Abstract
It is thought that the degeneration of germ cells associated with an increase in the temperature due to cryptorchidism involves oxidative stress. α-Tocopherol is a powerful antioxidant that prevents oxidation of polyunsaturated fats found in membranes and stabilizes peroxyl radicals. For this reason we were interested in determining the role of α-Tocopherol using experimental cryptorchidism, followed by orchidopexia in neonatal rats. Eighty-four, 10-day-postpartum (dpp) male rats (Wistar strain) were used and divided into 7 groups: healthy control, sham with α-Tocopherol treated with 30 or 100 mg/kg doses, sham vehicle, cryptorchidism treated with α-Tocopherol at 30 or 100 mg/kg doses and cryptorchidism vehicle. Cryptorchidism was surgically induced at 10 dpp. At 25 dpp the animals were treated with α-Tocopherol and the vitamin vehicle. Lipoperoxidation and testicular morphology was determined in half of the animals at 40 dpp (short term). The remaining animals underwent orchidopexia and fertility was determined at 90 dpp. Testicular morphology was determined at 120 dpp (long term) in these animals. A significant reduction of lipoperoxidation was observed in the cryptorchid group treated with α-Tocopherol compared to the untreated cryptorchid group, in addition to short-term histological alterations. At long term, we observed an increase in the area and maturation of the seminiferous epithelium, a decrease in apoptosis and histological alterations and an increase in fertility from α-Tocopherol treatment. α-Tocopherol treatment decreased lipoperoxidation, possibly stabilizing free radicals produced during cryptorchidism, reducing morphological testicular alterations and favoring fertility.
Keywords: cryptorchidism, fertility, male, oxidative stress and testicle
Cryptorchidism is present in more than 5% of newborn males. The index increases in premature and low-birth-weight infants. At 3 months of age, this index spontaneously decreases and is maintained at 1.6%. Subfertility is a recognized effect of cryptorchidism, even after testicular position has been corrected, owing to alterations in the maturation of germ cells (Cooper & Little 1985; Elder 1988; Lee & Coughlin 2001; Hutson et al. 2010), possibly caused by inguinal heat stress (DeFoor et al. 2004). Heat stress induces the generation of reactive oxygen species (ROS) in the testis (Ahotupa & Huhtaniemi 1992; Ikeda et al. 1999; DeFoor et al. 2004; Vigueras et al. 2009) and the reduction in endogenous antioxidant enzymes such as superoxide dismutase and catalase (Ahotupa & Huhtaniemi 1992). The superoxide anion, hydroxyl radical, nitric oxide and hydrogen peroxide are among the ROS generated (Zini & Schlegel 1997; Kumagai et al. 2002; Ishii et al. 2005). These ROS are transitory molecules with a high degree of chemical reactivity that could stimulate lipoperoxidation. They cause a deleterious change in cell membrane lipoprotein complexes in addition to testicular damage (Peltola et al. 1995), affecting spermatogonia for life and reducing sperm production and male fertility (El-Missiry 1999; Ghosh et al. 2002).
α-Tocopherol (αT) is an important antioxidant that localizes to cell membranes. It interrupts the lipoperoxidation chain reaction and traps the ROS created during univalent reduction in molecular oxygen (Traber & Kayden 1987). Therefore, it is important to determine whether αT prevents or reduces the damage caused by heat stress because of the testis’ inguinal position.
Material and methods
We used 84, 10-day-postpartum (dpp), male rats (Wistar strain) that stayed with their mothers until they weaned. Food and water were given on demand. All animals were handled according to the ethical principles and regulations specified by the Official Mexican Norm (NOM-062-200-1999). The protocol was approved by the Institutional Committee for the care and use of laboratory animals.
A total of 48 animals were used in both control and sham groups. The only intervention handling of animals from the sham group was surgical manipulation of the inguinal area without testicular fixation. The other 36 rats underwent surgical unilateral left cryptorchidism, and 12 animals were assigned randomly to each group which were defined in the following manner:
Group 1: Healthy control without surgical or pharmacological intervention (Control).
Group 2: Animals with simulated operation (sham) and daily administration of propylene glycol for 15 days, beginning at 25 dpp (Sham-Vehicle).
Group 3: Animals with sham operation and daily administration of 30 mg/kg i.p. of αT (Sigma-Aldrich, St Louis, MO, USA) for 15 days, beginning at 25 dpp (Sham-αT-30).
Group 4: Animals with sham operation and daily administration of 100 mg/kg i.p. of αT (Sigma-Aldrich) for 15 days, beginning at 25 dpp (Sham-αT-100).
Group 5: Animals with unilateral surgical cryptorchidism and daily administration of propylene glycol i.p. for 15 days in the 25 dpp (Cryptorchid-vehicle).
Group 6: Animals with unilateral surgical cryptorchidism and daily administration of 30 mg/kg i.p. of αT for 15 days, beginning at 25 dpp (Cryptorchid-αT-30).
Group 7: Animals with unilateral surgical cryptorchidism and daily administration of 100 mg/kg i.p. of αT for 15 days, beginning at 25 dpp (Cryptorchid-αT-100).
Surgical procedures
Cryptorchidism
Surgical cryptorchidism was performed as described by Shono et al. (1996): extra-abdominal exposition of the gubernaculums by means of a transversal inguinal incision, fixing the inguinal fascia with a 6-0 nylon suture, which impedes the testicle's descent.
Xylazine hydrochloride (10 mg/kg. i.p.) and ketamine (80 mg/kg. i.p.) were administered as anaesthetics. Dipyrone (5 mg/kg. i.p.) was administered as an analgesic.
At 40 days of age (short term), the lipoperoxidation and testicular morphology were determined in six animals from each group. Orchidopexy and orchidectomy of the contralateral testis, with the aim of preventing the participation of the healthy testicle in fertility test, were performed and were euthanized at 120 dpp (long term).
