Abstract
Myeloid-derived suppressor cells are important cell population with an immunoregulatory potential in both adaptive and innate immunity. Their immunosuppressive activity is widely accepted. However, emerging evidence suggests that this heterogeneous cell population can be, under some circumstances, immunostimulatory rather than suppressive. This finding can shed a new light on antitumour immunity which is believed to be impaired in immunosuppressive environments.
Keywords: antitumour immunity, immunoregulatory properties, myeloid-derived suppressor cells
Introduction
Myeloid-derived suppressor cells (MDSC), important innate regulators of immune response, have been investigated intensively in several recent studies. In mice, MDSC are a heterogeneous population of cells that express both CD11b and Gr-1 (Ly6G and Ly6C) and consists of early myeloid progenitors and immature myeloid cells [macrophages, granulocytes, dendritic cells (DC)] at different stages of differentiation. They lack or have reduced expression of markers of mature myeloid cells (Gabrilovich & Nagaraj 2009).
MDSC have the ability to suppress immune responses. The suppressive activity of MDSC is primarily associated with the metabolism of L-arginine, a substrate for inducible nitric oxide synthase, iNOS (NO generation) and arginase 1 (generation of urea and L-ornithine) and production of reactive oxygen species (ROS) (Mazzoni et al. 2002; Talmadge 2007, Kusmartsev et al. 2004). It has been proposed that they might play an important physiological role in preventing immune system from excessive activation and thereby limiting tissue damage during the immune response (Bronte & Zanovello 2005; Cripps et al. 2010; Dardalhon et al. 2010). In addition, MCSC accumulate in many pathological situations such as bacterial and parasitic infections, trauma, acute and chronic inflammation, autoimmunity (Goñi et al. 2002; Gómez-García et al. 2005; Ezernitchi et al. 2006; Makarenkova et al. 2006; Zhu et al. 2007; Iwata et al. 2010).
Numerous studies also point to a role of MDSC in cancer, where they negatively influence antitumour immunity. Although large body of evidence shows that MDSC accumulating in tumour bearers can inhibit immunosurveillance and contribute to tumour progression (both in patients and in tumour models) (Almand et al. 2001; Bunt et al. 2006; Nagaraj & Gabrilovich 2008; Liu et al. 2009), there emerge suggestions that myeloid CD11b+Gr-1+ cells could function paradoxically in an immunostimulatory way and even act against tumours (Table 2). The latter findings seem to be consistent with the fact that monocytes/macrophage lineage cells are highly plastic in nature. Their activities can be opposing depending on environmental influences (such as cytokines, prostaglandins, chemokines, hormones, pathogen-associated molecular patterns).
Table 2.
Examples of immunostimulatory function of myeloid CD11b+Gr-1+ cells
| Function of myeloid CD11b+Gr-1+cells | Experimental model | References | |
|---|---|---|---|
| 1 | Priming and expansion of antigen-specific B cells, optimal production of antibody | Immunization with alum | Jordan et al. 2004 |
| 2 | Inhibition of tumour growth during adoptive immunotherapy with CY | Ehrlich tumour | Peláez et al. 2001 |
| 3 | Activation of NK-cell effector functions | Lymphoma model RMA-S | Nausch et al. 2008 |
| 4 | MDSC-based vaccine increases survival time & protects from metastases | Colon adenocarcinoma | Ko et al. 2009 |
| 5 | Antigen-specific immunity and cross-priming | Ovarian carcinoma model | Tomihara et al. 2010 |
| 6 | CD11b+Ly-6C hi monocytes contributed to tumour growth inhibition | Chronic osteomyelitis in osteosarcoma model | Sottnik et al. 2010 |
| 7 | CD11b+Ly-6C+ cells differentiated into DC that were able to stimulate T cells | Diabetes | Caquard et al. 2010 |
| 8 | Enhancement of CTL response in vitro after differentiation into APC | Mammary adenocarcinoma | Bronte et al. 2000 |
APC, antigen-presenting cell; CTL, cytotoxic T lymphocytes; CY, cyclophosphamide; DC, dendritic cells; MDSC, myeloid-derived suppressor cells; NK, natural killer.
