Abstract
Application of adeno-associated virus (AAV) vector in large animal studies and clinical trials often requires high-titer and high-potency vectors. A number of currently used vector production methods, based on either transient transfection or helper virus infection of cell lines, have their advantages and limitations. We previously developed a 293-cell–based producer cell line method for high-titer and high-potency AAV2 vectors. Similar to several other methods, however, it requires multiple cloning steps for the vector and packaging plasmids and a two-step transfection and selection for stable cell lines. Here we report a simplified method with several key improvements and advantages: (1) a one-step cloning of AAV vector cassette into the serotype-specific packaging plasmid; (2) a single plasmid transfection and selection for stable AAV vector producer cell lines; (3) high vector yields of different serotypes, e.g., AAV2, 8, and 9, upon infection with an E1A/E1B-deleted helper adenovirus; (4) efficient packaging of both single-stranded and double-stranded (self-complementary) AAV vectors; and (5) efficient packaging of large AAV cassettes such as a mini-dystrophin vector (5.0 kb). All cell lines were stable with growth rates identical to the parental 293 cells. The vector yields were consistent among serotypes, with 5 × 1013 to 8 × 1013 vector genome particles per Nunc cell factory (equivalent to 40 15-cm plates). The vectors showed high potency for in vitro and in vivo transduction. In conclusion, the simple and versatile AAV producer cell line method can be useful for large scale AAV vector production in preclinical and clinical studies.
Yuan and colleagues describe a streamlined 293 cell-based producer cell line method for producing high-titer and high-potency AAV vectors. Using this method, the authors were able to produce AAV8 and AAV9 in addition to AAV2. This scalable method was found to be efficient in producing both singlestranded AAV and self-complementary AAV vectors.
Introduction
Adeno-associated virus (AAV) vectors are commonly used as a powerful tool for in vivo gene transfer studies. They have been successfully tested in animal models to establish efficient and long-term gene transfer in a variety of tissues and bodywide without apparent toxicities. The success of preclinical studies has led to clinical trials using AAV vectors to treat genetic diseases such as hemophilia (Margaritis and High, 2010), muscular dystrophy (Wang et al., 2000; Chamberlain, 2002; Mendell et al., 2009, 2010a,b), Leber's congenital amaurosis (Maguire et al., 2008, 2009), and alpha-1 antitrypsin deficiency (Brantly et al., 2009). Although the applications of AAV vectors offer great potential for many genetic diseases, current vector production methods still have room for improvement to meet the demands of clinical studies involving certain genetic diseases, particularly those that require large quantities of high-quality vectors. For example, gene therapy for muscular dystrophies requires whole-body gene transfer in muscle, which is the largest organ in the body. This prompted us to develop a high-yield, scalable production method to meet the demands.
For AAV vector production, a number of strategies differing in principles are being used (Wang et al., 2003). The most widely used is based on the helper-virus–free transient transfection method with all cis and trans components (vector plasmid and packaging plasmids, along with helper genes isolated from adenovirus) in host cells such as 293 cells (Xiao et al., 1998; Lock et al., 2010). While the transient-transfection method is simple in vector plasmid construction and generates high-titer AAV vectors that are free of adenovirus, it is not cost effective to scale up for clinical studies. A second strategy is the recombinant herpes simplex virus (rHSV)-based AAV production system, which utilizes rHSV vectors to bring the AAV vector and the Rep and Cap genes into the cells (Wu et al., 2002; Clement et al., 2009; Kang et al., 2009).The third method based on baculovirus system was developed in recent years and requires simultaneous infection of insect cells with several baculovirus vectors to deliver the AAV vector cassette and the Rep and Cap genes (Urabe et al., 2002; Kohlbrenner et al., 2005; Meghrous et al., 2005). For both the rHSV- and baculovirus-based AAV production systems, it is inconvenient to prepare large quantities of helper and vector viruses and maintain their purity and stability. The fourth method is based on the AAV producer cell lines derived from HeLa or A549, which stably harbored AAV Rep/cap genes. The AAV vector cassette was either stably integrated in the host genome (Clark et al., 1995) or introduced by an adenovirus that contained the cassette (Chadeuf et al., 2000; Wang et al., 2003). Although the cell line method is easy to scale up and produces relatively high titers of AAV vectors comparable to transient transfection method, these cell lines required wild-type adenovirus as the helper. Contamination of wild-type adenovirus in the final vector preparations is highly undesirable in view of vector safety.
To eliminate transient transfection step and avoid the use of wild-type helper adenovirus, we established AAV producer cell lines based upon the human 293 cells. These cells contain highly inducible AAV Rep and Cap genes and also the adenovirus E1A/E1B genes, able to use E1A/E1B-defective adenovirus for helper functions. Considering that E1A/E1B-defective adenovirus has been widely used as a gene therapy vector in humans, its safety profile is better than the wild-type adenovirus. However, the major difficulty in generating a 293-based AAV producer cell line is the E1A-mediated activation of AAV promoters p5 and p19, which control AAV Rep proteins. The latter are known to be cytostatic (Yang et al., 1994) and even cytotoxic (Schmidt et al., 2000) if constantly expressed. Additional difficulties in generating tightly regulated p19 is its location within the coding region of Rep78 and Rep68 (Im and Muzyczka, 1990). Previously, we developed a novel gene expression control system, termed dual slicing switch system, where an intron and three polyadenylation elements were inserted into the Rep gene-coding region disrupting all Rep transcripts. Upon induction of AAV Rep gene expression by Ad-cre (an E1A/E1B/E3-deleted adenovirus expressing the Cre gene), both DNA splicing by Cre-loxP and RNA splicing to remove the intron (dual splicing) reconstitute and activate Rep gene expression in the AAV producer cell lines. By using this tightly controlled system, we have successfully obtained the 293-based AAV packaging cell lines with both high stability and high vector yields (Qiao et al., 2002b). The 293 cell–based AAV packaging cell lines had higher or equivalent vector yields compared with the transfection method (Xiao et al., 1998) and HeLa cell–based lines (Clark et al., 1995; Qiao et al., 2002a).
There are several advantages to utilizing our previous 293-based cell lines, including high infectivity, high yields, and the capacity to scale up. However, a chief limitation is the multi-step and time-consuming procedure. Due to two steps of transfection and selections, several weeks to months are needed to produce a high-yield cell line (Qiao et al., 2002b). To establish our previous AAV producer cell line, the first step was to deliver the inducible Rep/Cap plasmid to the 293 cells to screen for parental inducible 293-Rep/Cap cell line without AAV vector sequences. The second step was to introduce the AAV vector component and additional copies of the inducible Rep and Cap genes to the Rep/Cap inducible parental cell line by using a different drug-resistant selection marker. Another limitation of this method is the large size of the second plasmid, which makes it very inconvenient to clone various vector cassettes into it due to very few choices of restriction enzyme sites.
