Abstract
Voluntary oral ethanol consumption in rodents is generally limited by strong taste-aversion in these species. Historically, this has been overcome by combining ethanol with a sweetener, typically sucrose or saccharine, and then slowly ‘fading’ away the sweetener. While useful in most instances, this approach has not proven as successful for some inbred strains of mice (e.g. DBA/2J) despite consistent evidence in the literature that these same strains express strong conditioned place preference for intraperitoneal- or intragastric-administered ethanol. Importantly, DBA/2J mice express a polymorphism in a ‘sweet’ taste receptor subunit gene that reduces the potency of sweet substances in these mice. We hypothesized that the presence of this polymorphism might help explain the contrasting behavioral findings of weak voluntary oral ethanol consumption following sucrose-fade yet robust conditioned place-preference for ethanol in this strain. To test this, we compared ethanol consumption initiated by either a ‘traditional’ sucrose-fade or a fade from an alternative tastant, monosodium glutamate (MSG). We found that in both C57BL/6J and DBA/2J mice the MSG-fade produced robust increases in home-cage ethanol consumption relative to the traditional sucrose-fade. This increased ethanol intake following MSG-fade was evident across a range of ethanol concentrations. Our findings suggest the potential utility of the MSG-fade to establish stable voluntary oral ethanol consumption in mice, particularly ethanol ‘non-preferring’ strains like DBA/2J, and lend additional support to the notion that ethanol consumption in DBA/2J mice is limited by pronounced taste aversion.
Keywords: drinking in the dark, two-bottle choice, umami, ethanol self-administration
Introduction
A central question in ethanol use/abuse is defining the central mechanisms that control the motivation to drink. Both taste and the subsequent post-ingestion pharmacological effects of the consumed ethanol are believed to be substantial contributors to the motivation to drink in non-dependent animals. C57BL/6J (B6) mice will voluntarily consume >10g/kg of 10% ethanol during 24hr home cage access when they prefer ethanol to water by ≈70% (Belknap et al., 1993; Yoneyama et al., 2008). Conversely DBA/2J (D2) mice drink ≈1g/kg of 10% ethanol and chose water over ethanol by ≥80% (Belknap et al., 1993; Yoneyama et al., 2008). Similar strain differences are evident in operant models of oral ethanol self-administration (Risinger et al., 1998). In contrast, D2 mice learn to discriminate IP ethanol from saline in almost half the time required by B6 mice (Shelton and Grant, 2002) and have more robust conditioned place preference for IP-administered ethanol (Cunningham et al., 1992; Gabriel and Cunningham, 2008). Both strains selectively respond for IV-administered ethanol at similar rates (Grahame and Cunningham, 1997). These findings suggest that the taste of ethanol may be a significant factor limiting oral ethanol consumption by inbred mouse strains like D2.
The combination of ethanol with naturally rewarding tastants can engender significantly greater ethanol consumption compared to ethanol alone. For example saccharin increases home cage ethanol consumption in both B6 and D2 mouse lines (Belknap et al., 1993; Yoneyama et al., 2008). The response to sweetened ethanol is dramatically strain-dependent (Yoneyama et al., 2008), suggesting that the biological mechanisms controlling ethanol and ethanol+sweet consumption may be genetically influenced (Phillips et al., 1994). B6 and D2 mice express distinct forms of the Tas1R3 gene which appears to govern the distinct saccharin preference in these mouse strains (Bachmanov et al., 2001; Max et al., 2001). Alterations in the TAS1R3 taste receptor protein reduces the sensitivity to sweet tastants (Inoue et al., 2007) and to ethanol (Blednov et al., 2008) but does not alter consumption of sodium chloride, quinine, or monosodium glutamate (Inoue et al., 2007). This suggests that reduced ‘behavioral’ efficacy of sucrose- or saccharine-related sweeteners to initiate ethanol consumption in D2 or other ‘non-drinking’ strains may be related to the diminished ‘biological’ efficacy of sweet tastants at taste receptors expressed in these strains.
Monosodium glutamate (MSG) is a unique tastant contributing to the ‘umami’ or savory flavor in protein-rich foods. MSG is preferred relative to water across a range of concentrations in two-bottle choice tests on different mouse lines (Bachmanov et al., 2000). Importantly, the biology of umami taste may involve numerous taste receptors including both the T1-family of receptors (Chen et al., 2009) and variants of the metabotropic glutamate receptors expressed by taste cells on the tongue (Monastyrskaia et al., 1999; San Gabriel et al., 2009). This suggests that genetic polymorphisms that reduce sweet taste might not similarly impact MSG consumption (Bachmanov et al., 2000). In the current study we test the hypothesis that MSG can be used to initiate ethanol consumption in inbred mouse lines.
Materials and Methods
Subjects
C57BL/6J (B6) and DBA/2J (D2) male mice were purchased at 5 weeks of age from the Jackson Laboratory (Bar Harbor, ME, USA) and housed individually on a reversed light/dark cycle (7am lights off). Food and water (exceptions noted below) were provided ad libitum. Animals were individually housed without any manipulation for four days to allow them to recover from any shipping-related stress. Animals were handled daily throughout the remainder of the study. All procedures were approved by the WFU IACUC and were consistent with the NIH Guide for the Care and Use of Animals.
Procedures
Two-bottle Choice
D2 or B6 male mice (n=16/strain) were individually housed and given access to 25ml graduated bottles (0.2ml accuracy) containing either water or water containing various concentrations of MSG (25mM, 50mM, 100mM, 200mM, and 400mM) as the only liquid source. Different concentrations of MSG were tested in ascending order on separate weeks. Water and tastant bottle positions were alternated each day. We made daily measures of the total volume consumed from each ‘bottle’ (to the nearest 0.2ml) and animal weight. Individuals whose consumption differed >25% on one side of the home cage versus the other were excluded from the two-bottle choice data analysis for that particular concentration (see Results). Total daily MSG and water intake were measured and used to calculate total fluid intake (MSG+water volume) and MSG preference ratio (MSG volume/total fluid volume).
For the ethanol drinking experiments, mice were randomly assigned to sucrose- or MSG-fade groups. Individuals were first tested using a four-day, 24hr two-bottle choice paradigm in the home cage similar to that described above. During this ‘choice’ period, the only fluids available for consumption on the home cage were water and either 10% sucrose in the sucrose-group or 100mM monosodium glutamate (MSG) in the MSG-group. 10% sucrose was chosen since it has been previously shown to engender near maximal consumption in both B6 and D2 mice (Lewis et al., 2005; Pothion et al., 2004). Prior to this study, MSG has not been as extensively studied in D2 mice (see Figure 1). Animals having any side bias (see above) during the two-bottle drinking period were excluded from the data shown in Table 1 but were kept in the ethanol drinking studies that followed (see below) since these latter experiments involved consumption from only a single bottle.
