Abstract
The tumor suppressor candidate gene RASSF1A encodes a microtubule-associated protein that is implicated in the regulation of cell proliferation, migration, and apoptosis. Several studies indicate that down-regulation of RASSF1A resulting from promoter hypermethylation is a frequent epigenetic abnormality in malignant melanoma. In this study, we report that compared with melanocytes in normal skins or benign skin lesions, RASSF1A is down-regulated in melanoma tissues as well as cell lines, and its expression negatively correlates with lymph node metastasis. Following ectopic expression in RASSF1A–deficient melanoma A375 cell line, RASSF1A reduces cell viability, suppresses cell cycle progression but enhances apoptotic cell death. In vivo, RASSF1A expression inhibits the tumorigenic potential of A375 cells in nude mice, which also correlates with decreased cell proliferation and increased apoptosis. On the molecular level, ectopic RASSF1A expression leads to differential expression of 209 genes, including 26 down-regulated and 183 up-regulated ones. Among different signaling pathways, activation of the apoptosis signal-regulating kinase 1 (ASK1)/p38 MAP kinase signaling is essential for RASSF1A-induced mitochondrial apoptosis, and the inhibition of the Akt/p70S6 kinase/eIF4E signaling is also important for RASSF1A-mediated apoptosis and cell cycle arrest. This is the first study exploring the biological functions and the underlying mechanisms of RASSF1A during melanoma development. It also identifies potential targets for further diagnosis and clinical therapy.
Keywords: RASSF1A, tumor suppressor gene, melanoma, apoptosis, cell cycle
Introduction
Malignant melanoma (MM) accounts for 80% of deaths from skin cancer and presents a low five-year survival rate of 14% (Miller and Mihm Jr, 2006). Several groups reported that promoter hypermethylation of the Ras association domain family 1, isoform A (RASSF1A) gene is frequently detected in MM cell lines, tissues and serum of MM patients (Hoon et al., 2004; Marini et al., 2005; Rastetter et al., 2007; Reifenberger et al., 2004; Spugnardi et al., 2003; Tanemura et al., 2009; Tellez et al., 2009), suggesting that aberrant expression of RASSF1A gene might be involved in melanoma progression. However, no previous studies have looked into the biological functions and underlying mechanisms of RASSF1A in melanoma. For lung cancers, it has been shown that introduction of RASSF1A into cancer cells lacking endogenous RASSF1A expression reduced colony formation in vitro and tumorigenesis in vivo, implying a tumor suppressive nature of this gene (Burbee et al., 2001; Dammann et al., 2000).
In this study, we observed that the expression level of RASSF1A was reduced in MM tissues and cell lines. Furthermore, ectopic expression of wild-type RASSF1A inhibited cell viability in vitro and tumorigenesis in vivo. These functions of RASSF1A were achieved by modulating cell cycle progression, apoptosis and signal transduction.
Materials and Methods
Clinical samples and immunohistochemistry
This study was approved by the Central South University Health Authority Joint Ethics Committee. Ten normal skin tissues, nine nevus pigmentosus (a benign skin lesion characterized by pigmented melanocytic proliferation) and twenty-three primary cutaneous MMs were collected in the Department of Dermatology at Xiangya Hospital (Changsha, China), with written consent obtained from all involved individuals. Based on the status of lymph node metastasis, the 23 cutaneous MMs were classified as those with lymph node metastasis (n=9) and those without (n=14).
Immunohistochemistry was performed as previously described (Goto et al., 2008) with the following antibodies: anti-RASSF1A monoclonal antibody (eBioscience, San Diego, CA), anti-S100 antibody, anti-Ki67 (Boster Biological Technology, Wuhan, China), anti-cleaved caspase 3 (Asp175) (Cell Signaling Technology, Beverly, MA). For RASSF1A staining, a staining index (range 0-9) was calculated as the product of the intensity of positive staining (scores: negative = 0, weak = 1, moderate =2, or strong = 3) and the proportion of positively stained cells (scores: <10% = 1, 10%-50% = 2, >50% = 3) (Zeng et al., 2007). Ki67 and cleaved caspase 3 staining were scored as the average ratio of positively stained cells to total tumor cells from four random areas at ×200 magnification.
Western blot analysis and cell fractionation
The following antibodies were used for Western blot analysis: anti-RASSF1A monoclonal antibody, anti-Akt, anti-phospho-Akt (Thr308), anti-p70S6K, anti-phospho-p70S6K (Thr398), anti-phospho-Rb (Ser780), anti-p38, anti-phospho-p38 (Thr180/Tyr182), anti-phospho-eIF4E (Ser209), anti-cleaved caspase-3 (Asp175) (Cell Signaling Technology), anti-α-tubulin, anti-β-actin, anti-caspase 3, anti-cytochrome c, anti-ASK1, anti-eIF4E, anti-cyclinD1 (Santa Cruz Biotechnology, Santa Cruz, CA),antimitochondrial porin (Sigma-Aldrich, St. Louis, MO). Mitochondrial and cytosolic fractions were obtained by differential centrifugation of cell homogenates as previously described (Yang et al., 1997). Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL). Western blot analysis was performed as previously reported (Zhou et al., 2008).
Plasmid
The open reading frame (ORF) of human RASSF1A gene (Ref Sequence: NM_007182.4) was amplified by PCR using human fetal brain cDNA library (CLONTECH, Palo Alto, CA) as template and the primers as follows: forward, 5′-atagatatctatgtcgggggagcctgag-3′, and reverse, 5′-atagaattctcacccaag ggggcaggc-3′. The resulting PCR fragment was purified and ligated into EcoRV/EcoRI-digested pIRESneo3 vector (CLONTECH) to generate the pIRES-RASSF1A construct. The ORF of human Akt1 gene (Ref Sequence: NM_005163.2) was amplified by PCR using human fetal brain cDNA library as template and the primers as follows: forward, 5′-atagaattcggatgagcgacgtggctatt-3′, and reverse, 5′-atactcgagtcaggccgtgccgctggc-3′. The PCR fragment was purified and ligated into EcoRI/XhoI digested pCMV-myc vector (CLONTECH), yielding the construct pCMV-myc/Akt1. The identities of recombinant constructs were confirmed by sequencing.
Cell culture and transfections
WM164, WM793, WM1341D, WM1552C and 1205Lu human melanoma cells were generously provided by Dr. Meenhard Herlyn (The Wistar Institute, University of Pennsylvania, Philadelphia, PA). A375 and A375SM cells were provided by Dr. Isiah Fidler (MD Anderson, Houston, TX). MeWo cells were purchased from ATCC (Manassas, VA). The M14 cell line was obtained from Developmental Therapeutics Program at the National Institutes of Health (USA). All WM cells and 1205Lu cells were maintained in a 4:1 MCDB 153/Leibovitz's L-15 medium supplemented with 5 μg/mL of insulin and 2% fetal bovine serum, A375, A375SM and M14 cells in DMEM supplemented with 10% fetal bovine serum, sodium pyruvate, and nonessential amino acids, and MeWo cells in RPMI medium supplemented with 10% fetal bovine serum.
