Abstract
The cancer-initiating capacity of most malignant tumours is considered to reside in a small subpopulation of cells. Therapeutical interventions should target these cells rather than the tumour mass. Numerous studies have shown that the carbohydrate antigen structure CD176 (Thomsen-Friedenreich antigen, core-1) is present in many types of cancer and absent in normal adult human tissues. In this study, we assessed whether CD176 is co-expressed with CD44 or CD133 [markers of cancer-initiating cells (CIC)] in human lung, breast and liver carcinoma. A variety of human cancer cell lines and surgical specimens of these malignancies were examined. It was found that in most cases the majority of tumour cells stained strongly for CD44 by immunohistochemistry and flow cytometry, whereas CD133 expression was found on a smaller, but varying proportion of cells. Co-expression of CD176 with CD44 was found at a surprisingly high percentage of cancer cells in vitro and in vivo. Co-expression of CD176 with CD133 was also detected, although at a lower rate. Tamoxifen treatment of MDA-435 breast cancer cells enhanced the CD44+/CD176+ phenotype. Evidence is provided through a new sandwich solid-phase enzyme-linked immunosorbent assay (ELISA) suggesting that CD44 is a carrier molecule for CD176 not only in colorectal cancer as previously reported, but also in lung, breast and liver cancer. The expression of CD176 in CIC suggests that it may represent an effective target for tumour therapies.
Keywords: cancer-initiating cells, CD133, CD176, CD44, co-expression, Thomsen-Friedenreich antigen
The theory that cancer-initiating cells (CIC) are a prerequisite for cancer ontogenesis is now widely accepted. Cancer-initiating cells exhibit low proliferative rates, self-renewing capacity, a propensity to differentiate into actively proliferating tumour cells, and show resistance to chemotherapy or radiation (Vander Griend et al. 2008).
Cancer-initiating cells were characterized based on the investigation of distinct surface marker patterns within primary tumours. CD44 was reported as a robust marker of CIC (Chu et al. 2009; Takaishi et al. 2009). A single CD44+ cell from a colorectal tumour could form a sphere in vitro and was able to generate a xenograft tumour resembling the properties of the primary tumour (Du et al. 2008). CD133 is also a widely recognized marker of CIC. CD133 was initially described as a surface antigen specific for human haematopoietic stem cells and as a marker for murine neuroepithelial and several other embryonic epithelia (Singh et al. 2004). In a number of recent studies, CD133 alone or in combination with other markers was used for the isolation of CIC from malignant tumours of colon, lung and liver (Haraguchi et al. 2008). CD133+ tumour cells repair radiation-induced DNA damage more effectively than CD133− tumour cells (Bao et al. 2006). CIC are also often resistant to chemotherapy and can account for chemotherapy failure (Sell & Leffert 2008). To design novel therapeutic agents against CIC, it will be desirable to seek targets of CIC that are absent from normal cells.
The Thomsen-Friedenreich antigen (TF, or CD176) is a tumour-associated carbohydrate epitope with the structure Galβ1-3GalNAcα1-O-. While this disaccharide is a ubiquitous core structure (core-1) found in a cryptic manner on many membrane glycoproteins of normal cells, its exposure on tumour cells is restricted to a few specific carrier proteins. It has been demonstrated that CD176 is expressed on the surface of various cancer cells, such as breast carcinomas (Springer 1997; Imai et al. 2001; Goletz et al. 2003), colorectal carcinomas (Cao et al. 1995), hepatocellular carcinomas (HCC) (Cao et al. 1999), several leukaemias (Cao et al. 2008) and other types of cancer, but absent from almost all normal adult cell types (Cao et al. 1996). As a functional moiety, CD176 on the surface of cancer cells is involved in the invasive and metastatic properties of the cells (Cao et al. 1995). An anti-CD176 antibody could induce apoptosis of leukaemic cells (Cao et al. 2008). As CD176 is strongly expressed on the surface of cancer cells and virtually absent from normal tissues, it is sensible to assume that this carbohydrate structure is a suitable target for cancer biotherapy. In addition to its presence on tumour cells, CD176 is known as a differentiation antigen that is generally expressed in human foetal epithelia (Barr et al. 1989). In this study, we have examined the possibility of coexpression of CD176 with CD44 and CD133 on lung, breast and liver cancer cell lines by flow cytometry and on surgical specimens from these tumours by immunohistochemistry in order to ask whether CD176 might be a marker of CIC. As it was found that the oestrogen receptor ligand tamoxifen (4-OHT) led to an increase in the number of mammary cancer stem cell-like cells in vitro with a CD44+/CD24− phenotype (Mani et al. 2008), we also wanted to know whether the number of CD176+ cells could be enhanced by treatment with 4-OHT. Furthermore, a new sandwich solid-phase enzyme-linked immunosorbent assay (ELISA) was used to investigate whether CD176 is carried directly by the CD44 glycoprotein in lung, breast and liver cancer.
A by-product of this study was the resolution of conflicting reports on the expression of CD176 in lung carcinomas (Takanami 1999; Toma et al. 1999).
Materials and methods
Antibodies
Antibodies applied were anti-CD44 mAb [G44-26 (mouse IgG2b); BD Biosciences, Franklin Lakes, NJ, USA], anti-CD133 mAb [ANC9C5 (mouse IgG); Ancell, Bayport, MN, USA], anti-CD176 mAb [NM-TF2 (mouse IgM); Glycotope, Berlin, Germany], and anti-MUC1 mAb [mPankoMab (mouse IgG1); Glycotope].
