Abstract
Objective:
The objective of the present study was to evaluate the cognitive enhancing and antioxidant activity of Hibiscus rosa sinensis.
Materials and Methods:
The learning and memory was impaired by administration of scopolamine (1 mg/kg, i.p.) in mice which is associated with altered brain oxidative status. The object recognition test (ORT) and passive avoidance test (PAT) were used to assess cognitive enhancing activity. Animals were treated with an ethyl acetate soluble fraction of the methanol extract of H. sinensis (25, 50 and 100 mg/kg, p.o).
Results:
The ethyl acetate soluble fraction of the methanol extract of H. sinensis (EASF) attenuated amnesia induced by scopolamine and aging. The discrimination index (DI) was significantly decreased in the aged and scopolamine group in ORT. Pretreatment with EASF significantly increased the DI. In PAT, scopolamine-treated mice exhibited significantly shorter step-down latencies (SDL). EASF treatment showed a significant increase in SDL in young, aged as well as in scopolamine-treated animals. The biochemical analysis of brain revealed that scopolamine treatment increased lipid peroxidation and decreased levels of superoxide dismutase (SOD) and glutathione reductase (GSH). Administration of extract significantly reduced LPO and reversed the decrease in brain SOD and GSH levels. The administration of H. sinensis improved memory in amnesic mice and prevented the oxidative stress associated with scopolamine. The mechanism of such protection of H. sinensis may be due to augmentation of cellular antioxidants.
Conclusion:
The results of the present study suggested that H. sinensis had a protective role against age and scopolamine-induced amnesia, indicating its utility in management of cognitive disorders.
Keywords: Cognitive enhancing, Hibiscus rosa sinensis, lipid peroxidation, oxidative stress, passive avoidance test, scopolamine
Introduction
Alzheimer's disease (AD) is a neurodegenerative disease causing memory loss and dementia, which mostly affects the elderly population.[1]The pathophysiology of AD is complex including defective beta-amyloid (Aβ) protein metabolism, abnormalities of glutaminergic, adrenergic, serotonergic and dopaminergic neurotransmission, and the potential involvement of inflammatory and oxidative pathways.[2]Currently, the treatments for AD are acetylcholinesterase (AChE) inhibitors, which increase the availability of acetylcholine (ACh) at cholinergic synapses and nootropic agents such as piracetam. However, the resulting side effects associated with these agents have limited their use. Since the cholinesterase inhibitors confer only modest benefits, additional non-cholinergic AD therapies are urgently needed. Multipotent agents aiming at diverse targets are expected to act better than the single target aiming counterparts in the fight against AD.[2] The impairment of memory in scopolamine-induced animal model is associated with altered status of brain oxidative stress.[3] Strong evidence supporting the involvement of oxidative stress within the forebrain cholinergic system has been suggested. The drugs with antioxidant effects might be beneficial for preserving brain function. Antioxidant enzymes are involved in the reduction of oxidative stress.[4] Antioxidant enzymes display the reduced activities in the affected brain region of Alzheimer's disease patients. Moreover, the reduction in the level of intracellular oxidized protein under these conditions has been associated with the improvement of cognitive and/or psychomotor functions. Thus, the efforts have been directed to find therapeutic agents that could reduce the oxidative damage and promote a functional recovery in degenerative disorders.
H. sinensis L. (Malvaceae) is an ornamental plant. It is native to India and China. Experimental studies reported that Hibiscus rosa sinensis possesses significant anti-complementary, antiphologistic,[5] anti-spermatogenic,[6] anti-tumor[7] and anticonvulsant activities.[8] The flowers and leaves of the plant were found to exhibit significant cardioprotective,[9] hypotensive,[10] lipid lowering,[11] and hypoglycemic activity.[12] Moreover, it has also been known to have a radical scavenging effect.[13] The pharmacological activities of H. sinensis were attributed due to the chemical constituents like quercetin, carotene, niacin, riboflavin, malvalic acid, gentisic acid, margaric acid, lauric acid, anthocyanin, and anthocyanidine.[14,15]
In spite of the reported antioxidant property of H. sinensis in a variety of models, there is no major investigative reports available pertaining to its cognitive enhancing effect. The objective of the study was to investigate the cognitive enhancing and antioxidant potential of H. sinensis in scopolamine-induced amnesic mice and in aged mice.