To overcome these limitations, we took advantage of the Gateway cloning technology (Suzuki et al., 2005) to simplify the cloning process. We also omitted the first step of parental AAV Rep/Cap cell cloning in the original protocol (Qiao et al., 2002b) and directly used the single plasmid containing the inducible Rep/Cap genes and AAV vector elements and a drug-resistant marker for a single transfection and selection step. This shortened more than half of the work load and process time. Furthermore, we have successfully tested the 293-based cell line strategy with different serotypes including AAV8 and AAV9 in addition to AAV2. Finally, these cell lines were found efficient in producing both single-stranded AAV(ssAAV) and double-stranded AAV(dsAAV) vectors. The improved method will provide a versatile and scalable AAV production system for preclinical and future clinical applications.
Materials and Methods
Construction of large plasmid for cell line establishment using Gateway system
The pENTR11 (Invitrogen, Carlsbad, CA) was chosen as the entry plasmid. To clone the AAV vector sequence into this plasmid, two restriction endonucleases that cut on opposite sites of the ccdB selection marker gene were used to replace the AAV vector sequence. For the construction of single-stranded AAV vector entry plasmid, the fragment containing the inverted terminal repeats (ITRs) and cytomegalovirus–green fluorescent protein (CMV-GFP) cassette was excised from pUF1-CMV-GFP (Wang et al., 2003) by Sse8387I digestion and filled in by Klenow enzyme, and then inserted into the pENTR11 plasmid, generating plasmid pENTR11-ss-CMV-GFP. For the construction of double-stranded AAV vector entry plasmid, the fragment containing the ITRs and CMV-GFP cassette was excised from the pdsAAV-CMV-GFP (Wang et al., 2003) plasmid by SwaI + EcoO109I double digestion and inserted into the PENTR11 plasmid, generating plasmid pENTR11-ds-CMV-GFP (Fig. 1a). For the construction of entry plasmid harboring the optimized human mini-dystrophin gene, plasmid pAAV-CMV-Opti-hDys3978 was digested by SwaI and ScaI, the fragment containing the CMV-hDys3978 flanked by ITRs was inserted between the XmnI and EcoRV site of pENTR11 vector to replace the ccdB gene, and therefore the entry plasmid pENTR11-CMV-opti-hDys3978 was obtained.
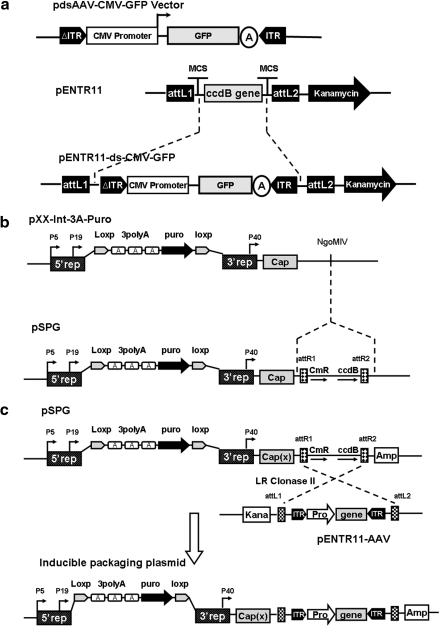
FIG. 1.
Plasmids construction and illustration of site-specific homologous recombination in the Gateway system. (a) Construction of the entry plasmid PENTR11-D(+)-CMV-GFP. The ccdB gene in pENTR11 was replaced by CMV-GFP-pA cassette flanked by adeno-associated virus (AAV) inverted terminal repeats (ITRs) from pAAV-D(+)-CMV-GFP. CMV, cytomegalovirus; GFP, green fluorescent protein. (b) Construction of the destination (packaging backbone) plasmid pSPG2. The reading frame cassette containing the chloramphenicol resistance gene (CmR) and the ccdB gene flanked by attR1 and attR2 sites was inserted into the NgoMIV site of plasmid pXX-Int-3A-Puro to generate the destination plasmid pSPG2. (c) The schematic diagram demonstrating the efficient generation of the inducible packaging plasmid by recombination of the destination plasmid pSPG and the entry plasmid pENTR11-AAV mediated by the LR clonase II. As a result, the large inducible packaging plasmid containing inducible rep, cap genes, and AAV vector sequences was obtained.
The pXX2-Int-3A-Puro (Qiao et al., 2002b) was chosen as a packaging backbone construct harboring the inducible Rep/Cap genes. A single restriction site of NgoMIV located in the noncoding area in this plasmid was utilized for insertion of recombination sites and and chloramphenicol resistance gene (CmR). The blunt end conversion cassette from the Gateway conversion kit (Invitrogen) (Campeau et al., 2009) was inserted into the NgoMIV site of the pXX2-Int-3A-Puro following the manufacture's protocol. The conversion cassette contains ccdB gene flanked by attR1 and attR2 site. Briefly, pXX2-Int-3A-Puro was first digested with NgoMIV and then blunted using Klenow fragment, and the linearized vector was then treated with calf intestinal alkaline phosphatase to prevent self-ligation. Subsequently, a ligation reaction was performed to insert the conversion cassette into the vector. After transforming the ligation product into an special E. coli strain (ccdB SurvivalTM 2 T1R Competent Cells, which tolerate the ccdB gene) (Invitrogen) and selecting the recombinant clone on the Luria-Bertani (LB) plates containing 30 μg/ml chloramphenicol and 50 μg/ml ampicillin, the destination plasmid pSPG2 for AAV2 was obtained (Fig. 1b). To construct the destination plasmid for establishment of other serotype AAV, the AAV2 Cap gene was replaced by Cap gene of other serotype AAV (for example: AAV8 or AAV9), and the obtained plasmid was named as pSPG8 or pSPG9, respectively.
To construct the inducible packaging plasmid for the establishment of cell line of a specific AAV serotype and vector, the ITR-containing vector cassette in a given entry plasmid was recombined efficiently into the destination plasmid of specific serotypes via the attL-attR(LR) recombination reaction. The recombinant product was then transformed into DH10B competent cells and plated onto LB agar plate containing 100 μg/ml ampicillin. Notably, the Entry and Destination vectors carried different drug resistance genes for selection in E. coli, kanamycin for the entry vector, and ampicillin for the destination vector. The parental destination plasmid contained ccdB gene and could not grow in DH10 strain. After recombination, the ccdB gene was removed from the destination vector and the recombined inducible packaging plasmid would grow in DH10B. As an example, recombination of pENTR11-ds-CMV-GFP and pSPG2 gave rise to an inducible plasmid named as pSPG2-ds-CMV-GFP (Fig. 1c), which was used for the establishment of dsAAV2-CMV-GFP–producing 293 cell line. By using the same strategy, we obtained a series of plasmids for the establishment of 293-based cell line producing different serotypes of ssAAV and dsAAV.
Cells and virus
Ad-GFP, an adenoviral vector harboring an EGFP gene under the control of CMV promoter, and Ad-Cre, an adenoviral vector harboring the Cre recombinase gene of P1 phage driven by CMV promoter, were acquired by a conventional method. These adenoviruses were E1A/E1B- and E3-defective first-generation adenoviral vectors (Jiang et al., 2001).