Cell lines and cell culture
A variety of human cancer cell lines derived from breast adenocarcinomas (MDA-MB-231, MDA-MB- 435, and MCF-7), from diverse lung cancers (SPC-A-1 and GLC-82, lung adenocarcinoma; NCI-H446, untypical small cell lung carcinoma; 801-D, giant cell lung carcinoma) and from HCC (Hep G2 and HuH-7) were used in this study. All cell lines were routinely cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 10% foetal calf serum. The cell lines were used at 3–4 passages after thawing.
Immunocytochemistry
The cultured cells were plated onto polylysine (Sigma, Saint Louis, MO, USA)-coated slides in DMEM/F12 medium containing 10% foetal calf serum overnight. Thereafter the medium was carefully aspirated, and the slides were air-dried. Wrapped slides could be stored at −80 °C until use. For immunofluorescence double staining, cells were fixed with cold (−20 °C) acetone for 15 min, blocked with 2% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 30 min, and incubated with anti-CD44 (G44-26) or anti-CD133 (ANC9C5) antibodies together with mAb CD176 (NM-TF2) in previously determined working dilutions for 60 min. The slides were subsequently incubated with a mixture of fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgM (μ-chain specific) (#F9259; Sigma) and Cy3-conjugated goat anti-mouse IgG (γ-chain specific) (#69732; Jackson Laboratories, West Grove, PA, USA) antibodies. Counterstaining was performed with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Beyotime Biotechnology, Jiangsu, China). Negative controls were performed with medium instead of the specific mAb. The slides were mounted with glycerol and analysed by confocal microscopy (Olympus, Tokyo, Japan) and fluorescence microscopy. The percentage of positive cells or double positive cells was counted on digital images.
Flow cytometry analysis
Cell suspensions of cell lines were prepared at 1 × 106 cells/100 μl. Cells were washed twice with PBS containing 2% BSA and incubated with the primary antibodies at appropriate dilutions at 4 °C for 20 min, followed by anti-mouse IgG-Cy3 (γ chain-specific) and anti-mouse IgM-FITC (μ chain-specific) second antibodies at appropriate concentrations at 4 °C for 20 min. Flow cytometry was performed with a FACScan (BD Biosciences). Collected data from 10,000 cells and WinMDI software were used in the analysis of FACS datafiles.
For 4-hydroxytamoxifen treatment, MDA-MB-231, MDA-MB-435 and MCF-7 breast adenocarcinoma cell lines were exposed to 4-OHT (#H6278; Sigma) at a final concentration of 20 nM for 24 h. Then flow cytometry analysis was performed as described earlier.
Tissues and immunohistochemistry
Twenty-one cases of lung carcinoma (13 adenocarcinomas and eight squamous cell carcinomas), 15 breast carcinomas, and 21 HCC specimens were obtained from patients who had undergone initial surgery. The samples were fully encoded to protect patient confidentiality and were approved by the local research ethics committees at all participating sites.
Fresh tissues were embedded carefully at −20 °C with optimal cutting temperature (OCT) compound in plastic mould, cut into 4–8-μm sections after equilibration in the cryostat chamber and fixed with cold (−20 °C) acetone for 15 min. For immunofluorescence double staining, the tissue sections were blocked with 2% BSA, incubated with the primary antibodies at appropriate dilutions for 60 min, and subsequently incubated with anti-IgG-Cy3 (γ-chain-specific) and anti-IgM-FITC (μ-chain-specific) secondary antibodies, counterstained with DAPI and mounted on glass slides with glycerol for microscopic analysis. For immunoperoxidase staining, the tissue sections were treated with 3% H2O2 for 30 min to block endogenous peroxidases, washed three times with PBS and blocked with 2% BSA. They were incubated with the primary antibody, and thereafter treated with peroxidase-labelled goat anti-mouse immunoglobulin antiserum (DAKO, Copenhagen, Denmark). Negative controls were performed with 2% BSA in PBS instead of the mAbs. The anti-MUC-1 mAb (mPankoMab) was used in lung carcinoma specimens as positive control in all batches. Colour was developed with the peroxidase substrate 3,3′-diaminobenzidine. Counterstaining was performed with haematoxylin. Cell numbers were counted at 100× magnification with a Nikon microscope.
Sandwich ELISA
One million cells were treated with 1 ml of 1% Triton X-100 (in 100 mM sodium phosphate pH 7.5, 150 mM NaCl) containing a mixture of protease inhibitors (#539134; Calbiochem, Darmstadt, Germany) and homogenized with oscillation at 4 °C for 30 min. After centrifugation for 10 min at 15,000 g, the supernatants were taken. Ninety-six-well polystyrene microtest plates were coated with the capture antibody against CD44 (G44-26, IgG2b) at a concentration of 1 μg/ml in coating buffer at 4 °C for 14 h. After blocking the remaining protein-binding sites with 5% BSA, 100 μl of supernatants of cell lysates were added to the wells and incubated at room temperature for 2 h. Then the plates were incubated with the anti-CD176 mAb (NM-TF2, IgM) followed by peroxidase-labelled goat anti-mouse IgM antibody (μ-chain specific) (SouthernBiotech, Birmingham, AL, USA). The colour reaction was developed with o-phenylenediamine dihydrochloride (OPD)/H2O2 solution at room temperature, and stopped with 2.5 M sulphuric acid. Negative controls were performed with 2% BSA in PBS instead of either the coating antibody, the protein extracts or the detecting antibody. The optical density of each well was determined within 30 min using a microplate reader (Bio-Rad, Hercules, CA, USA) at 492 nm.
