Abstract
Objectives:
To study the analgesic and anti-nociceptive activity of hydroethanolic extract of Drymaria cordata Willd.
Materials and Methods:
Wistar rats and Swiss albino mice were used for studying analgesic and anti-nociceptive activity of Drymaria cordata hydroethanolic extract (DCHE) at doses 50, 100 and 200 mg/kg p.o. Various models viz. acetic acid induced writhing model (female mice), Eddy's hot plate (mice) and tail flick model (rat) for analgesic study and formalin-induced paw licking model (mice) were used for anti-nociceptive study.
Results:
In acetic acid induced writhing model, effect of DCHE was better than the standard drug- indomethacin 10 mg/kg (p.o.). In the hot plate model, the maximum effect was observed at 60 min at a dose of 200 mg/kg p.o., which was higher than the standard drug morphine sulfate (1.5 mg/kg i.p.), whereas in the tail flick model, effect was comparable with morphine sulfate. In formalin-induced paw licking model, administration of DCHE completely abolished the early phase at 100 and 200 mg/kg p.o. and in the late phase, the effect of DCHE (200 mg/kg p.o.) was higher than indomethacin (10 mg/kg p.o.).
Conclusion:
DCHE was effective in both non-narcotic and narcotic models of nociception, suggesting its possible action via peripheral and central mechanism. It also abolished the early phase in formalin-induced paw licking model, suggesting complete inactivation of C-fiber at higher dose. The activity can be attributed to the phyto-constituents viz tannins, diterpenes, triterpenes and steroids present in the DCHE extract. In conclusion, DCHE can be developed as a potent analgesic and anti-nociceptive agent in future.
Keywords: Analgesic, acetic acid, Drymaria cordata, Eddy's hot plate, hydroethanol extract, tail flick
Introduction
The use of plant products is increasing in many segments of the population. According to an estimate, 80% of the world's population relies upon plants for their medication. Most of the synthetic drugs used at present for analgesic and anti- nociceptive effect cause many side and toxic effects. Plants still represent a large untapped source of structurally novel compounds that might serve as lead for the development of novel drugs.[1] Many medicines of plant origin with analgesic and anti-nociceptive activity had been used since long time without any adverse effect. North East India is considered as one of the “hotspots” for biodiversity in India, since out of the 1500 species of medicinal plants available in India, almost 350 species belong to Assam and many of these traditionally used plants have not yet been studied scientifically which can be developed as a potential drug after scientific validation.
Drymaria cordata Willd (Caryophyllaceae) locally known as “Laijabori” is one of the traditionally used plants of North East India for its analgesic, wound healing, anti-inflammatory activity and is also used as an antidote, appetizer, depurative, emollient, febrifuge, laxative and stimulant in both human and animals. It is a sprawling herb with procumbent and more or less ascending branched stems, often rooting at the lower nodes, quadrangular, glabrous or papillose especially in the upper internodes, which are slender, generally 2-6 cm long. The pounded leaf is applied to snake bites.[2]Studies on Drymaria cordata exhibited significant anti-tussive,[3]anti-bacterial[4] and anti-inflammatory[5] property. Hydroethanolic extracts of Drymaria cordata have been shown to possess significant anxiolytic activity.[6] We have also studied its anti-inflammatory, adaptogenic, anti-convulsant and anti-depressant activity (unpublished reports).
In our previous study, we have reported analgesic activity of the plant using methanol extract.[7] The present study aims to select the active extract responsible for analgesic activity using hydroethanolic extract and thereafter to isolate the phytoconstituent from the active fraction.
Materials and Methods
Collection of Plant material
The leaves of Drymaria cordata Willd were collected during the month of July to September, 2008 from the medicinal garden of the Department of Pharmacology and Toxicology, C.V.Sc. Khanapara, Guwahati and were identified by Botanical Survey of India, Shillong, Meghalaya. A voucher specimen (No AAU/CVSC/PHT/ 07-08/ 03) was deposited in the Herbarium of Botanical Survey of India, Shillong, Meghalaya.
Preparation of extract of Drymaria cordata
Fresh leaves of Drymaria cordata were cleaned and dried under shade in clean dust free environment, grinded and stored in an air-tight container. They were (250 g) soaked in 1000 ml of hydroethanol (50:50) for 72 h in separate beakers. It was stirred every 18 h using a sterile glass rod. The solvent was filtered every third day using muslin cloth and Whatman's filter paper No 1. The filtrate obtained was concentrated in rotary evaporator (Equitron, Roteva) at 50-60º C under reduced pressure leaving a dark brown residue. The Drymaria cordata hydroethanolic extract (DCHE), thus obtained, was transferred to a Petri dish and kept over water bath (50° C-) until the solvent gets completely evaporated. It was stored at 4° C for future use. Recovery was 18.06% (w/w).