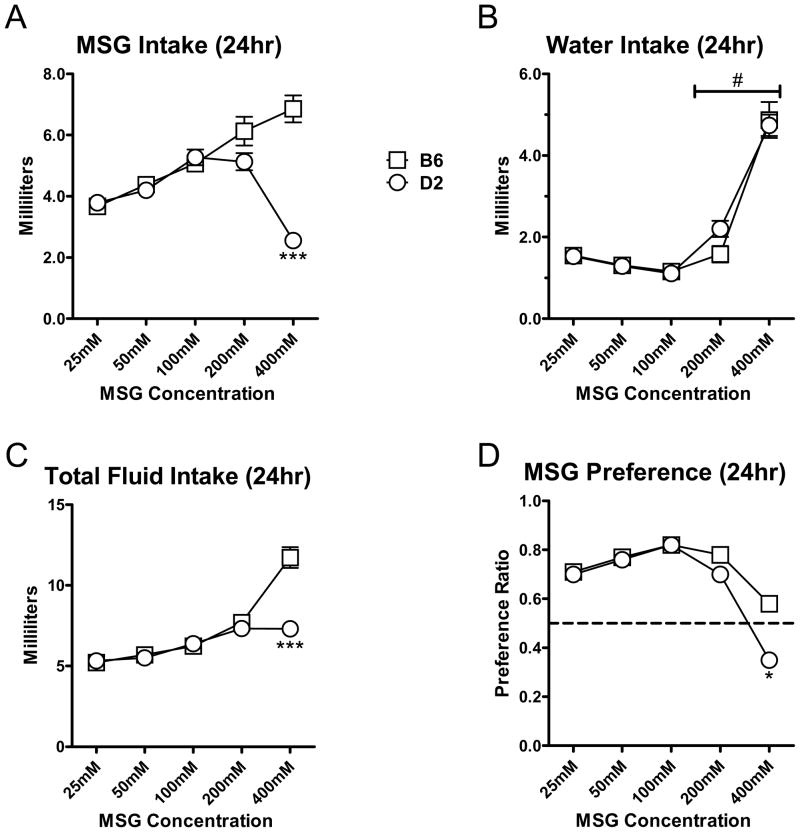
Figure 1. Continuous home-cage two bottle-choice access to increasing concentrations of monosodium glutamate (MSG) defines a ‘most palatable’ concentration.
(A) Average MSG consumption (in milliliters) across increasing concentrations illustrates a significant divergence in the volume consumed at higher MSG concentrations between C57BL/6J (B6, □) and DBA/2J (D2, ○) mice. There was a significant interaction between MSG concentration and strain (P<0.001, repeated measures two-way ANOVA). *** -- P<0.001, Tukey-Kramer post-hoc multiple comparison test across the strain-factor. (B) Average daily water consumption (milliliters) during the two-bottle choice showed no significant differences between strains and no significant interaction between strain and MSG concentration. However, water volumes consumed were significantly greater for the 200mM and 400mM concentrations compared lower MSG concentrations. # -- P<0.05, Tukey-Kramer post-hoc across the MSG concentrations. (C) Like MSG alone, there was a significant interaction between MSG concentration and strain for total fluid intake (in milliliters; MSG intake + water intake; P<0.001, repeated measures two-way ANOVA). *** -- P<0.001, Tukey-Kramer post-hoc test across the strain-factor. (D) The average ‘Preference Ratio’ was calculated as the volume of MSG consumed (in milliliters) divided by the total fluid consumed. The ratio generally followed the same concentration-dependent changes as MSG consumption (P<0.001, repeated measures two-way ANOVA, for strain × concentration interaction). * -- P<0.05, Tukey-Kramer post-hoc across the strain-factor.
Table 1.
Summary of Two-bottle Choice Drinkinga
| Strain | 10% Sucroseb | 100mM MSG | 2×2 Statisticc | |
|---|---|---|---|---|
| Tastant Intake (ml) | B6 | 16.8 ± 0.3 | 5.1 ± 0.2$$$ | Interaction (***) |
| D2 | 9.5 ± 0.4###d | 6.5 ± 0.3##,$$$ | ||
| Water Intake (ml) | B6 | 1.1 ± 0.1 | 2.8 ± 0.3$$$ | Interaction (**) |
| D2 | 1.7 ± 0.1# | 2.2 ± 0.2# | ||
| Total Fluid Intake (ml) | B6 | 17.8 ± 0.3 | 7.9 ± 0.3$$$ | Interaction (***) |
| D2 | 11.2 ± 0.4### | 8.7 ± 0.4$$$ | ||
| Preference (ml tastant/ml total) | B6 | 0.94 ± 0.03 | 0.66 ± 0.03$$$ | Interaction (***) |
| D2 | 0.85 ± 0.08### | 0.75 ± 0.02###,$$$ | ||
| Body Weight (g) | B6 | 19.1 ± 0.4 | 19.3 ± 0.5 | Strain (**) |
| D2 | 17.2 ± 0.5## | 18.3 ± 0.3 | ||
Three separate cohorts of C57BL6/J (B6) and DBA2/J mice (D2) for each tastant (n=16 total for each strain). Animals exhibiting ‘side preference’ for bottle position in the home cage are not included in this analysis (see text).
Mean ± SEM is shown for each strain and tastant. Values across test-days were averaged within each animal.
Repeated measures two-way ANOVA with strain and tastant as main factors.
-- P<0.01,
-- P<0.001
Tukey-Kramer post-hoc multiple comparison test of the strain-factor (#, within a tastant) and tastant factor ($, within a strain).
P<0.05,
P<0.01,
P<0.001
Limited Access Ethanol Self-administration in the Home Cage
For the sucrose- or MSG-fade and ethanol self-administration in the home cage, we used a variant of the single-bottle ‘drinking in the dark’ (DID) protocol (Rhodes et al., 2005). Two-hour access to the drinking ‘bottle’ (5ml serological pipette, 0.1ml accuracy) began 30min after the beginning the dark phase (lights-off at 7:00am) for 5 days a week with no access during the weekends. Standard home-cage drinking bottles were removed during this period and total volume consumed from 5ml drinking tubes was measured at the end of the access period. Food was kept in the home cage during the two-hour access, and standard water bottles were replaced immediately after the drinking session.
