Abstract
The present study investigated performance of unimanual and bimanual anti-phase and in-phase upper limb line drawing using three different types of cues. Fifteen Parkinson’s disease (PD) patients, 15 elderly, and 15 young adults drew lines away from and towards their body on a tabletop every 1000 ms for 30 s under three different cueing conditions: (1) verbal (‘up’, ‘down’); (2) auditory (high tone, low tone); (3) visual (target line switched from top to bottom). PD patients had larger and more variable amplitudes which may be related to the finding that they also produced more curvilinear movements than young and elderly adults. Consistent with previous research, when compared to the elderly and young adult group PD patients produced a mean relative phase which deviated more from the instructed coordination modes and they showed larger variability of relative phase in bimanual coordination, especially in anti-phase conditions. For all groups, auditory and verbal cues resulted in lower coefficient of variance of cycle time, lower variability of amplitude and lower variability of relative phase than visual cues. The benefit of auditory cues may be related to the timing nature of the task or factors related to the auditory cues (e.g., reduced attentional demands, more kinesthetic focus).
Keywords: Timing, Motor control, Drawing, Cues, Movement disorders
Parkinson’s disease (PD) results in sensorimotor deficits (see Abbruzzese & Berardelli , 2003). This is likely because PD is associated with basal ganglia dysfunction, which is part of the network associated with the generation of sensory related potentials (Ikeda et al., 1994), which have been shown to be reduced in PD patients (Thobois et al., 2000; Cunnington et al., 2001). This alteration in sensorimotor function has resulted in testing different cueing strategies that could be used to enhance motor function. It has been proposed that rhythmic cueing may circumvent the difficulties experienced by PD patients when performing voluntary initiated and continued rhythmic tasks (see for example Rubinstein, Giladi, & Hausdorff, 2002). Early research suggested that PD patients rely on vision (Cooke, Brown, & Brooks, 1978), however, a review examining several studies on the effects of external rhythmical cueing on gait in PD patients concluded that there was “strong evidence for improving walking speed with the help of auditory cues” but that there was “insufficient evidence for the effectiveness of visual and somatosensory cueing” (Lim et al., 2005, p. 695). However, the majority of research investigating the effects of external cues on motor tasks in PD patients has been focused on locomotion, rather than rhythmic timing and coordination of upper extremity tasks. Improvements in upper extremity motor function have the potential to greatly enhance PD patients quality of life as the vast majority of PD patients report having ‘clumsy hands’ (Jankovic, 1987). Successful performance of manual tasks, such as writing, dressing, eating, etc. are important activities of daily living which require complex coordination between upper limb segments. Despite the importance of upper extremity motor function, the impact of providing cues to improve upper extremity performance of PD patients has not been examined systematically.
Neuroanatomical (Kraaft et al., 2007; Wu, Wang, Hallett, & Chan, 2010) and animal (Kermadi, Liu, Tempini, Calciati, & Rouiller, 1998) studies have indicated that the basal ganglia contributes to supplementary motor area (SMA) function and bimanual coordination. Only a few studies have examined the influence of auditory cues on bimanual timing and coordination of the upper limbs. For example, Almeida, Wishart, and Lee (2002) examined PD patients’ bimanual in-phase and anti-phase rhythmic movements with and without an auditory cue and they concluded that coordination did not benefit significantly from an auditory cue. However, Johnson et al. (1998) reported that PD patients performing in-phase and anti-phase cranking movements, executed the in-phase movements with smaller differences between the hands (e.g., relative phase) and lower variability of relative phase using external auditory information, although no improvement in coordination occurred during the anti-phase movements. Thus, the benefits of auditory cues in rhythmical bimanual tasks are found to be inconsistent.
Other studies have investigated visual cues in bimanual movements in PD patients. Recently, Nieuwboer et al. (2009) examined repetitive anti-phase drawing with and without visual cues and found that visual cues decreased coordination variability in PD and controls. Byblow, Lewis, and Stinear (2003) examined continuous bimanual wrist flexion-extension movements in which one hand was passively driven by a motor and one hand was actively moved. They examined attention focus by manipulating vision. The vision conditions included vision of both hands, vision of the passive hand, vision of the active hand and no vision. They found decreases in amplitude variability with vision of the passive hand. Verheul and Geuze (2004) examined in-phase and anti-phase leading with left hand, and anti-phase leading with the right hand bimanual tapping movements and also manipulated visual feedback. Their results showed that PD patients had lower mean and variability of relative phase than a healthy comparison group when eliminating vision of both hands and when one hand was occluded it decreased coordination stability.
Research on rhythmic unimanual movements in PD is even sparser. Stegemoller, Simuni and MacKinnon (2009) examined unimanual tapping in which repetitive taps were performed opposite to an auditory cue. While this created a complex task, the cue was not used with the intention of improving performance. Ponsen et al. (2006) examined unimanual writing of sentences among other unimanual tasks and found decreased letter height as writing progressed in PD patients but not in the control group. This finding is consistent with symptoms of micrographia (Lewitt, 1983; van Gemmert, Teulings, & Stelmach, 2001) and hypometria (van Gemmert, Adler, & Stelmach, 2003). One study examined the influence of visual and verbal cues in rhythmic unimanual drawing of cursive l’s and found that while amplitude increased with both cues from a no cue comparison task, they did not find any differences between visual and verbal cueing. The cues in this study, however, were constantly given and were not related to the timing of the movements (Oliveira, et al. 1997). Taken together, the need emerges that more research is required to determine the influence of different cueing types on manual coordination in PD patients. To our knowledge there has not been a study to compare different cuing types (e.g., visual, auditory, verbal) among unimanual, bimanual in-phase and bimanual anti-phase movements within one study.