For stable transfection, A375 cells were transfected with Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol, followed by G418 selection. After two-week selection, the G418-resistant clones were isolated and pooled together. For silencing ASK-1, the siRNA sequences targeting ASK1 (siRNA-1: 5′-AAUUGCAGUCUGCACAGCCUUUCGG-3′; siRNA-2: 5′-AAAUGCGUAAUGAAACUUCACGUGG-3′) was synthesized as previously reported (Sekimukai et al., 2009). The ASK-1-specific siRNA and a scrambled oligonucleotide were chemically synthesized by GenePharma (Shanghai, China). For siRNAs transfection, RASSF1A/A375 cells were transfected with 100 nM of ASK1 siRNAs or scrambled siRNAs by Lipofectamine 2000 Reagent following the manufacturer's instructions.
Colony formation, MTT, apoptosis and cell-cycle analysis
For colony formation assay, A375 cells were seeded at 1,000 cells/well in six-well plate and maintained in sterile 37 °C, 5% CO2 incubator. After two weeks, visible cell colonies were fixed with methanol for 15 min and washed in PBS. The colonies were then stained with 0.1% crystal violet for 1 hour, rinsed with water, and finally counted manually. Each assay was performed in triplicate.
For MTT assay, A375 cells were seeded into 96-well plates at a density of 2×103 cells/well for 24, 48, 72 and 96 h, respectively. 20 μL of MTT stock solution (5 mg/mL) were added to each well to a final concentration of 0.5 mg/mL, and the cells were further incubated at 37°C for 4 h. The generated formazan was dissolved in dimethyl sulfoxide and measured at 570 nm using a Sunrise plate reader (Tecan Instruments, Switzerland).
Apoptotic cells were detected by Hoechst33258 staining or fluorescence activated cell sorting (FACS) for the sub-G1 population as previously described (Zhou et al., 2008). Cell cycle distribution was determined by FACS as previously reported (Zhou et al., 2008).
In vivo tumorigenicity assay
All animal experiments were approved by the Institutional Animal Care and Use Committee of the Central South University. A total of 1×106 cells were injected subcutaneously into the flank of male BALB/c nude mice (nu/nu) (4 mice/group). Every seven days, the shortest and longest diameter of the tumor were measured with calipers, and the tumor volume (mm3) was calculated as follows (Houchens et al., 1978): 0.5 × (the shortest diameter)2 ×(the longest diameter). Six weeks after cell injection, tumors were dissected and the wet weight of each tumor was determined. The in vivo experiment was repeated twice.
Microarray and quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted using TriZol reagent (Invitrogen, Carlsbad, CA). Preparation of cDNA and cRNA was conducted following instructions in the Affymetrix GeneChip Expression Analysis Manual (Affymetrix, Santa Clara, CA). cRNA was hybridized to an oligo-based array by using an HG_U133A chip, washed and scanned according to standard Affymetrix protocols. Data from the scanning of the Affymetrix Gene-Chips were gathered using the Affymetrix Microarray Suite v4.0 and exported to Microsoft Excel. The gene expression profile of the stable RASSF1A-expressing cells was compared to that of the vector-transfected control cells. Genes presenting a minimum of two-fold difference in expression level between the two cell populations were scored as differentially expressed ones. For qRT-PCR, cDNA was synthesized using the Reverse Transcription System (Promega, Madison, WI) according the manufacturer's protocol. The primers used for quantitative real-time PCR (qRT-PCR) are summarized in Supplementary Table 2.
Statistical Analysis
Differences in nonparametric variables were analyzed by the Fisher's exact test using SPSS 11.0 program (SPSS, Chicago, IL). Differences of parametric variables between groups were tested by Student's t test. Statistical analysis of xenograft tumor growth curve was performed using one-way ANOVA. A P value of < 0.05 was considered statistically significant.
Results
RASSF1A is down-regulated in MM samples and cell lines
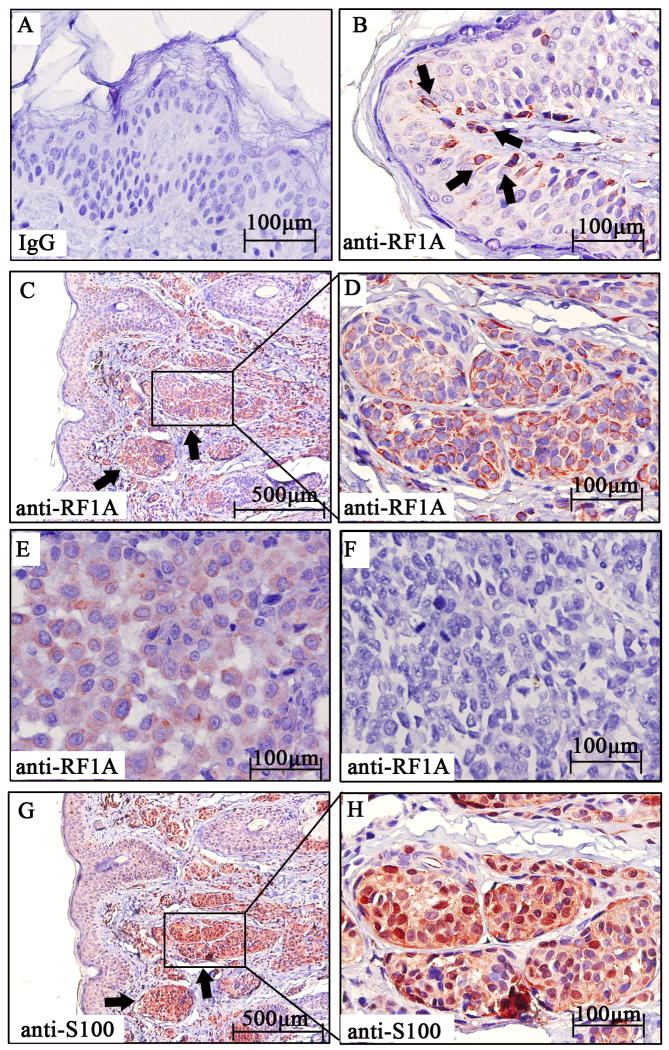
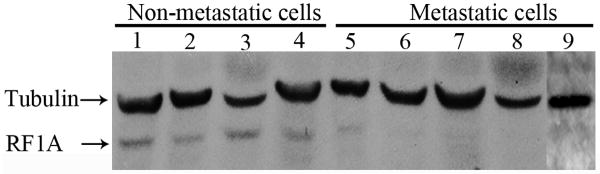
To examine the status of RASSF1A in MM, we first screened the expression levels of RASSF1A in MM tissues, which were compared with those in normal skin and nevus pigmentosus tissues. Normal mouse IgG was used as primary antibody, which serves as negative control for immunohistochemical analysis (Fig. 1A). By immunohistochemical analysis, 10 of 10 (100%) normal skin tissues showed strong cytoplasmic staining of RASSF1A in most melanocytes (Fig. 1B); 8 of 9 (88.9%) of nevus pigmentosus tissues showed strong cytoplasmic staining of RASSF1A in nevus nest (Fig. 1C and 1D); while only 8 of 14 (57.1%) MM samples without lymph node metastasis and 0 of 9 (0%) of those with lymph node metastasis showed weak to moderate staining of RASSF1A (Fig. 1E and 1F). The identity of melanocytes was further confirmed by S100 staining (Fig. 1G and 1H). Statistical analysis indicated that the staining intensity of RASSF1A in MM melanocytes was significantly lower than that in normal skin or benign lesions (Table 1, P < 0.01). Besides, there was a reverse correlation between RASSF1A intensity and the presence of lymph node metastasis (Table 1, P = 0.007). Next, we screened the expression levels of RASSF1A in several MM cell lines, including metastatic MM cells (1205Lu, MeWo, A375SM, M14 and A375) and non-metastatic MM cells (WM1552C, WM1341D, WM793 and WM164). By Western blot, RASSF1A expression was only detectable in non-metastatic but not in any metastatic MM cell lines (Fig. 2).
