Introduction
Oxytocin (OT) belongs to a highly conserved family of peptides that regulate social and reproductive behaviors in vertebrates. OT promotes female rat sexual and maternal behaviors and regulates male reproductive function. OT neurons in the male rat paraventricular nucleus of the hypothalamus (PVN) are activated (i.e., Fos-immunoreactive, -ir) following copulation or ex copula non-contact erections (Baskerville et al., 2009). Infusion of nanogram (ng) amounts of OT into the lateral ventricles stimulates copulation in male rats; these effects are blocked by pretreatment with an OT receptor (OTR) antagonist (OTA). Intracerebroventricular (icv) administration of an OTA increases intromission and ejaculation latencies and decreases mounts and intromissions. In addition, OT binding sites and varicosities have been identified in thoracolumbar and lumbosacral spinal segments that control penile erection in rats, and OTR is expressed in penile tissue in men. OT treatment (icv) induces ex copula penile erection in male rats; these effects are dependent, in part, on stimulation of OTRs in the PVN and CA1 region of the hippocampus. Male rats that fail to copulate to ejaculation have lower levels of OT mRNA in the PVN, compared to sexually vigorous males. OT can also restore copulation in males whose copulatory behavior had been impaired by chronic fluoxetine (Cantor et al., 1999). Plasma concentrations of OT increase during the sexual response in rats and humans; and a link between sexual impotence and reduced central OT production has been established in the male rat.
OT and the mesolimbic dopamine system interact to promote male rat sexual behavior. Microinjection of OT into the PVN, ventral tegmental area (VTA), ventral subiculum, or posteromedial cortical nucleus of the amygdala induces penile erection and increases dopamine levels in the nucleus accumbens (NAcc). OTA treatment (icv) reduces non-contact erections in the presence of a receptive female, and intraperitoneal (ip) injections of OT stimulate the onset of copulation in aging (20-month old) male rats. Taken together, the available data suggest that OT is involved in both the consummatory and appetitive phases of the sexual response in males.
Most pharmacological studies of the effects of OT on male sexual behavior have used icv administration of OT or OTA; the relatively few researchers who tested site-specific effects focused primarily on the PVN, VTA, hippocampus, and amygdala. Less attention has been given to the medial preoptic area (MPOA), a major site of integration for male sexual behavior. OT-producing neurons and OT binding sites have been identified in the MPOA of male rats, and OT-containing MPOA neurons are activated (Fos-ir) by copulation (Baskerville et al., 2009). In female rats, a few OT-ir neurons in the MPOA project indirectly to the clitoris and vagina, suggesting a role for OT in the MPOA in control of sexual function in females (Gelez et al., 2010). Thus, a major goal of the present study is to determine whether OT acts in the MPOA to facilitate male sexual behavior.
There is a great deal of variability in reproductive behavior of rodents. In female rats, levels of OTR binding are higher in high licking and grooming (LG) mothers than in low LG mothers. In addition, more maternally responsive females have higher levels of OTR binding in the amygdala, bed nucleus of the stria terminalis, and MPOA compared to mothers that are less responsive. We wished to test whether differences in OT or OTR activity in the MPOA may be associated with differences in male sexual behavior.
Using factor analysis,) condensed 10 measures of copulatory behavior into 4 factors. An initiation factor comprises latencies to the first mount and intromission. Intromission count factor corresponds to the number of intromissions required to elicit an ejaculation. Hit rate factor is the ratio of successful penile intromissions to total mount attempts. Copulatory rate factor includes the latency to the first ejaculation, latency to the resumption of copulation following an ejaculation, and average time interval between intromissions. Efficiency generally refers to the ability to accomplish a goal effectively, with minimal waste of time or resources. Therefore, we hypothesized that intra-MPOA injection of OT would improve sexual efficiency; that is, facilitate one or more of the above factors—i.e., increase hit rate factor and decrease initiation, copulatory rate, and intromission count factors, whereas injection of an OTA was expected to inhibit one or more of these factors. We also hypothesized that sexual efficiency would be correlated with OTR binding in the MPOA of sexually experienced male rats.
Methods
Overview of experiments
This study comprises three experiments. Experiment 1 tested the effects of intra-MPOA injections of OT on copulatory behaviors in sexually experienced male rats. Experiment 2a tested the effects of intra-MPOA injections of an OTA on copulatory behaviors in sexually experienced males. In Experiment 2b, the effects of intra-MPOA injection of an OTA on locomotor activity were assessed. In Experiment 3, the relationship between sexual efficiency and OTR binding in the MPOA was explored.
Animals and surgery
Adult Long-Evans/Blue-Spruce rats (Harlan, Indianapolis, IN) were housed singly in large plastic cages in a climate-controlled room, with food and water available ad lib. The light:dark cycle was 14:10, with lights off at 11:00 AM and on at 9:00 PM. Stimulus females of the same strain were housed in a different room. All procedures were in accordance with the National Institutes of Health Guidelines for the Use of Animals and were approved by the University’s Institutional Animal Care and Use Committee.
Stimulus females and ovariectomies (Experiments 1–3)
Females were ovariectomized, using bilateral flank incisions, under ketamine hydrochloride (75 mg/kg) and xylazine hydrochloride (10 mg/kg) anesthesia and allowed 2 weeks to recover. They were injected with 10 µg estradiol benzoate 48 h, and 500 µg progesterone 4 h, before a copulation test. Receptivity of the female was confirmed by allowing three intromissions by a stud male; only females that showed lordosis in response to the stud male were used for sexual experience sessions and behavioral testing.
Experimental males and stereotaxic surgeries (Experiments 1–2)
After becoming sexually experienced (i.e., achieving at least 4 ejaculations prior to behavioral testing), rats were anesthetized with ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (4 mg/kg) and then implanted with a 23g stainless steel guide cannula ending 1 mm above the MPOA. Stereotaxic coordinates (from bregma, AP +2.1; ML +.4; DV −.65 from dura) used for the anterior MPOA were adapted from. The guide cannula was secured in place by stainless steel screws and dental acrylic. A stainless steel stylet was then inserted into the guide cannula to prevent obstruction of the lumen.
Behavioral Testing
Sexual experience sessions (Experiments 1, 2a, 3)
In Experiments 1 and 2a, males were paired with receptive females for a combined total of 4–6 h over a 2 week period; only males that ejaculated at least 4 times during this time period were included in the study. In Experiment 3, 25 male rats had 11 30-min copulation sessions in their home cages over a 6 week period.