Materials and Methods
Plant material
The roots were collected in the month of August (2008) from local area of Nashik (India) and authenticated by P. S. N. Rao (Director, Botanical survey of India, Pune). A voucher specimen of the plant has been deposited at Botanical survey of India, Pune (Voucher Specimen No. NVHR3). The plant material was shade dried and coarsely powdered. The powdered plant material (1 kg) was defatted with petroleum ether (60-80ºC) by Soxhlet extractor. The defatted marc was further extracted with methanol for 72 h. Extract was filtered and concentrated under reduced pressure. The yield of the methanol extract of H. rosa sinensis roots was found to be 6.2% w/w. Further, the methanol extract of H. rosa sinensis was exhaustively extracted with ethyl acetate to obtain ethyl acetate soluble (EASF, 1.7 w/w) and ethyl acetate insoluble fractions (EAISF, 1.3 w/w). The ethyl acetate soluble fraction was suspended in Tween 80 (0.2% v/v) in distilled water and administered per orally (p.o.).
Animals
Swiss albino mice of either sex (young, age 8 weeks, 18-20 g and aged, age 32 weeks, 35-40 g) were used for the study. Animals were housed in polypropylene cages and maintained under the standard laboratory environmental conditions; temperature 25 ± 2ºC, 12 h light: 12 h dark cycle and 50 ± 5% relative humidity with free access to food and water ad libitum. Animals were acclimatized to laboratory conditions before the test. Each group consisted of five (n = 5) animals. All the experiments were carried out during the light period (08:00-16:00 h). The studies were carried out in accordance with the guidelines given by Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi (India). The Institutional Animal Ethical Committee of M.V.P.S College of Pharmacy, Nashik approved the protocol of the study (IAEC/2008/08 and 10).
Drugs and chemicals
Piracetam hydrochloride (UCB India Pvt. Ltd. Vapi, Gujarat), Donepezil hydrochloride (Glenmark Pharmaceutical, Mumbai), Scopolamine hydrobromide (Sigma Aldrich, USA). Distilled water was used as a vehicle.
Phytochemical screening of H. sinensis
Phytochemical screening of the EASF of the methanol extract of H. sinensis roots for the presence of flavonoids, glycosides, saponins, alkaloids, and sterols was carried out in accordance with procedures previously described.[16,17]
Treatment schedule
Animals (Young and aged mice) were divided into 16 groups having 5 animals in each group. EASF (25, 50 and 100 mg/kg, p.o.) was administered to young and aged mice of different groups. Amnesia was induced only in young mice by scopolamine. Groups I, II - Control (Young and aged, 0.2% v/v Tween 80, p.o respectively); Groups III, IV- Standard (Piracetam 200 mg/kg, i.p. in young and aged respectively); Group V- Scopolamine (1 mg/kg, i.p. in young); Group VI, VII, VIII-EASF (25, 50 and 100 mg/kg, p.o in young respectively); Groups IX, X, XI - EASF (25, 50 and 100 mg/kg, p.o in aged respectively); Groups XII, XIII, XIV- EASF (25, 50 and 100 mg/kg, p.o + Scopolamine in young respectively); Group XV- Piracetam (200 mg/kg, i.p. + Scopolamine in young); Group XVI- Donepezil (1 mg/kg, i.p. + Scopolamine in young).
