Abstract
Objective:
Leaves of Bichofia javanica (BJ) have been traditionally used for many ailments including cancer. In the present study, antileukemic activity of the leaf extract was evaluated on human leukemic cell lines.
Materials and Methods:
Human leukemic cell lines U937, K562, and HL60 were purchased from National Facility for Animal Tissue and Cell Culture, Pune, India. The cells were routinely maintained in RPMI 1640 medium supplemented with 10% heat inactivated fetal calf serum. Cultures were maintained at 37ºC in a humidified atmosphere containing 5% CO2 in air. The methanol extract of BJ (MEBJ) was dissolved in PBS and used at the concentrations of 5, 10, and 15 μg/ml for cell viability and cytotoxicity studies (MTT assay). Cell counts were made in quadruplicate samples at the interval of 24, 48, and 72 h and cytarabine (20 μg/ml) served as standard drug. The apoptotic pathway of cytotoxicity was assessed by DNA agarose gel electrophoresis technique and confirmed by fluorescence and confocal microscopic methods at the concentration of 10 μg/ml.
Results:
MEBJ showed significant cytotoxicity (P<0.001) in leukemic cell lines in the in-vitro cell proliferation assay. IC50 of MEBJ was very low (3.5 μg/ml) at 72 h in the HL60 cell line. The apoptotic pathway of cytotoxicity was observed at 10 μg/ml of MEBJ by the fragmented DNA pattern in the apoptosis assay, chromatin condensation, and apoptotic body formation as revealed in the fluorescence and confocal microscopic studies.
Conclusion:
The present findings support the ethno-medicinal use of BJ for cancer by mediating through the apoptosis pathway.
Keywords: Apoptosis pathway, Bischofia javanica, cytotoxicity
Introduction
Cancer is the second common cause of death in the developed countries next to cardiovascular diseases.[1] Leukemia is a heterogeneous hematological malignancy characterized by unregulated proliferation of the blood forming cells of the bone marrow. Medicinal plants and their phytoconstituents have always been a better choice for leukemia and nutraceuticals have been proved to have antileukemic activity in experimental studies.[2] One form of programmed cell death (PCD) is apoptosis, characterized by maintenance of intact cell membranes during the suicide process so as to allow adjacent cells to engulf the dying cell that does not release its content and trigger a local inflammatory reaction. Experimental studies have revealed that the apoptotic pathway of cell death is one of the most common mechanisms of action of anticancer agents.[3]
Bischofia javanica (BJ) Blume (Euphorbiaceae), commonly known as Bishop Wood, is widely distributed in India over the Sub-Himalayan region, Orissa, and south-West Coast from Konkan to Nilgiris. Traditionally, the bark is used for the treatment of tuberculosis, stomach ulcer, mouth ulcer, and athlete's foot.[4] Leaves are used in the treatment of stomachache and the leaf juice for cancerous wounds.[5] The major phytoconstituents are tannin, β amyrin, betulinic acid, friedelan-3α-ol, epifriedelinol, friedelin, luteolin and glucoside, quercetin, beta-sitosterol, stigmosterol, and ursolicacid.[6] The ethanolic extract of the leaves has also been shown to possess antimicrobial activity.[7] The bioassay-guided fractionated compounds including betulinic acid and its derivatives, betulonic acid, 3β-O-(Z)-coumaroyl betulinic acid, and 3β-O-(E)-coumaroyl betulinic acid, from the chloroform extract of the bark of BJ have been found to act as catalytic inhibitors of topoisomerase II activity with IC50 values ranging from 0.38 to 58 μM.[8] Anti-inflammatory and analgesic potency of BJ were studied by the authors in a previous research work.[9] Some anti-inflammatory chemopreventive agents have been found to suppress growth and proliferation of transformed or malignant cells through induction of programmed cell death or apoptosis.[10] Therefore, the present study explores ethnomedicinal utility of BJ for cancer on leukemic cell lines U937, K562, and HL60.
Materials and Methods
Drugs and Chemicals
RPMI-1640, fetal bovine serum (FBS), gentamycin, penicillin, streptomycin, trypan blue, agarose, tris, RNase A, proteinase K, propidium iodide (PI), acridine orange, ethidiumbromide, MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphSenyltetrazoliumbromide], and cytarabine (Ara-C) were purchased from Sigma (St. Louis, MO, USA). Other reagents were of analytical grade and procured locally.