Orchidopexy
After anaesthetizing the abdomen, an approximately 3-cm transverse inguinal incision was made in the skin and subcutaneous tissue. The cryptorchid testis was identified in the inguinal region and dissected from its attachments. If descended, it was fixed to the scrotal bag with a 6-0 prolene suture on the inferior pole of the vaginal tunic, intertegumentary subcutaneous tissue and fascia of the scrotal sac on the corresponding side. Once the testis’ permanence in the scrotal sac was verified, the incision was sutured on one plane with a 6-0 prolene suture.
Orchidectomy
A transversal inguinal incision was made through the different subcutaneous layers until the testicle was localized. Taking care not to cut the albugineous tunic, the testicle and epididymis were freed from adherences, identifying the sperm duct and tying it off with a double suture of absorbable 2-0 material and its elements separately (vessels, cremaster, etc.), removing the testicle and epididymis. The stoppage of bleeding was checked, and the inguinal wall sutured in layers with 6-0 prolene.
Fertility evaluation
Fertility was evaluated at 90 dpp by placing individual males from all groups with two fertile females for two oestrous cycles; vaginal swabs were performed in the morning to determine the presence of sperm. Once the presence of sperm was confirmed, females were separated to verify pregnancy, and if positive, until delivery to confirm the birth of healthy offspring.
Processing of biological material
All of the animals were sacrificed with an overdose of sodium pentobarbital (100 mg/kg) between 12:00 and 13:00 h to prevent circadian fluctuations and were examined post-mortem. Testicles were dissected, weighed and washed with saline solution (0.9%). For animals sacrificed at short term, half of each testicle was used to determine lipoperoxidation and the other half was distributed for inclusion in EPON 812 (Ted Pella, Inc., Redding, CA, USA) and paraffin. Tissue for lipoperoxidation was placed on ice. A sample of the left testicle of animals evaluated at long term was processed for inclusion in EPON and the rest for inclusion in paraffin. Tissue included in EPON was processed to determine the histopathological index (HI) of maturation or the Johnsen index (JI) and the epithelial area (EA), and material included in paraffin was used to determine the apoptosis index (AI) and cellular proliferation index (PI), as described below.
Lipoperoxidation through thiobarbituric acid-reactive substances
Production of thiobarbituric acid-reactive substances (TBARS) was measured according to the modified technique described for in vitro studies (Ríos & Santamaría 1991). A 1 ml aliquot containing the homogenized testis was added to 2 ml of the thiobarbituric acid (TBA) reagent (0.375 g of TBA, 15 g of trichloroacetic acid and 2.5 ml of concentrated HCl in 100 ml of distilled water), and the final solution (3 ml total volume) was heated in a boiling water bath for 30 min. Samples were cooled on ice and centrifuged at 3000 g for 15 min. The absorbance was measured in supernatants by spectrophotometry at 532 nm. Concentrations of TBARS were calculated by the interpolation of a periodic oxidation of malondialdehyde standard curve. The final result was expressed as nmol of TBARS per mg of protein.
Protein content in testis tissue samples was measured with the Folin phenol reagent (Lowry et al. 1951). The results of lipid peroxidation were normalized to the protein content in each sample.
Morphological evaluation
Tissue specimens were fixed overnight with Karnovsky's aldehyde solution (without Ca2+, pH 7.4) (Karnovsky 1965); postfixed with 1% OsO4, dehydrated in a graded series of ethanol and embedded in EPON 812 (Ted Pella). One-micron-thick sections were cut from EPON blocks using an Ultracut UCT microtome (Leica, Vienna, Austria) and stained with 0.5% toluidine blue.
Histological analysis of seminiferous tubes was performed using a BX 51 Olympus light microscope with fluorescence (Tokyo, Japan). Twenty to 30 transversal sections of seminiferous tubes per animal were analysed. Seminiferous epithelium area was determined by subtracting the internal area from the external area using an image analysing system (Image-Pro Plus 5.1, Media Cybernetics, INC. MD, USA) and was only assessed in transverse sections. Seminiferous epithelium degree of maturity was determined by means of the JI (Johnsen 1970). A score was assigned to each seminiferous tubule according to the main cell-type present, ranging from one (no cells) to ten (complete spermatogenesis with many late spermatids). The HI was determined by Vigueras et al. (2009).
Cellular apoptosis determined by TUNEL
Cellular apoptosis was determined using TUNEL (In situ Cell Death Detection Kit, Roche Diagnostic Corporation, IN, USA). Testes samples were fixed in 4% paraformaldehyde for 18 h and processed for embedding in paraffin. Transverse 4-μm-thick sections were cut with a microtome (Leica RM 2155; Microsystems, Nussloch Gmbh, Germany) and mounted on poly-l-lysine-coated slides (Sigma-Aldrich). The sections were deparaffinized and hydrated through a graded ethanol series. Sections were delineated by Dako pen (Dako, Carpinteria, CA, USA). To identify cells, slides were first contrasted with 4′,6-diamidino-2-phenylindole (DAPI) as follows: they were washed with Hanks’ balanced solution (Gibco, Grand Island, NY, USA) and incubated with DAPI (Sigma-Aldrich) at a concentration of 1 μg/ml, after which they were incubated with proteinase K (20 mg/ml) (Stratagene, La Jolla, CA, USA) for 30 min and placed in 0.3% H2O2 in methanol for 30 min. They were subsequently permeabilized in 0.1% Triton X-100 (Sigma-Aldrich) for 2 min and incubated in TUNEL solution (50 μl terminal deoxynucleotidyl transferase and 450 μl nucleotide mixture) for 1 h at 37 °C. As a control for staining specificity, some sections were processed throughout the incubation steps and treated with DNase (Stratagene) at a concentration of 1 μg/ml for 10 min at 37 °C, before incubation in TUNEL solution to induce DNA strand breaks.