Experimental methods
Object recognition test
The object recognition test (ORT) is a behavioral test that is widely used to examine animal's memory performance. Memory performance in the ORT is based on the natural tendency of animals to explore novel objects. The apparatus consists of an open white colored plywood box (70× 60× 30 cm) with a well-furnished floor. The apparatus is illuminated by a 60 W lamp suspended 50 cm above the box. The objects to be discriminated are made of plywood in two different shapes of 8 cm and colored black and white. The day before test, mice was given a habituation session where they were left to freely exploring the box for 2 min. No object was placed in the box during the habituation trial. On the day of test, two identical objects were presented in two opposite corner of the box during the first trial (T1), and the amount of time taken by each mouse to complete 20 s of object exploration was recorded. Exploration was considered as directing the nose at a distance less than 2 cm to the object and/or touching it with nose or forepaw. Turning around or sitting on the object was not considered as an exploratory behavior. During the second trial (T2, 90 min after T1), one of the objects presented in T1 (i.e., familiar object) was replaced by new object and mice was left in box for 5 min. The time spent (s) for exploration of the familiar (F) and new (N) object was recorded separately and the discrimination index (DI) was calculated. DI = N-F/ N + F, where DI= discrimination index, N = exploration of the new object, F= exploration of the familiar object.[18,19]
Scopolamine (1 mg/kg) was injected i.p. after 45 min of administration of EASF (25, 50 and 100 mg/kg, p.o) or piracetam (200 mg/kg, i.p.) or donepezil (1 mg/kg, i.p.) or vehicle in young mice and first trial was given 45 min after injection of scopolamine. After 45 min of administration of EASF (25, 50 and 100 mg/kg, p.o), the first trial was given in aged mice.
Passive avoidance paradigm/test
Passive avoidance behavior based on negative reinforcement was used to examine the long-term memory. The apparatus consisted of a box (27 × 27 × 27 cm) having three walls of wood and one wall of Plexiglass, featuring a grid floor (3 mm stainless steel rods set 8 mm apart), with a wooden platform (10 ×7 ×1.7 cm) in the center of the grid floor. The box was illuminated with a 15 W bulb during the experimental period. Electric shock (20 V AC) was delivered to the grid floor. Training was carried out in two similar sessions. Each mouse was gently placed on the wooden platform set in the center of the grid floor. When the mouse stepped down and placed all its paws on the grid floor, shock was delivered for 15 s and the step-down latency (SDL) was recorded. SDL was defined as the time taken by the mouse to step down from wood platform to grid floor with all its paws. Animals showing SDL in the range (2-15 s) during the first test were used for the second session and the retention test. The second session was carried out 90 min after the first test. When the animals stepped down before 60 s, electric shock was delivered for 15 s. During the second test, animals were removed from the shock-free zone if they did not step down for a period of 60 s. Retention was tested after 24 h in a similar manner, except that the electric shocks were not applied to the grid floor. Each mouse was again placed on the platform, and the SDL was recorded, with an upper cut-off time of 300 s. EASF (25, 50 and 100 mg/kg, p.o), piracetam (200 mg/kg, i.p.) and donepezil (1 mg/kg, i.p.) or vehicle were administered orally for 8 days and (SDL) was noted after 45 min of administration of last dose