Abstract
Despite the well-documented relation between estradiol (E2) and behavior, exposure to stressors may modify sensitivity to E2. The effects of E2 on behavior are, in part, likely related to their modulation of the serotonin (5HT) and oxytocin systems. The short allele (s-variant) polymorphism found in the promoter region of the SLC6A4 gene that encodes the 5HT transporter (5HTT) modulates responsivity to stressors. The current study used ovariectomized adult female rhesus monkeys to evaluate how exposure to the psychosocial stressor of social subordination and polymorphisms in the gene encoding 5HTT influence the behavioral effects of E2 and immunoreactive serum oxytocin. Dominant females had higher levels of oxytocin than subordinate animals even though E2 increased immunoreactive serum oxytocin in all females. E2 increased affiliative behaviors in all animals, with even more of these prosocial behaviors directed at dominant females. S-variant females, regardless of social status, were more aggressive towards more subordinate cage mates and these behaviors too were increased by E2. Subordinate s-variant females are most often involved in agonistic behavior, less affiliative behavior, and were less responsive to the anxiolytic action of E2. The results show that the short allele of the 5HTT gene synergizes with psychosocial stress exposure to affect the behavioral efficacy of E2 while confirming the actions of E2 for producing generalized behavioral arousal in females. Whether differences in the central action of 5HT and/or oxytocin are responsible for this effect requires further study.
Keywords: estradiol, subordination, serotonin transporter, behavior, cortisol, oxytocin, monkey
Introduction
Estradiol (E2) is a pleiotropic hormone that targets multiple neurochemical systems regulating a range of behaviors (McEwen, 2002; Pfaff et al., 2000) that likely enhances a female’s ability to attend to contextual demands of their environment (Morgan et al., 2004). Exposure to increased concentrations of E2 is generally accepted to be critical for the expression of female sexually motivated behavior in a number of animal models (Blaustein et al., 1987; Pope et al., 1987; Rissman et al., 1997; Wallen and Tannenbaum, 1997) as well as in women (Dennerstein et al., 1980). Despite this relation between E2 and behavior, exposure to stressors or stress hormones appears to disrupt the behavioral effects of E2. Restraint (Uphouse et al., 2005) or psychosocial stress (Pierce et al., 2008), as well as overexpression of corticotropin releasing-hormone (CRH) in the central nucleus of the amygdala (Keen-Rhinehart et al., 2009), reduces sexual behaviors in ovariectomized, hormone-primed rodent females, an effect that is overcome by higher doses of hormones (White and Uphouse, 2004). Imposition of subordination accomplished through harassment and noncontact aggression in macaque species is considered a potent psychosocial stressor (Sapolsky, 2005), as subordinate animals are hypercortisolemic due to diminished glucocorticoid negative feedback (Kaplan et al., 1984a; Shively, 1998; Wilson et al., 2008). In this context, proceptive behavior occurs more frequently in dominant compared to subordinate females (Shively et al., 1990) and at lower concentrations of circulating E2 (Wallen, 1990). While the consequences of psychosocial stress on E2-dependent changes in sexual behavior appear robust, it is unclear whether stressed-induced changes in signals from the limbic-hypothalamic-pituitary-adrenal (LHPA) axis affects the efficacy of E2 on other aspects of socio-emotional behaviors, including affiliation, aggression and anxiety-like behavior.
The effects of E2 on social behaviors are, in part, likely related to their modulation of the serotonin (5HT) neural system by increasing 5HT synthesis and modulating 5HT reuptake transporter (Bethea et al., 2002). The observations that stress or CRH administration reduces 5HT in the median raphe (Summers et al., 2003; Umriukhin et al., 2002) and decreases 5HT release to limbic structures (Price and Lucki, 2001; Thomas et al., 2003) provides a possible neuroanatomical support for the hypothesis that stress impairs E2 facilitation on behavior. Furthermore, reduced transcriptional activity of the SLC6A4 gene encoding the 5HT transporter (5HTT) due to naturally occurring polymorphisms in the length of the promoter region may also disrupt E2 action as the short promoter length (s-variant) of the SLC6A4 gene is associated with a range of behavioral phenotypes, including increased anxiety, aggression, and impulsivity in humans (see (Murphy et al., 2008)). These length variations with reduced transcriptional activity are also present in rhesus monkeys and the response to an acute psychosocial stressor appears greater in animals with a s-variant promoter length allele (l/s or s/s) compared to those with long promoter length alleles (l/l genotype) (Bennett et al., 2002; Lesch et al., 1997). It is not clear whether females with the s-variant of the 5HTT gene are less responsive to the behavioral effects of E2 and whether this is worsened by social subordination.
In addition to 5HT, another potential target of E2 in the brain for the prosocial and anxiolytic effects of E2 is oxytocin (Insel et al., 1998; Windle et al., 1997). In rodent models, E2 upregulates the expression of oxytocin (Lim and Young, 2006) and the oxytocin receptor (Choleris et al., 2003; Patisaul et al., 2003) and increases oxytocin binding density in limbic regions (McCarthy et al., 1996). While oxytocin may be important for attenuating hormonal markers of stress (Neumann et al., 2000; Nomura et al., 2003), chronic stressor exposure or corticosterone administration upregulates hypothalamic oxytocin levels (Laguna-Abreu et al., 2005; Paredes et al., 2006) and oxytocin receptor binding (Liberzon and Young, 1997) in rodents. These data would imply that the oxytocin system might be unregulated in socially subordinate females; however, it is not known how E2 would affect oxytocin activity in females exposed to different amounts of psychosocial stress.
The present study used ovariectomized adult female rhesus monkeys (Macaca mulatta) to determine how social subordination influences the behavioral effects of E2 and whether these effects were modified by 5HTT genotype. The study was designed to test the hypothesis that the prosocial behavioral effects of E2 would be diminished in subordinate females, particularly those with the s-variant allele in the gene encoding the 5HTT, and these differences may be associated with differences in oxytocin. Because evidence suggests that s-variant animals show more impulsive and aggressive behavior, we predicted that E2 would increase aggression significantly more in females with the s-variant 5HTT genotype. Finally, in order to test the hypothesis that differences in stress hormone action accounted for these status differences in the behavioral effects of E2, we predicted that administration of a CRH receptor antagonist would increase the prosocial effects of E2 in subordinate females.
Material and Methods
Subjects
Subjects were 37 adult female rhesus monkeys (Macaca mulatta) housed in indoor-outdoor runs at the Yerkes National Primate Research Center. Animals were fed a standard, low fat, high fiber diet (Ralston Purina Company, St. Louis MO) ad libitum and were supplemented daily with seasonal fresh fruit and vegetables. The Emory University Animal Care and Use Committee in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals” approved all procedures. All females had been previously ovariectomized and were genotyped for polymorphisms in the gene that encodes the 5HTT as either having both long promoter length alleles (l/l) or a short promoter length allele (l/s or s/s; (Jarrell et al., 2008)). Because the heterozygous short variant (l/s) produces a phenotype similar to that of the s/s genotype (Champoux et al., 2002), females with an l/s or s/s genotype were combined (s-variant).
Macaque social groups, regardless of size, are organized by a linear dominance hierarchy that functions to maintain group stability (Bernstein, 1970). Lower-ranking animals receive proportionately more aggression from higher-ranking group mates, and these subordinate animals terminate these interactions by emitting submissive behavior. Thus, control over an individual’s social and physical environment increases with higher dominance status (Sapolsky, 2005). Given the recurrent exposure to harassment from more dominant females, subordinate females have larger adrenal glands (Kaplan et al., 1984b) and show a greater cortisol response to social challenges (Cohen, 1999). In addition, pharmacological tests using a dexamethasone suppression test (Jarrell et al., 2008; Shively, 1998; Wilson et al., 2008) or ACTH challenge (Shively, 1998) show subordinate females are hypercortisolemic. The use of social subordination in macaques is a well established model to study the adverse effects of psychosocial stress on a number of health outcomes, including cardiovascular disease (Kaplan et al., 1996), addictive behavior (Morgan et al., 2002), reproductive compromise (Adams et al., 1985; Michopoulos et al., 2009a), immune changes (Gust et al., 1991; Paiardini et al., 2009), and appetite regulation (Wilson et al., 2008).
