Abstract
The nephroprotective activity of the ethanolic extract of Bauhinia variegata (Linn.) whole stem against cisplatin-induced nephropathy was investigated by an in vivo method in rats. Acute nephrotoxicity was induced by i.p. injection of cisplatin (7 mg/kg of body weight (b.w.)). Administration of ethanol extract at dose levels of 400 and 200 mg/kg (b.w.) to cisplatin-intoxicated rats for 14 days attenuated the biochemical and histological signs of nephrotoxicity of cisplatin in a dose-dependent fashion. Ethanol extract at 400 mg/kg decreased the serum level of creatinine (0.65 ± 0.09; P<0.001) and urea (32.86 ± 5.88; P<0.001) associated with a significant increase in body weight (7.16 ± 1.10; P<0.001) and urine volume output (11.95 ± 0.79; P<0.05) as compared to the toxic control group. The ethanol extract of B. variegata at 400 mg/kg (b.w.) exhibited significant and comparable nephroprotective potential to that of the standard polyherbal drug cystone. The statistically (one-way-ANOVA followed by Tukey-Kramer multiple comparison) processed results suggested the protective action of B. variegate whole stem against cisplatin-induced nephropathy.
Keywords: Cisplatin, nephrotoxicity, Kanchana
Introduction
Nephropathy is widely encountered among the people of entire world irrespective of the age, racial, environmental, and geographical variability. The etiology behind this complication is broad ranging from substance-induced to various metabolic and physiological disturbances, paneling nephropathy amongst the 10 leading causes of death across the world. Cisplatin (cis-diaminedichloroplatinum II, CDDP) is extensively used for the treatment of several cancers like testicular and lungs cancer. Unfortunately, the gracious drug cisplatin is conjoined with a brutal side effect since it induces nephrotoxicity.[1]
The plant Bauhinia variegata (BV) Linn., locally known as Kanchana,[2] belonging to the family Caesalpiniaceae, is a widely distributed plant throughout the subtropical and tropical regions of the world. It is used in abundance for medicinal and cattle feed purposes by the tribes of India[3] and also being used in various indigenous systems of medicine like Ayurveda[4] and Unani. Different parts of this plant are used traditionally in various ailments owing to its folkloric claims such as liver tonic, antibacterial,[5] to suppress the edema, dysentery, ulcers, eye disease, skin diseases, piles, and hemorrhoids.[6] The stem of this plant is reported to contain many active phytochemicals including stigmasterol, flavone glycosides, lupeol, kaempferol-3-glucoside, β-setosterol, etc.[7] In this present context, the in vivo nephroprotective activity of the ethanolic extract of B. variegata (BV) whole stem was evaluated in albino male rats (Wistar strain).
Material and Methods
The whole stem of BV was collected from young matured plant from Bharatpur Forest Range, Bhubaneswar, Orissa, India, during the month of November and December and identified by Dr. B. K. Jaysingh., Gopabandhu Ayurvedic College, Puri, Orissa. The plant specimen (Vide Voucher No.: 081) was deposited in herbarium of University Department of Pharmaceutical Sciences, Bhubaneswar, Orissa, India. After authentication fresh plant materials were collected in bulk, washed under running tap water to remove adherents, shade dried, and pulverized in a mechanical grinder to get coarse powder of BV whole stem.
Healthy albino male rats of Wistar strain weighing between 150 and 200 g were selected for the investigation. The animals were kept under maintained laboratory conditions with adequate supply of drinking water ad libitum and pallet diet. The experimental protocol was approved by the Institutional Animal Ethics Committee and the conditions in the animal house approved by Committee for Supervision on Experiments on Animals (Vide Registration No.: 990/c/06/CPCSEA).
