Abstract
Retinoic acid (RA) is purported to be required for expression of genes controlling proximodistal (Meis2) or anteroposterior (Shh) limb patterning. Embryos lacking RDH10, the primary enzyme synthesizing retinaldehyde during mouse development, survive until E14.5 with stunted forelimbs but apparently normal hindlimbs. Using embryos carrying the RARE-lacZ RA-reporter transgene, we show that endogenous RA activity in Rdh10trex/trex mutants is detected in neuroectoderm but not limbs during initiation and patterning. Treatment of Rdh10 mutants with 25nM RA restores RARE-lacZ activity to limb mesoderm, validating RARE-lacZ and verifying that RA is absent in mutant limbs. In Rdh10 mutants, hindlimbs exhibit normal Meis2/Shh expression and skeletal patterning, and although forelimbs are growth-retarded their Meis2 expression remains normal. Later in development, Rdh10 mutants lack interdigital RA activity and accordingly fail to exhibit normal loss of interdigital mesenchyme. These findings demonstrate that RA is unnecessary for limb patterning but required later for interdigital tissue loss.
Keywords: Limb bud patterning, interdigital development, retinoic acid synthesis, Rdh10, RARE-lacZ
INTRODUCTION
A role for retinoic acid (RA) signaling in limb patterning is a controversial issue and conflicting lines of evidence have yet to be resolved (Tabin and Wolpert, 2007; Zeller et al., 2009). Over the years, it has been suggested that RA is important for either A-P or P-D limb patterning. In the posterior limb mesenchyme, the zone of polarizing activity (ZPA) is recognized to be critical for establishing the A-P limb axis through the morphogen SHH (Riddle et al., 1993; Chiang et al., 2001; Ros et al., 2003). Early experiments in chick involving pharmacological doses of RA found that RA could induce the specification of ZPA cells, hence a posterior identity (Tickle et al., 1982; Wanek et al., 1991). However, a lower RA dose (0.01mg/ml still providing higher than physiological levels of RA (Horton and Maden, 1995)) has since been found to not have the same effect (Helms et al., 1994). High levels of RA were ultimately found to induce ectopic expression of Shh (Riddle et al., 1993), but endogenous RA was found to be unnecessary for limb Shh expression (Zhao et al., 2009). In the distal-most tip of the limb bud, signaling by FGF8 plus other redundant FGFs expressed in the apical ectodermal ridge (AER) is widely accepted to direct differentiation of underlying limb mesodermal progenitors, and is fundamental for P-D patterning and outgrowth (Lewandoski et al., 2000; Mariani et al., 2008). RA has been suggested to act from the proximal limb bud as an opposing signal to FGF to drive P-D patterning, through experiments that have demonstrated the capacity of RA to upregulate, and FGF to downregulate, the proximal limb markers Meis1 and Meis2 (Mercader et al., 2000; Yashiro et al., 2004). Whereas the requirement of FGF to repress Meis genes in the distal limb is supported by FGF loss-of-function studies (Mariani et al., 2008), the evidence for induction of Meis by RA is based on administration of pharmacological concentrations of RA or inhibitors (Mercader et al., 2000) or loss of Cyp26b1 function which increases RA activity distally (Yashiro et al., 2004). In contrast, induction of Meis2 by RA is not supported by Raldh2 loss-of-function studies that eliminate RA synthesis (Zhao et al., 2009).
The production of RA during mammalian embryogenesis is primarily controlled by tissue-specific expression of two enzymes in two distinct steps: retinol dehydrogenase-10 (RDH10) that synthesizes retinaldehyde from retinol, and retinaldehyde dehydrogenase-2 (RALDH2) that synthesizes RA from retinaldehyde (Duester, 2008). Additional enzymes contribute to RA synthesis in embryos or adult tissues (RALDH1, RALDH3, other RDHs, and alcohol dehydrogenases) but only Raldh2 and Rdh10 are embryonic lethal following genetic ablation (Niederreither et al., 1999; Sandell et al., 2007; Duester, 2008). Further control of RA distribution is governed by tissue-specific expression of cytochrome P450 enzymes (CYP26A1, CYP26B1, and CYP26C1) that metabolize RA and act to keep RA at bay in tissues where its influence is undesirable (Abu-Abed et al., 2002; Tahayato et al., 2003). RA can be detected in midgestation mouse embryos in many tissues due to the widespread (and sometimes overlapping) expression of Raldh2 (Niederreither et al., 1997) and Rdh10 (Cammas et al., 2007; Sandell et al., 2007). It is accepted that RA is a readily diffusible molecule and acts in a paracrine manner to regulate gene expression (Duester, 2008). Accordingly, not all tissues exhibiting RA presence necessarily require RA for a local signaling event. RA is present during limb bud outgrowth, initially throughout the entire early limb bud (Zhao et al., 2009), then shortly afterwards confined to the most proximal region due to the activity of CYP26B1 in the distal limb bud (MacLean et al., 2001). RA is not actually generated in the limb bud at these early stages, but diffuses from cells in the underlying trunk mesoderm that express Raldh2 (Niederreither et al., 1997; Zhao et al., 2009) and Rdh10 (Cammas et al., 2007; Sandell et al., 2007). At later stages, after the proximodistal (P-D) and anteroposterior (A-P) axes have been specified, RA is present in the interdigital mesenchyme generated from Raldh2 expressed in that tissue (Zhao et al., 2010).
Evidence from the Raldh2−/− mouse model (requiring maternal administration of RA at E7.5 in order to rescue early lethality) has questioned the role of RA in both A-P and P-D patterning. Raldh2−/− hindlimbs exhibit a normal Shh expression pattern, as well as normal expression of the P-D genes Fgf8 and Meis2, and have no detectable RA activity in the proximal limb bud or underlying mesoderm (Zhao et al., 2009). Stunted forelimbs observed in Raldh2−/− embryos were suggested to be due to ectopic FGF8 signaling extending into the trunk from its normal signaling centers in the primitive streak and heart in the absence of repression by trunk RA (Zhao et al., 2009). These observations suggest that Raldh2−/− stunted forelimbs are due to disruption of forelimb initiation and not patterning defects. Taken together, a plausible explanation for these findings is that the forelimb and hindlimb may rely on similar instructive signals other than RA to implement patterning, but the forelimb additionally requires RA for repression of trunk Fgf8 as a permissive signal to allow forelimb initiation to proceed normally. Later in development when the hindlimb initiates, evidence has been provided that the posterior Fgf8 boundary no longer requires RA (Sirbu and Duester, 2006); this observation may explain why loss of RA in Raldh2−/− embryos does not result in stunting of hindlimb growth.