Based on research with unimanual handwriting-like movements we predicted that PD would produce smaller amplitudes in line drawing in the direction of movement (e.g., the y dimension) than young adult and elderly comparison groups. Based on the fact that PD patients show increased movement variability (Romero, van Gemmert, Adler, Bekkering, & Stelmach, 2003; Teulings, Contreras-Vidal, Stelmach, & Adler, 1997; Sheridan, Flowers, & Hurrell, 1987), we predicted more variable cycle times and amplitudes for PD patients than young adult and elderly comparison groups. Based on several bimanual coordination studies (Almeida et al., 2002, 2003; Byblow, Summers, & Thomas, 2000), we predicted that for all groups, anti-phase tasks would have higher means and variability of relative phase than the in-phase task, and that this would be exaggerated in PD patients. Based on Johnson et al. (1998), we predicted that auditory cueing would improve PD patients mean relative phase (e.g., closer to instructed coordination pattern) and decrease variability of relative phase in the bimanual in-phase task so that their performance is similar to young adult and elderly comparison groups. However, based on the same study (Johnson et al.,1998), we also predicted that PD patients will not be able to change their relative phase to be closer to instructed coordination in the anti-phase task irrespective of the used cue. Based on Byblow et al. (2003), we predicted that visual cues may also improve PD patients’ means and variability of relative phase in bimanual coordination. Although we are among the first to examine the effects of verbal rhythmic presented cues on manual performance in PD patients, we predict that they may benefit from it even more than auditory cueing because it provides direction information which should make the anchor points of up and down more salient (Byblow, Carson, & Goodman, 1994).
2. Method
Participants
Forty-five right-handed people participated in this study; the first group consisted of 15 PD patients (M age = 71.1 yrs. SD age = 8.6 yrs.). PD participants performed while on medication and averaged 18.3 on subscale III (motor exam) of the Unified Parkinson’s Disease Rating Scale (UPDRS) (Fahn & Elton, 1987). Refer to Table 1 for PD participant characteristics. Two additional groups were used for comparison; one consisting of 15 elderly (M age = 69.3 yrs. SD age = 8.1 yrs.), and the second consisting of 15 young adults (M age = 25.6 yrs., SD age = 5.1 yrs.). All elderly participants met the cutoff score of 25 (out of a maximum of 30) on the Mini Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975). All participants indicated that they had normal or corrected-to-normal vision and/or hearing. To reliably assess handedness, participants were screened using six items of a handedness inventory (Oldfield, 1971). For this test, all participants wrote their name with a pen, drew a circle with a pen, used scissors to cut paper, threw a tennis ball, pretended to eat with a spoon, and pretended to brush their teeth. If, at least four of the six items were performed with the right hand, the participant was included in the experiment. Upon arrival participants either read, or were read, and signed the informed consent form. All protocols were approved by the Human Subjects Institutional Review Board of Arizona State University and in compliance with ethical principles for medical research involving human subjects as adopted in the declaration of Helsinki.
Table 1.
Participant Information
| Participant Number | Gender | Age | UPDRS-III (motor subscale) |
|---|---|---|---|
| 1 | M | 81 | 45 |
| 2 | M | 57 | 35 |
| 3 | M | 72 | 14 |
| 4 | M | 66 | 4 |
| 5 | M | 62 | 17 |
| 6 | M | 64 | 9 |
| 7 | M | 71 | Not available |
| 8 | F | 84 | 10 |
| 9 | F | 75 | 22 |
| 10 | M | 74 | 11 |
| 11 | M | 60 | 19 |
| 12 | M | 77 | 20 |
| 13 | M | 83 | 18 |
| 14 | M | 69 | 14 |
| 15 | M | 71 | Not available |
Tasks and Apparatus
All participants performed drawing movements on a digitizer tablet (Wacomtm Intuos 12 × 18 inches). The hands were covered by a box with an opening that faced the participants. Black material was draped over this opening so that the participants’ hands could enter and move under the box but participants could not see their hands. Although participants did not see their hands, real time tracings of the actual movement of the styli displayed on a computer screen in front of the participants which provided feedback about their hand movements. Movements of the styli were of exactly the same size as the on-line feedback on the computer monitor (i.e., one-to-one mapping). Previous research has shown that performance on this task is less variable in coordination while viewing response produced feedback than no vision or just vision of the hands (Ringenbach, Mulvey, van Gemmert, Stankus, & Maraj, 2009). In addition, there was a 2 cm wide piece of wood down the center of the box that separated the hands. This ensured that the styli participants held in their hands would not touch each other.
The task was to draw continuous lines away from and towards the body on a flat surface, The amplitude of the lines was specified by two horizontal target lines on a computer monitor in front of the participants which provided upper and lower limits of line drawing (e.g., y dimension) in the horizontal plane (further referred to as vertical lines). This was performed with a stylus in each hand. There were five tasks performed with a stylus in each hand 1) Unimanual Right 2) Unimanual Left 3) Bimanual In-phase: both hands following the cues at the same time 2) Bimanual Anti-phase Left: The left hand moving to cues and the right hand moving opposite the cues 3) Bimanual Anti-phase Right: The right hand moving to cues and the left hand moving opposite the cues. The cues indicated when to hit the horizontal target lines that were separated by 8 cm, with a 0.5 cm target width. The cue conditions were:
Visual: Top or bottom target line flashed on the computer monitor
Auditory: High pitch tone, low pitch tone heard from computer speakers
Verbal: Female voice saying “up”, “down” heard from computer speakers
All cues were presented for durations of 740 ms and there was 1000 ms between each cue presentation, which cycled for 30 s for each trial. The visual metronome was displayed at roughly eye level on a computer screen that was approximately 80 cm in front of the participants. For the auditory metronome, the direction was not specified for the high or low tones. Therefore depending on when and where the participant began moving, a high tone could have specified the up or down direction. In contrast, the verbal metronome specified a direction. The target lines on the computer were presented during the entire trial for the auditory and verbal conditions and either the top or the bottom line was presented during the visual metronome condition.
2.4. Procedure
All participants were seated in a chair at a table at a comfortable height. Next, the styli were placed in the left and right hands. The participants were asked to continuously draw lines for 30 s. Before the experiment started the participants practiced and were asked to demonstrate that they understood the task. The instructions emphasized keeping in time with the cues. The order of the cue conditions was counter-balanced across participants. A total of 45 trials were completed by each participant (3 trials for each cue of the 3 cue types and 5 tasks). There was individualized rest between trials. The testing session lasted approximately 60 minutes.
