Abstract
Hibernators exhibit a robust circannual cycle of body mass gain and loss primarily mediated by food intake, but the pathways controlling food intake in these animals have not been fully elucidated. Ghrelin is an orexigenic hormone that increases feeding in all mammals studied so far, but has not until recently been studied in hibernators. In other mammals, ghrelin stimulates feeding through phosphorylation and activation of AMP-activated protein kinase (AMPK). Activation of AMPK phosphorylates and deactivates acetyl Co-A carboxylase (ACC), a committed step in fatty acid synthesis. In order to determine the effects of exogenous ghrelin on food intake and metabolic factors (i.e. non-esterified fatty acids (NEFAs), and hypothalamic AMPK and ACC) in hibernators, ghrelin was peripherally injected into ground squirrels in all four seasons. Changes in food intake and body mass were recorded over a 2–6 hour period post injections, and squirrels were euthanized. Brains and blood were removed, and Western blots were performed to determine changes in phosphorylation of hypothalamic AMPK and ACC. A colorimetric assay was used to determine changes in concentration of serum NEFAs. We found that food intake, body mass, and locomotor activity significantly increased with ghrelin injections versus saline-injected controls, even in animals injected during their aphagic winter season. Injected ghrelin was correlated with increased phosphorylation of AMPK, but didn’t have an effect on ACC in winter. Ghrelin-injected animals also had increased levels of serum NEFAs compared with saline controls. This study is the first to show an effect of injected ghrelin on a hibernator.
Keywords: ghrelin, hibernator, behavior, food intake
1. Introduction
Hibernation is an energy-saving life history strategy employed by animals in many different genera. Mammals that hibernate (hibernators) undergo multi-day torpor bouts in winter during which food intake ceases and body temperature (Tb) drops to near ambient temperature (Ta). The golden-mantled ground squirrel (GMGS, Callospermophilus lateralis) is a diurnal hibernator with a robust annual cycle of body mass gain and loss primarily due to changes in food intake. During the hyperphagic prehibernation period (late July–September), GMGS double their food intake and increase body mass nearly 50%, mostly in the form of fat (Dark, 2005). During this autumnal period, animals also decrease metabolic rate and activity, which aids in building fat stores (Kenagy et al., 1989). During the winter hibernation period (October–March), GMGS cease food intake, and metabolic processes drop to very low rates. During each torpor bout throughout the winter, animals drop Tb to near Ta (usually around 5°C) for several days at a time, and then rewarm to a normal Tb of 36°C for a few hours (become euthermic) before returning to low Tb. During these euthermic inter-bout arousals (IBAs), animals do not eat and spend most of the time undergoing sleep (Torke and Twente, 1977; Heller and Ruby, 2004). Some male GMGS emerge from hibernation in late February, but females tend to remain torpid through mid-March. During the spring and summer months, GMGS reproduce and are euthermic and normophagic (Dark, 2005). GMGS tend to forage in the morning and evening, but are opportunistic feeders (Kenagy et al., 1989).
Little is known about the physiological controls of food intake in hibernators. Ghrelin is a recently discovered orexigenic gut/brain peptide that has various physiological effects; its effects on food intake and lipogenesis are well documented (Cummings, 2006; Strassburg et al., 2008), but it also appears to have effects on animal behavior that have not been clearly elucidated. Some of the evidence is apparently contradictory. For instance, in a 2006 study, ghrelin injected into lateral cerebral ventricles of rats increased exploratory behavior and spontaneous locomotor activity in rats (Jaszberenyi et al., 2006), contrary to the results of a 2005 study during which ghrelin injected into lateral cerebral ventricle of rats decreased spontaneous locomotor activity while increasing food intake (Castaneda et al., 2005). In the latter study, the increase in food intake was immediate, while the decrease in activity took some time to appear. This suggests that ghrelin first increases food-seeking behavior, but later increases energy saving behavior (Castaneda et al., 2005). Ghrelin injected peripherally in Siberian hamsters (Phodopus sungorus) increased foraging behavior, food hoarding, and food intake, but had no effect on spontaneous locomotor activity (Keen-Rhinehart and Bartness, 2005).
The ghrelin receptor, growth hormone secretogogue receptor 1 (GHSR1), is found in various areas of the central nervous system. The majority of expression is in the hypothalamus, with some expression in the cerebral cortex and the dorsal vagal complex of the medulla oblongata (Kojima et al., 1999; Cowley et al., 2003; Hou et al., 2006). In non-hibernators, one action of ghrelin is to stimulate phosphorylation and activation of AMP-activated protein kinase (AMPK) in the hypothalamus, which in turn phosphorylates and deactivates acetyl Co-A carboxylase (ACC), a committed step in fatty acid synthesis (Andersson et al., 2004, Kola et al., 2005, Kohno et al., 2008). There is some evidence that modification of these enzymes is required for the orexigenic effects of ghrelin (Kohno et al., 2008, Lage et al., 2010).
In addition to increasing food-seeking behavior, ghrelin has been shown to alter other behaviors. Ghrelin injected intraperitoneally (IP) and intracerebroventricularly increased food intake and anxiogenic behavior in rats (Asakawa et al., 2001; Carlini et al., 2002). Similarly, a study by Kodomari et al (2009) found that offspring of ghrelin-treated female mice exhibited increased stress behavior (measured as increased movement from the center of an open field).