Following introduction to the tastants during a four-day, continuous access, two-bottle choice period (above), mice were first given 2hr DID access to the tastant (100mM MSG or 10% sucrose) alone for two consecutive days. During the ethanol fade-in period (see Fig. 2), all mice were subsequently given access to mixtures of 100mM MSG or 10% sucrose plus increasing concentrations of ethanol (2, 4, and then 5%) with access at each concentration spanning at least two consecutive days. During the following tastant fade-out period MSG or sucrose concentrations in the 5% ethanol mixture were decreased to 75mM or 7.5% (respectively), to 50mM/5%, to 25mM/2.5%, to 10mM/1%, and finally to 5% ethanol alone for all mice in this experiment. Access to each tastant concentration + 5% ethanol was again given on at least two consecutive days. Novel ethanol/tastant combinations were never introduced on Mondays with some ethanol+tastant combinations therefore being given on three days interrupted by weekends where no drinking took place. Both the ethanol fade-in and the sucrose/MSG fade-out periods together lasted four weeks. At the end of the tastant fade-out period all animals were then given access to 5% ethanol alone, followed by 10% ethanol, then 15% ethanol for five consecutive days (Tue, Wed, Thur, Fri, and Mon) at each concentration. This procedure was performed on two different cohorts of animals (n=8 in each). The sucrose- and MSG-experiments in each strain were run in parallel.
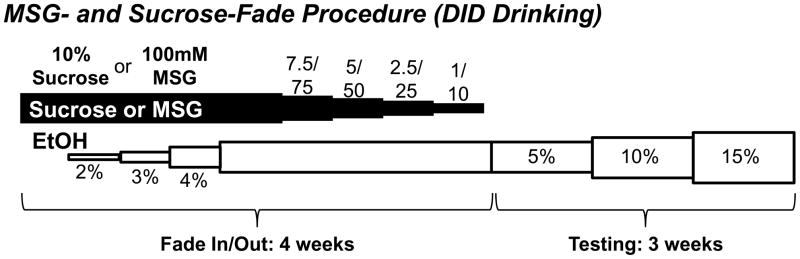
Figure 2. Fade procedure.
The fade procedure used a modified, single-bottle “Drinking in the Dark” paradigm according to published procedures (Rhodes et al., 2005). Animals were initially given a two bottle choice, continuous access exposure to the tastants prior to the limited access exposure (see Table 1). The modified fade-procedure increased ethanol concentrations (open bars) and tastant (MSG or sucrose; closed) concentrations independently from one another over a four-week period. Following the substitution procedure, animals were given limited DID access to increasing concentrations of ethanol in the absence of any tastants. See Methods section for details.
Statistics
Data were expressed as either mean ‘preference’, mean volume of liquid consumed (in milliliters), or as mean intake expressed as grams consumed per kilogram body weight per 2hr DID session (g/kg/2hr). Standard two-way ANOVA was used to analyze most data; and, the Tukey-Kramer multiple comparisons test was used post-hoc to identify significant differences across main factors where appropriate. Asterisks (*) and pound-signs (#) denote significant differences across individual factors that were identified with this post-hoc analysis. For some experiments, paired student’s t-test was used as noted in the results. Statistical significance for all analyses was defined as P<0.05.
Results
Two Bottle-Choice Drinking: MSG and Water
Since MSG drinking in the D2 strain has not been extensively explored, our initial experiment was designed to define an MSG concentration for future fade-studies that produces similar levels of intake/preference between B6 and D2 mice. In a standard two-bottle choice paradigm executed in the home cage where separate cohorts of 8 male mice from the D2 and B6 lines (n=16 for each strain) were given weekly access to water and water containing ascending concentrations of MSG (25mM, 50mM, 100mM, 200mM, and 400mM), we found that the volume of MSG consumed by both strains was very similar across MSG concentrations less than 200mM. Two-way ANOVA (repeated measures) found a significant interaction between strain and MSG concentration (P<0.001, F=52.5, DF=4). A Tukey-Kramer post-hoc multiple comparison test of these data found a significant difference between the strains at the 400mM MSG concentration (Fig. 1A, *** -- P<0.001). This later finding suggests that the significant interaction between strain and MSG was mainly related to the difference in consumption at this highest MSG concentration. There was also a significant increase in water consumption by both strains as MSG concentration increased (P<0.001, F=82.1, DF=4, repeated measures two-way ANOVA) but no significant strain×concentration interaction and no difference between B6 and D2 strains (P>0.05, F=0.08, DF=1 for strain and F=1.5, DF=4 for the interaction). MSG concentrations greater than 100mM significantly increased 24hr water intake (Fig. 1B, # -- at least P>0.05, Tukey-Kramer post-hoc). Given that water intake was not significantly difference between strains, total fluid consumption (MSG plus water, Fig. 1C) and MSG preference ratio (Fig. 1D) were very similar to MSG consumption alone. We subsequently selected 100mM MSG for the tastant-fade studies since it produced nearly identical consumption/preference measures in B6 and D2 mice and did not dramatically alter water consumption in either strain.
MSG- and Sucrose Fade to 5% Ethanol
Two-bottle Choice Drinking
For the ethanol drinking studies using the tastant-fade procedure, we familiarized mice with drinking from bottles and to the individual tastants themselves by initially exposing them to 10% sucrose or 100mM MSG using a four day, 24hr two-bottle choice test in the home cage. In the sucrose groups, only one out of sixteen D2 mice consistently drank more (>25%) from one side of the home cage than the other while none of the B6 mice expressed any observable ‘side preference’. In the MSG groups, one of the sixteen D2 mice and two of the sixteen B6 mice consistently had a side-preference. Animals exhibiting side-bias were excluded from the two-way analysis. With strain and tastant representing the two independent variables in this study (Table 1), we found significant interactions between these factors for several of the dependent variables including the volume of tastant consumed (P<0.001, F=210.4, DF=1), water intake (P<0.01, F=8.5, DF=1), total fluid intake (P<0.001, F=111.5, DF=1), and tastant preference ratio (P<0.001, F=34.7, DF=1). In general, these interactions appear to be driven by sucrose-groups which drank significantly larger volumes of the tastant compared to MSG-mice (Tukey-Kramer post-hoc test; P<0.001). This tended to reduce water intake to some degree but the effect on water intake was significant only for B6 mice (P<0.001, Tukey-Kramer). When measured at the end of the two-bottle choice period, sucrose-D2 mice weighed significantly less than their B6 counterparts. Importantly D2 mice also arrived from the vendor with substantially smaller body weights (14.9±0.3g; P<0.001, t=2.2, DF=62, t-test) compared to B6 mice (18.6±0.2g) despite being a similar age at the beginning of the study (~5 weeks).