Data processing
The x and y position of each stylus was sampled at 99 Hz with a resolution of 1000 lines per centimeter. The data in the y dimension represented the movement in the anterior-posterior (e.g., towards and away from the body) direction, which was the main direction of movement. Data also was collected in the x dimension in the medio-lateral (e.g., side-to-side) direction. The data obtained from the digitizer tablet were filtered using a dual-pass 5th order, 6 Hz cut-off, low-pass Butterworth filter. For differentiation of the filtered data, a three-point central difference technique was used1. All graphical and numerical techniques were completed using the Matlab™ mathematical programming environment.
Dependent Measures
The dependent measures used to describe the task characteristics included coefficient of variation of cycle time, mean and standard deviation (SD) of amplitude in the direction of movement (y dimension), and aspect ratio. A movement time for each cycle was computed using normalized y displacement data. An interactive computer graphic routine over-layed a criterion line on the displacement data at zero. This line could be moved during data analysis to eliminate small deviations or pauses2 at the direction changes that were not true cycles (see Robertson et al., 1999). This method has been used with typical adults and is used in this study to calculate accurate cycle times. Successive points of minimum displacements below this line were picked as markers, then sample numbers between these two points were converted into milliseconds as our measure of cycle time which included one away from the body and towards the body motion in the y dimension. Thus, this value was divided in half to calculate the average time to move up and down. The dependent measure of coefficient of variation of cycle time was calculated by dividing the SD of each cycle time by the mean of each cycle time, which was averaged over a 10 s trial. This was our main measure of timing because there were individual differences in cycle times among our groups, which alone influences cycle time variabilities, Using the same method, mean amplitude was calculated cycle by cycle in both the x and y dimensions and was then averaged over a trial. The mean amplitude in the y dimension was our main measure of movement size because it represented the amplitude in the instructed direction of movement. It was calculated as the difference from the largest amplitude point to the smallest amplitude point within one cycle and the within subject variability provided our measure of SD of amplitude. The mean amplitude in the x dimension was the difference from the farthest right side point to the farthest left side point within one cycle. The amplitude in the x dimension was only used in our mean aspect ratio calculation in which the amplitude in the x dimension was divided the amplitude in the y dimension. Thus, aspect ratio is our dependent measure that provides an indication of line straightness (Franz et al., 1991). Roughly, if the participant drew a straight line, the amplitude ratio would be close to zero.
For the bimanual tasks, relative phase was our dependent measure of between-hand coordination, which was calculated in the y dimension. To calculate a continuous estimate of relative phase, the displacement and velocity records for every cycle of each hand were normalized to the normal distribution. Relative phase was determined by calculating the absolute difference between the phase angle of the nondominant hand from the phase angle of the dominant hand at each sample. The mean relative phase within a trial was calculated. Based on previous research (see Carson et al., 1997; Robertson, 2001; Scholtz and Kelso, 1990), a range of ± 45° was used to represent specific coordination patterns. Relative phase values between 0 and 45° represented an in-phase coordination pattern (e.g., both hands moved away and towards the body simultaneously), values between 135 and 180° represented an anti-phase coordination pattern (e.g., when one hand moved away from the body the other hand moved towards the body), and 46 - 134° represented an intermediate-phase coordination pattern (i.e., one hand to lagged behind the other). SD of relative phase was also calculated to determine between hand coordination consistency.
Analyses
For all analyses, the first trial was eliminated as a practice trial and the remaining two trials were averaged. For individual hand analyses, a mixed-factorial ANOVA with a between-groups variable of group (young adult, elderly, PD) and three repeated measures variables of Task (unimanual, bimanual in-phase), cue type (auditory, verbal, visual), and hand (right, left) was conducted on each of the dependent variables of coefficient of variation of cycle time, mean and SD of amplitude in the y dimension, and aspect ratio.
For coordination analyses, a mixed ANOVA with a between-subject variable of group (young adult, elderly, PD) and two repeated measures of task (anti-phase right, anti-phase left, in-phase) and cue type (auditory, verbal, visual) were conducted on both mean and SD of relative phase.
Huynh-Feldt correction used to determine the significance of the ANOVA statistics are reported throughout. Tukey HSD procedures were used when between groups main effects or interactions proved to be significant. Unless otherwise stated, alpha level was set at p < .05.
3.0 Results
Individual hand measures
Coefficient of variation of cycle time (CV)
For CV, there was a main effect of cue type, F(2, 84) = 19.00, p = .000, η2 = .311. Post hoc analysis indicated that CV was lower (i.e., more consistent in timing) with the auditory cue than the visual and verbal cues and that the verbal cue CV was lower than the visual cue (Mauditory = 7.69, Mverbal = 9.04, Mvisual = 10.60). All other main effects of group, task, and hand and related interactions were non-significant p’s > .1.
Amplitude
For the measure of mean amplitude in the y dimension, which was the direction of movement, there were main effects of group, F(2, 42) = 3.33, p < .046, η2 = .137, and hand, F(1, 42) = 5.70, p = .022, η2 = .119, while the main effect of cue showed a trend, F(2, 84) = 3.02, p = .056, η2 = .067. Post hoc analysis indicated that movement amplitude was larger in the PD group than the young adult group (MPD = 12.7 cm, Madult = 11.3 cm, Melderly = 11.8 cm) and it was larger in the left hand (12.1 cm) than the right hand (11.7 cm). Post hoc analysis of the cue conditions showed that the approaching significance effect of cue occurred as result of a slightly larger amplitude for the visual cue than the amplitudes found for the conditions with the verbal and auditory cues (Mauditory = 11.8 cm, Mverbal = 11.9 cm, Mvisual = 12.1 cm). The hand main in effect was mediated by a task by hand interaction, F(1, 42) = 10.51,p = .002, η2 = .200, in which the amplitudes were similar for unimanual right and unimanual left, whereas when the participants performed bimanual in-phase line drawing the left hand movements were larger than the right hand movements. All other main effects and interactions were non-significant, all p’s > .1.