Figure 1. RASSF1A is down-regulated in MM samples.
(A). Staining of normal skin with normal mouse IgG as the primary antibody, which served as negative control for immunohistochemical staining. (B). Strong positive cytoplasmic staining of RASSF1A protein (red) in melanocytes (arrowhead) within normal skin. (C). Low-power magnification of nevus pigmentosus tissue stained with RASSF1A, which showed strong positive cytoplasmic staining of RASSF1A protein in nevus nest (arrowhead). (D). High-power magnification of the squared field from (C). (E). Weak positive staining of RASSF1A protein in melanoma tissues without lymph node metastasis. (F). Absence of RASSF1A protein in primary melanoma tissues with lymph node metastasis. (G). Low-power magnification of S100 staining in nevus pigmentosus, which showed strong positive nuclear and cytoplasmic signal (red) in nevus nest (arrowhead). (H). High-power magnification of the squared field from (G).
Table 1. Correlation between the clinicopathologic features and the expression of RASSF1A.
| Characteristics | RASSF1A expression | P Value | |||
|---|---|---|---|---|---|
| Negative (-) | Positive (+) | Moderate (++) | Strong (+++) | ||
| Normal skin(a) | 0 | 2 | 2 | 6 | |
| Melanocytes in navus(b) | 1 | 1 | 3 | 4 | Pb-c=0.006 |
| Malignant melanoma(c) | 15 | 6 | 2 | 0 | Pa-c=0.001 |
| Non-metastasis(d) | 6 | 5 | 3 | 0 | |
| Metastasis(e) | 9 | 0 | 0 | 0 | Pd-e=0.007 |
Figure 2. RASSF1A is down-regulated in MM cell lines.
Western blot analysis of RASSF1A in various melanoma cell lines. α-Tubulin was used as a loading control. 1: WM1552C; 2:WM1341D; 3: WM793; 4: WM164; 5:1205Lu; 6: MeWo; 7: A375SM; 8: M14; 9: A375.
Exogenous expression of RASSF1A suppresses melanoma cells viability in vitro
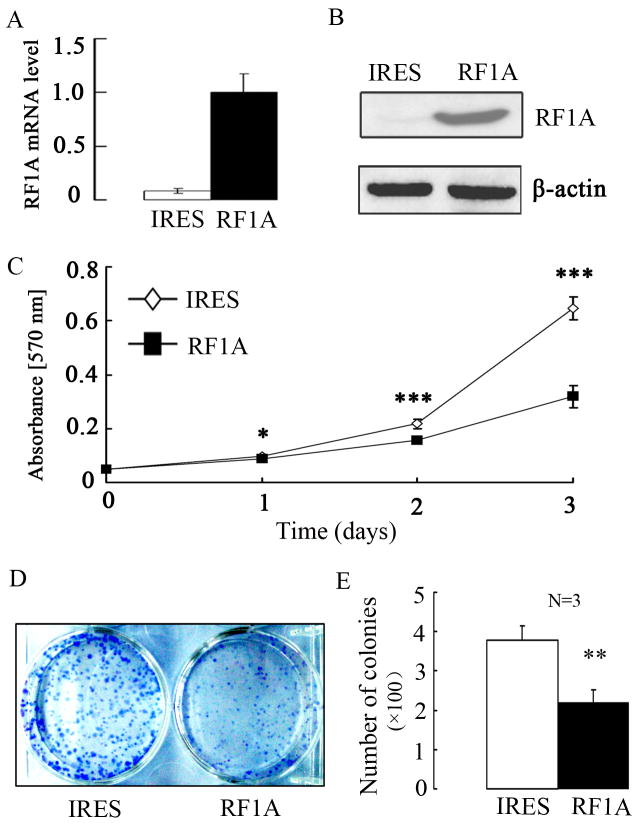
To further understand the biological functions of RASSF1A during melanoma development, we stably introduced the wild-type human RASSF1A via the pIRES-RASSF1A plasmid into human A375 MM cells that lack the endogenous expression of RASSF1A. As a control, we stably transfected the empty vector pIRESneo3 into the cells. For purpose of explanation, we will call these two cell populations RASSF1A cells and control cells, respectively. By both qRT-PCR and Western blot, RASSF1A expression was only detectable in RASSF1A but not control cells (Fig. 3A and 3B). We next examined the effect of RASSF1A on cell viability. From MTT assay, the growth rate of RASSF1A cells was lower when compared to that of control cells, which started to show significant difference from day 1 (P < 0.05) and reached maximum (50%) on day 3 (P < 0.001, Fig. 3C), implying RASSF1A inhibits cell viability in vitro. By another cell viability assay, colony formation assay, RASSF1A expression led to significantly reduced number of colonies (∼43% reduction, P = 0.005, Fig. 3D and 3E).
Figure 3. Exogenous expression of RASSF1A suppresses in vitro cell viability.