We have recently measured ghrelin levels in the serum of hibernating and winter euthermic GMGS, and found that ghrelin was still circulating in the blood even at low tissue temperature (Healy et al., 2010). In order to determine the effects of ghrelin on hibernators, we hypothesized that peripherally injected ghrelin would cause an increase in food intake and activity in GMGS during the three seasons during which they are euthermic and eating, but that ghrelin injected into aphagic GMGS in the winter season would have no effect. As ghrelin’s orexigenic effect may be dependent on the modification of AMPK and ACC, we expected that hypothalamic concentrations of these peptides would be low in the control animals, but would increase in reaction to injected ghrelin. We also hypothesized that since injected ghrelin should increase positive energy balance within the animal by stimulating food intake, animals injected with ghrelin would have an increased level of serum non-esterified fatty acids (NEFAs) when compared with saline-injected controls.
2. Methods and Materials
2.1. Animals
Adult GMGS of both sexes were trapped in Larimer County in the spring and early summers of 2008–2009 and kept in an animal facility at Colorado State University under an approved IACUC protocol. Animals were provided with cotton for nesting material, ad libitum food (Harlan Teklad 8640; Madison, WI, USA) and water, and maintained in a warm room (20°C) under natural photoperiod (Paragon Sun Tracker EC72ST; Invensys Controls, Carol Stream, IL, USA) until the beginning of November, when the temperature was reduced to 5°C and animals were kept in constant darkness (to mimic natural burrow conditions) for the remainder of the hibernation season.
2.2. Ghrelin injection experiment
Animals were randomly assigned to two groups, one group to be injected IP with ghrelin (n=18), and the other with saline (control, n=18). These two groups were then broken down into four smaller groups each: Summer (animals had been normophagic and consistently euthermic for 2 months, n=4/group), Autumn (animals were hyperphagic, at twice their normal food intake, n=4/group), Winter (animals had been aphagic and heterothermic for 2 months, n=7/group), and Spring (animals had been euthermic and normophagic for less than 1 month, n=3/group). All groups contained both male and female GMGS. Mouse/rat ghrelin (Bachem Corporation, H-4862) was dissolved in 1 ml sterile saline and used immediately. On one day of each season, 1-ml syringes with 25 gauge needles were prepared with either 50μl saline or 50μl ghrelin solution (at a dose of 50μg/kg, which is within the range used to elicit an orexigenic response in most rodents (Chen et al., 2004; Keen-Rhinehart and Bartness, 2005)). GMGS were weighed and were injected IP with saline or ghrelin solution. Cumulative food intake (in grams) was measured for each animal at 2 and 6 hours after injections (each animal was injected at 1000 hrs. and food intake was measured at 1200 and 1600 hrs.). All animals had food and water available ad libitum. Behavior was remotely monitored via video camera for 6 hours following the injections; at the end of this period, food intake was measured and animals were weighed again.
2.3. Hypothalamic dissection
Animals in the summer group were treated as described above, with the exception that behavior, food intake, and body mass were monitored for only two hours. Two hours after injections, animals were anesthetized with an intramuscular injection of ketamine-acepromazine-xylazine (75%-20%-5%). Blood samples were collected by cardiac puncture. Samples were allowed to coagulate before being centrifuged, and serum was removed and stored at −80°C until analysis. Animals were euthanized by decapitation; brains were removed, flash-frozen in 2-methylbutanol and stored at −80°C until use. Animals in the winter group were aroused from torpor to euthermia at 0700 hrs. and allowed to regain normal euthermic function for three hours, at which point saline or ghrelin was injected as described above. Two hours after injection, animals were euthanized as described above. Hypothalami from all brains were dissected out (using stereotaxic ground squirrel brain atlas by Joseph et al. (1966)) and homogenized in 0.5ml lysis buffer with protease inhibitor cocktail, centrifuged, and the supernatant removed. Homogenates were frozen at −80°C until assayed.
2.4. Neuropeptide analysis
Protein concentration was determined by BCA assay and Western Blots were performed on hypothalamus homogenate. Briefly, sample proteins were separated by SDS-PAGE and transferred to nitrocellulose. Equal amounts of protein were added to each gel, as confirmed by β-actin. The membranes were blocked in TBS with 5% milk powder and incubated overnight on an orbital shaker at 4°C in primary antibody (diluted 1:1000 for phosphorylated AMPK (pAMPK), total AMPK, phosphorylated ACC (pACC), total ACC, and β-actin). Antibodies were obtained from Cell Signaling (Phospho-AMPKα (Thr172) Rabbit mAb #2531, AMPKα (23A3) Rabbit mAb #2603, Phospho-Acetyl-CoA Carboxylase (Ser79) Antibody #3661, Acetyl-CoA Carboxylase (C83B10) Rabbit mAb # 3676, β-actin Antibody # 4967). After washing in TBST, membranes were incubated at room temperature for 1 hr in HRP-conjugated secondary antibody (1:2000) and HRP-conjugated anti-biotin antibody (1:1000). After further washing in TBST, membranes were developed by chemiluminescence (Amersham ECL Plus from GE Healthcare), and imaged on a STORM imager (GE Healthcare). Protein expression was quantified using ImageQuant (GE Healthcare) and normalized against β-actin.
2.5. Serum metabolites and hormones
Serum NEFA concentrations were determined by a commercially available colorimetric assay from Wako Chemicals (HR Series NEFA-HR (2)), using a BioTek Synergy HT microplate reader. Serum ghrelin was measured with an enzyme immunoassay from Phoenix Pharmaceuticals (EK031-31) as previously described (Healy et al., 2010).
2.6. Activity analysis
A small video camera (Sony Handycam DCR-HC20) was set approximately 6 feet from animal cages to stream directly into a laptop computer capturing the 6 hours after injections. After animal sacrifices, videos were analyzed for behavioral activity for each squirrel (only the initial two hours of post-injection behavior are reported here). Behaviors were categorized as inactive (animal in nest, not moving), non-specific activity (animal visibly moving around either in nest or outside of nest), nesting (animal moving cotton around cage or fluffing cotton in nest), feeding (animal observed putting food in mouth or keeping head in food bowl >5 seconds), grooming (animal observed licking, scratching, etc. either in or outside of nest), or drinking (animal’s mouth on water sipper or head in water bowl). Behavior in all videos was analyzed by one person to minimize variation. This individual was familiar with the behavioral ethogram but blind to animals’ experimental condition.