For SD of amplitude, there was a significant main effect of group, F(2, 42) = 5.65, p = .007, η2 = .212, and task, F(1, 42) = 24.77, p = .000, η2 = .371 , and again cue type approached significance, F(2, 84) = 3.08, p = .051, η2 = .068. The task main effect was mediated by a task by hand interaction, F(1, 42) = 4.74, p = .035, η2 = .101. Post hoc analysis revealed that similar to the mean amplitude results, overall the bimanual tasks had larger variability of amplitudes than the unimanual tasks and the left hand produced larger variability in amplitudes than the right hand in the bimanual in-phase task, whereas the variability of amplitudes were similar in unimanual right and unimanual left line drawing. The group and cue type main effects were mediated by a group by cue type interaction, F(4, 84) = 3.25, p =.016, η2 = .134. Overall PD patients had larger variability in mean amplitude than both comparison groups and auditory cues resulted in the least variability of movement amplitude. As can be seen in Fig. 1 and confirmed by post hoc analysis, PD patients produced less variability in movement amplitude following auditory cues than visual cues, whereas both comparison groups performed similarly across all cue types. All other main effects and interactions were non-significant, all p’s > .1.
Fig. 1.
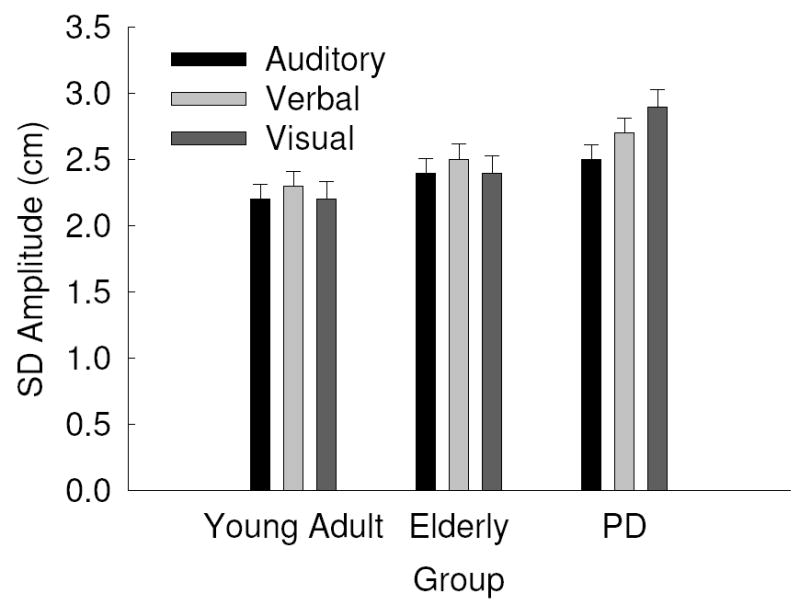
Standard Deviation (SD) of amplitude as a function of group by cue type with standard error bars.
Mean aspect ratio
The closer the ratio of the amplitude in the x and the y amplitudes is to 0.0, the straighter the line and the closer the aspect ratio is to 1.0, the closer the movement is to a circle. There was a main effect of group, F(2, 42) = 12.30, p = .000, η2 = .369. The group main effect was mediated by a trend of group by task interaction, F(2, 42) = 3.19, p = .051, η2 = .132. Post hoc analysis and Fig. 2 revealed that overall PD were more curvilinear in their movements than young adult and elderly comparison groups but that the young adult group produced more curvilinear movements during bimanual movements than unimanual movements, whereas both PD and elderly were similar in their movement path during both unimanual and bimanual tasks. All other main effects and interactions showed non-significant p’s (p > .1).
Fig. 2.
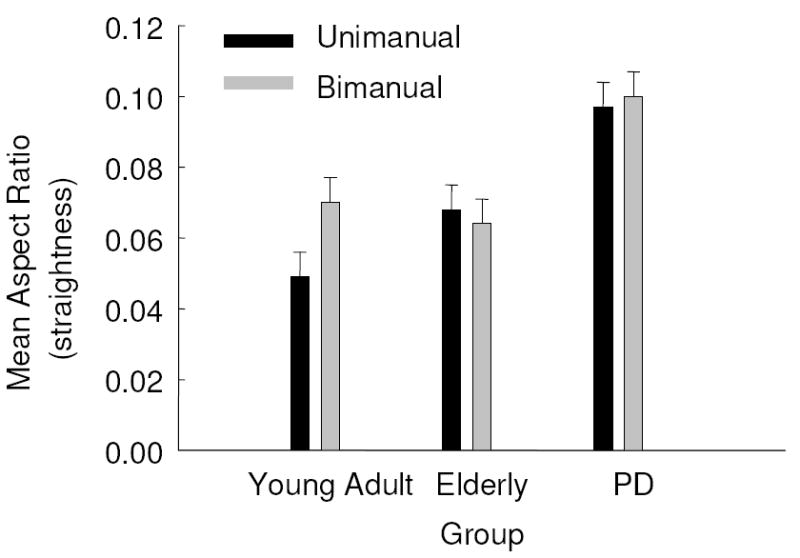
Mean aspect ratio as a function of group and task with standard error bars.
There were no significant differences for SD of aspect ratio, all p’s > .1.