For the present study, females were removed from their natal groups and formed into in eight small social groups of 4 to 5 monkeys each, as previously described (Jarrell et al., 2008). Briefly, all females were taken from the middle portions of the social hierarchy in their natal groups. The new groups were formed by sequentially adding unfamiliar females. These groups were established two years prior to the initiation of this study such that four groups contained females having an l/l genotype and four contained females having an s-variant genotype. Social status rankings, based on the outcome of dyadic agonistic interactions, in these small groups were stable and consistent. In accordance with previously established conventions (Shively et al., 1990), females ranked 1 and 2 were classified as dominant, while those of ranks 3, 4, and 5 were considered subordinate. Of the total 37 subjects, the genotype and status distributions were: 8 s-variant dominant, 12 s-variant subordinate, 8 l/l dominant, and 9 l/l subordinate.
Experimental design
All animals were studied in each of four treatment conditions, as previously described (Michopoulos et al., 2009a). The conditions each lasted one week each and were separated by a two-week, no treatment washout period. The order of treatments was counterbalanced across groups, with all females in a specific social group receiving the same treatment. Specifically, the four treatments consisted of control (placebo), E2 replacement (E2), CRH receptor analogue (CRHA), and E2 plus CRHA. A 0.25 ml sc injection of saline was administered at 0830 hours for five consecutive days as the control condition. E2 replacement was accomplished by implanting E2 filled Silastic capsules sc as previously described (Mook et al., 2005). Analysis of selected samples (days 4 and 7) during E2 replacement versus no E2 replacement conditions indicated that hormone replacement achieved mid-follicular phase concentrations (66.7 ± 2.1 vs. < 5.0 pg/ml). Capsules were implanted three days prior to the initiation of data collection and removed immediately following the end of the phase.
The CRHA utilized for the study was a CRH type 1 receptor antagonist CP154,526 (Pfizer, Groton CT), an analogue of the more widely used antalarmin (Seymour et al., 2003). CP154,526 was used as it crosses the blood brain barrier to bind to both peripheral and central CRH type 1 receptors (Seymour et al., 2003). Based on the existing literature in monkeys using antalarmin (Ayala et al., 2004; Broadbear et al., 2004; French et al., 2007; Habib et al., 2000), we chose to administer a dose of 10 mg/kg sc daily for five consecutive days at 0830 hours with the expectation that it would attenuate cortisol secretion. However, as described in Results and reported previously (Broadbear et al., 2004), this dose paradoxically stimulated cortisol secretion, allowing us to evaluate the impact of an increase in cortisol on behavior in female monkeys.
Outcome measures
The objective of this study was to assess how social subordination and 5HTT genotype may modify the effects of E2 on socio-emotional behavior. Data describing the effects of these manipulations on luteinizing hormone (LH) secretion are described elsewhere (Michopoulos et al., 2009a). Serum samples were collected at 0900 hours on days 4 and 7 to confirm E2 concentrations. Samples were collect on days 4 – 7 for morning cortisol analyses. While it is thought that peripheral levels of oxytocin do not reflect central oxytocin (Neumann, 2007), recent neuroanatomical evidence indicates magnocellular oxytocin neurons from the paraventricular nucleus of the hypothalamus (PVN) project to both the posterior pituitary and forebrain structures, suggesting serum oxytocin could be surrogate markers of centrally active oxytocin (Ross and Young, 2009). Consequently, we measured immunoreactive serum oxytocin on days 5 and 6 of each treatment condition to assess cumulative changes in peptide concentrations. All subjects had been previously habituated to removal from their groups for conscious venipuncture, with a particular group typically being sampled 10 minutes following entrance into the housing area thereby minimizing the arousal associated the sampling procedure (Blank et al., 1983; Walker et al., 1982).
Behavioral data were collected using an established ethogram (Jarrell et al., 2008). A 30-minute observation of each group was done five hours following saline or CRHA injection on each day of injection. Affiliative behavior was comprised of proximity and grooming; aggression was defined by threats, slaps, grabs, and bites; and submissive behavior was characterized by withdrawals, grimaces, and screams. Anxiety-like behavior consisted of body shakes, yawns, self-scratching, and self-grooming (Troisi, 2002). Data was recorded using a Palm PDA and the “Hand Obs” program developed by the Center for Behavioral Neuroscience (Graves and Wallen, 2006). Inter-observer reliability was greater than 92%.
Hormone assays
All assays were done in the Biomarkers Core Lab at the YNPRC. Selected samples were assayed for E2 to verify Silastic capsule efficacy using a modification of a previously validated commercial assay (Siemens/DPC; Los Angeles CA) (Pazol et al., 2004). Using 200 µl of serum, the assay has a sensitivity of 5 pg/ml and an intra- and inter-assay coefficient of variation (CV) of 5.2% and 11.1%, respectively. Serum levels of cortisol were determined by radioimmunoassay (RIA) with a commercially available kit (Beckman-Coulter/DSL, Webster TX). Using 25 µl, the assay has a range from 0.5 to 60 µg/dl with an inter- and intra-assay CV of 4.9% and 8.7%, respectively. Immunoreactive serum oxytocin was measured by EIA using a kit distributed by Enzo Life Sciences from Assay Designs (Ann Arbor MI). This assay measures immunoreactive oxytocin across a number of species in peripheral samples or cerebrospinal fluid. For rhesus monkeys, 50 µl of serum is diluted in 150 µl of assay buffer. With this protocol, the assay has a sensitivity of 15.6 pg/ml with an inter- and intra-assay CV of 7.5% and 10.2%, respectively. As described in the kit protocol, direct measurement of diluted rhesus monkey serum provides greater estimates of oxytocin than those samples preprocessed prior to assay (157%), the correlation of unextracted vs. extracted rhesus monkey serum is nonetheless strong (r = 0.89, n = 11, range of 56 – 252 pg/ml, extracted). Spiking rhesus monkey samples (n = 4) with 300 pg/ml of human oxytocin showed that resulting concentrations were, on average, 30% higher than expected. Given this degree of precision, we refer to the analysis of samples for oxytocin as immunoreactive serum oxytocin.
Statistical analysis
Data were summarized as mean ± standard error of the mean (SEM). In order to examine the categorical (status and genotype) and treatment (E2, CRHA), and day on treatment main effects and their interactions, data were analyzed with repeated measures analysis of variance in a 2 by 2 design to assess E2 (E2 and E2+CRHA) vs. non-E2 treatments (C and CRHA) and CRHA (CRHA and E2+CRHA) vs. non-CRHA treatments (C and E2). All statistical tests with p ≤ 0.05 were considered significant and post-hoc corrections were made for multiple comparisons when necessary.
Results
Social status categorizations
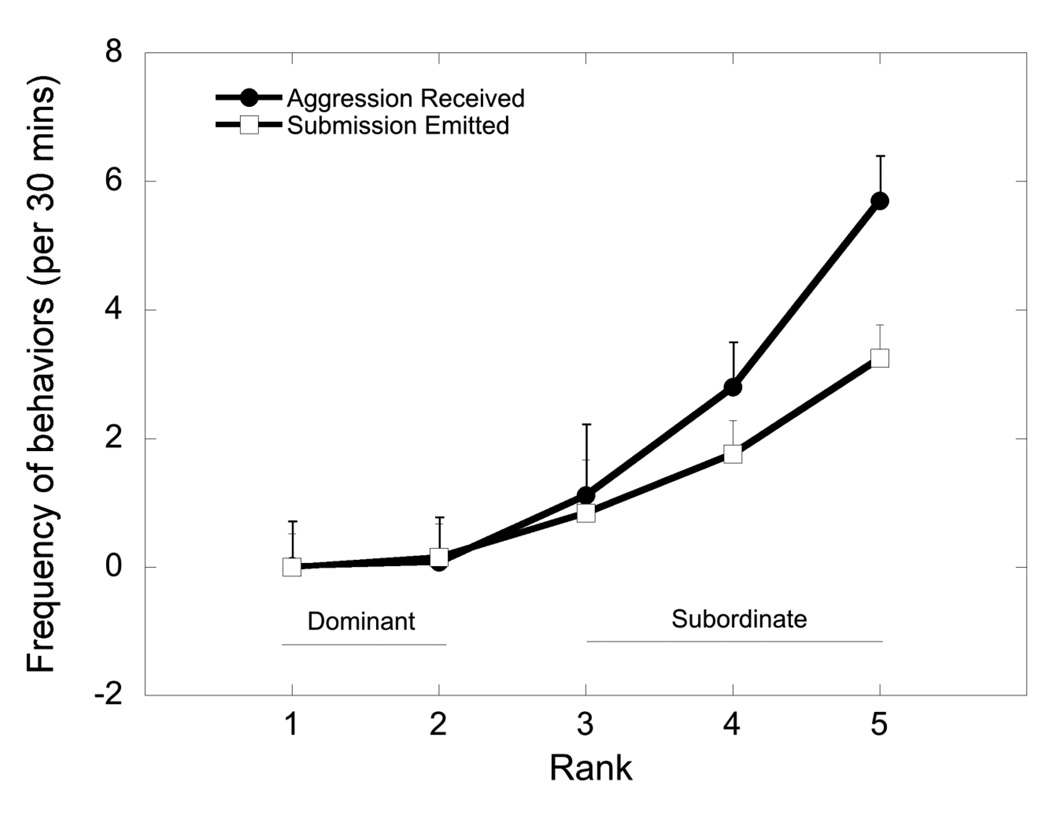
Figure 1 illustrates the mean frequency of aggression received and submissive behavior emitted across females at each rank collapsed across all treatment conditions and time. Data describing treatment effects on these behaviors is presented below. Females ranked 3 – 5 received significantly more aggression from higher-ranking group mates (F 4, 27 = 11.4, p < 0.001). This harassment was associated with rank-dependent, higher rates of submissive behavior (F 4, 27 = 6.56, p < 0.001).