Drug microinjections and copulation tests (Experiments 1 and 2a)
Behavioral tests started approximately one week after surgery and occurred between 12.00 and 17.00 h, once a week for 4 weeks (for a total of 4 copulation tests). Each male rat was first taken from the animal colony to another room. The stylet was removed from the guide cannula and replaced with an injection cannula, which was 1 mm longer than the guide cannula. Injections were administered at a rate of 0.5 µl/min using a Harvard infusion pump. After the injection of drug (OT or the OTA (d(CH2)51, Tyr(Me)2, Thr4, Orn8, Tyr-NH29)-vasotocin, Bachem Americas, Inc.) or vehicle, the injection cannula was left in place for 1 min to allow for adequate diffusion of solution. The injection cannula was then replaced with the stylet, and the animal was returned to its home cage. The animal was then taken to a testing room, and a receptive stimulus female was introduced into the male’s home cage approximately 5 min after the microinjection
Each test lasted for 30 min after the male’s first intromission, or for 30 min after introduction of the female if no intromission occurred. The following measures were recorded: frequency of anogenital investigation during the first 5 min of the 30-min copulation test (AGI), latency to first mount (ML), latency to first intromission (IL), latency from first intromission to first ejaculation (EL), postejaculatory interval (PEI, period of quiescence between first ejaculation and subsequent intromission), mount frequency preceding first ejaculation (MF1, first copulatory series), mount frequency preceding second ejaculation (MF2, second copulatory series), total mount frequency for 30-min test (MFT), intromission frequency preceding first ejaculation (IF1, first copulatory series), intromission frequency preceding second ejaculation (IF2, second copulatory series), total intromission frequency for 30-min test (IFT), intromission ratio for first copulatory series [IR1, IR1=IF1/(IF1 + MF1)], intromission ratio for second copulatory series [IR2, IR2=IF2/(IF2 + MF2)], intromission ratio for 30 min test [IRT, IRT=IFT/(IFT + MFT)], inter-intromission interval for the first copulatory series (III, III=EL/IF1), and total ejaculation frequency for the 30-min test (EF).
In Experiment 1, 17 sexually experienced males received 3 doses of OT (12.5 ng, 50 ng, and 100 ng) and saline in counterbalanced order. In Experiment 2a, 21 sexually experienced males received 3 doses of an OTA (50 ng, 200 ng, and 1 µg, all dissolved in 10% DMSO in saline) and vehicle (10% DMSO in saline) in counterbalanced order. OT-treated animals received 0.5 µl of solution (drug and saline) into the MPOA administered at a flow rate of 0.5 µl/min; OTA-treated males received 1 µl of solution (drug and 10% DMSO in saline) administered into the same area and at the same flow rate.
Drug microinjections and open field tests (Experiment 2b)
After the completion of Experiments 1 and 2a, 26 males (17 from Experiment 2a and 9 from other experiments) were used to test the effects of OTA on locomotor activity. A week after the last copulation test, animals were divided into two groups. Half of the animals were randomly assigned to the OTA condition and the other half were assigned to the vehicle condition. Animals in the drug condition received intra-MPOA injections of 1 µg OTA in 1 µl 10% DMSO in saline. Males in the control condition received 1 µl of vehicle (10% DMSO in saline). Five min after drug or vehicle treatment, animals were placed in the center of an open field, and the number of squares crossed in 10 min was recorded. The floor was cleaned with 70% ethanol between tests. The open field was made of plastic (56 cm × 56 cm × 20 cm), and the floor was divided into 16 squares (14 cm ×14 cm).
Sexual efficiency (Experiment 3)
Of the 11 copulation sessions for Experiment 3, only the last 3 sessions were scored. Averages for each male were calculated for intromission latency (IL), intromission frequency before the first ejaculation (IF1), latency to the first ejaculation (EL1), intromission interval before the first ejaculation (III1), postejaculatory interval 1 (PEI1) and intromission ratio before the first ejaculation (IR1). Behavioral measures were divided into 4 factors, based on Sach’s factor analysis. IL scores correspond to Sachs’s initiation factor. IF1 corresponds to the intromission count factor; IR1, to the hit rate factor; and the sum of EL1, PEI1, and III1, to the copulatory rate factor. For each factor, 2 groups of 5 animals were identified. With the exception of hit rate factor, the males with the lowest values were designated “efficient” copulators (n=5), and males with the highest values were assigned to the “inefficient” copulators group (n=5).
Histology (Experiments 1–3)
At the conclusion of each pharmacology experiment, animals were deeply anesthetized with sodium pentobarbital, after which the stylet was removed and replaced with an injection cannula, and a small amount of green food dye was injected into the target area. Animals were then decapitated and their brains were removed. Forty-µm sections were cut and then mounted on slides. Correct cannula placement was verified histologically, with the presence of damage and green food dye serving as indicators of placement. Only animals with correct cannula placements in the MPOA were included in data analyses. Data from animals with cannula placements outside of the MPOA or in the 3rd ventricle were combined and analyzed separately.
Receptor autoradiography (Experiment 3)
Subjects received a lethal dose of sodium pentobarbital, and brains were rapidly extracted and frozen. Twenty-µm brain sections were cut on a cryostat. Tissue containing MPOA was collected and processed for OTR autoradiographic binding using an established method. The OTR was labeled using 125I-OT ligand (PerkinElmer, USA). Slide-mounted sections were thawed for 30 minutes at room temperature, and preincubated in 50 mM Tris-HCl (pH 7.4) for 10 min ×2. Sections were then placed in incubation buffer consisting of 50 mM Tris-HCL (pH 7.4) with 10 mM MgCl2, 0.1% BSA, 0.05% bacitracin, and 50 pM of the OTR ligand. Following a 30-min incubation at room temperature, sections were rinsed 2×5 min in fresh cold buffer, dipped in cold ddH2O, and dried under a stream of cool air. Nonspecific binding was measured by incubating adjacent sections containing the caudal MPOA in solution containing the radiolabeled ligand for the OTR and an OTA, 50 nM Thr4Gly7-OT. Slides were exposed to BioMax MR film (Kodak, Rochester, NY). Optical densities from the resulting autoradiograms were obtained using ImageJ software.
Statistics
In Experiments 1 and 2a, a one-way repeated measures ANOVA followed by a post hoc Tukey’s test was used to compare behavioral effects of various doses of OT and OTA in experienced animals. In Experiment 2b, an independent-samples t-test was used to test the effects of OTA on locomotor activity. In Experiment 3, independent-samples t-tests were used to compare differences in behavior and receptor binding between efficient and inefficient copulators. Statistical significance was defined as p ≤ 0.05.