Coordination measures
Relative phase
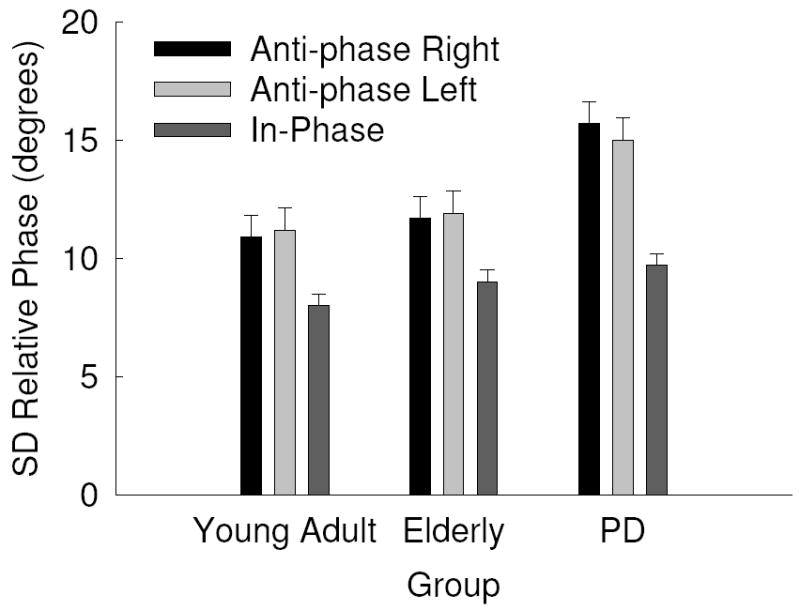
Relative phase values closer to 0.0 indicate that the right and left hand are at the same spatial position at the same point in time (i.e., symmetrical/in-phase). For the measure of mean relative phase, there were significant main effects for group, F(2, 42) = 4.28, p = .020, η2 = .169 and task, F(2, 84) = 26442.68, p = .000, η2 = .998. Overall, PD patients performed farther away from the target coordination mode than the young and elderly comparison groups and anti-phase tasks were performed with a different coordination pattern than the in-phase tasks. Both of these main effects were mediated by a group by task interaction, F(4, 84) = 4.32, p = .009, η2 = .171. As can be seen in Fig. 3 and confirmed by post hoc analysis, PD patients performed both anti-phase tasks farther from the instructed coordination pattern than both comparison groups. All other main effects and interactions were non-significant, all p’s > .1.
Fig. 3.
Standard Deviation (SD) of relative phase as a function of group and task with standard error bars.
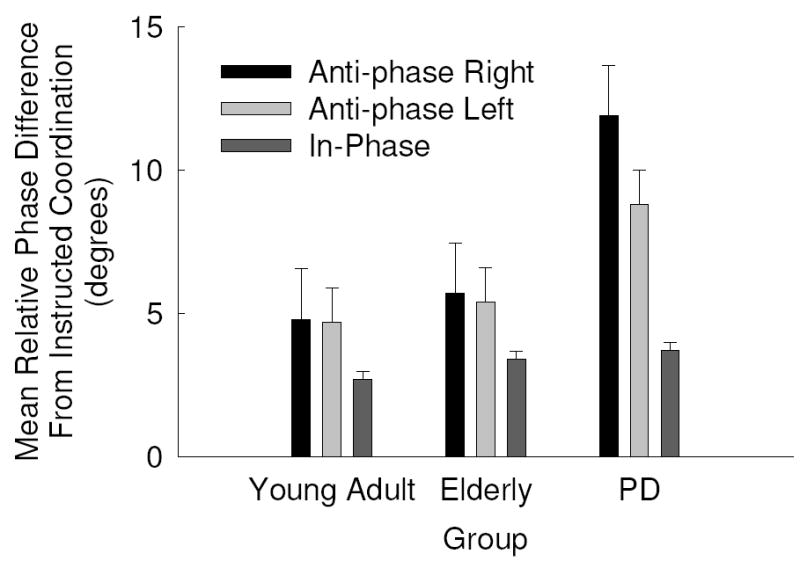
Overall the SD of relative phase results paralleled the mean relative phase results. There were significant main effects for group, F(2, 42) = 6.38, p = .004, η2 = .233, and task, F(2, 84) = 58.07, p = .000, η2 = .580, which were mediated by a group by task interaction, F(4, 84) = 3.70, p = .01, η2 = .150. Overall, PD patients produced more variability in bimanual coordination than the young and elderly comparison groups and all groups performed anti-phase tasks with more variability than the in-phase task. As can be seen in Fig. 4 and confirmed by post hoc analyses, PD were more variable in the anti-phase tasks than both comparison groups. In addition, the cue type main effect approached conventional levels of significance, F(2, 84) = 2.72, p = .075, η2 = .061, which revealed that coordination was performed more consistently using auditory cues than visual and verbal cues. All other main effects and interactions were non-significant p’s > .1.
Fig. 4.
Mean relative phase difference from instructed coordination mode as a function of group and task with standard error bars.
4.0 Discussion
The aim of this project was to determine the impact of auditory, visual and verbal cues on unimanual and bimanual anti-phase and in-phase performance among PD patients. Comparisons between the PD, older adult and young participants indicated PD patients’, actually showed greater relative improvements in bimanual motor performance following external movement cues. Improved upper extremity function, especially in tasks requiring a timing component, suggests cue-based interventions may be useful in improving upper extremity motor function in PD patients.
Hand differences
Unimanual tasks were produced with less variability in amplitude than bimanual in-phase movements. This is likely due to the similarity of the unimanual task to everyday tasks of writing and drawing. Surprisingly, there was a task by hand interaction in which there were no differences in amplitude between the right and left hands in the unimanual tasks, whereas the left hand had a larger amplitude than the right hand in the bimanual task. This result contradicts the concept of spatial assimilation in bimanual tasks (Franz et al., 1991) in which it is proposed that both hands are attracted or tend to move with similar spatial characteristics when performed together. Spatial assimilation has also been reported in PD patients and it was similar to typical adults performing bilateral arm pointing movements of varying distance and amplitudes (Stelmach & Worringham, 1988). Inspection of the means revealed that PD patients had the smallest difference in amplitude between their hands in the bimanual in-phase task which is consistent with Stelmach and Worringham’s (1998) findings in these patients. Because the young adult and elderly groups had larger differences between their hands, these groups are likely driving the task by hand interaction and hand differences in these groups should be researched further.
Also, the young adult group performed unimanual line drawing straighter than bimanual line drawing, whereas elderly and PD individuals performed similar strategies whether drawing with one hand or both hands. It is known that when an increase in the degrees of freedom necessary to perform a task, it increases the complexity and this has also been reported for individuals with PD (Seidler, Alberts, & Stelmach, 2001). Our results indicate that bimanual drawing is a more complex task for young adults; this was revealed in a strategy change, more curvilinear movements were used in the bimanual tasks when compared to unimanual tasks. This change in strategy was not found in older individuals and PD patients, whereas, unimanual drawing seem to be just as complex as bimanual drawing, suggesting that both tasks required similar strategies.