Fade-In to 5% Ethanol
Immediately following the two bottle choice test, all animals (n=32 in each strain, n=16 for each tastant per strain) were subjected to a modified ‘drinking in the dark’ (Rhodes et al., 2005) protocol for five days each week as described in the methods section. Animals were initially given limited access to the same tastant (sucrose or MSG) they experienced during the four-day two-bottle choice period and subsequently to ethanol concentrations increasing from 2% (v/v), 4%, and finally 5% (Fig. 2). For ethanol intake by D2 mice (g/kg/2hr), there was a significant interaction between the type of tastant mixed with the ethanol and ethanol concentration (P<0.01; F=5.7, DF=2, repeated measures two-way ANOVA). Importantly, ethanol intake was significantly less in MSG-D2 compared to sucrose-D2 at every ethanol concentration tested (Fig. 3A, *** -- P<0.001, Tukey-Kramer post-hoc multiple comparison test). There was also a significant increase in ethanol intake when moving from 2% to 4% ethanol (not shown, P<0.001, Tukey-Kramer) but only in the sucrose-D2 mice. Individual data points in Figure 3 represent mean intakes across individual animals averaged over the two-to-three days of consumption at each ethanol concentration (see Methods).
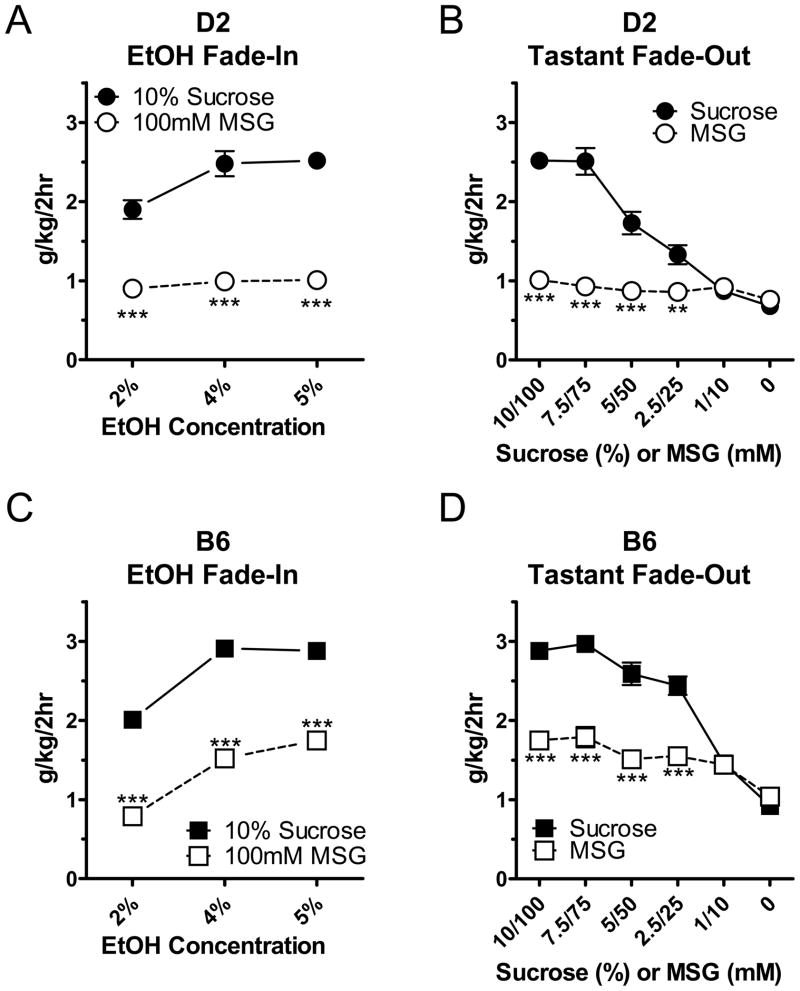
Figure 3. Increasing ethanol concentrations during the fade-in procedure limits intake in a strain- and tastant-dependent fashion.
(A) During the ethanol fade-in, MSG-D2 mice had significantly lower ethanol intakes (g/kg/2hr) compared to sucrose-D2 mice (n=16 in each experimental group). There was a significant interaction between ethanol concentration and the type of tastant (P<0.01, repeated measures two-way ANOVA). (B) Comparisons of 5% ethanol intake (g/kg/2hr) during the sucrose and MSG fade-out in D2 mice also revealed a significant interaction between both strain and tastant concentration (P<0.001, repeated measures two-way ANOVA) with values converging towards similar levels in the absence of tastant. (C) Ethanol intake (g/kg) in MSG-B6 mice was significantly lower than sucrose-B6 mice during the ethanol fade-in period (n=16 in each experimental group). There were significant main effects of the type of tastant (P<0.001) and ethanol concentration (P<0.001, repeated measures two-way ANOVA) but no interaction between these factors. (D) Like D2 mice, ethanol intake during the tastant fade-out in B6 mice indicated a significant interaction (P<0.001, repeated measures two-way ANOVA) between tastant concentration and the type of tastant. For all panels, individual data points represent mean intake across the groups with intakes from individual animals averaged over the two-to-three days of consumption at each tastant or ethanol concentration. ** -- P<0.01, *** -- P<0.001, Tukey-Kramer post-hoc test across the tastant-factor. Post-hoc results for the concentration factor are not shown (see text).
Compared to the D2 mice, a slightly different pattern of intake by B6 mice emerged across the increasing ethanol concentrations. As with the D2 data, the data points in Fig. 3C represent mean intakes or consumption levels across the 2–3 days B6 animals were given access to a particular ethanol concentration. Intakes (g/kg/2hr) increased for both MSG- and sucrose-fade B6 mice. Repeated measures two-way analysis did not find any significant interaction between the type of tastant and ethanol concentration, although there were significant main-effects of both ethanol concentration (P<0.001; F=122.3, DF=2) and the type of tastant used in the fade (P<0.001, F=229.8, DF=1). Along the concentration-factor, B6 ethanol intake initially increased when ethanol concentrations increased from 2% to 4% (P<0.001, Tukey-Kramer) and then stabilized when ethanol concentrations increased from 4% to 5% (P>0.05). Importantly, ethanol intakes were greater in sucrose-B6 mice compared to MSG-B6 mice at all three ethanol concentrations (Fig. 3C, *** -- P<0.001, Tukey-Kramer post-hoc).