As an independent genetic loss-of-function model to examine the consequences of losing limb RA activity, we studied Rdh10trex/trex mutant embryos which exhibit stunted forelimbs but apparently normal hindlimbs similar to RA-rescued Raldh2−/− embryos (Sandell et al., 2007). Embryos with the ethylnitrosourea-generated Rdh10trex/trex mutation have a major advantage over Raldh2−/− embryos in that there is no requirement of early RA treatment to rescue lethality, thus avoiding unknown influences of exogenous RA treatment. Although it was initially reported that this mutation resulted in lethality by E13.5, some mutant embryos survive as long as E14.5, allowing analysis of defects in development of limb digits. Here, we show that RA activity in Rdh10 mutants is present in the neural tube, but is completely absent in both forelimbs and hindlimbs during their initial development and patterning, including the proximal limb bud and underlying lateral plate and somitic mesoderm. Thus, RDH10 contributes the entire source of retinaldehyde needed for RA synthesis in somitic and lateral plate mesoderm in the vicinity of the limb. Although Rdh10 mutant forelimbs are stunted, they still express genes needed for patterning suggesting that their defect lies in the limb initiation phase of limb growth. Rdh10 mutant hindlimbs develop with normal expression of A-P and P-D patterning genes, and exhibit a normal complement of developing bone structures without any influence from RA activity. Only later in development do we find a requirement for RDH10 and an instructive role for RA in the hindlimb—in the process of interdigital mesenchyme tissue loss during digit formation.
RESULTS
Rdh10 is required for limb RA activity during limb bud initiation and early outgrowth
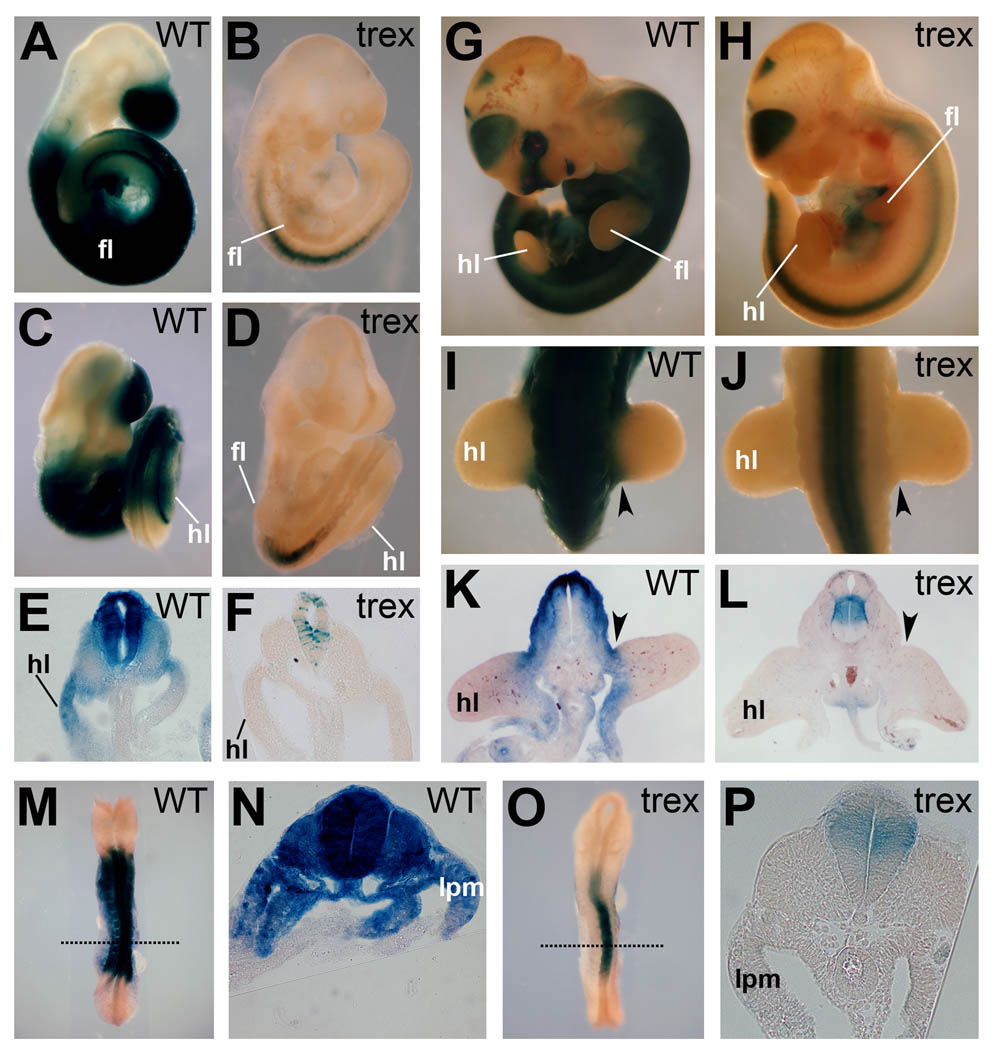
In order to assess the changes in RA activity in the developing hindlimb of Rdh10trex/trex mutant embryos we examined wild-type and Rdh10trex/trex mutant mice carrying the RARE-lacZ RA reporter transgene (Rossant et al., 1991) at the earliest stage that the hindlimb bud is visible (E9.5) and 24 hours later (E10.5) when the process of hindlimb patterning is in progress. At E9.5, RA activity in Rdh10 mutants was missing from the hindlimb and underlying mesoderm, but was detected in the neural tube (Fig. 1B,D,F); in wild-type embryos RA activity was detected throughout the whole of the early hindlimb bud plus neighboring lateral plate and somitic mesoderm (Fig. 1A,C,E). RA remained absent in hindlimbs and underlying lateral plate mesoderm of Rdh10 mutants at E10.5 (Fig. 1H,J,L), whereas RA activity in wild-type embryos was present in the proximal region of the limb bud and trunk mesoderm (Fig. 1G,I,K). These findings demonstrate that the Rdh10 mutant hindlimb lacks RA signaling from its earliest point of development until the point when P-D and A-P patterning has been established.
Fig. 1.
Rdh10 is required for limb RA activity during limb bud initiation and early outgrowth. RARE-lacZ staining in wild-type (WT) and Rdh10trex/trex mutant embryos at E9.5 (A–F), E10.5 (G–L), and E8.5 (M–P). Rdh10 mutants exhibit RA activity in the neural tube but not in mesoderm in or around the limb field. (E,F,K,L) Transverse sections through hindlimbs. (N,P) Transverse sections through presumptive forelimb region (indicated by dotted lines in M,O). Staining for 24h (shown in M–P) did not result in detection of RARE-lacZ in trunk mesoderm including that fated to become limb. hl, hindlimb bud; fl, forelimb bud; lpm, lateral plate mesoderm. Arrowheads indicate proximal hindlimb bud region.
In considering the possibility of early specification, whereby limb patterning might be determined in flank mesoderm prior to the onset of limb budding (Hasson et al., 2007; Naiche and Papaioannou, 2007), we evaluated expression of the RARE-lacZ reporter at E8.5 (12 somites). At this stage, presumptive hindlimb mesoderm has not yet formed, but the presumptive forelimb mesoderm is situated in the trunk lateral plate mesoderm centered at the level of somite-10. RA activity was not detected in lateral plate or somitic mesoderm at the level of the presumptive forelimb in Rdh10 mutants (Fig. 1O–P), compared to strong activity throughout wild-type mesodermal layers (Fig. 1M–N). This finding indicates RA signaling is absent from forelimb and hindlimb progenitors at a very early stage in Rdh10trex/trex embryos and rules out the possibility that these embryos possess an early phase of lateral plate mesodermal RA signaling that could affect limb patterning.