2.7. Statistics
Statistics were performed using Graph Pad Prism 5. Differences in food intake, body mass, NEFAs, and neuropeptide levels between control and experimental groups were determined by Student’s t-test. Differences between food intake and body mass between seasons were tested using a 1-way ANOVA with Student-Newman-Kuels post-test. Behavioral differences between seasons and experimental groups were tested using a 2-way ANOVA with Bonferroni post-test. All differences were considered significant at p≤0.05.
3. Results
3.1. Food intake
Animals injected with ghrelin significantly increased food intake compared with saline controls during all four seasons (Fig. 1). In spring, GMGS ate an average of 1.5g over a two hour period when injected with saline, but 4g per 2 hours when injected with ghrelin. In summer, saline-injected animals averaged 1.25g over a 2 hour period, while ghrelin-injected animals ate 6.5g. In autumn, animals injected with saline ate about 2g over 2 hours, while ghrelin-injected animals averaged 4.5g over the same time period. In winter, when hibernators are normally aphagic, saline-injected animals did not eat, but five of seven ghrelin-injected animals initiated food intake, eating a mean of 0.85g over a 2 hour period (Fig. 1).
Fig. 1.
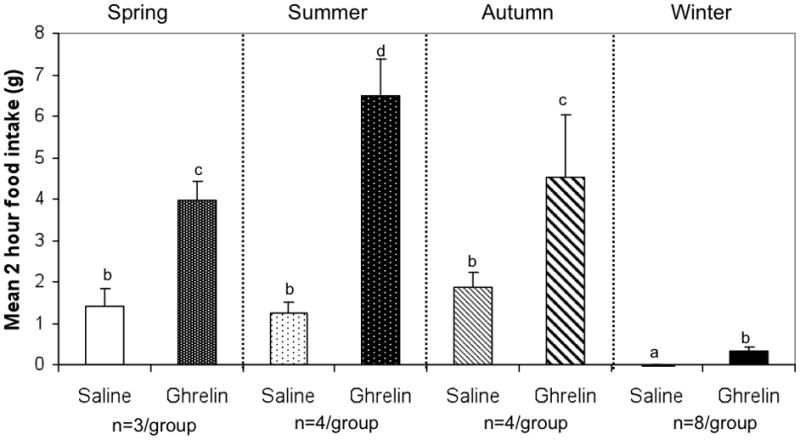
Ghrelin injected IP significantly increased mean cumulative food intake compared with saline injected controls 2 hrs after injection in spring, summer, autumn, and winter. Bars with different letters are statistically different (ANOVA with Student-Newman-Kuels post-test, p≤0.05).
3.2. Body mass
Animals injected with ghrelin either gained more mass or lost less mass than animals injected with saline in all seasons (Table 1). These differences were statistically significant in summer and autumn, but not different in spring and winter.
Table 1.
Changes in body mass 2 hours after injections of saline or ghrelin
| Treatment | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Saline | 1.11 ± 0.23 | −2.96 ± 0.34 | 0.50 ± 0.59 | 0 ± 0 |
| Ghrelin | 2.52 ± 0.92 | −0.89 ± 0.47* | 3.01 ± 1.17* | 0.33 ± 0.18 |
| P value | 0.07 | 0.006 | 0.05 | 0.06 |
Mean change in body mass per group ± SEM 2 hours post injection. Positive numbers indicate mass gain, and negative numbers indicate mass loss.
Differences between treatments by season are considered significant at p ≤ 0.05.
3.3. Neuropeptides
Western blots were performed to determine expression of total and phosphorylated forms of AMPK and ACC. In summer, ghrelin-injected animals had significantly increased levels of both total and phosphorylated AMPK and ACC when compared with saline controls (Fig. 2). Winter animals injected with ghrelin significantly increased the phosphorylated (activated) form of AMPK compared with saline controls (Fig. 3). There was no effect of ghrelin on total AMPK or phosphorylated or total ACC in winter animals.
Fig. 2.
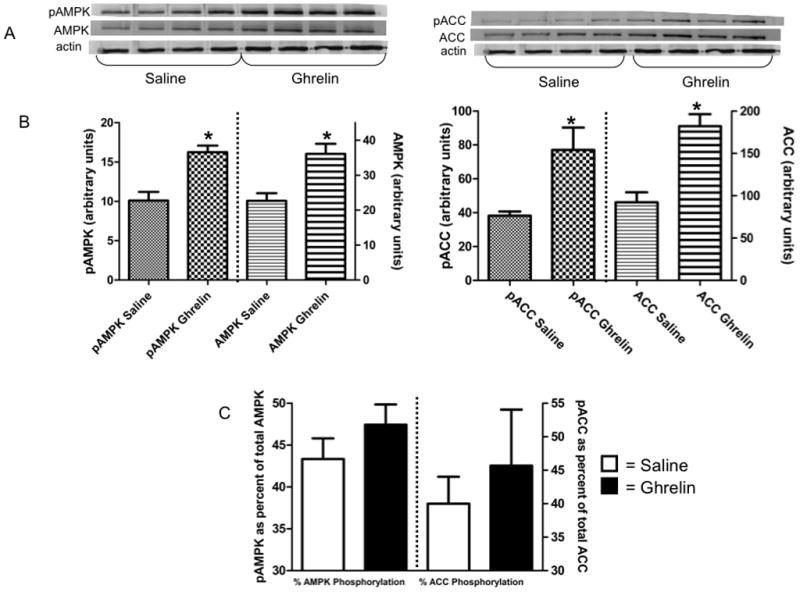
In total hypothalamus homogenate from normophagic summer animals, animals injected IP with ghrelin have statistically higher (p≤0.05) levels of total and phosphorylated AMPK and ACC compared with saline injected controls. (A) Representative Western blots of saline-injected (n=4) and ghrelin-injected (n=4) animals. Blots were probed for pAMPK and pACC, then stripped and reprobed for total AMPK and ACC with β-actin for a loading control. (B) Levels (normalized against actin) of phosphorylated vs. total AMPK and phosphorylated vs. total ACC. (C) Ratio of phosphorylated to total AMPK and ACC in hypothalamus after saline or ghrelin injection. * indicates significance between saline and ghrelin-injected animals at p≤0.05 (Student t-test).