293 Cell lines were propagated in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with heat-inactivated 10% fetal bovine serum (FBS; Invitrogen) and antibiotics. Stable transfection was performed in 293 cells by the calcium phosphate transfection method as previously described (Xiao et al., 1998). Briefly, the 293 cells were passed into a six-well plate at 1:4 ratio (area to area) with each well containing 1.5 ml of fresh DMEM supplemented with 10% FBS without antibiotics 1 day before transfection. The pSPG plasmid (3 μg) was linearized by AseI digestion followed by DNA precipitation. The linearized plasmid was dissolved in 10 to 20 μl of TE (pH 7.0) and added to an eppendorf tube containing 0.25 M CaCl2 and then quickly mixed with 125 μl of 2X HEPES-buffered saline and added to the 293 cells. At 12 hr after transfection, the medium was replaced with fresh DMEM containing 10% FBS and 2 μg/ml puromycin (Sigma). The transfected cells were trypsinized 48 hr later, serial diluted, and plated onto 15-cm-diameter dishes to allow for the growth of single-cell clones. The clones were picked using pipette and transferred to 12-well plate for continuous culture. To test the vector productivity of the cell lines, the helper Ad-cre at a multiplicity of infection (MOI) of 5 was added to the cells at confluence of 80%–90%. The cells and the supernatant were harvested 48 hr after infection and subjected to three freeze–thaw circles to release the AAV from cells and then incubated in the 56°C water incubator for 1 hr to inactive the helper Ad-cre virus. The titers of the vectors from each clonal celll lines were determined by the standard vector DNA dot blot method (Xiao et al., 1998) or by infection on 293 cells if the vector contained the GFP reporter gene.
AAV vector production and purification
For the production of AAV2, 8 and 9 from the inducible 293-based cell lines, the cells were simply infected with Ad-Cre at MOI of 5. At 2 days after infection, cells from 20 15-cm plates were pelleted by centrifugation and resuspended in suspension buffer (phosphate-buffered saline [PBS] with 25 mM HEPES and 150 mM NaCl). The viral particles were purified twice by cesium chloride density gradient ultracentrifugation, using the previously a published protocol (Ayuso et al., 2010). Vector titers were determined by the DNA dot-blot method.
To validate whether the cell line could retain a similar productivity while scaling up, Nunc Cell Factories (Nalge Nunc International, Roskilde, Denmark) were used to scale up the culture of the cell line for production of AAV9-CMV-Opti-hDys3978 vector according to the manufacturer's instructions. Each Nunc Cell Factory is equivalent to 40 15-cm plates. Ad-Cre infection, subsequent harvesting, and purification procedures were carried out similar to those described above.
Quantification of Rep gene copy number by using real-time polymerase chain reaction
For the quantification of Rep gene copy number in stable 293-based cell lines, we used SYBR green–based real-time quantitative assay (ABI PRISM 7700 Sequence Detector, Applied Biosystems, Foster City, CA). We designed the primers to amplify a 317-bp fragment of the Rep gene. The sequences of the forward and reverse primer are: rep-5′: 5′-GGG ATT ACC TCG GAG AAG CAG TGG-3′; and rep-3′: 5′-CTT CCC GGT AGT TGC AGG-3′. We also designed the primer to amplify a 300-bp fragment of the human glucagon gene as the internal cell copy number control. Sequences of the forward and reverse primers are as follows: human-glucagon-F: 5′-TGA GAG ACA TGC TGA AGG GAC-3′; human-glucagon-R: 5′-CTT TCA CCA GCC AAG CAA TG-3′.
Total cellular DNA was extracted from cells by using the DNeasy Tissue Kits (Qiagen, Valencia, CA). Copy numbers of the Rep gene detected by real-time polymerase chain reaction (PCR) were reported as Rep copies per cell.
Mice, AAV vectors administration, and immunostaining of dystrophin
The mdx mice were purchased from Jackson Laboratory (Bar Harbor, ME) and the animal protocols were approved by the Animal Care and Use Committee of University of North Carolina at Chapel Hill. The mdx mice (3-month-old males) were divided into two groups. One group was treated with the AAV9-CMV-opti-hDys3978 vector produced from the producer cell line, while the other group was given the same dosage of the AAV9-CMV-opti-hDys3978 produced by the triple plasmid transfection method. Specifically, 2.5 × 1010 vector genome (vg) (50 μl) of AAV vectors and 4 × 1010 vg (80 μl) of AAV vectors were directly injected into the tibialis anterior (TA) muscle and gastrocnemius (GAS) muscle respectively. Four weeks post injection, the mice were sacrificed and the injected muscles were carefully dissected and cryo-preserved. Then the muscle tissues were subjected to cryosectioning and immunofluorescent (IF) staining. The protocol for the IF analysis used here was described previously (Watchko et al., 2002). The primary polyclonal antibody (rabbit serum anti-human dystrophin R22 and R23 regions, 1:500) was produced in Xiao Xiao's lab, and the secondary antibody (Cy 3-conjugated AffiniPure Goat anti-Rabbit IgG, 1:500) was purchased from Jackson ImmunoResearch (West Grove, PA).
Neutral and alkaline gel electrophoresis of AAV DNA
To extract DNA from the purified AAV vector, 1 × 1011 vg of purified AAV vector was diluted into a final volume of 100 μl of DMEM containing 2 U of DNase I and incubated at 37°C for 30 min to digest any residual plasmid DNA. Then 100 μl of 2 × proteinase K buffer (20 mM Tris-HCl pH 8.0, 20 mM EDTA pH 8.0, 1% SDS) and 2 μl of proteinase K (20 mg/μl) were added and incubate at 55°C for 30 min. The AAV DNA was then precipitated by ethanol precipitation. The final DNA was dissolved in 50 μl of double-distilled H2O.
For neutral gel electrophoresis, the AAV DNA was run on regular 1% agarose gel. Alkaline gel electrophoresis of AAV DNA was performed according to the method described in published literature (McDonell et al., 1977).
Results
Construction of vector shuttle and packaging backbone plasmids using Gateway system
One of disadvantages of our previous system is that the size of the final plasmid for establishing a cell line is very large. It has very few convenient restriction enzyme sites to choose for subcloning the vectors with different reporter or therapeutic genes. To address this problem, we employed the Gateway system (Invitrogen) to facilitate the plasmid construction. Compared with traditional DNA cloning, Gateway cloning technology provides a rapid and efficient way for gene cloning into larger size plasmids without the requirement of restriction enzymes and gel purification and ligation steps (Suzuki et al., 2005).
First, we constructed a vector shuttle plasmid for AAV vector cassette (Fig. 1a). The plasmid contained Gateway attL recombination sites flanking the ITRs of the AAV vector. Shown in Fig. 1a is an example of constructing the vector shuttle plasmid for double-stranded AAV-CMV-GFP (dsAAV-CMV-GFP) vector (see Materials and Methods for details). The plasmid pENTR11-ds-CMV-GFP is ready to be recombined with the inducible Rep/Cap-containing plasmid below.
Next, we constructed a packaging backbone plasmid containing attR recombination sites for the shuttle vector plasmid to be recombined into. More importantly, the backbone plasmid contained the inducible Rep gene of AAV2 and Cap gene from different serotypes, e.g., AAV2, 8 and 9, respectively. Shown in Fig. 1b is an example of AAV2 packaging backbone plasmid. In detail, an open reading frame cassette containing the chloramphenicol resistance gene (CmR) and the ccdB gene flanked by attR1 and attR2 sites were inserted into the NgoMIV site of plasmid pXX-Int-3A-Puro, which is an AAV2 inducible Rep/Cap plasmid (Qiao et al., 2002b).