Figure 1.
Mean ± SEM rates of agonistic behavior collapsed across treatment conditions and genotype. Animals categorized as dominant (ranked 1 and 2) received less aggressive behavior (closed circle) than those categorized as subordinate (ranked 3–5) while subordinate animals emitted more submissive behaviors (open square) than dominant animals.
Serum Cortisol
As reported previously (Michopoulos et al., 2009a) and illustrated in Table 1, E2 decreased (F1, 33 = 14.90, p < 0.001) and the CRHA increased (F1,33 = 12.55, p < 0.01) morning cortisol values in all subjects. Serum cortisol was consistently lower throughout the week of E2 treatment compared to placebo (F3, 99 = 0.24, p = 0.87) but did increase progressively throughout the week during the CRHA treatment (data not shown; F3, 99 = 6.55, p < 0.01). Overall mean levels of cortisol were higher during CRHA even when combined with E2. Neither social status (F1, 33 = 2.08, p = 0.16), 5HTT genotype (F1, 33= 0.01, p = 0.96) nor the interaction of status and genotype (F1,33 < 0.01, p = 0.99) significantly influenced the effect of E2 on cortisol concentrations. Similarly, the CRHA–induced elevation in cortisol was unaffected by status (F3,99=0.96, p=0.41), genotype (F3,99 = 2.22, p = 0.90), or their interaction (F3,99=1.23, p = 0.30).
Table 1.
Mean ± SEM serum concentrations of morning cortisol (µg/dl) during each of the four treatment conditions in dominant females and subordinate females with an l/l or s-variant 5HTT genotype. Estradiol administration significantly attenuated serum cortisol (p < 0.001), indicated by different numbered superscripts. However, CRHA (corticotropin releasing-hormone receptor analogue) administration significantly increased serum cortisol (p = 0.001), indicated by a different lettered superscript. Serum cortisol did not differ significantly by status, genotype, or their interactions with treatments. See text for details.
| Group | Placebo1, A | CRHA1, B | Estradiol2, A | CRHA + Estradiol2, B |
|---|---|---|---|---|
| Dom, l/l | 27.9 ± 1.8 | 31.9 ± 2.4 | 23.0 ± 2.0 | 30.2 ± 2.3 |
| Dom, s-variant | 29.5 ± 2.6 | 34.6 ± 2.9 | 27.4 ± 1.9 | 30.6 ± 2.9 |
| Subordinate, l/l | 27.4 ± 1.7 | 32.1 ± 2.3 | 24.7 ± 1.8 | 28.4 ± 2.8 |
| Subordinate, s-variant | 28.6 ± 1.4 | 33.0 ± 1.9 | 26.3 ± 1.6 | 32.5 ± 2.4 |
Immunoreactive Serum Oxytocin
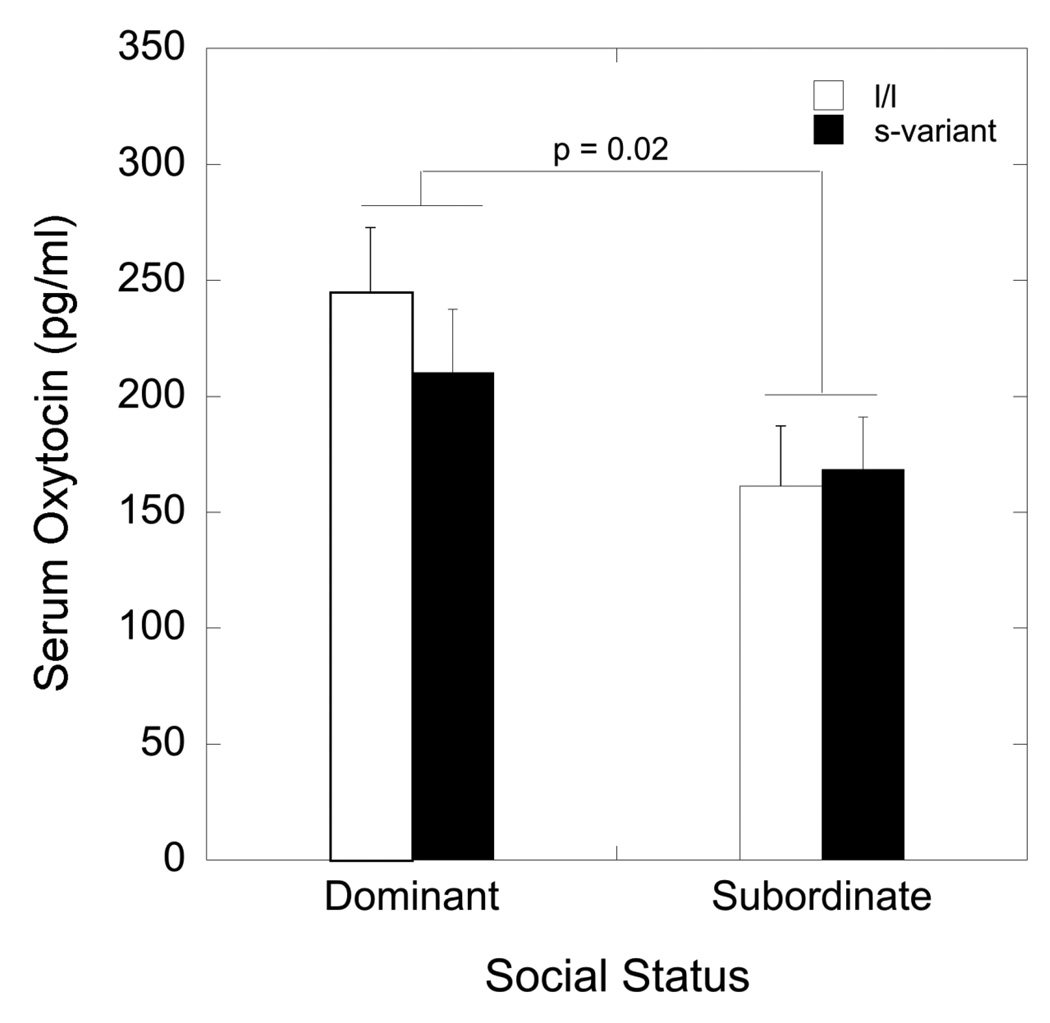
As illustrated in Figure 2, dominant females had significantly higher immunoreactive serum levels of oxytocin compared with subordinate animals regardless of treatment condition (F1,33= 5.87, p = 0.02) and this was unaffected by 5HTT genotype (Figure 2; F1,33 0.67, p = 0.42). In addition, E2 significantly (F1, 33 = 4.15, p = 0.05) increased overall immunoreactive serum oxytocin in all females (182 ± 14 vs. 209 ± 14) such that there was no status by E2 interaction on serum concentrations of immunoreactive oxytocin (F1,33 1.76, p = 0.19; data not shown). The administration of CRHA or its interaction with status or E2 treatment did not significantly affect immunoreactive serum oxytocin (p > 0.05; data not shown).
Figure 2.
Mean ± SEM serum concentrations of oxytocin in dominant and subordinate females with either an l/l (open bar) or s-variant (closed bar) 5HTT genotype. Values presented are overall oxytocin levels, collapsed across E2 (estradiol) and CRHA (corticotropin releasing-hormone receptor analogue) treatment conditions.