A classification of macrophages based mainly on the response to Th cytokines has been proposed. Classically activated macrophages (M1) develop under the influence of Th1 cytokines (e.g. IFN-γ) or bacterial products. They are capable of killing microorganisms and tumour cells. Presence of Th2 cytokines (e.g. IL-4, IL-13) mediates the development of alternatively activated macrophages (M2) that act by enhancing Th2 adaptive immune response and limiting Th1 immune response. In contrast to M1, M2 macrophages are involved in immunosuppression and tissue repair and were shown to exhibit protumour activity. Interestingly, phenotype of monocytes/macrophage lineage cells adapted to environmental signals may be reversible (Mantovani et al. 2004; Stout & Suttles 2004; Guiducci et al. 2005).
Suppressive activity of MDSC
The suppressive activity of MDSC has been clearly demonstrated. Most studies on their function has been performed in tumour models (both in vitro and in vivo), in which MDSC reduce antitumour immune response. Importantly, there are also studies which suggest that accumulation of MDSC in patients with cancer may correlate with defectiveness of immunological reactions against tumour (Almand et al. 2001; Bunt et al. 2006; Nagaraj & Gabrilovich 2008; Diaz-Montero et al. 2009; Liu et al. 2009). Collectively, MDSC downregulate innate immunity by impairment of natural killer cells (NK) activity (Liu et al. 2007) and decrease in macrophage production of IL-12 (Sinha et al. 2007) and regulate adaptive immunity by reduction of T-cell function. MDSC inhibit T-cell response by multiple pathways that include NO and ROS production, amino acid metabolism, T-regulatory cell (T reg) induction and secretion of inhibitory molecules such as IL-10 and TGF-β (Mazzoni et al. 2002; Terabe et al. 2003; Kusmartsev et al. 2004; Huang et al. 2006; Sinha et al. 2007; Srivastava et al. 2010).
Accumulation of MDSC is also linked to the impairment of development and function of DC, which are at the interface between innate and adaptive immunity (Greifenberg et al. 2009; Hu et al. 2010).
Immune stimulation and antitumour activity: another face of MDSC?
Myeloid cells with suppressive function were firstly discovered in the late 1970s when they were referred as natural suppressor (NS) cells. They were described as cells with ability to inhibit T-cell responses in vitro and in vivo (Strober 1984; Holda et al. 1985). Surprisingly, it was found that NS cells exhibit antiproliferative activity not only for lymphocytes but also for tumour cells. The mechanism of tumour growth inhibition by NS cells was not clarified by the investigators (Sugiura et al. 1990). NO, which was found to be produced by early myeloid suppressor cells (Angulo et al. 2000b) and which is believed to have a potent tumoricidal activity (Umansky & Schirrmacher 2001; Mocellin et al. 2007), could be responsible. The antitumor potential of early myeloid suppressor cells producing NO was investigated by Peláez et al. (2001). They used a model of combined adoptive therapy (tumour-specific lymphocytes) with cyclophosphamide (CY) that is known to cause transient MDSC accumulation in the spleen after injection into mice (Angulo et al. 2000a). They have proved that cells with features of MDSC derived from CY-treated mice indeed are able to inhibit tumour growth in vitro by generation of NO. Administration of NG-monomethyl-L-arginine (LMMA), an inhibitor of NO synthase, prevented nitrite production (end product of NO metabolism) and tumour cell growth inhibition. This study suggest that these effects may also occur in vivo; however, it has not been formally proved (Peláez et al. 2001).