Group differences
Our results are consistent with our prediction that PD patients would be more variable in movement performance. However, our results are not consistent with our prediction that PD patients would produce reduced amplitudes. In the present study, PD patients actually produced larger movement amplitudes than both comparison groups. The fact that PD patients’ amplitudes were not reduced may be caused by the fact that micrographia only occurs in 10-15% of the PD population (McLennan, Nakano, Tyler, & Schwab, 1972) and that the visual feedback in all tasks showed the drawn line, thus it encouraged participants to hit or cross the target lines. Similarly, other research has not observed reduced amplitudes among PD patients and comparison groups (e.g., Dounskaia, van Gemmert, Leis, & Stelmach, 2009). However, the fact that PD patients’ amplitudes were about 1 cm larger is interesting. The differences in amplitude is likely related to the aspect ratio findings in which PD patients produced significantly larger values, which would indicate that their drawing movements were more curvilinear (e.g., elliptical) than both comparison groups. One explanation for their more elliptical movements is that PD is known to result into a reduced capability to regulate fine muscular forces (Hallett & Khoshbin, 1980; Pfann, Buchman, Comella, & Corcos, 2001; Stelmach et al., 1989; van Gemmert et al., 1999) and that turning on and off muscles in multijoint movements is challenging (Kelly & Bastian, 2005). Producing a continuous up and down straight line of the length required in our study includes a distinct movement reversal which requires changing the muscle activations of the biceps and triceps quickly, whereas this strategy is reduced when drawing an ellipse. Line drawing and circle drawing have been shown to be two different tasks (Spencer & Zelaznik, 2003) and discontinuous loops in hand writing have been shown to be more difficult than continuous loops in young children (Wann & Kardirkamanathan, 1991). Dounskaia et al. (2009) suggested that individuals with PD have a tendency to select “simple” coordination patterns in which muscular effort is minimized. Perhaps PD patients are using a different strategy to overcome timing and muscular deficits. Further research is required to examine the muscular activations in these drawing movements in PD.
Overall our coordination results are consistent with our prediction and the literature that PD patients would have larger means and variability of relative phase in bimanual movements than the comparison groups and PD patients would have larger mean and variability of relative phase in the anti-phase tasks as compared to the in-phase task as found by several studies with PD patients (Almeida et al., 2002; Byblow et al., 2000; Johnson et al., 1998; Ponsen et al., 2006; Serrien, Steyvers, Debaere, Stelmach, & Swinnen, 2000). One explanation is that anti-phase bimanual coordination variability is higher in PD patients than comparison groups because of the added task complexity that requires different timing of muscle activation in each hand (Johnson et al., 1998). Another explanation of the results that anti-phase bimanual coordination was more variable in coordination in PD patients than comparison groups may be an early reflection of the asymmetric progression of the disease (Verheul & Geuze, 2004). In fact, it has been suggested that deficits in anti-phase bimanual coordination may be a sensitive clinical evaluation of early PD (Johnson et al., 1998; Ponsen et al., 2006).
Furthermore, our results are consistent with our prediction that antiphase right and antiphase left tasks would be similar for all groups and is consistent with previous research demonstrating the benefit of the instructed attentional focusing in which enhanced attentional cueing provided by focusing on one hand could have equated variability of relative phase in both anti-phase conditions (Amazeen, Amazeen, Treffner, & Turvey, 1997). One previous explanation suggested for deficits in anti-phase bimanual movements was that they would require attention switching between each hand that may be difficult for PD patients (Johnson et al., 1998). Our results showed that variability of relative phase in anti-phase right and anti-phase left were not different from each other suggesting that attention focus is possible in PD patients but it did not eliminate observed deficits in anti-phase bimanual coordination.
Cue type effects
Our results concerning the influence of different cue types on movement performance are somewhat consistent with our predictions and previous research. We found that auditory cues did result in lower CV of cycle time, smaller amplitudes, and less variability in amplitude and coordination than visual cues. The benefit of auditory cues is consistent with similar research, even the prediction based on Johnson et al.’s (1998) research that PD patients will have poor coordination in anti-phase tasks regardless of cue. However, we did not find a benefit of visual cues as was reported in previous research (Byblow et al., 2003). This may have occurred because in previous research visual and auditory cues were not compared to each other but were compared to no cue conditions. Although this explanation may have merit, we did not include a no cue condition, therefore we cannot determine if visual cues were better than no cues. In addition, we predicted that PD patients may benefit from verbal cueing more than auditory because of the added direction information. We found an advantage for the verbal cues when compared to the visual ones, but there seemed to be no direct evidence that benefits of the verbal cues differed from auditory ones. This leads us to believe that in all groups auditory or verbal cues enhance continuous movement performance when compared to visual cues.
One explanation for the benefit of the auditory and verbal cues could have been related to the modality appropriateness hypothesis (Welch, Duttonhurt, & Warren, 1986), which suggests that timing tasks benefit from auditory information and spatial tasks benefit from visual information. Because the line drawing task was a fairly pure timing task, auditory information may have been more appropriate and both the auditory and verbal instructions have an auditory component.
Another explanation for the benefit of auditory cues was that it was easier to follow the auditory and verbal cues than the visual cues because they were provided in a different modality than the real time visual tracings of the performance. The visual cue in the present study may have resulted in eye movements between the visual tracings of the styli and the visual cues, which resulted in less consistent cycle times, increased line length and increased line length and coordination variability than the verbal and auditory cues (Ringenbach, van Gemmert, Shill, & Stelmach, 2005). Furthermore, the auditory and verbal cues did not interfere with the monitoring of the visual feedback of the tracings.
A third explanation is that auditory cues may allow for a kinesthetic focus which has been shown to increase brain activation (i.e. contingent negative variation) in PD patients (Lim et al., 2006). Contingent negative variation (CNV) is a movement and sensory related potential, which occurs in response to two successive stimuli. PD is associated with basal ganglia dysfunction, which is part of the network associated with the generation of the late CNV (Ikeda et al., 1994). In Lim et al.’s study (2006) PD patients either focused on the feeling and sensations of a reach to a target, which they termed kinesthetic imagery, or focused on seeing the hand reach to a target, which they termed visual imagery. The results showed that kinesthetic imagery increased CNV in PD patients, but not in controls while visual imagery did not change CNV in either group. Based on this research perhaps the visual cue in the current study involved the focus on seeing the target which had no effect on CNV, whereas the auditory cues allowed a more kinesthetic focus on the sensation of the movement which may have improved cognitive and motor functioning in PD patients. Future research investigating timing and coordination in PD patients should include cognitive measures and continue to examine different cues as an aid to perform complex multi-joint movement tasks.