Statistical analysis
Data were analysed with either the chi-square test or Fisher's exact probability test. Pearson's correlation analysis of enumeration data was also performed. A P < 0.05 value was considered statistically significant.
Results
Expression of CD44 and CD133 in lung, breast and liver cancer cell lines
CD44 was detected on more than 60% of the cells in most of the cancer cell lines. One cell line, HuH-7 (HCC), showed a low percentage of CD44-positive cells. CD133-positive cells represented only a small subpopulation among the cell lines examined in this study (<1% of the cells) except for 801-D (giant cell lung carcinoma) and HuH-7 (HCC). These cell lines did consistently express CD133 at the surface of 15% and 6% of the cells, respectively.
Expression of CD176 in lung, breast and liver cancer cell lines
Through flow-cytometry and immunocytochemistry analysis with mAb NM-TF2, we found that CD176 was localized at the cellular surface and in the cytoplasm. The cell lines expressed CD176 at varying intensities: MDA-MB-231, MDA-MB-435 (breast adenocarcinoma), and HuH-7 (HCC) contained between 5% and 30% positive cells; SPC-A-1 (lung adenocarcinoma), 801-D (giant cell lung carcinoma), and HepG2 (HCC) revealed 30% to 60% positive cells; GLC-82 (lung adenocarcinoma), NCI-H446 (small cell lung carcinoma) and MCF-7 (breast adenocarcinoma) had more than 60% positive cells.
Co-expression of CD176 with CD44 or CD133 in lung, breast and liver cancer cell lines
Because CD44 and CD133 are accepted markers of CIC (Bao et al. 2006; Ponnusamy & Batra 2008; Chu et al. 2009), we examined whether one or both are coexpressed with CD176. Double immunofluorescence staining experiments with cell lines demonstrated that CD44 and CD176 were located at the cellular surface and exhibited coexpression of single cells or cell clusters (Figure 1a–i). Flow cytometry experiments revealed the following semi-quantitative coexpression data. MCF-7 (breast adenocarcinoma) contained about 7% of CD44+/CD176+ cells, SPC-A-1, 801-D and HepG2 contained from 30% to 60% of CD44+/CD176+ cells, and GLC-82 and NCIH446 had over 60% of CD44+/CD176+ cells (Table 1, Figures 1 and 2). In contrast, most cell lines contained only few cells with the CD133+/CD176+ phenotype (<1%). As an exception, 801-D and HuH-7 had more than 5% cells with the CD133+/CD176+ phenotype (Table 1).
Figure 1.
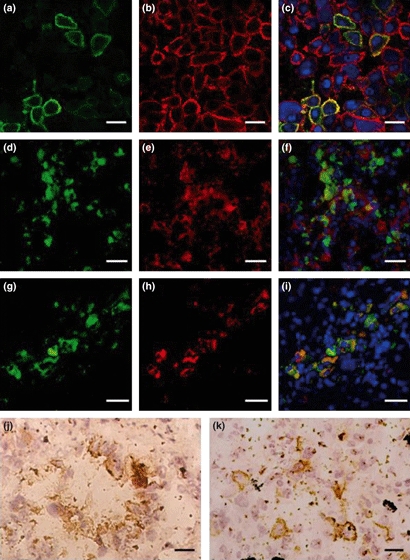
(a–c) Confocal microscopy analysis performed with the lung cancer cell line NCI-H446 (magnification ×400, bar: 20 μm). Cells were stained with monoclonal antibodies specific for CD44 (red) and CD176 (green). Nuclei were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (blue). CD44 is strongly expressed in most NCI-H446 cells (a). CD176 expression could be seen in the membrane of cell clusters (b). The mixed picture (c) demonstrates co-localization of CD44 and CD176 in these cell clusters (yellow). (d–i) Co-expression of CD44 and CD176 as well as of CD133 and CD176 in hepatocellular carcinoma tissues is shown by double immunofluorescence staining (magnification ×200, bar: 40 μm). Co-expression of CD176 (green, d)/CD44 (red, e) and CD133 (red, g)/CD176 (green, h) by overlay is found (yellow, f and i, respectively). (j,k) Expression of CD176 in lung cancer tissues as analysed by immunohistochemistry (magnification ×400, bar: 20 μm). CD176 is found at the cellular surface and in the cytoplasm of lung adenocarcinoma cells (j) and of lung squamous carcinoma cells (k).
Table 1.