Sections were mounted in Entellan (Merck, Darmstadt, Germany) and observed with a fluorescent microscope (Olympus DX51). The number of apoptotic cells per transversally cut seminiferous cord was calculated. Twenty to 30 transversal sections of seminiferous tubes per animal were analysed. All dilutions and washes between stages were performed using phosphate-buffered saline (PBS) unless otherwise specified.
Determination of cellular proliferation
Paraffin sections, prepared as above, were mounted on poly-l-lysine-coated slides (Sigma-Aldrich). Six sections from each testis were prepared. Slides were deparaffinized in xylene for 30 min. After rehydration through a graded ethanol series, sections were delineated by Dako pen (Dako). Endogenous peroxidase activity was blocked with 1% H2O2 (Merck) in methanol for 30 min, after which sections were washed with distilled water. Slides were incubated in 1% Triton X-100 solution (Sigma-Aldrich) for 10 min, blocked with 1% bovine serum albumin (Amersham Biosciences, Buckingham-shire, UK) for 2 h and incubated with a rabbit polyclonal antibody against histone-H3 (1:500 dilution) (Upstate Biotechnology, Lake Placid, NY, USA) for 18 h at 4 °C. Slides were then incubated with biotinylated anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA USA) at a 1:100 dilution for 1 h and then with streptavidin–peroxidase conjugate (Rabbit Immunocruz staining system; Santa Cruz Biotechnology) for 30 min in accordance with the manufacturer's instructions. Sections were incubated in a peroxidase substrate solution [1.6 ml distilled H2O, 20 μl 10× substrate buffer and 40 μl 50× diaminobenzidine chromogen (Santa Cruz Biotechnology)] for 6 min, counterstained with haematoxylin, dehydrated through a graded series of ethanol and cleared with xylene. PBS was used for all dilutions and washes between stages unless otherwise specified.
Control sections were processed in an identical manner with the exception that the primary antibody incubation step was omitted. Sections were mounted with Entellan mounting medium (Merck) and were examined using a light microscope. All tissue sections from control and experimental animals were processed at the same time to minimize any potential variance in the labelling procedure. Slides from both groups were randomized and coded in such a way that all subsequent analyses were conducted in a blinded manner. All histological examinations were performed by a single observer.
The PI was evaluated in transversally cut sections, and the number of cells stained with the anti-histone-H3 antibody was determined in relation to the total number of cells in the transversally cut tube. A total of 50 seminiferous tubules were evaluated per animal.
Stereology
The rest of the tissue included in paraffin was sectioned in a serial manner and cut at 15-μm-thick with a 150 μm separation. It was stained with haematoxylin-eosin-periodic acid-Schiff.
A systematic random procedure termed the optical fractionator (West et al. 1991) was used to count the number of spermatocytes and spermatids because of the fact that they are the germ cells most vulnerable to damage caused by ROS (Bauchéet al. 1994). The size of the counting frame was 35 × 35 μm, the sampling grid was 2500 × 2500 μm and the periodicity of the section was 20.
The height of the counting frame was 8 μm, and the guard zones were defined at 1 μm from the upper and lower borders of the counting frame. Nuclei cells that intersected with the upper guard zone and with the lateral exclusion boundaries of the counting frame were excluded from the count. The estimated total number of germ cells was calculated by using the Stereo investigator 9 program in a semi-automatic stereological system (MicroBrightField Inc., Williston, VT, USA). The program estimates the total number of cells considering the total area of the region of interest and the average volume of the tissue sampled in various sites randomly taken through the sampled section. The counting was carried out at 100×, and the coefficient of error (Gundersen, m = 1) was <0.07.
Statistical analysis
All parametric results were analysed using one-way analysis of variance test (anova) followed by the Tukey test. Non-parametric results were analysed using the ‘Kruskal–Wallis test’. Fertility was evaluated using a chi square test. A P < 0.05 was considered significant.
Results
Short term (40 dpp)
The testes of all animals in the control, sham and sham-αT groups were localized in the scrotum. In animals with unilateral cryptorchidism, the left testis was localized in an inguinal position and the contralateral testis in the scrotum.
The testicular weight of the groups with cryptorchid-vehicle and cryptorchid-αT (30 and 100 mg/kg) showed a decrease when compared to control, sham-vehicle and sham-αT groups (Table 1).
Table 1.
Effects of α-tocopherol on the lipoperoxidation and morphological parameters of cryptorchidism testes in 40-day-old rats
| Controls groups | Cryptorchid groups | ||||||
|---|---|---|---|---|---|---|---|
| Control | Sham-Vehicle | Sham αT-30 | Sham αT-100 | Vehicle | αT-30 | αT-100 | |
| Testis weight (g) | 0.88 ± 0.032† | 0.80 ± 0.031† | 0.79 ± 0.012† | 0.87 ± 0.043† | 0.62 ± 0.018* | 0.68 ± 0.016* | 0.54 ± 0.034*‡ |
| TBARS (nmoles of TBARS per mg of protein) | 0.14 ± 0.007† | 0.14 ± 0.002† | 0.12 ± 0.001† | 0.13 ± 0.001† | 0.20 ± 0.002* | 0.15 ± 0.001† | 0.17 ± 0.001* |
| HI | 0.80 ± 0.05† | 0.94 ± 0.02† | 0.56 ± 0.02† | 0.50 ± 0.02b | 8.31 ± 0.16* | 6.81 ± 0.18*† | 7.00 ± 0.08*† |
| EA (μm2) | 42.57 ± 0.82† | 38.79 ± 0.52*† | 41.63 ± 2.05† | 47.04 ± 1.08*† | 34.99 ± 0.88* | 32.17 ± 0.78* | 30.48 ± 0.68*† |
| AI | 1.37 ± 0.10† | 1.10 ± 0.07† | 1.24 ± 0.08† | 0.99 ± 0.13† | 10.93 ± 1.08* | 4.81 ± 0.49*† | 6.28 ± 0.32*† |
| PI | 7.76 ± 1.12 | 11.43 ± 1.46 | 9.92 ± 1.61 | 12.27 ± 1.49* | 5.56 ± 0.50 | 8.36 ± 0.84 | 9.41 ± 0.85 |
Data were collected from six animals per group and are expressed as the mean ± SEM.
