Abstract
The aim of this study was to examine whether Nedd4L (neural precursor cell expressed, developmentally down-regulated 4-like) participated in gallbladder carcinogenesis. We first immunohistochemically examined the expression of Nedd4L in various gallbladder tissue specimens. Weak immunoreactivity to Nedd4L-specific antibody was observed in normal or dysplastic epithelial cells. Cancer cells in non-invasive regions exhibited little immunoreactivity, whereas strong immunostaining was found in cytoplasm of many invasive cancers, especially at cancer invasive front with desmoplastic reaction. Notably, siRNA-mediated silencing of the Nedd4L gene significantly decreased the Matrigel-invasion activity and collagen invasion activity of cultured gallbladder cancer cells, without affecting the cell growth. The subtractive mRNA hybridization followed by RT-PCR and immunoblotting revealed that down-regulation of Nedd4L significantly decreased the expression of collagenases, matrix metalloproteinase (MMP)-1 and -13, in gallbladder cancer cells. Finally, immunohistochemical staining showed that many Nedd4L-expressing invasive gallbladder cancer cells co-expressed MMP-1 and MMP-13. These results indicated that over-expression of Nedd4L might lead to gallbladder cancer invasion by regulating the transcription of the MMP-1 and MMP-13 genes.
Keywords: cancer invasion, gallbladder cancer, metalloproteinase-1, metalloproteinase-13, Nedd4L
Gallbladder cancer is an aggressive cancer, often with high invasive activity, and therefore difficult to cure by conventional treatments. It is well known that the prognosis of patients with gallbladder cancer depends on the extent of surrounding tissue invasion of cancer cells. Despite the recent advances in cancer treatment, gallbladder cancer continues to carry a poor prognosis with the overall survival rate being less than 10% (Gourgiotis et al. 2008). Therefore, new therapeutic approaches based on pathobiological features of gallbladder carcinogenesis are needed.
It is believed that genotoxic stress, in association with chronic inflammation, is one of the major aetiological factors in gallbladder cancer (Zatonski et al. 1997; Schottenfeld & Beebe-Dimmer 2006). Many studies have linked early steps in gallbladder carcinogenesis to genetic mutations of p53 (Takagi et al. 1994; Wee et al. 1994; Diamantis et al. 1995), mutational activation of the K-ras proto-oncogene (Malats et al. 1995) and loss of cell-cycle regulation by mutations in CDK-INK4A (Yoshida et al. 1995). By contrast, the molecular mechanisms that are responsible for the robust invasive activity of gallbladder cancer are still largely unclear. A few studies examined the role of invasion-associated matrix metalloproteinase (MMP)-2 in gallbladder carcinogenesis; however, their results were not consistent with each other (Fan et al. 2002; Wu et al. 2009).
Neural precursor cell expressed, developmentally down-regulated 4-like (Nedd4L) (a homologue of the mouse Nedd4–2) is a HECT (homologous to E6-AP carboxyl terminus)-family ubiquitin ligase (Kamynina et al. 2001). It is well known that Nedd4L targets ENaC (epithelial Na+ channel) for proteasome degradation (Snyder et al. 2002). A mutation in the Nedd4L interacting region of ENaC increases the expression of ENaC on the cell membrane surface to cause an inherited form of hypertension, Liddle's syndrome (Schild et al. 1996; Staub et al.1996). Interestingly, recent studies have revealed that Nedd4L targets a broad range of molecules along with ENaC, and is thus thought to be involved in various biological properties other than regulating ion channels (Persaud et al. 2009; see review Yang & Kumar 2010). Nedd4, a ubiquitin ligase closely related to Nedd4L, is ubiquitously expressed in a broad range of tissues. By contrast, Nedd4L expression is restricted to the heart, brain, liver, kidney, and to a lesser extent to the lung (Kamynina et al. 2001). Interestingly, various cancer cell cultures expressed abundant Nedd4L (Chen & Matesic 2007); thus, Nedd4L might have an oncogenic property.
In the present study, we attempted to reveal the expression status of Nedd4L in gallbladder cancer cells, and subsequently identified the pathobiological property of Nedd4L in gallbladder carcinogenesis. The findings indicate that Nedd4L is over-expressed in many invasive gallbladder cancers, and may regulate the transcription of collagenase genes through its oncogenic properties. We believe that Nedd4L could be a novel target molecule for abrogating the invasion of gallbladder cancer.