Tastant Fade-out
After increasing the ethanol concentration to 5%, the amount of sucrose or MSG in the mixture was reduced across a range of defined concentrations. In D2 mice, we again found that ethanol intakes were relatively stable in MSG-mice compared to their sucrose counterparts. There was a significant interaction between the type of tastant and the tastant concentration (P<0.001, F=36.6, DF=5, repeated measures two-way ANOVA) with ethanol intakes converging towards similar levels. There were significant differences between MSG- and sucrose-D2 mice occurring only at the four highest tastant concentrations (Fig. 3B, ** -- P<0.01 and *** -- P<0.001, Tukey-Kramer post-hoc multiple comparison test), but not at the lowest two concentrations (P>0.05). Along the concentration-factor (data not shown), we found significant differences between the two highest sucrose concentrations (10% and 7.5%) and the lower sucrose concentrations (P<0.01 or greater, Tukey-Kramer) but no significant differences between the MSG concentrations tested here.
Despite greater ethanol intakes overall, the pattern of ethanol intake by B6 during the tastant fade-out appeared qualitatively similar to the D2 data. There was a significant interaction between tastant concentration and the type of tastant (P<0.001, F=40.4, DF= 5, repeated measures two-way ANOVA) with significant differences between sucrose and MSG at the four highest concentrations tested (Fig. 3D, *** -- P<0.001, Tukey-Kramer multiple comparison post-hoc). There was also a significant concentration-dependent decrease in ethanol intake along the concentration factor for both sucrose and MSG (not shown, P<0.05 or greater, Tukey-Kramer post-hoc).
Concentration-Dependent Ethanol Consumption
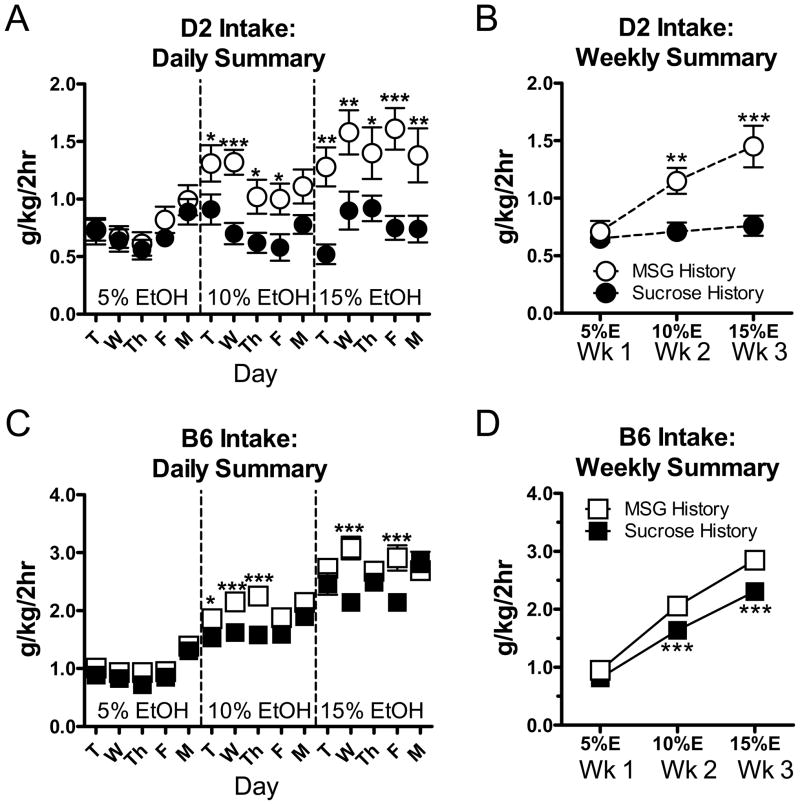
Immediately following the sucrose- and MSG-fade period, animals were given access to increasing concentrations of ethanol alone on consecutive weeks. As indicated in the Methods section, ethanol concentrations were changed on Tuesdays in a given week to avoid introducing a novel concentration immediately following a weekend when animals did not drink. For daily 5% ethanol drinking by D2 mice (Fig. 4A), ethanol intake (g/kg/2hr) was not significantly different across the different tastant-histories (closed symbol=Sucrose fade; open symbol=MSG-fade) and there was no significant interaction between tastant and day of the week (P>0.05, repeated measures two-way ANOVA). However, there was a significant effect of day of the week (P<0.001, F=11.7, DF=4, repeated measures two-way ANOVA) with drinking on Monday being significantly higher than any other day of the week (P<0.05 or greater; Tukey-Kramer multiple comparison post-hoc test). At higher ethanol concentrations, there were still no interactions between day of the week and tastant history although now there emerged significant main-effects of each factors for both 10% (P<0.01, F=10.0, DF=1 for tastant history; P<0.001, F=4.8, DF=4 for day) and 15% ethanol (P<0.01, F=11.6, DF=1 for tastant; P<0.01, F=3.9, DF=4 for day). Despite the significant main-effect of day at both ethanol concentrations, post-hoc multiple comparisons (Tukey-Kramer) of the 10% ethanol data did not identify any specific day of the week with significantly greater intake across each tastant (P>0.05). For 15% ethanol however, intake was significantly lower on Tues relative to Wed and Thur in only the sucrose-D2 animals (P<0.05). Importantly, ethanol intake by MSG-D2 mice was consistently larger compared to sucrose-D2 on most days for both 10% and 15% ethanol (Fig. 4A, * -- P<0.05, ** -- P<0.01, *** -- P<0.001, Tukey-Kramer).
Figure 4. Ethanol intakes are larger in MSG-fade mice and are concentration-dependent.
(A) Average daily ethanol intake (g/kg/2hr) in D2 mice (same individuals from Figure 3) was significantly greater at 10% and 15% ethanol following the MSG-fade compared to the sucrose-fade. For 5% ethanol, there was a significant effect of the day of the week (P<0.001, repeated measures two-way ANOVA, see text for details). For 10% and 15% ethanol intakes (g/kg/2hr), there were significant main-effects of both day of the week and the tastants (repeated measures two-way ANOVA, see text for details) with D2 animals from the MSG-fade drinking significantly more that sucrose-fade animals during several days at each concentration. * -- P<0.05, ** -- P<0.01, *** -- P<0.001, Tukey-Kramer post-hoc test across the ‘tastant history’-factor. (B) D2 daily intake values (g/kg/2hr) were averaged (excluding Monday, see text) across the weeks. For these data, there was a significant interaction (P<0.001, repeated measures two-way ANOVA) between tastant history and ethanol concentrations. Ethanol intakes by MSG-D2 mice were significantly greater than sucrose D2-mice at both the 10% and 15% ethanol concentration (** -- P<0.01, *** -- P<0.001, Tukey-Kramer post-hoc multiple comparison test). (C) Mean daily ethanol intakes in B6 mice following the MSG- and sucrose-fade. * -- P<0.05, *** -- P<0.001, Tukey-Kramer across the tastant-factor. Post-hoc analysis of day of the week is not shown (see text for details). (D) For mean weekly ethanol intakes in B6 mice, there was a significant interaction between ethanol concentration and fade history (P<0.01, repeated measures two-way ANOVA). Ethanol intakes with the 10% and 15% ethanol concentration were significantly greater in MSG-B6 mice compared to sucrose-B6 mice (*** -- P<0.01 Tukey-Kramer post-hoc multiple comparison test). Post-hoc comparisons across the concentration-factor are not shown (see text).