About 200 g of coarse powder of BV whole stem was taken in a soxhlet apparatus and extracted[8] successively with petroleum ether, chloroform, and ethanol. The extraction for each solvent was carried out for 18--20 h. The ethanol extract was collected by evaporating the solvent by slow heat treatment. Total 1.4 kg of pulverized stem was used to produce the required amount of test extract. Calculated amount of dried ethanol extract was suspended in 0.5% w/v of sodium CMC in a normal saline solution to prepare the test doses (200 and 400 mg/kg/ml.). The dose limits were selected on the basis of previously performed oral acute toxicity studies in mice, in accordance with the OECD guidelines.[9]
Total 30 Wistar rats were divided randomly into five groups of six animals each. Group I (normal control) received oral dose of 0.5% sodium CMC (1 ml each) for 14 days. Group II (toxic control) received single dose of cisplatin[10] (7 mg/kg of body weight; i.p.) on day 1. Group III (standard group) received standard polyherbal drug cystone[11] (5 ml/kg; p.o.) (Cystone Syrup, Himalaya Drug Company., Bangalore, India) for 14 days with single dose of cisplatin (7 mg/kg of body weight; i.p.) on day 1, group IV (EEBV200) and group V (EEBV400) received ethanolic extract 200 and 400 mg/kg b.w. once in a day for 14 days respectively along with the single dose of cisplatin (7 mg/kg of body weight; i.p.) on day 1. The treatment duration was considered for 14 days as documented by Yang et.al.[12]
Urine was collected over 24 h on 14th day by keeping the test animals in individual metabolic cages. The volume of collected urine samples was measured followed by estimation of biochemical parameters, namely urine creatinine and urine albumin. Blood samples were collected from the test animals under anesthesia (phenobarbiton sodium; 40 mg/kg of body weight; i.p) by cardiac puncture before sacrifice and serum parameters including creatinine, urea, albumin, and total protein were estimated.[13,14] The biochemical estimations were done in a Biochemical-semi-auto analyzer (Ebra-chem-5-plus, V2. West Germany) by standard procedures using commercial kits (Ecolin: Merck specialties, India). The kidneys were removed from the rats before sacrifice and organs were fixed[15] using a formosal solution (10% v/v of formaldehyde in normal saline), embedded with paraffin wax followed by preparation of tissue sections using a microtome for histopathology study.[16]
Statistics
Data obtained in the experiment were expressed in terms of mean ± SEM. Statistical significance of data was assessed by analysis of variance (one-way ANOVA) followed by a comparison between different groups using “Tukey-Kramer” multiple comparison test. The significance level was set at P<0.05. The toxic control group was compared with the normal control group and all other treatment groups were compared with the toxic control group.
Results
The results as cited in Table 1 includes change in body weight, kidney weight, urine volume along with the urine, and serum biochemistry data. Cisplatin administration-induced renal injury was prominent as evidenced by significantly depressed renal functions, body weight, and urine volume as compared to the normal control group.
Table 1.
Parameters studied for the nephroprotective effect of the ethanol extract of Bauhinia variegata
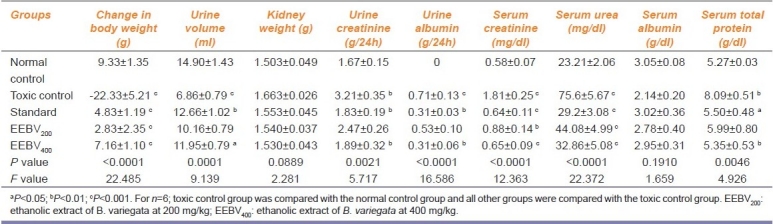
The EEBV400 group (ethanolic extract 400 mg/kg treated rat group) showed significant (P<0.001) elevation in body weight (7.16 ± 1.10) with a significant (P<0.05) increase in urine volume output (11.95 ± 0.79). However, the urine creatinine (1.81 ± 0.32) and albumin (0.31 ± 0.06) decreased significantly (P<0.01) as compared with the toxic control group. The serum creatinine (0.65 ± 0.09) and urea (32.86 ± 5.88) were found to be significantly (P<0.001) low when compared with the toxic control group.