Materials and methods
Tissue specimens, antibodies and immunohistochemical staining
Archival pathological tissue specimens used in this study have been described previously (Adachi et al. 2009). Briefly, 30 gallbladder carcinomas (age 54–76 years, average age 64.6 years; 24 women and six men) comprised advanced cases of gallbladder cancer [pT2 10, pT3 12, pT4 8; TNM staging according to the staging system of the American Joint Committee on Cancer (AJCC) was used]. Of these, eight cases (26.7%) were classified as well differentiated, 18 cases (60%) as moderately differentiated, and four cases (13.3%) as poorly differentiated adenocarcinomas. Ten gallbladder cholelithiasis tissues, five of which exhibited an incidental finding of dysplastic epithelia, were also examined in this study. All gallbladder tissue specimens were surgically obtained, fixed in 10% buffered formalin and paraffin-embedded.
A rabbit antibody specific to Nedd4L was purchased from ProteinTech Group Inc. (Chicago, IL, USA). Rabbit and mouse antibodies to MMP-1 and MMP-13, respectively, were purchased from Thermo Fisher Scientific Inc. (Fremont, CA, USA). Normal rabbit and mouse IgGs were prepared in our laboratory.
The procedures used for immunohistochemical staining were as described previously (Takeuchi et al. 2000). Briefly, deparaffinized sections were autoclaved for 15 min with 10 mm of citrate buffer (pH 6.0). Endogenous peroxidase was blocked by 0.3% hydrogen peroxide in methanol for 20 min. Next, the slides were incubated for 30 min in normal goat serum. The tissues were then immunostained with antibodies using ImmPRESS™, a polymerized reporter enzyme staining system (Vector laboratories Inc., Burlingame, CA, USA). The procedures were performed according to the manufacturer's protocol. Staining of more than 10% of the cells was considered as a positive result.
In several experiments, antibody to Nedd4L was preadsorbed with 293FT cell lysates, which were transfected with an expression vector containing Nedd4L cDNA or with empty vectors as described below. To verify the specificity of anti-Nedd4L antibody, we also performed Western blotting as described below.
Cell culture
NOZ (Horiuchi et al. 2003; Tekant et al. 2005) and OCUG-1 (Tekant et al. 2005) gallbladder cancer cells were obtained from the Japan Health Science Research Resources Bank (Osaka, Japan). TGBC1TKB (Tan et al. 2010) was obtained from RIKEN Cell Bank (Tsukuba, Japan). 293FT cells (Invitrogen, Carlsbad, CA, USA) were maintained in our laboratory. Cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) (Gibco Life Technologies, Grand Island, NY, USA) containing 10% heat-inactivated foetal bovine serum (FBS) and 50 μg/ml gentamycin (Gibco RL-Life Technologies).
Plasmids and transfection
The entire coding sequence of the human Nedd4L cDNA was amplified by PCR using the sense primer, 5′-GAAGATCTCATGGCGACCGGGCTCGGGGAG-3′ (BglII site underlined), and the antisense primer, 5′-GCTCTAGAATCCACCCCTTCAAATCCTTG-3′ (XbaI site underlined) from placenta cDNAs (Clontech, Palo Alto, CA, USA). After digestion with BglII and XbaI, the PCR product was subcloned into the p3xFLAG-CMV-24 expression vector (Sigma) at the BglII and XbaI restriction sites to generate the N-terminal FLAG- and C-terminal c-myc-tagged Nedd4L expression, and verified by sequencing. 293FT cells were transfected with the expression vector or empty vector using DOTAP transfection reagents (Boehringer Mannheim GmbH, Mannheim, Germany) according to the manufacturer's protocol and harvested after 48 h.
Western blotting
Western blotting was carried out according to the modified method of Towbin et al. (1979), as previously reported (Takeuchi et al. 2006). Briefly, equal amounts of proteins were electrophoresed on sodium dodecyl sulphate-polyacrylamide gels and electroblotted onto a polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA, USA). After blocking with bovine serum albumin, the membranes were incubated with antibodies. Western blotting chemiluminescence detection kit (Promega, Madison, WI, USA) was used for the detection of immunoreactivity.
siRNA-mediated RNA interference
The detailed procedure for siRNA-silencing of the target gene was performed as described previously (Adachi et al. 2009). In this study, we employed the siRNA duplex 5′-AACCACAACACAAAGUCACAG-3′ according to the method by Snyder et al. (2004). This sequence corresponds to nucleotides 1103–1125 of the human Nedd4L gene. We also used the GFP-siRNA duplex, target sequence 5′-CGGCAAGCUGACCCUGAAGUUCAU-3′, as a non-silencing control. siRNAs were transfected into gallbladder cancer cells using lipofectamine™ RNAiMAX according to the manufacturer's instructions (Invitrogen). At 48 h post-transfection, the cells were used for subsequent studies.