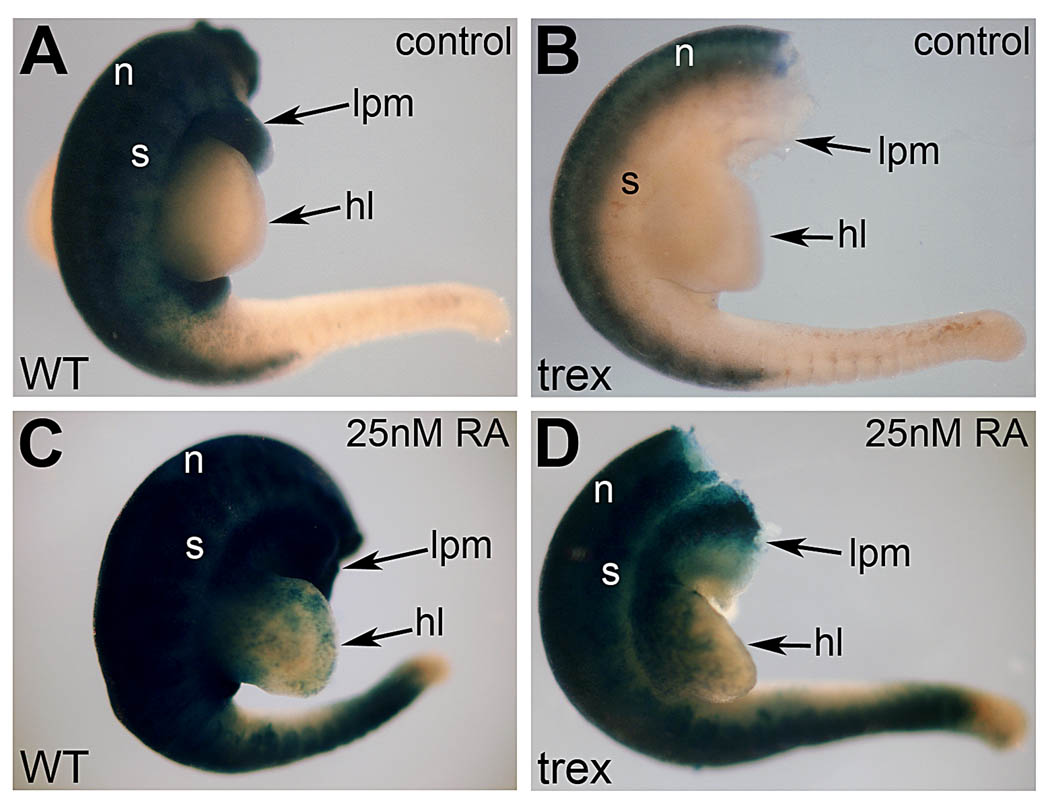
To validate the ability of RARE-lacZ transgenic mice to detect physiological levels of RA in the hindlimb bud, we performed an in vitro assay whereby E10.5 hindlimbs were cultured in 25nM RA overnight—below the 30nM reported to exist endogenously in mouse E10.5 limbs (Horton and Maden, 1995). Rdh10 mutant hindlimbs cultured in the absence of RA exhibited a pattern of RA activity typical of an Rdh10 mutant embryo with strong staining present in the neural tube, but absent in the limb and surrounding lateral plate mesoderm and somites (Fig. 2A–B). Following culture in 25nM RA, the Rdh10 mutant exhibited staining for RA activity in somites and lateral plate mesoderm in a manner similar to wild-type embryos, plus staining was now observed in the hindlimb bud (Fig. 2C–D). This clearly demonstrates that the RARE-lacZ reporter transgene is capable of detecting endogenous concentrations of RA in the hindlimb, and shows that the Rdh10 mutant hindlimb indeed lacks RA signaling. Our findings are consistent with previous studies demonstrated that the trex mutant version of RDH10 is a destabliized protein with no measurable retinol oxidation activity, suggesting that it may be a null mutant (Sandell et al., 2007).
Fig. 2.
Validation of RARE-lacZ as a sensitive RA reporter. RARE-lacZ staining in wild-type (A,C) and Rdh10trex/trex mutant (B,D) embryos cultured for 18h in the absence of RA (control) or presence of a low physiological dose of RA (25nM). RA treatment rescues RA activity in the proximal hindlimb bud and trunk mesoderm of the mutant. This observation demonstrates that RARE-lacZ can detect low levels of RA in limb buds and shows that Rdh10 mutant hindlimbs indeed lack RA signaling. hl, hindlimb bud; lpm, lateral plate mesoderm; n, neural tube; s, somitic mesoderm.
Hindlimb induction and patterning occurs normally in Rdh10 mutant embryos
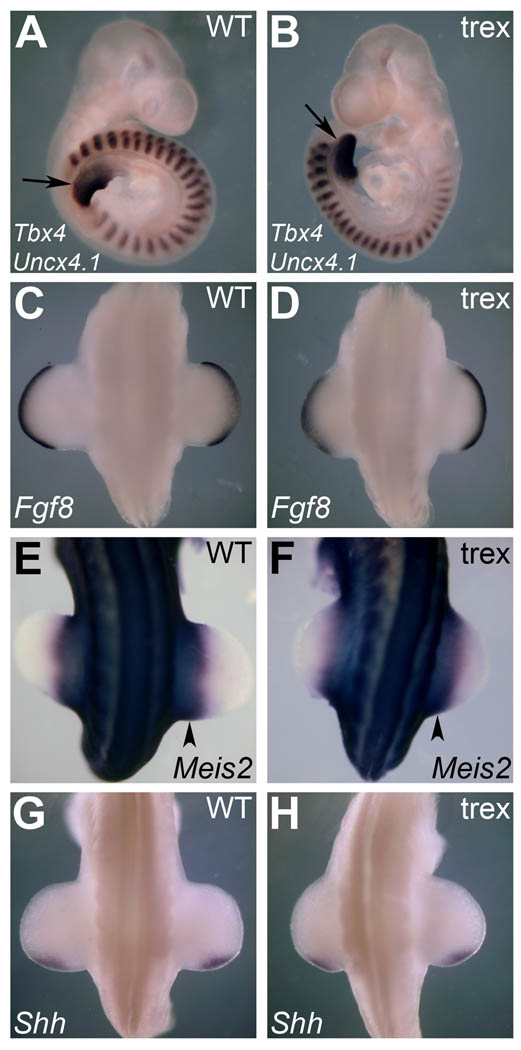
Having established that Rdh10 mutants develop hindlimb buds in the absence of RA activity in the limb fields, we examined the spatiotemporal expression of genes associated with hindlimb induction and patterning via in situ hybridization. An early indicator of hindlimb development is expression of Tbx4 in the lateral plate mesoderm around E9.5 (Gibson-Brown et al., 1996). Both the wild-type and Rdh10 mutant displayed equivalent Tbx4 expression at the same somite level (Fig. 3A–B). Furthermore, there was no observable difference in Tbx4 expression level, or size of the Tbx4 expression domain, between wild-type and mutant; demonstrating that RA is not required to obtain a normal Tbx4 expression domain in the hindlimb field.