Phytochemical screening
Freshly prepared DCHE was subjected to standard phytochemical screening tests for various constituents by standard methods.[8]
Animals and treatment regimes
Swiss albino mice (20-30 g) and Wistar rats (120-130 g) were used for the study. The animals had free access to food and water. They were fasted overnight before the experiment. They were housed in animal room, with alternating light-dark cycle of 12 h each. The animals were acclimatized to the laboratory conditions for at least five days prior to the experiments. All experiments were conducted between 0900 h and 1800 h.
Acute toxicity study was carried out according to the Organization of Economic Corporation Development (OECD) guidelines No. 425. DCHE was administered orally in doses of 100, 200, 400, 800, 1000 and 2000 mg/kg to the group of mice (n=3) and the percentage mortality was recorded for a period of 24 h. During the first 1 h after the drug administration, the mice were observed for any gross behavioral change and the parameters observed were hyperactivity, grooming, convulsions, sedation and loss of righting reflex, respiration, salivation, urination and defecation[9]
Based on the above toxicity study, direct limit test was done. Initially a particular dose, on the basis of the above study, was administered to single female rat and the rat was observed for 48 h with close surveillance up to initial 4 h (same as in case of first rat) after 48 h (of the second administration), same dose was administered in two more female rats and the observation was done same as for the previous rat. The rats were observed for 14 days and no adverse observation was found morphologically. The weight of the animals was recorded on 7th and 14th day.
Animals were divided into five groups of six animals each. The first group (Group I) served as a control group. The second (Group II) was used as the reference standard. Three groups (Group III, IV and V) received DCHE at three different doses (50, 100 and 200 mg/kg p.o.). The doses were selected on the basis of our preliminary screening. The research was conducted in accordance with the ethical rules on animal experimentation, approved by ethical committee, Guwahati Medical College, Guwahati (Registration numbers- 351).
Drugs
Indomethacin (Jagsonpal Pvt Ltd) and morphine sulfate (Drugs India Pvt. Ltd. Dispur, Guwahati-5) were used for the study. Distilled water was used as vehicle. All the chemicals and solvents were of analytical grade.
Analgesic and anti-nociceptive activity
Acetic acid induced writhing model in mice
In this model, the animals were pretreated with drugs 30 min before induction of writhing. The Group I animals received vehicle and Group II animals received the reference standard drug indomethacin (10 mg/kg p.o.). Analgesic activity of DCHE at the doses 50, 100 and 200 mg/kg p.o. (Group -III, IV and V) was assessed by counting the number of writhes induced by 0.7% acetic acid. The number of writhes per animal were counted for 20 min.[10] Percent reduction in writhing syndrome was calculated and compared with the standard drug. Percent reduction indicates the percentage protection against abdominal constriction which was taken as an index of analgesia.
It was calculated as:
{(Wc -Wt) × 100}/Wc
where, Wc = number of writhing of the control group
Wt = number of writhing of the treated group
Eddy's hot plate model in mice
In this model, the animals received vehicle (Group I) and standard drug indomethacin (10 mg/kg p.o.) (Group II). Analgesic activity of DCHE at doses 50, 100 and 200 mg/kg p.o. (Group III, IV and V) was assessed by observing the reaction time in the treated groups. The reaction time was noted at 0, 30, 60, 90 and 120 min following the administration of drugs.[11] A cut-off time of 20 sec was considered.
Tail flick model in rat
In this model, the animals received vehicle (Group I) and standard drug indomethacin (10 mg/kg p.o.) (Group II). Analgesic activity of DCHE at doses 50, 100 and 200 mg/kg p.o. (Group III, IV and V) was assessed by observing the reaction time in the treated groups.[12] Following the administration of drugs, the reaction time was noted at 0, 30, 60, 90 and 120 min.
Formalin- induced paw licking model in mice
In this model, the animals received vehicle (Group-I) and standard drug indomethacin (10 mg/kg p.o.) (Group II). Anti- nociceptive activity of DCHE at doses 50, 100 and 200 mg/kg, p.o. (Group III, IV and V) was assessed by observing the reaction time in the treated groups. Fifteen minutes after treatment, 20 μl of 1% formalin was injected subcutaneously under dorsal surface of the hind paw and the time spent for licking the paw injected with formalin was counted for 30 min post formalin injection and considered as indicative of the pain stimuli.[13] The formalin test has two distinctive phases possibly reflecting different types of pain. The first phase peaked at 5 min and the second phase at 20-30 min after formalin injection. This represented neurogenic and inflammatory responses, respectively.