on eighth day and again after 24 h i.e. on ninth day. In the scopolamine-treated group, scopolamine (1 mg/kg) was injected i.p. after 45 min of administration of EASF (25, 50 and 100 mg/kg) or piracetam or donepezil or vehicle and SDL was recorded after 45 min of injection of scopolamine on eighth day and after 24 h i.e. on ninth day. On the ninth day after measurement of SDL, the animals were sacrificed by cervical dislocation and antioxidant parameters such as lipid peroxidation (LPO), superoxide dismutase activity (SOD), glutathione reductase (GSH) levels in the brain were measured.[20]
Dissection and Homogenization
At the end of experiment, the mice of groups I, V, XII, XIII, XIV, XV and XVI were sacrificed by cervical dislocation and brains were taken out. They were rinsed thoroughly with ice-chilled 0.9% NaCl and weighed. A 10% (w/v) tissue homogenate was prepared in 0.1 M phosphate buffer (pH 7.4). The post nuclear fraction was obtained by centrifugation (Remi - C-30, Remi Industries Ltd, Mumbai, India) of the homogenate at 12000g for 60 min at 4ºC. A Shimadzu-160A spectrophotometer was used for subsequent assays.[21]
Biochemical analysis
Lipid peroxidation assay
The quantitative measurement of LPO in brain was done by the method of Wills (1966). The amount of malondialdehyde (MDA) formed was measured by reaction with thiobarbituric acid at 532 nm. The results were expressed as nanomole of MDA per milligram of protein, using the molar extension coefficient of chromophore (1.56 × 105 M-1 cm-1 ).[22]
Superoxide dismutase activity
Superoxide dismutase activity (SOD) was assayed according to the method of Kono (1978), wherein the reduction of nitroblue tetrazolium chloride (NBT) was inhibited by the superoxide dismutase which was measured at 560 nm spectrophotometrically. Briefly the reaction was initiated by the addition of hydroxylamine hydrochloride to the reaction mixture containing NBT and post nuclear fraction of brain homogenate. The results were expressed as % inhibition.[23]
Estimation of reduced glutathione
Reduced glutathione (GSH) in the brain was estimated according to the method of Ellman (1959). A 0.1 ml of sample of homogenate was precipitated with 0.75 ml of 4% sulfosalicylic acid. The assay mixture contained 0.5 ml of supernatant and 4.5 ml of DTNB in 0.1 M phosphate buffer, pH 8.0. The yellow color developed was read immediately at 412 nm. The results were expressed as nanomole of GSH per milligram of protein.[24]
Protein estimation
The protein content was measured according to the method of Lowery et al. (1951), using bovine serum albumin as standard and expressed as μg protein/mg of tissue.[25]
Statistical analysis
Results are expressed as mean ± S.E.M., and the statistical analysis of data was done using one-way analysis of variance (ANOVA) followed by Dunnett's test. The probability level less than 0.05 was considered statistically significant.
Result
Phytochemical screening
The phytochemical screening of EASF of H. sinensis revealed the presence of glycosides, flavonoids, tannins and saponins.
Object Recognition Test
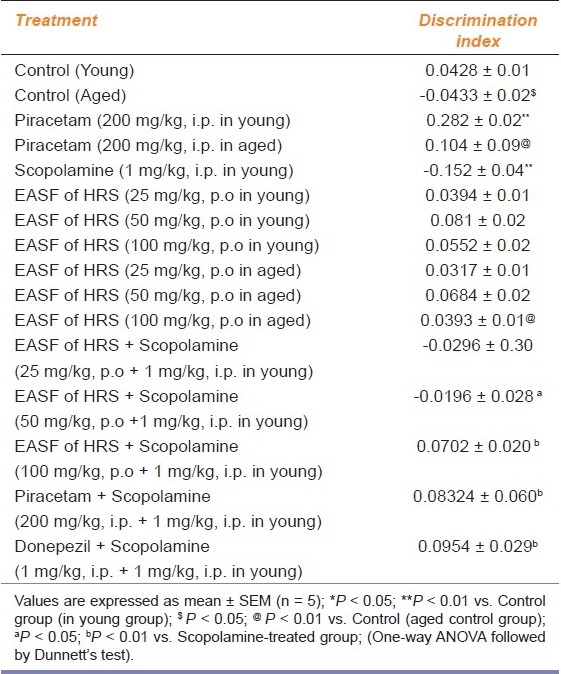
The discrimination index was significantly (P < 0.05; P < 0.01) decreased in the aged and scopolamine group as compared to control indicating impairment of memory in object recognition test. Pretreatment with EASF of H. sinensis (25, 50 and 100 mg/kg) significantly increased the DI in the aged and scopolamine-treated group, indicating improvement in short-term memory and reversal of amnesia induced by scopolamine. Piracetam and donepezil also significantly improved short-term memory in the aged and scopolamine-treated group (P < 0.01) [Table 1].
Table 1.