Fig. 3.
Hindlimb induction and patterning occurs normally in Rdh10trex/trex mutant embryos. In situ hybridization of hindlimb induction and patterning genes at E9.5 and E10.5 reveals no difference between wild-type and mutant. (A–B) E9.5; Tbx4 representing the early hindlimb (arrows) and Uncx4.1 marking the somites. (C–D) E10.5; Fgf8 expression distally in hindlimb AER. (E–F) E10.5; Meis2 expression in the proximal hindlimb bud region (arrowheads). (G–H) E10.5; Shh expression posteriorly in the hindlimb ZPA.
At E10.5 we went on to examine gene expression patterns that signify A-P and/or P-D patterning within the hindlimbs. By this stage a number of key patterning genes are expressed that demonstrate the progression of patterning across the different limb axes. At the distal tip of the limb bud, expression of Fgf genes from the AER is known to be crucial for normal limb bud outgrowth and for establishing a normal complement of correctly patterned skeletal elements along the P-D axis (Lewandoski et al., 2000; Mariani et al., 2008). While the mechanism of the AER’s influence on the limb bud remains controversial, Fgf8 is well established as the principal gene that exerts this influence (Mariani et al., 2008). Rdh10 mutant embryos exhibited normal Fgf8 expression in the AER at E10.5, and outgrowth of the hindlimb was very similar to that of wild-type embryos of a similar size (Fig. 3C–D).
RA activity in the proximal limb bud has been suggested to act as an opposing signal to that of FGF8 in the AER—via experiments that have demonstrated upregulation of the P-D patterning marker Meis2 when RA levels are increased (Mercader et al., 2000; Yashiro et al., 2004). We show here that removal of all traces of endogenous RA activity in the hindlimb and surrounding tissues by RDH10 inactivation has no effect on Meis2 expression at E10.5 (in the proximal limb bud or neighboring mesoderm), demonstrating that this P-D patterning marker is activated independently of RA (Fig. 3E–F).
At E10.5, Shh expression in the posterior mesenchyme of the limb bud defines a zone of polarizing activity (ZPA) and is pivotal in establishing A-P identity (Riddle et al., 1993). While RA has been suggested to be a key upstream factor in ZPA function and the limb SHH signaling pathway (Tickle et al., 1982; Riddle et al., 1993), we found that Shh expression in Rdh10 mutant hindlimbs was maintained in the correct spatiotemporal manner in the absence of RA (Fig. 3G–H). This shows that RA is not required to act upstream to establish the Shh posterior limb expression domain. Taken together, we show that in the absence of RA detection in the limb or underlying tissue, gene expression patterns representing the initiation of the hindlimb bud and establishment of A-P and P-D polarity are normal and therefore not dependant on RDH10 or RA signaling.
Rdh10 mutant forelimbs express genes important for patterning
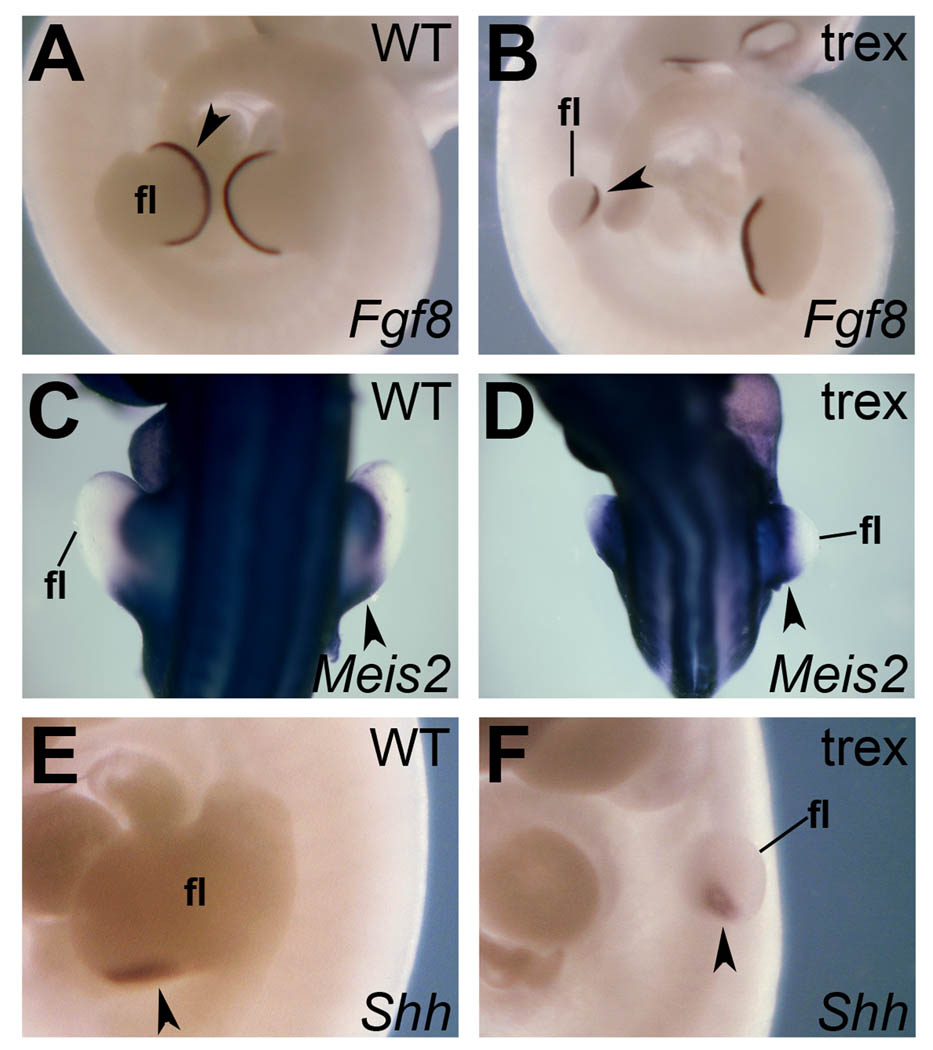
To complement the spatiotemporal mRNA expression analysis on the hindlimb, we conducted the same in situ analyses at E10.5 for patterning genes in the forelimb. While the forelimb in the Rdh10 mutant is clearly stunted, we still observed a small domain of Fgf8 expression in the distal forelimb (AER) (Fig. 4A–B), normal Meis2 expression in the proximal limb bud (Fig. 4C–D), and a domain of Shh expression in the posterior limb mesenchyme that extended a little further distal than normal (Fig. 4E–F). These findings show that RA is not required for induction of these patterning genes, nor to limit their expression to roughly the normal location after taking into account the small size of the Rdh10 mutant forelimb. These results are more consistent with a primary role for RA in forelimb growth with affects on patterning being secondary to the growth defect (Zhao et al., 2009).