Even though some reports suggest that MDSC have an ability to inhibit tumour cell proliferation by releasing NO, the majority of studies show the opposite effect. This could be partially explained by a dual role for this molecule. NO at high concentration can cause apoptosis of tumour cells, but on the other hand, at low concentration it can act as tumour promoter (indirectly promoting DNA damage) (Mocellin et al. 2007; Umansky & Schirrmacher 2001). Furthermore, tumour cells can acquire resistance to NO-induced apoptosis (Wang & MacNaughton 2005). Additionally, important effectors of antitumour immunity, lymphocytes, are sensitive to NO (Mazzoni et al. 2002). The presence of NO and superoxide anions leads to the generation of highly toxic peroxynitrate. Production of peroxynitrite during direct contact of MDSC with T cells results in the nitration of TCR and CD8 molecule, thus making T-cell unresponsive to antigen-specific stimulation (Nagaraj et al. 2010). Therefore, NO secreted by MDSC has a tumoricidal potential, but alone may not be sufficient to destroy tumour masses and its prolonged production can be more harmful than beneficial.
Interestingly, despite the strong evidence that MDSC can make lymphocytes defective, some studies demonstrate that the presence of MDSC is not necessarily related to a hampered function of T cells. Srivastava et al. (2008) observed that even if proliferation of CD4+ cells from patients with lung cancer is initially inhibited by MDSC, MHC II positive lung cancer vaccines can prime and boost tumour-specific CD4+ cells, which secrete IFN-γ. Similarly, Watanabe et al. have demonstrated that MDSC strongly inhibit the proliferation of T cells, but production of IFN-γ by these cells was still detected. Importantly, the same author has shown that MDSC do not inhibit function of T cells in vivo at the effector phase. After cotransfer of MDSC with activated T cells into tumour-bearing mice, tumour regression could still be observed (Watanabe et al. 2008). However, neither interaction between transferred lymphocytes and MDSC in vivo nor the phenotype of MDSC after cotransfer was investigated.
Apart from T cells, MDSC also affect NK cells, the second important effector cells in antitumour immunity. Several studies suggest that MDSC inhibit NK cells function by different mechanisms (Liu et al. 2007; Hoechst et al. 2009; Li et al. 2009). However, one study using NK-sensitive tumour model, RMA-S lymphoma, unravelled an activating role for CD11b+Gr-1+F4/80+ MDSC on NK cells. These suppressor cells express RAE-1, a ligand for the activating receptor NKG2D, and induce NK to produce IFN-γ. Depletion of MDSC (using anti-Gr-1 antibody) in this tumour model accelerates tumour growth (Nausch et al. 2008) in contrast to studies using other tumour models (Gabrilovich & Nagaraj 2009; Li et al. 2009). This report proves that even though MDSC are suppressive, they can also be immunostimulatory under some circumstances.
Development of immunostimulatory properties in MDSC could occur when these cells are placed in a proper cytokine environment. In the 1990s it was reported that a suppressive population of granulocyte-macrophage progenitor cells could be differentiated by using a combination of low doses of IFN-γ and TNF-α (Pak et al. 1995). The finding of Bronte et al. suggested that the same cytokines can drive differentiation of MDSC into antigen-presenting cell (APC)-like cells. They have demonstrated that suppressive CD11b+Gr-1+CD31+ cells in the presence of IFN-γ and TNF-α or IL-12 get transformed into stimulatory cells (with upregulation of CD86) that enhance cytotoxic T lymphocytes (CTL) responses in vitro (Bronte et al. 2000). Similarly, Narita et al. (2009) showed that CD11b+Gr-1+ cells from tumour-bearing mice can differentiate in vitro into either functional CD11c+ cells (which are able to generate CTL) or F4/80+ suppressive macrophages depending on the environment (Th1 cytokines vs. tumour-derived factors). Myeloid-derived suppressor cell-like cells have also been shown to have ability to differentiate into immunostimulatory