Preparation of the Plant Extract
The leaves of BJ were collected from Melli region of Sikkim, India, in the month of June 2005. The plant material was identified and authenticated at Botanical Survey of India (BSI), Sikkim. The collected leaves were shade dried for 15 days and ground in a laboratory grinder. The powdered leaves were extracted with methanol in soxhlet apparatus at 50--60º C. After exhaustive extraction, the extract was concentrated by distilling the solvent for further use. The concentrated extract was kept in desiccators. The yield was determined as 5.0% w/w. The extract was dissolved in phosphate buffer saline and employed for antileukemic studies.
Phytochemical Analysis
The methanol extract was subjected to phytochemical analysis for constituent identification using a standard protocol.[11]
Human Leukemic Cell Lines
U-937 (myeloid leukemic cell line), K-562 (erythro leukemic cell line), and HL-60 (acute myeloblastic leukemic cell line) were obtained from National Facility for Animal Tissue and Cell culture, Pune, India. The growth of the cell lines were maintained in the Drug Development Division, Indian Institute of Chemical Biology, Kolkata, India in RPMI-1640 supplemented with 10% heat inactivated fetal bovine serum (FBS) and gentamycin (40 μg/ml), penicillin (100 units/ml), and streptomycin (10 μg/ml). They were grown at 37ºC in a humidified atmosphere of 5% CO2 , 95% air in a CO2 incubator.
Determination of Cell Proliferation
a) Cell Viability Studies
The log phase cell suspensions of U937, K562, and HL60 cells at a concentration of 105 /ml in RPMI- 1640 (with 10% FBS) were used for the experiment in a 96-well microtitre sterile plate. In each well 100 μl of cell suspension was taken and the methanol extract of Bischofia javanica (MEBJ) was added at different concentrations (5, 10, and 15 μg/ml). Cytarabine (Ara-C) (20 μg/ml) was used as the standard antileukemic drug. The cell viable count was done using inverted microscope (Olympus CKX41). The calculation was done by adopting trypan blue exclusion principle[12] for 24, 48, and 72 h of treatment. For each concentration of drugs, the readings were taken in quadruplicate.
b) Cytotoxicity Studies
The cells were treated with different concentrations of MEBJ (5, 10, and 15μg/ml), Ara-C (20 μg/ml) and incubated for 24 h. The MTT solution [3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide] was added to each well to make the final concentration to 400 μg/ml and further incubated at 37ºC in a CO2 incubator (5% CO2) for 3 h.[13] The reaction resulted in the reduction of MTT by the mitochondrial dehydrogenase of viable cells to a purple formazan product. The MTT--formazan product was dissolved in DMSO and estimated by measuring the absorbance at 492 nm in an ELISA plate reader (Lab system MS). The IC50 for MEBJ was determined after 24, 48, and 72 h of incubation.
Apoptosis Assay by Analysis of DNA Fragmentation and Agarose Gel Electrophoresis
U937, K562, and HL60 cells were treated with 10 μg/ml MEBJ for 24 h and then DNA was isolated from the control and treated groups. Leukemic cells at a concentration of 2.5×105 after treatment were harvested from each control and treated group, washed with PBS, and isolated by following the general phenol–chloroform extraction procedure[14] and dissolved in TE buffer (50mM Tris–HCl at pH 7 and 10 mM EDTA at pH 7.5). The DNA was electrophoresed in a gel electrophoresis apparatus (Horizontal-Atto India) at 20 V for 12 h in 1.5% agarose gel and stained with 0.5 μg/ml ethidium bromide and observed in a UV transilluminator.
Detection of Apoptosis by Fluorescence Microscopy
U937, K562, and HL60 cell suspensions at a concentration of 105 /ml were taken in a Petri dish and treated with 10 μg/ml of MEBJ for 24 h. To distinguish the living cells from apoptotic and dead cells, they were washed with PBS and stained with a combination of acridine orange (100 μg/ml): ethidium bromide (100 μg/ml) 1:1 ratio for 10 min and 10 μl of the cell suspension was taken on a slide and images were scanned[15] using a fluorescence microscope (Leica, USA). Images were obtained and rescaled identically in Adobe Photoshop 6.0.