Combined flow-cytometric and immunofluorescence analysis of co-expression of CD176 with CD44 or CD133 on cancer cell lines (per cent of cells belonging to the respective group)
| Tissue derivation | Cell lines | CD44−/CD176− (%) | CD44−/CD176+ (%) | CD44+/CD176− (%) | CD44+/CD176+ (%) | CD133−/CD176− (%) | CD133−/CD176+ (%) | CD133+/CD176− (%) | CD133+/CD176+ (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lung cancer | |||||||||
| Adenocarcinoma | SPC-A-1 | ∼6 | ∼20 | ∼5 | >60 | ∼11 | >60 | <1 | <1 |
| Adenocarcinoma | GLC-82 | <1 | ∼4 | ∼1 | >60 | ∼7 | >60 | <1 | <1 |
| Small cell carcinoma | NCIH446 | ∼10 | ∼8 | ∼17 | >60 | ∼15 | >60 | <1 | <1 |
| Giant cell carcinoma | 801-D | ∼23 | ∼6 | ∼46 | ∼23 | ∼60 | ∼25 | ∼11 | ∼4 |
| Breast cancer | |||||||||
| Adenocarcinoma | MDA-231 | >60 | ∼2 | ∼43 | ∼1 | >60 | ∼3 | <1 | <1 |
| Adenocarcinoma | MDA-435 | >60 | 16 | ∼10 | ∼4 | >60 | ∼20 | <1 | <1 |
| Adenocarcinoma | MCF-7 | >60 | ∼4 | ∼24 | ∼7 | >60 | ∼11 | <1 | <1 |
| Liver cancer | |||||||||
| HCC | HepG-2 | ∼6 | ∼17 | ∼23 | ∼54 | ∼29 | >60 | <1 | <1 |
| HCC | HuH-7 | >60 | ∼13 | <1 | <1 | >60 | ∼13 | ∼2 | ∼4 |
HCC, hepatocellular carcinoma.
Figure 2.
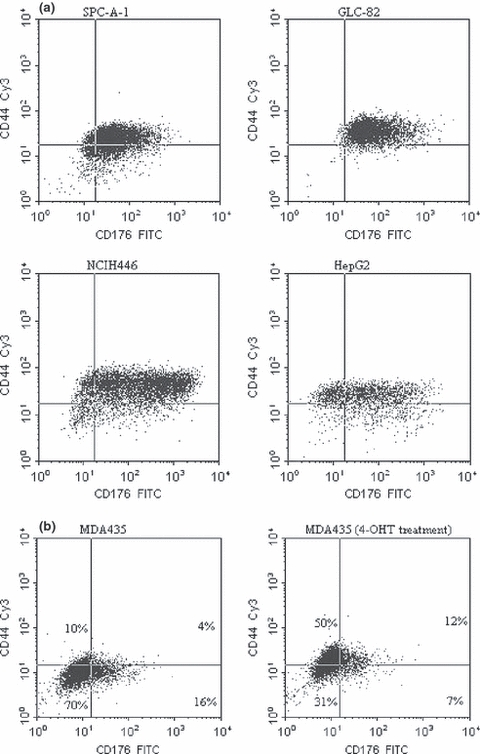
(a) FACS analysis of co-expression of CD44 and CD176 in cells of the cell lines SPC-A-1, GLC-82 (lung adenocarcinoma) and HepG2 (hepatocellular carcinoma). Cells were incubated with anti-CD44 (IgG) and anti-CD176 (IgM) antibodies, followed by incubation with anti-IgG-Cy3 (γ-chain specific) and anti-IgM-fluorescein isothiocyanate (μ-chain specific) second antisera. A minimum of 10,000 events were collected per sample. The percentage of cells with the respective combination of markers is indicated in each section of the graph, demonstrating a strong correlation of staining of both markers. Data shown are representative of several independent experiments. (b) FACS analysis of co-expression of CD44 and CD176 before and after 4-OHT treatment of MDA-MB-435 (breast carcinoma) cells. The percentage of cells with the respective combination of markers is indicated in each section of the graph. The number of CD44+/CD176+ cells is increased after treatment with 4-OHT for 24 h. Data shown are representative of several independent experiments.
To assess whether CD44 and CD176 expression was affected simultaneously by exogeneous treatment, we added the oestrogen receptor ligand tamoxifen (4-OHT) to breast cancer cells (20 nM for 24 h). The results indicate that MDA-MB-231 and MCF-7 had no significantly increased numbers of CD44+/CD176+ expressing cells (as shown by flow cytometry), whereas in MDA-MB-435 the percentage of CD44+/CD176+ cells was increased after treatment (Figure 2b).
Expression of CD176 in lung carcinoma tissues
We also investigated the expression of CD176 in lung carcinoma tissues employing mAb NM-TF2. The tissues examined from 21 patients included 13 adenocarcinomas and eight squamous cell carcinomas. CD176 was distributed at the cellular surface and in the cytoplasm. Examples are shown in Figure 1j,k. The percentage of CD176-positive cells in lung carcinomas is shown in Table 2. Four cases revealed over 50% positive cells. These data confirm the expression of CD176 in lung cancer tissues in contrast to an earlier paper (Toma et al.1999). CD176 expression was compared with clinicopathological features by means of statistical analysis. Cases showing >5% of positive cells were defined as positive. The results show a correlation between the status of CD176 and the tumour grade as well as the presence of metastases in patients with lung carcinoma (P < 0.05). There were no statistically significant correlations between the expression of CD176 and other clinicopathological features including patient age, sex or histological subtypes (adenocarcinomas or squamous cell carcinomas).
Table 2.