Results
Experiment 1. Effects of intra-MPOA OT on copulation
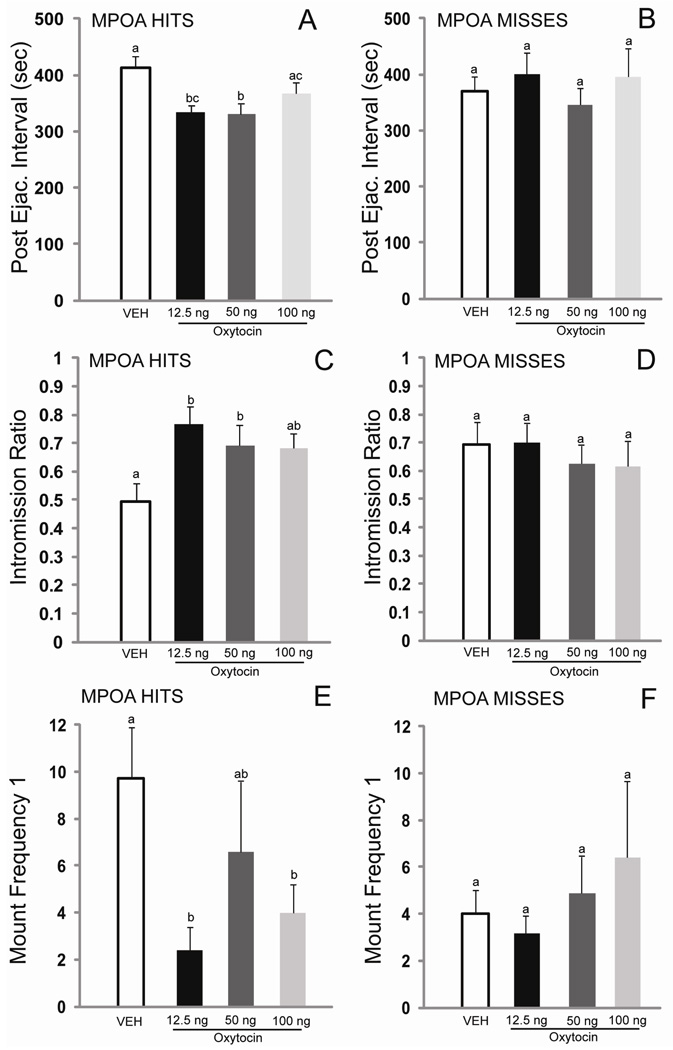
Of the initial 17 males that were cannulated, 10 had correct cannula placements in the MPOA and were included in all data analyses (Figure 1). The 7 males with cannulae outside the MPOA were analyzed separately. There were significant effects of treatment on the following behaviors: postejaculatory interval 1 (PEI1, F(3, 27) = 6.16, p < 0.01), mount frequency 1 (MF1, F(3, 27) = 3.74, p < 0.05), intromission ratio 1 (IR1, F(3, 27) = 3.72, p < 0.05), mount frequency total (MFT, F(3, 27) = 4.18, p < 0.01), and intromission ratio total (IRT, F(3, 27) = 4.47, p < 0.01). Trends for drug-induced decreases in inter-intromission interval 1 (III1, F(3, 27) = 2.70, p = 0.07) and increases in ejaculation frequency (EF, F(3, 27) = 2.34, p = 0.09) were also observed. Post hoc comparisons revealed that the 12.5 ng dose of OT was the most effective dose, significantly decreasing PEI1, MF1, and MFT, and significantly increasing IR1 and IRT, compared to the saline condition. The 50 ng dose of OT significantly decreased PEI1, and significantly increased IR1, compared to the saline condition. The 100 ng dose of OT significantly decreased MF1 and MFT and significantly increased IRT. For animals with placements outside of the MPOA or in the 3rd ventricle (n=7), there was no effect of OT on copulation. Figure 2 provides comparisons of the behavioral effects of OT on PEI, IR, and MF1 when injected inside and outside of the MPOA.
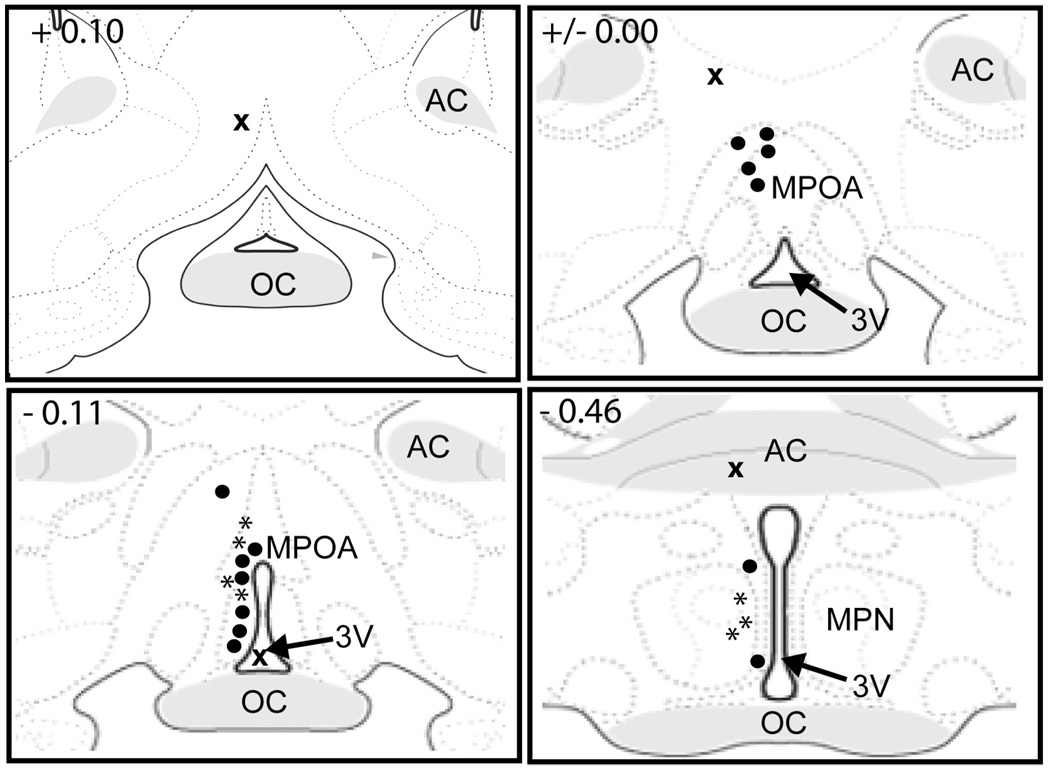
Fig. 1. Representative histology showing cannula placements for intra-MPOA injections of oxytocin.