Detection of Apoptosis by Confocal Microscopy
U937, K562, and HL60 cells were grown to about 70% confluence and then 105 cells were taken in a Petri dish and treated with 10 μg/ml MEBJ for 24 h. The cells were then washed with PBS and stained with 10 μg/ml propidium iodide (PI) for 5 min and a 10μl cell suspension was taken on slide and fluorescent images were scanned using a confocal laser scanning microscope [Leica DM-TRB].[16] Images of PI were acquired from a argon/krypton laser and a UV laser line using a 590 nm long pass filter for PI and a 450 nm band pass filter for UV images. The diameter of the detection pinhole corresponded to one airy unit of 512×512 and pixel images were obtained and rescaled identically in Adobe Photoshop 6.0.
Statistical Analysis
Statistical analysis of the data was performed by Student's t-test and P<0.05 was considered statistically significant. The IC50 value was calculated by using linear regression analysis.
Results
Phytochemical analysis of MEBJ revealed the presence of alkaloids, sterols, triterpenoids, carbohydrates, proteins, flavanoids, tannins, and saponins . In-vitro cell proliferation studies showed that MEBJ produced a significant (P<0.001) reduction in the cell count of all the leukemic cell lines U937, K562, and HL60 in a concentration-dependent manner [Figure 1a–c]. MEBJ at 15 μg/ml reduced the viable cells more effectively than the standard drug cytarabine (Ara-C). The sensitivity of K562 cell line to MEBJ was relatively less when compared to U937 and HL60 cell lines at 24 h of incubation. The MTT assay revealed that MEBJ and Ara-C have significant (P <0.001) cytotoxicity at 72 h of incubation in all the three cell lines [Figure 2a–c]. In the K562 cell line, the agents showed less cytotoxicity as compared to U937 and HL60. The IC50 values of MEBJ revealed that is more effective (IC50 of 3.5μg/ml) against HL60 leukemic cells at 72 h compared to K562 cell lines (IC50 of 12.9 μg/ml) [Table 1].
Figure 1(a).
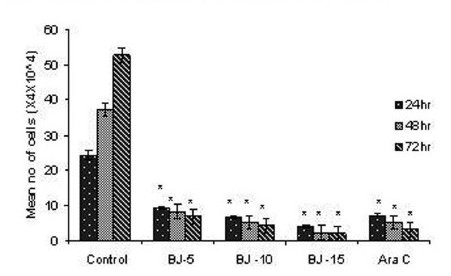
Effect of MEBJ on cell viability of the U-937 cell line by the trypan blue exclusion method. Each value represents quadruplicate samples of mean± SEM. *P<0.001 as compared to control.
Figure 1(c).
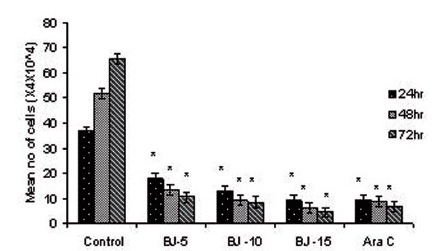
Effect of MEBJ on cell viability of the HL-60 cell line by the trypan blue exclusion method. Each value represents quadruplicate samples of mean± SEM. *P<0.001 as compared to control
Figure 2(a).
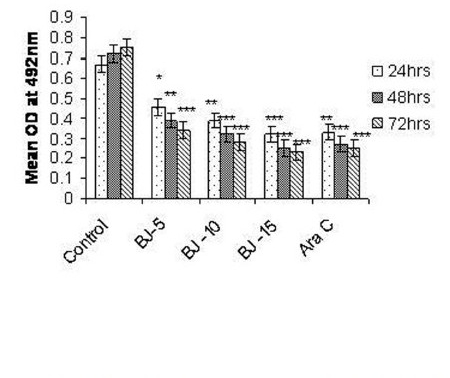
Effect of MEBJ on cytotoxicity of the U-937 cell line by the MTT assay method. Each value represents means of quadruplicate determinations mean ± SEM. *P<0.05,**P<0.01,***P<0.001 as compared to control
Figure 2(c).
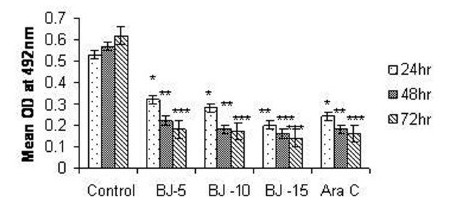
Effect of MEBJ on cytotoxicity of the HL-60 cell line by the MTT assay method. Each value represents means of quadruplicate determinations mean±SEM .*P<0.05, **P<0.01, ***P<0.001
Table 1.
