Abstract
The prefrontal cortex (PFC) and posterior parietal cortex (PPC) are critical neural substrates for working memory. Neural activity persists in these regions during the maintenance of a working memory representation. Persistent activity, therefore, may be the neural mechanism by which information is temporarily maintained. However, the nature of the representation or what is actually being represented by this persistent activity is not well understood. In this review, we summarize the recent functional magnetic resonance imaging (fMRI) studies conducted in our laboratory that test hypotheses about the nature of persistent activity during a variety of spatial cognition tasks. We find that the same areas in the PFC and PPC that show persistent activity during the maintenance of a working memory representation also show persistent activity during the maintenance of spatial attention and the maintenance of motor intention. Therefore, we conclude that persistent activity is not specific to working memory, but instead, carries information that can be used generally to support a variety of cognitions. Specifically, activity in topographically organized maps of prioritized space in PFC and PPC could be read out to guide attention allocation, spatial memory, and motor planning.
Keywords: working memory, attention, motor intention, fMRI, prefrontal cortex, parietal cortex
Introduction
Working memory (WM) is the process by which organisms maintain information no longer present in the immediate environment but necessary for future adaptive behavior. Since WM is a fundamental component of almost all high level cognitions, ranging from reading (Conway et al., 2005) to decision making (Curtis & Lee, 2010), it is not surprising that extensive efforts have been made to understand the neural mechanisms supporting WM.
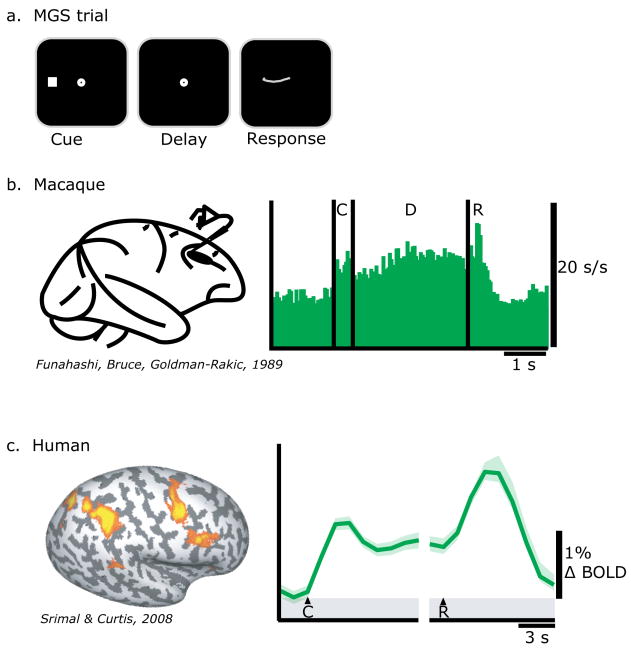
Persistent neural activity during the delay period between a sensory cue (e.g., the position of a briefly flashed spot of light) and a later motor response (e.g., a shift of gaze to the remembered location) is the most compelling evidence that this activity reflects some form of a memory representation (Fuster & Alexander, 1971; Kubota & Niki, 1971). Neurons in the monkey prefrontal and posterior parietal cortex (PFC; PPC) show persistent activity during the delay period of an oculomotor variant of a delayed response task called a memory-guided saccade (MGS) task (Fig 1ab) (Funahashi et al., 1989; Gnadt & Andersen, 1988). Recently, Srimal and Curtis (2008) used functional magnetic resonance imaging (fMRI) to measure delay period activity in a MGS task. As can be seen in Figure 1c, neural activity persists above baseline levels during the delay period in the PFC and PPC similar to the way elevated firing rates persist in monkey electrophysiology studies.
Fig 1.
a. Trial schematic of a memory guided saccade (MGS) task. A subject must maintain the location of a briefly presented visual cue over a memory delay and then make a saccade to the past cue's location. b. A spike histogram from a neuron in the monkey PFC during a MGS task (Funahashi et al., 1989). c. BOLD signal time course from the human PFC during a MGS task (Srimal & Curtis, 2008). Notice that in both species neural activity persists during the memory delay period. C=cue, D=delay, and R=response.