Fig. 4.
Expression of patterning genes in Rdh10trex/trex forelimbs. In situ hybridization was used to examine forelimbs in E10.5 wild-type and Rdh10 mutant embryos. (A–B) Fgf8 expression is observed distally in forelimb AER. (C–D) Meis2 expression is seen in the proximal forelimb bud region. (E–F) Shh expression is observed in the posterior/distal region of the forelimb. fl, forelimb bud. Arrowheads mark the relevant expression pattern.
The Rdh10 mutant hindlimb has a normal complement of skeletal elements at E14.5
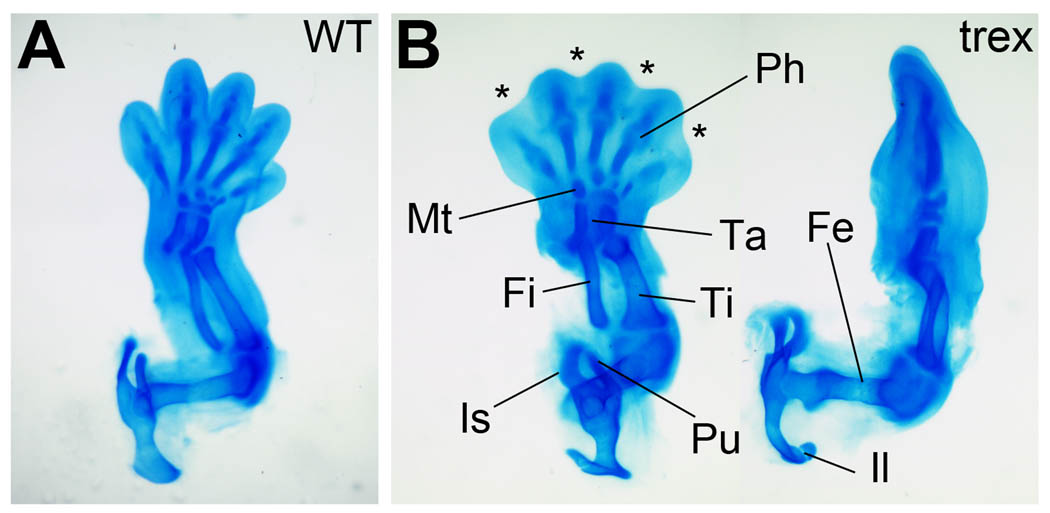
Following expression of a normal compliment of limb patterning genes at E10.5, we examined the hindlimb at E14.5 in Rdh10 mutant embryos to determine whether patterning had completed correctly. At this stage, five days following hindlimb initiation, the three major regions of the limb are evident (stylopod, zeugopod and autopod) and their respective skeletal elements have taken on their specific identity which can be detected via Alcian Blue staining of cartilage (Hogan et al., 1994). All the skeletal elements of the hindlimb in the Rdh10 mutant, from the pelvis to the phalanges, were present and exhibited relatively normal 3-dimensional size, shape, and spatial association to each other confirming the correct patterning of all three hindlimb axes (Fig. 5A–B). The Rdh10 mutant hindlimb appears to be slightly smaller than wild-type, likely due to the fact that E14.5 is the very limit of survival for this mutant and this observation is consistent with a more general growth retardation that can be observed in mutants of this stage.
Fig. 5.
The Rdh10trex/trex mutant hindlimb has a normal complement of skeletal elements at E14.5. Alcian blue staining of cartilage showing all the skeletal elements of the hindlimb. (A) Wild-type; ventral view. (B) Mutant; ventral view (left) and anterior view (right) to more easily observe femur. Ph, phalanges; Mt, metatarsals; Ta, tarsals; Fi, fibula; Ti, tibia; Fe, femur; Pu, pubis; Is, ischium; Il, ilium. Asterisks mark regions of interdigital webbing retained by mutant autopod.
Rdh10 is essential for interdigital RA-activity and loss of interdigital mesenchyme
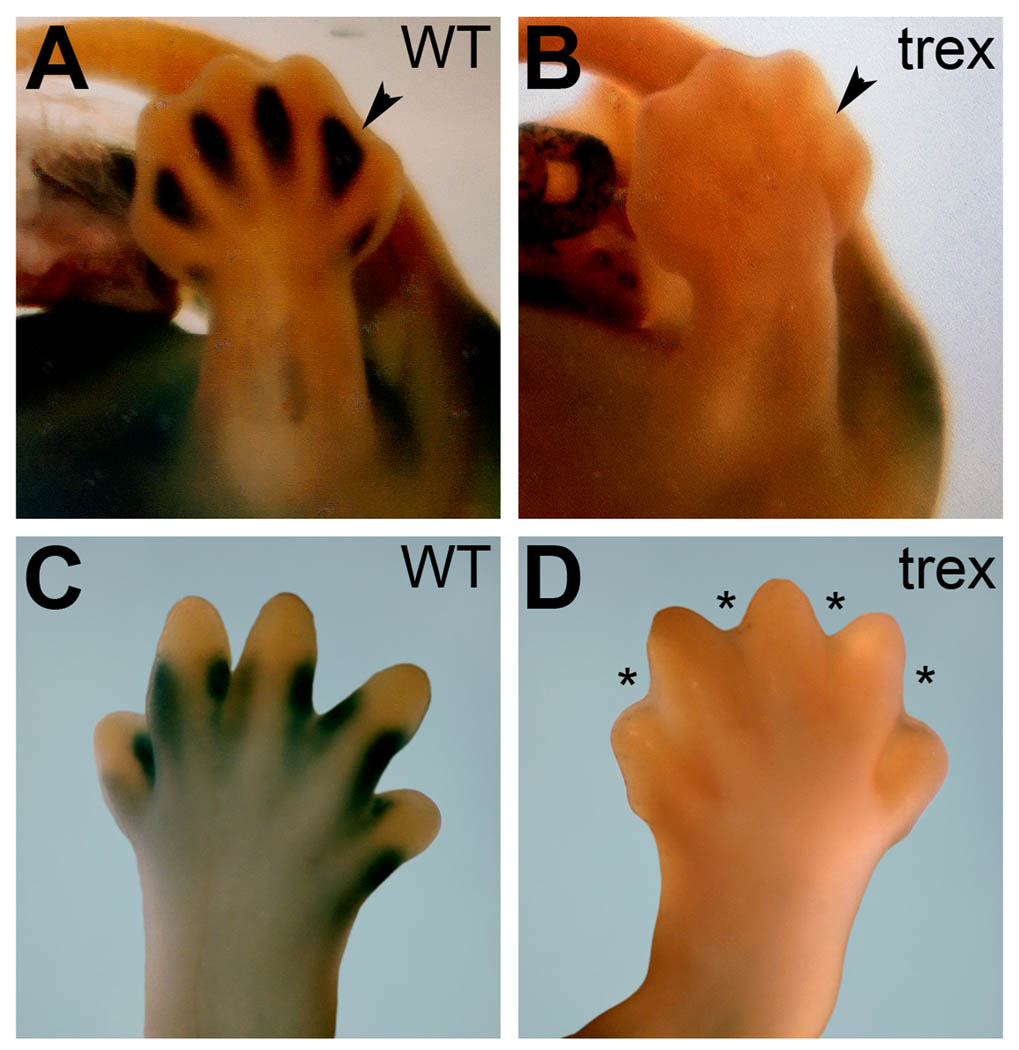
Beginning around E12.5, Raldh2 expression and RA activity are readily detected in the interdigital mesenchyme, a tissue that is destined to undergo apoptosis in order to separate the digits in a process that requires RA receptors and RALDH2 (Ghyselinck et al., 1997; Dupé et al., 1999; Zhao et al., 2010). Rdh10 has also been reported to be expressed in interdigital mesenchyme (Cammas et al., 2007). We examined interdigital RA activity using mice carrying the RARE-lacZ reporter transgene to assess the potential contribution of Rdh10 in driving this process at stages immediately prior to and after observable hindlimb interdigital tissue loss. We found that Rdh10 mutants display no RA activity in any part of the hindlimb autopod at E13.5 or E14.5 including the interdigital mesenchyme (Fig. 6A–D). Rdh10 mutants exhibited a webbed autopod at E14.5 in contrast with wild-type autopods that displayed clearly definable digits at this stage as observed in either lacZ-stained hindlimbs (Fig. 6C–D) or Alcian blue-stained hindlimbs (Fig. 5A–B). These findings are very similar to the interdigital webbing phenotype previously described in RARβ/RARγ compound mutants (Ghyselinck et al., 1997; Dupé et al., 1999). Together with previous studies showing that RA generated by RALDH2 is required for interdigital tissue loss and (Zhao et al., 2010), these findings demonstrate that RDH10 and RALDH2 function sequentially to metabolize retinol to RA in the interdigital mesenchyme to stimulate RA signaling needed for loss of interdigital webbing.
Fig. 6.
Rdh10 is essential for interdigital RA-activity and loss of interdigital mesenchyme. RARE-lacZ expression is lacking in Rdh10trex/trex mutant autopods. (A–B) RARE-lacZ expression at E13.5 shows interdigital staining in wild-type versus no staining in the same region in the mutant (arrowheads). (C–D) RARE-lacZ expression at E14.5 shows staining at the digit-interdigit junction in wild-type versus no staining in the mutant. Asterisks mark regions of interdigital webbing in the mutant due to a failure of normal interdigital tissue loss.
DISCUSSION