Dark circles represent placements within the MPOA (n=10); an “x” represents a placement outside of the MPOA (n=7). MPOA, medial preoptic area; MPN, medial preoptic nucleus; AHA, anterior hypothalamic area; AC, anterior commissure; OC, optic chiasm; 3V, 3rd ventricle; OPT, optic tract. Images adapted and modified from Swanson (2003), with permission from Elsevier.
Fig. 2. Oxytocin decreases postejaculatory interval and mount frequency 1, and increases intromission ratio when injected into the MPOA (A, C), but not when injected outside of the MPOA or into the 3rd ventricle (B, D).
Values are given as mean (±SEM). Bars with different letters indicate a significant difference between treatments. p< 0.05.
Experiment 2a. Effects of intra-MPOA OTA on copulation
Of the 21 cannulated males, 14 had correct cannula placements in the MPOA and were included in all data analyses (Figure 3). Four males had cannula placements in the 3rd ventricle or outside of the MPOA, and they were analyzed separately. Three males were excluded because of problems with cannula tubing during copulation tests. In animals with correct cannula placement there were significant effects of treatment on intromission latency (IL, F(3, 36) = 3.94, p < 0.05), anogenital investigation (AGI, F(3, 39) = 3.38, p < 0.05), and intromission ratio 2 (IR2, F(3, 15) = 5.62, p < 0.01) (See Table 1). Trends were observed for mount latency (ML, F(3, 36) = 2.35, p = 0.09), mount frequency 2 (MF2, F(3, 15) = 2.65, p = 0.09), and intromission frequency 2 (IF2, F(3, 15) = 2.85, p = 0.07). Post hoc comparisons revealed that the 1 µg dose of an OTA significantly increased intromission latency compared to the vehicle condition. There was also a significant effect of OTA treatment on anogenital investigation (AGI). Specifically, the 1 µg dose significantly increased AGI compared to the 50 ng, and 200 ng doses, and there was a non-significant reduction (p= 0.08) in AGI in response to the 200 ng dose of an OTA compared to the vehicle condition. All doses of the OTA significantly decreased IR2 compared to the vehicle condition. Results of the post hoc comparisons are in Table 1. Among the 4 males with misplaced cannulae, 2 copulated on only 3 of the 4 tests; therefore, the method of unweighted means was used to calculate values for the missing data points. Analysis of the adjusted data revealed that there was a significant difference only on AGI (F(3, 9) = 4.36, p < 0.05).
Fig. 3. Representative histology showing cannula placements for intra-MPOA injections of an OTA.
Dark circles represent placements within the MPOA (n=14); an “x” represents a placement outside of the MPOA (n=4); stars represent placements in the MPOA for additional animals that were used in the locomotor experiment (n=7). MPOA, medial preoptic area; MPN, medial preoptic nucleus; AC, anterior commissure; OC, optic chiasm; 3V, 3rd ventricle. Images adapted and modified from Swanson (2003), with permission from Elsevier.
Table 1.
Intra-MPOA injections of an OTA inhibit certain aspects of copulation in sexually experienced male rats.
| Treatment | Intromission latency (s) |
Frequency of AGI |
Intromission ratio (2) |
|---|---|---|---|
| Vehicle | 46.5 ± 20.0 | 2.1 ± 0.9 | 0.82 ± 0.11 |
| O 50ng | 64.9 ± 30.2 | 1.2 ± 0.4 | 0.41 ± 0.09*a |
| T 200ng | 65.9 ± 37.0 | 0.6 ± 0.3#a | 0.56 ± 0.06**a |
| A 1µg | 362.7 ± 148.2*a#bc | 3.0 ± 0.9*b**c | 0.50 ± 0.05*a |
Note. Values are given as mean ± SEM.
s, seconds; AGI, anogenital investigation; (2), second copulatory series; MPOA, medial preoptic area; OTA, oxytocin receptor antagonist.
p < 0.10.
p < 0.05.
p < 0.01.
Different from vehicle.
Different from 50 ng OTA.
Different from 200 ng OTA.
Experiment 2b. Effects of intra-MPOA OTA on locomotor activity
Of the initial 26 cannulated males, 21 had correct cannula placements in the MPOA and were included in all data analyses (Figure 3, 14 from Experiment 2 and 7 from other experiments). Administration of an OTA into the MPOA had no significant effect on locomotor activity compared to controls, as measured in an open field, but there was a trend toward an increase (t(19) = 1.73, p = 0.10).
