Abstract
Objective:
To assess the anticataleptic and antiepileptic activity of leaves of Mucuna pruriens in albino rats.
Materials and Methods:
Haloperidol-induced catalepsy (HIC), maximum electro-shock (MES) method, pilocarpine-induced Status epilepticus (PISE) and single-dose effect of M. pruriens were employed.
Results:
M. pruriens (100 mg/kg) had significant anticataleptic and antiepileptic activity in HIC, MES, and PISE.
Conclusions:
M. pruriens extract has the potential to be an anticataleptic and antiepileptic drug. Dopamine and 5-HT may have a role in such activity.
Keywords: Catalepsy, dopamine, epilepsy, Mucuna pruriens
Introduction
Catalepsy is a nervous condition characterized by muscular rigidity and fixity of posture regardless of external stimuli as well as decreased sensitivity to pain. Catalepsy can appear in schizophrenia, Parkinsonism, and epilepsy. Symptoms include rigid body, rigid limbs, and limbs staying in the same position when moved.[1]
Epilepsy is defined as a group of disorders of central nervous system (CNS) characterized by paroxysmal cerebral dysrhythmia, manifesting as brief episodes (seizures) of loss or disturbances of consciousness with or without characteristic body movement (convulsion), sensory or psychiatric phenomena.[2] Epilepsy is the third most common neurological disorder affecting the worldwide population.[3]
The plant Mucuna pruriens (family: Fabaceae) is an annual plant found in India. It has high content of dopamine in seeds. The plant also contains 5-HT.[4] The leaves of M. pruriens (MP) are also reported to contain dopamine.[5] Dopamine is used for treatment of catalepsy.[6] It also acts as a neuroprotective agent in epilepsy.[7] The seeds of MP are reported to possess anti-cataleptic activity.[8] However, literature survey does not reveal any such activity in leaves of MP. Therefore the present study was undertaken to investigate the anti-cataleptic and antiepileptic effects of ethanolic extracts of leaves of MP.
Materials and Methods
Animals
Adult albino rats of either sex (weighing 150-175g) bred in the animal house, Siksha ‘O’ Anusandhan University, Bhubaneswar, were used for this study. Animals were housed under a standard 12 h:12 h light/dark cycle and were provided with food and water ad libitum. Animals were acclimatized to laboratory conditions before testing. Each animal was used once. The experimental protocol was approved by Institutional Animal Ethics committee, SOA University, (reg. no. 1171/c/08/CPCSEA) and the study was conducted according to the CPCSEA guidelines for the use and care of experimental animals.
Preparation of alcoholic extract
The leaves of MP were collected from Chandaka forest near Bhubaneswar after due authentication and were shade dried. The leaf powder was subjected to successive soxhlet extraction for 12 h with petroleum ether followed by chloroform and finally with 95% ethanol. The ethanolic extract thus obtained is used in the study.
Drugs and dosage
The test drug, ethanolic extract of leaves of MP (50, 100, 200 and 400 mg/kg), and standard drugs levodopa (100 mg/kg i.p.), phenytoin (25 mg/kg i.p.), diazepam (5 mg/kg i.p.), haloperidol (1 mg/kg i.p.), and pilocarpine (350 mg/kg i.p.) were suspended or dissolved in distilled water and administered.
Experimental Design
Haloperidol-induced catalepsy
Catalepsy was induced with haloperidol (1 mg/kg i.p.) and assessed at 30 min intervals until 120 min by means of a standard bar test. Haloperidol was chosen so that it could elicit a moderate degree of catalepsy.[9] Catalepsy was assessed in terms of points given to imposed position of limbs (front) extended and resting on a 3 and 9 cm high wooden bar (1 cm diameter). The end of catalepsy was considered to occur when both front paws were removed from the bar or if the animal moved its head in an exploratory manner.[10] Levodopa (100 mg/kg) was used as standard treatment. Ethanolic extract of MP was administered to three different groups (n=6 in each group) at a dose of 50, 100, 200 mg/kg p.o. respectively 1 h prior to haloperidol administration.
