Abstract
We have recently shown that co-administration of conventional single-stranded adeno-associated virus 2 (ssAAV2) vectors with self-complementary (sc) AAV2-protein phosphatase 5 (PP5) vectors leads to a significant increase in the transduction efficiency of ssAAV2 vectors in human cells in vitro as well as in murine hepatocytes in vivo. In the present study, this strategy has been further optimized by generating a mixed population of ssAAV2-EGFP and scAAV2-PP5 vectors at a 10:1 ratio to achieve enhanced green fluorescent protein (EGFP) transgene expression at approximately 5- to 10-fold higher efficiency, both in vitro and in vivo. This simple coproduction method should be adaptable to any ssAAV serotype vector containing transgene cassettes that are too large to be encapsidated in scAAV vectors.
Previous studies by this group have shown that coadministration of conventional single-stranded adenoassociated virus serotype 2 (ssAAV2) vectors with self-complementary (sc) AAV2–protein phosphatase-5 (PP5) vectors leads to a significant increase in the transduction efficiency of ssAAV2 vectors in human cells in vitro as well as in murine hepatocytes in vivo. Here, Ma and colleagues expand on those previous findings and describe a quadruple-plasmid transfection protocol that results in the production of ssAAV vectors that transduce cells up to 9-fold more efficiently than in the absence of PP5 coexpression.
Introduction
Recombinant vectors based on a nonpathogenic human parvovirus, the adeno-associated virus (AAV), have gained attention for gene transfer and gene therapy because of their safety and/or clinical efficacy in a number of Phase I/II clinical trials in humans (Flotte et al., 1996, 2004; Kay et al., 2000; Aitken et al., 2001; Marshall, 2001; Wagner et al., 2002; Manno et al., 2003; Conlon and Flotte, 2004; Snyder and Francis, 2005). However, the conventional AAV vectors contain a single-stranded DNA genome, which is transcriptionally inactive. Our group and others have documented that viral second-strand synthesis is a major rate-limiting step in AAV vector–mediated transgene expression (Muzyczka, 1992; Ferrari et al., 1996; Fisher et al., 1996; Qing et al., 1997, 1998; Mah et al., 1998; McCarty et al., 2001; Wang et al., 2003; Zhong et al., 2004a; Zhao et al., 2007). Although double-stranded DNA–containing AAV vectors, termed self-complementary AAV (scAAV), have been developed that bypass the requirement for viral second-strand DNA synthesis (McCarty et al., 2001), their packaging capacity is reduced by approximately one half (Grieger and Samulski, 2005; Wu et al., 2007). Thus, scAAV vectors containing large genes, such as the human coagulation factor VIII (hF.VIII) for the potential gene therapy for hemophilia A, are unlikely to be generated (Sarkar et al., 2003; Jiang et al., 2006), and strategies to improve the transduction efficiency of conventional ssAAV vectors need to be developed.
In pursuit of one such strategy, we focused our studies on FKBP52, a 52-kDa host cell protein that binds to the immunosuppressive drug, FK-506. Phosphorylated forms of FKBP52 interact specifically with the D-sequence within the inverted terminal repeat (ITR) of the AAV genome (Qing et al., 1997, 2001). FKBP52 can be phosphorylated at both tyrosine (Tyr) and serine/threonine (Ser/Thr) residues, with phosphorylation strongly inhibiting the viral second-strand DNA synthesis, thereby negatively impacting AAV-mediated transgene expression (Qing et al., 1998, 2001, 2003; Zhong et al., 2004a,b,c). We also identified two key cellular phosphatases, T-cell protein tyrosine phosphatase (TC-PTP) and protein phosphatase 5 (PP5), which catalyze dephosphorylation of FKBP52 at Tyr and Ser/Thr residues, respectively, and thus prevent binding of FKBP52 to the D-sequence, leading to efficient viral second-strand DNA synthesis and AAV-mediated transgene expression (Qing et al., 2003; Zhong et al., 2004b; Zhao et al., 2007). Subsequently, we also developed scAAV-TC-PTP and scAAV-PP5 vectors (Zhong et al., 2004a; Zhao et al., 2007; Jayandharan et al., 2008) and demonstrated that co-infection or co-administration with these scAAV vectors leads to a significant increase in the transduction efficiency of ssAAV vectors, both in vitro and in vivo (Jayandharan et al., 2008, 2010).