Experiment 3. Relation of OTR binding in the MPOA to sexual efficiency
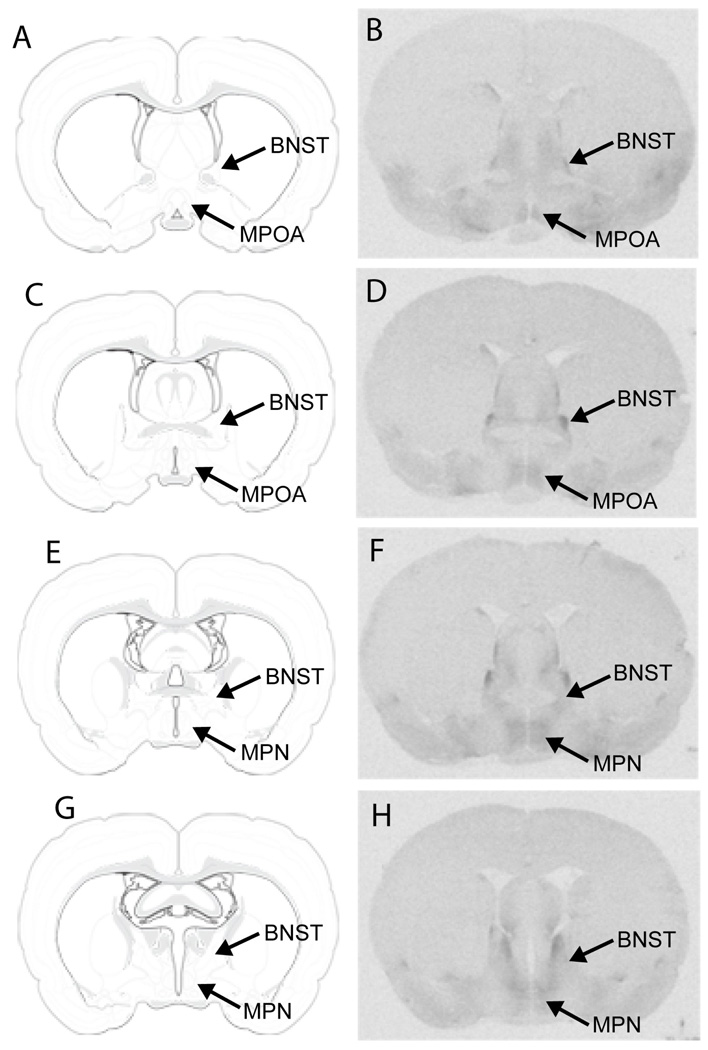
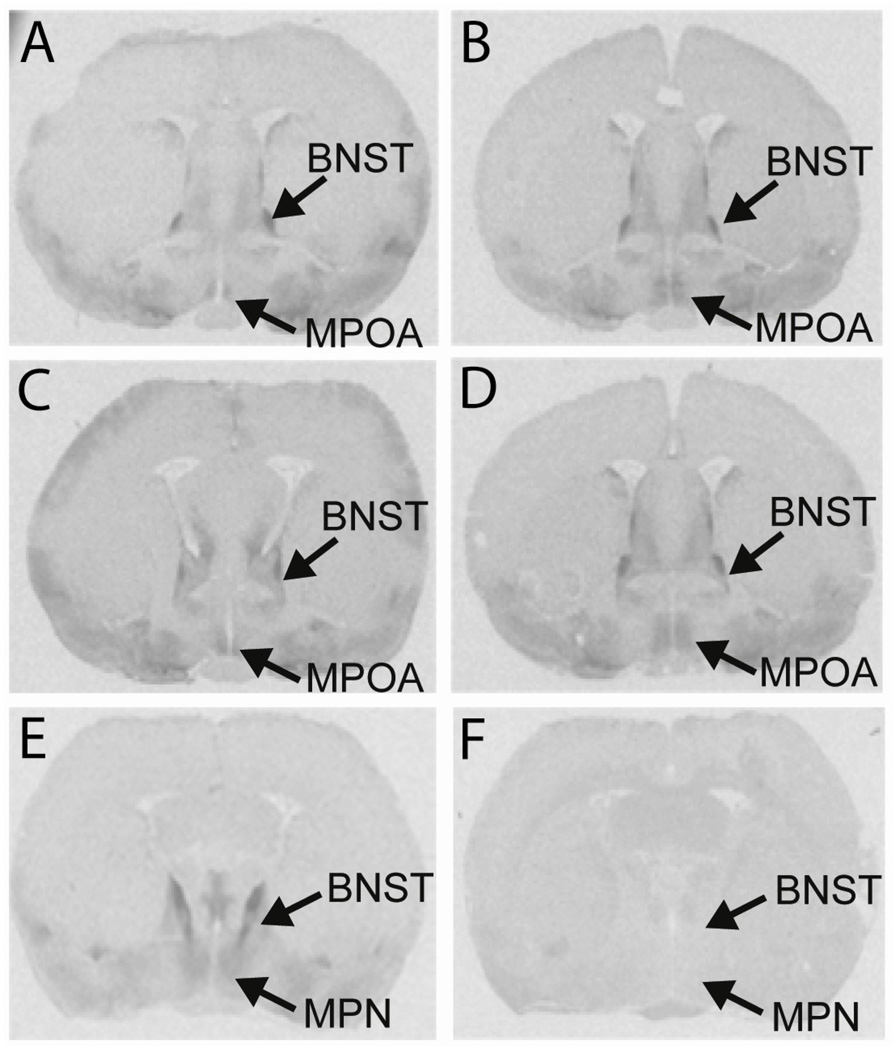
The differences in behavior between efficient and inefficient animals were significant for all four of Sach’s copulatory factors (initiation, t(8) = 4.57, p < 0.01; intromission count, t(8) = 11.60, p < 0.001; hit rate, t(8) = 14.42, p < 0.001; and copulatory rate, t(8) = 9.03, p < 0.001). Detectable OTR binding was observed in all areas of the MPOA—from the most rostral area to the most caudal (Figure 4). A relationship between sexual efficiency and OTR binding in the anterior portion of the MPOA was detected for two factors. For hit rate factor (t(7) = 2.27, p < 0.05) and copulatory rate factor (t(8) = 6.47, p < 0.01), efficient copulators had lower levels of OTR binding in the rostral MPOA compared to inefficient copulators (Table 2). There were no significant differences in receptor binding between groups for initiation factor and intromission count factor. There was no relationship between sexual efficiency and OTR binding in the BNST or caudal MPOA. Figure 7 shows autoradiograms from two males, one efficient (Figure 5A, C) and one inefficient (Figure 5B, D). Non-specific binding is shown in sections containing the caudal MPN (Figure 5E, F).
Fig. 4. Autoradiograms showing oxytocin receptor binding in the MPOA.
rostal MPOA 1 (B), rostral MPOA 2 (D), MPN (F), and caudal MPN (H). Illustrations adapted from Swanson (2003), with permission from Elsevier, showing: rostral MPOA 1 (A), rostral MPOA 2 (C), MPN (E), and caudal MPN (G). MPOA, medial preoptic area; MPN, medial preoptic nucleus; BNST, bed nucleus of the stria terminalis.
Table 2.
Differences in oxytocin receptor binding between efficient and inefficient males
| Behavior/Brain Region | Efficient | Inefficient | |
|---|---|---|---|
| Initiation Factor: | |||
| Rostral MPOA 1 | 65.22 ± 10.81 | 76.42 ± 9.54 | |
| Rostral MPOA 2 | 64.85 ± 9.53 | 88.76 ± 27.04 | |
| MPN | 186.10 ± 12.35 | 161.91 ± 11.45 | |
| Caudal MPN | 107.24 ± 11.99 | 95.41 ± 15.82 | |
| Intro. Count Factor: | |||
| Rostral MPOA 1 | 75.29 ± 16.83 | 81.87 ± 20.25 | |
| Rostral MPOA 2 | 75.46 ± 9.39 | 85.96 ± 13.99 | |
| MPN | 156.16 ± 11.72 | 176.17 ± 7.85 | |
| Caudal MPN | 94.27 ± 17.03 | 105.22 ± 8.18 | |
| Hit Rate Factor: | |||
| Rostral MPOA 1 | 50.16 ± 5.23 | 77.88 ± 9.95* | |
| Rostral MPOA 2 | 77.05 ± 12.33 | 91.27 ± 11.97 | |
| MPN | 174.86 ± 26.80 | 167.74 ± 16.29 | |
| Caudal MPN | 99.82 ± 10.04 | 83.22 ± 11.81 | |
| Cop. Rate Factor: | |||
| Rostral MPOA 1 | 49.45 ± 4.12 | 84.53 ± 3.53** | |
| Rostral MPOA 2 | 69.58 ± 8.90 | 99.57 ± 5.61* | |
| MPN | 157.94 ± 17.68 | 177.74 ± 12.55 | |
| Caudal MPN | 99.02 ± 10.27 | 93.40 ± 14.76 | |
Note. Values represent integrated optical densities and are given as mean ± SEM.