(A). The expression of RASSF1A mRNA was analyzed by qRT-PCR in stable RASSF1A (RF1A) and control (IRES) cell lines. GAPDH was used as internal control. (B). The exogenous expression of RASSF1A protein was analyzed by Western blot analysis. β-Actin was used as a loading control. (C). For growth curves, RASSF1A and control cells were seeded into 96-well plates, with cell viability at indicated time points determined by MTT assay. Data are presented as the mean±SEM of values from 3 independent experiments. (D). A typical microphotograph of colony derived from RASSF1A and control cells. (E). Quantification of the colonies formed in colony formation assay. Data are presented as the mean±SEM of values from 3 independent experiments. * P<0.05, ** P<0.01, and *** P<0.001, as compared with control cells.
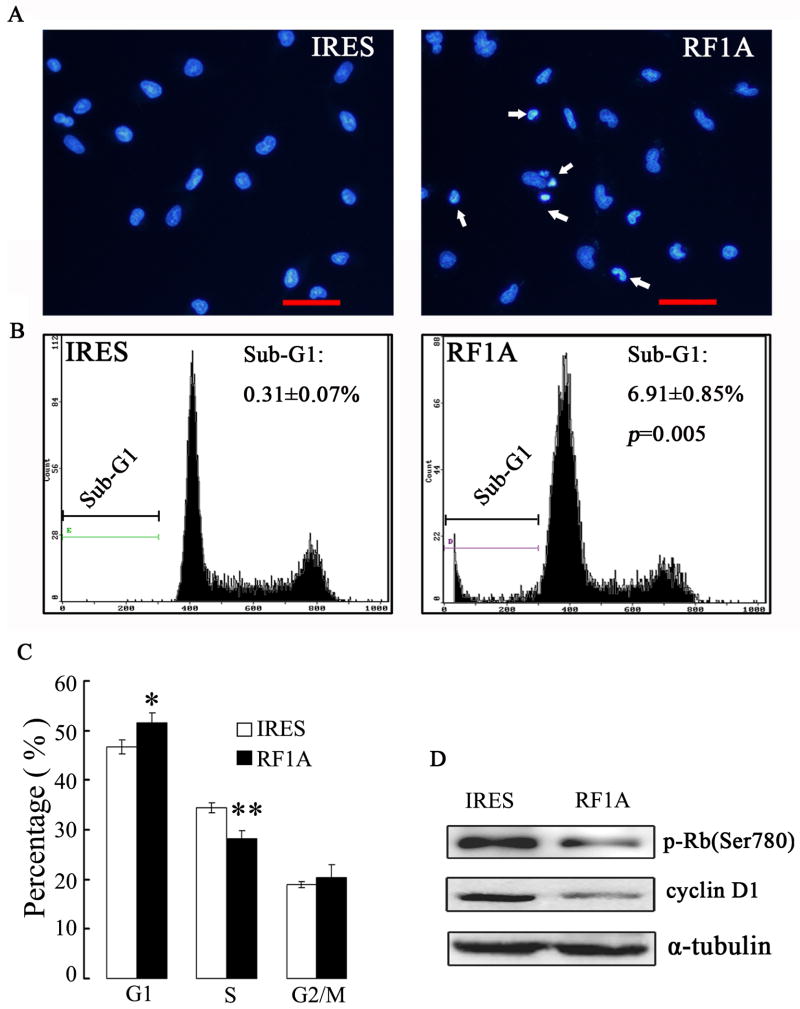
Exogenous expression of RASSF1A induces apoptosis and cell cycle G1-S phase arrest in melanoma cells in vitro
Since cell viability is the net result of cell proliferation and apoptosis, to characterize the putative antioncogenic properties of RASSF1A on melanoma cells, we examined the status of apoptosis and cell cycle progression between RASSF1A and control cells. By Hoechst33258 nuclear staining (Fig. 4A) and FACS analysis for sub-G1 cell population (Fig. 4B), RASSF1A cells exhibited significantly higher level of apoptosis, as compared to control cells (P=0.005). The FACS analysis also revealed that exogenous expression of RASSF1A resulted in an increase of cells in the G1 phase while a decrease in the S phase, as compared to control cells (Fig. 4C). Consistently, decreased levels of cyclin D1 and phosphorylated Rb were detected in RASSF1A cells (Fig. 4D).
Figure 4. Exogenous expression of RASSF1A induces apoptotic cell death and G1-S cell cycle arrest in A375 cells.
(A). Respresentative microphotographs of Hoechst33258 staining showed exogenous expression of RASSF1A enhanced apoptotic cell death in A375 cells. IRES, control cells; RF1A, RASSF1A cells. The arrows indicated apoptotic cells, which showed condensed DNA. Scale bar, 50 μm. (B). Representative cell-cycle distribution analysis by FACS on RASSF1 and control cells. The percentage of sub-G1 population was indicated. (C). Quantification of three independent cell-cycle distribution analysis as performed in (B) * P < 0.05, as compared to control cells. (D). The expressions of cyclin D1 and phospho-Rb were analyzed by Western blot analysis. α-tubulin was used as an internal control.