In an attempt to further reduce the labor and production costs associated with packaging ssAAV and scAAV vectors separately, we reasoned that the two steps could be combined to generate a mixed population of the two vectors. Here we describe a quadruple-plasmid transfection protocol that results in the production of ssAAV vectors that transduce cells up to ninefold more efficiently than in the absence of PP5 co-expression. This simple strategy should be applicable for generating more efficient ssAAV vectors containing large genes that exceed the packaging capacity of scAAV vectors.
Materials and Methods
Plasmids and vectors
Standard cloning techniques were used to construct all recombinant AAV-based plasmids. Recombinant expression plasmids containing the Rous sarcoma virus (RSV) promoter–driven human PP5 cDNA was generously provided by Drs. David J. Chen and Benjamin P.C. Chen (University of Texas Southwestern Medical Center at Dallas). The scAAV2 plasmids containing the RSV promoter–driven human PP5 (pdsAAV-RSV-PP5) was constructed by standard cloning methods as described previously (Zhong et al., 2004a; Zhao et al., 2007). The scAAV plasmid containing the chicken beta-actin promoter (CBAp) in the vector backbone of plasmid pdsAAV-CBAp-EGFP was a kind gift from Dr. X. Xiao, University of North Carolina at Chapel Hill, and the recombinant AAV plasmid containing the human cytomegalovirus immediate-early gene promoter (CMVp)-driven hrGFP was purchased from Stratagene. Recombinant AAV helper plasmid pACG2-RC was generously provided by Dr. R.J. Samulski, University of North Carolina at Chapel Hill. The adenovirus-helper plasmid, pAd-Helper, was purchased from Stratagene. The CMVp-hrGFP expression cassette was replaced by the CBAp-EGFP expression cassette to generate a recombinant plasmid designated pBL-18.
Highly purified stocks of conventional recombinant ssAAV2 vector were generated by either the triple-plasmid transfection protocol as described previously (Auricchio et al., 2001) or modified to include RSV-PP5 in a quadruple-plasmid protocol under identical conditions. The physical particle titers of recombinant vector stocks were determined by quantitative DNA slot-blot analyses using 32P-labeled enhanced green fluorescent protein (EGFP)- or PP5-specific DNA probes as described previously (Kube and Srivastava, 1997).
Recombinant AAV vector transduction studies in vitro
Human 293 cells were infected with 2 × 103 to 5 × 103 vector genomes (vgs) of ssAAV2-EGFP or ssAAV2-EGFP+scAAV2-PP5 vectors at 37°C for 1 hr, and transgene expression was visualized using an Axiovert 25 fluorescence microscope (Carl Zeiss, Inc.). Images from five visual fields were analyzed quantitatively by ImageJ analysis software (National Institutes of Health). Transgene expression (mean ± SD) was assessed as total area of green fluorescence (pixel2) per visual field. ANOVA was used to compare between test results and the control and they were determined to be statistically significant.
Animal handling
All animal experiments were performed according to the guidelines for animal care specified by the Animal Care Services at the University of Florida. Ten-week-old male C57BL/6J mice were purchased from Jackson Laboratory and maintained at the University of Florida College of Medicine. The Institutional Animal Care and Use Committee approved all protocols for the care and use of these mice.
Recombinant AAV vector transduction studies in vivo
Approximately 1 × 1010 vgs of ssAAV2-EGFP vectors were injected into C57BL/6J mice intravenously via the tail vein (n = 2, per group). Phosphate-buffered saline (PBS)-injected mice were used as an appropriate control. Liver sections from three hepatic lobes of the PBS-injected and vector-injected mice 2 weeks post-injection were evaluated for the transduction efficiency of the ssAAV2-EGFP vector. The EGFP expression was measured by imaging using an Axiovert 25 fluorescence microscope (Carl Zeiss, Inc.). Images from four visual fields of mock- and vector-administered hepatocytes were analyzed quantitatively by ImageJ analysis software. Transgene expression (mean ± SD) was assessed as total area of green fluorescence (pixel2) per visual field. ANOVA was used to compare between test results and the control and they were determined to be statistically significant.