Finally, we recombined the vector shuttle plasmid and packaging backbone plasmids to obtain the final inducible vector and packaging plasmid for the generation of 293 cell lines (Fig. 1c). This step had no enzyme digestion and ligation involved. After recombining the vector shuttle plasmid pENTR11-ds-CMV-GFP with packaging backbone plasmid pSPG2 in the test tube by LR Clonase II, the recombination product was transformed into the DH10B competent E. coli cells and selected on the plate containing Amp antibiotics. The recombination efficiency was greater than 95% mainly due to the negative selection marker ccdB gene and different antibiotic resistant gene selection.
By utilizing the Gateway system, it was much easier for us to establish an inducible AAV plasmid containing different promoters, genes of interest, and alternative AAV serotypes. For example, only the vector shuttle plasmid needs modification for new promoter and new genes of interest. Similarly, only the packaging backbone plasmid needs to be modified to obtain the Cap gene of different AAV serotypes.
Generation of stable and high-titer 293 cell–based dsAAV2 cell line by one-step strategy
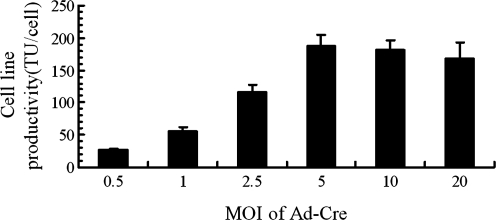
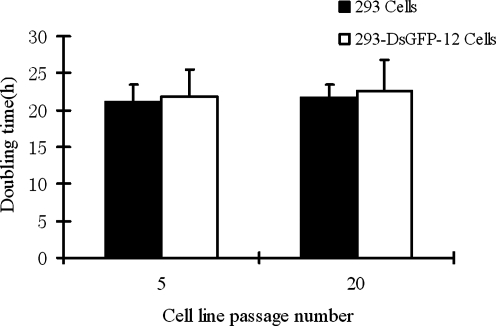
Previously, two steps of transfection and selection were required to establish the high-yield AAV-producer 293 cell lines. Here we used the one-step procedure with the final plasmid pSPG2-ds-CMV-GFP containing inducible AAV2 packaging genes, AAV2 ITRs, and CMV-GFP reporter gene cassette. One of the ITRs contained the deletion of the D-sequence and therefore enabled production of dsAAV (Wang et al., 2003). This plasmid was transfected into 293 cells and selected for puroR-resistant colonies. Among 48 clones tested for productivity, more than 10 of them demonstrated AAV vector yields of >109 transducing units (TU, GFP-positive cells) from each 10-cm plate of producer cells (equivalent to 200 TU/cell). The yield of one cell line, the 293-dsGFP-12, was as high as 9 × 109 TU per 10-cm plate (8 × 1011 vg, equivalent to 1.6 × 105 vg/cell). Optimization of helper Ad-Cre MOI ranging from 0.1 to 20 showed that MOI of 5 was optimal for AAV vector production (Fig. 2). Furthermore, the AAV packaging cell lines were very stable with normal growth rate and morphology indistinguishable from the parental 293 cells (Fig. 3). Importantly, the productivity also remained the same after consecutive multiple passages (data not shown), consistent with our previously published data (Qiao et al., 2002b).
FIG. 2.
Optimization of the multiplicities of infection (MOI) of Ad-Cre for the production of AAV2-D(+)-CMV-GFP from cell line 293-DsGFP-12. Different groups of cells were cultured in 10-cm plate (n = 4 for each group). At 70% to 90% confluence, the cells were infected with different MOI of Ad-Cre. When clear CPE was evident at around 48 hr post infection, all the cells were harvested and centrifuged at 2000 rpm for 10 min. Then the supernatant was removed and 1 ml of virus suspension buffer (see Material and Method for details) was added to the pellets, which contained AAV vector and Ad-Cre virus. After three freeze–thaw cycles in a dry ice–ethanol bath, tubes were subjected to centrifugation again and the supernatant containing final viruses were used for titration. All the viruses were incubated at 56 for 30 min to inactivate Ad-Cre before titration. The yield of the cell line was determined by quantifying the transduction unit per production cell (TU/cell). Mean values and standard deviations are displayed in the figure.
FIG. 3.
The doubling time of 293-dsAAV2-GFP-12 cell line at passages 5 and 20. The parental 293 cell line was used as a control. Both cell lines were seeded in a six-well plate at a plating density of 5 × 105 cells per well and cultivated in a cell incubator. Every 24 hr (i.e., 24, 48, 72 post seeding), three wells of each kind of cell were used to determine the cell numbers by counting the cells using a hematocytometer. The cell doubling time was calculated using the algorithm provided by http://www.doubling-time.com/compute.php?lang=en
Generation of 293-based cell lines for AAV serotypes 8 and 9
Our previous cell lines were all AAV2 vector producers mainly because it was the most used serotype at the time (Clark et al., 2005; Qiao et al., 2002a). However, lower efficiency of gene transfer in vivo and high prevalence of pre-existing immunity have hampered the use of AAV2-based vectors (Gao et al., 2000). Novel serotypes and engineered AAV capsids (Gao et al., 2002; Yang et al., 2009) have been generated and explored, including AAV8 and AAV9.
To establish AAV8 and AAV9 cell lines, we modified the AAV2 packaging plasmid pSPG2 and replaced the AAV2 Cap gene with that of AAV8 or AAV9, generating pSPG8 and pSPG9, respectively (Fig. 1c). By recombination of these packaging backbone plasmids with the vector shuttle plasmid, we obtained the inducible vector and packaging plasmids for the production of AAV8 and AAV9 vectors. Following the same procedure for the establishment of the AAV2 producer cell lines, cell lines for the production of dsAAV8-CMV-GFP and dsAAV9-CMV-GFP were established.
Creating the 293-based cell line packaging a large-sized gene
Previous attempts to generate high-titer AAV-mini-dystrophin vector with a baculovirus-based system were not successful, possibly due to the oversize of the vector (unpublished data), which was around 5 kb and slightly over the size of the wild-type AAV (4.8 kb). Here we tried to generate high-yield AAV producer cell line for the mini-dystrophin construct. We cloned the AAV-CMV-Opti-hDys3978 vector cassette into the vector shuttle plasmid containing attL recombination sites. The shuttle plasmid was recombined with the pSPG9 plasmid to generate the final inducible AAV9 packaging plasmid pSPG9-CMV-opti-hDys3978. As described before, the AAV9-CMV-Opti-hDys3978 cell line clones were randomly picked and subjected to productivity test by using dot blot assay. Among the 48 cell clones tested, more than 10 yielded greater than 1 × 1012 vg per 10-cm plate (equivalent to 2 × 105 vg/cell). One of them, 293-Dys3978-14, yielded as high as 2.6 × 1012 vg per 10-cm plate (equivalent to 5.2 × 105 vg/cell) and was then selected for large-scale production.