Evaluation of immunohistochemical positivity in lung, breast, liver cancer tissues
| Per cent CD44+ cells | Per cent CD133+ cells | Per cent CD176+ cells | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | +* | ++ | +++ | ++++ | + | ++ | +++ | ++++ | + | ++ | +++ | ++++ |
| Lung carcinoma (NSCLC) | 0/10† | 0/10 | 0/10 | 10/10 | 11/21 | 6/21 | 1/21 | 1/21 | 5/21 | 9/21 | 3/21 | 4/21 |
| Breast carcinoma | 0/15 | 1/15 | 6/15 | 8/15 | 10/15 | 2/15 | 0/15 | 0/15 | 2/15 | 12/15 | 1/15 | 0/15 |
| Liver cancer (HCC) | 0/21 | 0/21 | 9/21 | 12/21 | 11/15 | 3/15 | 0/15 | 0/15 | 10/21 | 7/21 | 4/21 | 0/21 |
NSCLC, non-small cell lung carcinoma; HCC, hepatocellular carcinoma.
*Score: +, <5%; ++, 5–30%; +++, 30–60%; ++++, >60%.
No. of relevant cases /total no. of cases examined.
Coexpression of CD176 with CD44 or CD133 in lung, breast and HCC tissues
The immunohistochemical data for CD44, CD133 and CD176 expression in the tissues in question are shown in Table 2. Similar to the cancer cell lines, CD44 was expressed strongly and diffusely in all carcinomas and at a high percentage (more than 60% of the total number of cancer cells). CD133+ cells were found in most cases, but only in a subpopulation of cells (<10% of the total number of cancer cells). Two cases of lung carcinomas, three of breast carcinomas and one HCC did not express CD133 at all. The CD133+ cells were present either in a scattered or in a clustered pattern. CD133 expression did not show any statistically significant correlation with sex, age or histological subtypes in lung carcinomas.
Coexpression of CD176 with CD44 or CD133 in the clinical samples was examined (see Table 3; the given values are the mean of the samples studied.). The double staining results in the cancerous tissues revealed that 5-30% of CD44+ cells expressed simultaneously CD176, and most of the CD133+ cells also expressed CD176. However, we also noted that coexpression of CD176 with CD44 or CD133 was not seen in every cell positive for one of the markers. Some CD44+ and CD133+ cells did not express CD176 and vice versa. The HCC had significantly more cells with a CD133+/CD176+ phenotype than lung or breast carcinoma sections. There was a statistically significant correlation between the status of CD176 and CD133 in lung carcinoma (P < 0.05). Correlations between clinicopathological features and the CD44+/CD176+ phenotype were not seen in this study (see Table 4).
Table 3.
Immunofluorescence analysis of CD44, CD133 and CD176 expression in lung, breast and liver cancer tissues (per cent of carcinoma cells belonging to the respective group)
| Cancer type | CD44−/CD176− (%) | CD44−/CD176+ (%) | CD44+/CD176− (%) | CD44+/CD176+ (%) | CD133−/CD176− (%) | CD133−/CD176+ (%) | CD133+/CD176− (%) | CD133+/CD176+ (%) |
|---|---|---|---|---|---|---|---|---|
| Lung carcinoma (NSCLC) | 1–5 | <1 | 30–60 | 5–30 | >60 | 5–30 | 1–5 | 1–5 |
| Breast carcinoma | 5–30 | 1–5 | 5–30 | 5–30 | >60 | 5–30 | 1–5 | 1–5 |
| Liver carcinoma (HCC) | 1–5 | 1–5 | 30–60 | 5–30 | >60 | 1–5 | 1–5 | 5–30 |
NSCLC, non-small cell lung carcinoma; HCC, hepatocellular carcinoma.
Table 4.
Comparison of clinicopathological features to co-expression of CD176 and CD44†
| CD176 | CD44 | CD176±CD44± | ||||
|---|---|---|---|---|---|---|
| Tumour and clinicopathological features | 5–30% | >30% | 5–30% | >30% | 5–15% | >15% |
| Lung carcinoma (21 cases) | ||||||
| Histological type | ||||||
| ACA | 5/13 (38) | 4/13 (31) | 0/13 | 13/13 (100) | 6/13 (46) | 5/13 (38) |
| SCC | 4/8 (50) | 3/8 (38) | 0/8 | 8/8 (100) | 3/8 (38) | 2/8 (25) |
| TNM stage | ||||||
| I + II | 6/12 (50) | 6/12 (50)* | 0/12 | 12/12 (100) | 5/12 (42) | 5/12 (42) |
| III + IV | 3/9 (30) | 1/9 (11)* | 0/9 | 9/9 (100) | 3/9 (33) | 2/9 (22) |
| Differentiation | ||||||
| Poor | 5/6 (83) | 5/6 (83)* | 0/6 | 6/6 (100) | 4/6 (67) | 3/6 (50) |
| Well or Moderate | 4/15 (27) | 2/15 (13)* | 0/15 | 6/15 (100) | 4/15 (27) | 5/15 (34) |
| Breast carcinoma (15 cases) | ||||||
| Histological type | ||||||
| IC | 8/10 (80) | 1/10 (10) | 0/10 | 10/10 (100) | 6/10 (60) | 2/10 (20) |
| NIC | 4/5 (80) | 0/5 | 1/5 (20) | 4/5 (80) | 3/5 (60) | 0/5 |
| TNM stage | ||||||
| I + II | 5/5 (100) | 1/5 (20) | 1/5 (20) | 4/5 (80) | 4/5 (80) | 1/5 (20) |
| III + IV | 7/10 (70) | 0/10 | 0/10 | 10/10 (100) | 5/10 (50) | 1/10 (10) |
| Differentiation | ||||||
| Poor | 5/6 (83) | 1/6 (17) | 1/6 (17) | 5/6 (83) | 4/6 (67) | 1/6 (17) |
| Well or Moderate | 7/9 (78) | 0/9 | 0/9 | 9/9 (100) | 5/9 (56) | 1/9 (11) |
| HCC (21 cases) | ||||||
| TNM stage | ||||||
| I + II | 3/11 (27) | 3/11 (27)* | 0/11 | 11/11 (100) | 3/11 (27) | 5/11 (45) |
| III + IV | 4/10 (40) | 1/10 (10)* | 0/10 | 10/10 (100) | 3/10 (30) | 2/10 (20) |
| Differentiation | ||||||
| Poor | 3/5 (60) | 2/5 (40) | 0/5 | 5/5 (100) | 2/5 (40) | 2/5 (40) |
| Well or moderate | 4/16 (66) | 2/16 (33) | 0/16 | 16/16 (100) | 3/16 (19) | 5/16 (31) |
Values are no. of cases applicable / total no. of cases examined (with per cent in parentheses). The statistical differences were compared using the Fisher's exact test (two-tailed).