To provide a more concise picture of ethanol concentration-dependent drinking engendered by the sucrose- and MSG-fade, we analyzed mean intake data for individual animals across Tuesday through Friday during weekly drinking at a given ethanol concentration. Mondays were excluded from this analysis to avoid confounds related to any ‘weekend off’ changes in consumption. Analysis of these ‘weekly’ intake (g/kg) data in D2 mice revealed a significant interaction (P<0.001, repeated measures two-way ANOVA) between ethanol concentration and the type of tastant used for the fade. Specifically MSG-D2 mice had significantly greater ethanol intakes (g/kg/2hr) than sucrose-D2 mice at both the 10% (P<0.01) and 15% (P<0.001) concentrations (Fig. 4B, Tukey-Kramer post-hoc multiple comparison test). Across the concentration factor, intakes were significantly greater in the MSG-D2 mice as ethanol concentration increased (not shown, P<0.001, Tukey-Kramer); however intakes in sucrose-D2 mice appeared concentration-independent (P>0.05).
Like the D2 mice, the MSG-fade also increased ethanol intake in B6 mice relative to the sucrose-fade. For daily drinking summaries at 5% ethanol (Fig. 4C), we again found a significant main-effect of day of the week (P<0.001, F=31.2, DF=4, repeated measures two-way ANOVA) but not tastant history nor any interaction between these factors (P>0.05). Like the D2 mice, Monday intakes following a weekend of no drinking were significant larger than intakes during the rest of the week for both sucrose- and MSG-B6 mice (not shown; P<0.001, Tukey-Kramer post-hoc multiple comparisons test). For 10% ethanol (g/kg/2hr), the significant main-effect of day remained (P<0.05); and there emerged a significant main-effect of tastant history (P<0.001) without any interaction between factors (P>0.05, repeated measures two-way ANOVA). The main-effect of day appeared to be driven primarily by the significant intake differences between Monday and the preceding Tuesday (not shown; P<0.05, Tukey-Kramer). Importantly, there were also significant differences between ethanol intake of sucrose- and MSG-B6 mice on three out of five drinking days (Fig. 4C; * -- P<0.05, ** -- P<0.01, *** -- P<0.001, Tukey-Kramer). Finally, during daily sessions with 15% ethanol, there was a significant interaction between day and tastant history (P<0.001). Post-hoc multiple comparisons found significant differences between tastants on two out of five drinking days (Fig. 4C; *** -- P<0.001, Tukey-Kramer). ‘Weekly’ ethanol intake across the tastant- and ethanol concentration-variables likewise had a significant interaction between these factors (P<0.01, repeated measures two-way ANOVA). Along with a significant increase in ethanol intake as concentration increased across the concentration factor (not shown, P<0.001, Tukey-Kramer post-hoc multiple comparison test), ethanol intakes were also significantly greater in MSG-B6 mice relative to sucrose-B6 animals at both the 10% and 15% ethanol concentrations (Fig. 4D; *** -- P<0.001, Tukey-Kramer post-hoc).
The significant strain-dependent difference in the body weights noted in the two-bottle choice dataset was also present at the end of the 15% ethanol access period (not shown, P<0.001, two-way ANOVA). However, there was no significant difference in body weight between sucrose-D2 (24.2±0.5grams) and MSG-D2 mice (24.8±0.6grams; P>0.05) or sucrose-B6 (27.6±0.5 grams) and MSG-B6 animals (27.6±0.4 grams; P>0.05, Tukey-Kramer).
Discussion
In the current study we have shown that an alternative tastant, monosodium glutamate (MSG), can be used during a traditional fade-out procedure to initiate limited-access ethanol drinking in the home cage by C57BL/6J (B6) and DBA/2J (D2) male mice. In our hands, the MSG-fade produces higher levels of ethanol intake than a sucrose-fade in both inbred strains. This suggests that the MSG-fade might be a very useful tool to produce increased ethanol self-administration in mouse strains traditionally considered to be ‘ethanol non-preferring’. For example, the level of ethanol consumption in D2 mice with a MSG-history in our study was substantially greater than intakes reported for this strain in the literature even with much longer DID limited access (Rhodes et al., 2007).
During the extended two bottle choice experiment between varying concentrations of MSG and water (Fig. 1), we were able to identify a MSG concentration (100mM) that engendered similar consumption volumes and preferences in both B6 and D2 mice. For the B6 mice, our results are similar to previous work with the related C57BL/6ByJ and C57BL/6-CrSLC stains (Bachmanov et al., 2000; Yamamoto et al., 2009). MSG drinking by D2 or related strains of mice has not been as extensively studied. Klotus and Blizard reported that D2 mice did not prefer MSG to water in a 6-hour limited-access two bottle choice test (Kotlus and Blizard, 1998). However, this study employed a slightly higher MSG concentrations (150mM) than that defined as producing the optimum preference (100mM) in the current work. The older mice used in the former study might have also contributed to any differences between our studies.
The four-day two bottle choice exposure prior to the ethanol fade largely paralleled the MSG intake data from the long term study. This dataset clearly shows that both 10% sucrose and 100mM MSG were more preferred than water in both B6 and D2 mice. However, sucrose preference/intake was much more robust than MSG preference/intake in both strains. We initially chose 10% sucrose for our studies because it engendered similar preferences between B6 and D2 in the literature (Lewis et al., 2005; Pothion et al., 2004). Although the four day two bottle choice exposure might have directly influenced the subsequent fading results or the ethanol self-administration findings, the differences in preference between sucrose and MSG in either strain did not ultimately predict the level of ethanol intake. For example, sucrose was clearly the most preferred tastant in both strains yet the MSG-fade produced higher levels of ethanol intake in each. Likewise, D2 mice had a greater MSG preference than B6, while B6 had a greater sucrose preference that D2. B6 mice ultimately had higher ethanol intake levels regardless of their tastant-history. Finally, sucrose and MSG intake or preference during the four day initial exposure did not correlate with ethanol intake. This suggests to us that the initial tastant preference or any tastant-specific nutritive contribution ultimately makes a relatively minor contribution to the consumption of ethanol alone following the fade.