Results
Co-transfection of plasmids containing ssAAV2-EGFP and scAAV2-PP5 leads to the production of a mixed-population of both vectors
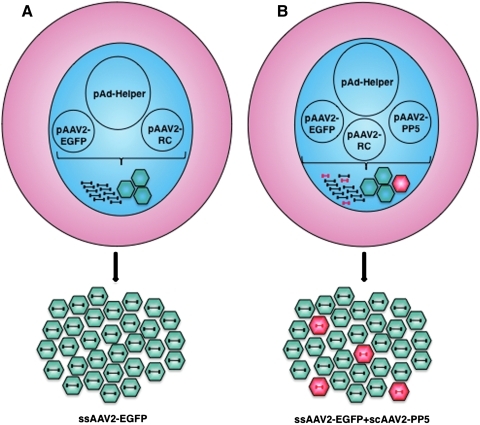
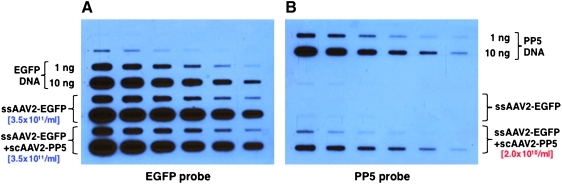
We recently reported that co-administration of scAAV2-PP5 vectors resulted in a significant increase in the transduction efficiency of ssAAV2-EGFP vectors in murine hepatocytes in vivo (Jayandharan et al., 2008, 2010). Since these vectors were generated separately using the standard triple-plasmid transfection protocol (shown schematically in Fig. 1A), and then admixed, we wished to examine whether it was feasible to generate the two vectors simultaneously using a quadruple-plasmid transfection protocol (depicted schematically in Fig. 1B). To this end, in addition to the pAAV2-RC and pAd-Helper plasmids, the pBL-18 (AAV2-CBAp-EGFP) and pAAV2-RSVp-PP5 plasmids were co-transfected at a 10:1 ratio. The rest of the steps were the same as the standard triple-plasmid transfection protocol, which was also employed under identical conditions to generate the two sets of vector stocks. The vector titers were determined using two identical quantitative DNA slot-blots containing twofold serial dilutions of the appropriate plasmid DNA controls. The blots were probed with 32P-labeled EGFP- and PP5-specific DNA probes, respectively. These results are shown in Fig. 2. It is evident that co-transfection with the PP5 plasmid did not affect the titers of ssAAV2-EGFP vectors (Fig. 2A), and that scAAV2-PP5 vectors were packaged at approximately 17-fold lower titers (Fig. 2B).
FIG. 1.
Schematic representation of generation of single-stranded adeno-associated virus 2 (ssAAV2) vectors by the (A) conventional triple-plasmid transfection and (B) quadruple-plasmid transfection protocols. The latter protocol would be expected to yield a mixed-vector stock at 10:1 ratio. See text for details. EGFP, enhanced green fluorescent protein; PP5, protein phosphatase 5. Color images available online at www.liebertonline.com/hum
FIG. 2.
Quantitative DNA slot-blots for determining the vector titer stocks. Twofold serial dilutions of the two vector stocks generated by the triple-plasmid and the quadruple-plasmid transfection protocols, respectively, were analyzed on two identical blots probed with 32P-labeled (A) EGFP-specific DNA probe and (B) PP5-specific DNA probe. Recombinant EGFP and PP5 plasmids (top two rows in each blots, respectively) were also used as appropriate controls. scAAV2, self-complementary adeno-associated virus 2. Color images available online at www.liebertonline.com/hum
ssAAV2-EGFP+scAAV-PP5 coproduced vectors transduce human cells more efficiently in vitro
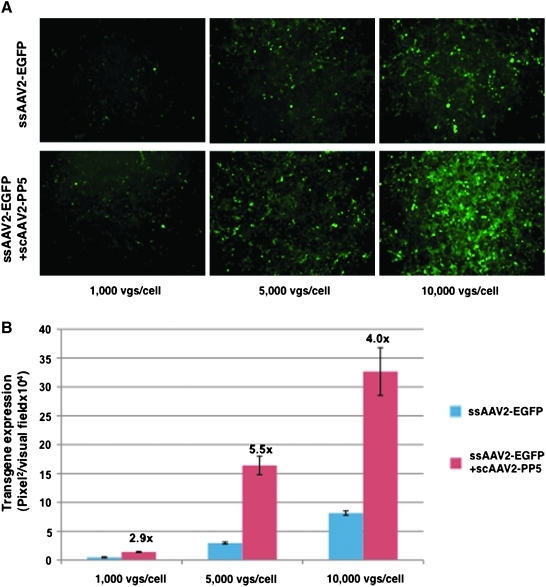