IC50 values of methanol extract of Bischofia javanica (MEBJ) on leukemic cell lines
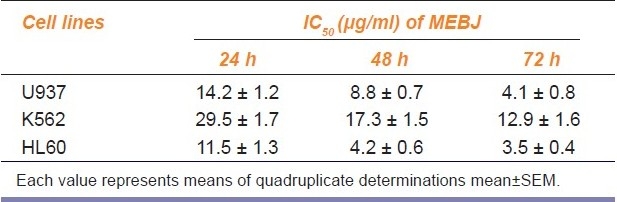
Figure 1(b).
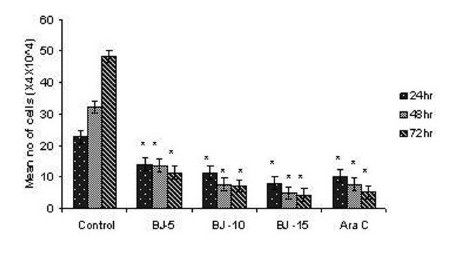
Effect of MEBJ on cell viability of the K-562 cell line by the trypan blue exclusion method. Each value represents quadruplicate samples of mean± SEM. *P<0.001 as compared to control.
Figure 2(b).
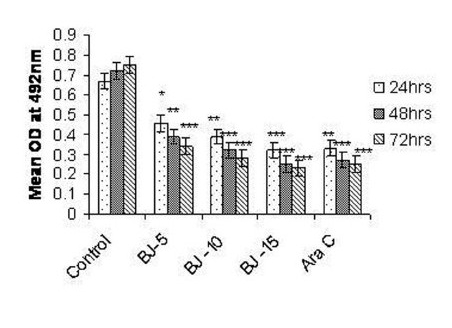
Effect of MEBJ on cytotoxicity of the K-562 cell line by the MTT assay method. Each value represents means of quadruplicate determinations mean ± SEM. *P<0.05,**P<0.01 as compared to control
The DNA isolated from U937, K562, and HL60 cells when electrophoresed showed an intact band of DNA in control, whereas degraded DNA was found as ladder formation in the case of MEBJ-treated cells [Figure 3a–c]. Ara-C-treated DNA also showed the same gel pattern. DNA fragmentation is the hallmark of apoptosis. It is due to cleavage of nuclear DNA at inter-nucleosomal linking sites yielding DNA fragments in multiples of 180 bp, which upon electrophoresis yields a ladder pattern. The test and standard drugs did not show ladder formation prominently in the K562 cell line. Fluorescence and confocal microscopic investigations revealed that MEBJ induced cell death in U937, K562, and HL60 cell lines by apoptosis [Figures 4 and 5]. Phenotypically, apoptosis is characterized by cell shrinkage, chromatin compaction, plasma membrane blebbing, DNA fragmentation, and collapse of the cell into small intact fragments (apoptotic bodies). Figure 5 shows the morphology of U937, K562, and HL60 cells treated with MEBJ and stained with PI (morphology of corresponding control cells are also shown). The confocal images showed that while the untreated cells possessed intact nuclei, cells treated with MEBJ caused the formation of clear apoptotic bodies and membrane blebbing.
Figure 3(a).
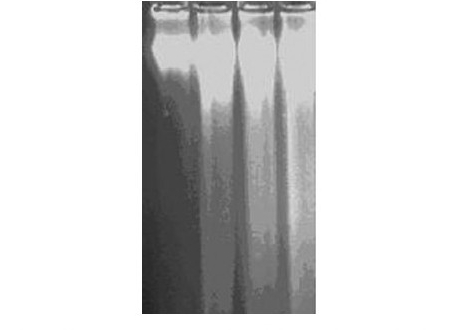
DNA fragmentation assay of MEBJ on leukemic cell lines by the agarose gel electrophoresis method: (a) U937 cell line Lanes 1, 2, 3 and 4 were U-937 control, cytarabine 20 μg/ml, MEBJ 10 μg/ ml, and 15 μg/ml treated
Figure 3(c).

DNA fragmentation assay of MEBJ on leukemic cell lines by agarose gel electrophoresis method (c) HL60 cell line. Lanes 9, 10, 11, and 12 were HL-60 control, cytarabine 20 μg/ml, MEBJ 10 μg/ml and 15 μg/ml treated
Figure 4.