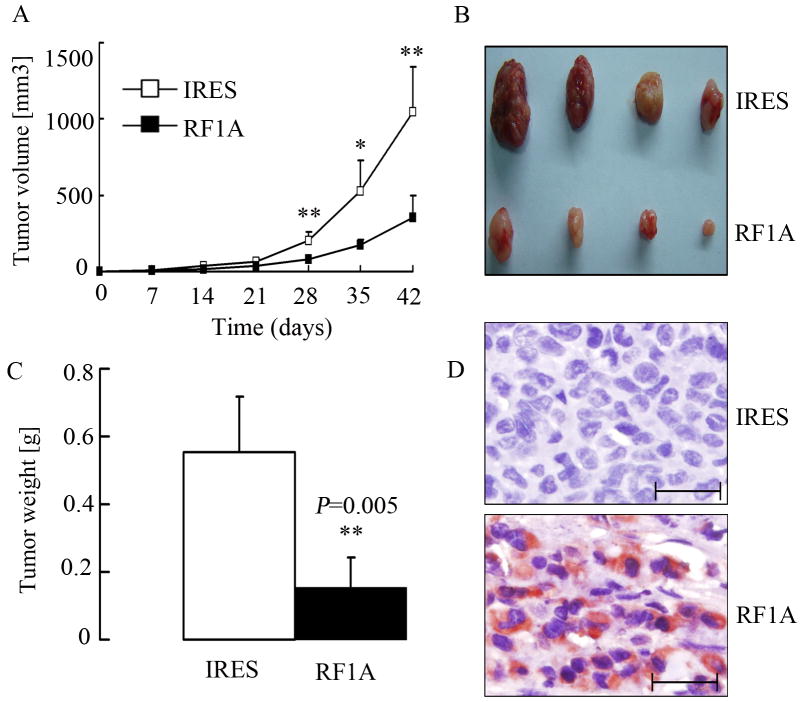
Exogenous expression of RASSF1A suppresses in vivo tumorigenesis of melanoma cells
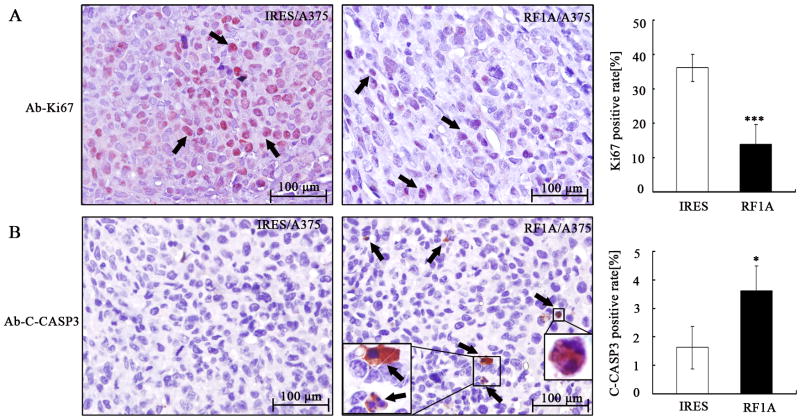
Besides the in vitro activity, we also examined the RASSF1A and control cells for their potential on in vivo tumorigenesis. As shown in Fig. 5A to 5C, RASSF1A cells produced dramatically smaller and lighter tumors, as compared to control cells (P = 0.005). Consistent with the results in vitro, tumors derived from RASSF1A cells showed elevated expression of RASSF1A protein as compared with those from control cells (Fig. 5D). We next examined whether the suppressed tumor growth was associated with decreased proliferation and/or increased apoptosis in vivo. Immunohistochemistry assay showed the proportion of Ki67 (a marker for cell proliferation)-positive cells in tumors from RASSF1A cells ((13.7±5.7)%) was significantly lower than in those from control cells ((36.1±3.9)%; P < 0.001; Fig. 6A). In contrast, apoptosis, as revealed by positive cleaved-caspase 3 staining, was higher in tumors from RASSF1A cells ((3.6±0.8)%) than in those from control cells ((1.6±0.7)%, P < 0.05, Fig. 6B). These results suggested that the inhibition of tumor growth following RASSF1A expression was attributable to decreased cell proliferation as well as increased apoptosis in vivo.
Figure 5. Exogenous expression of RASSF1A suppresses in vivo tumorigenesis.
(A). Tumor growth curve. Control (IRES) and RASSF1A (RF1A) cells were injected s.c. into BALB/c nude mice (N=4/group), and tumors were monitored every 7 days with the volume calculated. * P<0.05, ** P<0.01, as compared to tumors from control cells. (B). Photograph of tumors after isolation. (C). The wet weight of tumors was measured after excision. * P<0.01, as compared to tumors from control cells. (D). Tumors were subjected to immunohistochemistry assays for the expression of RASSF1A protein, which showed strong positive staining (red) in tumors from RASSF1A cells. Scale bar, 50 μm.
Fig. 6. Exogenous expression of RASSF1A suppresses cell proliferation and induces apoptosis in vivo.
Xenograft tumors derived from control (IRES) and RASSF1A (RF1A) cells (N=4/group) were subjected to immunohistochemical analysis on Ki67 (A) and cleaved casp3 (B) for assessing proliferation and apoptosis, respectively. . Arrows indicate positively stained cells. *** P < 0.001; * P < 0.05, as compared to tumors from control cells.
Ectopic RASSF1A leads to differential gene expression
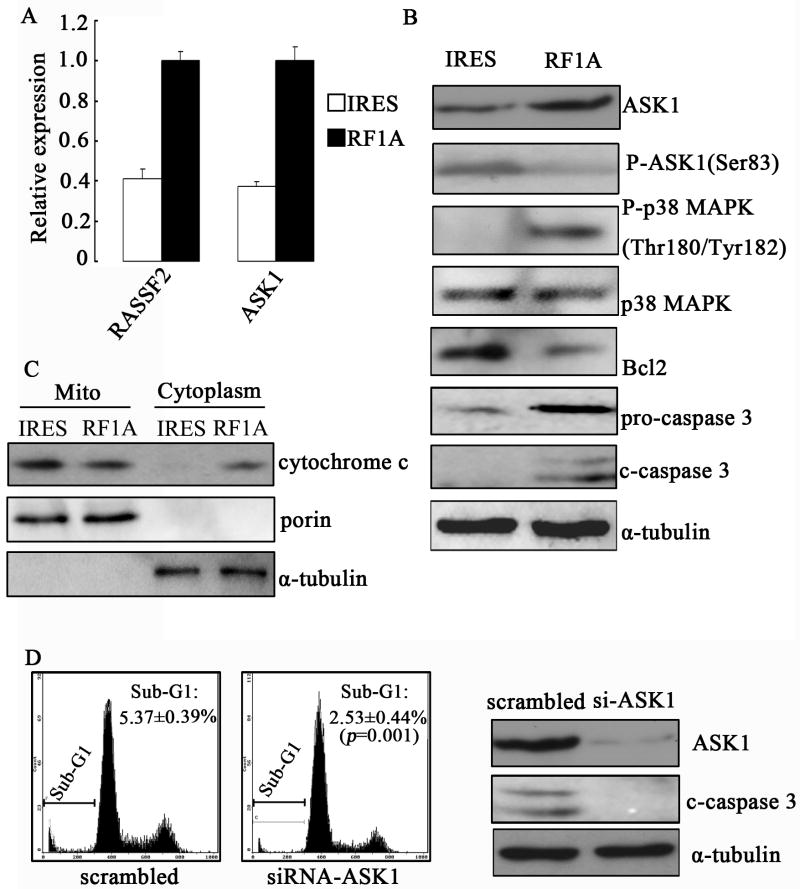
In order to identify molecular changes induced by RASSF1A in melanoma, the expression profile of RASSF1A cells was investigated and compared with that of the control cells. High-density oligonucleotide microarray revealed 209 differentially expressed genes with a minimum two-fold difference between the two cell populations, among which, 26 were down-regulated whereas 183 up-regulated in RASSF1A cells as compared to control cells. Some of the differentially expressed genes are involved distinct biological processes such as transcriptional regulation, cell signaling, cell adhesion and immune responses (supplementary Table 1). Consistent with the observed phenotypes both in vitro and in vivo, some of the differentially expressed genes are key regulators for apoptosis, such as apoptosis signal-regulating kinase 1 (ASK1), macrophage stimulating 1 (MST1) and Ras association domain-containing protein 2 (RASSF2), and some are for cell cycle progression including cyclin D2 and growth-arrest-specific 1 (GAS1).
