Abstract
Objectives:
Conventional polyethoxylated castor oil (PCO)-based paclitaxel is associated with major adverse drug reactions (ADRs). Nanoxel, a nanoparticle-based formulation, may improve its tolerability by removing the need for PCO vehicle, and also permit its use in a higher dose. We conducted intensive monitoring of the ADR profile of Nanoxel in comparison with conventional paclitaxel in a public tertiary care set-up.
Materials and Methods:
ADR data were collected from 10 patients receiving Nanoxel and 10 age-matched controls receiving conventional paclitaxel in this longitudinal observational study, conducted in a medical oncology ward over 18 months. Severity was graded as per US National Cancer Institute Common Terminology Criteria for Adverse Events.
Results:
The groups had comparable demography at baseline. The median disease duration and per cycle median dose of paclitaxel were greater in the Nanoxel arm. Total 119 ADRs were noted with Nanoxel and 123 with conventional paclitaxel. Of these, 25 (21.0%, 95% CI 13.69–28.33%) in the Nanoxel and 20 (16.2%, 95% CI 9.74–22.78%) in paclitaxel group were of grade 3/4 severity. Common events included myalgia, nausea, anemia, paresthesia, alopecia, diarrhea, and vomiting with Nanoxel, and paresthesia, anemia, myalgia, anorexia, alopecia, vomiting, diarrhea, stomatitis, and nausea with paclitaxel. Of the less common events (<5%), grade 2 or 3 arthralgia was seen exclusively with Nanoxel while motor neuropathy with muscular weakness was more frequent and severe with conventional paclitaxel. Hypersensitivity reactions were not encountered in either arm, although no antiallergy premedication was employed for Nanoxel.
Conclusions:
Despite its ADR profile being statistically comparable to conventional paclitaxel, this observational study suggests that Nanoxel tolerability could be better, considering that a significantly higher dose was employed. This hypothesis needs confirmation through an interventional study.
Keywords: Adverse drug reaction, nanoparticle, observational study, paclitaxel
Introduction
Paclitaxel is used for the treatment of ovarian, breast, and lung (non-small-cell) cancers.[1]It acts by binding to the β-tubulin subunit of microtubules, promoting polymerization and inappropriately stabilizing the microtubules, leading to mitotic arrest and cell death.[1]The usual dose is 135–175 mg/m2 intravenously (IV) every 3 weeks.
Conventional paclitaxel infusion is formulated in a vehicle of 50% ethanol and 50% polyethoxylated castor oil (PCO), commercially known as “Cremophor EL,” to overcome the problem of poor water solubility.[2,3]However, this vehicle has been implicated in a number of adverse drug reactions (ADRs) associated with paclitaxel, including acute hypersensitivity manifesting as respiratory distress, hypotension, angioedema, urticaria, and rash.[4,5]Minor allergic reactions (e.g., flushing and rash) occur in about 40% of patients, while nearly 3% experience potentially life-threatening reactions, despite premedication with corticosteroids and antihistamines.[2,6]Similar reactions have been induced in dogs by the PCO vehicle, implying that the vehicle, rather than the drug, is the culprit.[7,8]Another concern with PCO is the risk of peripheral neuropathy.[3]Neuropathy has been reported with IV but not oral cyclosporine –; another drug whose IV formulation contains PCO.[9,10]These toxicities restrict the dose of paclitaxel that can be administered, potentially compromising efficacy.
Attempts are being made to develop novel PCO-free formulations of paclitaxel so as to increase efficacy while reducing vehicle-associated toxicity. Paclitaxel albumin-bound nanoparticle (“nab” paclitaxel) has been approved by the United States Food and Drug Administration in 2005 and several other novel formulations (e.g., paclitaxel polyglumex, liposomal, and microsphere delivery systems) are currently undergoing evaluation.[11,12]However, none of these are available in the Indian market.
