Abstract
Objective:
The purpose of this study has been to assess the efficacy of duloxetine, a selective inhibitor of serotonin and norepinephrine reuptake, in the treatment of stress urinary incontinence (SUI) in women.
Materials and Methods:
The study included 50 women aged above 18 years with a predominant symptom of stress urinary incontinence (SUI). The case definition included a predominant symptom of SUI with a weekly incontinence episode frequency (IEF) of seven or greater and a positive cough stress test. All the patients received duloxetine 20 mg twice daily for 12 weeks. The primary outcome variables included the IEF and improvement in quality of life. Paired Student's ‘t’ test was used to analyze changes in IEF.
Results:
The improvement with duloxetine treatment was found in 40 out of 50 patients. Remaining 10 patients did not show any improvement with duloxetine and discontinued the treatment. In 40 patients, the mean baseline IEF was 12.5/week. At the end of three months treatment, IEF was six/week. This shows a statistically significant reduction in the IEF. Also, there was a good improvement in quality of life with 65% of patients in the “very much better” and “much better” categories according to PGI-I scale. In the remaining 10 patients, there was no significant improvement after one month of treatment and patients underwent surgery.
Conclusions:
The findings support duloxetine as a potential treatment for women with stress urinary incontinence.
Keywords: Duloxetine hydrochloride, norepinephrine serotonin reuptake inhibitor, quality of life, stress urinary incontinence
Introduction
Stress urinary incontinence (SUI) is the involuntary loss of urine associated with physical activities such as running, jumping or lifting or with sneezing and coughing.[1] Urinary incontinence (UI) can have a significant impact on quality of life. Social isolation and loneliness, increased health care utilization and significant cost are associated with stress urinary incontinence.[2] The mechanisms underlying SUI are inadequately understood. Multiple factors are involved, including muscle and ligament support of the bladder base, absence of bladder spasms (detrusor overactivity) during bladder filling, integrity of neurological innervation and preservations of the urethra's endothelial cushion and sealing ability which involve urothelial, vascular, connective tissue and neurological components.[3] However, even with a mildly compromised sphincter mechanism, continence can be maintained by keeping bladder volume below the threshold at which leakage occurs. This can be accomplished by adjusting fluid intake and voiding frequency and by minimizing physical stress through treating cough.[4] Various surgical options are available for women with more severe or frequent stress urinary incontinence including retro pubic colposuspension and pubo vaginal sling placement.[1] Another non pharmacological treatment option for women with SUI is pelvic floor muscle training, which can be effective in highly motivated individuals with less severe incontinence.[1] The musculature of the levator ani complex provides substantial support for the urethra, vagina and rectum as they descend through the pelvic floor. These muscle groups can be rehabilitated through exercise and physical therapy.[5] Physical therapy will not cure or improve all cases of stress incontinence however, pelvic muscular rehabilitation can play a significant role in the treatment of SUI.[6]
Older drug therapy like α agonists, β agonists, tricyclic antidepressants, anticholinergic drugs etc. for SUI have unimpressive clinical efficacy and are associated with significant adverse events.[7] The use of pharmacotherapy is often considered to provide symptomatic relief for women who are not suitable candidates for conservative strategies or surgery.[7]
Numerous studies have implicated 5-HT and NE systems in the neural control of lower urinary tract function.[8] The parasympathetic, sympathetic and somatic spinal cord nuclei that control lower urinary tract function and the spinal areas that contain terminals of lower urinary tract primary afferent fibers densely are innervated by 5-HT and NE terminals.[9] Duloxetine is an orally administered, balanced dual serotonin and nor epinephrine reuptake inhibitor.[10,11] In this context, balance is defined from preclinical pharmacologic data that demonstrated little difference in the relative affinity for duloxetine in binding to the NE and 5-HT transport sites.[12]
There are few studies which have compared the effect of duloxetine with placebo in the treatment of SUI. As there are no Indian studies regarding duloxetine in the treatment of SUI, this study was done to evaluate the effect of duloxetine in SUI.
Materials and Methods
A prospective study was conducted from 01-10-2005 to 31-08-2006 on patients with SUI. Fifty patients were included in the study. The patients with SUI attending Urology department were recruited from R.L.Jalappa Hospital and Research centre, attached to Sri Devaraj Urs medical college, Kolar.