There are several features of persistent activity that strongly suggest it is a mechanism for WM maintenance in humans and non-human primates. First, BOLD signal persists in human brain areas homologous to non-human primate brain areas in which neuronal spiking persists, most notably in the PFC and PPC (Corbetta, Kincade, & Shulman, 2002; Curtis & D'Esposito, 2003; Funahashi et al., 1989; Goldberg, Bisley, Powell, Gottlieb, & Kusunoki, 2002; Snyder, Batista, & Andersen, 1997). Second, delay period activity is coupled to task performance. It scales with the duration of the delay period. In other words, activity persists as long as the subject actively maintains the WM representation. Additionally, greater delay period activity predicts better WM performance. Using a MGS task, Curtis et al (2004) used the distance between the memory guided saccade and the actual cued location as a continuous measure of WM accuracy. The magnitude of BOLD activity in PFC and PPC predicted the accuracy of the upcoming saccade. Similarly, neuronal spiking is higher and persistent through the delay period of correct compared to error MGS trials in the monkey PFC (Funahashi et al., 1989). Thus, it is thought that delay period activity can reflect the fidelity of a WM representation. Third, just as early visual neurons have receptive fields, neurons in higher level areas, like the PFC and PPC, have receptive/response fields (RF) (Bisley & Goldberg, 2003; Bruce & Goldberg, 1985; Snyder et al., 1997; Thompson, Hanes, Bichot, & Schall, 1996). Electrophysiological studies have shown that delay period activity is spatially selective; neuronal delay period activity is enhanced when the location of the memoranda falls within the neuron's RF, which is most often in the contralateral visual hemifield. Although BOLD imaging cannot resolve a neuron's RFs, one can predict a coarser spatial selectivity, a gross contralateral bias. Indeed, during memory delays BOLD activity is greater in the hemisphere contralateral to the visual field of the memoranda (Curtis & D'Esposito, 2006; Srimal & Curtis, 2008). Moreover, delay period activity shows a contralateral bias during the maintenance of spatial WM representations in retinotopically defined subregions of PPC (Schluppeck, Curtis, Glimcher, & Heeger, 2006).
Together these results strongly indicate that the neural mechanism supporting working memory may be contingent upon persistent activity in the PFC and PPC. Nonetheless, the nature of the mechanism remains unknown. It is thought that persistent activity forms a bridge across time linking the prior stimulus cue with its contingent response (Fuster, 2001). Within a traditional working memory framework, persistent activity may reflect the active maintenance of the past stimulus. Neurons that are selective for and stimulated by the presentation of the cue remain in an active state through persistent activation through the retention interval. In the context of a MGS task, persistent delay period activity may reflect a maintained representation of the cued location. However, delay period activity could just as easily reflect the maintenance of spatial attention directed towards the prior location of the flashed cue (i.e., covert attention). Similarly, the delay period activity could reflect the preparation of forthcoming saccade to the cued location (i.e., motor intention). Indeed, persistent activity in the PFC and PPC has been reported during intervals in which animals are attending covertly or preparing a motor response (Andersen & Buneo, 2002; Armstrong, Chang, & Moore, 2009; Corbetta et al., 2002; Goldberg et al., 2002; Serences & Yantis, 2007). Therefore, delay period activity that has traditionally been thought to be mnemonic in nature may alternatively reflect the maintenance of attention or motor intention. Separate subregions of the PFC and PPC may support different spatial cognitive operations, like WM, attention, and intention. Alternatively, the same portion of the PFC and PPC may support a variety of spatial cognitive abilities. In testing among these alternatives, we aim to constrain how we model the neural mechanisms of WM. In this review, we summarize the results from our lab's fMRI studies designed to test hypotheses about the nature of persistent activity in the PFC and PPC.