Anxiety-like behavior
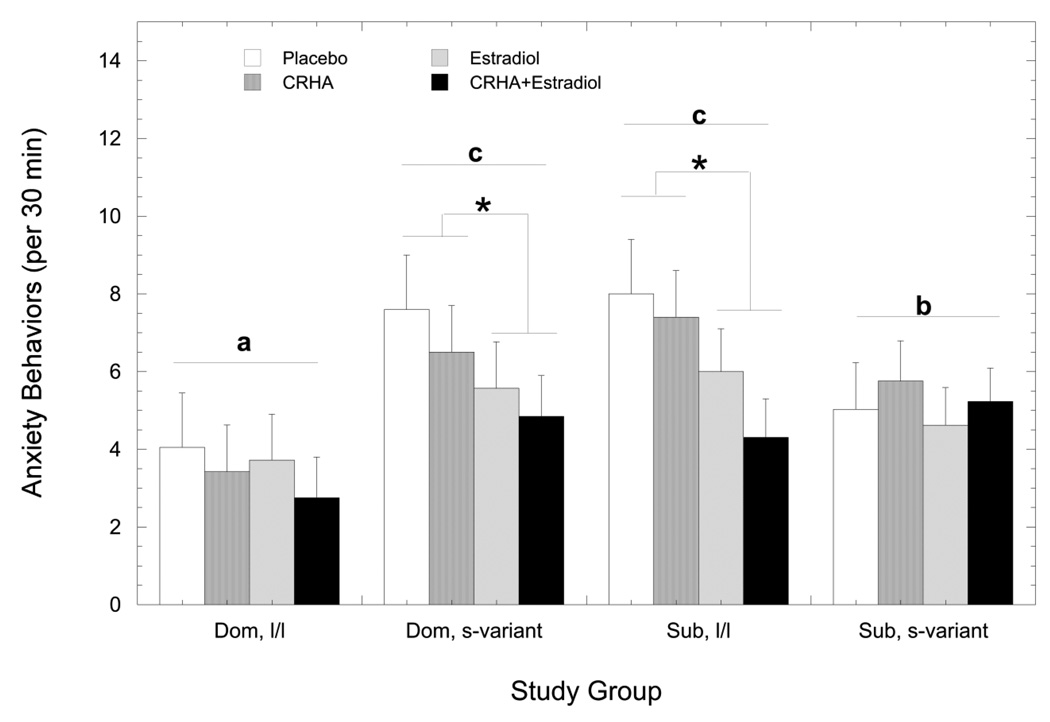
Figure 3 shows E2 significantly attenuated anxiety-like behavior compared to placebo (6.07 ± 0.61 vs. 4.45 ± 0.49 per 30 min; F 1, 33 = 12.03, p < 0.01). However, this anxiolytic effect of E2 was significantly modified by status and genotype (F 1, 33 = 5.22, p = 0.03). Dominant females with an l/l 5HTT genotype had the lowest rates of anxiety-like behavior compared with other females (p < 0.05) and the decrease due to E2 replacement (13%) was not significant (p > 0.05). The higher rates of anxiety-like behavior were attenuated by E2 in dominant s-variant (25%) and subordinate l/l females (39%; p < 0.05) but not in subordinate s-variant females (9%; p > 0.05). Finally, anxiety-like behaviors were not affected by treatment with the CRHA (F1, 33 = 1.10, p = 0.30) or its interaction with E2, status, or genotype (p > 0.05).
Figure 3.
Mean ± SEM rates of anxiety-like behavior in dominant females and subordinate females with either an l/l or s-variant 5HTT genotype during the placebo (open bar), estradiol (hatched bar), corticotropin releasing-hormone receptor analogue (CRHA; grey bar), and combined estradiol and CRHA (closed bar) treatments. Different letters among the four study groups reflect significantly different rates of anxiety-like behavior collapsed across treatment conditions. The asterisk indicates estradiol replacement significantly attenuated anxiety-like behaviors in those study groups.
Affiliative behavior
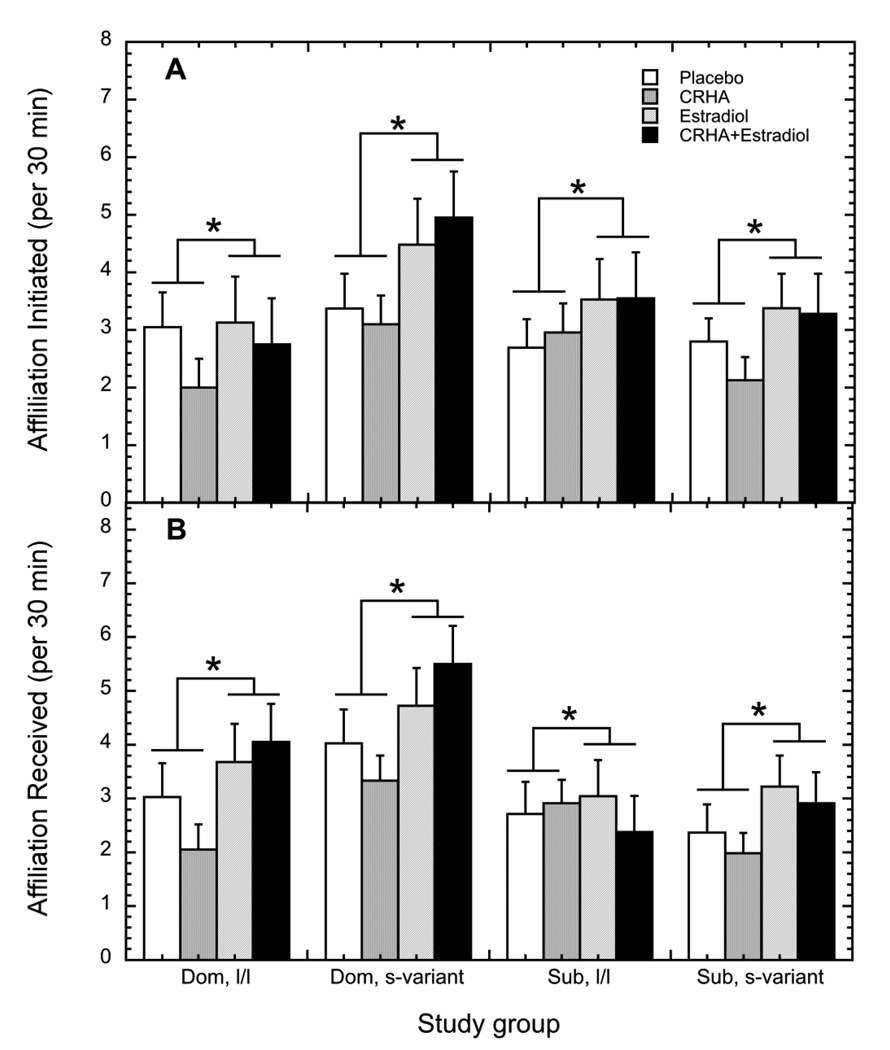
Overall, there were no main effects of status, 5HTT genotype, or a status by genotype interaction on affiliation directed toward others (p > 0.05). However, as shown in Figure 4A, treatment with E2 significantly increased affiliative behavior directed towards others compared to non-E2 treatment conditions (3.63 ± 0.36 vs. 2.76 ± 0.23 per 30 min; F1, 33 = 4.91, p = 0.03). While the effect of E2 on increasing affiliation initiated by dominant, l/l females appeared to be less compared to other groups, there was no status by genotype by E2 treatment interaction on the amount of affiliative behavior initiated by females (F1, 33 = 0.35, p = 0.56). Finally, affiliative behavior directed toward others was not significantly affected by CRHA treatment or its interaction with E2, status, or genotype (p > 0.05).
Figure 4.
Mean ± SEM rates for affiliative behavior (A) directed towards others and (B) received from others during the placebo (open bar), estradiol (hatched bar), corticotropin releasing-hormone receptor analogue (CRHA; grey bar), and combined estradiol and CRHA (closed bar) treatments in dominant females and subordinate females with either an l/l or s-variant 5HTT genotype. Asterisks indicate the significant elevation in behavior induced by estradiol replacement.
Corresponding to significantly higher rates of affiliation initiated by females during E2 treatments (Figure 4A), rates of affiliation received were also increased by E2 (2.80 ± 0.22 vs. 3.69 ± 0.30; Figure 4B; F1, 33 = 7.24, p = 0.01). Dominant females were most often the targets of this behavior compared with subordinates regardless of treatment condition (3.80 ± 0.30 vs. 2.69 ± 0.28 per 30 min; Figure 4B; F1, 33 = 7.07, p = 0.01). While it appeared the E2-induced increase in affiliation received was less in subordinates compared to dominant females, there was no status by E2 interaction (F1, 33 = 2.23, p = 0.15). However, the effect of E2 varied significantly by status in the context of CRHA treatments (Figure 4B; F1, 33 = 6.52, p = 0.02). In the absence E2, CRHA decreased affiliation received by dominant females whereas rates were unchanged in subordinates. In contrast, treatment with CRHA reduced the rate of received affiliation during E2 treatment in subordinate females whereas it increased rates during E2 for dominant females. Finally, 5HTT genotype did not modify the effects of status or E2 treatment whether females were targets of affiliation (P > 0.05).