Ever since classical developmental biology experiments identified the AER and ZPA as mediators of P-D and A-P patterning, subsequent studies were undertaken to pinpoint the morphogens responsible for implementing their influence (Tabin and Wolpert, 2007; Zeller et al., 2009). We describe here a genetic loss-of-function study demonstrating that the loss of physiological levels of RA in hindlimbs of Rdh10trex/trex embryos has no effect on hindlimb patterning, but does abolish interdigital mesenchyme tissue loss. Our studies with the RARE-lacZ RA-reporter transgene have demonstrated that this transgene is sensitive to low physiological levels of RA. Previous HPLC studies demonstrated that endogenous RA levels in various tissues of E10.5 mouse embryos range from 10–100 nM (Horton and Maden, 1995) and that the average amount of RA in an E10.5 embryo is 25 nM (Mic et al., 2003). Here, we have shown that E10.5 Rdh10 mutant hindlimbs indeed lack RA activity, as RARE-lacZ expression can be recovered in the hindlimb when treated with a physiological level of RA (25 nM). Our studies show that previous evidence advocating roles for RA in either A-P or P-D patterning actually demonstrate the teratogenic traits of the RA molecule but do not establish an endogenous requirement in vivo for limb patterning.
During the search for the ZPA morphogen, RA was originally suggested to play this role; pharmacological doses of RA applied to anterior chick limb bud regions were shown to induce specification of ZPA-like cells, which when engrafted to other limbs resulted in A-P limb duplication (Tickle et al., 1982; Wanek et al., 1991). However, subsequent investigation revealed that lower RA doses could not replicate the same effect (Helms et al., 1994), and RA was soon after discarded in favor of SHH in implementing A-P polarity from the ZPA. Nevertheless, RA has sometimes been placed in a role upstream of SHH due to its ability to upregulate Shh expression (Riddle et al., 1993), although we argue that this effect has only been demonstrated using doses approximately 1000 times higher than the endogenous RA concentration. Our data clearly shows no spatiotemporal change in hindlimb Shh expression following loss of hindlimb RA activity in Rdh10 mutants.
More recently, the emphasis on research into a potential role for RA in limb patterning has switched to the P-D axis. While the influence of FGF8 signaling from the AER is unchallenged in its role as a principle P-D organizer (Mariani et al., 2008), controversy remains as to how this influence is specifically implemented (Tabin and Wolpert, 2007; Zeller et al., 2009). Originally, a ‘progress zone’ model was put forward stating that as cells migrate away from the AER, they exit the influence of FGF signaling from the AER and take on increasingly more distal fates over time; however, no evidence for a molecular clock mechanism has been found to validate this hypothesis. The ‘early specification model’—supported by conditional FGF loss-of-function studies—states that FGFs establish the different progenitor pools (needed for development of the stylopod, zeugopod, and autopod) early, and not progressively, although no evidence for early differential gene expression in the limb has been described. Finally a ‘two signal’ model has been put forward (without bias toward either of the other two models) suggesting RA in the proximal limb bud acts as a proximalizing signal in opposition to distal FGF signaling from the AER to control P-D outgrowth. This model was derived from evidence demonstrating the capacity of ectopic RA to upregulate the proximal limb marker Meis2 in distal limb tissue (Mercader et al., 2000; Yashiro et al., 2004). When exogenous RA treatment has been used to induce Meis2, pharmacological doses were used (Mercader et al., 2000), the lowest being 33µM which is still 1000x more concentrated than the accepted endogenous RA level in limbs at around 30nM (Horton and Maden, 1995). In Cyp26b1−/− embryos, ectopic Meis2 expression in more distal regions of the limb bud was attributed to direct upregulation by endogenous RA which was shown with RARE-lacZ to extend ectopically into the distal limb. However, we found no loss of Meis2 expression in Rdh10 mutant limbs shown here to be devoid of endogenous RA activity using RARE-lacZ that was validated as a sensitive marker of endogenous RA activity. As such, we conclude that Meis2 does not require RA for its proximal expression in the limb. We propose that ectopic RA in the limb bud indirectly causes Meis2 upregulation via a general disruption in patterning, perhaps by obstructing FGF signaling. This conclusion is supported by studies showing that loss of FGF signaling results in distal expansion of Meis gene expression (Mariani et al., 2008), plus other studies showing that ectopic RA in the distal limb can disrupt AER morphology in chick (Tickle et al., 1989) and downregulate Fgf4 expression in the AER of mouse Cyp26b1 mutants (Yashiro et al., 2004). Thus, we conclude that factors other than RA induce limb Meis expression, while distal FGF signaling restricts Meis expression to a proximal location.