Nanoxel, a biodegradable nanoparticle-based paclitaxel delivery system, developed by M/s Dabur Pharma Ltd., was approved by the Drugs Controller General of India on August 23, 2006, for use in metastatic breast cancer. The polymer is an amphiphilic entity that forms nanometer-sized micelles (mean particle size 80 nm) when exposed to water. The hydrophobic core region serves as a drug reservoir where the highly hydrophobic paclitaxel molecules are incorporated, surrounded by hydrophilic groups in the periphery. The formulation is water soluble and obviates the need for PCO. The drug is released slowly by surface erosion of the polymer.[13,14]Besides avoiding PCO, it also allows targeted drug delivery. The particle size permits selective entry into tumor cells, utilizing the enhanced vascular permeability associated with tumorigenesis, while sparing normal tissue. This is expected to further minimize toxicity, along with an increase in the antitumor activity, due to selective accumulation of the drug in tumor tissue.[14]
Considering the novelty of the Nanoxel delivery system, we undertook a longitudinal observational study to monitor the ADR profile of Nanoxel vis-à-vis conventional paclitaxel.
Materials and Methods
The study was conducted in the medical oncology ward of a government teaching hospital. Institutional ethics committee approval and written informed consent were taken. During March 2007 to September 2008, adult patients of either sex, judged to be suitable for paclitaxel therapy (either monotherapy, or in combination with other anticancer agents) were included if they had not received paclitaxel earlier. Patients with significant impairment of liver, kidney, heart, lung, or other vital organs and those with history of substance abuse were excluded.
The investigators had no role in deciding whether an individual patient received either conventional or nanoparticle formulation of paclitaxel. However, for each patient enrolled in the Nanoxel arm, an age-matched (within ±5 years) subject fulfilling the selection criteria was enrolled in the conventional paclitaxel arm. During the study period, 10 patients could be recruited into each study arm.
Paclitaxel chemotherapy requires four to six cycles at 21-day intervals. However, patients in the concerned ward tend to drop out owing to various socioeconomic issues and in a few instances, the chemotherapeutic regimen is changed by the oncologist due to lack of tumor response. Thus all patients may not receive the full course (four to six cycles) of chemotherapy. It was decided a priori to enroll only patients who can be followed up for at least two cycles. In case the regimen remained unchanged and the patient was available, follow-up was to be extended for the full course of chemotherapy.
For each patient, the ADR profile was noted through detailed history, clinical examination, and scanning of source documents (e.g., bed head tickets and laboratory test reports) during the 1- or 2-day hospital stay for a chemotherapy cycle and again after 3 weeks when readmitted for the subsequent cycle. ADR data were also sought in case the subject visited the outpatient department between scheduled hospital admissions. The data were captured on predesigned case report forms that included details on the demographic profile, date of onset of event, its nature, severity and outcome, concomitant medications received, dechallenge and rechallenge information, etc. ADR severity was graded as per the US National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.[15]ADR causality was assessed by the World Health Organization-Uppsala Monitoring Centre standardized case causality assessment criteria.[16]
ADR profiles have been summarized as percentages. Baseline demographic and disease profile of the two groups were compared using the Mann-Whitney U-test for numerical nonparametric data and Fisher's exact test for categorical data. The incidence of individual reactions was compared by Fisher's exact test.
Results
Apart from use in breast carcinoma in 3 of the 10 cases, Nanoxel was also used for the treatment of ovarian carcinoma in 2 cases, and carcinomas of lung, larynx, rectum, broad ligament, and retroperitoneal liposarcoma in 1 case each. In the 10 corresponding age-matched subjects, conventional paclitaxel was used for breast cancer in 2 patients, lung cancer in 4 and for cancers of ovary, tongue, esophagus, and malignant pleural effusion in 1 each.
Patients in the Nanoxel group received either Nanoxel monotherapy (n = 3), combination therapy of Nanoxel on day 1 plus carboplatin 450-600 mg IV on day 2 (n = 6), or Nanoxel on day 1 plus gemcitabine 1.2 g IV on day 2 and day 9 (n = 1). In the other group, regimens were either paclitaxel on day 1 plus carboplatin 450 mg IV on day 2 (n = 3), paclitaxel on day 1 plus cisplatin 100–155 mg IV on day 2 (n = 5), or paclitaxel on day 1 plus doxorubicin 70 mg IV on day 2 (n = 2). Nanoxel was infused at a median dose of 330 mg IV over 1 h and conventional paclitaxel at 260 mg IV over 3 h. Both infusions used in-line filters.