Fig. 3.
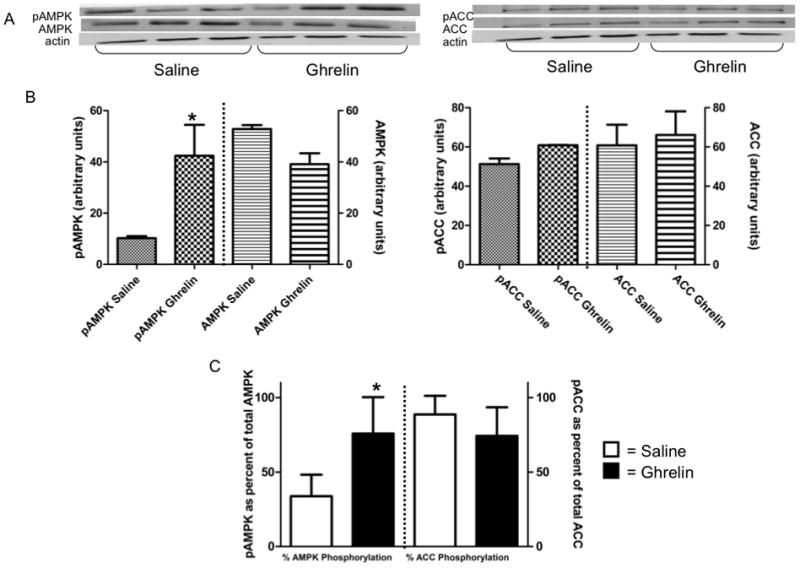
In total hypothalamus homogenate from aphagic winter animals, animals injected IP with ghrelin have statistically higher (p≤0.05) levels of phosphorylated AMPK compared with saline injected controls, but there were no differences in total AMPK or phosphorylated and total ACC. (A) Representative Western blots of saline-injected (n=3) and ghrelin-injected (n=3) animals. Blots were probed for pAMPK and pACC, then stripped and reprobed for total AMPK and ACC with β-actin for a loading control. (B) Levels (normalized against actin) of phosphorylated vs. total AMPK and phosphorylated vs. total ACC. (C) Proportion of phosphorylated to total AMPK and ACC in hypothalamus after saline or ghrelin injection. * indicates significance between saline and ghrelin-injected animals at p≤0.05 (Student t-test).
3.4. Serum ghrelin and NEFAs
Animals injected with ghrelin had increased serum ghrelin concentrations when compared with saline control, both in summer and winter (Fig. 4).
Fig. 4.
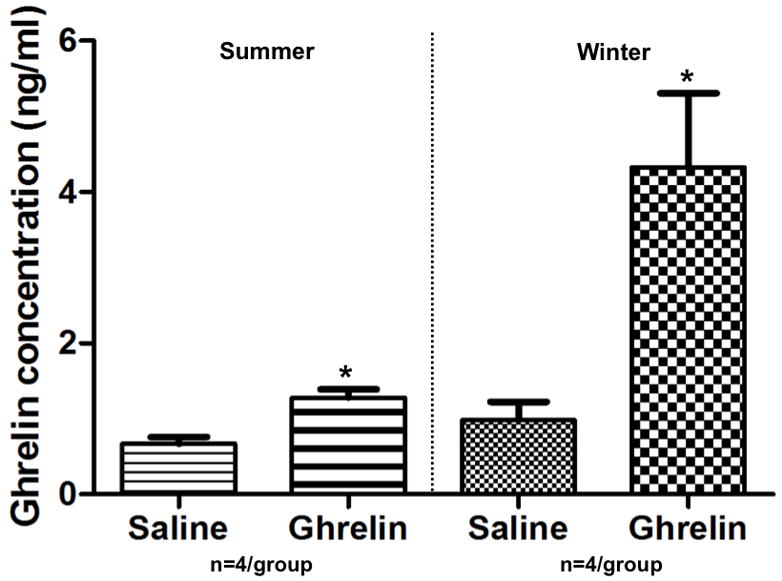
Animals injected IP with ghrelin had statistically higher serum ghrelin concentrations than saline-injected animals in summer and winter. * indicates significance between saline and ghrelin-injected animals at p≤0.05.
Ghrelin-injected winter animals also showed higher NEFA concentrations than saline-injected controls (Fig. 5).
Fig. 5.