Visuospatial working memory
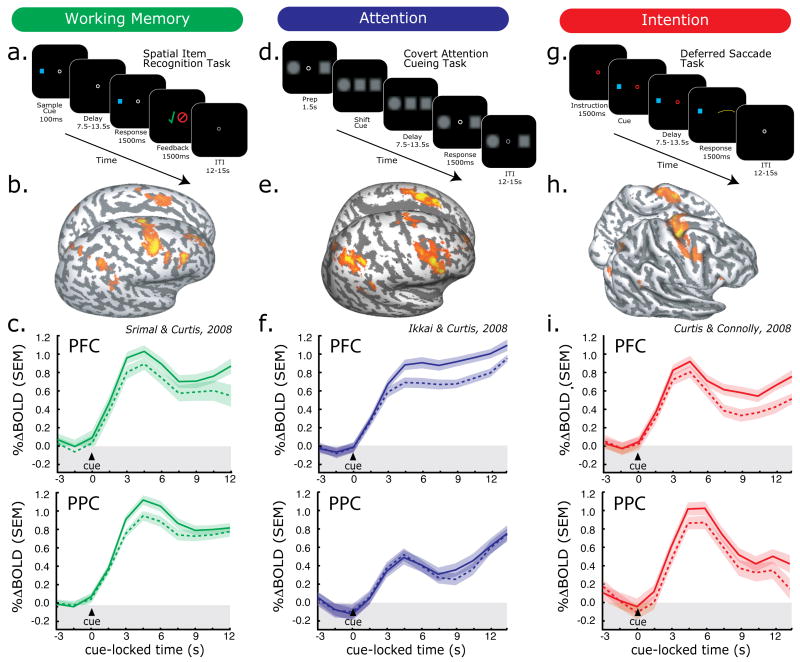
As discussed above, in both monkeys and humans activity in the PFC and PPC persists during the delay periods of MGS tasks. During the delay, subjects know the exact metrics of the forthcoming saccade – move eyes from central fixation to the location of the visual cue. Therefore, persistent activity could reflect the maintenance of a saccade plan (i.e., a prospective code) instead of the maintenance of the past sensory event (i.e., a retrospective code). To test between these alternatives, Srimal and Curtis (2008) compared delay period activity when subjects were performing a standard MGS (Fig 1a), where the upcoming memory-guided response was known during the delay, and a spatial item recognition task (Fig 2a), where the memory-guided response was unpredictable. Both tasks involved remembering the location of a briefly flashed visual cue across a long retention interval. After the delay period of the MGS task, subjects made a saccade to the remembered location. After the delay period of the spatial item recognition task, subjects indicated with a button press whether a new visual probe matched the location cued before the delay. No eye-movements were made during this condition. Briefly, in order to identify regions with significant delay activity, we first modeled each experimental event (e.g. cue, delay, response) separately with impulse convolved with canonical hemodynamic response function. Inter-trial interval (ITI) served as a baseline. A GLM was used to estimate the delay period parameter fits and a permutation test was used to identify significant clusters across subjects. For time-series analyses, we first drew anatomical regions of interest (ROIs) on each subject's anatomical images, and chose 20 voxels within the anatomical ROIs that showed the greatest task relation, regardless of the trial event (cue, delay, response) or sign of activation (positive or negative).
Fig 2.
Task and results summary from working memory (WM), attention, and intention studies. Trial schematics are shown for each task (a., d., and g.). Statistical parametric maps of significant delay period specific activity are projected onto the surface of a subject's cortical sheet for each task (b., e., and h.). Time courses (average, SEM) from the PFC (c., f., i. top panels) and PPC (bottom panels) are shown time locked to the presentation of the cue. Solid lines represent trials in which the memoranda (c.), locus of attention (f.), or direction of antisaccade (i.) was in hemifield contralateral to the cortical hemisphere and dashed lines represent ipsilateral trials. Notice that both PFC and PPC show delay period activation, that this activation persists throughout the delay period, and it shows a contralateral bias.
Activity persisted at the same level in the exact same regions of the PFC and PPC during the delay periods of both conditions (Fig 1c, Fig 2b & c). From these results, we can conclude that persistent activity is not specifically tied to the maintenance of metrics of the planned saccades since only the MGS task required saccades. Both tasks shared the need to maintain the location of the flashed visual cue. Therefore, the most parsimonious explanation is that persistent activity reflects the maintenance of the cued location, a working memory representation, which could be used to guide saccades (MGS) or aid match/non-match spatial judgments.