Effect of EASF of H. sinensis on discrimination index in young and aged mice in object recognition test
Passive Avoidance Paradigm/Test
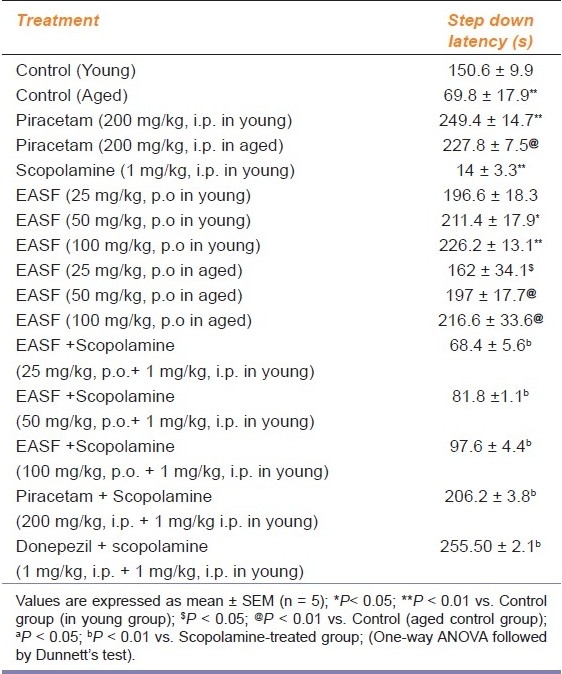
SDL of second day (ninth day of drug treatment) indicated the long-term memory of animals. The SDL was significantly decreased (P < 0.01) in the aged and scopolamine-treated group as compared to the control group. EASF of H. sinensis (50 and 100 mg/kg) administered to young and aged mice for 8 days showed an increase in SDL as compared to the respective control groups (P < 0.05; P < 0.01). Administration of EASF of H. sinensis for 8 days reversed memory deficits due to scopolamine and aging-induced amnesia. Piracetam and donepezil also showed improvement in memory in the young, aged as well as scopolamine-treated groups (P < 0.01) [Table 2].
Table 2.
Effect of EASF of H. sinensis on transfer latencies of young and aged mice in passive avoidance test
Biochemical effect
Lipid peroxidation assay
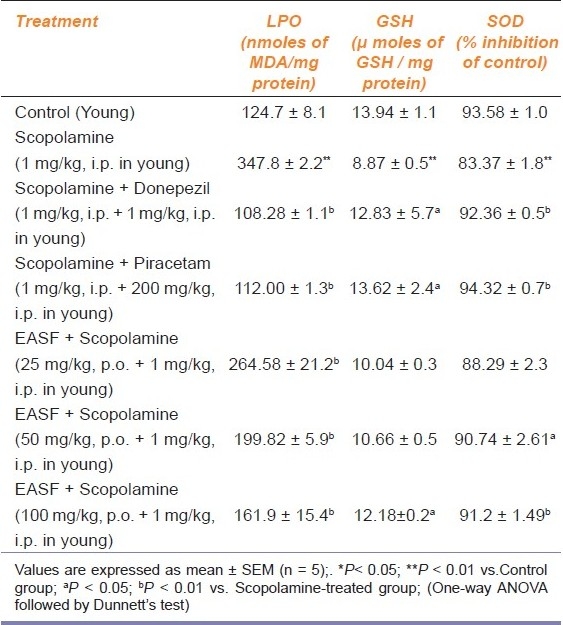
On the ninth day after measurement of SLD, the level of MDA was investigated. The level of MDA was significantly increased (P < 0.01) in the scopolamine group, as compared with the control group, while administration of EASF of H. sinensis (25, 50 and 100 mg/kg) significantly (P < 0.01) brought down the level of MDA, compared with the scopolamine group. The MDA level was also significantly decreased in the piracetam and donepezil group (P < 0.01) [Table 3].
Table 3.
Effect of EASF on antioxidant parameters in scopolamine-induced oxidative stress
Effect on brain SOD level
The level of the defensive antioxidant enzyme SOD was significantly decreased in the scopolamine group (P < 0.01) as compared to the control group. Pretreatment with EASF of H. sinensis (100 mg/kg) resulted in elevation of SOD (P < 0.01) as compared to the scopolamine-treated group. The SOD level was also significantly increased in the piracetam and donepezil group (P < 0.05) [Table 3].