RASSF1A activates mitochondrial apoptotic pathway through the up-regulation of ASK1 in melanoma cells
To characterize the apoptotic signaling activated by exogenous RASSF1A, we focused on ASK1, an MAP kinase kinase kinase (MAPKKK) important for stress- and cytokine-induced apoptosis. We first confirmed the microarray data by qRT-PCR, which showed increased ASK1 level in RASSF1A cells as compared to control cells (Fig. 7A). Consistently, a higher protein level of ASK1 was also detected in RASSF1A cells, but the level of phosphorylated ASK1, which represents inactivated ASK1, decreased (Fig. 7B). It has been demonstrated that ASK1 promotes apoptosis through activating both stress-activated protein kinase (SAPK, also known as c-Jun amino-terminal kinase (JNK)) and p38 MAP kinase, (Ichijo et al., 1997). Hence we hypothesized that the up-regulation of ASK1 following ectopic RASSF1A expression may contribute to apoptosis through activating JNK and p38 MAP kinases. By Western blot, we found a higher phosphorylated p38 level in RASSF1A cells than in control cells, while the total p38 protein levels in both cells were the same (Fig. 7B). In contrast, phosphorylated JNK, the active form of JNK, was not detectable in either cell population (data not shown). Besides p38, we also detected reduced Bcl2 level and enhanced cleaved caspase 3 level in RASSF1A cells (Fig. 7B). Since Bcl2 is an essential element for maintaining mitochondrial membrane integrity and inhibiting cytochrome c release to induce mitochodrial apoptosis, we further examined the release of cytochrome c by subcellular fractionation. As shown in Fig. 7C, there was a higher cytoplasmic c level in RASSF1A cells than in control cells, implying an enhanced level of mitochondrial apoptosis. To assess the importance of ASK1 in RASSF1A-mediated apoptosis, we knocked down the expression of endogenous ASK1 by siRNA in RASSF1A cells (Fig. 7D right). Compared to the control siRNA-transfected cells, silencing ASK1 led to reduced apoptosis as identified by FACS assay (Fig. 7D left) and cleaved caspase 3 level as indicated by Western blot (Fig. 7D right). These data indicate that ASK1 mediates RASSF1A-induced apoptosis in MM cells.
Figure 7. Exogenous expression of RASSF1A mediates mitochondrial apoptosis through up-regulation of ASK1.
(A). qRT-PCR of RASSF2 and ASK1 expression in control (IRES) or RASSF1A (RF1A) cells. GAPDH was used as internal control. (B). The expressions of ASK1, phospho-ASK1, p38, phospho-p38, Bcl2, caspase 3 and cleaved-caspase 3 were analyzed by Western blotting. (C). The subcellular distribution of cytochrome c was analyzed by Western blot. (D). Apoptosis in RASSF1A cells transfected with either control (scrambled) or ASK1-specific siRNA (siRNA-ASK1) was determined by FACS (left two panels, sub-G1 population) or Western blotting on cleaved casp-3 (right panel). Representative images from three independent experiments are shown.
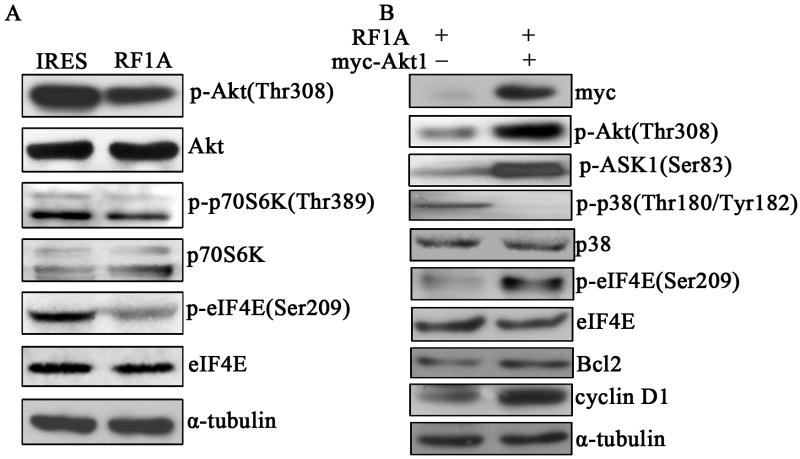
Exogenous expression of RASSF1A blocks the Akt-p70S6K-eIF4E pathway
Akt has been shown to inhibit cell apoptosis in part by phosphorylating proapoptotic kinase ASK1 and inhibiting ASK1 activity (Zhang et al., 2005). As we observed RASSF1A inhibited phosphorylation of ASK1, we are interested in understanding whether RASSF1A could affect the activation of Akt signaling pathway in melanoma cells. As expected, we observed a reduced phosphorylated Akt level in RASSF1A cells as compared to control cells (Fig. 8A), which is consistent with a previous finding that RASSF1A down-regulates the expression of phosphorylated/activated Akt (Thaler et al., 2009). Furthermore, we followed two other downstream molecules of Akt, p70S6K and eIF4E, and found that the activation/phosphorylation of both molecules were lower in RASSF1A cells than in control cells (Fig. 8A). To evaluate the significance of Akt in RASSF1A-induced apoptosis and cell cycle progression, we ectopically introduced pCMV-myc empty vector or myc-tagged Akt into RASSF1A cells. As shown in Fig. 8B, transfection of myc-tagged Akt increased the level of phosphorylated Akt, phosphorylated ASK1, phosphorylated eIF4E, as well as Bcl2 and cyclin D1, but decreased the level of phosphorylated p38, suggesting that RASSF1A inhibits Akt signaling pathway in A375 cells, which at least partially contributes to the down-regulation of anti-apoptosis protein Bcl2 and cell cycle regulator cyclin D1, and subsequently induces apoptosis and G1-S cell cycle arrest.
Figure 8. Exogenous expression of RASSF1A inhibits Akt/p70S6K/eIF4E signaling pathway.
(A). The expressions of phospho-Akt (p-Akt), Akt, p-p70S6K, p70S6K, p-eIF4E, and eIF4E were analyzed by Western blottingin control (IRES) and RASSF1A (RF1A) cells. (B). The expressions of myc, p-Akt, p-ASK1, p-p38, p38, p-eIF4E, eIF4E, Bcl2 and cyclin D1 were determined by Western blotting in RASSF1A cells transfected with either empty (-) or Akt1-expressing vector (+). α-tubulin was used as an internal control.A single representative from three independent experiments is shown.