Visuospatial attention
In another study, we tested the alternative explanation that the persistent activity during a WM delay reflects the maintenance of attention, not memory. Following the presentation of the sample cue of a MGS task, subjects may covertly orient their attention to the location of the cue (Awh, Armstrong, & Moore, 2006). Maintaining attention at the cued location over the delay may be responsible for delay period activity. Behaviorally, spatial attention and WM are functionally tightly linked, such that spatial attention is obligatorily allocated to the memorized location. Performance in attention demanding tasks is enhanced when the attended location matches the location held in WM (Awh, Jonides, & Reuter-Lorenz, 1998; Awh, Smith, & Jonides, 1995)(see Olivers & Elmer, 2011). On the other hand, attention away from the memorized location disrupts spatial WM performance, but not object WM (Awh et al., 1998; Smyth, 1996; Smyth & Scholey, 1994), suggesting that attention may be the means by which we maintain spatial WM representations. Moreover, lesion/inactivation of PFC or PPC also impairs performance on attention demanding tasks (Kalla, Muggleton, Cowey, & Walsh, 2009; Li, Mazzoni, & Andersen, 1999; Mesulam, 1999; Muggleton, Juan, Cowey, & Walsh, 2003; O'Shea, Muggleton, Cowey, & Walsh, 2004; Peers et al., 2005; Posner, Walker, Friedrich, & Rafal, 1984; Wardak, Ibos, Duhamel, & Olivier, 2006)
We asked if BOLD activity would persist in the PFC and PPC during the maintenance of covert attention, as in does during spatial WM maintenance (Ikkai & Curtis, 2008). Subjects maintained attention at one of two peripheral locations covertly such that they could detect the slight dimming in an attended stimulus (Fig 2b). This procedure ensured focused and sustained attention at the cued location, and required no motor response until the delay was over. Significant BOLD activity was observed over PFC and PPC during the covert attention delay (Fig 2e). BOLD activity in the PFC and PPC persisted well above baseline throughout the maintenance period and showed a contralateral bias, as it does during WM maintenance (Fig 2f). These results demonstrate that the maintenance of covert attention alone is sufficient to induce persistent activity even in the absence of WM. Furthermore, persistence during WM maintenance may be explained by maintenance of attention at the memoranda's location.
Motor intention
There is still another alternative that we tackled in a third study. Delay period activity during a MGS task could reflect the location of the upcoming memory guided saccade. In effect, as soon as the visual cue is flashed one could cue up a saccade to acquire the cue and maintain that plan or intention throughout the delay. Such a strategy is efficient and plausible. Delay period activity would arise from the activity of neurons involved in planning a saccade to the cued location, as opposed to those involved in maintaining a WM representation of the cue's location or maintaining covert attention at the cue's location.
In order to test this alternative, we asked subjects to plan a saccade away from a continuously visible cued location (“antisaccade”) in a deferred saccade task (Fig 2g) (Curtis & Connolly, 2008). Importantly, subjects only executed the antisaccade after a long delay interval during which they were trained to maintain the saccade plan and remain in a state of oculomotor readiness. In both the MGS task and the deferred saccade task, subjects planned and executed saccades to blank space guided by internal representations. In the case of the MGS, it was guided by memory. In the case of the deferred saccade task, it was guided by a simple mirrored transformation of the visible cue's location but not WM. Additionally, the attention demands were quite low compared to our covert attention task. Activity persisted throughout the delay in the PFC and PPC (Fig 2h) despite the fact that WM was not required and attention demands were low (Fig 2i). BOLD signal also showed a spatial bias, but this time it was greater in the hemisphere opposite the direction of the planned saccade, not the visual cue. Therefore, delay period activity during a traditional MGS task could be driven by the activity of neurons that code for the forthcoming saccade, a prospective motor code (Curtis & D'Esposito, 2006). This interpretation contradicts the traditional notion that delay period activity reflects the memory of the cued location. This notion is strengthened by the contraversive bias observed in the BOLD time courses.
Common activation during WM, attention, and intention
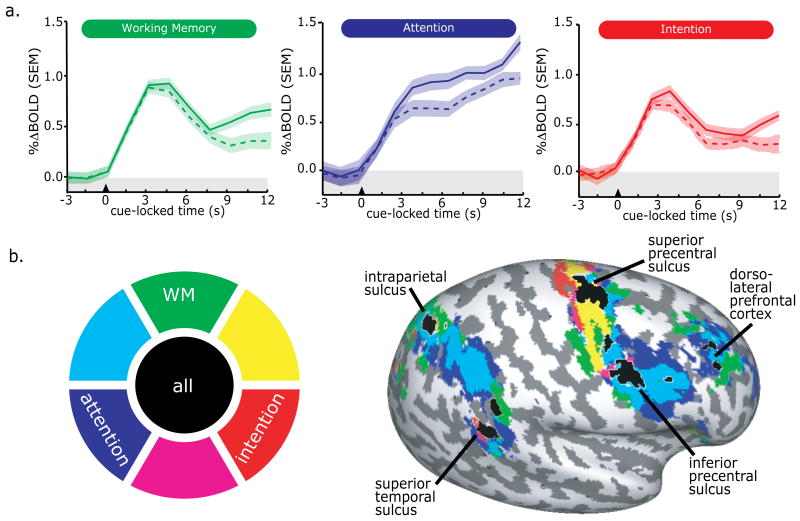
In three separate studies we demonstrated that the human PFC and PPC show sustained delay period activity while maintaining spatial WM representations, maintaining covert spatial attention, and while maintaining an oculomotor intention. At face value, the location of the activations in the PFC and PPC look similar and the profile of the time courses look similar (Fig 2, middle and bottom panels). As planned, five subjects were in all three studies, allowing us to more rigorously and quantitatively compare their data across tasks.