Premedication with dexamethasone, diphenhydramine, and ondansetron was undertaken prior to conventional paclitaxel infusion, whereas in the Nanoxel group only domperidone was given without any steroids or antihistamines. The Nanoxel arm had two diabetic and two hypertensive subjects, while the conventional paclitaxel arm included one and three such patients, respectively. However, these comorbidities were well controlled and subjects continued their regular medicines (insulin, oral hypoglycemics, amlodipine, atenolol, or losartan) throughout the duration of chemotherapy.
At baseline, the demographic and disease-related profiles (age, sex, weight, number of chemotherapy drugs per cycle, and number of follow-up visits) were comparable between the two groups, except for a significantly longer (P < 0.05) disease duration and a higher total dose per cycle (P < 0.01) in the Nanoxel group, compared to conventional paclitaxel [Table 1].
Table 1.
Baseline demographic and disease profile of study subjects

Every patient experienced one or more ADRs. A total of 119 reactions were noted in the Nanoxel arm and 123 in the conventional paclitaxel arm. The median number of ADRs per patient was 11.5 (interquartile range [IQR] 9) with Nanoxel, versus 12 (IQR 8) with conventional paclitaxel; the median number of ADR types per patient was 8 (IQR 4) and 7 (IQR 2.2) in the two groups, respectively. These differences were not statistically significant.
Myalgia, nausea, anemia, paresthesia, alopecia, diarrhea, and vomiting (in that order) were the most frequently encountered (incidence ≥ 5%) ADRs with Nanoxel whereas paresthesia, anemia, myalgia, anorexia, alopecia, vomiting, diarrhea, stomatitis, and nausea were the most common ADRs with conventional paclitaxel. Among these common ADRs, anemia, alopecia, and diarrhea had a nearly similar incidence in either group; myalgia and nausea were more common with Nanoxel, whereas paresthesia, anorexia, vomiting, and stomatitis were more frequent with conventional paclitaxel [Table 2]. Of the less frequently encountered ADRs (incidence < 5%), four cases of arthralgia (grade 2 or 3) involving knee, ankle, and shoulder were noted in the Nanoxel group; no such event was encountered with conventional paclitaxel. On the other hand, four cases of grade 2 or 3 motor neuropathy, resulting in muscular weakness of limbs, were noted with paclitaxel versus only one case of grade 1 weakness with Nanoxel [Table 2]. Again, these differences were not statistically significant.
Table 2.
Spectrum of suspected adverse drug reactions of nanoparticle versus conventional paclitaxel formulation
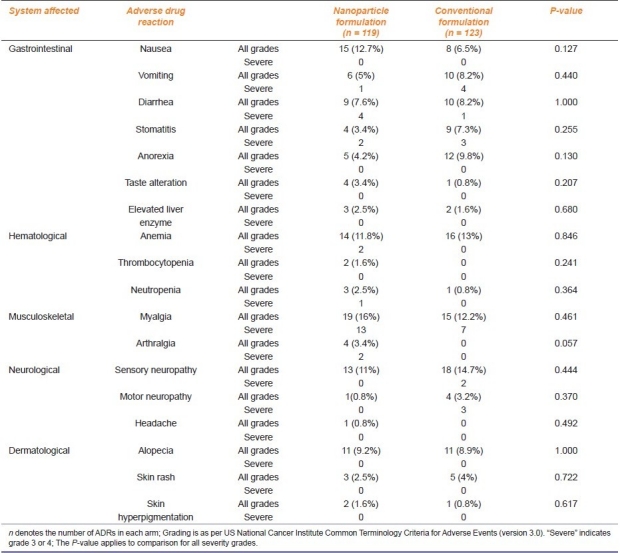
A total of 25 events (21%, 95% confidence interval [CI] 13.69–28.33%) in the Nanoxel group and 20 events (16.26%, 95% CI 9.74–22.78%) in the conventional paclitaxel arm were considered “severe” [Table 2]. Except for diarrhea, which included both grade 3 and 4 events, other “severe” events were restricted to grade 3 by NCI CTCAE criteria.