We hypothesized that rapid expression of PP5 from scAAV vectors would lead to dephosphorylation of FKBP52, resulting in efficient second-strand synthesis of the conventional ssAAV-EGFP vectors. Approximately 5 × 105 293 cells were plated in each well in six-well plates and incubated at 37°C for 12 hr. Cells were washed once with Dulbecco's modified Eagle's medium (DMEM) and then either mock-infected or infected at 37°C for 1 hr with various multiplicities of infection (MOI) of the two vector stocks under identical conditions. Cells were incubated in complete DMEM containing 10% fetal bovine serum and 1% antibiotics. Seventy-two hours post-infection, cells were visualized under a fluorescence microscope. As can be seen in Fig. 3A, up to about a sixfold increase was observed in the transgene expression from ssAAV2 vectors that also contained the scAAV2-PP5 vectors at a 10:1 ratio (Fig. 3B). These data correlate well with our previous studies in which co-infection of both ssAAV2-EGFP and scAAV2-PP5 vectors augmented the transduction efficiency by about five- to sevenfold in human cells (Jayandharan et al., 2008). These results further corroborate that scAAV2-PP5 vectors serve as helper viruses to augment the transduction efficiency of ssAAV2-EGFP vectors by facilitating the viral second-strand DNA synthesis.
FIG. 3.
ssAAV vector-mediated transduction of human 293 cells. (A) Vector stocks generated by the triple-plasmid and the quadruple-plasmid transfection protocols, respectively, were used to transduce cells at various indicated multiplicities of infection, and transgene expression was detected by fluorescence microscopy 72 hr post-transduction. Representative images are shown. (B) Quantitative analyses of the data from (A). Images from five visual fields were analyzed quantitatively by ImageJ analysis software. Transgene expression (mean value) was assessed as total area of green fluorescence (pixel2) per visual field. Analysis of variance (ANOVA) was used to compare test results with the control EGFP expression. vgs, vector genomes. Color images available online at www.liebertonline.com/hum
scAAV2-PP5 vector-mediated ssAAV2-EGFP viral second-strand DNA synthesis occurs in the target cell
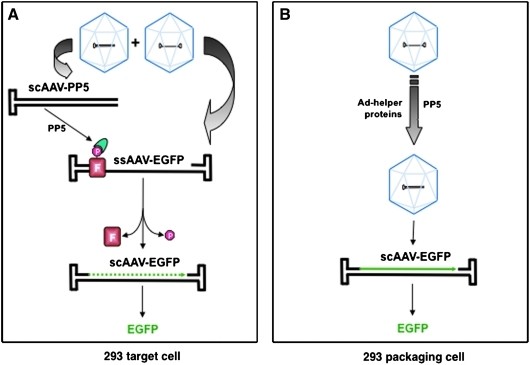
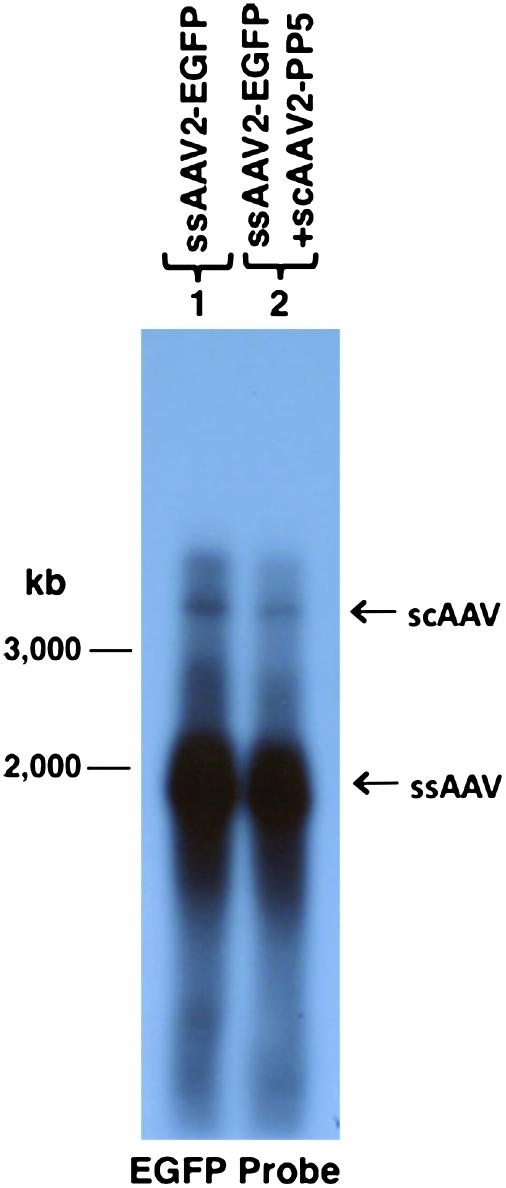
We next wished to distinguish whether the ssAAV2-EGFP vector genomes underwent second-strand DNA synthesis following transduction of the target cell, or whether scAAV2-EGFP vectors were assembled in the packaging cell, especially since the size of the ssAAV2-EGFP genome was less than 2.0 kb. These two possibilities are shown schematically in Fig. 4. Thus, it was crucial to determine the nature of the encapsidated AAV2-EGFP genomes in the vector stocks. To this end, equal amounts of ssAAV2-EGFP and ssAAV2-EGFP+scAAV-PP5 vectors were denatured, and the DNA samples were electrophoresed on alkaline agarose gels and autoradiographed using a 32P-labeled EGFP-specific DNA probe. These results, shown in Fig. 5, clearly document that in both vectors, the viral genomes were present largely as single-stranded DNA, although a low-level encapsidation of scAAV genomes also occurred, which is consistent with previously published reports (Muzyczka, 1992; Ferrari et al., 1996; Fisher et al., 1996; Qing et al., 1997, 1998; Mah et al., 1998; McCarty et al., 2001; Wang et al., 2003; Zhong et al., 2004a; Zhao et al., 2007).