A proforma containing detailed information on each patient was prepared according to the protocol designed for the study. Informed consent was taken from all the patients included in the study. Ethical clearance was obtained from institutional ethics committee. Women aged >18 years with stress urinary incontinence with ≥ 7 incontinence episodes per week were included in the study. Duration of symptom of at least three–four months was included. Patients with urge incontinence, nocturnal enuresis and with occult vesico – vaginal fistula or uretero – vaginal fistula were excluded from the study. There were no other urologic exclusion criteria. Patients only with SUI without any psychiatric co morbidities were included. Relevant data was collected from newly detected cases of SUI in women. The data included name and age of the patient, socioeconomic status, dates of admission and discharge for inpatients, duration of stress incontinence and dates of follow-up. No pharmacological and nonpharmacological pre treatment was allowed.
Duloxetine 20 mg twice daily was prescribed for each patient. The study was conducted for a period of 12 weeks and follow up was done once in four weeks. Objective testing was used to confirm SUI. Patients were asked to come with full bladder. Cough stress test was performed to these patients. Visualization of urine leakage concurrent with a cough was required to diagnose SUI.
There were two outcome measures.
1. Incontinence episode frequency/week (IEF/week): The primary efficacy measure was the IEF, recorded on daily dairies for one week. Three diaries were completed during the active treatment phase of the study. The patients were asked to write the number of incontinence episodes in the diary.
2. A quality of life measure, the patient global impression on improvement scale (PGI-I), has been validated as an assessment tool in psychopharmacologic research. This validated question measures a subject's self perceived change in her condition with treatment across seven categories from `very much better’ to `very much worse’.
PGI-I scale: Check the one number that best describes how you have felt overall since you began receiving this medication. (psychiatric stem and lower urinary tract stem)
Very much better.
Much better.
A little better.
No change.
A little worse.
Much worse.
Very much worse.
The patients were asked to check the number during each visit. Cough stress test was performed during each visit. Response to the drug was monitored by noting decrease in symptoms and improvement in patient's quality of life. The decrease in incontinence episodes was analyzed by using paired ‘t’ test. Quality of life was assessed by using PGI-I scale. This is expressed as percent improvement in patient's quality of life.
Results
Out of the 50 women enrolled in the study, there were 10 dropouts after one month of starting duloxetine treatment. These 10 women did not show any clinical improvement with the drug treatment and were taken for surgery. Remaining 40 women completed the entire treatment schedule of three months. The outcome measures of this study were the IEF per week and quality of life measure using patient global impression of improvement (PGI-I) scale. Table 1 shows the effect of duloxetine on IEF at baseline and after treatment.
Table 1.
Shows the effect of Duloxetine on IEF at baseline and after treatment
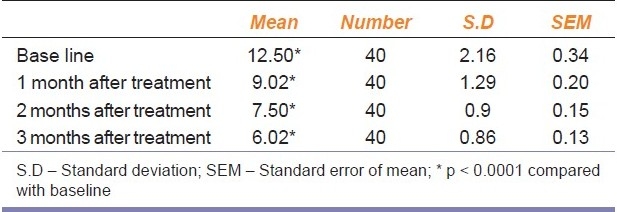
The mean IEF at baseline was 12.50 ± 2.2 [Table 1], and 9.02 ± 1.3, 7.50 ± 0.9, 6.02 ± 0.86 at the end of one month, two months and three months of treatment with duloxetine respectively. This shows that there was a statistically significant reduction in the IEF at the end of each month when compared to the baseline. Table 2 shows the comparison between patients improving and patients not responding to treatment.
Table 2.
Shows the comparision between patients improving and patients not responding to treatment
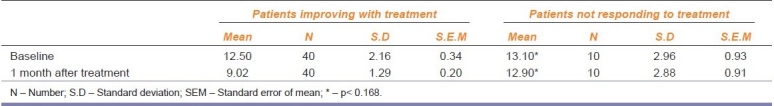
In patients showing improvement, the mean IEF at baseline was 12.50 ± 2.2 and 9.02 ± 1.3 [Table 2] at the end of one month of treatment. This shows a statistically significant reduction in the IEF at the end of one month. In patients not responding to treatment, the mean IEF at baseline was 13.10 ± 2.96 and 12.90 ± 2.88 at the end of one month of treatment with duloxetine. There was no statistically significant difference between baseline and after one month of treatment (P< 0.168) in IEF in these 10 patients. Hence, these patients discontinued treatment with duloxetine and underwent surgery.