The histological features found from the tissue sections of different groups are mentioned in Table 2 and the photomicrographs of tissue sections are presented in Figure 1a--d. The histopathology of tissue sections suggest that the toxic control group had encountered vast histological damages as evidenced by the glomerular and tubular congestion with abnormal Bowman's capsule, blood vessel congestion, epithelial cell desquamation, and presence of tubular cast with few inflamatory cells. The histological features of the EEBV400 group showed minimal cellular damage in contrast to the toxic control group. The EEBV400 group showed normal glomerular and tubular arrangements with normal Bowmen's capsule. Congestion of blood vessels was minimal and tubular cast were not present.
Table 2.
Histological features found from L.S of kidneys of different groups
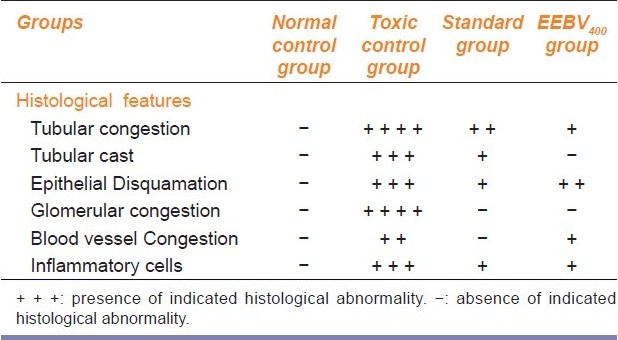
Figure 1.
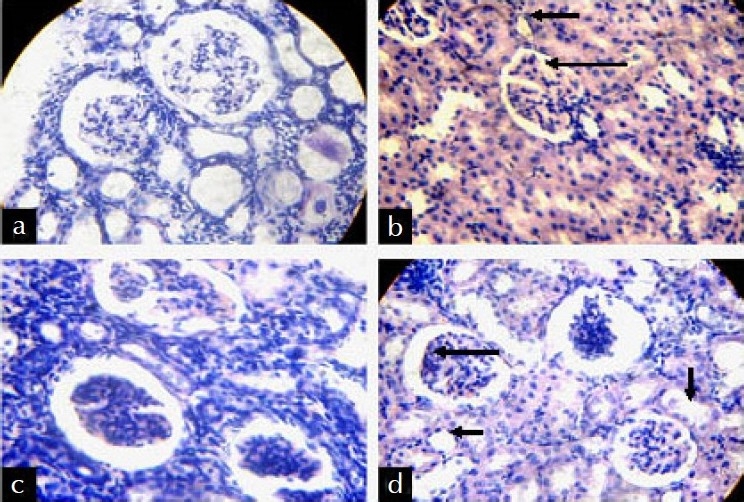
Photomicrographas of L.S. of kidney of different groups (a) Normal Control Group, (b) Toxic Control Group, (c) Standard Group, (d) EEBV400 Group.
Discussion
The present study aimed to evaluate the protective effect of whole stem extract (ethanol) of BV Linn. plant against cisplatin-induced nephropathy in rats. Cisplatin-administered rats (toxic control group) had encountered acute kidney dysfunction as evidenced by elevation in serum urea and creatinine, decreased urine output and body weight with multiple histological damages. Treatment with the ethanol extract of BV at the dose level of 400 mg/kg b.w. for 14 days (EEBV400 group) significantly lowered the serum level of creatinine and urea, decreased urine creatinine and albumin with a significant weight gain, and increased urine output when compared with the toxic group. The histological damages in the BV extract-treated group were minimal in contrast to the toxic rats. The statistical significance of the nephroprotective activity of BV-treated group and the polyherbal drug cystone (standard group)-treated group (both the groups were compared against toxic control) were found almost equal as both groups gained same level of significance (P<0.001) against the toxic group in most of the parameters including serum urea and creatinine.
The results of our study suggest that the ethanolic extract of BV possesses nephroprotective potential depending on the dose levels. Extensive and multidimensional further research is needed to elucidate the exact mechanism of nephroprotective action of this plant extract.