MPOA, medial preoptic area; MPN, medial preoptic nucleus; Intro., intromission; Cop, copulatory.
p < 0.05.
p < 0.001.
Fig. 5. Autoradiograms showing differences in oxytocin receptor binding between groups and non-specific binding.
Efficient males (A, C) have lower levels of binding in the rostral MPOA compared to inefficient (B, D) rats. Sections containing the caudal MPN that were incubated with oxytocin receptor radioligand show high levels of binding (E). Adjacent sections also containing the caudal MPN, from the same animal, that were incubated with the OTR radioligand and an OTA showed almost no binding (F). MPOA, medial preoptic area; MPN, medial preoptic nucleus; BNST, bed nucleus of the stria terminalis.
Discussion
Microinjection of OT into the MPOA facilitated copulation in sexually experienced male rats, whereas an OTA, also injected into the MPOA, inhibited certain aspects of copulation. These inhibitory effects were not likely the result of inhibition of general locomotor activity by drug treatment, as injection of OTA into the MPOA had no significant effect on locomotor activity in an open field. Contrary to expectation, males with higher copulatory rates and hit rates had lower levels of OTR binding in the rostral MPOA compared to inefficient animals.
OT injected into the lateral ventricles, PVN, hippocampus, amygdala, or VTA has induced ex copula penile erections in male rats. In copulation tests, intravenous (iv) administration of OT facilitated the intromission count factor; i.e., decreased the number of intromissions preceding the first and subsequent ejaculations. Systemic and icv administration of OT also decreased the latency to the first ejaculation (EL) and the postejaculatory interval of sexual quiescence (PEI). The results reported here are consistent with these previous findings. Specifically, intra-MPOA injection of OT significantly decreased PEI1 and produced a trend for decreased III1 (copulatory rate factor). All doses of OT injected into the MPOA improved the hit rate preceding the first ejaculation, as well as for the entire 30-minute copulation test, and there was a trend for an increase in the number of ejaculations (EF). The most effective dose was 12.5 ng, as it significantly facilitated five copulatory measures, with trends for two more. The 50 ng and 100 ng doses also facilitated some behaviors, but not as many as the 12.5 ng dose. OT had no significant effect on behavior when injected into the 3rd ventricle or into areas just outside of the MPOA. Interestingly, males that received previous injections of OT into the MPOA performed differently when they received vehicle compared to vehicle-treated males that received previous injections of OT outside of the MPOA or into the third ventricle. It should be noted, however, that animals received three doses of OT and saline in counterbalanced order. Collectively, these data suggest that OT facilitates male sexual behavior, in part, by acting in the MPOA.
Previously, i.c.v. administration of an OTA reduced penile erections, decreased mounts and intromissions, and abolished ejaculation in male rats. In the present study, intra-MPOA injection of an OTA inhibited certain aspects of male sexual behavior. Blocking OTRs in the MPOA decreased anogenital investigation, suggesting that OTR stimulation in the MPOA facilitates this behavior. It has been reported that OT in the MPOA facilitates social recognition in male rats, and being able to recognize a previously encountered conspecific is dependent on the appropriate processing of olfactory cues, which can be obtained via AGI. Therefore, OT in the MPOA may promote general investigation behaviors in both social and sexual contexts. However, the fact that OTA delivered through cannulae in the third ventricle or an area adjacent to the MPOA also decreased AGI suggests that OT in several brain areas may regulate AGI. The most salient behavioral effect of OTA treatment was the significant increase in intromission latency (IL) by the 1 µg dose. It is interesting that OT in the MPOA did not decrease IL in Experiment 1. However, these males were sexually experienced, and the inability of OT to decrease IL further may be attributed to a floor effect for this behavior. The delayed initiation of copulation in response to OTA treatment may have been due, in part, to inhibition of penile erections, which is consistent with previous findings that OT contributes to the regulation of erectile function in the rat. There was also a trend toward a longer ML in response to the OTA, which suggests that OTR stimulation in the MPOA may promote sexual motivation or interest. This interpretation of the data is consistent with previous findings that the MPOA is involved in sexual motivation. However, additional tests that specifically measure sexual motivation are needed to determine whether OT in the MPOA promotes sexual motivation, since longer ML and IL may reflect a general reduction in sexual efficiency and/or erectile function that is independent of motivation. After males achieved an intromission, intra-MPOA injection of an OTA had no effect on behaviors preceding the first ejaculation (i.e., first copulatory series). This was unexpected, as injection of OT into the MPOA facilitated sexual behavior during the first copulatory series in Experiment 1. Microinjection of OTA into the MPOA did decrease sexual efficiency during the second copulatory series. This result is interesting, because the first ejaculation typically decreases the number of intromissions and the time before subsequent ejaculations. In the present study, OTR antagonism in the MPOA prevented this facilitation.