Acknowledgments
This research was supported by NINDS NS40266 and NS39352.
Footnotes
Patients with excessive resting tremor of > 2 on the UPDRS were excluded from data analysis and none of the patients exhibited action or intention tremor.
Qualitative observation of all movement traces did not reveal any differences in pauses among the three groups.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Shannon D. R. Ringenbach, Email: shannon.ringenbach@asu.edu.
Arend W. A. Van Gemmert, Email: gemmert@lsu.edu.
Holly A. Shill, Email: holly.shill@bannerhealth.com.
George E. Stelmach, Email: stelmach@asu.edu.
References
- Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Movement Disorders. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- Almeida QJ, Wishart LR, Lee TD. Bimanual coordination deficits with Parkinson’s disease: The influence of movement speed and external cueing. Movement Disorders. 2002;17:30–37. doi: 10.1002/mds.10030. [DOI] [PubMed] [Google Scholar]
- Almeida QJ, Wishart LR, Lee TD. Disruptive influences of a cued voluntary shift on coordinated movement in Parkinson’s disease. Neuropsychologia. 2003;41:442–452. doi: 10.1016/s0028-3932(02)00155-0. [DOI] [PubMed] [Google Scholar]
- Amazeen EL, Amazeen PG, Treffner PJ, Turvey MT. Attention and handedness in bimanual coordination dynamics. Journal of Experimental Psychology; Human Perception and Performance. 1997;23:1552–1560. [Google Scholar]
- Byblow WD, Carson R, Goodman D. Expressions of asymmetries and anchoring in bimanual coordination. Human Movement Science. 1994;13:3–28. [Google Scholar]
- Byblow WD, Lewis GN, Stinear JW. Effector-specific visual information influence kinesthesis and reaction time performance in Parkinson’s disease. Journal of Motor Behavior. 2003;35:99–108. doi: 10.1080/00222890309602125. [DOI] [PubMed] [Google Scholar]
- Byblow WD, Summers JJ, Thomas J. Spontaneous and intentional dynamics of bimanual coordination in Parkinson’s disease. Human Movement Science. 2000;19:223–249. [Google Scholar]
- Cooke JD, Brown JD, Brooks VB. Increased dependence on visual information for movement control in patients with Parkinson’s disease. Canadian Journal of Neurological Sciences. 1978;5:413–415. doi: 10.1017/s0317167100024197. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Egan GF, O’Sullivan JD, Hughes AJ, Bradshaw JL, Colebatch JG. Motor imagery in Parkinson’s disease: A PET study. Movement Disorders. 2001;16:849–857. doi: 10.1002/mds.1181. [DOI] [PubMed] [Google Scholar]
- Dounskaia N, van Gemmert AWA, Leis BC, Stelmach GE. Biased wrist and finger coordination in Parkinsonian patients during performance of graphical tasks. Neuropsychologia. 2009;47:2504–2514. doi: 10.1016/j.neuropsychologia.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton RL. UPDRS program members. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s Disease. Vol. 2. Florham Park, NJ: Macmillan Healthcare Information; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State – practical method for grading cognitive state of patients for clinical. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Franz EA, Zelaznik HN, McCabe G. Spatial topological constraints in a bimanual task. Acta Psychologica. 1991;77:137–151. doi: 10.1016/0001-6918(91)90028-x. [DOI] [PubMed] [Google Scholar]
- Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Shibasaki H, Nagamine T, Terada K, Kaji R, Fukuyama H. Dissociation between contingent negative variation and Bereitschafts potential in a patient with cerebellar efferent lesion. Electroencephalaograpy Clinical Neurophysiology. 1994;90:359–364. doi: 10.1016/0013-4694(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Pathophysiology and clinical assessment of motor symptoms in Parkinson’s disease. In: Koller WC, editor. Hanbook of Parkinson’s disease. New York: Marcel Dekker; 1987. pp. 99–126. [Google Scholar]
- Johnson KA, Cunnington R, Bradshaw JL, Phillips JG, Iansek R, Rogers MA. Bi-manual co-ordination in Parkinson’s disease. Brain: A Journal of Neurology. 1998;121:743–753. doi: 10.1093/brain/121.4.743. [DOI] [PubMed] [Google Scholar]
- Kelly VE, Bastian AJ. Antiparkinson medications improve agonist activation but not antagonist inhibition during sequential reaching movements. Movement Disorders. 2005;20:694–704. doi: 10.1002/mds.20386. [DOI] [PubMed] [Google Scholar]
- Lewitt PA. Micrographia as a focal sign of neurological disease. Journal of Neurology, Neurosurgery and Psychiatry. 1983;46:1152–1153. doi: 10.1136/jnnp.46.12.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Van Wegen E, De Goede C, Deutekom M, Nieuwboer A, Willems A, Jones D, Rochester L, Kwakkel G. Effects of external rhythmical cueing on gait in patients with Parkinson’s disease: a systematic review. Clinical Rehabilitation. 2005;19:695–713. doi: 10.1191/0269215505cr906oa. [DOI] [PubMed] [Google Scholar]
- Lim VK, Polych MA, Hollander A, Byblow WD, Kirk IJ, Hamm JP. Kinesthetic but not visual imagery assists in normalizing the CNV in Parkinson’s disease. Clinical Neurophysiology. 2006;117:2308–2314. doi: 10.1016/j.clinph.2006.06.713. [DOI] [PubMed] [Google Scholar]
- McLennan JE, Nakano K, Tyler HR, Schwab RS. Micrographia in Parkinsons disease. Journal of the Neurological Sciences. 1972;15:141–152. doi: 10.1016/0022-510x(72)90002-0. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swimmen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson’s disease. European Journal of Neuroscience. 2009;29:1422–1430. doi: 10.1111/j.1460-9568.2009.06681.x. [DOI] [PubMed] [Google Scholar]