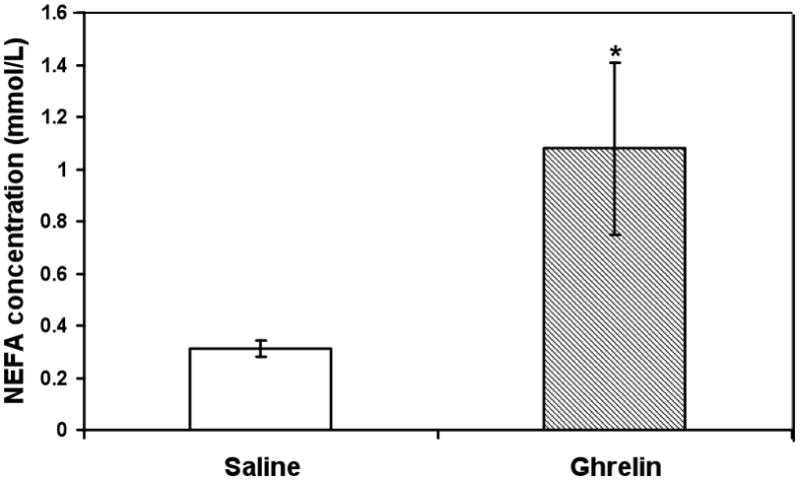
Animals injected IP with ghrelin had statistically higher serum NEFA concentrations than saline-injected animals in winter. * indicates significance between saline and ghrelin-injected animals at p≤0.05 (Student t-test).
3.5. Behavior
Videos recording the two hours after injections were analyzed to determine what effects peripherally injected ghrelin would have on behavior. In general, animals injected with ghrelin were more active than those injected with saline, especially in feeding and nesting behaviors, but these differences were not always statistically significant (Table 2).
Table 2.
Behavioral changes by season after injection with saline or ghrelin
| Treatment | Inactivity | N.S. Activity | Feeding | Grooming | Nesting | Drinking |
|---|---|---|---|---|---|---|
| Saline | ||||||
| Summer (n=4) | 6971 ± 6971 (92%) a | 65.75 ± 65.75 (0.9%) f | 37.5 ± 37.5 (0.5%) i | 118 ± 118 (1.6%) k | 7.5 ± 7.5 (0.1%) m | 0 ± 0 (0%) o |
| Autumn (n=3) | 5281.3 ± 615.8 (73%) b | 1595.7 ± 679.3 (22%) f | 31.3 ± 31.3 (0.4%) i | 15.7 ± 11.1 (0.2%) k | 249 ± 78 (3.5%) m | 27 ± 20.8 (0.4%) o |
| Winter (n=3) | 7081 ± 119 (98.4%) a | 0 ± 0 (0%) f | 0 ± 0 (0%) i | 0 ± 0 (0%) k | 119 ± 119 (1.6%) m | 0 ± 0 (0%) o |
| Spring (n=4) | 2955 ± 974 (41%) c | 3625 ± 833 (50.3%) g | 34 ± 12.5 (0.5%) i | 416 ± 187 (5.8%) k | 142.75 ± 49.7 (2%) m | 27 ± 16.3 (0.4%) o |
| Ghrelin | ||||||
| Summer (n=4) | 4818 ± 4818 (66.9%) de | 1726 ± 1726 (24%) h | 153.5 ± 153.5 (21%) j | 386.25 ± 386.25 (5.4%) l | 89 ± 89 (1.2%) n | 26.5 ± 26.5 (0.4%) p |
| Autumn (n=3) | 4498 ± 1064.5 (62.5%) de | 1292 ± 598 (17.9%) h | 228 ± 164.1 (3.2%) j | 327.7 ± 316.2 (4.5%) l | 845.3 ± 249.1* (11.7%) n | 9 ± 5.2 (0.1%) p |
| Winter (n=3) | 6325.7 ± 94* (87.9%) d | 161 ± 161 (2.2%) h | 14.7 ± 7.3* (0.2%) j | 0 ± 0 (0%) l | 698.7 ± 179* (9.7%) n | 0 ± 0 (0%) p |
| Spring (n=4) | 2613.25 ± 890 (36.3%) e | 3593.5 ± 748* (49.9%) h | 230.5 ± 88.3* (3.2%) j | 413.25 ± 150 (5.7%) l | 301.5 ± 109.2 (4.2%) n | 48 ± 19.8 (0.7%) p |
N.S. Activity = non-specific activity. Mean time (in seconds) per group ± SEM (percent of total time in parentheses calculated out of 7200 seconds (2 hours) post injection).
ghrelin different from saline (same season, same activity). Numbers with differing letters (eg. a,b,c) indicate significant difference between seasons within an activity and treatment type. All differences are considered significant at p ≤ 0.05
In summer, animals injected with ghrelin showed no significant differences in behavior from those injected with saline. In autumn, ghrelin-injected animals spent significantly more time nesting than saline controls. In winter, ghrelin-injected animals spent significantly less time inactive, and more time feeding and nesting than did the controls. In spring, ghrelin-injected animals spent significantly more time than controls in non-specific activities (generally moving around the cage) and feeding.
By season, in the two hours after injection, control animals were most active in spring, less active in autumn, and least active in summer and winter (Table 2). Ghrelin injected animals were most active in spring and least active in winter, but total time active did not differ between summer and autumn. Animals generally spent more time in non-specific activity in spring than in other seasons.
Ghrelin also appeared to alter the percentage of active time that animals were engaged in specific behaviors (Fig. 6). In spring, summer, and autumn, animals spent the majority of their active time engaged in non-specific behavior (group means ranged from 29–85%), with considerable amounts of time spent grooming (1–52%) and nesting (3–31%). Time spent feeding was between 1% and 16% of total time active. In winter, the majority of time active was spent nesting (100% for saline injected animals and 80% for ghrelin-injected animals), with 18% of time spent in random activity and 2% spent feeding in the ghrelin-injected animals (Fig. 6). In all groups, time spent drinking was minimal and did not differ by treatment or season, and as such was excluded from Fig. 6.
Fig. 6.