Figure 1 shows the decrease in median IEF. There was a decrease of 30% in first month, around 40-45% at the end of second month and over 50% decrease in IEF in third month. This shows that there was a significant decrease in IEF in these patients at the end of three months of treatment.
Figure 1.
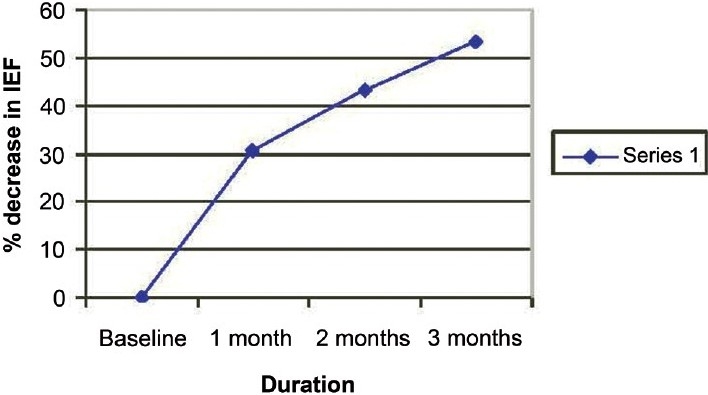
Percentage decrease in median IEF
Figure 2 shows the number of women with improvement in psychiatric and lower urinary tract stem using PGI-I. It shows that 27.5% were in ‘very much better’ category, 37.5% in ‘much better’category and 35% women were in a ‘little better’ category.
Figure 2.
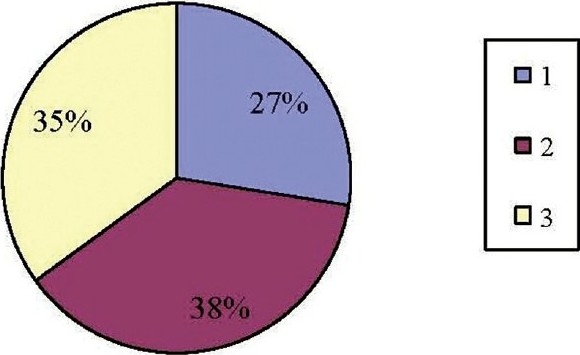
Percentage of women showing improvement in psychiatric and lower urinary tract stem using PGI-I
Only few patients complained of adverse effects like nausea (10 patients) and dry mouth (six patients) indicating that duloxetine was very well tolerated with least incidence of side effects.[13]
Discussion
There are a few studies conducted on duloxetine which have compared the efficacy of duloxetine with placebo in SUI.[14,15] In a study, duloxetine was given at different doses of 20 mg, 40 mg, and 80 mg twice daily and was compared with placebo.[14] More adverse effects were reported when duloxetine was given in the dosages of 40 mg and 80 mg, but not with 20 mg.[14] Hence in our study, we used 20 mg of duloxetine which is found to be safe and effective.
The age of women included in the study was between 20 and 60 years. Forty women completed three months therapy with duloxetine. Other 10 patients did not show any improvement in IEF at the end of one month (P<0.168). The PGI score in these patients at the end of one month was 4 which means that there is no change in the urinary tract condition. These patients were advised surgery. The other 40 patients continued to receive 20 mg of duloxetine twice daily. The IEF per week decreased significantly at the end of one month which was maintained throughout the study period. Statistically significant improvement was seen in IEF/week (P<.0001) after the patient received 20 mg of duloxetine twice daily per day. Our study shows the median percentage decrease in IEF was 53.84% at the end of three months, which is comparable to the other studies.[14,15] The decrease in IEF was 53.84% at the end of three months in our study [as shown in Figure 1] as compared to 59.5% in another study.[13]
By using PGI-I scale, we have analyzed psychiatric and lower urinary stem during every visit. Psychiatric stem describes how the patient felt since she began receiving the treatment. Lower urinary tract stem describes how the patient's urinary tract condition is now compared to how it was before she began receiving the treatment.