The inverse relationship between OTR binding in the rostral MPOA and sexual efficiency was unexpected. In female rats, there is a positive relationship between OTR binding in the MPOA and maternal licking and grooming and responsivity to pups. Both male sexual behavior and maternal behavior are regulated by a similar chemosensory circuit, which includes the medial amydala, bed nucleus of the stria terminalis, and MPOA. One possible explanation is that efficient males have OTR binding levels in the rostral MPOA that are more male-typical, while inefficient males have levels that are less male-typical. The rostral MPOA contains the anteroventral periventricular nucleus (AVPV), which is a sexually dimorphic area that is larger in females. Therefore, the differences in OTR binding between efficient and inefficient males may simply reflect differences in masculinization and/or defeminization. A more likely explanation for the inverse relationship is that efficient males may have higher levels of OT in the rostral MPOA, which could, in theory, lead to internalization or transcriptional suppression of OTRs (reviewed in Gimpl and Fahrenholz, 2001). This interpretation of the present data is supported by previous findings that sexually vigorous males have higher levels of OT mRNA in the PVN, compared to males that fail to copulate to ejaculation. In addition, in female rats, OTR binding in the PVN is dramatically reduced during lactation, and this, the authors suggest, is likely due to receptor down-regulation caused by high levels of OT in lactating rats. A major question, which cannot be answered with the available data, is whether OTRs in the rostral MPOA contribute to the expression of male sexual behavior. The inverse association between OTR binding in the rostral MPOA and sexual efficiency does not provide any information about a possible causal link. It may be that the behavioral effects of OT reported here are primarily due to OT and OTR activity in other areas of the MPOA (e.g., the medial preoptic nucleus, MPN), and the inability to detect a relationship between sexual efficiency and OTR binding levels in other areas of the MPOA may simply reflect a limitation of the behavioral paradigm or the number of animals used. Therefore, it may be possible that OTR binding levels in the MPN and/or caudal MPOA are (positively) correlated with sexual efficiency, but a larger number of animals may be needed to detect differences between groups.
An interesting possibility is that OT may stimulate dopaminergic activity in the MPOA, which has been shown to facilitate copulation and sexual motivation. OT and dopamine have been shown to interact in the PVN and the mesolimbic dopamine system to enhance genital function and mating (Baskerville et al., 2009;. OT and dopamine also interact in the nucleus accumbens to promote pair bonding in female prairie voles. Although OT neurons in the MPOA were activated (Fos-ir) by mating and also contained D2, D3, and D4 receptors, antagonists to those receptors did not affect Fos-ir, suggesting that those receptor subtypes do not mediate the mating-induced activation of MPOA OT neurons (Baskerville et al., 2009). Those authors did not test the effects of D1 or D5 antagonists; however, earlier research implicates D1-like receptors in the MPOA in both in copula and ex copula touch-based erections (reviewed in Hull et al., 2007). It is interesting that MPOA OT neurons were activated by mating but not by ex copula noncontact erections, whereas PVN OT neurons were activated by ex copula noncontact erections, but not by mating (Baskerville et al., 2009). Similarly, OT injected into the MPOA did not increase spontaneous penile erection episodes in male rats, but OT in the PVN did increase such episodes (Melis et al., 1986). Thus, as Sachs suggested, the mechanisms governing erection are context-specific (2000).
In summary, OT activity in the MPOA is not necessary for copulation in sexually experienced male rats, but it is sufficient to facilitate their copulatory behavior and improve sexual efficiency. These data also suggest that OTR activity in the MPOA stimulates anogenital investigation, facilitates the initiation of copulation, and facilitates ejaculations after the first. Thus, OT activity in the MPOA may regulate certain aspects of sexual behavior and not others.
Acknowledgements
This work was supported by NIH grant MH040826 to E.M. Hull. We would like to thank Drs. Zuoxin Wang, Mohamed Kabbaj, and Yan Liu for their advice and technical assistance. This research was part of the doctoral dissertation of M. Gil.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argiolas A, Collu M, Gessa GL, Melis MR, Serra G. The oxytocin antagonist d(CH2)5Tyr(Me)-Orn8-vasotocin inhibits male copulatory behaviour in rats. Eur. J. Pharmacol. 1988;149:389–392. doi: 10.1016/0014-2999(88)90675-9. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Gessa GL. Central functions of oxytocin. Neurosci. Biobehav. Rev. 1991;15:217–231. doi: 10.1016/s0149-7634(05)80002-8. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR, Gessa GL. Intraventricular oxytocin induces yawning and penile erection in rats. Eur. J. Pharmacol. 1985;117:395–396. doi: 10.1016/0014-2999(85)90018-4. [DOI] [PubMed] [Google Scholar]
- Arletti R, Bazzani C, Castelli M, Bertolini A. Oxytocin improves male copulatory performance in rats. Horm. Behav. 1985;19:14–20. doi: 10.1016/0018-506x(85)90002-9. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. Sexual behavior of aging male rats is stimulated by oxytocin. Eur. J. Pharmacol. 1990;179:377–381. doi: 10.1016/0014-2999(90)90178-9. [DOI] [PubMed] [Google Scholar]
- Arletti R, Benelli A, Bertolini A. Oxytocin involvement in male and female sexual behavior. Ann. N. Y. Acad. Sci. 1992;652:180–193. doi: 10.1111/j.1749-6632.1992.tb34354.x. [DOI] [PubMed] [Google Scholar]
- Arletti R, Calza L, Giardino L, Benelli A, Cavazzuti E, Bertolini A. Sexual impotence is associated with a reduced production of oxytocin and with an increased production of opioid peptides in the paraventricular nucleus of male rats. Neurosci. Lett. 1997;233:65–68. doi: 10.1016/s0304-3940(97)00478-3. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Allard J, Wayman C, Douglas AJ. Dopamine-oxytocin interactions in penile erection. Eur. J. Neurosci. 2009;30:2151–2164. doi: 10.1111/j.1460-9568.2009.06999.x. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Brain vasotocin pathways and the control of sexual behaviors in the bullfrog. Brain Res. Bull. 1997;44:345–350. doi: 10.1016/s0361-9230(97)00213-x. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Jirikowski GF, Greer ER, Pedersen CA. Medial preoptic area oxytocin and female sexual receptivity. Behav. Neurosci. 1989;103:655–662. doi: 10.1037//0735-7044.103.3.655. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Johns JM, Faggin BM, Senger MA, Pedersen CA. Infusion of an oxytocin antagonist into the medial preoptic area prior to progesterone inhibits sexual receptivity and increases rejection in female rats. Horm. Behav. 1994;28:288–302. doi: 10.1006/hbeh.1994.1024. [DOI] [PubMed] [Google Scholar]