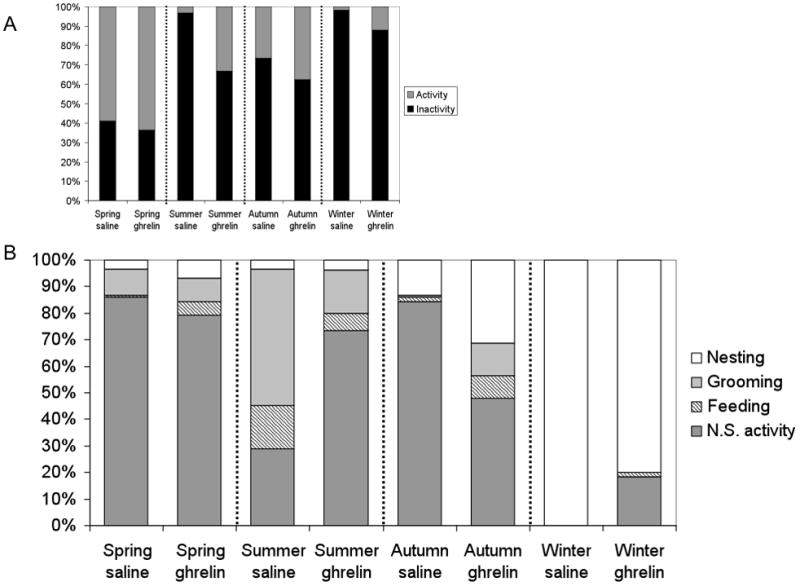
Ghrelin injection increased mean total activity compared with saline-injected controls in spring, summer, autumn, and winter. (A) Percent of 2 hrs after saline or ghrelin injections spent active (grey bars) or inactive (black bars) by season. (B) Percent of time spent performing specific behaviors (e.g. non-specific activity, feeding, grooming, and nesting) during total time active for each season after saline or ghrelin injections by season (=time (seconds) spent in specific behavior/total time active (seconds)*100). Drinking was omitted due to extremely small percentages.
4. Discussion
The orexigenic effect of ghrelin in most mammals is well documented, but this study is the first to show an effect of ghrelin on winter-acclimated golden-mantled ground squirrels. We injected ghrelin peripherally into squirrels during all four seasons and measured changes in food intake, body mass, and activity levels for 6 hours post-injection. We observed an increase in food intake in all squirrels injected with ghrelin—animals injected with ghrelin ate 2–4 times as much as the saline controls, even during winter when animals are normally aphagic. Animals aroused to euthermia were induced to eat by injection of ghrelin, while animals injected with saline remained aphagic, and indeed attempted to return to torpor within two hours of being aroused. A similar food intake response was recently elicited in another hibernating sciurid (Marmota flaviventris), which showed that central infusion of the AMPK agonist 5-aminoimidazole-4- carboxamide 1 B-D-ribofuranoside (AICAR) in winter aphagic marmots caused an initiation of food intake and cessation of torpor bouts while saline-infused animals remained aphagic and reentered torpor even when held at high Ta (Florant et al., 2010).
Both male and female squirrels were included in each group of ghrelin or saline injected animals. There was no clear sex difference in response to ghrelin injection in any of our experiments, possibly due to our small sample size. Further experimentation is needed to clarify whether sex differences affect response to ghrelin during different seasons.
Animals injected with ghrelin generally had higher body masses than saline-injected controls two hours after injection, probably due to the increase in food intake. These increases were not always significant, most likely an artifact of the small sample size. Body mass in GMGS tends to fluctuate widely throughout the day (by 2–10 grams, Florant lab unpub. data) based on animals’ activity levels and timing of food intake, especially in summer when ground squirrels maintain a consistently high metabolic rate (Armitage and Shulenberger, 1972). Both saline-injected and ghrelin-injected groups of summer animals lost body mass over the two hour post-injection period, but the mass loss was attenuated by ghrelin-induced food intake.
Ghrelin-injected animals had higher serum ghrelin concentrations than those injected with saline in both winter and summer, but the ghrelin injections led to a proportionally greater increase in serum ghrelin in the winter than in summer. However, winter ghrelin-injected animals ate far less than summer ghrelin-injected animals, so it is possible that the lower metabolic and circulation rates of the winter squirrels delayed the absorption and distribution of the injected ghrelin bolus, leading to higher circulating levels when animals were sacrificed two hours after injection. In other mammals, ghrelin stimulates food intake by binding to the GHSR1 receptor (in the hypothalamus or on afferent neurons of the vagus nerve) and stimulating the release of NPY and AgRP from neurons in the hypothalamus (Kamegai et al., 2001; Kojima & Kangawa, 2008; Seoane et al., 2003). The majority of ghrelin receptors are found in the ARC of the hypothalamus, but ghrelin-containing neurons are also found in the paraventricular nucleus of the hypothalamus (Kojima & Kangawa, 2008). Usually, circulating ghrelin concentrations are directly correlated with food intake (Cummings, 2006; Keen-Rhinehart and Bartness, 2005), but in our animals, circulating serum ghrelin appears to have a diminished effect on the food intake of aphagic winter-acclimated ground squirrels compared with normophagic animals. This is similar to effects seen recently in another seasonal rodent, the photoperiodic Siberian hamster (Phodopus sungorus), which decreases food intake and undergoes shallow torpor bouts when acclimated to short-day length (SD). SD acclimated hamsters showed decreased sensitivity to ghrelin when compared with long-day (LD) acclimated animals (Bradley et al., 2010).
The increase in food intake exhibited by GMGS after ghrelin injection was accompanied by an increase of the phosphorylated (active) form of AMPK in both summer and winter, as seen in previous research (Kohno et al., 2008). Summer ghrelin-injected animals had increased levels of both total and phosphorylated AMPK when compared with saline-injected controls, suggesting that in summer hibernators, ghrelin injection stimulates AMPK release, but does not increase the rate of phosphorylation. In winter ghrelin-injected animals, we saw an increase in the phosphorylated form of AMPK in the hypothalamus, but there was no difference in total levels of AMPK, which suggests that ghrelin stimulated a larger proportion of total AMPK to be phosphorylated and activated.