Scoring methods
If the animal maintained the imposed posture for at least 10 s it was considered to be cataleptic.
Stage I – Rat moves normally when placed on the table, score = 0.
Stage II – Rat moves only when touched or pushed, score = 0.5.
Stage III – Rat placed on the table with front paws set alternately on a 3 cm high block fails to correct the posture in 10 s, score = 0.5, for each paw with a total, score = 1, for this stage.
Stage IV – Rat fails to remove when the front paws are placed alternately on a 9 cm high block, score = 1, for each paw with a total score = 2 for this stage.[11]
Maximum electroshock method
MES seizures were evoked through transauricular electrodes with a current of 150 mA for duration of 0.2 s. The ethanolic extract of MP was administered to a group of rats (n=6) in a dose of 100 mg/kg, p.o, 1 h before application of electroshock. The duration of tonic hind limb extensor was noted.[12] The percentage of protection was calculated. Phenytoin (25 mg/kg) was used as standard.
Pilocarpine-induced status epilepticus
The albino rats were divided into group of six each. Status epilepticus was induced by administration of pilocarpine at a dose of 350 mg/kg, i.p. Atropine 1 mg/kg i.p. was administered 30 min prior to pilocarpine to reduce the peripheral cholinergic effects of pilocarpine. The ethanolic extract of MP 100 mg/kg was given orally 1 h before injection of pilocarpine nitrate. The severity of status epilepticus was observed every 15 min till 90 min and thereafter every 30 min till 180 min using the following scoring system. Diazepam (5 mg/kg) was used as standard.[13]
Stage 0 – No response.
Stage 1 – Fictive scratching.
Stage 2 – Tremor.
Stage 3 – Head nodding.
Stage 4 – Forelimb clonus.
Stage 5 – Rearing and falling back.
Statistical Analysis
The data were expressed as mean ± SEM. The results were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett's t-test. P<0.01 was considered as significant.[11]
Results
Effect of the ethanolic extract of leaves of MP on HIC
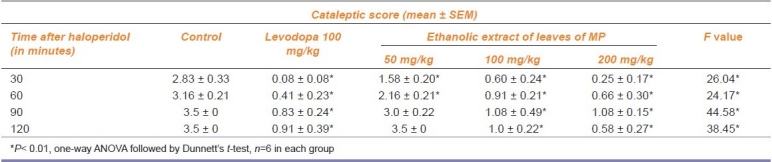
The cataleptic score in rats treated with ethanolic extract of leaves of MP (100 mg/kg) showed a significant (P<0.01) reduction like levodopa (100 mg/kg) against HIC [Table 1] . The control group showed a maximum cataleptic score of 3.5 after 90 min of haloperidol administration.
Table 1.
Anticataleptic effect of Mucuna pruriens (MP) on haloperidol-induced catalepsy in rats.
Effect of ethanolic extract of leaves of MP on tonic hind limb extension in MES
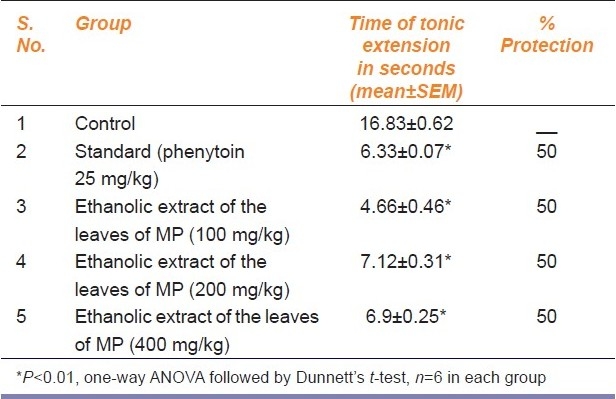
The duration of tonic hind limb extension in rats treated with vehicle was 16.83±0.62 s. The ethanolic extract of leaves of MP showed a significant (P<0.01) reduction in tonic extension phase (4.66±0.46 s) at a dose of 100 mg/kg. It completely abolished the tonic extension phase in three out of six rats (50% protection). At higher doses also the response was similar [Table 2.]