Effect on brain GSH level
The content of GSH was depleted significantly (P < 0.01) in the scopolamine-treated group, as compared with the control group, indicating the neurotoxicity induced by scopolamine in mice. On the other hand, the GSH level was found to be elevated significantly (P < 0.05; P < 0.01) after administration of EASF of H. sinensis (50 and 100 mg/kg) as compared with the scopolamine-treated group. Piracetam and donepezil also significantly increased the level of GSH [Table 3].
Discussion
In the present study, EASF of H. sinensis (25, 50 and 100 mg/kg) improved learning and memory of mice significantly in interoceptive behavioral models employed. The simultaneous analysis or a distinction between reference and working memory is well established through ORT and PAT. Scopolamine, a non-selective muscarinic antagonist blocks cholinergic signaling and produce memory deficit that are similar to those found in age related senile CNS dysfunction. Scopolamine interferes with memory and cognitive function and subsequently causes impairment of reference (long term) and working (short term) memories. In this study, mice were given scopolamine to induce memory impairment at a dose of 1 mg/kg.
Many clinical studies have reported strong evidence that oxidative stress is involved in the pathogenesis of Alzheimer's disease. The oxygen-free radicals are implicated in the process of age related decline in the cognitive performance may be responsible for the development of Alzheimer's disease in elderly persons.[20] El-Sherbiny et al. (2003) reported that memory impairment in the scopolamine-induced animal model is associated with the increased oxidative stress within rat brain. An increased oxidation of lipids, proteins and deoxyribonucleic acid, alterations in mitochondrial function and a possible role of amyloid beta and its precursor protein in oxidative reaction in experimental models of Alzheimer's disease are demonstrated. Moreover, strong evidence supporting the involvement of oxidative damage in neurodegenerative disease has been suggested by various clinical studies.[26] The drugs with antioxidant effects might be beneficial for preserving brain function. Antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase as well as glutathione reductase (GSH) and ascorbate are involved in the reduction of oxidative stress. Antioxidant enzymes display the reduced activities in the affected brain region of patients of Alzheimer's disease. Moreover, the reduction in the level of intracellular oxidized protein under these conditions has been associated with the improvement of cognitive and/or psychomotor functions.[3] Augmentation of endogenous antioxidants by therapeutic substances has recently evoked scientific interest because any such a property of a therapeutic agent can be expected to cause significant improvement in the endogenous defense against oxidative stress.[9] These agents also reduce the oxidative damage and promote a functional recovery in degenerative disorders. In the course of searching natural products with memory enhancing activity using scopolamine-induced amnesic mouse as an experimental model for Alzheimer's disease, it was found that the EASF of H. sinensis showed a significant memory enhancing activity in ORT and PAT. The memory impairment induced by acute administration of scopolamine is associated with altered level of SOD and GSH in the brain.[3] More specifically, the entire brain of patients with Alzheimer's disease (AD) was shown to be subjected to an oxidative challenge. Such a peroxidation process and the overproduction of free radicals may lead to consumption of detoxifying endogenous antioxidants such as SOD and GSH. The animals exposed to conditioned fear, treated with scopolamine (1 mg/kg) exhibited elevated brain MDA level, while SOD and GSH levels were reduced.
In the present study, the effect of EASF was examined on the performance of mice in an object recognition task that has been considered to be a pure working memory task. Mice are able to discriminate between a familiar object and a new object 1 h or less, but not 24 h, after the presentation of the familiar object.[27] The effect of EASF was investigated on the acquisition of the information and on the consolidation of memory that takes place shortly after the acquisition and on the restitution of the information. The results indicated that mice spend more time in exploring a new object than a familiar object in the aged and scopolamine-treated group when pretreated with EASF (50 and 100 mg/kg). The DI was significantly decreased in the aged and scopolamine-treated group. Pretreatment with EASF (50 and 100 mg/kg) significantly increased the DI when compared with respective control. There was no significant effect on the DI in young mice treated with EASF.