Comparing time-courses of activation
Within each subject, functional ROIs created in the original 3 studies were first transformed into a common space, and the significant voxels that overlapped across tasks were selected for further analysis. It is important to note that selected voxels were task related, significantly modulated by the task regardless of direction (i.e., activation or deactivation), trial epoch (i.e, cue, delay, response), and task (i.e., WM, attention, intention). This procedure yielded for each subject ROIs in the PFC and PPC where there were common activations across the three tasks. The time courses from the PFC ROI are shown in Figure 3a. In the same subjects, in the exact same brain tissue, delay period activity persisted during the maintenance of WM, attention, and intention. Moreover, a contralateral bias in the BOLD signal can be seen during the delay in each of the tasks (solid line = contralateral; dashed line = ipsilateral). These results indicate that there are portions of the PFC and PPC, which do not appear to be specialized for WM or any of the tasks. In contrast, they suggest that the same subregion of the PFC and PPC may support all of these spatial cognitions.
Fig 3.
Delay period activity from subjects who participated in all three of the studies. (N=5). a. BOLD time-series from the exact same voxels in superior precentral sulcus (sPCS) across the three studies. Notice that delay period activity persists during the WM, attention, and intention delay periods and shows a contralateral bias. b. Cortex with significant delay period activity projected on an inflated cortical sheet of the right hemisphere. The color wheel is the legend for the delay period activity. Areas that show both activation for attention and intention would be depicted in magenta. Areas that show delay period activation during all three tasks are depicted in black and those areas are labeled.
Comparing localization of delay period activity
Our ROI based time course analyses described above allowed us to test if there were common brain areas in PFC and PPC that might show delay period activity during one or more of the tasks. And as described above, we did find evidence for such areas in the PFC and PPC. Next, we wanted to test the opposite. We asked whether there might be areas in the PFC and/or PPC that appear to only show delay period activity for one of the three tasks. In essence, we were testing to see if there might be areas that are specialized for WM, attention, or intentions. For each of the five subjects we mapped delay period activity during the WM, attention, and intention tasks, using the procedures described above (see “Visuospatial working memory”) onto a standard template cortical surface. We computed statistical parametric maps on the cortical surfaces for each of the tasks. We projected significant delay period activity for each task in a different color (Figure 3b). As one can see, delay period activity shows a striking pattern of overlap in the PFC and PPC. Several subregions of the PFC and PPC show significant delay period activity in all three tasks, shown in black. Specifically, delay period activity for all three tasks was found in the dorsolateral PFC, superior and inferior portions of the precentral sulcus, the dorsal portion of the intraparietal sulcus, and the superior temporal sulcus. The superior precentral sulcus and intraparietal sulcus foci line up well with the loci of the ROIs used in the time course analyses. Overall, there were no areas that appear to show delay period activity for only one of the tasks. The hints here and there in the map are most likely due to thresholding artifacts. For example, the amount of delay period activity in the very simple intention task was often lower than the other two tasks, which may be responsible for blue-green spread in inferior precentral sulcus, though this region show significant above-baseline delay activity in the original study with N = 12 (Curtis & Connolly, 2008). Nonetheless, these results clearly show that neural activity in subregions of the PFC and PPC persists during the maintenance of a spatial WM represent, the maintenance of covert attention, and the maintenance of an oculomotor intention. We have demonstrated this in the same subjects using similarly structured task designs and data analytic methods allowing us to make straightforward inferences. Next, we turn to the theoretical implications of these findings.
A common mechanism supporting spatial WM, attention and intention?