Statistical analysis
The statistical analysis of data was done using one-way analysis of variance by using the SPSS software (version 11.5). P< 0.01 was considered as highly significant.
Results
Phytochemical screening
The phytochemical screening of DCHE showed the presence of diterpenes, tannins by ferric chloride and gelatin test; triterpenes by Salkowski's and Liberman Buchardt's test and steroids by Salkowski's and Liberman Buchardt's test.
Acute toxicity studies
Oral administration of DCHE up to 2 g/kg did not produce any toxic effects in the normal behavior of the mice. No mortality was observed and the extract was found to be safe at the given dose.
Analgesic and anti-nociceptive activity
Acetic acid induced writhing model in mice
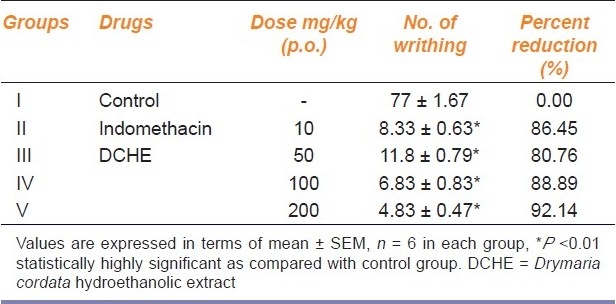
The DCHE produced significant (P<0.01) reduction in the number of writhing in mice in dose dependent manner. At 100 and 200 mg/kg oral dose, the percent reduction of writhing was 88.89% and 92.14% respectively, as compared to the control group, whereas the standard drug indomethacin (10 mg/kg p.o.) showed a reduction of 86.45% [Table 1].
Table 1.
Analgesic activity of DCHE in female albino mice in acetic acid induced writhing method
Eddy's hot plate model in mice
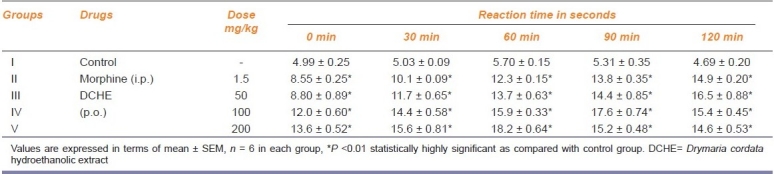
In this model, the reaction time in DCHE treated group increased significantly (P<0.01) in comparison to the control group. The maximum effect was observed at the highest dose viz. 200 mg/kg p.o. at 60 min which showed a reaction time of 18.2 sec, whereas the standard drug morphine (1.5 mg/kg i.p.) showed a reaction time of 12.3 sec. The extract also showed dose and time dependent activity [Table 2].
Table 2.
Analgesic activity of DCHE in Eddy's hot plate model in mice
Tail flick model in rat
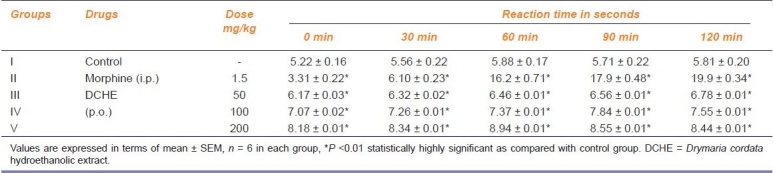
In the tail flick test, increase in the reaction time was significant (P<0.01) as compared to the control group. Maximum effect was 8.94 sec at 60 min post treatment with 200mg/kg p.o. of DCHE, whereas in the vehicle treated control group the reaction time was 5.88 sec at 60 min, clearly indicating the analgesic property of the plant extract [Table 3].
Table 3.
Analgesic activity of DCHE in Wistar rat by tail flick method
Formalin- induced paw licking model in mice
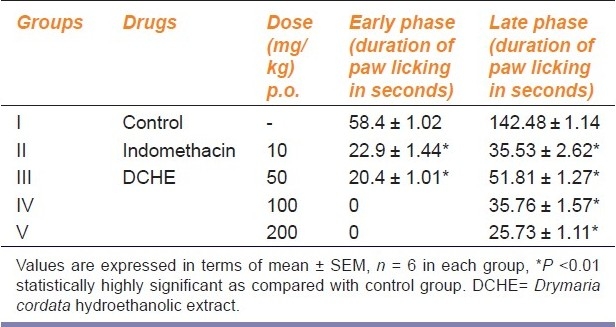
Administration of DCHE at 50 mg/kg p.o. caused reduction in duration of paw-licking (20.4 sec) as compared to the control group (58.4 sec). Higher doses of DCHE at 100 and 200 mg/kg p.o. showed complete abolishment of the early phase indicated by absence of paw licking after the formalin injection. However, the standard drug- indomethacin (10 mg/kg p.o.) exhibited a reduction of paw licking time of 22.9 sec only in the early phase. In late phase, the administration of DCHE decreased the duration of paw licking dose dependently from 51.81 sec at 50 mg/kg p.o. to 25.73 sec at 200 mg/kg p.o. in the late phase. On the other hand, the standard drug- indomethacin (10 mg/kg p.o.) exhibited a reduction of paw licking time of 35.53 sec in the late phase. The effect of DCHE at 200 mg/kg p.o. was better than the standard drug [Table 4].