Thus, the results demonstrates that EASF (50 and 100 mg/kg) improved retention in mice subjected to object recognition task in the aged and scopolamine-treated group. EASF improves the consolidation and possibly the acquisition phase of working memory that is altered in interoceptive memory deficit models, i.e. age and scopolamine. Piracetam and donepezil, the established nootropic agents used as a standard in the present study also significantly improved the DI.
The ameliorative effects of EASF on learning and memory were investigated in the passive avoidance task. Scopolamine-treated mice exhibited significantly shorter step-down latencies. EASF (25, 50 and 100 mg/kg) treatment showed a significant increase in SDL in young as well as aged animals. Pretreatment with EASF (25, 50, and 100 mg/kg) significantly decreased SDL in the scopolamine-treated group. Thus, EASF significantly reversed the deficit produced by scopolamine. Donepezil (1 mg/kg) and piracetam (200 mg/kg) used as positive control, also increased the SDL, which is consistent with previous reports.
The EASF meet the criteria for nootropic activity. The pretreatment with EASF for 8 days protected the animals from memory deficit produced by scopolamine in PAT. Administration of scopolamine significantly increased the MDA level, an important marker for LPO and reduced both GSH and SOD activities in mice brain. Administration of EASF for 8 days produced a significant fall in MDA and restored the GSH and SOD activities in mice brain. Extract may exert a protective effect against oxidative damage induced by scopolamine by diminishing the reduction in the activities of GSH and SOD in mice brain. Joshi and Parle reported nootropic activity of Hibiscus sabdariffa Linn. The memory improving activity of H. sabdariffa may be attributed to its antioxidant, neuroprotective, pro-cholinergic, and anti-acetylcholine esterase, suggesting the species of Hibiscus might have chemical constituents which possess nootropic activity.[20] It has been known that neuroprotective compounds showed the significant anti-amnesic activity in memory deficit induced by age and scopolamine. The above behavioral and biochemical results suggest that EASF has the ability to improve or ameliorate spatial long term and working memory by the regulation of the antioxidant system. The observed beneficial effects of H. rosa sinensis may be attributed to its diversified chemical components namely glycosides, flavonoids, tannins and saponins.
Conclusion
Our investigation indicates that combination of antioxidant and neuroprotective role could be responsible for a cognitive enhancing effect. Hence, Hibiscus rosa sinensis may be useful in the treatment or prevention of various cognitive disorders.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: A review of progress. J Neurol Neurosurg. 1999;66:137–47. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang SY, Lee KY, Koo KA, Yoon JS, Lima SW, Kima YC, et al. ESP-102, a standardized combined extract of Angelica gigas, Saururus chinensis and Schizandra chinensis, significantly improved scopolamine-induced memory impairment in mice. Life Sci. 2005;76:1691–05. doi: 10.1016/j.lfs.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 3.El-Sherbiny DA, Khalifa AE, Attia AS, Eldenshary Eel-D. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnesic dose of scopolamine. Pharmacol Biochem Behav. 2003;76:525–33. doi: 10.1016/j.pbb.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 4.Wilson JX. Antioxidant defense of the brain: A role for astrocyte. Can J Physiol Pharmacol. 1997;75:1149–63. [PubMed] [Google Scholar]
- 5.Mhaskar KS, Blatter E, Calus JF. Shri Satguru Publication; India: Shri Satguru Publication; 2000. Kirtikar and Basu's illustrated Indian Medicinal Plant their usage in Aurveda and Unani Medicine; pp. 462–64. [Google Scholar]
- 6.Reddy CM, Murthy DR, Patil SB. Antispermatogenic and androgenic activities of various extracts of Hibiscus rosa sinensis in albino mice. Indian J Exp Biol. 1997;35:1170–74. [PubMed] [Google Scholar]