αT, α-Tocopherol; TBARS, thiobarbituric acid-reactive substances; HI, histopathological index; EA, epithelial area; AI, apoptosis index; PI, proliferation index.
P < 0.05 (vs. control).
P < 0.001 (vs. cryptorchid-vehicle).
P < 0.001 (cryptorchid-αT-30 vs. cryptorchid-αT-100).
Lipoperoxidation in different groups was determined using the TBARS technique, which was present in low concentrations in the testes of the control, sham-vehicle and sham-αT (30 and 100 mg/kg) (P > 0.05) (Table 1). The values increased significantly (+42%, P < 0.05) in testes from the cryptorchid-vehicle group compared to the control group. In cryptorchidism, animals that had been administered αT (30 and 100 mg/kg), lipoperoxidation was reduced by −25% (P < 0.05) and −15%, respectively (Table 1), in comparison with the cryptorchid-vehicle group. The cryptorchid-αT with 30 mg/kg showed no significant differences compared to the control group; however, the criptorchid-αT-100 group was significantly different from the control group.
Histologically, the testicles of the control, sham-vehicle and sham-αT groups showed normal cytoarchitecture, characterized by seminiferous tubules in different stages of the seminiferous epithelial cycle and by germ cells in different stages of development from spermatogonia to round spermatids. Some tubules showed elongated spermatids (Fig. 1a,b,c). Testicles of animals in sham-vehicle and sham-αT groups (30 and 100 mg/kg) showed no significant differences, compared to the control group, in most of the histological parameters evaluated (HI, EA, AI and PI) (Fig. 2a,d; Table 1).
Figure 1.
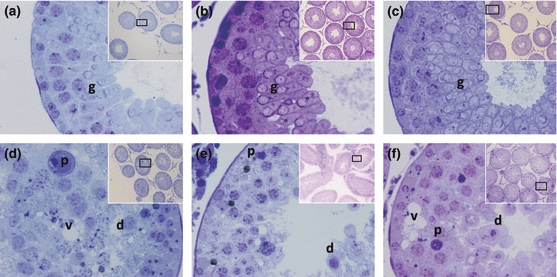
Histological images of seminiferous tubules from 40 dpp animals in different experimental conditions. (a–c) Healthy control, sham-vehicle and sham-αT-100, respectively, in which normal cytoarchitecture is observed with abundant germ cells (g). (d) Cryptorchid-vehicle showing cellular desquamation (d), pyknosis (p) and vacuolization (v). (e) Cryptorchid-αT-30 showing cellular desquamation (d), pyknosis (p) and spermatogenesis detention. (f) Cryptorchid-αT-100 showing cellular desquamation (d), pyknosis (p) and vacuolization (v) (60×). The inset shows a panoramic view of seminiferous tubules (10×), toluidine blue.
Figure 2.
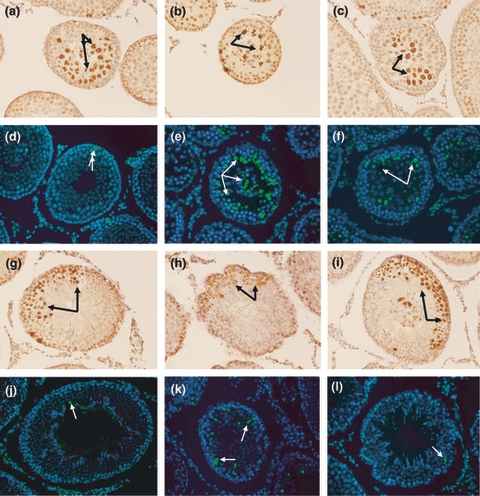
(a–f) Seminiferous tubules from 40 dpp animals with different experimental conditions. (a–c) Control, cryptorchid-vehicle and cryptorchid-αT-30, respectively, proliferating cells (arrow). (d) Apoptotic control cells (arrow). (e) Cryptorchid-vehicle showing a large number of apoptotic cells (arrow), both desquamated cells and spermatocytes. (f) Cryptorchid-αT-30 showing some spermatocytes and spermatids in apoptosis (arrow). (g–l) Seminiferous tubules from 120 dpp animals under different experimental conditions. (g–i) Control, cryptorchid-vehicle and cryptorchid-αT-30, respectively, showing a large amount of proliferative cells (arrow), even in the cryptorchid-vehicle group. (j) Seminiferous tubules with an apoptotic cell (arrow) from the control group. (k) Spermatocytes undergoing apoptosis (arrow) from cryptorchid-vehicle group. (l) Cryptorchid-αT-30 with a smaller number of apoptotic cells (arrow) compared to the cryptorchid-vehicle group. Immunocytochemistry (20×) using the H3S anti-histone antibody and TUNEL technique to detect apoptosis.
The seminiferous tubules of animals from cryptorchid-vehicle and cryptorchid-αT groups (30 and 100 mg/kg) showed cell desquamation, folding of the basal lamina, vacuolization of germ and Sertoli cells, pyknosis, giant cells and hypoplasia of germ cells (Fig. 1d,e,f). In these three groups, the HI, EA and AI were significantly affected with respect to the control group (Table 1). The cryptorchidism-vehicle group had a significant increase in HI and AI with respect to cryptorchidism-αT-30 and 100 mg/kg groups (Fig. 2b,c,e,f). The most advanced germ cells were the round spermatids (Table 1 and Fig. 1d–f).