In mammals, the phosphorylation of AMPK usually causes the concomitant phosphorylation and deactivation of ACC. Since ACC is a committed step in fatty acid synthesis, its deactivation effectively causes a switch from fatty acid synthesis to fatty acid oxidation. We measured total and phosphorylated levels of ACC in the hypothalami of ghrelin-injected and saline-injected animals. In summer, ghrelin-injected animals had increased levels of both phosphorylated and total ACC, as seen in previous research (Kohno et al., 2008), but we saw no difference in either total or phosphorylated ACC between groups in the winter. Since winter hibernators are typically aphagic and lipolytic, it is possible that ghrelin stimulated the phosphorylation of AMPK to cause an increase in appetite, but since animals had ceased fatty acid synthesis prior to entering hibernation, ACC was already maximally phosphorylated.
We also found a significant increase in circulating serum NEFA concentrations after peripheral ghrelin injection, suggesting that ghrelin increased available substrate for mitochondrial oxidation at the same time as activating AMPK. Since ghrelin’s primary role is to stimulate appetite, and secondarily increase fatty acid synthesis (over the long term), it seems plausible that an increase in circulating ghrelin might initially raise circulating NEFAs concurrently with increasing food intake, but eventually cause a decrease in circulating NEFAs as fatty acid synthesis was stimulated (Theander-Carrillo et al., 2006). It is also possible that due to the acute and transitory nature of the peripheral injection, and the short time after injections in which the animals were sacrificed, that the observed increase in NEFAs after ghrelin injection was due to the breakdown of the food recently consumed (some food was found in the stomachs of ghrelin-injected animals). More experimentation is necessary to clarify these results, but they seem to demonstrate that ghrelin injection increases circulating endogenous energy availability, as seen previously in other rodents (Andrews et al., 2008).
Activity levels of GMGS generally increased with ghrelin injections, especially random activity and feeding/food seeking behaviors. Ghrelin has been shown to increase random locomotor activity levels in rats and mice (Jazberenyi et al., 2006; Jerlhag et al., 2006), in apparent contradiction to its well-known lipogenic effects. This effect on locomotor activity may be due to ghrelin’s amplifying effect on central dopamine (DA) neurons (Jerlhag et al., 2006). However, this increase in locomotor activity appears to be dose dependent—as various behaviors are stimulated by different areas of the brain (e.g. hypothalamus controls ghrelin’s effect on feeding behavior; cerebral cortex controls ghrelin’s effect on memory), these brain regions are also differently sensitive to ghrelin level (see Ferrini et al., 2009 for a review). It is likely that the increase in active behavior resulting from ghrelin injections in our experiment is due to the intensified appetite stimulus usually associated with injection of this peptide.
Like ghrelin, the hormone orexin is involved with regulation of feeding, the sleep-wakefulness cycle, and energy homeostasis (including thermoregulation and locomotor activity) (Toshinai et al., 2003; Verty et al., 2010). It appears that ghrelin’s effects on food intake in most rodents occur through the action of the orexin system (Szentirmai et al., 2007; Toshinai et al., 2003). There is very little known about the presence or action of orexin in hibernators, but it is possible that the effects of ghrelin are mediated through the orexin pathway.
Hibernators represent a unique animal model for the study of human obesity and a ‘natural knock-out’ for research on controls of food intake. These animals are normally completely aphagic during their winter hibernation season, so it is significant that injection of a single peptide induced GMGS to commence food intake. Further experiments are needed to elucidate ghrelin’s effect on hibernators during the aphagic hibernation season and during the summer hyperphagic season. Nonetheless, this initial study indicates that ghrelin does have an effect on hibernators, even during a period of time when they do not normally eat.
Acknowledgments
Thanks to Cassandra Gearhart, Kendra Burdett, Ashley Fenn, Yvonne Diaz, and Thomas Barnett for help with trapping and animal care. We thank Denise Pearson of Fox Acres Country Club for allowing us to trap ground squirrels on their property. This work was supported by a Sigma Xi Grant-in-aid of research to JEH and an NIH grant (DK 067017-3) to GLF.