Our hypothesis that RA is unnecessary for hindlimb patterning most likely also pertains to forelimb patterning. The Rdh10 mutant model shows that when RA is missing from limb buds only the forelimbs exhibit a growth defect, but it is unlikely that the forelimb requires proximal or posterior RA signaling centers for its patterning when the hindlimb does not. Indeed, in our analysis of the stunted Rdh10 mutant forelimb, we observed expression of Meis2, Shh, and Fgf8 in roughly the normal position after taking into account the fact that the forelimb is much smaller than normal. More credible is a forelimb-specific RA requirement unrelated to patterning such as a role in forelimb initiation which occurs about one day before hindlimb initiation. Studies with RA-rescued Raldh2−/− embryos have shown that RA synthesized in early trunk mesoderm from somite stages 1–10 functions to repress Fgf8 in trunk tissues anterior of the primitive streak and posterior of the heart, consequently permitting proper body axis extension and forelimb bud initiation which occurs in the developing trunk during this period (Sirbu et al., 2008; Zhao et al., 2009). Accordingly, loss of RA signaling results in ectopic FGF signaling in early trunk mesoderm that may be responsible for the observed delay of forelimb initiation in RA-rescued Raldh2−/− embryos (Zhao et al., 2009). Thus, we propose that the underlying cause of forelimb skeletal defects previously reported for Rdh10 mutants (Sandell et al., 2007) is not loss of an instructive RA signal needed for forelimb patterning but loss of RA needed to prevent a defect in forelimb growth that makes the forelimb too small to develop completely normal expression patterns of key patterning genes such as Fgf8 and Shh. Previous studies on Rdh10 mutant forelimbs reported that Shh exhibited a posterior domain located more distal than normal (as we observed here), and that AER Fgf8 exhibited a markedly reduced expression level more severe than we observed (Sandell et al., 2007). We attribute this difference in AER Fgf8 expression to variation in the severity of forelimb retardation, which ranges from a very small and narrow protrusion to a more significant bud, but always smaller than normal. This variation is likely coupled to how well each Rdh10 mutant embryo is able to use alternative enzymes to generate retinaldehyde and produce RA activity that is necessary for survival and growth, although such RA activity does not occur in limb field mesoderm. In contrast to forelimbs, we observe that hindlimbs do not require RA signaling for their initial growth, but other studies have demonstrated that early hindlimb growth is dependent upon Pitx1 and Pitx2 (Marcil et al., 2003).
Our studies here with Rdh10 mutants describe a simpler genetic model than RA-rescued Raldh2 mutants (Zhao et al., 2009) that does not require rescue with RA to avoid early lethality. Rdh10 mutants provide further support for our hypothesis that loss of RA signaling affects growth of forelimbs (which may indirectly affect patterning) but has no effect on hindlimb growth or patterning, Together, these findings provide strong evidence that RA is not required as an instructive signal for A-P or P-D patterning. As it is evident that limb buds need to be clear of RA in all but the most proximal region to avoid undesirable teratogenic consequences, we suggest that CYP26B1 functions simply to keep RA out of the majority of the limb bud to prevent disruption of limb development rather than functioning to establish proximodistal patterning.
Raldh2 and Rdh10 are expressed in overlapping domains encompassing the somites, intermediate mesoderm and lateral plate mesoderm. RA synthesis in early trunk mesoderm has been shown to be crucial for instructive neural tube RA signaling (such as induction of 3'-Hox genes), but trunk RA signaling also performs a permissive function important for mesoderm development through repression of FGFs and potentially other signaling ligands in regions where their influence is undesirable (Duester, 2008). But RA itself is undesirable in some locations including the distal limb. We suggest that in order for RA to perform its essential functions in neural tube and trunk mesoderm, some RA is able to enter the limbs but that it is unnecessary in that location and is mostly eliminated by CYP26B1. Later in development, Raldh2 and Rdh10 expression in the interdigital mesenchyme leads to an instructive RA signaling event in that tissue, which is crucial in the process of interdigital apoptosis (Ghyselinck et al., 1997; Dupé et al., 1999; Zhao et al., 2010). RA has for a long time been suggested to function as an instructive signal for either A-P or P-D limb patterning, but the genetic evidence presented here and previously (Zhao et al., 2009) warrants a change of the paradigm to a model in which RA is not required for limb patterning and is actively removed to ensure that it does not disrupt limb development.
EXPERIMENTAL PROCEDURES
Mouse strains
Rdh10trex/trex mice were generated via ethylnitrosourea mutagenesis as described previously (Sandell et al., 2007). Rdh10 mutant embryos were readily detected by the presence of a stunted forelimb, and were verified by DNA sequencing of a PCR product overlapping the mutation (Sandell et al., 2007). Rdh10 mutants were crossed with RARE-lacZ RA-reporter transgenic mice (Rossant et al., 1991), and these mice (on a mixed genetic background) were used throughout the study maintained on standard mouse chow. All mouse studies conformed to the regulatory standards adopted by the Animal Research Committee at the Sanford-Burnham Medical Research Institute.
In situ hybridization, lacZ detection, sectioning, and cartilage staining
Whole mount in situ hybridization was used to detect mRNA transcripts as previously described (Wilkinson, 1992). RARE-lacZ, encoding β-galactosidase, was detected in embryos by staining 8–10h with X-gal (5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside) as previously described (Rossant et al., 1991); in some cases staining was performed for 24h, but no additional sites of lacZ detection were observed. Stained embryos were incubated in a gelatin-BSA solution (0.5% Gelatin, 30% BSA, 20% Sucrose in 1xPBS; Sigma) for 1 hour, then embedded in fresh gelatin-BSA solution polymerized with 1.75% glutaraldehyde (Sigma), and sectioned at 40µm with a vibratome. Alcian blue staining of cartilage was performed as previously described (Hogan et al., 1994).
In vitro culture of RA-treated embryos
Wild-type and Rdh10 mutant E10.5 embryos were cut in half, transversely, between the fore- and hindlimbs. The posterior half, encompassing the hindlimbs, was cultured for 18 hours in serum-free (retinoid-free) DMEM/F-12 culture media (Gibco-Life Technologies) at 37°C in 5% CO2 and in Millicell culture plate inserts (Millipore), either in the presence or absence of 25nM all-trans-retinoic acid (Sigma Chemical).