Long term (120 dpp)
The testicles of all the animals were localized in the scrotum. The testicular weight of the animals in the chryptorchid-vehicle group was significantly reduced when compared with control (−30%), sham-vehicle (−28%), sham-αT-30 (−28%) and sham-αT-100 (−32%) groups. However, in the cryptorchid-αT-30 group, the reduction in the testicular weight was approximately of −12% without a significant difference when compared to control, sham-vehicle and sham-αT groups. Although it also did not show any difference with the cryptorchid-vehicle group, the testicular weight in the criptorchid-αT-100 group showed significant difference with the control group and no difference with the cryptorchid-vehicle group (Table 2).
Table 2.
Effects of α-tocopherol on the lipoperoxidation and morphologic parameters of cryptorchidism testes in rats at 120 days of age
| Controls groups | Cryptorchid groups | ||||||
|---|---|---|---|---|---|---|---|
| Control | Sham-Vehicle | Sham αT-30 | Sham αT-100 | Vehicle | αT-30 | αT-100 | |
| Testis weight (g) | 1.84 ± 0.063† | 1.78 ± 0.056† | 1.80 ± 0.017† | 1.90 ± 0.035† | 1.28 ± 0.096* | 1.61 ± 0.028 | 1.49 ± 0.142*‡ |
| HI | 0.25 ± 0.04† | 0.33 ± 0.05† | 0.31 ± 0.04† | 0.27 ± 0.05† | 3.08 ± 0.21* | 0.92 ± 0.08*† | 1.49 ± 0.11*†‡ |
| EA (μm2) × 103 | 79.91 ± 1.10† | 74.75 ± 8.94† | 74.73 ± 8.41† | 74.51 ± 8.24† | 45.71 ± 2.76* | 75.22 ± 1.57† | 68.93 ± 0.82*†‡ |
| AI | 1.00 ± 0.13† | 1.19 ± 0.12† | 1.08 ± 0.11† | 0.53 ± 0.04† | 3.24 ± 0.26* | 1.07 ± 0.11† | 1.48 ± 0.16† |
| PI | 12.21 ± 1.64 | 11.68 ± 1.78† | 12.65 ± 1.21† | 15.71 ± 1.14 | 10.0 ± 1.21 | 12.67 ± 1.33 | 11.68 ± 1.78 |
| JI | 10.0 ± 0† | 9.74 ± 0.09† | 9.93 ± 0.25† | 10 ± 0.01† | 8.43 ± 0.38* | 9.83 ± 0.07† | 9.74 ± 0.07† |
| Spermatocytes and spermatids number (×106) | 82.64 ± 5.52† | 78.37 ± 14.44† | 71.20 ± 1.48† | 78.41 ± 2.91† | 34.30 ± 3.67* | 60.51 ± 3.98 | 51.52 ± 2.44* |
| Pregnant female/total evaluated female | 12/12† | 8/12† | 10/12† | 12/12† | 2/12* | 6/12 | 5/12* |
The data were collected from six animals by group and are expressed as the mean ± SEM.
αT, α-tocopherol; TBARS, thiobarbituric acid-reactive substances; HI, histopathological index; EA, epithelial area; AI, apoptosis index; PI, proliferation index; JI, Johnsen index.
P < 0.05 (vs. control).
P < 0.001 (vs. cryptorchid-vehicle).
P < 0.001 (cryptorchid-αT-30 vs. cryptorchid-αT-100).
At this age, the seminiferous tubules of control and sham animals (vehicle, αT-30 and 100 mg/kg) had normal cytoarchitecture with abundant germ cells in different stages of the seminiferous epithelium cycle and spermatids in different phases of development: from round to elongated (Fig. 3a–c). The HI, EA, AI and PI of sham groups showed no differences in comparison with the control group (Table 2, Fig. 2g,j).
Figure 3.
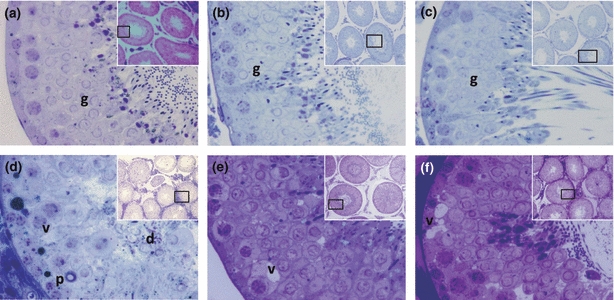
Histological tubules from 120 dpp animals with different experimental conditions. (a–c) Control, sham-vehicle and sham-αT-100, respectively, in which complete spermatogenesis is appreciated, as well as abundant germ cells (g) with normal cytoarchitecture. (d) Cryptorchid-vehicle showing obvious histological alterations such as cellular desquamation (d), epithelial vacuolization (v), pyknosis (p) with spermatogenesis detention in spermatide. (e) Cryptorchid-αT-30 with an obvious reduction in histological alterations compared to the cryptorchid-vehicle group, although some epithelial vacuoles still persist (v), complete spermatogenesis is appreciated. (f) Cryptorchid-αT-100 showing a clear reduction in histological alterations, although vacuoles (v) persist (60×). The inset shows a panoramic view of seminiferous tubules (10×), toluidine blue.
The seminiferous tubules of the animals from the cryptorchid-vehicle group showed histological alterations such as pyknosis, cellular desquamation, vacuolization of Sertoli cells and creasing of the basal lamina, until seminiferous tubules with only Sertoli cells and spermatogonia (Fig. 3d). The JI and EA of this group were significantly reduced, and the HI and AI increased in comparison with the rest of the groups (Table 2).