To test whether the cell line can be adopted to the scalable production, Nunc cell factory (equals to 40 of 15-cm plates) was used to culture three cell lines (293-dsAAV2-GFP-12, 293-ssAAV2-GFP-145, 293-ssAAV9-Dys3978-14). After the purification of the AAV vector from the cell line, the productivity for each cell line was analyzed by dot blot assay. These cell lines showed similar productivity in Nunc cell factory as in plates. The yield of each cell line was about 5 × 1013 to 8 × 1013 vg particles per Nunc cell factory (equivalent to 0.9 × 105 to 1.3 × 105 vg/cell) (Table 1).
Table 1.
Vector Yields From Different Cell Lines via Large-Scale Productiona
| Cell line | Vector produced | Purified vector (vg) from Nunc cell factory |
|---|---|---|
| 293-ssAAV2-GFP-145 | ssAAV2-CMV-GFP | 8.0 × 1013 |
| 293-dsAAV2-GFP-12 | dsAAV2-CMV-GFP | 6.4 × 1013 |
| 293-ssAAV9-Dys3978-14 | dsAAV9-CMV-hDys3978 | 5.6 × 1013 |
The Nunc cell factory (equivalent to 40 15-cm plates) was utilized to scale up AAV production based upon cell lines. Ad-Cre infection and sebsequent AAV vector harvesting and purification processes were carried out as described in Material and Methods. vg, vector genome.
Amplification of Rep/Cap genes in high-yield producer cell lines upon Ad helper infection
To investigate the relationship of cell line productivity with the Rep/Cap gene copy number integrated in cells, the Rep/Cap gene copy numbers in several 293-based cell lines were quantitatively determined by using real-time PCR technology (Table 2). The range was between 10 and 50 copies per 293 cell. We did not find an obvious relationship between the integrated Rep/Cap gene copy number and vector productivity. We have previously documented that the Rep and Cap genes were amplified during the vector production phase, upon Ad-Cre helper virus infection of the producer cells (Qiao et al., 2002b). However, the previous producer cells contained high-copy number of Rep and Cap genes that were introduced separately by two rounds of stable transfection and selection. It is unclear which component (or both) was amplified during the production phase.
Table 2.
Integrated Rep Gene Copy Numbers in 293-Based Cell Lines and Vector Yieldsa
| Cell line | Vector produced | Rep copies/cell | Purified vector (vg) from 20 × 15-cm plates |
|---|---|---|---|
| 293-ssAAV2-GFP-145 | ssAAV2-CMV-GFP | 34 ± 1.63 | 2.8 × 1013 |
| 293-dsAAV2-GFP-12 | dsAAV2-CMV-GFP | 17 ± 4.74 | 2.0 × 1013 |
| 293-dsAAV8-GFP-23 | dsAAV8-CMV-GFP | 28 ± 3.56 | 1.2 × 1013 |
| 293-dsAAV9-GFP-34 | dsAAV8-D(CMV)-GFP | 23 ± 3.23 | 1.5 × 1013 |
| 293-ssAAV9-Dys3978-14 | dsAAV9-CMV-hDys3978 | 36 ± 5.25 | 2.5 × 1013 |
The copy numbers displayed in the table were those in the stable cell lines before Ad-Cre infection. SYBR Green–based real-time PCR methods were utilized here (see Material and Methods for details).
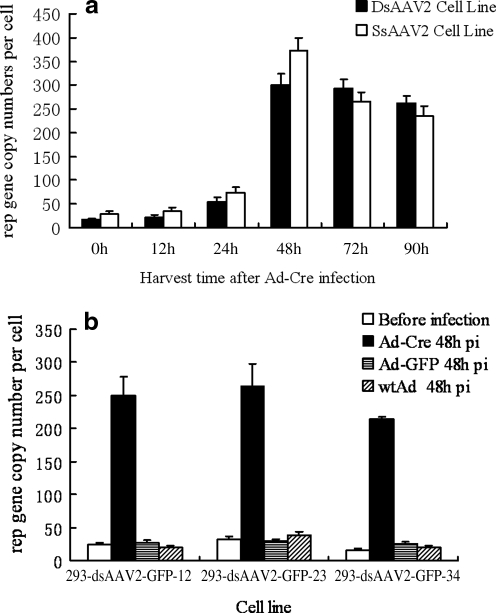
To see if the Rep and Cap genes were also amplified in the new generation producer cells that contained the genes introduced at a single time, we analyzed the Rep and Cap copy numbers in those cell lines before and after Ad-Cre infection. Quantitative real-time PCR analysis was performed on the total DNA extracted from those cells. As shown in Fig. 4a, Rep/Cap gene of 293-dsAAV2-GFP-12 was amplified around 14-fold, starting from 17 copies per cell to approximately 236 copies at 48 hr after Ad-Cre infection. Similarly, amplification of Rep/Cap genes was also observed in the other 293-based cell line, such as single-stranded AAV packaging cell line, the 293-ssAAV2-GFP-145 cells (Fig. 4a); AAV8 producer cells, the 293-dsAAV8-GFP-23 (Fig. 4b); and the AAV9 producer cells, the 293-dsAAV9-GFP-34 (Fig. 4b). In general, the inducible Rep/Cap genes were amplified 10–20 times after Ad-Cre infection. Importantly, this amplification was dependent on the activation of Rep gene by Ad-Cre. The same cell line infected with Ad-GFP and wild-type adenovirus did not achieve any amplification of the AAV Rep/Cap genes (Fig. 4b) because these two helper Ad could not activate the Rep gene that had three polyA sites inserted in the middle of the coding region (Fig. 1). These results show that amplification of the Rep and Cap genes is responsible in part for the high vector yields in the cell lines.
FIG. 4.
The amplification of Rep gene after Ad-cre infection in 293-based AAV producer cell line. (a) Real-time PCR analysis of AAV rep gene amplification in cell lines producing double-stranded AAV2-ds-CMV-GFP vector (solid column) and single-stranded AAV2-CMV-GFP (open column) at different time points post Ad-Cre infection at MOI of 5. Total cellular DNA was extracted from different cell lines at different time point post Ad-Cre infection, then real-time PCR was performed to determine the rep gene copy numbers. The final copy number was calculated as (vector copy numbers/human glucagon gene internal control) × 2. (b) Real-time PCR analysis of AAV rep gene amplification in cell lines producing AAV2-ds-CMV-GFP, AAV8-ds-GFP, and AAV9-ds-GFP. The Ad-Cre, Ad-GFP and wild-type adenoviruses were utilized at an MOI of 5, and the viruses were harvested at 48 hr post infection.