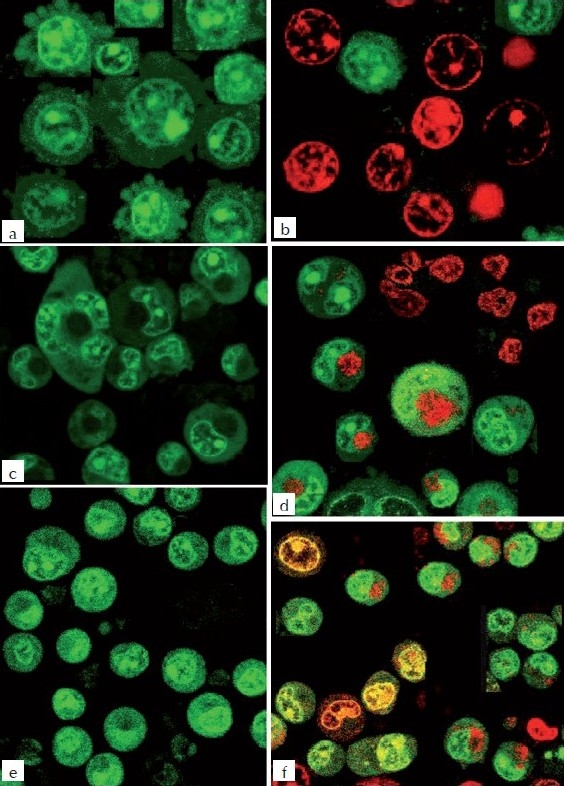
Fluorescence microscopic study of MEBJ on U937, K562, and HL60 leukemic cell lines stained with a combination of acridine orange (100 ƒÊg/ml): ethidium bromide (100 μg/ml) 1:1 ratio a) U-937 Control b) U-937 MEBJ10 μg/ml treated c) K-562 Control d) K-562 MEBJ10 μg/ml treated e) HL- 60 Control f) Hl-60 MEBJ10 μg/ml treated treated
Figure 5.
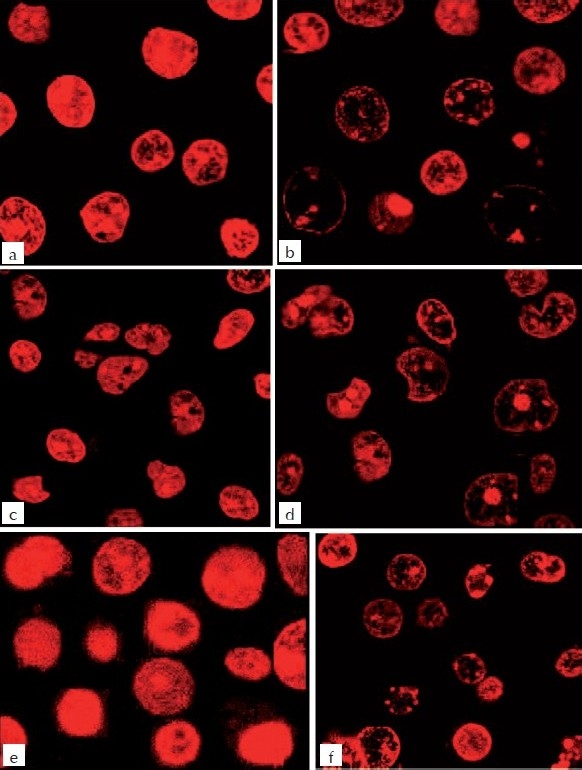
Confocal microscopic study of MEBJ on U937, K562, and HL60 leukemic cell lines stained with 10 μg/ml propidium iodide. (a) U937- control, (b) U937 – MEBJ 10 μg/ml treated, (c) (5b1) K562-Control (d) (b2) K562 – MEBJ 10 μg/ml treated, (e) HL60-control, (f) (5c2) HL60 – MEBJ 10 μg/ml treated
Figure 3(b).