Aggressive Behavior
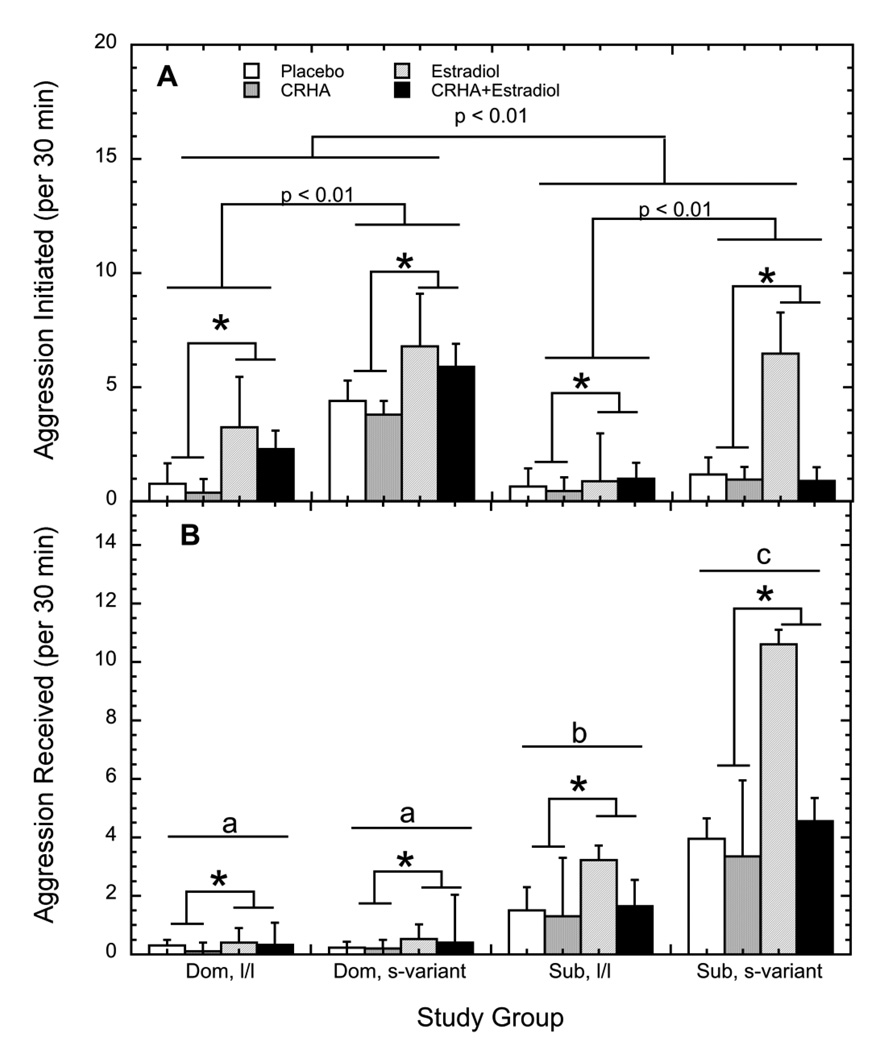
As expected, dominant females initiated significantly more aggression than subordinate monkeys (3.44 ± 0.65 vs. 1.56 ± 1.56; Figure 5A; F1, 33 = 4.78, p = 0.04). However, s-variant females showed significantly higher rates of aggressive compared with l/l subjects (3.76 ± 0.59 vs. 1.21 ± 0.62; F1, 33= 8.94, p < 0.01). There was no status by genotype interaction (F1, 33 = 1.25, p = 0.27). Importantly, as shown in Figure 5A rates of aggression directed toward others were significantly increased during treatment with E2 in all females (3.43 ± 0.65 vs. 1.57 ± 0.36; F1, 33 = 9.35, p < 0.01). In contrast, CRHA attenuated aggression (1.96 ± 0.31 vs. 3.04 ± 0.63; F 1, 33 = 5.00, p = 0.03) regardless of E2, status or genotype (p > 0.05).
Figure 5.
Mean ± SEM rates for aggressive behavior (A) initiated and (B) received during the placebo (open bar), estradiol (hatched bar), corticotropin releasing-hormone receptor analogue (CRHA; grey bar), and combined estradiol and CRHA (closed bar) conditions in dominant females and subordinate females with either an l/l or s-variant 5HTT genotype. Asterisks indicate the significant increase in behavior induced by estradiol replacement. Different letters in panel B indicate groups different significantly from one another in aggression received regardless of treatment phase (p < 0.05).
Not surprisingly, subordinate females were most often the target of aggression (Figure 5B; F1, 33 = 7.67, p < 0.01). Again, this status effect was modified by genotype (F 1, 33 = 4.63, p = 0.04), as subordinate s-variant females received more aggression than subordinate l/l females (5.01 ± 1.14 vs. 2.10 ± 0.41). Corresponding to the higher rates of aggression initiated during E2 replacements, rates of aggression received were also higher during E2 (F1, 33 = 5.00, p = 0.03). The apparent decrease in aggression received by subordinates during CRHA treatment was not statistically significant (F1, 33 = 1.85, p = 0.18).
Discussion
Our current findings showed that mid-follicular phase levels of E2 increased behavioral activity, reflected in agonistic and affiliative behaviors, but provided support for the notion that exposure to stressors and upregulation of the stress axis attenuates the behavioral effects of E2 (Keen-Rhinehart et al., 2009; Pierce et al., 2008; Uphouse et al., 2005) particularly in individuals that may be genetically more reactive to stressor exposure (Murphy et al., 2008). While immunoreactive serum levels of oxytocin were lower in subordinate compared to dominant animals, regardless of 5HTT genotype, E2 increased oxytocin levels in all females. However, s-variant subordinate females showed highest rates of agonistic behavior and lowest rates of affiliation that occurred coincident with a reduced sensitivity to the anxiolytic actions of E2, suggesting that this 5HTT polymorphism synergizes with psychosocial stress exposure to affect the behavioral effects of E2. These findings are important for understanding the factors that modulate the behavioral response to E2 and for identifying mechanisms responsible for female emotional sociality and emotional well-being.
The present study extends previous observations of increased depressive- (Shively et al., 2006; Shively et al., 1997) and anxiety-like behaviors (Wilson et al., 2008) related to social status in female macaques. While the lowest rates of anxiety-like behaviors were observed in dominant females with the l/l 5HTT genotype, dominant s-variant females had rates of anxiety indistinguishable from l/l subordinates. S-variant subordinates expressed anxiety behaviors at a higher rate than dominant l/l females but less than other groups. The higher rates of anxiety-like behaviors in subordinates may be an adaptive response to their unpredictable social environment (Huhman, 2006), specifically potential threats of aggression (Troisi, 2002). Coupled with the higher rates of harassment, subordinates were less often recipients of affiliation and this too could contribute to the higher anxiety behaviors. However, this explanation does not hold for the dominant s-variant females, as their access to resources is unimpeded. Rather, their increased rates of anxiety may be a consequence of their propensity to aggress more frequently with cage mates, as reduced expression of 5HTT characteristic of the short allele of the 5HTT gene is associated with increased anxiety and impulsive behavior (Murphy et al., 2008).
The well-established anxiolytic effects of E2 (Bernardi et al., 1989; Galea et al., 2001; Okada et al., 1997; Rocha et al., 2005; Walf et al., 2004) were significantly affected by social status and genotype. Rates of anxiety were decreased by E2 only in dominant s-variant and subordinate l/l females. It is possible that these behaviors could not be reduced further by E2 in the dominant l/l females, as their baseline level of anxiety-like behaviors were lowest compared to other groups. In contrast, the higher rates anxiety-like behaviors in s-variant subordinate females were unaffected by E2. This could be explained by a disruption of E2 efficacy in these animals, as limbic estrogen receptor levels are decreased in individuals with stress-induced affective disorders (Perlman et al., 2005; Perlman et al., 2004). However, this seems unlikely, as the s-variant subordinate females showed an increase in affiliative behavior during E2 replacement. It is possible that the neurochemical targets, notably 5HT, that mediate these anxiolytic effects of E2 are altered in some fashion in females exposed to the stress of subordination (Summers et al., 2003) and exacerbated by the s-variant polymorphism. Differences in response to the anxiolytic effects of E2 cannot be attributed to differences in oxytocin, as there was no genotype difference in immunoreactive serum oxytocin in subordinate females. E2 dose-response studies on 5HT responsivity or central manipulation of the oxytocin system can better address these status - genotype differences in anxiety behavior.