Cell proliferation, Matrigel invasion and collagen-gel invasion assay
Cell proliferation was evaluated by counting the number of viable cells, as previously described (Takeuchi et al. 2006). Briefly, 1 × 105 cells were cultured on standard 60-mm tissue culture dishes (BD Falcon, San Jose, CA, USA) in triplicate. After 24 and 48 h, live cells were counted.
Matrigel-invasion activity was evaluated using six-well BD BioCoat Matrigel Invasion Chamber plates (BD Biosciences, CA) according to the manufacturer's protocol. The detailed procedure has been described previously (Takeuchi et al. 2006). Briefly, 1 × 105 cells were placed in the upper compartment of an invasion chamber. After 24 h of incubation with DMEM containing 10% (lower chamber) or 0% (upper chamber) FCS, the cells on the lower surface of the filter were counted.
Collagen-gel-based cell invasion assay was carried out using collagen solution (Nitta Gelatin, Osaka, Japan) as described previously (Adachi et al. 2009). Briefly, 100 μl of collagen gel was set within a 6.5-mm diameter and 8-μm pore filter transwell (Corning Costar, Corning, NY, USA). Subsequently, 2 × 104 cells were plated in 100 μl of serum-free DMEM and 1 ml of DMEM supplemented with 10% FBS, and recombinant hepatocyte growth factor (final concentration 20 μg/ml) was applied added underneath the filter. After 48 h, the cells were collected from beneath the well plates.
These assays were carried out in triplicate and repeated twice. Statistical analysis was performed by Student's t-test for unpaired observations. Findings of P < 0.01 were considered significant.
Subtractive hybridization
The PCR-SelectTM cDNA subtraction assay (Takara Bio Inc., Otsu, Japan) was performed with the cDNA samples from Nedd4L-down-regulated and control TGBC1TKB cells according to the manufacturer's protocols as described before (Adachi et al. 2010). Briefly, poly(A) + RNA was extracted from Nedd4L-specific siRNA-treated and control GFP-siRNA-treated TGBC1TKB cells using RNAzol (Invitrogen) and the Oligo-dT mRNA purification kit (Takara Bio Inc.). Poly(A) + RNAs were converted to first-stranded cDNAs by incubation with reverse transcriptase and cDNA synthesis primer, 5′-TTTTGTACAAGCTT30 N1N-3′ (The RsaI cutting region is underlined). Subsequently, double-stranded cDNA (ds cDNA) was synthesized and digested to blunt-ended fragments by treatment with RsaI. Only ds cDNA from control GFP-siRNA-treated Nedd4L-expressing cells was ligated with oligonucleotides: 5′-CTAATACGACTCACTATAGGGCTCGAGCGGCCGCCCGGGCAGGT-3′ (adapter1) or 5′-CTAATACGACTCACTATAGGGCAGCGTGGTCGCGGCCGAGGT-3′ (adapter2R). For the first hybridization step, the respective adapter-ligated cDNA was hybridized with an excessive amount of cDNA from Nedd4L-siRNA-treated cells. After the second hybridization, in combination with each of the solutions obtained from the first hybridization step, cDNAs obtained from control GFP-siRNA-treated cells were amplified by PCR using the 1st primer, 5′-CTAATACGACTCACTATAGGGC-3′, 2nd nested primer1, 5′-TCGAGCGGCCGCCCGGGCAGGT-3′, and 2nd nested primer2, 5′-AGCGTGGTCGCGGCCGAGGT-3′. These cDNA fragments, which were predominantly expressed in Nedd4L-expressing cells, were subcloned into the pMD20-T vector (Takara Bio Inc.), transfected into JM109 cells (Promega), and their sequences were determined using the ABI 310 auto-sequencer (Perkin-Elmer, Waltham, MA, USA). Nucleotide sequences were examined using the NCBI database (http://www.ncbi.nlm.nih.gov/).
Reverse transcriptase-polymerase chain reaction (RT-PCR)
RT-PCR was performed as previously described (Takeuchi et al. 2006). Briefly, total cellular RNA was prepared from cell lysates using RNA-zol B (Biotex Laboratory, Houston, TX, USA). cDNA synthesis from total RNA and subsequent PCR were performed using an RNA LA (long and accurate) PCR kit (Takara Bio Inc.). The procedure was performed according to the manufacturer's protocol. The primer sets used in this study were sense 5′-CAGTGGAGATTTGTGAACAGGG-3′ and antisense 5′-CTAGAATCCACCCCTTCAAATCCTTG-3′ for Nedd4L, and sense 5′-ACCACAGTCCATGCCATCAC-3′ and antisense 5′-TCCACCACCCTGTTGCTGTA-3′ for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primer sets used to detect MMPs are summarized in Table 1. The PCR-amplified products were electrophoretically separated on 2% agarose gel.