For the three patients who received Nanoxel monotherapy, the ADRs having either “certain” or “probable/likely” association included myalgia (grade 2 and 3), nausea (grade 1), vomiting (grade 2), diarrhea (grade 2 and 3), alopecia (grade 2), myelosuppression (grade 1 anemia and grade 2 thrombocytopenia), sensory neuropathy (grade 1 and 2), and hyperpigmentation of skin (grade 1). No hypersensitivity reaction (anaphylaxis, rash, or urticaria) was noted in either arm. Febrile neutropenia and injection site reactions were also not encountered.
Discussion
Though indicated for the same malignancies, Nanoxel was primarily used for cases refractory to conventional paclitaxel or for the recurrence of malignancy following previous therapy, which explains the significantly higher disease duration in the Nanoxel arm. The much higher cost price of Nanoxel probably precluded its use as first-line paclitaxel chemotherapy in our setting.
Reactions encountered in the conventional paclitaxel arm (mostly in combination with carboplatin or cisplatin) match the known ADR profile of such combinations.[17,18] Motor neuropathy, resulting in proximal muscular weakness of upper and lower limbs, was more common and severe with conventional paclitaxel than Nanoxel. Although less common than sensory neuropathy, motor neuropathy is known to occur with conventional paclitaxel.[19,20]
Sensory neuropathy, muscular weakness of limbs and vomiting, and stomatitis of all grades as well as of severe grade were more common with conventional paclitaxel than with Nanoxel. Although these differences were not statistically significant, when viewed in the light of the fact that the per cycle dose of Nanoxel was significantly higher than conventional paclitaxel [Table 1], this might indicate a better tolerability profile of Nanoxel. All grade and severe grade myalgia, neutropenia, and arthralgia were, however, more common with Nanoxel which may be a consequence of the higher dose. The absence of hypersensitivity reactions with the nanoparticle formulation, even without antiallergy premedication, is worth noting.
In addition to the small sample size and limited ethnic and geographic coverage, our study had another limitation of being an observational pharmacovigilance study. Causality assessment was hampered by the fact that most patients were on combination therapy and the toxicities of paclitaxel overlapped with those of the other drugs used to a substantial extent. Although we reviewed reports of tests that were ordered by the oncologist, there was no scope to undertake additional investigations, such as nerve conduction studies, to confirm suspected reactions or to unearth additional ones. Finally, since no long-term follow-up has been undertaken, delayed onset ADRs have not been captured.
Notwithstanding these limitations, we can conclude that the ADR profiles of Nanoxel and conventional paclitaxel appear to be similar from a statistical viewpoint and events such as myalgia, paresthesia, anorexia, nausea, vomiting, diarrhea, stomatitis, alopecia, and myelosuppression occur with either formulation. However, despite a significantly higher dose, the incidence and severity of many of these events are comparatively less with Nanoxel, suggesting that Nanoxel may actually be better tolerated, even in higher doses. Myalgia and arthralgia, however, tend to occur more frequently with Nanoxel. The lack of hypersensitivity reactions despite skipping prophylactic premedication suggests that the nanoparticle formulation is probably less allergic. A larger study, in the form of a focused postmarketing surveillance study, or a randomized controlled trial, is needed to confirm the trends shown by this observational study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Chabner BA, Amrein PC, Druker BJ, Michaelson MD, Mitsiades CS, Goss PE. Antineoplastic agents. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill; 2006. pp. 1352–4. [Google Scholar]