Characterization of AAV vectors from cell lines in vitro and in vivo
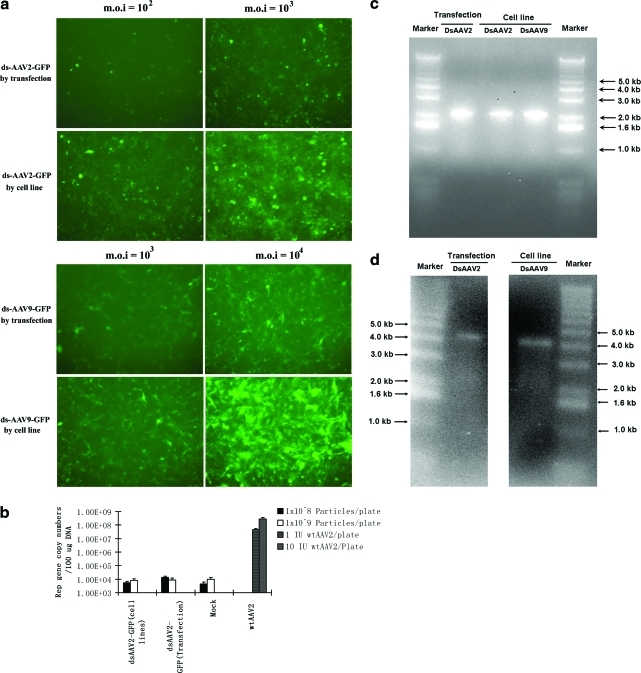
We next examined the cell line–produced vectors for their infectivities in vitro and in vivo, wild-type–like AAV contamination, and the integrity of the virus genome, particularly for the double-stranded vectors. The ratios of viral genome particles versus the transducing units (vg/TU) is usually used as an indicator of AAV2 vector infectivity in vitro. We thus compared the vg/TU ratio of dsAAV2-GFP vectors produced by the methods of cell lines or triple-plasmid transient transfection side by side. The vg titer was determined by vector DNA dot blot, whereas the TU titer was determined by infecting 293 cells in the absence of adenovirus. Our results showed that the ratio of vg/TU of AAV2 vectors produced from the 293-DsGFP-12 cell line was 85.4 ± 11.2, whereas the ratio of the vg/TU of AAV2 vectors derived from the triple transfection method was 480.8 ± 67.2. Additionally, we performed in vitro infection assay to compare the transduction efficiency of the viruses produced from different methods. As shown in Fig. 5a, both AAV2 and AAV9 vectors produced from cell line method demonstrated better infectivity than the double-stranded vectors produced from transfection method, which is consistent with the previously published data on ssAAV2-GFP vectors (Qiao et al., 2002b).
FIG. 5.
Characterizaiton of AAV vectors produced from cell lines in vitro. (a) Comparison of the transduction efficiency of AAV vectors produced by the transfection method or cell line method. 293 Cells were used for the AAV2 vector transduction efficiency test. U87 cells were used for the AAV9 vector. Original magnification × 100. (b) Real-time quantitative PCR analysis of replication competent AAV (rcAAV) in different AAV GFP vectors. The primers were the same as described in quantificaiton of Rep gene copy numbers in Material and Methods. Up to 109 viral genome particles of highly purified AAV vectors produced from different methods were used to infect 10-cm plates of 293 cells, which were coinfected with wild-type adenovirus at an MOI of 5 to provide helper function for rcAAV amplification. As a positive control, wild-type AAV was also used to infect the 293 cells at a total dose ranging from 1 to 10 infectious units per 10-cm plate along with wild-type adenovirus coinfection. (c) Electrophoresis of vector DNA produced from the cell line and transfection methods on the neutral gel. Two double-stranded vectors, AAV2-ds-GFP and AAV9-ds-GFP, produced from cell lines displayed double-stranded hairpin of 2.2 kb on neutral gel, similar to the dsAAV genome DNA generated from the transfection method. (d) Electrophoresis of vector DNA on alkaline gel. The dsAAV hairpin DNA from different methods was denatured into a 4.4-kb linear ssDNA. No detectable linear single-stranded monomers of 2.2 kb were present in the alkaline gel, indicating that most of the packaged genome in dsAAV particles is the dimer hairpin DNA. Color images available online at www.liebertonline.com/hum
Since the generation of replication-competent AAV (rcAAV, also termed wild-type–like AAV) is a potential safety concern for clinical applications of AAV vectors, we set out to examine the wild-type–like AAV in the cell line–produced AAV vectors using the quantitative real-time PCR method. We chose to use an infection-based viral amplification assay with wild-type adenovirus helper to detect the rcAAV (Qiao et al., 2002b). Real-time PCR analysis of the viral DNA isolated after two rounds of amplification in 293 cells revealed no detectable AAV coding sequences (similar to background level) from the AAV vector stocks produced by both cell line and triple plasmid transduction methods (Fig. 5b). These results indicate that there is no detectable rcAAV in up to 109 viral genome particles of AAV vector produced by the 293-based cell lines.
We also examined the integrity of the scAAV genomes produced by the cell line method, since other labs have reported that a mixture of double-stranded and single-stranded genomes could be packaged into AAV virons during the process of vector production even using a transient transfection method (Wu et al., 2002). In addition, an HSV helper system was unable to produce the scAAV vectors without a vast majority of the vector DNA genomes being in the ss form when assayed on alkaline denature gel (Wu et al., 2002). To confirm that the AAV genomes packaged in the scAAV vectors derived from the 293-based cell line were indeed predominantly in the hairpin-like self-complementary form as observed before (Wang et al., 2003), the DNA extracted from both single- and double-stranded vectors produced from cell lines was subjected to neutral and alkaline gel electrophoresis. On neutral agarose gel, the dsAAV DNA produced from both AAV2 and AAV9 cells lines displayed a double-stranded hairpin of 2.2 kb, similar to the dsAAV genome DNA generated from transfection method (Fig. 5c). Consistently, the dsAAV hairpin DNAs were denatured into a 4.4-kb linear ssDNA when running on the alkaline agarose gel (Fig. 5d). No significant linear single-stranded monomers of 2.2 kb were present in the alkaline gel, indicating that the vast majority of the packaged genome in dsAAV particles from both production methods were indeed in the form of double-stranded hairpin DNA.
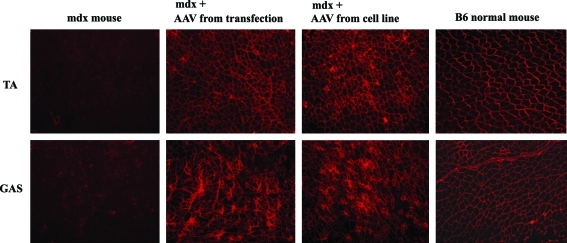
Finally, we analyzed the vector infectivity in vivo. AAV9-CMV-Opti-hDys3978 vectors produced by the transfection method or cell line method were directly injected in dystrophin-deficient mdx mice in the TA muscle (2.5 × 1010 vg of vector in 50 μl) and GAS muscle (4 × 1010 vg of vector in 80 μl), respectively. The treated mice were sacrificed 4 weeks post delivery. Dystrophin expression was evaluated by immunofluorescent staining on cryo-thin sections of the muscles. Greater than 80% of the myofibers in the TA and GAS muscles of mdx mice injected with vector from cell line method showed strong dystrophin staining, similar to the muscles injected with AAV9-CMV-Opti-hDys3978 made by triple transfection method (Fig. 6). This result suggests that the vector produced from our 293-based cell line is also highly infectious in vivo.
FIG. 6.