Overall aggressive behavior was influenced independently by both status and 5HTT genotype. Specifically, dominant females were more aggressive towards subordinate cage mates. This is not surprising, as in such hierarchical social organizations, most individuals cannot avoid aggression received from higher-ranking group members and thus must emit submissive behaviors to terminate the aggression (Bernstein and Gordon, 1974). Furthermore, under the stable group situation of the present study, s-variant females were more aggressive than l/l subjects. Dysfunction of the 5HT system is linked to increased incidences of aggressive behavior, as 5HT usually acts to inhibit aggression (Summers et al., 2005) and limit impulsivity (Hollander and Rosen, 2000). Previous studies indicate 5HT tone is lower in individuals with an s-variant genotype (Hoffman et al., 2007; Manuck et al., 2004; Reist et al., 2001) and reduced central 5HT activity is associated with increased impulsivity and aggression (Higley and Linnoila, 1997; Hollander and Rosen, 2000; Manuck et al., 2003; Westergaard et al., 2003; Westergaard et al., 1999), as well as hostility in humans (Reist et al., 2003; Williams et al., 2003). Our data support the hypothesis that females with an s-variant 5HTT genotype are more aggressive in a stable social group situation.
Our observation that dominant animals received more affiliation than subordinate animals was associated with overall higher immunoreactive oxytocin levels present in dominant animals. Indeed, studies have shown that oxytocin facilitates affiliative behavior (Campbell, 2008; Donaldson and Young, 2008) and promotes adaptive responses to challenging social situations (Lee et al., 2009). In addition, engaging in affiliative behavior enhances peripheral oxytocin levels (Carter et al., 2008; Paredes et al., 2006). E2 replacement increased both affiliative and aggressive behaviors in all females regardless of status and genotype. The increase in affiliative behaviors such as proximity and grooming by E2 is consistent with previous observations (Shively et al., 2007; Wallen and Tannenbaum, 1997). Furthermore, our data show that, in addition to increasing affiliative behavior, E2 also increased immunoreactive serum oxytocin in all females compared to the placebo condition. Our findings should be considered preliminary as E2-induced increases in OT have been reported only in the hypothalamus (Patisaul et al., 2003) and the link between changes in immunoreactive serum oxytocin and affiliative behavior is only correlational. These observations need to be confirmed with E2 dose-response changes in both peripheral and central oxytocin using an assay platform, such as mass spectrometry, that is independent of possible confounds associated with antibody affinity and specificity inherent in immunoassays.
Replacement of E2 to ovariectomized female rhesus monkeys also decreased morning cortisol levels, consistent with other data indicating E2 decreases basal or stress-induced activation of the LHPA axis (Patchev and Almeida, 1996; Saltzman et al., 2006; Young et al., 2001). However, other studies show that E2 increases activation of the LHPA axis (Viau and Meaney, 1991) by decreasing glucocorticoid negative feedback (Patchev and Almeida, 1996; Wilson et al., 2005); increasing adrenal sensitivity to ACTH (Figueiredo et al., 2007) and enhancing diurnal (Gudmundsson et al., 1999; Smith and Norman, 1987) or morning cortisol secretion (Giussani et al., 2000; Stavisky et al., 2003). While this discrepancy in the literature surrounding E2’s ability to alter LHPA function might reflect differential access to social support (Barbosa and Mota, 2009; Doyle et al., 2008), a more parsimonious explanation is that a single morning cortisol sample was the only parameter of LHPA activity collected and not sufficient to adequately describe the effects of E2 on LHPA function. While the lack of a social status difference in morning cortisol levels during the placebo treatment could be considered a limitation of the current study, these data are consistent with previous studies suggesting that using a single measure of morning cortisol is not sufficient measure of LHPA activity in subordinate monkeys (Michopoulos et al., 2009a; Michopoulos et al., 2010). Assessing LHPA negative feedback by dexamethasone administration is necessary to show hypercortisolemia in subordinate females (Kaplan et al., 1984a; Sapolsky, 2005; Shively, 1998; Wilson et al., 2008).
As we have reported in a companion paper (Michopoulos et al., 2009a), administration of a 10 mg/kg dose of the CRH type 1 receptor antagonist CP154,526, an analog of the widely used antalarmin, for five consecutive days paradoxically increased serum cortisol levels in all females even in the presence of E2. Previous uses of CP154,526 on glucocorticoid levels and anxiety behaviors are inconsistent, in both rodents (Arborelius et al., 2000; Bornstein et al., 1998) and non-human primates (Ayala et al., 2004; French et al., 2007; Habib et al., 2000). While the effects of the CRHA on behavior were associated with increased cortisol levels, we cannot rule out the possibility that the CRHA is having a more direct effect on modulating social behaviors via a central mechanism, and thus this data should be considered preliminary. Further studies are necessary to determine the mechanism by which increased cortisol levels mediate aggression and affiliation in female rhesus monkeys.
In summary, the data reported here add support to the long-standing notion that E2 has potent effects on female socio-emotional behavior and is consistent with the hypothesis that E2 induces generalized behavioral arousal, allowing the female to adapt to and cope with environmental challenges (Ribeiro et al., 2009). However, the data extend these findings by showing how social subordination and 5HTT genotype may modify these effects. The attenuated anxiolytic response to E2 in subordinate s-variant females supports data showing exposure to stressor may disrupt E2 regulation of behavior sensitivity to E2 and further implicates the s-variant of the 5HTT polymorphism as a predisposing factor in increased individual vulnerability to adverse consequence due to psychosocial stress exposure. Furthermore, the present data suggest that social status differences in immunoreactive serum oxytocin, as a surrogate measure of central concentrations, may be one of several neurochemical factors that mediate the expression of these social behaviors. We must also emphasize that, in social living animals, socio-emotional behaviors do not occur in isolation but rather reflect a female’s response to her social environment and biological condition. Thus, an evaluation of the hormonal regulation of these behaviors must take that of a multi-variable approach into consideration. Dose – response studies with E2 are needed to better define the parameters and neurochemical basis of reduced sensitivity to E2 in this model of psychosocial stress.