Table 1.
PCR primer sets to detect MMP molecules
| mRNA target | Primers | Product length |
|---|---|---|
| MMP-1 | Forward: 5′-CGACTCTAGAAACACAAGAGCAAGA-3′ | 787 bp |
| Reverse: 5′-AAGGTTAGCTTACTGTCACACGCTT-3′ | ||
| MMP-2 | Forward: 5′-CAGCCTGGGACTGCCCCCTGAT-3′ | 400 bp |
| Reverse: 5′-CAGGCCCCTCCGGGTCCTTCTC-3′ | ||
| MMP-3 | Forward: 5′-GAACAATGGACAAAGGATACAACA-3′ | 719 bp |
| Reverse: 5′-TTCTTCAAAAACAGCATCAATCTT-3′ | ||
| MMP-9 | Forward: 5′-GCATCGCGGAGATTGGGAACC-3′ | 398 bp |
| Reverse: 5′-CGGGGAACATCCGGTCCACCTC-3′ | ||
| MMP-10 | Forward: 5′-ACTCTACAACTCATTCACAGAGCT-3′ | 408 bp |
| Reverse: 5′-CTTGGATAACCTGCTTGTACCTCAT-3′ | ||
| MMP-11 | Forward: 5′-GAGCAGGTGCGGCAGACGA-3′ | 350 bp |
| Reverse: 5′-CGAAAGGTGTAGAAGGCGGACA-3′ | ||
| MMP-12 | Forward: 5′-TTTGTTCCTCACTGCTGTTCAC-3′ | 780 bp |
| Reverse: 5′-CTAACAACCAAACCAGCTATTGC-3′ | ||
| MMP-13 | Forward: 5′-CATCACCATTCAAGATGCATCCAGGGGTC-3′ | 718 bp |
| Reverse: 5′-TCCTTGGAGTGGTCAAGACCTAA-3′ | ||
| MMP-19 | Forward: 5′-ACCATGAACTGCCAGCAGCTGTGGCTGGGC-3′ | 482 bp |
| Reverse: 5′-CCATGGAAGGAGAGGCGGATGTCAGCCGC-3′ | ||
| MMP-28 | Forward: 5′-CCAGCCCGCGGAGCGCGGA-3′ | 787 bp |
| Reverse: 5′-CCCATACAGGCTCTGCACGGCCAGC-3′ |
Results
Nedd4L was expressed in cultured gallbladder cancer cells and invasive gallbladder cancers
We started experiments by examining Nedd4L expression in cultured gallbladder cancer cells. RT-PCR assay showed that all the three cultured gallbladder cancer cells under study expressed Nedd4L mRNA.
Subsequently, we performed immunohistochemical examination of the expression of Nedd4L in archival pathological gallbladder tissue specimens, which comprised normal, regenerative, metaplastic, dysplastic and cancerous epithelial cells. The representative results are shown in Figure 1. Little or no immunoreactivity with anti-Nedd4L antibody was found in non-cancerous, regenerative, metaplastic or dysplastic epithelial cells. Little immunoreactivity was also observed in non-invasive foci of the gallbladder cancers examined. By contrast, strong immunoreactivity was found in invasive foci of 23 of the 30 gallbladder cancer tissue specimens. Notably, immunoreactivity of anti-Nedd4L was not ubiquitously observed in positive cases, but was focal and more strongly observed at the cancer invasive front with desmoplastic reaction.
Figure 1.

Representative immunohistochemical staining of various gallbladder tissues. Weak Nedd4L immunoreactivity was observed in non-cancerous epithelium (a) dysplastic epithelial cells (b) or non-invasive foci of gallbladder cancer (c, d). In contrast, invasive gallbladder cancer cells were focally immunostained with anti-Nedd4L antibody (e), but not with control preadsorbed antibody, as described in Materials and methods (f).
Anti-Nedd4L antibody detected a band corresponding to the molecular weight of Nedd4L protein on Western blots (Figure 2). Preadsorption of the antibody with lysates of Nedd4L-expressing 293FT cells entirely diminished the immunoreactivity; however, similar diminution of immunoreactivity was not observed in the case of 293FT cells not expressing Nedd4L. These findings verified the specificity of the present immunostaining procedure. No significant immunoreactivity was found with control rabbit IgGs.