Analysis of the potency of the AAV9-CMV-opti-hDys3978 vector produced from cell line in vivo. The AAV9-CMV-Opti-hDys3978 vectors produced from either transfection or cell line method were delivered into the tibialis anterior (TA) muscle and gastrocnemius (GAS) muscle of mdx mice (three mice per group) via direct intramuscular (IM) injection. Three weeks post vector delivery, the delivered muscles were harvested and cryo-preserved. The muscles were then subjected to cryosectioning and immunofluorescent staining against dystrophin (see Material and Method for details). TA and GAS from a normal B6 mouse and an mdx mouse without treatment were utilized as postive and negative controls. Original magnification: × 100. Color images available online at www.liebertonline.com/hum
Discussion
To meet the demand for high quality and large quantity AAV vectors required in preclinical large animal studies and for clinical trials, extensive efforts have been made to improve the methodology for AAV production. We have previously made strides in developing the first stable 293-cell–based high yield AAV2 producer cell lines. In addition to the avoidance of the transient tranfection step during vector production, the major advantage of using the 293-based cell line is the ability to avoid the use of wild-type adenovirus as the helper. However, the major drawback of our previous method is the multiple steps of molecular cloning for construction of a new vector and an alternative serotype plasmid and the two-stage stable-transfection steps to obtain the final AAV producer cell lines. In this study, we streamlined the cloning process by using Gateway technology, which utilizes site-specific recombination instead of endonuclease and ligase to insert a gene of interest into an expression vector. It offers two major advantages: first, the recombination is efficient and rapid, making the cloning process much easier especially for oversized vectors. Second, it offers flexibility and convenience for swapping different transgene cassette or using alternative serotypes for new producer cells. In this study, we also established the 293-based cell lines of a few different AAV serotypes using the one-step transfection process. High-yield producers could be consistently obtained with normal and stable growth rate identical to their parent 293 cells. Thus, the improved and simplified method can significantly save time and effort in generating producer cell lines.
Using the simplified protocol, we have successfully established 293-based high-yield AAV producer cell lines for GFP-expressing vectors packaged in AAV2, AAV8, and AAV9 capsids. The GFP vectors were also in the form of double-stranded (self-complementary) DNA genomes. The same GFP vector backbone, however, turned out to be very difficult to produce with an HSV helper virus–based production system (X. Wu, personal communication). The dsAAV produced by the HSV system (Wu et al., 2002) contained mostly ssAAV genomes when assayed on an alkaline denaturing gel. For oversized AAV vectors, we had no difficulty in establishing high-yield cell lines, e.g., the cell line producing AAV9-mini-dystrophin vector, which has a size of over 5 kb. However, the same vector backbone did not generate high-yield vector in the baculovirus system even though a few attempts had been made. This was most likely due to the large vector size or toxic sequence for baculovirus (unpublished results and personal communications). To carry out large-scale production, the cell line approaches have distinct advantages with the use of bioreactors. For the 293-based cell lines, it is easy to adapt to suspension culture. The technology for suspension culture of 293 cells has already been well established. Serum-free media for suspension culture are readily available from commercial sources. Furthermore, in this study, we used the Nunc cell factory to perform the large-scale AAV production. The productivity of the cells cultured in Nunc cell factory remained similar to the cells cultured in small plates, reaching to 5 × 1013 to 8 × 1013 vg/Nunc (equivalent to 0.9 × 105 to 1.3 × 105 vg/cell) after purification, suggesting the productivity of the cell lines were stable for scaling up.
To find out why the cell lines could produce high-yield vectors, we looked at the copy numbers of the stably integrated Rep/Cap genes and the copy numbers during vector production. We found that the Rep/Cap genes in all of our cell lines were amplified 10–20 times after Ad-Cre helper infection, reaching several hundreds of copies per cell. This amplification was only observed by infection with Ad-Cre for the induction of Rep expression, but not by infection with Ad-GFP or wild-type adenovirus. This finding indicated that the amplification was Rep protein dependent. The mechanism of the application remains unclear, but it is possibly through the p5 promoter that was reportedly able to act as an origin for limited DNA replication in a Rep-dependent manner (Francois et al., 2005). The amplification of the Rep/Cap genes was observed in our previous 293 cell lines for AAV2 vectors (Qiao et al., 2002b) and was also observed in HeLa-based wild-type adenovirus-inducible AAV producer cell lines (Nony et al., 2001; Tessier et al., 2001), suggesting that the amplification of Rep/Cap gene may be essential to the higher productivity of all AAV producer cell lines (Liu et al., 2000).
A chief advantage of the 293-based AAV producer cell line over other cell lines is the use of replication-defective adenovirus Ad-Cre, rather than the wild-type adenovirus. The replication-defective E1-deleted adenoviral vectors have been widely used for gene therapy in clinical settings for vaccine and cancers and have shown a generally safe profile in clinical trials aside from a single fatal adverse event more than 10 years ago. Nevertheless, even the replication-deficient adenovirus should be thoroughly inactivated and eliminated from AAV preparations in view of safety and immunity. The commonly used method is to thermally inactivate the helper adenovirus. However, this method has some drawbacks because it can not completely inactivate the adenovirus. To address this problem, a novel method using high hydrostatic pressure (HHP) to specifically and completely inactivate helper adenovirus has been developed (Leonard et al., 2007). This HHP method leads to thorough elimination of adenovirus from the AAV preparations without affecting AAV vector potency. Furthermore, utilization of two-column chromotography purification system should be effective in removing the helper adenovirus (Gao et al., 2000). This easily scalable purification scheme starts with cationic exchange chromatography to capture AAV2 virions, followed by a purifying step with anionic exchange chromatography, resulting in highly efficient recovery of AAVs from crude lysates with substantially improved purity and potency. Column purification methods have also been sucessfully utilized for other AAV serotypes such as AAV1 (Smith et al., 2008) and AAV8 (Davidoff et al., 2004). The combination of our Gateway-mediated single-step 293-based AAV producer cell line with the column chromatography purification system should facilitate AAV vector production for preclinical and clinical applications.
Research in AAV-mediated gene therapy has been greatly advanced over the last decade. Novel developments, such as natural isolates of primate AAVs (Gao et al., 2002), direct evolution of tissue-tropic AAV capsids (Maheshri et al., 2006; Li et al., 2008; Yang et al., 2009; Asokan et al., 2010), and double-stranded AAV vectors (McCarty et al., 2003; Wang et al., 2003), have greatly expanded the potential of AAVs as a gene therapy vector in a variety of clinical applications. Future success in AAV-mediated clinical gene therapy requires large-scale production of potent AAV vectors with high titer and purity.
Acknowledgment
This work was supported by NIH grants AR 45967 and 56953 to X.X.
Author Disclosure Statement
No competing financial interests exist.