cells in vivo (Caquard et al. 2010). These cells had the CD11b+Ly-6G−Ly-6C+ phenotype and were generated after CY injection into NOD mice (anti-Gr-1 antibody recognizes two epitopes: Ly-6G is expressed mainly on granulocytic fraction of MDSC and Ly-6C expressed mainly on monocytic fraction of MDSC). They resemble MDSC functionally as they exhibit immunosuppressive activity in vitro but have the morphology of inflammatory monocytes. In the model of prediabetic NOD/SCID, adoptive transfer of this cell population unexpectedly caused their conversion into CD11c+ cells in vivo. When cultured with GM-CSF and IL-4, CD11b+Ly-6C+ cells were also able to differentiate into DC-like cells that were capable of stimulating T-cell response. Culture of CD11b+Ly-6C+ cells with IFN-γ or IL-4 results in the upregulation of NOS2 or ARG1 respectively (Caquard et al. 2010). This study, in agreement with previous studies (Bronte et al. 2000; Narita et al. 2009), demonstrates that cells with features of MDSC exhibit plasticity. This observation was used by Ko et al. (2009) who created an APC-based vaccine from MDSC. As it is known that NKT cells have potential to reduce the suppressive activity of MDSC (De Santo et al. 2008), α-galactosylceramide (αGalCer), the ligand for NKT cells, was used for the preparation of the vaccine. Thus the MDSC presenting tumour antigen (Ag) and αGalCer after injection into tumour-bearing mice were converted into APC and prolonged survival time. This vaccine was shown to lead to antitumour immunity, as illustrated by generation of CTL and NK responses (Ko et al. 2009). This suggests that not only cytokine milieu alone (Table 1) but also NKT cells can deliver signals to MDSC, thereby changing their function from immunosuppressive into immunostimulatory (Figure 1).
Table 1.
Cytokines that drive differentiation of CD11b+ Gr-1+ cells into immunostimulatory cells in vitro
| Cytokines | Phenotype after differentiation | Refs |
|---|---|---|
| IFN-γ + TNF-α | CD11c−CD86+ MHC II+ | Bronte et al. 2000 |
| IL-12 | CD11c−CD86+ MHC II− | Bronte et al. 2000 |
| GM-CSF + IL-4 | CD11c+ MHC II+ | Caquard et al. 2010 |
| IFN-γ + IL-12 + GM-CSF + IL-3 | CD11c+MHC I+ MHC II+ | Narita et al. 2009 |
Figure 1.
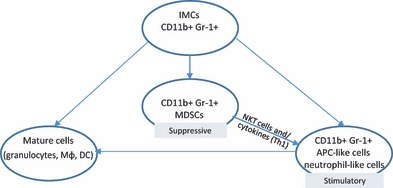
Simplified model for possible routes of differentiation of myeloid CD11b+Gr-1+ cells. (IMCs, immature myeloid cells; MDSCs, myeloid-derived suppressor cells; Mϕ, macrophages; DC, dendritic cells; APC, antigen-presenting cells).
Myeloid cells with markers characteristic for MDSC were also found in the ascites of epithelial ovarian carcinoma in mice. However, these cells lack suppressive activity and resemble neutrophils, so they cannot be regarded as MDSC. Surprisingly, they are highly phagocytic and cross-prime Ag-specific T cells in vivo. Adoptive transfer of these cells delayed tumour development (Tomihara et al. 2010). Antitumour activity of CD11b+Gr-1+ neutrophils was also described in another study, where they were shown to infiltrate tumour site after TGF-β blockade (Fridlender et al. 2009). It is not known whether CD11b+Gr-1+ neutrophils could differentiate from MDSC.
Conditions for the differentiation of immune cells towards antitumour effector cells can be created during infection with microorganisms. The beneficial properties of bacterial infection in patients with cancer were already observed in the 19th century by William Coley as quoted in (McCarthy 2006) and constitute the basis for Salmonella-based cancer immunotherapies. These have shown to be very promising with impressive responses in preclinical trials (Pawelek et al. 1997; Avogadri et al. 2005; Bereta et al. 2007; Chen et al. 2009). Interestingly, it was observed that CD11b+Gr-1+ cells infiltrate a tumour tissue in tumour-bearing mice injected with Salmonella. The presence of CD11b+Gr-1+ cells at tumour site was correlated with the areas of tumour necrosis (Avogadri et al. 2005). However, the exact role of these cells has not been investigated, and it is not known whether these CD11b+Gr-1+ cells can be regarded as MDSC or MDSC-derived cells.