ACKNOWLEDGMENTS
We thank J. Rossant for providing RARE-lacZ RA-reporter mice, and the following for mouse cDNAs used to prepare in situ hybridization probes: P. Gruss (Meis2), G. Martin (Fgf8), A. McMahon (Shh), and V. Papaioannou (Tbx4). Research support was provided by grants from the National Institutes of Health (G.D. and P.T) and by the Stowers Institute for Medical Research (P.T.).
Grant sponsors: National Institutes of Health; GM062848 (G.D.) and DE016082 (P.T.).
REFERENCES
- Abu-Abed S, MacLean G, Fraulob V, Chambon P, Petkovich M, Dollé P. Differential expression of the retinoic acid-metabolizing enzymes CYP26A1 and CYP26B1 during murine organogenesis. Mech.Dev. 2002;110:173–177. doi: 10.1016/s0925-4773(01)00572-x. [DOI] [PubMed] [Google Scholar]
- Cammas L, Romand R, Fraulob V, Mura C, Dolle P. Expression of the murine retinol dehydrogenase 10 (Rdh10) gene correlates with many sites of retinoid signalling during embryogenesis and organ differentiation. Dev. Dyn. 2007;236:2899–2908. doi: 10.1002/dvdy.21312. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF. Manifestation of the limb prepattern: Limb development in the absence of sonic hedgehog function. Dev Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupé V, Ghyselinck NB, Thomazy V, Nagy L, Davies PJA, Chambon P, Mark M. Essential roles of retinoic acid signaling in interdigital apoptosis and control of BMP-7 expression in mouse autopods. Dev Biol. 1999;208:30–43. doi: 10.1006/dbio.1998.9176. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Dupé V, Dierich A, Messaddeq N, Garnier J-M, Rochette-Egly C, Chambon P, Mark M. Role of the retinoic acid receptor beta (RARb) during mouse development. Int. J. Dev. Biol. 1997;41:425–447. [PubMed] [Google Scholar]
- Gibson-Brown JJ, Agulnik SI, Chapman DL, Alexiou M, Garvey N, Lee SM, Papaioannou VE. Evidence of a role for T-box genes in the evolution of limb morphogenesis and the specification of forelimb/hindlimb identity. Mech. Dev. 1996;56:93–101. doi: 10.1016/0925-4773(96)00514-x. [DOI] [PubMed] [Google Scholar]
- Hasson P, Del Buono J, Logan MP. Tbx5 is dispensable for forelimb outgrowth. Development. 2007;134:85–92. doi: 10.1242/dev.02622. [DOI] [PubMed] [Google Scholar]
- Helms J, Thaller C, Eichele G. Relationship between retinoic acid and sonic hedgehog, two polarizing signals in the chick wing bud. Development. 1994;120:3267–3274. doi: 10.1242/dev.120.11.3267. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo. Second Edition. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1994. [Google Scholar]
- Horton C, Maden M. Endogenous distribution of retinoids during normal development and teratogenesis in the mouse embryo. Dev. Dyn. 1995;202:312–323. doi: 10.1002/aja.1002020310. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- MacLean G, Abu-Abed S, Dollé P, Tahayato A, Chambon P, Petkovich M. Cloning of a novel retinoic-acid metabolizing cytochrome P450, Cyp26B1, and comparative expression analysis with Cyp26A1 during early murine development. Mech. Dev. 2001;107:195–201. doi: 10.1016/s0925-4773(01)00463-4. [DOI] [PubMed] [Google Scholar]
- Marcil A, Dumontier É, Chamberland M, Camper SA, Drouin J. Pitx1 and Pitx2 are required for development of hindlimb buds. Development. 2003;130:45–55. doi: 10.1242/dev.00192. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401–405. doi: 10.1038/nature06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Piedra ME, Martínez-A C, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc. Natl. Acad. Sci. USA. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Tbx4 is not required for hindlimb identity or post-bud hindlimb outgrowth. Development. 2007;134:93–103. doi: 10.1242/dev.02712. [DOI] [PubMed] [Google Scholar]
- Niederreither K, McCaffery P, Dräger UC, Chambon P, Dollé P. Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mech. Dev. 1997;62:67–78. doi: 10.1016/s0925-4773(96)00653-3. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Ros MA, Dahn RD, Fernandez-Teran M, Rashka K, Caruccio NC, Hasso SM, Bitgood JJ, Lancman JJ, Fallon JF. The chick oligozeugodactyly (ozd) mutant lacks sonic hedgehog function in the limb. Development. 2003;130:527–537. doi: 10.1242/dev.00245. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguére V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Sandell LL, Sanderson BW, Moiseyev G, Johnson T, Mushegian A, Young K, Rey JP, Ma JX, Staehling-Hampton K, Trainor PA. RDH10 is essential for synthesis of embryonic retinoic acid and is required for limb, craniofacial, and organ development. Genes Dev. 2007;21:1113–1124. doi: 10.1101/gad.1533407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Duester G. Retinoic acid signaling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol. 2006;8:271–277. doi: 10.1038/ncb1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev. Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C, Wolpert L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007;21:1433–1442. doi: 10.1101/gad.1547407. [DOI] [PubMed] [Google Scholar]
- Tahayato A, Dollé P, Petkovich M. Cyp26c1 encodes a novel retinoic acid-metabolizing enzyme expressed in the hindbrain, inner ear, first branchial arch and tooth buds during murine development. Gene Exp Patt. 2003;3:449–454. doi: 10.1016/s1567-133x(03)00066-8. [DOI] [PubMed] [Google Scholar]
- Tickle C, Alberts BM, Wolpert L, Lee J. Local application of retinoic acid to the limb bud mimics the action of the polarizing region. Nature. 1982;296:564–565. doi: 10.1038/296564a0. [DOI] [PubMed] [Google Scholar]
- Tickle C, Crawley A, Farrar J. Retinoic acid application to chick wing buds leads to a dose-dependent reorganization of the apical ectodermal ridge that is mediated by the mesenchyme. Development. 1989;106:691–705. doi: 10.1242/dev.106.4.691. [DOI] [PubMed] [Google Scholar]
- Wanek N, Gardiner DM, Muneoka K, Bryant SV. Conversion by retinoic acid of anterior cells into ZPA cells in the chick wing bud. Nature. 1991;350:81–83. doi: 10.1038/350081a0. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In Situ Hybridization: A Practical Approach. Oxford: IRL Press; 1992. pp. 75–83. [Google Scholar]
- Yashiro K, Zhao X, Uehara M, Yamashita K, Nishijima M, Nishino J, Saijoh Y, Sakai Y, Hamada H. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing limb. Dev Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nature Rev Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- Zhao X, Brade T, Cunningham TJ, Duester G. Retinoic acid controls expression of tissue remodeling genes Hmgn1 and Fgf18 at the digit-interdigit junction. Dev. Dyn. 2010;239:665–671. doi: 10.1002/dvdy.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Sirbu IO, Mic FA, Molotkova N, Molotkov A, Kumar S, Duester G. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr. Biol. 2009;19:1050–1057. doi: 10.1016/j.cub.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]