FIG. 4.
Schematic representation of (A) viral second-strand DNA synthesis in the target and (B) the packaging 293 cells. FKBP52 (F), phosphorylated at serine/threonine residues (red symbol), which strongly inhibits the second-strand DNA synthesis of a conventional ssAAV vector, is dephosphorylated at serine/threonine residues by PP5 (blue semi-oval) expressed from scAAV-PP5 vectors, which allows more efficient viral second-strand DNA synthesis of conventional ssAAV vector, and consequently, leads to more efficient transgene expression in the target cell. Alternatively, deliberate overexpression of adenovirus-helper proteins and/or PP5 leads to the generation of scAAV-EGFP genomes that are encapsidated in vector capsids in the packaging cell. See text for details. Color images available online at www.liebertonline.com/hum
FIG. 5.
Southern blot analysis of the nature of the AAV DNA genomes in vector stocks generated by the two protocols. Equivalent amounts of DNA samples were denatured at 65°C for 30 min and electrophoresed on an alkaline-agarose gel and probed with 32P-labeled EGFP-specific DNA probe. Standard DNA Mr markers and the EGFP DNA insert were used as appropriate controls. Color images available online at www.liebertonline.com/hum
ssAAV2-EGFP+scAAV-PP5 coproduced vectors also transduce murine hepatocytes more efficiently in vivo
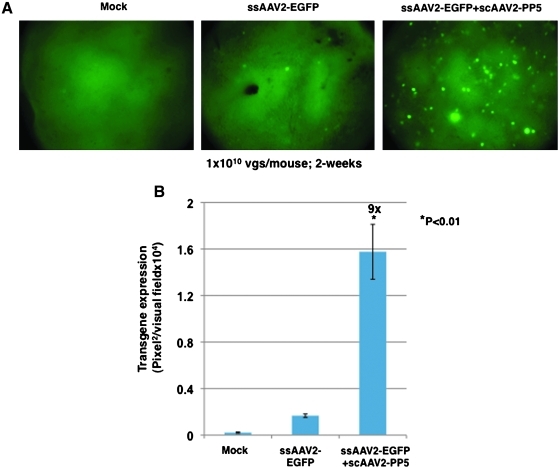
Finally, it was also of interest to evaluate the transduction efficiency of ssAAV2-EGFP+scAAV-PP5 vectors in murine hepatocytes in vivo. Approximately 1 × 1010 physical particles of ssAAV2-EGFP vectors alone or the mixed-population of ssAAV2-EGFP+scAAV2-PP5 vectors were intravenously injected into male C57BL/6J mice via the tail vein. PBS-injected mice were used as an appropriate control. Liver sections from three hepatic lobes of the PBS-injected and vector-injected mice 2 weeks after injection were examined for the transduction efficiency of the ssAAV2-EGFP vector. These results are shown in Fig. 6. Consistent with previously published studies (Ponnazhagan et al., 1997; Snyder et al., 1997; Nakai et al., 2000; Chen et al., 2001; Song et al., 2001; Zhong et al., 2004a; Jayandharan et al., 2008), little green fluorescence occurred in hepatocytes 2 weeks after injection of conventional ssAAV2-EGFP vectors alone. However, injection of ssAAV2-EGFP+scAAV2-PP5 vectors led to an approximately ninefold increase in the transduction of hepatocytes. The injection of scAAV2-PP5 vectors did not lead to major histological abnormalities in the liver. Both PBS- and helper virus–injected groups were grossly normal. The liver tissue from all PBS- or helper virus–injected animals had no evidence of any toxicity or any pathological lesions upon examination by an experienced pathologist certified by the American College of Veterinary Pathologists. Since FKBP52 is present predominantly in Ser/Thr-phosphorylated form in murine hepatocytes, these studies suggest that PP5-mediated dephosphorylation of FKBP52 is necessary and sufficient to augment the viral second-strand DNA synthesis of, and consequently, transgene expression from, ssAAV-EGFP vectors (Jayandharan et al., 2008, 2010).