Acknowledgments
We would like to thank Jennifer Whitley, Shannon Bounar, and Jeff Fisher for their expert technical assistance. The CP154526 was gift from Pfizer (Groton CT). This work was funded by HD046501, MH079100, RR00165, and F31MH085445 (VM). The YNPRC is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MR, Kaplan JR, Koritnik DR. Psychosocial influences on ovarian endocrine and ovulatory function in Macaca fascicularis. Physiology & behavior. 1985;35:935–940. doi: 10.1016/0031-9384(85)90262-8. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Skelton KH, Thrivikraman KV, Plotsky PM, Schulz DW, Owens MJ. Chronic administration of the selective corticotropin-releasing factor 1 receptor antagonist CP-154,526: behavioral, endocrine and neurochemical effects in the rat. The Journal of pharmacology and experimental therapeutics. 2000;294:588–597. [PubMed] [Google Scholar]
- Ayala AR, Pushkas J, Higley JD, Ronsaville D, Gold PW, Chrousos GP, Pacak K, Calis KA, Gerald M, Lindell S, Rice KC, Cizza G. Behavioral, adrenal, and sympathetic responses to long-term administration of an oral corticotropin-releasing hormone receptor antagonist in a primate stress paradigm. The Journal of clinical endocrinology and metabolism. 2004;89:5729–5737. doi: 10.1210/jc.2003-032170. [DOI] [PubMed] [Google Scholar]
- Barbosa MN, Mota MT. Behavioral and hormonal response of common marmosets, Callithrix jacchus, to two environmental conditions. Primates; journal of primatology. 2009;50:253–260. doi: 10.1007/s10329-009-0137-2. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, Champoux M, Suomi SJ, Linnoila MV, Higley JD. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bernardi M, Vergoni AV, Sandrini M, Tagliavini S, Bertolini A. Influence of ovariectomy, estradiol and progesterone on the behavior of mice in an experimental model of depression. Physiology & behavior. 1989;45:1067–1068. doi: 10.1016/0031-9384(89)90238-2. [DOI] [PubMed] [Google Scholar]
- Bernstein I, Gordon T. The function of aggression in primate societies. American Science. 1974;62:304–311. [PubMed] [Google Scholar]
- Bernstein IS. Primate status hierachies. In: Rosenblum LA, editor. Primate Behavior: Developments in Field and laboratory Research. New York: Academic Press; 1970. pp. 71–109. [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in neuroendocrinology. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta endocrinologica. 1983;102:190–195. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Finkbohner R, Delville Y. Estrogen-induced and estrogen-facilitated female rat sexual behavior is not mediated by progestin receptors. Neuroendocrinology. 1987;45:152–159. doi: 10.1159/000124717. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Webster EL, Torpy DJ, Richman SJ, Mitsiades N, Igel M, Lewis DB, Rice KC, Joost HG, Tsokos M, Chrousos GP. Chronic effects of a nonpeptide corticotropin-releasing hormone type I receptor antagonist on pituitary-adrenal function, body weight, and metabolic regulation. Endocrinology. 1998;139:1546–1555. doi: 10.1210/endo.139.4.5938. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Rivier JE, Rice KC, Woods JH. Corticotropin-releasing hormone antagonists, astressin B and antalarmin: differing profiles of activity in rhesus monkeys. Neuropsychopharmacology. 2004;29:1112–1121. doi: 10.1038/sj.npp.1300410. [DOI] [PubMed] [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biological psychology. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Progress in brain research. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6192–6197. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. Social status and susceptibility to respiratory infections. Annals of the New York Academy of Sciences. 1999;896:246–253. doi: 10.1111/j.1749-6632.1999.tb08119.x. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Burrows GD, Wood C, Hyman G. Hormones and sexuality: effect of estrogen and progestogen. Obstet Gynecol. 1980;56:316–322. [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science (New York, N.Y. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Baker KC, Cox LD. Physiological and behavioral effects of social introduction on adult male rhesus macaques. American journal of primatology. 2008;70:542–550. doi: 10.1002/ajp.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo HF, Ulrich-Lai YM, Choi DC, Herman JP. Estrogen potentiates adrenocortical responses to stress in female rats. Am J Physiol Endocrinol Metab. 2007;292:E1173–E1182. doi: 10.1152/ajpendo.00102.2006. [DOI] [PubMed] [Google Scholar]
- French JA, Fite JE, Jensen H, Oparowski K, Rukstalis MR, Fix H, Jones B, Maxwell H, Pacer M, Power ML, Schulkin J. Treatment with CRH-1 antagonist antalarmin reduces behavioral and endocrine responses to social stressors in marmosets (Callithrix kuhlii) American journal of primatology. 2007;69:877–889. doi: 10.1002/ajp.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Barr AM. Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behavioural brain research. 2001;122:1–9. doi: 10.1016/s0166-4328(01)00170-x. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Farber DM, Jenkins SL, Yen A, Winter JA, Tame JD, Nathanielsz PW. Opposing effects of androgen and estrogen on pituitary-adrenal function in nonpregnant primates. Biology of reproduction. 2000;62:1445–1451. doi: 10.1095/biolreprod62.5.1445. [DOI] [PubMed] [Google Scholar]
- Graves FC, Wallen K. Androgen-induced yawning in rhesus monkey females is reversed with a nonsteroidal anti-androgen. Hormones and behavior. 2006;49:233–236. doi: 10.1016/j.yhbeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gudmundsson A, Goodman B, Lent S, Barczi S, Grace A, Boyle L, Ershler WB, Carnes M. Effects of estrogen replacement therapy on the circadian rhythms of serum cortisol and body temperature in postmenopausal women. Exp Gerontol. 1999;34:809–818. doi: 10.1016/s0531-5565(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav Immun. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, McCann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Linnoila M. Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Annals of the New York Academy of Sciences. 1997;836:39–56. doi: 10.1111/j.1749-6632.1997.tb52354.x. [DOI] [PubMed] [Google Scholar]
- Hoffman JB, Kaplan JR, Kinkead B, Berga SL, Wilson ME. Metabolic and reproductive consequences of the serotonin transporter promoter polymorphism (5-HTTLPR) in adult female rhesus monkeys (Macaca mulatta) Endocrine. 2007;31:202–211. doi: 10.1007/s12020-007-0017-8. [DOI] [PubMed] [Google Scholar]
- Hollander E, Rosen J. Impulsivity. Journal of psychopharmacology (Oxford, England) 2000;14:S39–S44. doi: 10.1177/02698811000142S106. [DOI] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50:640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Insel TR, Winslow JT, Wang Z, Young LJ. Oxytocin, vasopressin, and the neuroendocrine basis of pair bond formation. Adv Exp Med Biol. 1998;449:215–224. doi: 10.1007/978-1-4615-4871-3_28. [DOI] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiology & behavior. 2008;93:807–819. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J, Adams M, Clarkson T, Kortinik D. Psychosocial influences on female "protection" among cynomolgus macaques. Atherosclerosis. 1984a;53:283–295. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female 'protection' among cynomolgus macaques. Atherosclerosis. 1984b;53:283–295. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosomatic Medicine. 1996;58:598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, Davis M, Owens MJ, Nemeroff CB, Wilson ME. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Molecular psychiatry. 2009;14:37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laguna-Abreu MT, Koenigkam-Santos M, Colleta AM, Elias PC, Moreira AC, Antunes-Rodrigues J, Elias LL, Castro M. Time course of vasopressin and oxytocin secretion after stress in adrenalectomized rats. Horm Metab Res. 2005;37:84–88. doi: 10.1055/s-2005-861159. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88:127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flugge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mossner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: alternative biallelic variation in rhesus monkeys. Rapid communication. J Neural Transm. 1997;104:1259–1266. doi: 10.1007/BF01294726. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Young EA. Effects of stress and glucocorticoids on CNS oxytocin receptor binding. Psychoneuroendocrinology. 1997;22:411–422. doi: 10.1016/s0306-4530(97)00045-0. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ. Neuropeptidergic regulation of affiliative behavior and social bonding in animals. Hormones and behavior. 2006;50:506–517. doi: 10.1016/j.yhbeh.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Muldoon MF. Socio-economic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter gene-linked polymorphic region. Psychoneuroendocrinology. 2004;29:651–668. doi: 10.1016/S0306-4530(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Kaplan JR, Rymeski BA, Fairbanks L, Wilson ME. Approach to a stranger is associated with low CNS serotonergic responsivity in female cynomolgus monkeys. American journal of primatology. 2003 doi: 10.1002/ajp.10118. in press. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, McDonald CH, Brooks PJ, Goldman D. An anxiolytic action of oxytocin is enhanced by estrogen in the mouse. Physiology & behavior. 1996;60:1209–1215. doi: 10.1016/s0031-9384(96)00212-0. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009a;81:1154–1163. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Loucks TL, Berga SL, Rivier J, Wilson ME. Increased ghrelin sensitivity and calorie consumption in subordinate monkeys is affected by short-term astressin B administration. Endocine. 2010 doi: 10.1007/s12020-010-9378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mook D, Felger J, Graves F, Wallen K, Wilson ME. Tamoxifen fails to affect central serotonergic tone but increases indices of anxiety in female rhesus macaques. Psychoneuroendocrinology. 2005;30:273–283. doi: 10.1016/j.psyneuen.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nature neuroscience. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, Pfaff DW. Estrogens and non-reproductive behaviors related to activity and fear. Neuroscience and biobehavioral reviews. 2004;28:55–63. doi: 10.1016/j.neubiorev.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID. Stimuli and consequences of dendritic release of oxytocin within the brain. Biochem Soc Trans. 2007;35:1252–1257. doi: 10.1042/BST0351252. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. Journal of neuroendocrinology. 2000;12:235–243. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- Nomura M, Saito J, Ueta Y, Muglia LJ, Pfaff DW, Ogawa S. Enhanced up-regulation of corticotropin-releasing hormone gene expression in response to restraint stress in the hypothalamic paraventricular nucleus of oxytocin gene-deficient male mice. Journal of neuroendocrinology. 2003;15:1054–1061. doi: 10.1046/j.1365-2826.2003.01095.x. [DOI] [PubMed] [Google Scholar]