References
- Asokan A. Conway J.C. Phillips J.L., et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 2010;28:79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso E. Mingozzi F. Montane J., et al. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17:503–510. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- Brantly M.L. Chulay J.D. Wang L., et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau E. Ruhl V.E. Rodier F., et al. A versatile viral system for expression and depletion of proteins in mammalian cells. PLoS One. 2009;4:e6529. doi: 10.1371/journal.pone.0006529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadeuf G. Favre D. Tessier J., et al. Efficient recombinant adeno-associated virus production by a stable rep-cap HeLa cell line correlates with adenovirus-induced amplification of the integrated rep-cap genome. J. Gene Med. 2000;2:260–268. doi: 10.1002/1521-2254(200007/08)2:4<260::AID-JGM111>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Chamberlain J.S. Gene therapy of muscular dystrophy. Hum Mol Genet. 2002;11:2355–2362. doi: 10.1093/hmg/11.20.2355. [DOI] [PubMed] [Google Scholar]
- Clark K.R. Voulgaropoulou F. Fraley D.M. Johnson P.R. Cell lines for the production of recombinant adeno-associated virus. Hum. Gene Ther. 1995;6:1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- Clement N. Knop D.R. Byrne B.J. Large-scale adeno-associated viral vector production using a herpesvirus-based system enables manufacturing for clinical studies. Hum. Gene Ther. 2009;20:796–806. doi: 10.1089/hum.2009.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff A.M. Ng C.Y. Sleep S., et al. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J. Virol. Methods. 2004;121:209–215. doi: 10.1016/j.jviromet.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Francois A. Guilbaud M. Awedikian R., et al. The cellular TATA binding protein is required for rep-dependent replication of a minimal adeno-associated virus type 2 p5 element. J. Virol. 2005;79:11082–11094. doi: 10.1128/JVI.79.17.11082-11094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G. Qu G. Burnham M.S., et al. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum. Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- Gao G.P. Alvira M.R. Wang L., et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U. S. A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D.S. Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990;61:447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- Jiang X.C. Qin S. Qiao C., et al. Apolipoprotein B secretion and atherosclerosis are decreased in mice with phospholipid-transfer protein deficiency. Nat. Med. 2001;7:847–852. doi: 10.1038/89977. [DOI] [PubMed] [Google Scholar]
- Kang W. Wang L. Harrell H., et al. An efficient rHSV-based complementation system for the production of multiple rAAV vector serotypes. Gene Ther. 2009;16:229–239. doi: 10.1038/gt.2008.158. [DOI] [PubMed] [Google Scholar]
- Kohlbrenner E. Aslanidi G. Nash K., et al. Successful production of pseudotyped rAAV vectors using a modified baculovirus expression system. Mol. Ther. 2005;12:1217–1225. doi: 10.1016/j.ymthe.2005.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J.N. Ferstl P. Delgado A. Schaffer D.V. Enhanced preparation of adeno-associated viral vectors by using high hydrostatic pressure to selectively inactivate helper adenovirus. Biotechnol. Bioeng. 2007;97:1170–1179. doi: 10.1002/bit.21355. [DOI] [PubMed] [Google Scholar]
- Li W. Asokan A. Wu Z., et al. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol. Ther. 2008;16:1252–1260. doi: 10.1038/mt.2008.100. [DOI] [PubMed] [Google Scholar]
- Liu X. Voulgaropoulou F. Chen R., et al. Selective Rep-Cap gene amplification as a mechanism for high-titer recombinant AAV production from stable cell lines. Mol. Ther. 2000;2:394–403. doi: 10.1006/mthe.2000.0132. [DOI] [PubMed] [Google Scholar]
- Lock M. Alvira M. Vandenberghe L.H., et al. Rapid, simple and versatile manufacturing of recombinant adeno-associated virus vectors at scale. Hum. Gene Ther. 2010;21:1259–1271. doi: 10.1089/hum.2010.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M. High K.A. Auricchio A., et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M. Simonelli F. Pierce E.A., et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshri N. Koerber J.T. Kaspar B.K. Schaffer D.V. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 2006;24:198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- Margaritis P. High K.A. Gene therapy in haemophilia—going for cure? Haemophilia. 2010;3(16 Suppl):24–28. doi: 10.1111/j.1365-2516.2010.02256.x. [DOI] [PubMed] [Google Scholar]
- McCarty D.M. Fu H. Monahan P.E., et al. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10:2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- McDonell M.W. Simon M.N. Studier F.W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J. Mol. Biol. 1977;110:119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Meghrous J. Aucoin M.G. Jacob D., et al. Production of recombinant adeno-associated viral vectors using a baculovirus/insect cell suspension culture system: from shake flasks to a 20-L bioreactor. Biotechnol. Prog. 2005;21:154–160. doi: 10.1021/bp049802e. [DOI] [PubMed] [Google Scholar]
- Mendell J.R. Campbell K. Rodino-Klapac L., et al. Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 2010a;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J.R. Rodino-Klapac L.R. Rosales-Quintero X., et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann. Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J.R. Rodino-Klapac L.R. Rosales X.Q., et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann. Neurol. 2010b;68:629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nony P. Tessier J. Chadeuf G., et al. Novel cis-acting replication element in the adeno-associated virus type 2 genome is involved in amplification of integrated rep-cap sequences. J. Virol. 2001;75:9991–9994. doi: 10.1128/JVI.75.20.9991-9994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C. Li J. Skold A., et al. Feasibility of generating adeno-associated virus packaging cell lines containing inducible adenovirus helper genes. J. Virol. 2002a;76:1904–1913. doi: 10.1128/JVI.76.4.1904-1913.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao C. Wang B. Zhu X., et al. A novel gene expression control system and its use in stable, high-titer 293 cell-based adeno-associated virus packaging cell lines. J. Virol. 2002b;76:13015–13027. doi: 10.1128/JVI.76.24.13015-13027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. Afione S. Kotin R.M. Adeno-associated virus type 2 Rep78 induces apoptosis through caspase activation independently of p53. J. Virol. 2000;74:9441–9450. doi: 10.1128/jvi.74.20.9441-9450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.H. Yang L. Kotin R.M. Chromatography-based purification of adeno-associated virus. Methods Mol. Biol. 2008;434:37–54. doi: 10.1007/978-1-60327-248-3_4. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Kagawa N. Fujino T., et al. A novel high-throughput (HTP) cloning strategy for site-directed designed chimeragenesis and mutation using the Gateway cloning system. Nucleic Acids Res. 2005;33:e109. doi: 10.1093/nar/gni103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier J. Chadeuf G. Nony P., et al. Characterization of adenovirus-induced inverted terminal repeat-independent amplification of integrated adeno-associated virus rep-cap sequences. J. Virol. 2001;75:375–383. doi: 10.1128/JVI.75.1.375-383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urabe M. Ding C. Kotin R.M. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum. Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- Wang B. Li J. Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Ma H.I. Li J., et al. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Watchko J. O'Day T. Wang B., et al. Adeno-associated virus vector-mediated minidystrophin gene therapy improves dystrophic muscle contractile function in mdx mice. Hum. Gene Ther. 2002;13:1451–1460. doi: 10.1089/10430340260185085. [DOI] [PubMed] [Google Scholar]
- Wu Z. Wu X. Cao H., et al. A novel and highly efficient production system for recombinant adeno-associated virus vector. Sci. China C Life Sci. 2002;45:96–104. doi: 10.1360/02yc9011. [DOI] [PubMed] [Google Scholar]
- Xiao X. Li J. Samulski R.J. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. Jiang J. Drouin L.M., et al. A myocardium tropic adeno-associated virus (AAV) evolved by DNA shuffling and in vivo selection. Proc. Natl. Acad. Sci. U. S. A. 2009;106:3946–3951. doi: 10.1073/pnas.0813207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q. Chen F. Trempe J.P. Characterization of cell lines that inducibly express the adeno-associated virus Rep proteins. J. Virol. 1994;68:4847–4856. doi: 10.1128/jvi.68.8.4847-4856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]