Acknowledgments
The authors are thankful to Head of the Dept., University Department of Pharmaceutical Sciences, Utkal University, Bhubaneswra, Orissa for providing laboratory facilities to carryout this research work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Zhang JG, Lindup WE. Role of mitochondria in Cisplatin-induced oxidative damage exhibited by rat renal cortical slices. Biochem Pharmacol. 1993;45:2215–22. [PubMed] [Google Scholar]
- 2.Chopra RN, Nayer SL, Chopra IC. Glossary of Indian Medicinal Plants. India: Council of Industrial and Scientific Research (CSIR); 1956. p. 35. [Google Scholar]
- 3.Kirtikar KR, Basu BD. Indian Medicinal Plant. Dehradun: International Book Distributor; 1999. pp. 892–901. [Google Scholar]
- 4.The Ayurvedic Pharmacopoeia of India. Ministry of Health and family Welfare, Government of India. 1990:10. [Google Scholar]
- 5.Chanda S, Parekh J, Karathia NN. Evaluation of antibacterial activity and phytochemical analysis of Bauhinia variegata L. bark. Afr J Biomed Res. 2006;9:53–6. [Google Scholar]
- 6.Mali RG, Mahajan SG, Mehta AA. Rakta Kanchan (Bauhinia variegata): Chemistry, Traditional and Medicinal uses- a review. Pharmacogn Rev. 2007;1:314–19. [Google Scholar]
- 7.Gupta AK, Vidyapati TJ, Chauhan JS. Chemical examination of the stem of Bauhinia variegata. Planta Med. 1980;38:174–6. [Google Scholar]
- 8.Suftness M, Douras J. Drugs of plant origin -methods in cancer research. New York: Academic Press; 1979. p. 548. [Google Scholar]
- 9.OECD. Acute oral toxicity- Acute oral toxic class method, Gudieline 423. Paris: Organization for economic co-operation and development; 2000. adopted on 23.03.1996. In 11th addendum to the OECD guidelines for testing of chemicals. [Google Scholar]
- 10.Suzuki CA, Cherian MG. Interaction of cis-diaminedichloroplatinum with metallothionein and glutathione in rat liver and kidney. Toxicology. 1990;64:113–27. doi: 10.1016/0300-483x(90)90129-5. [DOI] [PubMed] [Google Scholar]
- 11.Rao M, Rao MN. Protective effect of Cystone, a polyherbal Ayurvedic Preparation on Cisplatin induced Renal toxicity in Rats. J Ethnopharmacol. 1998;62:1–6. doi: 10.1016/s0378-8741(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 12.Yang HK, Yong WK, Young JO, Nam IB, Sun AC, Hae GC, et al. Protective effect of the ethanol extract of the roots of Brassica rapa on cisplatin-induced nephrotoxicity in LLC-PK1 cells and rats. Biol Pharm Bull. 2006;29:2436–41. doi: 10.1248/bpb.29.2436. [DOI] [PubMed] [Google Scholar]
- 13.Cheesbrough M. District Laboratory Practice in Tropical Countries. England: Cambridge University Press; 2003. pp. 310–95. [Google Scholar]
- 14.Tietz . Text book of Clinical Chemistry. 3rd ed. Phailadelphia: W.B Saunders Company; 1999. pp. 617–721. [Google Scholar]
- 15.Sood R. Medical Laboratory Technology-Methods and Interpretation. 4th ed. India: Jaypee Bros Publications; 2002. pp. 224–6. [Google Scholar]
- 16.Ogeturk M, Kus I, Colakoglu N, Zararsiz I, Ilhan N, Sarsilmaz M. Caffeic acid phenethyl ester protects kidneys against CCl4 toxcicity in rats. J Ethnopharmacol. 2005;97:273–80. doi: 10.1016/j.jep.2004.11.019. [DOI] [PubMed] [Google Scholar]