The strong interpretation of our findings is that there is a single neural mechanism dependent upon persistent activity in the PFC and PPC that is common to maintaining WM representations, attention, and intentions, and perhaps a host of additional spatial cognitions. These results, combined with existing theories, help us constrain how we model the common mechanism. We propose that subregions in the PFC and PPC contain populations of neurons that together form maps of space. These maps of space may dynamically code for the priority or importance of locations defined by the behavioral context (Ipata, Gee, Bisley, & Goldberg, 2009; Serences & Yantis, 2006; Thompson & Bichot, 2005). Early visual neurons feed forward information about the physical features of stimuli. Prioritized maps of space could be shaped by the saliency of bottom up feed forward information (Itti & Koch, 2001) combined with goal-relevant top-down information from higher association cortices (Serences & Yantis, 2006). Presumably, the population of neurons comprising the map of prioritized space is organized in a retinal topographic manner, with each neuron coding for different but overlapping portions of the visual field. Persistent activity among neurons with RFs that include the behaviorally relevant location may be the mechanism by which prioritized space remains prioritized over delay periods. Computational models that simulate such neuronal networks are able to maintain stable patterns of activity as “bump” attractors (Compte, Brunel, Goldman-Rakic, & Wang, 2000). Importantly, the prioritized map of space may only contain information about the location of behaviorally relevant event. For instance, the prioritized map during a WM delay in which one is maintaining the position of a cue that was presented 10 degree to the left may be identical to the map during the maintenance of covert attention 10 degrees to the left and identical to the map during the maintenance of a saccade plan to a location 10 degrees to the left. Specifically, the pattern of activity within the map of space may be agnostic to the conditions that led to the prioritized map. Other brain areas could then read out the general map of prioritized space to fulfill the specific behavioral demands. For instance, downstream oculomotor areas (e.g. superior colliculus and brainstem saccade generator) may read out the priority maps of space in the PFC and PPC to compute the metrics of saccades, both visually and memory guided. In addition, a read out of the same priority map by posterior visual areas may enhance the allocation of attention (Carrasco, 2006; Liu, Abrams, & Carrasco, 2009; Posner, 1980) by exciting neurons with overlapping RFs (Moore & Armstrong, 2003), suppressing neurons with non-overlapping RFs (Moore & Armstrong, 2003), or reshaping the RFs of visual neurons (Anton-Erxleben, Stephan, & Treue, 2009; Womelsdorf, Anton-Erxleben, & Treue, 2008)
The superior portion of the precentral sulcus (sPCS) in the PFC and the dorsal portion of the intrparietal sulcus (IPS) in the PPC consistently shows the most robust delay period activity across subjects, tasks, studies, and labs. These two areas are the likely human homologues or evolved variants of monkey areas FEF and LIP and are our best candidates for priority maps. In addition to the findings from our lab showing that BOLD activity in the sPCS and IPS persists during WM, attention, and intention delay periods, findings from other studies reinforce their candidacy as priority maps. Both areas are densely interconnected with visual areas and have important projections to oculomotor structures (Averbeck & Seo, 2008; Barbas, 1992; Cavada & Goldman-Rakic, 1989a, 1989b; Gaymard, Ploner, Rivaud, Vermersch, & Pierrot-Deseilligny, 1998; Lynch, Graybiel, & Lobeck, 1985; Petrides & Pandya, 2006; Schall, 2002). In other words, they are ideally situated to receive the kind of inputs necessary to construct a priority map that could be accessed by a many brain areas to influence variety of spatially guided behaviors. Functional MRI studies have demonstrated that both the sPCS and IPS are topographically organized (Sereno, Pitzalis, & Martinez, 2001; Silver & Kastner, 2009; Silver, Ress, & Heeger, 2005) supporting the notion that populations of neurons with varying RFs together compose a topographic map of space. Electrical microstimulation of the monkey FEF at levels too low to evoke a saccade lowers the accuracy of memory guided saccades (Opris, Barborica, & Ferrera, 2005), affects visual discrimination in a manner similar to covert spatial attention (Moore & Fallah, 2004), and alters the trajectory of self generated saccades (McPeek, 2006). Importantly, these distortions in memory, visual attention, and saccade planning are entirely consistent with a priority map model if one assumes that microstimulation perturbs the map's pattern of activity used to tag the behaviorally relevant location. Similar effects in humans have been reported following transcranial magnetic stimulation (TMS) over the sPCS in the putative human FEF (Grosbras & Paus, 2002; Muri, Vermersch, Rivaud, Gaymard, & Pierrot-Deseilligny, 1996; Smith, Jackson, & Rorden, 2005). Together, there is converging evidence in support of the priority map model.