Table 2.
Antiepileptic effect of acute administration of the ethanolic extract of leaves of Mucuna pruriens (MP) on maximal electric-shock (MES)-induced seizures in rats.
Effect of ethanolic extract of leaves of MP on pilocarpine-induced status epilepticus
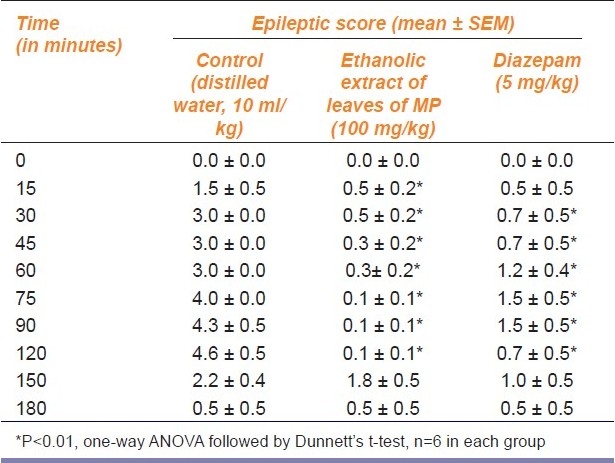
The ethanolic extract of leaves of MP (100 mg/kg) significantly (P<0.01) reduced the epileptic score in pilocarpine-induced status epilepticus model as compared to control [Table 3] . Diazepam (5 mg/kg) also significantly reduced the severity of seizures.
Table 3.
Antiepileptic effect of ethanolic extract of leaves of Mucuna pruriens (MP) (100 mg/kg) on pilocarpine-induced status epilepticus in rats
Discussion
The alcoholic extract showed no signs of lethality up to 2000 mg/kg. So initially three doses i.e. 50, 100, and 200 mg/kg were selected for the evaluation of anticataleptic activity using the HIC model. At a dose of 100 mg/kg the ethanolic extract showed a significant reduction (P<0.01) of the cataleptic score. So for the evaluation of MES and PISE, the same dose of 100 mg/kg was used. However, for evaluation of dose-dependent antiepileptic activity, two more doses of 200 and 400 mg/kg were used in MES.
The HIC model is used to assess anticataleptic drugs. Inhibition of cataleptic score predicts activity of drug against Parkinson's disease. So the ethanolic extract of MP may be useful in catalepsy.[14]
The MES is probably the best validated method for assessment of antiepileptic drugs in general tonic clonic seizure. Inhibition of the MES test predicts activity against seizure and cortical focal seizure. So the MP extract may be useful in generalized tonic clonic and cortical focal seizure.[15] However, this antiepileptic activity is not dose dependent.
Status epilepticus induced by pilocarpine-treated rats has served as a useful model of temporal lobe epilepsy and reproduces some of the key features of temporal lobe epilepsy in human beings. Since MP extract significantly (P<0.01) reduces the epileptic score than control, it may be effective in temporal lobe epilepsy.[16]
The leaves of MP are reported to contain dopamine and 5-HT. Dopamine is used for treatment of catalepsy. Dopamine acts as a neuroprotective agent in epilepsy. Increased serotonin immunoreactivity has been reported in human epileptic brain tissue resected for the control of epilepsy.[17] 5-HT1A receptors are reduced in the ipsilateral hippocampus in temporal-lobe epilepsy patients.[18] So dopamine and 5-HT might play an important role in the anticataleptic and antiepileptic activity of the ethanolic extract of MP.