Figure 2.

The Western blot demonstrating the specificity of the Nedd4L antibody used in this study. Expression vector, harbouring the entire coding region of human Nedd4L cDNA, or empty vector alone, was transfected into 293FT cells using the DOTAP reagent. After 48 h, the cells were harvested and used for Western blotting (lane 1, 3: empty vector alone; lane 2, 4: expression vector containing Nedd4L cDNA.) Note the Nedd4L band, which was detected with anti-Nedd4L (lane 4) and anti-FLAG (Sigma-Aldrich, St. Louis, MO, USA) antibodies (lane 2).
siRNA-mediated silencing of Nedd4L decreased invasive activity of cultured gallbladder cancer cells in Matrigel-invasion assay and collagen-gel-based assay, without affecting cell growth
We investigated whether the down-regulation of Nedd4L affected the proliferation of gallbladder cancer cells. Significant silencing of Nedd4L expression was observed in TGBC1TKB and OCUG-1 cells with the use of Nedd4L-specific siRNA, but not control siRNA (representative findings using TGBC1TKB cells are shown in Figure 3a). Interestingly, siRNA-silencing of Nedd4L decreased collagen-gel invasion activity and Matrigel-invasion activity of TGBC1TKB and OCUG-1 cells, without altering cell growth (representative findings using TGBC1TKB cells are shown in Figure 3b–d).
Figure 3.
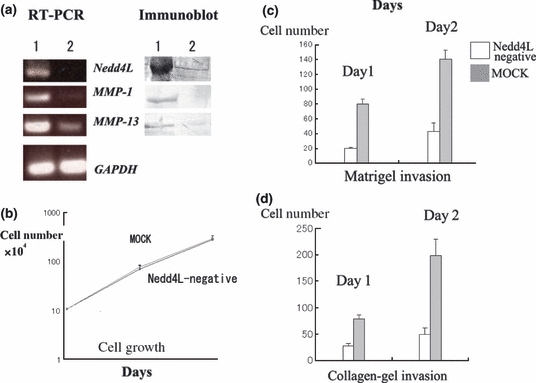
siRNA-silencing of Nedd4L decreased gallbladder cancer invasion without affecting cell growth. (a) Nedd4L was significantly downregulated with use of specific siRNA for Nedd4L (lane 2), but not control siRNA (lane 1) in TGBC1TKB cells (left: RT-PCR, right: Western blotting). (b) There was no significant difference in cell growth between Nedd4L-specific siRNA-treated and control TGBC1TKB cells (c) and (d): In contrast, Nedd4L-negative TGBC1TKB cells exhibited much weaker invasive activity than control cells (P < 0.01) on Matrigel (c) and collagen-gel invasion assay (d). Bars represent standard errors of the mean.
Combined with the results of immunohistochemical staining, it is plausible that Nedd4L is over-expressed in many gallbladder cancer cells in order to increase their invasive activity.
Down-regulation of Nedd4L decreased the transcripts of collagenases, MMP-1 and MMP-13 genes
Nedd4L is a ubiquitin ligase and mediates proteasome degradation of various molecules. Current metaproteome study isolated over 100 substrates of Nedd4L using proteome arrays (Persaud et al. 2009); however, we could not find any candidate substrate that directly links Nedd4L to cancer invasion.
Interestingly, it is also known that Nedd4 family molecules, including Nedd4L, potentiate activation of transcription, at least partially, in a manner independent of the ubiquitin-proteasome system (Imhof & McDonnell 1996; Kuratomi et al. 2005).
Therefore, we speculated that Nedd4L could modulate the transcription of cancer invasion-related genes. We performed a subtractive mRNA hybridization assay and found that the transcription of several genes was decreased by siRNA-mediated silencing of Nedd4L molecules in TGBC1TKB gallbladder cancer cells (Table 2). We noted that siRNA-mediated down-regulation of Nedd4L decreased the expression of collagenases, MMP-1 and MMP-13, the expression of which is important for cancer invasion.