DNA fragmentation assay of MEBJ on leukemic cell lines by the agarose gel electrophoresis method. (b) K562 cell lineLanes 5, 6, 7 and 8 were K-562 control, cytarabine 20 μg/ml, MEBJ 10 μg/ ml and 15 μg/ml treated
Discussion
Cancer is a disease characterized by uncontrolled proliferation of cells. Tumor development is accelerated by disruption of the balance between cell proliferation and cell death, which is maintained through regulation of various signal transduction pathways. Chemoprevention, which refers to the use of non-toxic chemical substances to inhibit, delay, and/or reverse cellular events associated with carcinogenesis, is regarded as a promising alternative strategy for the management of cancer.[17] A vast variety of naturally occurring substances have been shown to protect against experimental carcinogenesis and it is becoming increasingly evident that phytochemicals of different plants possess marked cancer chemo-preventive properties.[18] Numerous experimental, epidemiological, and clinical studies have revealed that NSAIDs are having promising anticancer activities.[19] The mechanism responsible for the anti-tumor activity of NSAIDs is still unknown. But the antineoplastic effects of NSAIDs may also include activation of apoptosis, inhibition of angiogenesis, or direct inhibition of cancer cell growth by blocking signal transduction pathways responsible for cell proliferation.[20] Our previous study showed that MEBJ had a marked anti-inflammatory property.[9] A number of COX-independent targets have been proposed to mediate the cancer chemo-preventive properties of NSAIDs, including 15-lipoxygenase, Ras, PPAR, NF-κB, PDK-1/Akt, and phosphodiesterase.[21]
The antiproliferative, cytotoxic, and DNA synthesis inhibitory effect of MEBJ were supported by the cell count study by trypan blue exclusion principle and MTT-assay. MEBJ was reported to have phytoconstituents like triterpenoids, flavonoids, and tannins. Most of the triterpenoids and flavonoids have been reported to have appreciable anticancer activity both in animal models and in cell lines.[22,23] It is well known that apoptosis in recent years has become an important issue in biomedical research and the chemopreventive agents, which can modulate apoptosis, may be able to affect the steady state cell population that are often useful targets in the management and therapy for cancer. The present study has shown that MEBJ produced a concentration- and time-dependent decrease in cell count in U937, K562, and HL60 cell lines and so it was subsequently investigated to find out whether the cytotoxic effect is mediated via an apoptotic mechanism or not. Apoptosis is a tightly regulated process, which involves changes in the expression of a distinct set of genes. Two of the major genes responsible for regulating the mitochondrial apoptosis pathway are antiapoptotic Bcl-2 and proapoptotic bax.[24] Cells undergoing apoptosis usually exhibit fragmentation of the cell into membrane-bound apoptotic bodies, nuclear and cytoplasmic condensation and endolytic cleavage of the DNA into small oligonucleosomal fragments.[25] Hence, the apoptosis assays by analysis of DNA fragmentation and examination of apoptotic bodies by fluorescence and confocal microscopy were carried out. The results confirmed that MEBJ induced DNA ladder formation, chromatin condensation, and externalization of a membrane that are characteristic features of apoptosis. Hence, this research work has explored the potent anticancer activity of leaves of MEBJ on human leukemic cell lines and rationalized its ethno-medicinal use for cancer.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Jaggi OP. Cancer causes, Prevention and Treatment. New Delhi: Orient Paper Backs Publishers; 2005. p. 155. [Google Scholar]
- 2.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–9. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 3.Piazza GA, Pohm AL, Krutzsch M, Sprel G, Praranza NS, Gross PH. Antineoplastic drugs sulindac sulfide and sulfone inhibit cell growth by inducing apoptosis. Cancer Res. 1995;55:3110–6. [PubMed] [Google Scholar]
- 4.Nayar C, Chopra R. Glossary of Indian Medicinal Plants. India, New Delhi: CSIR; 1970. p. 37. [Google Scholar]