Concluding remarks
Taking together the data, despite the widely accepted immunosuppressive capacities of MDSC, a new function has emerged which is immune stimulation and antitumour activity. Why can MDSC be both immunosuppressive and immunostimulatory? Factors which should be responsible for their contradictory functions include the known dual role of NO, the cytokine milieu, interaction with NKT cells and the tumour microenvironment. Furthermore, we should also include heterogeneity and the presence of cells at different stages of maturation within a population of MDSC. This raises a possibility that under diverse conditions different subsets of MDSC can be expanded. We can expect that MDSC accumulating during pathologic situations can involve not only cells with phenotypic but also cells with functional diversity. It is known that some subpopulations of MDSC are more suppressive than others (e.g. the monocytic fraction of MDSC is generally more suppressive than the granulocytic one) and some do not show suppressive activity at all (e.g. the eosinophil subpopulation) (Ribechini et al. 2010). Examples introduced earlier do not indicate one specific subpopulation of MDSC that could exhibit stimulatory activity. Myeloid CD11b+Gr-1+cells phenotypically resembling both inflammatory monocytes and granulocytes were demonstrated to have this capacity.
There are still many open questions and challenges in research on MDSC. Lack of specificity of markers, (CD11b and Gr-1), and heterogeneity make this population of cells complex and difficult to analyse. The precise conditions for stimulatory activity of MDSC in vivo are not known. The exact role of NKT cells on MDSC is not clear, and future experiments should provide answers. Finally, the stimulatory abilities of MDSC have not been yet detected in humans.
This review presents our view, supported by some evidence, that MDSC can be successfully converted into stimulatory cells even if they were initially inhibitory (Figure 1). This is in contrast to another known population of suppressive cells, the regulatory T cells (T reg). Further detailed investigation into the phenomenon of dual differentiation ability of MDSC may reveal novel strategies for overcoming immunosuppression (which is detrimental in some pathological situations, e.g. in cancer) this would be without the need for elimination of suppressive cells, instead, it would involve redirecting them into beneficial cells.
Acknowledgments
The authors thank Prof. Gunter Hammerling from DKFZ, Heidelberg, for critical reading the manuscript and valuable advices. We also thank Dr Rafał Biedroń and Dr Maria Ptak (Jagiellonian University College of Medicine) for general assistance. This work was supported by the grant of Jagiellonian University College of Medicine: No K/ZBW/000549.
References
- Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- Angulo I, de las Heras FG, García-Bustos JF, Gargallo D, Muñoz-Fernández MA, Fresno M. Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood. 2000a;95:212–220. [PubMed] [Google Scholar]
- Angulo I, Rullas J, Campillo JA, et al. Early myeloid cells are high producers of nitric oxide upon CD40 plus IFN-gamma stimulation through a mechanism dependent on endogenous TNF-alpha and IL-1alpha. Eur. J. Immunol. 2000b;30:1263–1271. doi: 10.1002/(SICI)1521-4141(200005)30:5<1263::AID-IMMU1263>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Avogadri F, Martinoli C, Petrovska L, et al. Cancer immunotherapy based on killing of Salmonella-infected tumor cells. Cancer Res. 2005;65:3920–3927. doi: 10.1158/0008-5472.CAN-04-3002. [DOI] [PubMed] [Google Scholar]
- Bereta M, Hayhurst A, Gajda M, et al. Improving tumor targeting and therapeutic potential of Salmonella VNP20009 by displaying cell surface CEA-specific antibodies. Vaccine. 2007;25:4183–4192. doi: 10.1016/j.vaccine.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005;5:641–654. doi: 10.1038/nri1668. Review. [DOI] [PubMed] [Google Scholar]
- Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- Caquard M, Ferret-Bernard S, Haurogné K, et al. Diabetes acceleration by cyclophosphamide in the non-obese diabetic mouse is associated with differentiation of immunosuppressive monocytes into immunostimulatory cells. Immunol. Lett. 2010;129:85–93. doi: 10.1016/j.imlet.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Chen G, Wei DP, Jia LJ, et al. Oral delivery of tumor-targeting Salmonella exhibits promising therapeutic efficacy and low toxicity. Cancer Sci. 2009;100:2437–2443. doi: 10.1111/j.1349-7006.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps JG, Wang J, Maria A, Blumenthal I, Gorham JD. Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology. 2010;52:1350–1359. doi: 10.1002/hep.23841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Anderson AC, Karman J, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. J. Immunol. 2010;185:1383–1392. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santo C, Salio M, Masri SH, et al. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J. Clin. Invest. 2008;118:4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezernitchi AV, Vaknin I, Cohen-Daniel L, et al. TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J. Immunol. 2006;177:4763–4772. doi: 10.4049/jimmunol.177.7.4763. [DOI] [PubMed] [Google Scholar]
- Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-García L, López-Marín LM, Saavedra R, Reyes JL, Rodríguez-Sosa M, Terrazas LI. Intact glycans from cestode antigens are involved in innate activation of myeloid suppressor cells. Parasite Immunol. 2005;27:395–405. doi: 10.1111/j.1365-3024.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- Goñi O, Alcaide P, Fresno M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+)immature myeloid suppressor cells. Int. Immunol. 2002;14:1125–1134. doi: 10.1093/intimm/dxf076. [DOI] [PubMed] [Google Scholar]
- Greifenberg V, Ribechini E, Rössner S, Lutz MB. Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur. J. Immunol. 2009;39:2865–2876. doi: 10.1002/eji.200939486. [DOI] [PubMed] [Google Scholar]
- Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- Hoechst B, Voigtlaender T, Ormandy L, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holda JH, Maier T, Claman HN. Murine graft-versus-host disease across minor barriers: immunosuppressive aspects of natural suppressor cells. Immunol. Rev. 1985;88:87–105. doi: 10.1111/j.1600-065x.1985.tb01154.x. Review. [DOI] [PubMed] [Google Scholar]
- Hu CE, Gan J, Zhang RD, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand. J. Gastroenterol. 2010 doi: 10.3109/00365521.2010.516450. in press. [DOI] [PubMed] [Google Scholar]
- Huang B, Pan PY, Li Q, et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Furuichi K, Kitagawa K, et al. Involvement of CD11b(+) GR-1 (low) cells in autoimmune disorder in MRL-Fas (lpr) mouse. Clin. Exp. Nephrol. 2010;14:411–417. doi: 10.1007/s10157-010-0309-9. [DOI] [PubMed] [Google Scholar]
- Jordan MB, Mills DM, Kappler J, Marrack P, Cambier JC. Promotion of B cell immune responses via an alum-induced myeloid cell population. Science. 2004;304:1808–1810. doi: 10.1126/science.1089926. [DOI] [PubMed] [Google Scholar]
- Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J. Immunol. 2009;182:1818–1828. doi: 10.4049/jimmunol.0802430. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 2009;182:240–249. doi: 10.4049/jimmunol.182.1.240. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu S, Kappes J, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Wang YM, Wang CL, et al. Population alterations of L: – arginase – and inducible nitric oxide synthase-expressed CD11b(+)/CD14 (-)/CD15 (+)/CD33 (+) myeloid-derived suppressor cells and CD8 (+) T lymphocytes in patients with advanced-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2009;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J. Immunol. 2006;176:2085–2094. doi: 10.4049/jimmunol.176.4.2085. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. Review. [DOI] [PubMed] [Google Scholar]