- Oliveira RM, Gurd JM, Nixon P, Marshall JC, Passingham RE. Micrographia in Parkinson’s disease: the effect of providing external cues. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:429–433. doi: 10.1136/jnnp.63.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfann KD, Buchman AS, Comella CL, Corcos DM. Control of movement distance in Parkinson’s disease. Movement Disorders. 2001;16:1048–1065. doi: 10.1002/mds.1220. [DOI] [PubMed] [Google Scholar]
- Ponsen MM, Daffertshofer A, van den Heuvel E, Wolters ECh, Beek PJ, Berendse HW. Bimanual coordination dysfunction in early, untreated Parkinson’s disease. Parkinsonism & Related Disorders. 2006;12:246–252. doi: 10.1016/j.parkreldis.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Ringenbach SDR, Mulvey GM, van Gemmert AWA, Stankus A, Maraj BKV. Sensory feedback is beneficial for performance of continuous bimanual tasks for adults with Down syndrome. Down Syndrome Quarterly. 2009;11:3–9. [Google Scholar]
- Ringenbach SDR, van Gemmert AWA, Shill HA, Stelmach GE. Cue benefits on drawing tasks in Parkinsonian patients depend on task requirements. Society for Neuroscience Abstracts (Program No. 757.9) 2005 Online. [Google Scholar]
- Robertson S, Zelaznik HN, Lantero D, Gadacz-Bojczyk K, Spencer R, Doffin J, Schneidt T. Correlations for timing consistency among tapping and drawing tasks: Evidence against a single timing process for motor control. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1316–1330. doi: 10.1037//0096-1523.25.5.1316. [DOI] [PubMed] [Google Scholar]
- Robertson SD. The development of bimanual skill: The search for stable patterns of coordination. Journal of Motor Behavior. 2001;33:114–126. doi: 10.1080/00222890109603144. [DOI] [PubMed] [Google Scholar]
- Romero DH, van Gemmert AWA, Adler CH, Bekkering H, Stelmach GE. Altered aiming movements in Parkinson’s disease patients and elderly adults as a function of delays in movement onset. Experimental Brain Research. 2003;151:249–261. doi: 10.1007/s00221-003-1452-2. [DOI] [PubMed] [Google Scholar]
- Rubinstein TC, Giladi N, Hausdorff JM. The power of cueing to circumvent dopamine deficits: a review of physical therapy treatment of gait disturbances in Parkinson’s disease. Movement Disorders. 2002;17:1148–1160. doi: 10.1002/mds.10259. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Alberts JL, Stelmach GE. Multijoint movement control in Parkinson’s disease. Experimental Brain Research. 2001;140:335–344. doi: 10.1007/s002210100829. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Steyvers M, Debaere F, Stelmach GE, Swinnen SP. Bimanual coordination and limb-specific parameterization in patients with Parkinson’s disease. Neuropsychologia. 2000;38:1714–1722. doi: 10.1016/s0028-3932(00)00086-5. [DOI] [PubMed] [Google Scholar]
- Sheridan MR, Flowers KA, Hurrell J. Programmingand executionof movement in Parkinsons-disease. Brain. 1987;110:1247–1271. doi: 10.1093/brain/110.5.1247. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Zelaznik HN. Weber (slope) analyses of timing variability in tapping and drawing tasks. Journal of Motor Behavior. 2003;35:371–382. doi: 10.1080/00222890309603157. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–1439. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- Stegemoller EL, Simuni T, MacKinnon CD. The effects of Parkinson’s disease and age on syncopated finger movements. Brain Research. 2009;1290:12–20. doi: 10.1016/j.brainres.2009.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelmach GE, Teasdale N, Phillips J, Worringham CJ. Force production characteristics in Parkinsons-disease. Experimental Brain Research. 1989;76:165–172. doi: 10.1007/BF00253633. [DOI] [PubMed] [Google Scholar]
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Experimental Neurology. 1997;146:159–170. doi: 10.1006/exnr.1997.6507. [DOI] [PubMed] [Google Scholar]
- Thobois S, Dominey PF, Decety J, Pollak P, Gregoire MC, Le Bars D, Broussolle E. Motor imagery in normal subjects and in asymmetrical Parkinson’s disease. Neurology. 2000;55:996–1002. doi: 10.1212/wnl.55.7.996. [DOI] [PubMed] [Google Scholar]
- van Gemmert AWA, Adler CH, Stelmach GE. Parkinson’s disease patients undershoot target size in handwriting and similar tasks. Journal of Neurology, Neurosurgery, and Psychiatry. 2003;74:1502–1508. doi: 10.1136/jnnp.74.11.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gemmert AWA, Teulings HL, Contreras-Vidal JL, Stelmach GE. Parkinson’s disease and the control of size and speed in handwriting. Neuropsychologia. 1999;37:685–694. doi: 10.1016/s0028-3932(98)00122-5. [DOI] [PubMed] [Google Scholar]
- van Gemmert AWA, Teulings HL, Stelmach GE. Parkinsonian patients reduce their stroke size with increased processing demands. Brain and Cognition. 2001;47:504–512. doi: 10.1006/brcg.2001.1328. [DOI] [PubMed] [Google Scholar]
- Verheul MHG, Geuze RH. Inter-limb coupling in bimanual rhythmic coordination in Parkinson’s disease. Human Movement Science. 2004;23:503–525. doi: 10.1016/j.humov.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Wann JP, Kardirkamanathan M. Variability in Childrens’ Handwriting: Computer diagnosis of writing difficulties. In: Wann JP, Wing AM, Sovik N, editors. The development of graphic skills. Academic; London: 1991. pp. 223–236. [Google Scholar]
- Welch RB, Duttonhurt LD, Warren DH. Contributions of audition and vision to temporal rate perception. Perception and Psychophysics. 1986;39:294–300. doi: 10.3758/bf03204939. [DOI] [PubMed] [Google Scholar]