In our study, we observed that 37.5% of women scored ‘1’ which means there was ‘very much better’ improvement in the symptoms. 27.5% of the women scored ‘2’ which means there was ‘much better’ improvement in the symptoms. 35% of the women scored ‘3’ which comes under ‘a little better’ category [as shown in Figure 2] . Hence in our study, 65% women showed improvement in PGI-I score in our study as compared to 73.6% in another study.[16] Studies have shown improvement in PGI-I score at the end of three months. Only few patients complained of adverse effects like nausea (10 patients) and dry mouth (six patients) indicating that duloxetine was very well tolerated with least incidence of side effects. This is reteriated in published literature too.[13] Hence pharmacotherapy with duloxetine may prove to be effective and relatively safe option in women with SUI.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Zinner NR, Koke SC, Viktrup L. Pharmacotherapy for stress urinary incontinence : Present and future options. Drugs. 2004;64:1503–16. doi: 10.2165/00003495-200464140-00001. [DOI] [PubMed] [Google Scholar]
- 2.Fultz NH, Herzog AR. Self-reported social and emotional impact of urinary incontinence. J Am Geriatr Soc. 2001;49:8929. doi: 10.1046/j.1532-5415.2001.49179.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernard M. Human Physiology. England: Springer; 1987. Physiology of the lower urinary tract; pp. 333–50. [Google Scholar]
- 4.Resnick NM, Griffiths DJ. Expanding treatment options for stress urinary incontinence in women. JAMA. 2003;290:3957. doi: 10.1001/jama.290.3.395. [DOI] [PubMed] [Google Scholar]
- 5.Wall LL, Davidson TG. The role of muscular re-education by physical therapy in the treatment of genuine stress urinary incontinence. Obstet Gynecol Surv. 1992;47:32231. doi: 10.1097/00006254-199205000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Benvenuti F, Caputo GM, Bandinelli S, Mayer F, Biagini C, Sommavilla A. Reeducative treatment of female genuine stress incontinence. Am J Phys Med. 1987;66:15568. [PubMed] [Google Scholar]
- 7.Blaivas JG, Olsson CA. Stress incontinence: Classification and surgical approach. Urol J. 1988;139:72931. doi: 10.1016/s0022-5347(17)42611-5. [DOI] [PubMed] [Google Scholar]
- 8.Kojima M, Taksuchi Y, Goto M, Sano Y. Immunohistochemical study on the distribution of serotonin fibres in the spinal cord of the dog. Cell Tissue Res. 1982;226:47791. doi: 10.1007/BF00214778. [DOI] [PubMed] [Google Scholar]
- 9.McCormack PL, Keating GM. Duloxetine: In stress urinary incontinence. Drugs. 2004;64:256773. doi: 10.2165/00003495-200464220-00005. [DOI] [PubMed] [Google Scholar]
- 10.Raskin J, Goldstein DJ, Mallinckrodt CH, Ferguson MB. Duloxetine in the long-term treatment of major depressive disorder. J Clin Psychiatry. 2003;64:123744. doi: 10.4088/jcp.v64n1015. [DOI] [PubMed] [Google Scholar]
- 11.Thor KB. Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: Implications for treating stress urinary incontinence. Urology. 2003;62:39. doi: 10.1016/s0090-4295(03)00754-4. [DOI] [PubMed] [Google Scholar]
- 12.Wong DT, Bymaster FP. Dual serotonin and noradrenaline uptake inhibitor class of antidepressants potential for greater efficacy or just hype? Prog Drug Res. 2002;58:16922. doi: 10.1007/978-3-0348-8183-8_5. [DOI] [PubMed] [Google Scholar]
- 13.Oelke M, Roovers JP, Michel MC. Safety and tolerability of duloxetine in women with stress urinary incontinence. BJOG. 2006;113:226. doi: 10.1111/j.1471-0528.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- 14.Millard RJ, Moore K, Rencken R, Yalcin I, Bump RC. Duloxetine vs placebo in the treatment of stress urinary incontinence: A four-continent randomized clinical trial. BJU Int. 2004;93:3118. doi: 10.1111/j.1464-410x.2004.04607.x. [DOI] [PubMed] [Google Scholar]
- 15.van Kerrebroeck P, Abrams P, Lange R, Slack M, Wyndaele JJ, Yalcin I, et al. Duloxetine versus placebo in the treatment of European and Canadian women with stress urinary incontinence. BJOG. 2004;111:249–57. doi: 10.1111/j.1471-0528.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- 16.Dmochowski RR, Miklos JR, Norton PA, Zinner NR, Yalcin I, Bump RC. Duloxetine versus placebo for the treatment of North American women with stress urinary incontinence. J Urol. 2003;170:125963. doi: 10.1097/01.ju.0000080708.87092.cc. [DOI] [PubMed] [Google Scholar]