- Okada M, Hayashi N, Kometani M, Nakao K, Inukai T. Influences of ovariectomy and continuous replacement of 17beta-estradiol on the tail skin temperature and behavior in the forced swimming test in rats. Japanese journal of pharmacology. 1997;73:93–96. doi: 10.1254/jjp.73.93. [DOI] [PubMed] [Google Scholar]
- Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, Wilson ME. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain Behav Immun. 2009;23:286–293. doi: 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes J, Szeto A, Levine JE, Zaias J, Gonzales JA, Mendez AJ, Llabre MM, Schneiderman N, McCabe PM. Social experience influences hypothalamic oxytocin in the WHHL rabbit. Psychoneuroendocrinology. 2006;31:1062–1075. doi: 10.1016/j.psyneuen.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Almeida OF. Gonadal steroids exert facilitating and "buffering" effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci. 1996;16:7077–7084. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. Journal of neuroendocrinology. 2003;15:787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- Pazol K, Kaplan JR, Abbott D, Appt SE, Wilson ME. Practical measurement of total and bioavailable estradiol in female macaques. Clin Chim Acta. 2004;340:117–126. doi: 10.1016/j.cccn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Tomaskovic-Crook E, Montague DM, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. Alteration in estrogen receptor alpha mRNA levels in frontal cortex and hippocampus of patients with major mental illness. Biological psychiatry. 2005;58:812–824. doi: 10.1016/j.biopsych.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biological psychiatry. 2004;56:844–852. doi: 10.1016/j.biopsych.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Pfaff DW, Vasudevan N, Kia HK, Zhu YS, Chan J, Garey J, Morgan M, Ogawa S. Estrogens, brain and behavior: studies in fundamental neurobiology and observations related to women's health. Journal of Steroid Biochemistry & Molecular Biology. 2000;74:365–373. doi: 10.1016/s0960-0760(00)00114-x. [DOI] [PubMed] [Google Scholar]
- Pierce BN, Hemsworth PH, Rivalland ET, Wagenmaker ER, Morrissey AD, Papargiris MM, Clarke IJ, Karsch FJ, Turner AI, Tilbrook AJ. Psychosocial stress suppresses attractivity, proceptivity and pulsatile LH secretion in the ewe. Hormones and behavior. 2008;54:424–434. doi: 10.1016/j.yhbeh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Pope NS, Wilson ME, Gordon TP. The effect of season on the induction of sexual behavior by estradiol in female rhesus monkeys. Biology of reproduction. 1987;36:1047–1054. doi: 10.1095/biolreprod36.4.1047. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21:2833–2841. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist C, Mazzanti C, Vu R, Tran D, Goldman D. Serotonin transporter promoter polymorphism is associated with attenuated prolactin response to fenfluramine. American journal of medical genetics. 2001;105:363–368. doi: 10.1002/ajmg.1360. [DOI] [PubMed] [Google Scholar]
- Reist C, Nakamura K, Sagart E, Sokolski KN, Fujimoto KA. Impulsive aggressive behavior: open-label treatment with citalopram. The Journal of clinical psychiatry. 2003;64:81–85. [PubMed] [Google Scholar]
- Ribeiro AC, Pfaff DW, Devidze N. Estradiol modulates behavioral arousal and induces changes in gene expression profiles in brain regions involved in the control of vigilance. Eur J Neurosci. 2009;29:795–801. doi: 10.1111/j.1460-9568.2009.06620.x. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Hormones & Behavior. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Fleischer R, Schaeffer JM, Rohrer SP, Hickey GJ. 17beta-Estradiol-induced antidepressant-like effect in the Forced Swim Test is absent in estrogen receptor-beta knockout (BERKO) mice. Psychopharmacology. 2005 doi: 10.1007/s00213-004-2078-1. [DOI] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Frontiers in neuroendocrinology. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman W, Hogan BK, Horman BM, Abbott DH. Social suppression of cortisol in female marmosets: role of luteinizing hormone/chorionic gonadotropin. General and comparative endocrinology. 2006;149:90–99. doi: 10.1016/j.ygcen.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science (New York, N.Y. 2005;308:648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Seymour PA, Schmidt AW, Schulz DW. The pharmacology of CP-154,526, a nonpeptide antagonist of the CRH1 receptor: a review. CNS drug reviews. 2003;9:57–96. doi: 10.1111/j.1527-3458.2003.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, Henderson JA, Smith MA, Buchheimer N. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Archives of general psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Manuck SB, Kaplan JR, Koritnik DR. Oral contraceptive administration, interfemale relationships, and sexual behavior in Macaca fascicularis. Archives of sexual behavior. 1990;19:101–117. doi: 10.1007/BF01542226. [DOI] [PubMed] [Google Scholar]
- Shively CA, Wood CE, Register TC, Willard SL, Lees CJ, Chen H, Sitruk-Ware RL, Tsong Y-Y, Cline JM. Hormone therapy effects on social behavior and activity levels of surgically postmenopausal cynomolgus monkeys. Psychoneuroendocrinology. 2007;32:981–990. doi: 10.1016/j.psyneuen.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Norman RL. Influence of the gonads on cortisol secretion in female rhesus macaques. Endocrinology. 1987;121:2192–2198. doi: 10.1210/endo-121-6-2192. [DOI] [PubMed] [Google Scholar]
- Stavisky RC, Watson SL, Anthony MS, Manuck SB, Adams MR, Kaplan JR. Influence of estradiol on cortisol secretion in ovariectomized cynomolgus macaques (Macaca fascicularis) American journal of primatology. 2003;60:17–22. doi: 10.1002/ajp.10076. [DOI] [PubMed] [Google Scholar]
- Summers CH, Kampshoff JL, Ronan PJ, Lowry CA, Prestbo AA, Korzan WJ, Renner KJ. Monoaminergic activity in subregions of raphe nuclei elicited by prior stress and the neuropeptide corticotropin-releasing factor. Journal of neuroendocrinology. 2003;15:1122–1133. doi: 10.1111/j.1365-2826.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- Summers CH, Korzan WJ, Lukkes JL, Watt MJ, Forster GL, Overli O, Hoglund E, Larson ET, Ronan PJ, Matter JM, Summers TR, Renner KJ, Greenberg N. Does serotonin influence aggression? comparing regional activity before and during social interaction. Physiol Biochem Zool. 2005;78:679–694. doi: 10.1086/432139. [DOI] [PubMed] [Google Scholar]
- Thomas E, Pernar L, Lucki I, Valentino RJ. Corticotropin-releasing factor in the dorsal raphe nucleus regulates activity of lateral septal neurons. Brain research. 2003;960:201–208. doi: 10.1016/s0006-8993(02)03882-9. [DOI] [PubMed] [Google Scholar]
- Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress (Amsterdam, Netherlands) 2002;5:47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- Umriukhin AE, Wigger A, Singewald N, Landgraf R. Hypothalamic and hippocampal release of serotonin in rats bred for hyper- or hypo-anxiety. Stress (Amsterdam, Netherlands) 2002;5:299–305. doi: 10.1080/1025389021000061200. [DOI] [PubMed] [Google Scholar]
- Uphouse L, Selvamani A, Lincoln C, Morales L, Comeaux D. Mild restraint reduces the time hormonally primed rats spend with sexually active males. Behavioural brain research. 2005;157:343–350. doi: 10.1016/j.bbr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacology, biochemistry, and behavior. 2004;78:523–529. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- Wallen K. Desire and ability: hormones and the regulation of female sexual behavior. Neuroscience and biobehavioral reviews. 1990;14:233–241. doi: 10.1016/s0149-7634(05)80223-4. [DOI] [PubMed] [Google Scholar]
- Wallen K, Tannenbaum PL. Hormonal modulation of sexual behavior and affiliation in rhesus monkeys. Annals of the New York Academy of Sciences. 1997;807:185–202. doi: 10.1111/j.1749-6632.1997.tb51920.x. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Cleveland A, Trenkle MK, Lussier ID, Higley JD. CSF 5-HIAA concentration as an early screening tool for predicting significant life history outcomes in female specific-pathogen-free (SPF) rhesus macaques (Macaca mulatta) maintained in captive breeding groups. J Med Primatol. 2003;32:95–104. doi: 10.1034/j.1600-0684.2003.00013.x. [DOI] [PubMed] [Google Scholar]
- Westergaard GC, Suomi SJ, Higley JD, Mehlman PT. CSF 5-HIAA and aggression in female macaque monkeys: species and interindividual differences. Psychopharmacology. 1999;146:440–446. doi: 10.1007/pl00005489. [DOI] [PubMed] [Google Scholar]
- White S, Uphouse L. Estrogen and progesterone dose-dependently reduce disruptive effects of restraint on lordosis behavior. Hormones and behavior. 2004;45:201–208. doi: 10.1016/j.yhbeh.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Williams RB, Barefoot JC, Schneiderman N. Psychosocial risk factors for cardiovascular disease: more than one culprit at work. Jama. 2003;290:2190–2192. doi: 10.1001/jama.290.16.2190. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiology & Behavior. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Legendre A, Pazol K, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic-hypothalamic- pituitary-adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26:89–97. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- Windle RJ, Shanks N, Lightman SL, Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138:2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M, Parkison V, Shastry S. Effects of estrogen antagonists and agonists on the ACTH response to restraint stress in female rats. Neuropsychopharmacology. 2001;25:881–891. doi: 10.1016/S0893-133X(01)00301-3. [DOI] [PubMed] [Google Scholar]