Table 2.
mRNAs accumulating in Nedd4L-expressing vs. -negative TGBC1TKB cells
| A | Aliases | Known or putative function |
|---|---|---|
| 1. RPL9 | Ribosomal protein L9 | A component of the 60S subunit of ribosome |
| 2. RPL41 | Ribosomal protein L41 | A component of the 60S subunit of ribosome |
| 3. Aconitase 1 | IREB1* | Iron sensor |
| 4. PTMA | Prothymosin, alpha | Possible mediator of immune function |
| 5. S100A1 | S100 alpha | S100 family protein |
| 6. S100 A6 | Calcyclin | S100 family protein |
| 7. NFE2L1 | Nuclear factor (erythroid-derived 2)-like 1 | Transcription factor |
| 8. MBTPS1 | Membrane-bound transcription factor peptidase, site 1 | Regulation of lipid metabolism |
| 9. GORASP2 | Golgi reassembly stacking protein 2, 55 kDa | Assembly and membrane stacking of the Golgi cisternae |
| 10. MMP-1 | Matrix metalloproteinase 1 Collagenase 1 | Cleaves the interstitial collagens, types I, II and III. |
| 11. MMP-13 | Matrix metallopeptidase 13 Collagenase 3 | Cleaves type II collagen more efficiently than types I and III |
Iron regulatory element binding protein 1
By virtue of RT-PCR, we confirmed that down-regulation of Nedd4L decreased the transcripts of collagenase, MMP-1 and -13 genes in TGBC1TKB and OCUG-1 cells (Figure 3a). Western blotting experiments further confirmed that down-regulation of Nedd4L decreased the amounts of MMP-1 and -13 molecules secreted in culture supernatants of gallbladder cancer cells (Figure 3a).
These findings indicate that down-regulation of Nedd4L could suppress cancer invasion through down-regulating the transcription of MMP-1 and -13 genes in cultured gallbladder cancer cells.
Expression of MMP-1 and MMP-13 in gallbladder cancer tissues
We could not find any reports that investigated the MMP-1 and/or MMP-13 expression in gallbladder tissues or cancers. Hence, we studied the expression of MMP-1 or MMP-13 in gallbladder cancer tissues, especially focusing on its relationship with Nedd4L expression. As demonstrated in Figure 4, MMP-1 and MMP-13 were strongly expressed in gallbladder cancer cells, especially at the invasive front, where cancer cells over-expressed Nedd4L. Matrix metalloproteinase-1 and MMP-3 were strongly expressed in invasive foci of 18 and 16 out of 30 gallbladder cancer cases respectively. Notably, all of the MMP-1 and/or MMP-13 expressing invasive gallbladder cancer tissue specimens were also stained for Nedd4L at cancer invasion foci. By contrast, MMP-1 or MMP-13 was weakly expressed in non-cancerous gallbladder epithelial cells, or non-invasive gallbladder cancer cells, which also showed low expression of Nedd4L.
Figure 4.
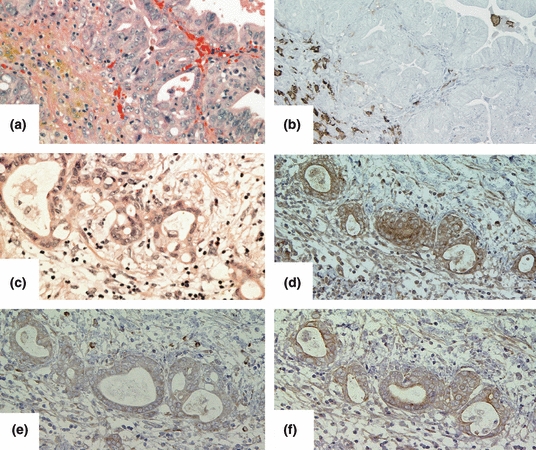
Correlation of expression of Nedd4L, MMP-1 and MMP-13 in human tissues. Representative immunohistochemical staining of non-invasive gallbladder cancer foci (a: HE staining) with anti-MMP-1 antibody (b). Note the staining in infiltrating stromal cells, including macrophage, whereas non-invasive gallbladder cancer cells did not exhibit staining. Invasive gallbladder cancer cells (c: HE staining) were stained with anti-Nedd4L (d), anti-MMP-1 (e), and anti-MMP-13 antibodies (f). Note that Nedd4L-expressing cancer cells expressed both MMP-1 and MMP-13.
These results indicated that Nedd4L expression can be correlated with MMP-1 and MMP-13 expression in gallbladder cancer tissues.
Discussion
The data presented in this article indicate that Nedd4L is over-expressed in many gallbladder cancers and may promote invasion of gallbladder cancer through the upregulation of MMP-1 and MMP-13 expression. We also investigated whether transcription of any other MMPs genes was altered by down-regulation of Nedd4L in gallbladder cancer cells; however, no significant alteration of mRNA expression in other MMPs was detected by RT-PCR using the primer sets listed in Table 1. Matrix metalloproteinase-1 and MMP-13 have a unique property to cleave the triple helix of collagen; as a result, the polypeptide chains become susceptible to further degradation by other MMPs. In normal human tissues, the gene expression of MMP-1 and MMP-13 is tightly regulated by transcription factors (see review Vincenti & Brinckerhoff 2002), whereas MMP-1 and MMP-13 are over-expressed in various malignant tumours at an early step of tumour invasion (Westermarck & Kähäri 1999; Ala-aho & Kähäri 2005). To our knowledge, this is the first demonstration of Nedd4L-mediated regulation of MMP-1 and MMP-13 expression at the transcriptional level.