FIG. 6.
Comparative analysis of ssAAV2-EGFP vector-mediated transduction efficiency in hepatocytes of normal C57BL/6 mice injected with vector stocks generated by the two protocols. (A) Transgene expression was detected by fluorescence microscopy 2 weeks post-injection of 1 × 1010 ssAAV2-EGFP vector particles per animal via the tail vein. Representative images are shown. (B) Quantitative analyses of the data from (A). Transgene expression was assessed as described in the legend to Fig. 2. *p < 0.01. Color images available online at www.liebertonline.com/hum
Discussion
It is now firmly established that the viral second-strand DNA synthesis, which is strongly inhibited by phosphorylated forms of a cellular chaperone protein, FKBP52, is a major rate-limiting step that impacts the transduction efficiency of ssAAV vectors (Ferrari et al., 1996; Fisher et al., 1996; Qing et al., 1997, 1998, 2001, 2003; Mah et al., 1998; Zhong et al., 2004b). Although the development of scAAV vectors can circumvent this problem (McCarty et al., 2001, 2003; Wang et al., 2003), the packaging capacity of these vectors is severely limited (Grieger and Samulski, 2005; Wu et al., 2007), and scAAV vectors containing large genes cannot be generated. Thus, there is a need to develop alternative strategies to achieve efficient transgene expression from ssAAV vectors. Indeed, we have devised a dual-vector approach in which scAAV-TC-PTP and/or the scAAV-PP5 vectors are admixed with a ssAAV vector, and efficient transgene expression ensues (Zhong et al., 2004a; Jayandharan et al., 2008, 2010). However, this involves the production of at least two separate vector stocks, which is both labor intensive and expensive.
In this article, we describe the usefulness of a simple approach to generate two AAV vectors simultaneously in the same production-run by using quadruple-plasmid transfection. Based upon our recent studies, in which we admixed ssAAV vectors containing the human coagulation factor IX (hF.IX) and scAAV-PP5 vectors at a 10:1 ratio and achieved therapeutic levels of F.IX expression at otherwise subtherapeutic doses of hF.IX vector (Jayandharan et al., 2010), we used the same ratio of the ssAAV-EGFP and scAAV-PP5 plasmids. Although the optimal ratio needs to be determined experimentally, these studies suggest that it may be possible to further reduce the plasmid concentrations to lower the vector production costs. A potential complication involving the possibility of homologous recombination events between the two ITR-containing vectors following co-transfection in the packaging cell line would appear to be inconsequential both because of the 10:1 ratio of the two plasmids used, and no significant cross-hybridization signals were observed on DNA slot-blots (Fig. 2).