Table 4.
Anti-nociceptive activity of DCHE in albino mice in formalin induced paw-licking test
Discussion
Any injury or tissue damage is associated with pain and inflammation. Analgesics can act on peripheral or central nervous system. Peripherally acting analgesics act by blocking the generation of impulses at chemoreceptors site of pain, while centrally acting analgesics not only raise the threshold for pain, but also alter the physiological response to pain and suppress the patient's anxiety and apprehension. Pain and inflammation are an essential prelude to the repair process.[14]
Acetic acid induced writhing response in mice is not only simple and reliable but also affords rapid evaluation of peripheral type of analgesic action. Acetic acid causes inflammatory pain by inducing capillary permeability and liberating endogenous substances that excite pain nerve ending. Acetic acid is also known to increase PGE1 and PGE2 peripherally.[15] NSAIDs can inhibit COX in peripheral tissues and therefore interfere with the mechanism of transduction of primary afferent nociceptors.[16] The mechanism of analgesic activity of DCHE could be probably due to the blockade of the effect or the release of endogenous substances that excite pain nerve endings similar to that of indomethacin and NSAIDs. Thus, the reduction in the number of writhing indicates that DCHE might exert anti-nociceptive activity by inhibition of prostaglandin synthesis or action of prostaglandin.
In the hot plate model, nociceptive reaction toward thermal stimuli in mice is a well-validated model for detection of opiate analgesics as well as several types of analgesics drugs from spinal origin.[17] The tail flick model is also used to evaluate analgesic agents acting through central nervous system. DCHE was found to be effective in both the models.
The formalin-induced paw licking model comprises of early phase and late phase. The early phase (immediately after injection) seems to be caused by C-fiber activation due to the peripheral stimulus. The late phase (starting approximately 20 min after formalin injection) appears to depend on the combination of an inflammatory reaction, activation of NMDA and non-NMDA receptors and NO cascade[18] in the peripheral tissue and the functional changes in the dorsal horn of the spinal cord.[19] In our study, DCHE completely abolishes the early phase at the dose 100 and 200 mg/kg p.o. suggesting complete inactivation of C- fiber in the early phase. DCHE decreased the reaction time in dose dependent manner in the late phase also which might suggest that DCHE causes partial inactivation of NMDA and non-NMDA receptors.
Our previous studies have shown anxiolytic[6] activity in the hydroethanol extract and analgesic activity in the methanol extract.[7] Analgesic activity of hydroethanol extract of Drymaria cordata in the present study showed better effect in both narcotic and non narcotic models, whereas in the methanol extract, the activity was more prominent in non narcotic models (acetic acid induced writhing) only. The methanol extract contains flavonoids, steroids, triterpenes, diterpenes, alkaloids and tannins whereas the hydroethanol extract contains steroids, triterpenes, diterpenes and tannins. Hence, significant analgesic activity in hydroethanolic extract might be attributed to the presence of these bioactive principles.
In conclusion, it can be interpreted that DCHE possesses promising analgesic and anti-nociceptive properties, which are probably peripherally mediated via inhibition of prostaglandin synthesis as well as central inhibitory mechanism and may be of potential benefit for management of pain. Further studies on isolation and fractionation of the active components from the leaf of Drymaria cordata and study on its mechanism of action to ascertain their analgesic and anti-nociceptive properties will throw light on mode of action.
Acknowledgments
The authors are grateful to National Medicinal Plant Board, Govt. of India, New Delhi for providing financial assistance to carry out this work. Physical facility provided by the Director of Research (Vety), C.V.Sc is also gratefully acknowledged.
Footnotes
Source of Support: National Medicinal Plant Board, Govt. of India, New Delhi.
Conflict of Interest: None declared.