The MSG or sucrose/ethanol fade procedure yielded a number of novel observations that deserve additional discussion. First, MSG-D2 mice appear more sensitive to increasing ethanol concentrations since they were able to maintain constant ethanol intakes across a relatively narrow range of ethanol concentrations (Fig. 3A). Given that both strains have higher intakes with the sucrose/ethanol mixture compared to the MSG/ethanol mixture, this data might suggest that sucrose better ‘masks’ the taste of ethanol. This interpretation would complement our findings of higher preference levels for sucrose compared to MSG in both strains (Table 1). Finally, it is also noteworthy that the ethanol intake in D2 mice during the MSG fade-out was relatively constant (Fig. 3B) throughout the same period where ethanol intake in MSG-B6 decreased in a concentration-dependent fashion (Fig. 3D). This strain difference could be regulated by a number of distinct neurobehavioral mechanisms. For example, a number of important studies from the Cunningham laboratory have shown that D2 mice more readily acquire conditioned place preference for IP- administered ethanol (Cunningham et al., 1992; Gabriel and Cunningham, 2008; Gremel et al., 2006). This suggests that the pharmacological salience of ethanol may be more pronounced in the D2 strain relative to B6.
Finally, we found that the ethanol intakes by B6 and D2 mice following the sucrose- and MSG-fade were dramatically altered by the ethanol concentrations that animals were allowed to consume. During this study, we noted that 5% ethanol consumption on Mondays following a weekend of no drinking produced significantly greater intakes in both strains. Although similar results were not forth-coming with the 10% and 15% ethanol drinking, this pattern of consumption appears similar to the ‘alcohol deprivation effect’ where ethanol consumption/self-administration is increased following brief periods of experimentally forced abstinence (Geiger and Barker, 1976; Khisti et al., 2006; Sparta et al., 2009). Conversely, while there was no difference between MSG- and sucrose-groups at the 5% ethanol concentration, both B6 and D2 mice with a MSG-fade history had statistically larger ethanol intakes than sucrose-fade mice during drinking of the higher (10–15%) of ethanol concentrations. It is also apparent from our data that ethanol intakes by B6 mice were much more robust than by D2 mice, but only at ethanol concentrations higher than 5%. This finding is generally consistent with a large body of literature describing differences in voluntary ethanol consumption in these strains across different self-administration paradigms (Belknap et al., 1993; Mittleman et al., 2003; Rhodes et al., 2007; Risinger et al., 1998; Yoneyama et al., 2008).
An interesting difference between ethanol intake in the current study and the available literature is that the MSG-fade appears to produce a proportionally larger consumption relative to sucrose in the D2 mice. Although the reasons for this are unclear, it is worth noting that the MSG-fade procedure itself may have provided more prolonged exposures to lower ethanol concentrations than procedures used in the literature. This prolonged exposure to low ethanol concentrations might be particularly suited for D2 mice to ‘overcome’ their well-known robust ethanol conditioned taste aversion-learning (Horowitz and Whitney, 1975). Supporting this, unpublished findings from Dr. Judy Grisel (Furman University) show that an extended drinking experience with mixtures of non-alcoholic beer and ethanol result in similar levels of 7% ethanol intake by B6 and D2 mice (personal communication). Similarly, ‘pre-exposure’ to ethanol is known to reduce subsequent conditioned taste aversion in D2 mice (Risinger and Cunningham, 1995).
There are a number of limitations in the current experimental design that we must also acknowledge. We did not measure blood ethanol concentrations (BECs) following the drinking sessions in any of the mice used in this study. There is therefore no direct evidence that either B6 or D2 mice drank quantities of ethanol sufficient to produce a pharmacological effect. Previous work from another lab suggests that meaningful BECs may only be evident in the 2hr DID paradigm when ethanol intakes greatly exceed 1g/kg (see Figure 3A in (Rhodes et al., 2005)) or when the access period is much longer than 2hr (Rhodes et al., 2007). Given the robust ethanol metabolism in mice and the fact that food was available throughout the daily access periods, it is conceivable that one would have to know the precise pattern of ethanol consumption in a given strain in order to sample peak blood ethanol concentrations during a 2hr access period. Finally, the limited access paradigm with a single bottle also restricts interpretation of the drinking data. Additional studies with ethanol preference, direct BEC measures after longer access periods, or post-consumption behavioral assessments are needed to test the hypothesis that increased drinking following an MSG fade results in an overt pharmacological effect.
Because of these limitations, the exact mechanisms regulating increased ethanol intake following the MSG-fade remain to be established. One possibility is that MSG might act directly on the nervous system to increase ethanol consumption in the home cage. High doses of intra-peritoneal MSG can be neurotoxic to adult mouse hypothalamic neurons (Dawson, 1983; Park et al., 2000). However the MSG dose required for neurotoxicity (4mg/g) is considerably larger than that being ingested orally during at typical limited access period (≤1.3mg/g). Since glutamate is a non-essential amino acid whose uptake sites in the gut readily adapt to dietary levels, it is probable that only a fraction (<10%; (Bourdel et al., 1981)) of the ingested glutamate would likely be found in the circulation following a limited access drinking session. It is also noteworthy that a single D2 mouse in the sucrose-fade group did drink 15% ethanol at levels (0.4–1.3 g/kg/2hr) similar to the MSG-fade D2 mice. This suggests that sucrose-fade D2 mice possess the capacity to ingest quantities of ethanol similar the MSG-fade D2 mice but simply fail to do so. Finally, the history of MSG- or sucrose-exposure might alter metabolic or peripheral processes which ultimately contribute to ethanol consumption in the absence of the tastants themselves. However, any tastant-dependent changes in peripheral processes must last for many weeks in order to influence ethanol consumption during the latter phases of our ethanol drinking experiment. One would imagine such effects would be evident in the 5% ethanol intake data which did not indicate any differences between the tastants. We favor the alternative hypothesis that the MSG-ethanol mixture, unlike sucrose-ethanol, may avoid ‘confusion’ between the ethanol and tastant cues during the fade-out (Fig. 3B). Since D2 mice readily generalize conditioned taste aversion of bitter but not sweet solutions to 10% ethanol (Blizard, 2007), high sucrose concentrations may merely mask the more ‘bitter-like’ taste of ethanol experienced by D2 mice and ultimately disadvantage positive associations between ethanol taste and pharmacology.
In summary, we have shown that using monosodium glutamate as a tastant produces a greater level of ethanol intake than does the more traditional tastant sucrose during a tastant-fade procedure. The magnitude of this effect was particularly robust in male DBA/2J mice although MSG was also effective in male C57BL/6J mice. Together these findings might suggest the utility of a MSG-fade procedure for the initiation of measurable limited-access ethanol drinking in mouse strains traditionally considered to be ethanol ‘non-preferring’.