- 5.Von Reis S, Lipp F. New plant sources for drugs and Foods from the New York Botanical Garden Herbarium. Cambridge: Massachuestts Harvard University Press; 1982. p. 221. [Google Scholar]
- 6.Cambie RC, Ash J. Fijian Medicinal Plants. Australia: CSIRO Publications; 1984. p. 124. [Google Scholar]
- 7.Khan MR, Kihara M, Omoloso AD. Anti-microbial activity of Bidens pilosa, Bischofia javanica, Elmerillia papuana and Sigesbekia orientalis. Fitoterapia. 2001;72:662–5. doi: 10.1016/s0367-326x(01)00261-1. [DOI] [PubMed] [Google Scholar]
- 8.Wada S, Tanaka R. Betulinic acid and its derivatives, potent DNA Topoisomerase II inhibitors from the bark of Bischofia javanica. Chem Biodivers. 2005;2:689. doi: 10.1002/cbdv.200590045. [DOI] [PubMed] [Google Scholar]
- 9.Sutharson L, Nath LK, Kar PK, Shila EB, Rajan JV. Anti-inflammatory and antinociceptive activities of methanolic extract of leaves of Bischofia javanica blume on experimental animals. Asian J Chem. 2007;7:5150–6. [Google Scholar]
- 10.Samaha HS, Kelloff GJ, Steele V. Modulation of apoptosis by sulindac, curcumin, phenylethyl-3-methylcaffeate and 6-phenylhexyl isothiocyanate: Apoptotic index as a biomarker in colon cancer chemoprevention and promotion. Cancer Res. 1997;57:1301–5. [PubMed] [Google Scholar]
- 11.Harborne JB. Phytochemical Methods. London: Chapman and Hall; 1984. p. 120. [Google Scholar]
- 12.Sur P, Chatterjee SP, Roy P, Sur B. 5-Nitrofuran derivatives of fatty acid hydrazides induce differentiation in human myeloid leukemic cell lines. Cancer Letter. 1995;94:27–32. doi: 10.1016/0304-3835(95)03819-i. [DOI] [PubMed] [Google Scholar]
- 13.Kawada M, Amemiya M, Ishizuka M, Takeuchi M. Differential induction of apoptosis in B 16 Melanoma E1-4 lymphoma cells by cytostatin and bactobolin. Jpn J Cancer Res. 1999;90:219–25. doi: 10.1111/j.1349-7006.1999.tb00736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann A, Lorenz HM, Voll R, Grunke M, WoithT , Kalden JR. A rapid and simple method for the isolation of DNA fragments. Nucleic Acid Res. 1994;22:5506–7. doi: 10.1093/nar/22.24.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorman A, McCarthy J, Finucane D, Reville W, Cotter T. Morphological assessment of apoptosis. In: Cotter TG, Martin SJ, editors. Techniques in Apoptosis. A user's guide. New York: Portland Press; 1994. p. 333. [Google Scholar]
- 16.Pawley JB. Handbook of Biological Confocal Microscopy. New York: Plenum Publishing Corporation; 1995. p. 56. [Google Scholar]
- 17.Benner SE, Hong WK. Clinical chemoprevention: Developing a cancer prevention strategy. J Natl Cancer Inst. 1993;85:1446–7. doi: 10.1093/jnci/85.18.1446. [DOI] [PubMed] [Google Scholar]
- 18.Geneive EH, Lynn SA, Jose CR, Helen J, David H, Navindra PS. Kaurene diterpenes from Laetia thamnia inhibit the growth of human cancer cells in vitro. Cancer Letters. 2006;244:190–4. doi: 10.1016/j.canlet.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Thun MJ, Henley SJ, Patrono C. Non steroidal anti-inflammatory drugs as anticancer agents: Mechanistic, pharmacologic, and clinical issues. J Natl Cancer Inst. 2002;94:252–66. doi: 10.1093/jnci/94.4.252. [DOI] [PubMed] [Google Scholar]
- 20.Hussan SS, Szabo JL, Tamawski S. Possible mechanism of action of NSAIDs for cancer. Am J Gastroentrol. 2002;97:13–21. [Google Scholar]
- 21.Eling TE, Baek SJ, Shim M, Lee CH. NSAID activated gene (NAG-1), a modulator of tumorigenesis. J Biochem Mol Biol. 2006;39:649–55. doi: 10.5483/bmbrep.2006.39.6.649. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Umeda T, Yagi A. Triterpenoid saponins from the roots of tea plant (Camellia sinensis var.assamica) Phytochemistry. 2000;53:941–6. doi: 10.1016/s0031-9422(99)00559-2. [DOI] [PubMed] [Google Scholar]
- 23.Tae JL, On HK, Yeoun HK, Jun HL, Shin K, Jong WP, Taeg KK. Quercetin arrests G2/M phase and induce caspase-dependent cell death in U937 cells. Cancer Letters. 2006;240:234–42. doi: 10.1016/j.canlet.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 24.Tong XH, Lin SG, Fujii M, Hou DX. Molecular mechanisms of echinocystic acid-induced apoptosis in HepG2 cells. Biochem Biophys Res Commun. 2004;321:539–46. doi: 10.1016/j.bbrc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Thatte U, Bagadey S, Dahanukar S. Modulation of programmed cell death by medicinal plants. Cell Mol Biol. 2000;46:199–214. [PubMed] [Google Scholar]