The precise molecular mechanism by which Nedd4L increased the expression of MMP-1 and MMP-13 is currently unknown. In general, the expression of majority of the MMPs is normally low in tissues and is induced when remodelling of extracellular matrix (ECM) is required. Matrix metalloproteinase-1 and MMP-13 are also inducible in response to growth factors, cytokines, and contact with ECM. Expression of MMP-1 and MMP-13 is increased by stimuli through phosphorylation events of mitogen-activated protein kinase (MAPK) group molecules of the signal transduction pathway (Vincenti & Brinckerhoff 2002). A recent metaproteome analysis (Persaud et al. 2009) shows that Nedd4L targets many mitogen-activated protein kinase group molecules, i.e. mitogen-activated protein kinase-activated protein kinase (MAPKAPK) 3, MAPKAPK 5, mitogen-activated protein kinase kinase kinase (MAP3K) 3, and MAP3K8. It could be possible that down-regulation of Nedd4L leads to the repression of MMP-1 and MMP-13 through MAPK pathways that are not yet characterized.
Apart from cancer pathobiology, MMP-1 and MMP-13 gene expression has been a prime target for the development of a new arthritis therapy. This is because MMP-1 and MMP-13 degrade type II collagen in the cartilage, thus participating in the progression of rheumatoid arthritis and osteoarthritis (Vincenti & Brinckerhoff 2002). Whether silencing of Nedd4L expression could decrease MMP-1 and MMP-13 expression in synoviocyte is currently being explored.
In conclusion, this study highlights Nedd4L as an invasion-associated molecule in gallbladder cancer. We speculate that a hitherto uncharacterized molecular pathway, which links Nedd4L to MMP-1 and MMP-13, could be a molecular target for developing a new therapeutic approach for patients with gallbladder cancer and can be possibly used in patients with arthritis.
References
- Adachi Y, Takeuchi T, Nagayama T, Ohtsuki Y, Furihata M. Zeb1-mediated T-cadherin repression increases the invasive potential of gallbladder cancer. FEBS Lett. 2009;583:430–436. doi: 10.1016/j.febslet.2008.12.042. [DOI] [PubMed] [Google Scholar]
- Adachi Y, Takeuchi T, Nagayama T, Furihata M. T-cadherin modulates tumor-associated molecules in gallbladder cancer cells. Cancer Invest. 2010;28:120–126. doi: 10.3109/07357900903124472. [DOI] [PubMed] [Google Scholar]
- Ala-aho R, Kähäri VM. Collagenases in cancer. Biochimie. 2005;87:273–286. doi: 10.1016/j.biochi.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Chen C, Matesic LE. The Nedd4-like family of E3 ubiquitin ligases and cancer. Cancer Metastasis Rev. 2007;26:587–604. doi: 10.1007/s10555-007-9091-x. [DOI] [PubMed] [Google Scholar]
- Diamantis I, Karamitopoulou E, Perentes E, Zimmermann A. p53 protein immunoreactivity in extrahepatic bile duct and gallbladder cancer: correlation with tumor grade and survival. Hepatology. 1995;22:774–779. [PubMed] [Google Scholar]
- Fan YZ, Zhang JT, Yang HC, Yang YQ. Expression of MMP-2, TIMP-2 protein and the ratio of MMP-2/TIMP-2 in gallbladder carcinoma and their significance. World J. Gastroenterol. 2002;8:1138–1143. doi: 10.3748/wjg.v8.i6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourgiotis S, Kocher HM, Solaini L, Yarollahi LA, Tsiambas E, Salemis NS. Gallbladder cancer. Am. J. Surg. 2008;196:252–264. doi: 10.1016/j.amjsurg.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Kawamata H, Fujimori T, Kuroda Y. A MEK inhibitor (U0126) prolongs survival in nude mice bearing human gallbladder cancer cells with K-ras mutation: analysis in a novel orthotopic inoculation model. Int. J. Oncol. 2003;23:957–963. [PubMed] [Google Scholar]
- Imhof MO, McDonnell DP. Yeast RSP5 and its human homolog hRPF1 potentiate hormone-dependent activation of transcription by human progesterone and glucocorticoid receptors. Mol. Cell. Biol. 1996;16:2594–2605. doi: 10.1128/mcb.16.6.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamynina E, Dedonneville C, Dens M, Vandewalle A, Staub O. A novel mouse Nedd4 protein suppresses the activity of the epithelial Na+ channel. FASEB J. 2001;15:204–214. doi: 10.1096/fj.00-0191com. [DOI] [PubMed] [Google Scholar]