References
- 1.Ahmad F, Khan RA, Rasheed S. Study of analgesic and anti-inflammatory activity from plant extracts of Lactuca scarliola and Artemsia absinthium. J Int Acad Sci. 1992;5:111–14. [Google Scholar]
- 2.Saklani A, Jain SK. Cross cultural ethno botany of North East India. New Delhi: Deep publishers; 1994. p. 97. [Google Scholar]
- 3.Mukherjee PK, Bhattacharya S, Saha K, Giri SN, Pal M, Saha BP. Studies on anti-tussive activity of Drymaria cordata Willd (Caryophylaceae) J Ethnopharmacol. 1997;56:74–7. doi: 10.1016/s0378-8741(97)01512-2. [DOI] [PubMed] [Google Scholar]
- 4.Mukherjee PK, Saha K, Bhattacharya S, Giri SN, Pal M, Saha BP. Studies on anti bacterial activity of Drymaria cordata Willd (Caryophylaceae) Phytother Res. 1998;11:249–50. doi: 10.1016/s0378-8741(97)01512-2. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee PK, Mukherjee K, Bhattacharya S, Saha BP, Pal M. Studies on the anti- inflammatory effects of Drymaria cordata Willd. Nat Prod Sci. 1998;4:91–4. [Google Scholar]
- 6.Barua CC, Roy JD, Buragohain B, Barua AG, Borah P, Lahkar M. Anxiolytic activity of hydroethanolic extract of Drymaria cordata Willd. Indian J Exp Biol. 2009;47:969–73. [PubMed] [Google Scholar]
- 7.Baruah CC, Pal SK, Baruah AG, Roy JD, Buragohain B, Bora RS, Lahon LC. Analgesic activity of methanolic extract of Drymaria cordata Willd Cayophyllaceae. Pharmacologyonline. 2009;2:470–6. [Google Scholar]
- 8.Harborne JB. Phytochemical Screening - Guide to modern techniques of plant analysis. 2nd ed. NewYork: Chapman and Hall; 1991. p. 653. [Google Scholar]
- 9.Vogel HG. Drug discovery and evaluation: Pharmacological Assay. New York: Berlin Heidelberg; 2002. p. 385. [Google Scholar]
- 10.Witkin LB, Herbner CF, Gaddi F, O’Keefe E, Spitaletta P, Plumer AJ. Pharmacology of 2-aminoindane hydrochloride (SU- 8629).A potent non- narcotic analgesic. J Pharmacol Exp Ther. 1961;133:400–8. [PubMed] [Google Scholar]
- 11.Eddy NB, Leimbach D. Systematic analgesics II.Diethyl butenyl and diethienylbutyl amines. J Pharmacol Exp Ther. 1953;107:387–93. [Google Scholar]
- 12.Turner RA. Screening methods in pharmacology. New York: Academic Press; 1965. p. 158. [Google Scholar]
- 13.Hunskaar S, Hole K. The formalin test in mice-dissociation between inflammatory and non- inflammatory pain. J Pain. 1997;30:103–4. doi: 10.1016/0304-3959(87)90088-1. [DOI] [PubMed] [Google Scholar]
- 14.Shreedhara CS, Vaidya VP, Vagdevi HM, Latha KP, Muralikrishna KS, Krupanidhi AM. Screening of Bauhinia purpurea Linn.for analgesic and anti-inflammatory activities. Indian J Pharmacol. 2009;41:75–9. doi: 10.4103/0253-7613.51345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar V, Singh PN, Bhattacharya SK. Anti- inflammatory and analgesic activity of Indian Hypericum perforatum L. Indian J Exp Biol. 2001;19:339–43. [PubMed] [Google Scholar]
- 16.Adzu B, Amos S, Kapo SD, Gamaniel KS. Anti-inflammatory and anti-nociceptive effects of Sphaeranthus senegalensis. J Ethnopharmacol. 2003;84:169–73. doi: 10.1016/s0378-8741(02)00295-7. [DOI] [PubMed] [Google Scholar]
- 17.Alhaider AA, Lei SZ, Wilcox GL. Spinal 5-HT mediated anti-nociception possible release of GABA. J Neurosci. 1991;11:1881–8. doi: 10.1523/JNEUROSCI.11-07-01881.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson EM, Carlton SM. Intraplanter injection of dextrophan, Ketamine or memantine attenuates formalin induced behaviors. Brain Res. 1998;785:136–42. doi: 10.1016/s0006-8993(97)01396-6. [DOI] [PubMed] [Google Scholar]
- 19.Abbott FV, Franklin KB, Westbrook RF. The formalin test scoring properties of the first and second phase of the pain response in the rats. J Pain. 1995;60:91–102. doi: 10.1016/0304-3959(94)00095-V. [DOI] [PubMed] [Google Scholar]