Application of αT in animals with cryptorchidism led to a reduction in HI and AI and an increase in JI and EA, as well as the presence of elongated spermatids, compared to the cryptorchid-vehicle group (Table 2, Fig. 3d–f). The HI in cryptorchid αT groups was significantly reduced by −70% and −51% with 30 and 100 mg/kg of αT, respectively, compared to the cryptorchid-vehicle group. The AI was also greatly reduced by −67% and −54% with 30 and 100 mg/kg of αT respectively. The JI increased by +16% and +15% with 30 and 100 mg/kg of αT, respectively, and the EA significantly increased by +64% and +50% with 30 and 100 mg/kg of αT respectively. Cellular proliferation did not seem to be modified in any of the groups. Favourable αT outcome was more evident with a 30 mg/kg dose, but only in some parameters (like AI and JI) were the values of the control group reached (Table 2, Fig. 2h,i,k,l).
The sterologic study showed that the germinal cell population was reduced significantly in the cryptorchid-vehicle compared with control (−58%), sham-vehicle (−56%), sham-αT-30 (−51%) and sham-αT-100 (−56%) groups. This reduction in the germinal cell population was less compared with control in the groups with cryptorchidism and administration of vitamin (cryptorchid-αT-30 of -26% and cryptorchid-αT-100 of −37%), and only the administration of 30 mg/kg was the one that reached the level of the control group, without showing a significant difference (P > 0.05). It is important to point out that the cryptorchid-αT-30 and αT-100 groups did not show a difference with the cryptorchid-vehicle group (Table 2).
All females in the control group were pregnant. There were fewer pregnant females in the sham-vehicle and sham-αT (30 and 100 mg/kg) groups. The number of pregnant females was also reduced in the cryptorchid-vehicle group, but the administration of αT led to an increase in pregnant females (at least 50%) in the two doses used (Table 2).
The fertility of the cryptorchid-vehicle group was significantly reduced compared with control, sham-vehicle and sham-αT groups. The administration of the vitamin in the cryptorchid-αT-30 and αT-100 groups increased the fertility to more than +50% without difference in the cryptorchid-vehicle group although and without achieving the fertility level of the control group (Table 2).
Discussion
It has been suggested that cryptorchidism is associated with a decrease in antioxidant enzyme activity (Zini & Schlegel 1997) or an increase in the production of ROS, such as superoxide anion, hydroxyl radical, nitric oxide and hydrogen peroxide (Zini & Schlegel 1997; Kumagai et al. 2002; Ishii et al. 2005), which stimulates lipoperoxidation (Janero 1990). Antioxidant treatments could increase a cell's endogenous antioxidant defense system, inhibiting ROS production or working to trap free radicals and impede lipoperoxidation (Tilly & Tilly 1995). Attempts have been made to decrease the damage caused by free radicals in cryptorchidism (Kumagai et al. 2002; DeFoor et al. 2004), but there are no reports on the use of αT as a powerful antioxidant in this pathology.
αT is an important antioxidant, found mainly in cell membranes, that acts by interrupting the chain reaction involved in lipid peroxidation and traps ROS generated during the univalent reduction in molecular oxygen (Traber & Kayden 1987; Sen Gupta et al. 2004; Shirpoor et al. 2007; Aybek et al. 2008). αT has been shown to decrease lipoperoxidation of different organs undergoing oxidative stress (Nemmiche et al. 2007).
The results of this study confirm that the generation of lipoperoxidation plays an important role in the cryptorchid testicular damage mechanism. The administration of αT significantly reduces lipoperoxidation because of the αT action mechanism. This mechanism has been suggested to decrease lipoperoxidation by ceding hydrogen from the hydroxyl group in position 6 of its ring, in addition to its capacity to stabilize radicals produced during this process (Traber & Kayden 1987), reducing the ROS and the histological damage caused by them, leading to the conservation of spermatogenesis and increasing fertility. It has also been reported that αT reduces damage caused by ROS generated by exposure to metals and toxic substances inside the testicle (Lucesoli & Fraga 1999; Jedlinska-Krakowska et al. 2006; Acharya et al. 2008).
Short-term observation of animals with cryptorchidism without the administration of αT had a number of alterations similar to those described previously in testicles subjected to elevated temperature (Huff et al. 1989; Brehm & Steger 2005), such as the reduction in the EA, maturation index and testicular weight. These alterations are possibly owing to the fact that spermatocytes and spermatids are the germ cells most vulnerable to damage caused by ROS because, even though they are capable of converting superoxide anion to hydrogen peroxide, they have difficulty metabolizing peroxide, which saturates its protective system against peroxide that becomes a highly toxic hydroxyl radical (Bauchéet al. 1994).
In our study, αT was shown to partially protect rat testicles exposed to cryptorchidism, which was evaluated at short term because of the extrascrotal position of the testicle at this age. Protection by αT was more obvious at 120 dpp (long term), because of the testicle's position in the scrotum and at the 30 mg/kg/day dose, wherein there was an increase in JI, EA, testis weight, number of spermatocytes and spermatids and a decrease in HI and AI.
In the cryptorchid-vehicle group, even with orchidopexia, histological alterations were similar to those mentioned previously. Desquamation of their germ cells was probably due to the destabilization of Sertoli cell membranes caused by lipoperoxidation, which altered the cell union with germ cells. By administering αT, lipoperoxidation of the membrane was impeded, conserving cell union and increasing the amount of germ cells in the seminiferous epithelium, resulting in a greater EA and JI.
Cryptorchidism is associated with high levels of apoptosis in germ cells (Shikone et al. 1994). In this work, we showed that αT reduced apoptosis in germ cells. The mechanism through which this is believed to occur is through αT inhibition of the production of lipoxygenases, enzymes that initiate membrane lipoperoxidation and activate apoptosis (Maccarrone et al. 2001), promoting cell survival and reducing histological alterations to the seminiferous epithelium.