- Cantor JM, Binik YM, Pfaus JG. Chronic fluoxetine inhibits sexual behavior in the male rat: reversal with oxytocin. Psychopharmacology. 1999;144:355–362. doi: 10.1007/s002130051018. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J. Clin. Endocrinol. Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 2000;12:1145–1148. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Klein MJ. Oxytocin receptors on oxytocin neurones: histoautoradiographic detection in the lactating rat. J. Physiol. 1994;480(Pt 1):155–161. doi: 10.1113/jphysiol.1994.sp020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelez H, Poirier S, Facchinetti P, Allers KA, Wayman C, Alexandre L, Giuliano F. Neuroanatomical evidence for a role of central melanocortin-4 receptors and oxytocin in the efferent control of the rodent clitoris and vagina. J. Sexual Med. 2010;7:2056–2067. doi: 10.1111/j.1743-6109.2010.01760.x. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol. Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM, Muschamp JW. The neurochemistry of male sexual behavior. In: Lajtha A, editor. Behavioral Neuroendocrinology, Neurochemistry, and Molecular Neurobiology. New York: Springer Verlag; 2007. pp. 37–94. [Google Scholar]
- Hull EM, Meisel RL, Sachs BD. Male Sexual Behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain, and Behavior. Vol. 1. San Diego: Academic Press; 2002. pp. 3–137. [Google Scholar]
- Hull EM, Rodriguez-Manzo G. Male Sexual Behavior. In: Pfaff DW, editor. Hormones, Brain, and Behavior. 2nd ed. Vol. 1. Elsevier Press: Amsterdam; 2009. pp. 5–65. [Google Scholar]
- Kleitz-Nelson HK, Dominguez JM, Cornil CA, Ball GF. Is sexual motivational state linked to dopamine release in the medial preoptic area? Behav. Neurosci. 2010;124:300–304. doi: 10.1037/a0018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Mahlmann S, Meyerhof W, Hausmann H, Heierhorst J, Schonrock C, Zwiers H, Lederis K, Richter D. Structure, function, and phylogeny of [Arg8]vasotocin receptors from teleost fish and toad. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1342–1345. doi: 10.1073/pnas.91.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis MR, Argiolas A, Gessa GL. Oxytocin-induced penile erection and yawning: site of action in the brain. Brain. Res. 1986;398:259–265. doi: 10.1016/0006-8993(86)91485-x. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, Boi A, Ferri GL, Argiolas A. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. Eur. J. Neurosci. 2007;26:1026–1035. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- Melis MR, Spano MS, Succu S, Argiolas A. The oxytocin antagonist d(CH2)5Tyr(Me)2-Orn8-vasotocin reduces non-contact penile erections in male rats. Neurosci. Lett. 1999;265(3):171–174. doi: 10.1016/s0304-3940(99)00236-0. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Sanna F, Boi A, Argiolas A. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur. J. Neurosci. 2009;30:1349–1357. doi: 10.1111/j.1460-9568.2009.06912.x. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav. Neurosci. 1994;108:1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. 2nd ed. New York: Academic Press; 1979. [Google Scholar]
- Pfaus JG, Phillips AG. Role of dopamine in anticipatory and consummatory aspects of sexual behavior in the male rat. Behav. Neurosci. 1991;105:727–743. doi: 10.1037//0735-7044.105.5.727. [DOI] [PubMed] [Google Scholar]
- Pittman QJ, Spencer SJ. Neurohypophysial peptides: gatekeepers in the amygdala. Trends Endocrinol. Metab. 2005;16:343–344. doi: 10.1016/j.tem.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur. Neuropsychopharmacol. 1991;1(4):555–560. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- Sachs BD. Conceptual and Neural Mechanisms of Masculine Copulatory Behavior. In: McGill TE, Dewsbury DA, Sachs BD, editors. Sex and Behavior. New York and London: Plenum Press; 1978. pp. 267–295. [Google Scholar]
- Sachs BD. Contextual approaches to the physiology and classification of erectile function, erectile dysfunction, and sexual arousal. Neurosci. Biobehav. Rev. 2000;24:541–560. doi: 10.1016/s0149-7634(00)00022-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Organization and regulation of sexually dimorphic neuroendocrine pathways. Behav. Brain. Res. 1998;92:195–203. doi: 10.1016/s0166-4328(97)00191-5. [DOI] [PubMed] [Google Scholar]
- Stoneham MD, Everitt BJ, Hansen S, Lightman SL, Todd K. Oxytocin and sexual behaviour in the male rat and rabbit. J. Endocrinol. 1985;107:97–106. doi: 10.1677/joe.0.1070097. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Melis T, Boi A, Argiolas A, Melis MR. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extra-cellular dopamine in the nucleus accumbens: involvement of central oxytocin. Neuropharmacology. 2007;52:1034–1043. doi: 10.1016/j.neuropharm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Cocco C, Melis T, Boi A, Ferri GL, Argiolas A, Melis MR. Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: role of nitric oxide and cyclic GMP. Eur. J. Neurosci. 2008;28:813–821. doi: 10.1111/j.1460-9568.2008.06385.x. [DOI] [PubMed] [Google Scholar]
- Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S. Oxytocin receptors in the central nervous system. Distribution, development, and species differences. Ann. N. Y. Acad. Sci. 1992;652:29–38. doi: 10.1111/j.1749-6632.1992.tb34343.x. [DOI] [PubMed] [Google Scholar]
- Veronneau-Longueville F, Rampin O, Freund-Mercier MJ, Tang Y, Calas A, Marson L, McKenna KE, Stoeckel ME, Benoit G, Giuliano F. Oxytocinergic innervation of autonomic nuclei controlling penile erection in the rat. Neuroscience. 1999;93:1437–1447. doi: 10.1016/s0306-4522(99)00262-6. [DOI] [PubMed] [Google Scholar]
- Vignozzi L, Filippi S, Luconi M, Morelli A, Mancina R, Marini M, Vannelli GB, Granchi S, Orlando C, Gelmini S, Ledda F, Forti G, Maggi M. Oxytocin receptor is expressed in the penis and mediates an estrogen-dependent smooth muscle contractility. Endocrinology. 2004;145:1823–1834. doi: 10.1210/en.2003-0962. [DOI] [PubMed] [Google Scholar]
- Wang ZX, Liu Y, Young LJ, Insel TR. Hypothalamic vasopressin gene expression increases in both males and females postpartum in a biparental rodent. J. Neuroendocrinol. 2000;12:111–120. doi: 10.1046/j.1365-2826.2000.00435.x. [DOI] [PubMed] [Google Scholar]
- Warner RK, Thompson JT, Markowski VP, Loucks JA, Bazzett TJ, Eaton RC, Hull EM. Microinjection of the dopamine antagonist cis-flupenthixol into the MPOA impairs copulation, penile reflexes and sexual motivation in male rats. Brain Res. 1991;540:177–182. doi: 10.1016/0006-8993(91)90505-p. [DOI] [PubMed] [Google Scholar]
- Witt DM, Insel TR. Increased Fos expression in oxytocin neurons following masculine sexual behavior. J. Neuroendocrinol. 1994;6:13–18. doi: 10.1111/j.1365-2826.1994.tb00549.x. [DOI] [PubMed] [Google Scholar]