampal injection of testosterone enanthate (an androgen receptor agonist) and anisomycin (protein synthesis inhibitor) on spatial learning and memory in adult, male rats. Behav. Brain Res. 2005a;156:263–268. doi: 10.1016/j.bbr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Mohaddess G, Khamnei S, Arjomand M. No significant difference between intact and testosterone depleted or administrated male rats in spatial learning and memory. IJPR. 2005b;1:29–32. [Google Scholar]
- Naghdi N, Nafisy N, Majlessi N. The effects of intrahippocampal testosterone and flutamide on spatial localization in the Morris water maze. Brain Res. 2001;897:44–51. doi: 10.1016/s0006-8993(00)03261-3. [DOI] [PubMed] [Google Scholar]
- Nishimura JI, Endo Y, Kimura F. A long-term stress exposure impairs maze learning performance in rats. Neurosci. Lett. 1999;273:125–128. doi: 10.1016/s0304-3940(99)00645-x. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Fluttert M, Sutanto W, de Kloet ER. Continuous blockade of brain glucocorticoid rececptors facilitates spatial learning and memory in rats. Eur. J. Neurosci. 1998;10:3759–3766. doi: 10.1046/j.1460-9568.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Olton DS, Papas BC. Spatial memory and hippocampal function. Neuropsychologia. 1979;17:669–682. doi: 10.1016/0028-3932(79)90042-3. [DOI] [PubMed] [Google Scholar]
- Osborne DM, Edinger KL, Frye CA. Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. Age. 2009;31:191–198. doi: 10.1007/s11357-009-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Kohlmaier JR, Alexander GM. Posttraining intrahippocampal estradiol injections enhance spatial memory in male rats: interactions with cholinergic systems. Behav. Neurosci. 1996;110:626–632. doi: 10.1037//0735-7044.110.3.626. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Larson P, Kratz K, Thiebaux M, Bluestein B, Buckwalter JG, Rizzo AA. Sex differences in mental rotation and spatial rotation in a virtual environment. Neurospychologia. 2004;42:555–562. doi: 10.1016/j.neuropsychologia.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Kostenuik MA, Ossenkopp KP, Kavaliers M. Sex differences in performance in the Morris water maze and the esfects of initial nonstationary hidden platform training. Behav. Neurosci. 1996;110:1309–1320. doi: 10.1037//0735-7044.110.6.1309. [DOI] [PubMed] [Google Scholar]
- Postma A, Jager G, Kessels RPC, Kippeschaar HPF, van Honk J. Sex differences for selective forms of spatial memory. Brain Cogn. 2004;54:24–34. doi: 10.1016/s0278-2626(03)00238-0. [DOI] [PubMed] [Google Scholar]
- Puts DA, Cardenas RA, Bailey DH, Burriss RP, Jordan CL, Breedlove SM. Salivary testosterone does not predict mental rotation performance in men or women. Horm. Behav. 2010;58:282–289. doi: 10.1016/j.yhbeh.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Roof RL, Havens MD. Testosterone improves maze performance and induces development of a male hippocampus in females. Brain Res. 1992;572:310–313. doi: 10.1016/0006-8993(92)90491-q. [DOI] [PubMed] [Google Scholar]
- Roof RL, Stein DG. Gender differences in Morris water maze performance depend on task parameters. Physiol. Behav. 1999;68:81–86. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm. Behav. 2006;50:18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Sarihi A, Motamedi F, Naghdi N, Rashidy-Pour A. Lidocaine reversible inactivation of the median raphe nucleus has no effect on reference memory but enhances working memory versions of the Morris water maze task. Behav. Brain Res. 2000;114:1–9. doi: 10.1016/s0166-4328(00)00176-5. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Jacobson TK, Markus E. Hippocampal and striatal dependent navigation: sex differences are limited to acquisition. Horm. Behav. 2009;56:199–205. doi: 10.1016/j.yhbeh.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav. Neurosci. 1995;109:1063–1073. doi: 10.1037//0735-7044.109.6.1063. [DOI] [PubMed] [Google Scholar]
- Seymoure P, Dou H, Juraska JM. Sex differences in radial maze performance: influence of rearing environment and room cues. Psychobiology. 1996;24:33–37. [Google Scholar]
- Silverman I, Kastuk D, Choi J, Phillips K. Testosterone levels and spatial ability in men. Psychoneuroendocrinology. 1999;24:813–822. doi: 10.1016/s0306-4530(99)00031-1. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LAM. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev. Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Gill M, Weinberg A, Galea LAM. Castration differentially affects spatial working and reference memory in male rats. Archiv. Sex. Behav. 2008;37:19–29. doi: 10.1007/s10508-007-9264-2. [DOI] [PubMed] [Google Scholar]
- Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male. 2003;6:13–17. [PubMed] [Google Scholar]
- Thilers PP, MacDonald SWS, Herlitz A. The association between endogenous free testosterone and cognitive performance: A population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology. 2006;31:565–576. doi: 10.1016/j.psyneuen.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Thompson WR, Wright JS. “Persistence” in rats: effects of testosterone. Physiol. Psychol. 1979;7:291–294. [Google Scholar]
- Tonkiss J, Shultz P, Galler JR. Long-Evans and Sprague-Dawley rats differ in their spatial navigation performance during ontogeny and maturity. Dev. Psychobiol. 1993;25:567–579. doi: 10.1002/dev.420250804. [DOI] [PubMed] [Google Scholar]
- Ulubaev A, Lee DM, Purandare N, Pendleton N, Wu FCW. Activational effects of sex hormone on cognition in men. Clin. Endocrinol. 2009;71:607–623. doi: 10.1111/j.1365-2265.2009.03562.x. [DOI] [PubMed] [Google Scholar]
- van Hest A, van Haaren F, van de Poll NE. Perseverative responding in male and female wister rats: effects of gonadal hormones. Horm. Behav. 1989;23:57–67. doi: 10.1016/0018-506x(89)90074-3. [DOI] [PubMed] [Google Scholar]
- Vaughan C, Goldstein FC, Tenover JL. Exogenous testosterone alone or with finasteride does not improve measurements of cognition in older men with low serum testosterone. J. Androl. 2007;28:875–881. doi: 10.2164/jandrol.107.002931. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J. Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress in mediated by the medial preoptic area. J. Neurobiol. 1996;16:1866–1876. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav. Neurosci. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Preut R, Hellhammer DH, Kudielka BM, Schurmeyer TH, Krischbaum C. Testosterone and cognition in elderly men: a single testosterone injection blocks the practice effect in verbal fluency, but has no effect on spatial or verbal memory. Biol. Psychiatry. 2000;47:650–654. doi: 10.1016/s0006-3223(99)00145-6. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jordan CL. Sex differences, laterality, and hormonal regulation of androgen receptor immunoreactivity in rat hippocampus. Horm. Behav. 2002;42:327–336. doi: 10.1006/hbeh.2002.1822. [DOI] [PubMed] [Google Scholar]
- Ziegler SG, Thornton JE. Low luteinizing hormone enhances spatial memory and has protective effects on memory loss in rats. Horm. Behav. doi: 10.1016/j.yhbeh.2010.07.002. In Press. [DOI] [PubMed] [Google Scholar]