At long term, it is not enough to have performed an effective orchidopexia in addition to αT treatment because altered seminiferous tubules are seen in the testicles of these rats, besides a partial recovery of testicular weight and the number of germinal cells that although they did not show a significant difference with the control group, a significant increase was not seen when compared to the criptorchid-vehicle group. It is possible that there is a high degree of damage at the spermatogonia level that is probably permanent.
There is the possibility that the ROS or another damage way generates irreversible alterations in Sertoli cells, which inhibit the production of factors (including epidermal growth factors) that maintain the survival of germ cells. This factor has been used successfully for fertility in animal models with cryptorchidism and orchidopexy (Kurokawa et al. 2005).
The partial fertility recovery of the groups with cryptorchid and αT makes us think on an alternate damaging mechanism to the epididymis level. De Miguel et al. (2001) using immunohistochemical and morphometric techniques showed a reduced development of the human cryptorchid epididymis that could explain that surgical descent would probably not be able to completely reverse the fertility alterations.
It attracted our attention that by administering a 100 mg/kg/day dose of αT in the cryptorchidism group, the lipoperoxidation was not as reduced as in the 30 mg/kg/day group. This could be attributed to the saturation of the lipoperoxidation regeneration system. αT, by ceding hydrogen, oxidizes and gives rise to a new stable free radical (Burton & Traber 1990). However, αT reduction, or regeneration, is caused by ascorbic acid and glutathione peroxidase (Pita 1997). It is possible that the reduction in glutathione peroxidase seen in cryptorchid (Bauchéet al. 1994), and an elevated dose of αT (100 mg/kg/day), caused an accumulation of the αT radical (as there are less enzymes to reduce it) leading to an increase in the amount of free radicals.
Lastly, our results have been pointed out by different authors (Hadziselimovic 2008). Orchidopexia alone is not enough to completely restore spermatogenesis, justifying the search for new germinal epithelium protective substances in the cryptorchid testicle at a stage before orchidopexia.
Conclusion
Treatment with αT prior to orchidopexia, particularly at 30 mg/kg, partially protects the undescended testicle from damage caused by ROS (demonstrated by the significant improvement in seminiferous epithelium damage), restored spermatogenesis and increased fertility.
Acknowledgments
We are grateful to Pedro Medina Granados and Edgar Daniel Cervantes Arias for technical assistance. We thank Julieta Mendoza Torreblanca and Leticia Granados Rojas for their technical support from the Instituto Nacional de Pediatría, Stereological Unit. This work was supported by 12/2006 INP grants.
References
- Acharya UR, Mishra M, Patro J, Panda MK. Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod. Toxicol. 2008;25:84–88. doi: 10.1016/j.reprotox.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Ahotupa M, Huhtaniemi I. Impaired detoxification of reactive oxygen and consequent oxidative stress in experimentally cryptorchid rat testis. Biol. Reprod. 1992;46:1114–1118. doi: 10.1095/biolreprod46.6.1114. [DOI] [PubMed] [Google Scholar]
- Aybek H, Aybek Z, Rota S, Şen N, Akbulut M. The effects of diabetes mellitus, age, and vitamin E on testicular oxidative stress. Fertil. Steril. 2008;90:755–760. doi: 10.1016/j.fertnstert.2007.01.101. [DOI] [PubMed] [Google Scholar]
- Bauché F, Fouchard MH, Jégou B. Antioxidant system in rat testicular cells. FEBS Lett. 1994;349:392–396. doi: 10.1016/0014-5793(94)00709-8. [DOI] [PubMed] [Google Scholar]
- Brehm R, Steger K. Regulation of Sertoli cell and germ cell differentiation. Adv. Anat. Embryol. Cell Biol. 2005;181:1–93. [PubMed] [Google Scholar]
- Burton GW, Traber MG. Vitamin E: antioxidant activity, bioavailability. Annu. Rev. Nutr. 1990;10:357–382. doi: 10.1146/annurev.nu.10.070190.002041. [DOI] [PubMed] [Google Scholar]
- Cooper BJ, Little TM. Orchidopexy: theory and practice. Br. Med. J. (Clin. Res. Ed.) 1985;291:706–707. doi: 10.1136/bmj.291.6497.706-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miguel M, Mariño JM, González PP, Nistal M, Regadera J. Epididymal growth and differentiation are altered in human cryptorchidism. J. Androl. 2001;22:212–225. [PubMed] [Google Scholar]
- DeFoor WR, Kuan CY, Pinkerton M, Sheldon CA, Lewis AG. Modulation of germ cell apoptosis with a nitric oxide synthase inhibitor in a murine model of congenital cryptorchidism. J. Urol. 2004;172:1731–1735. doi: 10.1097/01.ju.0000138846.56399.de. [DOI] [PubMed] [Google Scholar]
- Elder JS. The undescended testis: hormonal and surgical management. Surg. Clin. North Am. 1988;68:983–1005. doi: 10.1016/s0039-6109(16)44633-5. [DOI] [PubMed] [Google Scholar]
- El-Missiry MA. Enhanced testicular antioxidant system by ascorbic acid in alloxan diabetic rats. Comp. Biochem. Physiol. Pharmacol. Toxicol. Endocrinol. 1999;124:233–237. doi: 10.1016/s0742-8413(99)00070-5. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Das UB, Misro M. Protective role of alpha-tocopherol-succinate(provitamin-E) in cyclophosphamide induced testicular gametogenic and steroidogenic disorders: a correlative approach to oxidative stress. Free Radic. Res. 2002;36:1208–1218. doi: 10.1080/1071576021000016472. [DOI] [PubMed] [Google Scholar]