Discussion
Methylation of the RASSF1A gene is a common epigenetic aberrations described thus far in human tumors. Previous studies reported down-regulation of RASSF1A protein following promoter hypermethylation in melanoma cells and tissues (Hoon et al., 2004; Marini et al., 2005; Rastetter et al., 2007; Reifenberger et al., 2004; Spugnardi et al., 2003; Tanemura et al., 2009; Tellez et al., 2009). These findings raise the possibility that RASSF1A is functionally involved in the tumorigenesis of MM. Here we confirmed that RASSF1A is down-regulated in both MM cell lines and tissues. Importantly, lower level of RASSF1A protein correlates with higher lymph node metastasis of melanoma. Although not tested in this study, we speculated that promoter hypermethylation is a potential mechanism for reduced expression of RASSF1A in melanoma, which therefore, can be used as a molecular marker for melanoma progression.
Advanced from the clinical significance, we further characterized the biological functions of RASSF1A gene. We showed that ectopic expression of RASSF1A in A375 cells reduced cell viability in vitro and tumorigenesis in vivo. These findings strongly supported the role of RASSF1A as a bona fide tumor suppressor gene in melanoma development. Although we only focused the effect of RASSF1A on cell viability and the underlying molecular mechanisms in this study, the reverse correlation between RASSF1A expression and lymph node metastasis revealed by the correlation analysis implies that this gene may also regulate tumor cell invasion and motility, which requires further investigations.
Evading apoptosis is an essential biological feature acquired by tumor cells during cancer development. In this study, we found that exogenous expression of RASSF1A enhanced apoptosis in A375 cells, as demonstrated by Hoechst 33258 staining and flow cytometry analysis. Microarray analysis revealed that RASSF1A induced the expression of several key apoptosis modulaters, such as ASK1, RASSF2 and MST1. ASK1 is a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways to induce apoptosis (Ichijo et al., 1997). Kei Tobiume reported that ASK1 is required for sustained activations of JNK and p38 MAP kinases and apoptosis (Tobiume et al., 2001). In this study, exogenous RASSF1A increased ASK1 level while decreased phospho-ASK1, which in turn activated p38 but not JNK MAP kinase, even though the microarray data suggested that c-Jun mRNA level was increased following overexpression of RASSF1A. Another study implicated the inhibition of JNK pathway as a mechanism by which RASSF1A regulates the level of cyclin D1 (Whang et al., 2005). However, we did not observe this connection in MM models (data not shown), potentially due to the differences in cell types.
On the molecular level, we observed the reduction of Bcl2 protein in RASSF1A cells, even though both microarray and qRT-PCR (data not shown) analysis did not identify such alterations on the mRNA level, suggesting that the modulation of Bcl2 by RASSF1A likely occurs post-transcriptionally, such as on mRNA translation and/or protein stability. Concomitant with Bcl2 reduction, we also identified enhanced cytoplasmic release of cytochrome c and cleavage of caspase 3 in response to RASSF1A expression, indicating the activation of mitochondrial apoptotic signaling pathway. Further mechanistic study revealed ASK1 as an mediator for RASSF1A-induced apoptosis, given that down-regulation of ASK1 by siRNA decreased the level of cleaved-caspase 3 and partially rescued RASSF1A-expressing cells from apoptosis. This observation is consistent with previous literature that ASK1 induces apoptosis mainly via the mitochondria-dependent caspase activation (Hatai et al., 2000). Collectively these data provide a model that RASSF1A up-regulates ASK1, which in turn activates p38 MAP kinase and then alters the expression of multiple components in mitochondrial-dependent apoptosis pathway to induce apoptosis.
Previous studies on the role of RASSF1A in cell cycle progression demonstrated that exogenous expression of RASSF1A induced cell cycle arrest at the G1 phase by down-regulating cyclin D1 (Whang et al., 2005). In agreement with this report, we also observed that the ectopic expression of RASSF1A blocked G1-S transition in melanoma cells. This growth arrest correlates with inhibition of cyclin D1 and p-Rb protein accumulation, which likely prevents the progression of RASSF1A-expressing cells from Rb-controlled cell cycle restriction point to S phase. On the other hand, Chow LS, et al. have reported that exogenous expression of RASSF1A gene has no effect on cell cycle progression or cyclin D1 expression in the neural progenitor cells (NPC) C666-1 (Chow et al., 2004). These controversial results, again, may arise from different cancer types, suggesting cell-type-specific functions of RASSF1A, which should be further investigated
The role of Akt in oncogenic transformation has been well demonstrated. Hyperactivation of Akt is associated with resistance to apoptosis, increase in cell growth, cell proliferation, and cellular energy metabolism (Hay, 2005). Akt has been shown to inhibit cell apoptosis in part by phosphorylating proapoptotic kinase ASK1 and inhibiting ASK1 activity (Zhang et al., 2005). In this study, we observed that RASSF1A increased ASK1 mRNA and protein level but inhibited phosphorylation of ASK1 (inactivation of ASK1). Another critical downstream effector of Akt, which contributes to tumorigenesis, is mTOR. Upon activation, mTOR, which forms a rapamycin-sensitive complex with regulatory-associated protein of mTOR (Raptor), increases mRNA translation via activation of S6-kinase and eukaryotic translation initiation factor 4E (eIF4E)(Hay and Sonenberg, 2004). In this study, we observed that RASSF1A inhibited phosphorylation of Akt, the downstream phosphorylation of ASK1, as well as the activation of p70S6 kinase and eIF4E, all of which were restored in response to exogenous expression of myc-tagged Akt1, and in turn up-regulated the expression of cyclin D1 and Bcl2. Therefore, suppression of Akt/mTOR signaling pathway by RASSF1A is another potential mechanism leading to decreased cyclin D1 and Bcl2 levels and subsequent apoptosis and G1-S cell cycle arrest.
In summary, we found that RASSF1A protein is down-regulated in MM tissues and cell lines, and the reduction of RASSF1A protein is associated with more significant lymph node metastasis. We also provided functional evidence that RASSF1A is a potential tumor suppressor gene in MM by inducing apoptosis and inhibiting cell cycle progression both in vitro and in vivo. These functions are achieved at least partially through the activation of ASK1/p38-mediated mitochondrial apoptotic signaling and suppression of the Akt-p70S6K-eIF4E signaling. Therefore, this study not only improved our understanding on the biological significance of RASSF1A during melanoma development, but also provided potential targets for the development of novel diagnostic strategies and anticancer therapies.