One advantage of this vector production system is that this approach should be applicable to any ssAAV transgene cassette and can be adapted to any of the additional available AAV serotype vector since the same ssAAV2 genome is cross-packaged in different serotype vectors (Gao et al., 2002, 2006). Because deliberate expression of PP5 has thus far not been shown to be deleterious in human cells in vitro (Zhao et al., 2007; Jayandharan et al., 2008) or in murine hepatocytes in vivo (Jayandharan et al., 2010), including in PP5-transgenic mice (unpublished results), it is tempting to speculate that this system could also be adapted to generate AAV serotype vectors for their safe and efficacious use in the potential gene therapy in humans.
Acknowledgments
We thank Drs. David Chen and Benjamin Chen for their kind gift of PP5 expression plasmid and Drs. R. Jude Samulski and Xiao Xiao for generously providing the pACG2-RC and pdsCBAp-EGFP plasmids, respectively. This research was supported in part by Public Health Service grants HL-076901, HL-097088, and P01 DK-058327 (Project 1) from the National Institutes of Health (to AS). GRJ was supported in part by an Overseas Associate Fellowship-2006 from the Department of Biotechnology, Government of India.
Author Disclosure Statement
No competing financial interests exist.
References
- Aitken M.L. Moss R.B. Waltz D.A., et al. A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum. Gene Ther. 2001;12:1907–1916. doi: 10.1089/104303401753153956. [DOI] [PubMed] [Google Scholar]
- Auricchio A. Hildinger M. O'Connor E., et al. Isolation of highly infectious and pure adeno-associated virus type 2 vectors with a single-step gravity-flow column. Hum. Gene Ther. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- Chen S.J. Tazelaar J. Wilson J.M. Selective repopulation of normal mouse liver by hepatocytes transduced in vivo with recombinant adeno-associated virus. Hum. Gene Ther. 2001;12:45–50. doi: 10.1089/104303401450951. [DOI] [PubMed] [Google Scholar]
- Conlon T.J. Flotte T.R. Recombinant adeno-associated virus vectors for gene therapy. Expert Opin. Biol. Ther. 2004;4:1093–1101. doi: 10.1517/14712598.4.7.1093. [DOI] [PubMed] [Google Scholar]
- Ferrari F.K. Samulski T. Shenk T. Samulski R.J. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J. Virol. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher K.J. Gao G.P. Weitzman M.D., et al. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J. Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T. Carter B. Conrad C., et al. A phase I study of an adeno-associated virus-CFTR gene vector in adult CF patients with mild lung disease. Hum. Gene Ther. 1996;7:1145–1159. doi: 10.1089/hum.1996.7.9-1145. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Brantly M.L. Spencer L.T., et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum. Gene Ther. 2004;15:93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G.P. Lu Y. Sun X., et al. High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J. Virol. 2006;80:6192–6194. doi: 10.1128/JVI.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger J.C. Samulski R.J. Packaging capacity of adeno-associated virus serotypes: impact of larger genomes on infectivity and postentry steps. J. Virol. 2005;79:9933–9944. doi: 10.1128/JVI.79.15.9933-9944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayandharan G.R. Zhong L. Li B., et al. Strategies for improving the transduction efficiency of single-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2008;15:1287–1293. doi: 10.1038/gt.2008.89. [DOI] [PubMed] [Google Scholar]
- Jayandharan G.R. Zhong L. Sack B.K., et al. Optimized adeno-associated virus (AAV)-protein phosphatase-5 helper viruses for efficient liver transduction by single-stranded AAV vectors: therapeutic expression of factor IX at reduced vector doses. Hum. Gene Ther. 2010;21:271–283. doi: 10.1089/hum.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. Lillicrap D. Patarroyo-White S., et al. Multiyear therapeutic benefit of AAV serotypes 2, 6, and 8 delivering factor VIII to hemophilia A mice and dogs. Blood. 2006;108:107–115. doi: 10.1182/blood-2005-12-5115. [DOI] [PubMed] [Google Scholar]