P < 0.05.
ACA, adenocarcinoma; SCC, squamous cell carcinoma; IC, infiltrative carcinoma; NIC, non-infiltrative carcinoma; HCC, hepatocellular carcinoma.
Detection of CD44 as carrier protein of CD176
Potential glycoprotein carrier molecules of CD176 were analysed by a sandwich ELISA in several carcinoma cell lines. We used polystyrene microplates coated with anti-CD44 antibody to capture CD44 glycoprotein from whole-cell lysates. The plates were then incubated with NM-TF2 (anti-CD176) as detection antibody. As shown in Table 5, captured CD44 was found to react with the anti-CD176 mAb in all (4) lung and (2) liver cancer cell lines as well as in two of three breast cancer cell lines, indicating that the CD44 glycoprotein apparently is a common carrier of CD176 in these cancer cases.
Table 5.
Binding of CD176 antibody in sandwich ELISA with catcher antibody: CD44
| Tissue derivation | Cell lines | CD176+ on CD44+ |
|---|---|---|
| Lung cancer | ||
| Adenocarcinoma | SPC-A-1 | + |
| Adenocarcinoma | GLC-82 | + |
| Small cell carcinoma | NCIH446 | (+) |
| Giant cell carcinoma | 801-D | (+) |
| Breast cancer | ||
| Adenocarcinoma | MDA-231 | − |
| Adenocarcinoma | MDA-435 | + |
| Adenocarcinoma | MCF-7 | (+) |
| Liver cancer | ||
| HCC | HepG-2 | + |
| HCC | HuH-7 | (+) |
Score according to OD492 values minus blank as follows: +, >0.2; (+), 0.1–0.2; −, <0.1.
HCC, hepatocellular carcinoma.
Discussion
Numerous publications have led to a general acceptance of the view that initiation, maintenance, and metastatic spread of most if not all tumour types is essentially because of a subpopulation of cancer cells called CIC or cancer stem cells (Dalerba et al. 2007; Chu et al. 2009).
Cancer-initiating cells, besides their capacity of self-renewal, exhibit the propensity to differentiate into actively proliferating and finally into more or less mature tumour cells. They are obviously different from the majority of cells of the tumour mass. Therefore, they should specifically be targeted in cancer therapies. A great number of more or less specific markers of CIC have been described during recent years. The crux of these markers is that there are too many of them, and that they are not sufficiently consistent. Among the markers most widely accepted is CD44 (Shipitsin et al. 2007; Ponnusamy & Batra 2008). Another membrane glycoprotein, CD133, has also been proposed to be capable of identifying a cancer initiating population in brain, colon, lung and other solid tumours (Tirino et al. 2008). CD133 is an independent prognostic marker that correlates with poor overall survival in patients with malignancies (Horst et al. 2008). However, a recent report showed that CD133 negative cells were also capable of long-term tumourigenesis in NOD/SCID mice (Shmelkov et al. 2008).
In this study, we have evaluated the expression of CD44 and CD133 in cell lines and in clinical samples from lung, breast and liver cancer. We found that a majority of tumour cells stained positively for CD44. CD133 was consistently expressed in lung, breast and liver cancer tissues at varying intensities. In contrast, most of the cancer cell lines studied revealed only few cells with CD133 expression. Other authors have reported that CD133 was expressed on about 2.5% and 6–29% of the total number of tumour cells in colon cancer (Ricci-Vitiani et al. 2007), non-small cell lung cancer (Tirino et al. 2009) and brain tumours (Singh et al. 2004), respectively.
We then examined the coexpression of these two markers of CIC, CD44 and CD133, with CD176. There are so far no reports on the occurrence of this antigen on CIC, and in particular on CIC of solid tumours. We here report on a major percentage of cells coexpressing CD176 and CD44, especially in lung carcinoma cell lines and in HepG2, and a significant number in all other examined cell lines. In the examined lung, breast and liver cancer tissues, the number of cells coexpressing CD176 and CD44 amounted to 5–30%. The number of cells coexpressing CD176 and CD133 was much lower in all cases (as was the total number of CD133 expressing cells), but coexpressing cells were never completely absent. There is no reason to expect coexpression of CD176 on all CD44- or CD133-positive cells. Coexpressing cells may represent a subpopulation of CIC, or the expression of CD176 (as an intermediate in the glycan biosynthesis) may be a dynamic or fluctuating event.