Acknowledgments
This work was supported by NIH/NIAAA awards R01 AA014445 and U01 AA016671 (B.A.M.) and the Translational Center for the Neurobehavioral Study of Alcohol (AA017056).
Footnotes
Author Contributions: AC was responsible for the execution of all the experiments and assisted with the data analysis and editing the final and revised manuscripts. BM was responsible for the conceptualization and design of all experiments, data analysis, and manuscript preparation/revision.
References
- Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK. Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses. 2001;26:925–933. doi: 10.1093/chemse/26.7.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmanov AA, Tordoff MG, Beauchamp GK. Intake of umami-tasting solutions by mice: a genetic analysis. J Nutr. 2000;130:935S–941S. doi: 10.1093/jn/130.4.935S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav. 2008;7:1–13. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blizard DA. Sweet and bitter taste of ethanol in C57BL/6J and DBA2/J mouse strains. Behav Genet. 2007;37:146–159. doi: 10.1007/s10519-006-9121-4. [DOI] [PubMed] [Google Scholar]
- Bourdel G, Kande J, Robin D, Robin P. Quantitative and qualitative circadian variations of amino acid intestinal efflux in mixed-fed and in protein-meal-fed rats. J Nutr. 1981;111:1528–1535. doi: 10.1093/jn/111.9.1528. [DOI] [PubMed] [Google Scholar]
- Chen QY, Alarcon S, Tharp A, Ahmed OM, Estrella NL, Greene TA, Rucker J, Breslin PA. Perceptual variation in umami taste and polymorphisms in TAS1R taste receptor genes. Am J Clin Nutr. 2009;90:770S–779S. doi: 10.3945/ajcn.2009.27462N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Niehus DR, Malott DH, Prather LK. Genetic differences in the rewarding and activating effects of morphine and ethanol. Psychopharmacology (Berl) 1992;107:385–393. doi: 10.1007/BF02245166. [DOI] [PubMed] [Google Scholar]
- Dawson R., Jr Acute and long lasting neurochemical effects of monosodium glutamate administration to mice. Neuropharmacology. 1983;22:1417–1419. doi: 10.1016/0028-3908(83)90235-6. [DOI] [PubMed] [Google Scholar]
- Gabriel KI, Cunningham CL. Effects of maternal strain on ethanol responses in reciprocal F1 C57BL/6J and DBA/2J hybrid mice. Genes Brain Behav. 2008;7:276–287. doi: 10.1111/j.1601-183X.2007.00349.x. [DOI] [PubMed] [Google Scholar]
- Geiger JF, Barker LM. Alcohol consumption by rat and mouse strains. Functions of taste and alcohol deprivation. J Stud Alcohol. 1976;37:950–958. doi: 10.15288/jsa.1976.37.950. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Cunningham CL. Intravenous ethanol self-administration in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 1997;21:56–62. [PubMed] [Google Scholar]
- Gremel CM, Gabriel KI, Cunningham CL. Topiramate does not affect the acquisition or expression of ethanol conditioned place preference in DBA/2J or C57BL/6J mice. Alcohol Clin Exp Res. 2006;30:783–790. doi: 10.1111/j.1530-0277.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- Horowitz GP, Whitney G. Alcohol-induced conditioned aversion: genotypie specificity in mice (Mus musculus) J Comp Physiol Psychol. 1975;89:340–346. doi: 10.1037/h0076803. [DOI] [PubMed] [Google Scholar]
- Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics. 2007;32:82–94. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol. 2006;40:119–126. doi: 10.1016/j.alcohol.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlus BS, Blizard DA. Measuring gustatory variation in mice: a short-term fluid-intake test. Physiol Behav. 1998;64:37–47. doi: 10.1016/s0031-9384(98)00016-x. [DOI] [PubMed] [Google Scholar]
- Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85:546–556. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Max M, Shanker YG, Huang L, Rong M, Liu Z, Campagne F, Weinstein H, Damak S, Margolskee RF. Tas1r3, encoding a new candidate taste receptor, is allelic to the sweet responsiveness locus Sac. Nat Genet. 2001;28:58–63. doi: 10.1038/ng0501-58. [DOI] [PubMed] [Google Scholar]
- Mittleman G, Van Brunt CL, Matthews DB. Schedule-induced ethanol self-administration in DBA/2J and C57BL/6J mice. Alcohol Clin Exp Res. 2003;27:918–925. doi: 10.1097/01.ALC.0000071930.48632.AE. [DOI] [PubMed] [Google Scholar]
- Monastyrskaia K, Lundstrom K, Plahl D, Acuna G, Schweitzer C, Malherbe P, Mutel V. Effect of the umami peptides on the ligand binding and function of rat mGlu4a receptor might implicate this receptor in the monosodium glutamate taste transduction. Br J Pharmacol. 1999;128:1027–1034. doi: 10.1038/sj.bjp.0702885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Choi SH, Piao Y, Kim S, Lee YJ, Kim HS, Jeong SJ, Rah JC, Seo JH, Lee JH, Chang K, Jung YJ, Suh YH. Glutamate and aspartate impair memory retention and damage hypothalamic neurons in adult mice. Toxicol Lett. 2000;115:117–125. doi: 10.1016/s0378-4274(00)00188-0. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155:135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res. 1998;22:677–684. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. Genetic differences in ethanol-induced conditioned taste aversion after ethanol preexposure. Alcohol. 1995;12:535–539. doi: 10.1016/0741-8329(95)00040-2. [DOI] [PubMed] [Google Scholar]
- San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. Am J Clin Nutr. 2009;90:743S–746S. doi: 10.3945/ajcn.2009.27462I. [DOI] [PubMed] [Google Scholar]
- Shelton KL, Grant KA. Discriminative Stimulus Effects of Ethanol in C57BL/6J and DBA/2J Inbred Mice. Alcohol Clin Exp Res. 2002;26:747–757. [PubMed] [Google Scholar]
- Sparta DR, Ferraro FM, 3rd, Fee JR, Knapp DJ, Breese GR, Thiele TE. The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcohol Clin Exp Res. 2009;33:31–42. doi: 10.1111/j.1530-0277.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Watanabe U, Fujimoto M, Sako N. Taste preference and nerve response to 5′-inosine monophosphate are enhanced by glutathione in mice. Chem Senses. 2009;34:809–818. doi: 10.1093/chemse/bjp070. [DOI] [PubMed] [Google Scholar]
- Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008;42:149–160. doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]