- 7.Sharma N, Khan N, Sulthana S. Study on prevention of two-stage skin carcinogenesis by Hibiscus rosa sinensis extract and the role of its chemical constituent, gentisic acid, in the inhibition of tumor promotion response and oxidative stress in mice. Eur J Cancer Prev. 2004;13:53–64. doi: 10.1097/00008469-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Kasture VS, Chopde CT, Deshmukh VK. Anticonvulsive activity of Albizzia lebbeck, Hibiscus rosa sinensis and Butea monosperma in experimental animals. J Ethnopharmacol. 2000;71:65–75. doi: 10.1016/s0378-8741(99)00192-0. [DOI] [PubMed] [Google Scholar]
- 9.Gauthaman KK, Saleem MT, Thanislas PT, Prabhu V, Krishnamoorthy KK, Devaraj NS. Cardioprotective effect of the Hibiscus rosa sinensis flowers in an oxidative stress model of myocardial ischemic reperfusion injury in rat. BMC Complement Altern Med. 2006;6:32–9. doi: 10.1186/1472-6882-6-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui AA, Wani SM, Rajesh R, Alagarsamy V. Phytochemical and pharmacological investigation of Hibiscus rosa sinensis. Indian J Pharm. 2008;68:588–93. [Google Scholar]
- 11.Gomathi N, Malarvili T, Mahesh R, Begun VH. Lipids lowering effect of Hibiscus rosa sinensis flower petals on monosodium glutamate induced obese rats. Pharmacologyonline. 2008;1:400–09. [Google Scholar]
- 12.Sachdewa A, Khemani LD. Effect of ethanolic extract of flowers of Hibiscus rosa sinensis L.on blood glucose and lipid profile in streptozotocin induced diabetes in rats. J Ethnopharmacol. 2003;89:61–66. doi: 10.1016/s0378-8741(03)00230-7. [DOI] [PubMed] [Google Scholar]
- 13.Masaki HS, Sakaki S, Atsumi T, Sakurai H. Active oxygen scavenging activity of plant extracts. Biol Pharm Bull. 1995;18:162–66. doi: 10.1248/bpb.18.162. [DOI] [PubMed] [Google Scholar]
- 14.Nadkarni AK. Indian Materia Medica. India. 1976:1199. [Google Scholar]
- 15.Anonymous. New Delhi, India: CSIR; 1956. The Wealth of India.A Dictionary of Indian Raw Materials and Industrial Products; pp. 91–2. [Google Scholar]
- 16.Trease GD, Evans WC. Pharmacognosy. New York: Harcourt Brace and Company; 1997. p. 275, 343, 571. [Google Scholar]
- 17.Kokate CK. Practical Pharmacognosy. India: Vallabh Prakashan; 1994. pp. 104–11. [Google Scholar]
- 18.Ennaceure A, Delacour J. A new one-trial test for neurobiological studies of memory in rats: Behavioral data. Behav Brain Res. 1998;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 19.Dhingra D, Parle M, Kulkarni SK. Memory enhancing activity of Glycyrrhiza glabra in mice. J Ethnopharmacol. 2004;91:361–65. doi: 10.1016/j.jep.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Joshi H, Parle M. Pharmacological evidences for antiamnesic potentials of Phyllanthus amarus in mice. Afr J Biomed Res. 2000;10:165–73. [Google Scholar]
- 21.Naidu P, Singh A, Shrinivas K. Effect of Withania Somnifera root extract on haloperidol-induced orofacial dyskinesia: Possible mechanisms of action. J Med Food. 2003;6:107–14. doi: 10.1089/109662003322233503. [DOI] [PubMed] [Google Scholar]
- 22.Wills ED. Mechanism of lipid peroxide formation in animal tissues. Biochem. 1966;99:667–76. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and and assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 24.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1978;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Lowery OH. Protein measurements with the Folin-phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 26.Jeong EU, Lee KY, Kim SH, Sung SH, Kim YC. Cognitive enhancing and antioxidant activities of iridoid glycoside from Scrophularia buergeriana in scopolamine treated mice. Eur J Pharmacol. 2008;288:78–84. doi: 10.1016/j.ejphar.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 27.Deschaux O, Bizot JC, Goyffon M. Apamine improves learning in an object recognition task in rats. Neurosci Lett. 1997;222:159–62. doi: 10.1016/s0304-3940(97)13367-5. [DOI] [PubMed] [Google Scholar]