Footnotes
From a thesis submitted to the Academic Faculty of Colorado State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem. 2004;279:12005–12008. doi: 10.1074/jbc.C300557200. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage KB, Shulenberger E. Evidence for a circannual metabolic cycle in Citellus tridecemlineatus, a hibernator. Comp Biochem Physiol. 1972;42:667–688. doi: 10.1016/0300-9629(72)90445-8. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Fujimiya M, Katsuura G, Makino S, Fujino MA, Kasuga M. A role of ghrelin in neuroendocrine and behavioral responses to stress in mice. Neuroendocrinol. 2001;74:143–147. doi: 10.1159/000054680. [DOI] [PubMed] [Google Scholar]
- Bradley SP, Pattullo LM, Patel PN, Prendergast BJ. Photoperiodic regulation of the orexigenic effects of ghrelin in Siberian hamsters. Horm Behav. 2010;58:647–652. doi: 10.1016/j.yhbeh.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlini VP, Monzon ME, Varas MM, Cragnolini AB, Schiöth HB, Scimonelli TN, de Barioglioa SR. Ghrelin increases anxiety-like behavior and memory retention in rats. Biochem Biophys Res Commun. 2002;299:739–743. doi: 10.1016/s0006-291x(02)02740-7. [DOI] [PubMed] [Google Scholar]
- Castaneda TR, Jurgens H, Wiedmer P, Pfluger P, Diano S, Horvath TL, Tang-Christensen M, Tschop MH. Obesity and the neuroendocrine control of energy homeostasis: the role of spontaneous locomotor activity. J Nutr. 2005;135:1314–1319. doi: 10.1093/jn/135.5.1314. [DOI] [PubMed] [Google Scholar]
- Chen HY, Trumbauer ME, Chen AS, Weingarth DT, Adams JR, Frazier EG, Shen Z, Marsh DJ, Feighner SD, Guan XM, Ye Z, Nargund RP, Smith RG, Van der Ploeg LH, Howard AD, MacNeil DJ, Qian S. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology. 2004;145:2607–2612. doi: 10.1210/en.2003-1596. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smith RG, Diano S, Tschop M, Pronchuck N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML, Garcia-Segura LM, Nillni EA, Mendez P, Low MJ, Sotonyi P, Friedman JM, Liu H, Pinto S, Colmers WF, Cone RD, Horvath TL. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Dark J. Annual lipid cycles in hibernators: integration of physiology and behavior. Annu Rev Nutr. 2005;25:469–497. doi: 10.1146/annurev.nutr.25.050304.092514. [DOI] [PubMed] [Google Scholar]
- Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Current Neuropharmacology. 2009;7:37–49. doi: 10.2174/157015909787602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florant GL, Fenn AM, Healy JE, Wilkerson GK, Handa RJ. To eat or not to eat: the effect of AICAR on food intake regulation in yellow-bellied marmots (Marmota flaviventris) J Exp Biol. 2010;213:2031–2037. doi: 10.1242/jeb.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy JE, Ostrom CE, Wilkerson GK, Florant GL. Serum ghrelin concentrations change with physiological state in a sciurid hibernator (Spermophilus lateralis) Gen Comp Endocrinol. 2010;166:372–378. doi: 10.1016/j.ygcen.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller HC, Ruby NF. Sleep and circadian rhythms in mammalian torpor. Annu Rev Physiol. 2004;66:275–289. doi: 10.1146/annurev.physiol.66.032102.115313. [DOI] [PubMed] [Google Scholar]
- Hou Z, Miao Y, Gao L, Pan H, Zhu S. Ghrelin-containing neuron in cerebral cortex and hypothalamus linked with the DVC of brainstem in rat. Regul Pept. 2006;134:126–131. doi: 10.1016/j.regpep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Jászberényi M, Bujdosó E, Bagosi Z, Telegdy G. Mediation of the behavioral, endocrine and thermoregulatory actions of ghrelin. Horm Behav. 2006;50:266–273. doi: 10.1016/j.yhbeh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- Joseph SA, Knigge KA, Kalejs LM, Hoffman RA, Reid P. A stereotaxic atlas of the brain of the 13-line ground squirrel (Citellus tridecemlineatus) Edgewood, MD: Edgewood Arsenal Special Publications; 1966. [Google Scholar]
- Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes. 2001;50:2438–2443. doi: 10.2337/diabetes.50.11.2438. [DOI] [PubMed] [Google Scholar]
- Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging, and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- Kenagy GJ, Sharbaugh SM, Nagy KA. Annual cycle of energy and time expenditure in a golden-mantled ground squirrel population. Oecologia. 1989;78:269–282. doi: 10.1007/BF00377166. [DOI] [PubMed] [Google Scholar]
- Kodomari I, Maruoka T, Yamauchi R, Wada E, Wada K. Ghrelin alters postnatal endocrine secretion and behavior in mouse offspring. Neurochem Int. 2009;54:222–228. doi: 10.1016/j.neuint.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Kohno D, Sone H, Minokoshi Y, Yada T. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun. 2008;366:388–392. doi: 10.1016/j.bbrc.2007.11.166. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Structure and function of ghrelin. Results Probl Cell Differ. 2008;46:89–115. doi: 10.1007/400_2007_049. [DOI] [PubMed] [Google Scholar]
- Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- Lage R, Vazquez MJ, Varela L, Saha AK, Vidal-Puig A, Nogueiras R, Dieguez C, Lopez M. Ghrelin effects on neuropeptides in the rat hypothalamus depend on fatty acid metabolism actions on BSX but not on gender. FASEB J. 2010;24:2670–2679. doi: 10.1096/fj.09-150672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane LM, Lopez M, Tovar S, Casanueva F, Senaris R, Dieguez C. Agouti- related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinol. 2003;144:544–551. doi: 10.1210/en.2002-220795. [DOI] [PubMed] [Google Scholar]
- Strassburg S, Anker S, Castaneda T, Burget L, Perez-Tilve D, Pfluger PT, Nogueiras R, Halem H, Dong JZ, Culler M, Datta R, Tschop MH. Long-term effects of ghrelin and ghrelin receptor agonists on energy balance in rats. Am J Physiol Endocrinol Metab. 2008;295:E78–E84. doi: 10.1152/ajpendo.00040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentirmai E, Kapas L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R575–R585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schurmann A, Szanto I, Tschop MH, Rohner- Jeanrenaud F. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest. 2006;116:1983–1993. doi: 10.1172/JCI25811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torke KG, Twente JW. Behavior of Spermophilus lateralis between periods of hibernation. J Mamm. 1977;58:385–390. [PubMed] [Google Scholar]
- Toshinai K, Date Y, Murakami N, Shimada M, Mondal MS, Shimbara T, Guan JL, Wang QP, Funahashi H, Sakurai T, Shioda S, Matsukura S, Kangawa K, Nakazato M. Ghrelin-induced food intake is mediated via the orexin pathway. Endocrinol. 2003;144:1506–151. doi: 10.1210/en.2002-220788. [DOI] [PubMed] [Google Scholar]
- Verty ANA, Allen AM, Oldfield BJ. The endogenous actions of hypothalamic peptides on brown adipose tissue thermogenesis in the rat. Endocrinol. 2010;151:4236–4246. doi: 10.1210/en.2009-1235. [DOI] [PubMed] [Google Scholar]