The CD176 antigen (core-1, or Thomsen-Friedenreich antigen) is a carbohydrate antigen with the structure Galβ1-3GalNAcα1-, which is O-glycosidically linked to threonines and serines of membrane glycoproteins. CD176 is masked in normal and benign adult human tissues by prolonged carbohydrate chains, and finally by sialic acid, but is uncovered during the process of malignant transformation (Cao et al. 1997; Springer 1997). It was also observed that CD176-positive primary tumours have a nearly fourfold risk for liver metastasis compared to CD176-negtive tumours in primary colorectal cancer (Cao et al. 1995). More recent studies demonstrated that an anti-CD176 antibody–induced apoptosis of CD176+ leukaemia cells (Cao et al. 2008). The outstanding tumour specificity of CD176 of various carcinomas renders it a suitable target for tumour therapy (Springer 1997; Goletz et al. 2003; Franco 2005). The data presented here demonstrating that CD176 is not only expressed on mature cancer cells but obviously also or even preferably on CIC of solid tumours, add additional weight on CD176 as an attractive therapeutic target. We also wanted to know whether the number of CD176+ breast cancer cells would be enhanced after treatment with tamoxifen (4-OHT). Tamoxifen was reported to induce G0/G1 growth arrest and to inhibit the proliferation of breast cancer cells. A recent study showed that tamoxifen treatment increased the number of mammary cancer stem cell-like cells (Mani et al. 2008). In our study, tamoxifen treatment of breast cancer cells enhanced the CD44+/CD176+ phenotype in one of three cell lines (MDA-MB-435). We consider this as supporting evidence for the assumption that this phenotype identifies CIC.
Correlations to clinicopathological features have been described for both CD44 and for CD176 as single markers. Whether the coexpression of both markers is of additional pathophysiological importance is an open question, and not very likely. Our limited data give no evidence for such an assumption.
According to the cancer stem cell theory, recurrences and metastases of cancer depend on CIC (Dalerba et al. 2007). The existence of a population of such cells with properties different from the tumour mass may explain why conventional therapies, e.g. treatment with tamoxifen, are only able to suppress cancer but often cannot completely eradicate it. On the contrary, this treatment may even enhance the number of CIC (Mani et al. 2008). On the other side, the elimination of CIC could actually prevent recrudescences of tumours. The development of new therapeutic approaches to target CIC may therefore have a profound impact on cancer therapy. The identification of CD176 on CIC of solid tumours is an important argument for the development of CD176-based immunotherapies, and may explain the success of Georg Springer's early vaccination attempts (Springer 1997).
Demasking of CD176 seems to be a selective process that involves only a few among all possible candidate glycoproteins present at the cell membrane. The most prominent carrier molecule of CD176 identified in epithelial cells so far is the polymorphic epithelial mucin MUC-1, for example in breast and colorectal carcinoma (Barr et al. 1989; Cao et al. 1997; Baldus et al. 1998). Another study showed that a splice variant of CD44 is a carrier of CD176 on colorectal carcinomas (Singh et al. 2001). In the present study, we examined whether CD44 might also be the carrier molecule for core-1 in lung, breast and liver carcinoma cells. To this end, we applied a special sandwich ELISA and examined with it lung, breast and liver cancer. Our data suggest that CD176 is indeed carried by CD44 in tumours other than colorectal carcinomas. In other words, it is a more general phenomenon.
The CD176 antigen has been found to be a useful marker of prognosis in lung patients (Takanami 1999), but there remain conflicting reports on its expression in this tissue (Toma et al. 1999). In our study, we re-examined this issue by employing the monoclonal antibody NM-TF2, which is well characterized and suited for immunohistochemistry. High CD176 expression was observed in most lung cancer cell lines. In clinical lung cancer samples, we found that more than 50% of the cases were CD176+ above the cut-off value of 5% positive cells.
In summary, CD176 (Thomsen-Friedenreich antigen, core-1) expression was observed in human lung, breast and liver carcinomas and in cell lines derived from these malignancies. Coexpression of CD44 and CD176, as well as of CD133 and CD176 in human lung, breast and liver carcinoma indicated that CD176 is expressed on CIC, and can be considered to be a novel (carbohydrate) marker of these cells. This makes CD176, which is almost absent in normal and benign adult human tissues, an even more promising target for tumour therapies.
Acknowledgments
We thank the First Affiliated Hospital of Kunming Medical College and the Kunming First People Hospital for providing patient samples and gratefully acknowledge the technical assistance of Ms Fan Xiao-Na. This research was supported by a grant from Yunnan Province Science and Technology Department (2008CCO15).