- Mazzoni A, Bronte V, Visintin A, et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- McCarthy EF. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006;26:154–158. [PMC free article] [PubMed] [Google Scholar]
- Mocellin S, Bronte V, Nitti D. Nitric oxide, a double edged sword in cancer biology: searching for therapeutic opportunities. Med. Res. Rev. 2007;27:317–352. doi: 10.1002/med.20092. Review. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Gabrilovich DI. Tumor escape mechanism governed by myeloid-derived suppressor cells. Cancer Res. 2008;68:2561–2563. doi: 10.1158/0008-5472.CAN-07-6229. Review. [DOI] [PubMed] [Google Scholar]
- Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J. Immunol. 2010;184:3106–3116. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita Y, Wakita D, Ohkur T, Chamoto K, Nishimura T. Potential differentiation of tumor bearing mouse CD11b+Gr-1+ immature myeloid cells into both suppressor macrophages and immunostimulatory dendritic cells. Biomed. Res. 2009;30:7–15. doi: 10.2220/biomedres.30.7. [DOI] [PubMed] [Google Scholar]
- Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak AS, Ip G, Wright MA, Young MR. Treating tumor-bearing mice with low-dose gamma-interferon plus tumor necrosis factor alpha to diminish immune suppressive granulocyte-macrophage progenitor cells increases responsiveness to interleukin 2 immunotherapy. Cancer Res. 1995;55:885–890. [PubMed] [Google Scholar]
- Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- Peláez B, Campillo JA, López-Asenjo JA, Subiza JL. Cyclophosphamide induces the development of early myeloid cells suppressing tumor cell growth by a nitric oxide-dependent mechanism. J. Immunol. 2001;166:6608–6615. doi: 10.4049/jimmunol.166.11.6608. [DOI] [PubMed] [Google Scholar]
- Ribechini E, Greifenberg V, Sandwick S, Lutz MB. Subsets, expansion and activation of myeloid-derived suppressor cells. Med. Microbiol. Immunol. 2010;199:273–281. doi: 10.1007/s00430-010-0151-4. [DOI] [PubMed] [Google Scholar]
- Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- Sottnik JL, U'Ren LW, Thamm DH, Withrow SJ, Dow SW. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol. Immunother. 2010;59:367–378. doi: 10.1007/s00262-009-0755-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol. Immunother. 2008;57:1493–1504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J. Leukoc. Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober S. Natural suppressor (NS) cells, neonatal tolerance, and total lymphoid irradiation: exploring obscure relationships. Annu. Rev. Immunol. 1984;2:219–237. doi: 10.1146/annurev.iy.02.040184.001251. Review. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Inaba M, Ogata H, et al. Inhibition of tumor cell proliferation by natural suppressor cells present in murine bone marrow. Cancer Res. 1990;50:2582–2586. [PubMed] [Google Scholar]
- Talmadge JE. Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clin. Cancer Res. 2007;13:5243–5248. doi: 10.1158/1078-0432.CCR-07-0182. Review. [DOI] [PubMed] [Google Scholar]
- Terabe M, Matsui S, Park JM, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J. Exp. Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomihara K, Guo M, Shin T, et al. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b(+)Gr-1(+) cells. J. Immunol. 2010;184:6151–6160. doi: 10.4049/jimmunol.0903519. [DOI] [PubMed] [Google Scholar]
- Umansky V, Schirrmacher V. Nitric oxide-induced apoptosis in tumor cells. Adv. Cancer Res. 2001;82:107–131. doi: 10.1016/s0065-230x(01)82004-2. Review. [DOI] [PubMed] [Google Scholar]
- Wang H, MacNaughton WK. Overexpressed beta-catenin blocks nitric oxide-induced apoptosis in colonic cancer cells. Cancer Res. 2005;65:8604–8607. doi: 10.1158/0008-5472.CAN-05-1169. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Deguchi K, Zheng R, et al. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J. Immunol. 2008;181:3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- Zhu B, Bando Y, Xiao S, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 2007;179:5228–5237. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]