- 2.ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. Pharmacological effects of formulation vehicles: Implications for cancer chemotherapy. Clin Pharmacokinet. 2003;42:665–85. doi: 10.2165/00003088-200342070-00005. [DOI] [PubMed] [Google Scholar]
- 3.Hennenfent KL, Govindan R. Novel formulations of taxanes: A review.Old wine in a new bottle? Ann Oncol. 2006;17:735–49. doi: 10.1093/annonc/mdj100. [DOI] [PubMed] [Google Scholar]
- 4.Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8:1263–8. doi: 10.1200/JCO.1990.8.7.1263. [DOI] [PubMed] [Google Scholar]
- 5.Wiernik PH, Schwartz EL, Strauman JJ, Lipton RB, Dutcher JP, Paietta E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47:2486–93. [PubMed] [Google Scholar]
- 6.Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol) Semin Oncol. 1993;20:1–15. [PubMed] [Google Scholar]
- 7.Lorenz W, Reimann HJ, Schmal A, Dormann P, Schwarz B, Neugebauer E, et al. Histamine release in dogs by Cremophor EL and its derivatives: Oxethylated oleic acid is the most effective constituent. Agents Actions. 1977;7:63–7. doi: 10.1007/BF01964882. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein BJ. Docetaxel as an alternative to paclitaxel after acute hypersensitivity reactions. Ann Pharmacother. 2000;34:1332–5. doi: 10.1345/aph.19383. [DOI] [PubMed] [Google Scholar]
- 9.de Groen PC, Aksamit AJ, Rakela J, Forbes GS, Krom RA. Central nervous system toxicity after liver transplantation.The role of cyclosporine and cholesterol. N Engl J Med. 1987;317:861–6. doi: 10.1056/NEJM198710013171404. [DOI] [PubMed] [Google Scholar]
- 10.Brat DJ, Windebank AJ, Brimijoin S. Emulsifier for intravenous cyclosporin inhibits neurite outgrowth, causes deficits in rapid axonal transport and leads to structural abnormalities in differentiating N1E.115 neuroblastoma. J Pharmacol Exp Ther. 1992;261:803–10. [PubMed] [Google Scholar]
- 11.Perez EA. Novel enhanced delivery taxanes: An update. Semin Oncol. 2007;34:1–5. doi: 10.1053/s0093-7754(07)00088-7. [DOI] [PubMed] [Google Scholar]
- 12.Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235:179–92. doi: 10.1016/s0378-5173(01)00986-3. [DOI] [PubMed] [Google Scholar]
- 13.New Delhi: Dabur Pharma Ltd; 2006. NANOXEL Injection Package Insert [Version Number IND/00/2006 dated December, 2006] [Google Scholar]
- 14.Matsumura Y. Polymeric micellar delivery systems in oncology. Jpn J Clin Oncol. 2008;38:793–802. doi: 10.1093/jjco/hyn116. [DOI] [PubMed] [Google Scholar]
- 15.United States: National Cancer Institute; [accessed on 2010 Apr 7]. Common Terminology Criteria for Adverse Events v 3.0 (CTCAE). [monograph on the Internet] Available from URL: http://www. ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. [Google Scholar]
- 16.The use of the WHO-UMC system for standardized case causality assessment [monograph on the Internet] Uppsala: The Uppsala Monitoring Centre; 2005. [accessed on 2010 Apr 7]. Available from URL: http://www.who-umc. org/graphics/4409.pdf.
- 17.Okamoto I, Moriyama E, Fujii S, Kishi H, Nomura M, Goto E, et al. Phase II study of carboplatin-paclitaxel combination chemotherapy in elderly patients with advanced non-small cell lung cancer. Jpn J Clin Oncol. 2005;35:188–94. doi: 10.1093/jjco/hyi059. [DOI] [PubMed] [Google Scholar]
- 18.Nagata N, Kimura M, Hirabayashi N, Tuburaya A, Murata T, Kondo K, et al. Phase II study of weekly paclitaxel and cisplatin combination therapy for advanced or recurrent gastric cancer. Hepatogastroenterology. 2008;55:1846–50. [PubMed] [Google Scholar]
- 19.Freilich RJ, Balmaceda C, Seidman AD, Rubin M, DeAngelis LM. Motor neuropathy due to docetaxel and paclitaxel. Neurology. 1996;47:115–8. doi: 10.1212/wnl.47.1.115. [DOI] [PubMed] [Google Scholar]
- 20.Kuroi K, Shimozuma K. Neurotoxicity of taxanes: Symptoms and quality of life assessment. Breast Cancer. 2004;11:92–9. doi: 10.1007/BF02968010. [DOI] [PubMed] [Google Scholar]