References
- Baldus SE, Hanisch FG, Kotlarek GM, et al. Coexpression of MUC-1 mucin peptide core and the Thomsen-Friedenreich antigen in colorectal neoplasms. Cancer. 1998;82:1019–1027. doi: 10.1002/(sici)1097-0142(19980315)82:6<1019::aid-cncr3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:687–688. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Barr N, Taylor CR, Young T, Springer GF. Are pancarcinoma T and Tn differentiation antigens? Cancer. 1989;64:834–841. doi: 10.1002/1097-0142(19890815)64:4<834::aid-cncr2820640413>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Cao Y, Karsten UR, Liebrich W, Haensch W, Springer GF, Schlag PM. Expression of Thomsen-Friedenreich-related antigens in primary and metastatic colorectal carcinomas. A reevaluation. Cancer. 1995;76:1700–1708. doi: 10.1002/1097-0142(19951115)76:10<1700::aid-cncr2820761005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cao Y, Stosiek P, Springer GF, Karsten U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem. Cell Biol. 1996;106:197–207. doi: 10.1007/BF02484401. [DOI] [PubMed] [Google Scholar]
- Cao Y, Schlag PM, Karsten U. Immunodetection of epithelial mucin (MUC1, MUC3) and mucin-associated glycotopes (TF, Tn, and Sialosyl-Tn) in benign and malignant lesions of colon epithelium: apolar localization correspond to the malignant transformation. Virchows Arch. 1997;431:159–166. doi: 10.1007/s004280050083. [DOI] [PubMed] [Google Scholar]
- Cao Y, Karsten U, Otto G, Bannasch P. Expression of MUC1, Thomsen-Friedenreich antigen, Tn, sialosyl-Tn, and 2,6-linked sialic acid in hepatocellular carcinomas and preneoplastic hepatocellular lesions. Virchows Arch. 1999;434:503–509. doi: 10.1007/s004280050375. [DOI] [PubMed] [Google Scholar]
- Cao Y, Merling A, Karsten U, et al. Expression of CD175 (Tn), CD175s (sialosyl-Tn), and CD176 (Thomsen-Friedenreich antigen) on malignant human hematopoietic cells. Int. J. Cancer. 2008;123:89–99. doi: 10.1002/ijc.23493. [DOI] [PubMed] [Google Scholar]
- Chu P, Clanton DJ, Snipas TS, et al. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. Int. J. Cancer. 2009;124:1312–1321. doi: 10.1002/ijc.24061. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu. Rev. Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- Du L, Wang H, He L, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin. Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]
- Franco A. CTL-based cancer preventive/therapeutic vaccines for carcinomas: role of tumour-associated carbohydrate antigens. Scand. J. Immunol. 2005;61:391–397. doi: 10.1111/j.1365-3083.2005.01596.x. [DOI] [PubMed] [Google Scholar]
- Goletz S, Cao Y, Danielcyk A, Ravn P, Schoeber U, Karsten U. Thomsen-Friedenreich antigen: the ‘hidden’ tumor antigen. Adv. Exp. Med. Biol. 2003;535:147–162. doi: 10.1007/978-1-4615-0065-0_10. [DOI] [PubMed] [Google Scholar]
- Haraguchi N, Ohkuma M, Sakashita H, et al. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann. Surg. Oncol. 2008;15:2927–2933. doi: 10.1245/s10434-008-0074-0. [DOI] [PubMed] [Google Scholar]
- Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br. J. Cancer. 2008;99:1285–1289. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai J, Ghazizadeh M, Naito Z, Asano G. Immunohistochemical expression of T, Tn and sialyl-Tn antigens and clinical outcome in human breast carcinoma. Anticancer Res. 2001;21:1327–1334. [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy MP, Batra SK. Ovarian cancer: emerging concept on cancer stem cells. J. Ovarian Res. 2008;1:4. doi: 10.1186/1757-2215-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Sell S, Leffert HL. Liver cancer stem cells. J. Clin. Oncol. 2008;26:2800–2805. doi: 10.1200/JCO.2007.15.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipitsin M, Campbell LL, Argani P, Weremowicz S. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T. CD133 expression is not restricted to stem cells, and both CD133+ and CD133− metastatic colon cancer cells initiate tumors. J. Clin. Invest. 2008;118:2021–2024. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Campbell BJ, Yu LG, et al. Cell surface-expressed Thomsen-Friedenreich antigen in colon cancer is predominantly carried on high molecular weight splice variants of CD44. Glycobiology. 2001;11:587–592. doi: 10.1093/glycob/11.7.587. [DOI] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Springer GF. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- Takaishi S, Okumura T, Tu S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanami I. Expression of Thomsen-Friedenreich antigen as a marker of poor prognosis in pulmonary adenocarcinoma. Oncol. Rep. 1999;6:341–344. doi: 10.3892/or.6.2.341. [DOI] [PubMed] [Google Scholar]
- Tirino V, Desiderio V, d'Aquino R, et al. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PLoS ONE. 2008;3:e3469. doi: 10.1371/journal.pone.0003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirino V, Camerlingo R, Franco R, et al. The role of CD133 in the identification and characterisation of tumour-initiating cells in non-small-cell lung cancer. Eur. J. Cardiothorac. Surg. 2009;36:446–453. doi: 10.1016/j.ejcts.2009.03.063. [DOI] [PubMed] [Google Scholar]
- Toma V, Sata T, Vogt P, Komminoth P, Heitz P, Roth J. Thomsen-Friedenreich glycotope in developing human lung and in lung carcinoma. Cancer. 1999;85:2151–2159. [PubMed] [Google Scholar]
- Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–9711. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]