Acknowledgments
The authors are thankful to the Vice Chancellor, Siksha O Anusandhan University for providing the research facilities.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Rasmussen K, Hsu MA, Noone S, Johnson BG, Thompson LK, Hemrick-Luecke SK. The treatment of extra pyramidal symptoms. Schizophr Bull. 2007;33:1291–7. doi: 10.1093/schbul/sbm087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tripathy KD. Essentials of medical pharmacology. 5th ed. New Delhi: Jaypee Publishers; 2003. [Google Scholar]
- 3.Gardiner RM. Impact of our understanding of genetic aetiology of epilepsy. J Neurol. 2000;247:327–34. doi: 10.1007/s004150050598. [DOI] [PubMed] [Google Scholar]
- 4.Hussain G, Manyam BV. The phytochemistry, toxicity and food potentials of velvet bean. Phytother. 1997;11:419–23. [Google Scholar]
- 5.Manyam BV, Dhanasekaran M, Hare TA. Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phytother Res. 2004;18:706–12. doi: 10.1002/ptr.1514. [DOI] [PubMed] [Google Scholar]
- 6.Katzenschlager R, Evans A, Manson A. Mucuna pruriens in Parkinson's disease: A double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75:1672–77. doi: 10.1136/jnnp.2003.028761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bozzi Y, Vallone D, Borrelli E. Neuroprotective Role of Dopamine in CNS. J Neurosci. 2000;20:8643–9. doi: 10.1523/JNEUROSCI.20-22-08643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manyam BV, Dhanasekaran M, Hare TA. Effect of antiparkinson drug HP-200 (Mucuna pruriens) on the central monoaminergic neurotransmitters. Phytother. 2004;18:97–101. doi: 10.1002/ptr.1407. [DOI] [PubMed] [Google Scholar]
- 9.Silva SR, Futuro-Neto HA, Pires JG. Effects of 5-HT3 receptor antagonists on neuroleptic-induced catalepsy in mice. Neuropharmacol. 1995;34:97–9. doi: 10.1016/0028-3908(94)00146-j. [DOI] [PubMed] [Google Scholar]
- 10.Ferre S, Guix T, Prat G, Jane F, Casas M. Is experimental catalepsy properly measured? Pharmac Biochem Behav. 1990;35:753–7. doi: 10.1016/0091-3057(90)90354-k. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni SK. Hand book of experimental pharmacology. 3rd ed. New Delhi: Vallabh Prakashan; 1999. [Google Scholar]
- 12.Ambawade SD, Kasture VS, Kasture SB. The anticonvulsant activity of roots and rhizomes of Glycyrrhiza glabra. Indian J Pharmacol. 2002;34:251–5. [Google Scholar]
- 13.Cavalheiro EA, Santos NF, Priel MR. The pilocarpine model of epilepsy in mice. Epilepsia. 1996;37:1015–9. doi: 10.1111/j.1528-1157.1996.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 14.Rao C, Lalit M, Gopalkrishna HN. The effect of NR-ANX-C (a polyherbal product) on HIC. Indian J Physiol Pharmacol. 2004;48:108–9. [Google Scholar]
- 15.Mc Donald RL, Kelly KM. Antiepileptic drugs and Mechanism of action. Epilepsia. 1993;34:81–8. [Google Scholar]
- 16.Ormandy GC, Jope RS, Snead OC. Anticonvulsant action of MK-801 on Pilocarpine-model of Status epilepticus in rats. Exp Neurol. 1989;106:172–80. doi: 10.1016/0014-4886(89)90091-5. [DOI] [PubMed] [Google Scholar]
- 17.Trottier S, Evrard B, Vignal JP, Scarabin JM, Chauvel P. The serotonergic innervations of the cerebral cortex in man and its changes in focal cortical dysplasia. Epilep Res. 1996;25:79–106. doi: 10.1016/0920-1211(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 18.Clinckers R, Smolders I, Meurs A, Ebinger G, Michotte Y. Anticonvulsant action of hippocampal dopamine and serotonin is independently mediated by D and 5-HT receptors. J Neurochem. 2004;89:834–43. doi: 10.1111/j.1471-4159.2004.02355.x. [DOI] [PubMed] [Google Scholar]