- Kay M.A. Manno C.S. Ragni M.V., et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat. Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Kube D.M. Srivastava A. Quantitative DNA slot blot analysis: inhibition of DNA binding to membranes by magnesium ions. Nucleic Acids Res. 1997;25:3375–3376. doi: 10.1093/nar/25.16.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah C. Qing K. Khuntirat B., et al. Adeno-associated virus type 2-mediated gene transfer: role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. J. Virol. 1998;72:9835–9843. doi: 10.1128/jvi.72.12.9835-9843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C.S. Chew A.J. Hutchison S., et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- Marshall E. Gene therapy. Viral vectors still pack surprises. Science. 2001;294:1640. doi: 10.1126/science.294.5547.1640. [DOI] [PubMed] [Google Scholar]
- McCarty D.M. Monahan P.E. Samulski R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr. Top. Microbiol. Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- Nakai H. Storm T.A. Kay M.A. Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat. Biotechnol. 2000;18:527–532. doi: 10.1038/75390. [DOI] [PubMed] [Google Scholar]
- Ponnazhagan S. Mukherjee P. Yoder M.C., et al. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene. 1997;190:203–210. doi: 10.1016/s0378-1119(96)00576-8. [DOI] [PubMed] [Google Scholar]
- Qing K. Hansen J. Weigel-Kelley K.A., et al. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. J. Virol. 2001;75:8968–8976. doi: 10.1128/JVI.75.19.8968-8976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K. Khuntirat B. Mah C., et al. Adeno-associated virus type 2-mediated gene transfer: correlation of tyrosine phosphorylation of the cellular single-stranded D sequence-binding protein with transgene expression in human cells in vitro and murine tissues in vivo. J. Virol. 1998;72:1593–1599. doi: 10.1128/jvi.72.2.1593-1599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K. Li W. Zhong L., et al. Adeno-associated virus type 2-mediated gene transfer: role of cellular T-cell protein tyrosine phosphatase in transgene expression in established cell lines in vitro and transgenic mice in vivo. J. Virol. 2003;77:2741–2746. doi: 10.1128/JVI.77.4.2741-2746.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing K. Wang X.S. Kube D.M., et al. Role of tyrosine phosphorylation of a cellular protein in adeno-associated virus 2-mediated transgene expression. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10879–10884. doi: 10.1073/pnas.94.20.10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R. Xiao W. Kazazian H.H., JR. A single adeno-associated virus (AAV)-murine factor VIII vector partially corrects the hemophilia A phenotype. J. Thromb. Haemost. 2003;1:220–226. doi: 10.1046/j.1538-7836.2003.00096.x. [DOI] [PubMed] [Google Scholar]
- Snyder R.O. Francis J. Adeno-associated viral vectors for clinical gene transfer studies. Curr. Gene Ther. 2005;5:311–321. doi: 10.2174/1566523054065066. [DOI] [PubMed] [Google Scholar]
- Snyder R.O. Miao C.H. Patijn G.A., et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat. Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- Song S. Embury J. Laipis P.J., et al. Stable therapeutic serum levels of human alpha-1 antitrypsin (AAT) after portal vein injection of recombinant adeno-associated virus (rAAV) vectors. Gene Ther. 2001;8:1299–1306. doi: 10.1038/sj.gt.3301422. [DOI] [PubMed] [Google Scholar]
- Wagner J.A. Nepomuceno I.B. Messner A.H., et al. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum. Gene Ther. 2002;13:1349–1359. doi: 10.1089/104303402760128577. [DOI] [PubMed] [Google Scholar]
- Wang Z. Ma H.I. Li J., et al. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Wu J. Zhao W. Zhong L., et al. Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Hum. Gene Ther. 2007;18:171–182. doi: 10.1089/hum.2006.088. [DOI] [PubMed] [Google Scholar]
- Zhao W. Wu J. Zhong L. Srivastava A. Adeno-associated virus 2-mediated gene transfer: role of a cellular serine/threonine protein phosphatase in augmenting transduction efficiency. Gene Ther. 2007;14:545–550. doi: 10.1038/sj.gt.3302886. [DOI] [PubMed] [Google Scholar]
- Zhong L. Chen L. Li Y., et al. Self-complementary adeno-associated virus 2 (AAV)-T cell protein tyrosine phosphatase vectors as helper viruses to improve transduction efficiency of conventional single-stranded AAV vectors in vitro and in vivo. Mol. Ther. 2004a;10:950–957. doi: 10.1016/j.ymthe.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Zhong L. Li W. Yang Z., et al. Improved transduction of primary murine hepatocytes by recombinant adeno-associated virus 2 vectors in vivo. Gene Ther. 2004b;11:1165–1169. doi: 10.1038/sj.gt.3302283. [DOI] [PubMed] [Google Scholar]
- Zhong L. Li W. Yang Z., et al. Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells. Hum. Gene Ther. 2004c;15:1207–1218. doi: 10.1089/hum.2004.15.1207. [DOI] [PubMed] [Google Scholar]