- Kuratomi G, Komuro A, Goto K, et al. Neural precursor cell expressed, developmentally down-regulated 4-2 (NEDD4-2) negatively regulates transforming growth factor-β (TGF-β) signaling by inducing ubiquitin-mediated degradation of Smad2 and TGF-β type I receptor. Biochem. J. 2005;386:461–470. doi: 10.1042/BJ20040738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malats N, Porta M, Pinol JL, Corominas JM, Rifa J, Real FX. Ki-ras mutations as a prognostic factor in extrahepatic bile system cancer PANK-ras Project Investigators. J. Clin. Oncol. 1995;13:1679–1686. doi: 10.1200/JCO.1995.13.7.1679. [DOI] [PubMed] [Google Scholar]
- Persaud A, Alberts P, Amsen EM, et al. Comparison of substrate specificity of the ubiquitin ligases Nedd4 and Nedd4-2 using proteome arrays. Mol. Syst. Biol. 2009;5 doi: 10.1038/msb.2009.85. art. No. 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC. Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach. Proc. Natl Acad. Sci. USA. 1996;93:15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J. Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel L. J. Biol. Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- Snyder PM, Steines JC, Olson DR. Relative contribution of Nedd4 and Nedd4-2 to ENaC regulation in epithelia determined by RNA interference. J. Biol. Chem. 2004;279:5042–5046. doi: 10.1074/jbc.M312477200. [DOI] [PubMed] [Google Scholar]
- Staub O, Dho S, Henry PC, et al. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle's syndrome. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Naito E, Yamanouchi H, Ohtsuka H, Kominami R, Yamamoto M. Mutation of the p53 gene in gallbladder cancer. Tohoku J. Exp. Med. 1994;172:283–289. doi: 10.1620/tjem.172.283. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Misaki A, Liang SB, et al. Expression of T-cadherin (CDH13, H-cadherin) in human brain and its characteristics as a negative growth regulator of EGF in neuroblastoma cells. J. Neurochem. 2000;74:1489–1497. doi: 10.1046/j.1471-4159.2000.0741489.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Adachi Y, Sonobe H, Furihata M, Ohtsuki Y. A ubiquitin ligase, skeletrophin, is a negative regulator of melanoma invasion. Oncogene. 2006;25:7059–7069. doi: 10.1038/sj.onc.1209688. [DOI] [PubMed] [Google Scholar]
- Tan FL, Ooi A, Huang D, et al. p38delta/MAPK13 as a diagnostic marker for cholangiocarcinoma and its involvement in cell motility and invasion. Int. J. Cancer. 2010;126:2353–2361. doi: 10.1002/ijc.24944. [DOI] [PubMed] [Google Scholar]
- Tekant Y, Davydova J, Ramirez PJ, Curiel DT, Vickers SM, Yamamoto M. Oncolytic adenoviral therapy in gallbladder carcinoma. Surgery. 2005;137:527–535. doi: 10.1016/j.surg.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–164. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee A, The M, Raju GC. Clinical importance of p53 protein in gallbladder carcinoma and its precursor lesions. J. Clin. Pathol. 1994;47:453–456. doi: 10.1136/jcp.47.5.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- Wu W, Wang R, Liu H, et al. Prediction of prognosis in gallbladder carcinoma by CD147 and MMP-2 immunohistochemistry. Med. Oncol. 2009;26:117–123. doi: 10.1007/s12032-008-9087-6. [DOI] [PubMed] [Google Scholar]
- Yang B, Kumar S. Nedd4 and Nedd4-2: closely related ubiquitin-protein ligases with distinct physiological functions. Cell Death Differ. 2010;17:68–77. doi: 10.1038/cdd.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Todoroki T, Ichikawa Y, et al. Mutations of p16Ink4/CDKN2 and p15Ink4B/MTS2 genes in biliary tract cancers. Cancer Res. 1995;55:2756–2760. [PubMed] [Google Scholar]
- Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case–control study of the SEARCH Program of the International Agency for Research on Cancer. J. Natl Cancer Inst. 1997;89:1132–1138. doi: 10.1093/jnci/89.15.1132. [DOI] [PubMed] [Google Scholar]