- Hadziselimovic F. Successful treatment of unilateral cryptorchid boys risking infertility with LH-RH analogue. Int. Braz. J. Urol. 2008;34:319–326. doi: 10.1590/s1677-55382008000300009. [DOI] [PubMed] [Google Scholar]
- Huff DS, Hadziselimovic F, Snyder HM, 3rd, Duckett JW, Keating MA. Postnatal testicular maldevelopment in unilateral cryptorchidism. J. Urol. 1989;142:546–548. doi: 10.1016/s0022-5347(17)38811-0. [DOI] [PubMed] [Google Scholar]
- Hutson J, Balic A, Nation T, Southwell B. Cryptorchidism. Semin. Pediatr. Surg. 2010;19:215–224. doi: 10.1053/j.sempedsurg.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Kodama H, Fukuda J, et al. Role of radical oxygen species in rat testicular germ cell apoptosis induced by heat stress. Biol. Reprod. 1999;61:393–399. doi: 10.1095/biolreprod61.2.393. [DOI] [PubMed] [Google Scholar]
- Ishii T, Matzuki S, Luchi Y, et al. Accelerated impairment of spematogenic cells in SOD1-knockout mice under heat stress. Free Radic. Res. 2005;39:697–705. doi: 10.1080/10715760500130517. [DOI] [PubMed] [Google Scholar]
- Janero DR. Malondialdehyde and thiobarbituric acid reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990;9:515–540. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- Jedlinska-Krakowska M, Bomba G, Jakubowski K, Rotkiewicz T, Jana B, Penkowski A. Impact of oxidative stress and supplementation with vitamins E and C on testes morphology in rats. J. Reprod. Dev. 2006;52:203–209. doi: 10.1262/jrd.17028. [DOI] [PubMed] [Google Scholar]
- Johnsen SG. Testicular biopsy score count – a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 1965;27:137A. [Google Scholar]
- Kumagai A, Kodama H, Kumagai J. Xanthine oxidase inhibitors suppress testicular germ cell apoptosis induced by experimental cryptorchidism. Mol. Hum. Reprod. 2002;8:118–123. doi: 10.1093/molehr/8.2.118. [DOI] [PubMed] [Google Scholar]
- Kurokawa S, Kojima Y, Mizuno K, Nakane A, Hayashi Y, Kohri K. Effect of epidermal growth factor on spermatogenesis in the cryptorchid rat. J. Urol. 2005;174:2415–2419. doi: 10.1097/01.ju.0000180414.81767.68. [DOI] [PubMed] [Google Scholar]
- Lee PA, Coughlin MT. Fertility after bilateral cryptorchidism. Evaluation by paternity, hormone, and semen data. Horm. Res. 2001;55:28–32. doi: 10.1159/000049960. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lucesoli F, Fraga CG. Oxidative stress in testes of rats subjected to chronic iron intoxication and α-tocopherol supplementation. Toxicology. 1999;132:179–186. doi: 10.1016/s0300-483x(98)00152-8. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Melino G, Finazzi-Agrò A. Lipoxygenases and their involvement in programmed cell death. Cell Death Differ. 2001;8:776–784. doi: 10.1038/sj.cdd.4400908. [DOI] [PubMed] [Google Scholar]
- Nemmiche S, Chabane-Sari D, Guiraud P. Role of alpha-tocopherol in cadmium-induced oxidative stress in Wistar rat's blood, liver and brain. Chem. Biol. Interact. 2007;170:221–230. doi: 10.1016/j.cbi.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Peltola V, Huhtaniemi I, Ahotupa M. Abdominal position of the rat testis is associated with high level of lipid peroxidation. Biol. Reprod. 1995;53:1146–1150. doi: 10.1095/biolreprod53.5.1146. [DOI] [PubMed] [Google Scholar]
- Pita RG. Funciones de la vitamina E en la nutrición humana. Rev. Cuba. Aliment. Nutr. 1997;11:46–57. [Google Scholar]
- Ríos C, Santamaría A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem. Res. 1991;16:1139–1143. doi: 10.1007/BF00966592. [DOI] [PubMed] [Google Scholar]
- Sen Gupta R, Sen Gupta E, Dhakal BK, Takhur AR, Ahnn J. Vitamin C and vitamin E protect the rat testes from cadmium-induced reactive oxygen species. Mol. Cells. 2004;17:132–139. [PubMed] [Google Scholar]
- Shikone T, Billing H, Hsueh AJ. Experimentally induced cryptorchidism increases apoptosis in rat testis. Biol. Reprod. 1994;51:865–872. doi: 10.1095/biolreprod51.5.865. [DOI] [PubMed] [Google Scholar]
- Shirpoor A, Ansari MH, Salami S, Pakdel FG, Rasmi Y. Effect of vitamin E on oxidative stress status in small intestine of diabetic rat. World J. Gastroenterol. 2007;13:4340–4344. doi: 10.3748/wjg.v13.i32.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shono T, Zakaria O, Imajima T, Suita S. Extra-abdominal fixation of the gubernaculum inhibits testicular descent in newborn rats. J. Pediatr. Surg. 1996;31:503–506. doi: 10.1016/s0022-3468(96)90483-2. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology. 1995;136:242–252. doi: 10.1210/endo.136.1.7828537. [DOI] [PubMed] [Google Scholar]
- Traber MG, Kayden HJ. Tocopherol distribution and intracellular localization in human adipose tissue. Am. J. Clin. Nutr. 1987;46:488–495. doi: 10.1093/ajcn/46.3.488. [DOI] [PubMed] [Google Scholar]
- Vigueras VRM, Molina OD, Reyes TG, et al. Effect of allopurinol on damage caused by free radicals to cryptorchid testes. Acta Histochem. 2009;111:127–137. doi: 10.1016/j.acthis.2008.05.004. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zini A, Schlegel PN. Cu/Zn superoxide dismutase, catalase and gluthatione peroxidase mRNA expression in the rat testis after surgical cryptorchidism and efferent duct ligation. J. Urol. 1997;158:659–663. [PubMed] [Google Scholar]