Supplementary Material
Acknowledgments
This study is supported by the following grants: Grant 2006cb910502 from the State Key Science Research Program of China, Grant 2007AA02Z170 from the National “863” High Technology Program of China, Grant 81000883 from the National Natural Science Foundation of China, Project 111-2-12 from the National “111” Project, Grant 201012200017 from the The Free Exploration Program of Central South University of China and Grant R01 CA082295 from the National Institutes of Health of United States.
Footnotes
Competing interests: The authors declare no competing interests.
References
- Burbee DG, Forgacs E, Zochbauer-Muller S, Shivakumar L, Fong K, Gao B, Randle D, Kondo M, Virmani A, Bader S. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. JNCI Journal of the National Cancer Institute. 2001;93(9):691. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LSN, Lo KW, Kwong J, To KF, Tsang KS, Lam CW, Dammann R, Huang DP. RASSF1A is a target tumor suppressor from 3p21. 3 in nasopharyngeal carcinoma. International Journal of Cancer. 2004;109(6):839–847. doi: 10.1002/ijc.20079. [DOI] [PubMed] [Google Scholar]
- Dammann R, Li C, Yoon JH, Chin PL, Bates S, Pfeifer GP. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21. 3. Nature genetics. 2000;25(3):315–319. doi: 10.1038/77083. [DOI] [PubMed] [Google Scholar]
- Goto Y, Ferrone S, Arigami T, Kitago M, Tanemura A, Sunami E, Nguyen SL, Turner RR, Morton DL, Hoon DSB. Human high molecular weight-melanoma-associated antigen: utility for detection of metastatic melanoma in sentinel lymph nodes. Clinical Cancer Research. 2008;14(11):3401. doi: 10.1158/1078-0432.CCR-07-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatai T, Matsuzawa A, Inoshita S, Mochida Y, Kuroda T, Sakamaki K, Kuida K, Yonehara S, Ichijo H, Takeda K. Execution of apoptosis signal-regulating kinase 1 (ASK1)-induced apoptosis by the mitochondria-dependent caspase activation. Journal of Biological Chemistry. 2000;275(34):26576. doi: 10.1074/jbc.M003412200. [DOI] [PubMed] [Google Scholar]
- Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8(3):179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes & development. 2004;18(16):1926. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hoon DSB, Spugnardi M, Kuo C, Huang SK, Morton DL, Taback B. Profiling epigenetic inactivation of tumor suppressor genes in tumors and plasma from cutaneous melanoma patients. Oncogene. 2004;23(22):4014–4022. doi: 10.1038/sj.onc.1207505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchens DP, Ovejera AA, Barker AD. Proc Sym the Use of Athymic (nude) Mice in Cancer Research. New York: Gustav fischer; 1978. The therapy of human tumors in athymic (nude) mice; pp. 267–280. [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275(5296):90. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Marini A, Mirmohammadsadegh A, Nambiar S, Gustrau A, Ruzicka T, Hengge UR. Epigenetic inactivation of tumor suppressor genes in serum of patients with cutaneous melanoma. Journal of Investigative Dermatology. 2005;126(2):422–431. doi: 10.1038/sj.jid.5700073. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Mihm MC., Jr Melanoma. The New England journal of medicine. 2006;355(1):51. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- Rastetter M, Schagdarsurengin U, Lahtz C, Fiedler E, Marsch WC, Dammann R, Helmbold P. Frequent intra-tumoural heterogeneity of promoter hypermethylation in malignant melanoma. Histology and histopathology. 2007;22(9):1005. doi: 10.14670/HH-22.1005. [DOI] [PubMed] [Google Scholar]
- Reifenberger J, Knobbe CB, Sterzinger AA, Blaschke B, Schulte KW, Ruzicka T, Reifenberger G. Frequent alterations of Ras signaling pathway genes in sporadic malignant melanomas. International Journal of Cancer. 2004;109(3):377–384. doi: 10.1002/ijc.11722. [DOI] [PubMed] [Google Scholar]
- Sekimukai D, Honda S, Negi A. RNA interference for apoptosis signal-regulating kinase-1 (ASK-1) rescues photoreceptor death in the rd1 mouse. Mol Vis. 2009;(15):1764–1773. [PMC free article] [PubMed] [Google Scholar]
- Spugnardi M, Tommasi S, Dammann R, Pfeifer GP, Hoon DSB. Epigenetic inactivation of RAS association domain family protein 1 (RASSF1A) in malignant cutaneous melanoma. Cancer research. 2003;63(7):1639. [PubMed] [Google Scholar]
- Tanemura A, Terando AM, Sim MS, van Hoesel AQ, de Maat MFG, Morton DL, Hoon DSB. CpG island methylator phenotype predicts progression of malignant melanoma. Clinical Cancer Research. 2009;15(5):1801. doi: 10.1158/1078-0432.CCR-08-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez CS, Shen L, Estécio MRH, Jelinek J, Gershenwald JE, Issa JPJ. CpG island methylation profiling in human melanoma cell lines. Melanoma Research. 2009;19(3):146. doi: 10.1097/cmr.0b013e32832b274e. [DOI] [PubMed] [Google Scholar]
- Thaler S, Hahnel PS, Schad A, Dammann R, Schuler M. RASSF1A mediates p21Cip1/Waf1-dependent cell cycle arrest and senescence through modulation of the Raf-MEK-ERK pathway and inhibition of Akt. Cancer research. 2009;69(5):1748. doi: 10.1158/0008-5472.CAN-08-1377. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO reports. 2001;2(3):222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whang YM, Kim YH, Kim JS, Yoo YD. RASSF1A suppresses the c-Jun-NH2-kinase pathway and inhibits cell cycle progression. Cancer research. 2005;65(9):3682. doi: 10.1158/0008-5472.CAN-04-2792. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275(5303):1129. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- Zeng ZY, Zhou YH, Zhang WL, Xiong W, Fan SQ, Li XL, Luo XM, Wu MH, Yang YX, Huang C. Gene expression profiling of nasopharyngeal carcinoma reveals the abnormally regulated Wnt signaling pathway. Human pathology. 2007;38(1):120–133. doi: 10.1016/j.humpath.2006.06.023. [DOI] [PubMed] [Google Scholar]
- Zhang R, Luo D, Miao R, Bai L, Ge Q, Sessa WC, Min W. Hsp90–Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24(24):3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zeng Z, Zhang W, Xiong W, Wu M, Tan Y, Yi W, Xiao L, Li X, Huang C. Lactotransferrin: A candidate tumor suppressor-Deficient expression in human nasopharyngeal carcinoma and inhibition of NPC cell proliferation by modulating the mitogen-activated protein kinase pathway. International Journal of Cancer. 2008;123(9):2065–2072. doi: 10.1002/ijc.23727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.