PFC and PPC influence on other cortical areas during attention and WM tasks
Above we suggest that a read out of a priority map of space by visual cortex could be used to guide spatial attention by enhancing the activity of neurons with RFs that match the prioritized location on the map and perhaps suppressing the activity of neurons with non-matching RFs. Spatially directed attention and the maintenance of WM representations modulate early visual neurons as a function of whether or not the locus of attention or WM overlaps with the visual neuron's RF. For instance, activity in early visual neurons increases depending on whether or not attention is directed within its RF (Luck, Chelazzi, Hillyard, & Desimone, 1997; Motter, 1993). In human neuroimaging studies, attention enhances neural activity in the retinotopic portions of the visual cortex corresponding to the locus of attention (Chawla, Rees, & Friston, 1999; Kastner, Pinsk, De Weerd, Desimone, & Ungerleider, 1999; Offen, Schluppeck, & Heeger, 2009; Ress, Backus, & Heeger, 2000; Silver, Ress, & Heeger, 2007; Stokes, Thompson, Nobre, & Duncan, 2009), and BOLD signal decreases when attention is allocated elsewhere (Silver et al., 2007). Similarly, the maintenance of a WM representation enhances the neural activity in the retinotopic portions of early visual areas that correspond to the location of a memoranda in both monkeys (Super, Spekreijse, & Lamme, 2001) and humans (Awh et al., 1999; Geng, Ruff, & Driver, 2009; Munneke, Heslenfeld, & Theeuwes, 2010; Pessoa, Gutierrez, Bandettini, & Ungerleider, 2002; Postle, Awh, Jonides, Smith, & D'Esposito, 2004). These results and others clearly demonstrate that visuospatial attention and WM affect the activity of early visual cortex. One would like to conclude from these results that top-down signals from PFC and PPC are the source of this influence, however, direct evidence is scant. Perhaps the most direct evidence comes from microstimulation studies. As briefly mentioned above, electrical microstimulation of monkey FEF neurons with currents too low to evoke saccades increases the monkey's sensitivity to detect contrast changes in targets that spatially overlap with the neuron's RF similar to heightened sensitivity of attended locations (Moore & Fallah, 2004). In another study, sub-saccade threshold microstimulation of FEF neurons increased the firing rates of V4 neurons with overlapping RFs (Moore & Armstrong, 2003). FEF microstimulation also increases the stimulus discriminability of V4 neurons (Armstrong & Moore, 2007). Similarly in humans, TMS over FEF increased the BOLD activity of early visual areas, and also enhanced perceptual performance (Ruff et al., 2006). TMS over FEF also decreased reaction times to detect a peripheral target and modulated occipital ERPs during the maintenance of attention (Taylor, Nobre, & Rushworth, 2007). Therefore, FEF activity may enhance visual perception through its feedback projections to visual areas in a retinotopic manner. These results allow one to easily imagine a priority map composed of a topographically organized population of neurons in the FEF that are reciprocally connected to neurons in early visual areas with matching RFs. Bottom-up information from the visual cortex could be used to build a prioritized map of space that could be maintained for periods of time or modified by current goals and then read out by visual cortex to focus processing in relevant portions of the visual field.
Summary and Conclusions
Neural activity persists in subregions of the PFC and PPC during the maintenance of WM representations. Persistent activity is thought to be the neural mechanism by which information is temporarily maintained. In this review, we used findings from our recent fMRI studies to test hypotheses about the nature of persistent activity during a variety of spatial cognition tasks. We found that the same areas in the PFC and PPC that show persistent activity during the maintenance of a WM representation also show persistent activity during the maintenance of spatial attention and the maintenance of motor intention. We conclude that persistent activity in the PFC and PPC carries information that can be used generally to support a variety of spatial cognitions. We propose that activity in topographically organized priority maps in PFC and PPC could be read out by other brain areas to guide attention allocation, spatial memory, and motor planning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Anton-Erxleben K, Stephan VM, Treue S. Attention reshapes center-surround receptive field structure in macaque cortical area MT. Cereb Cortex. 2009;19(10):2466–2478. doi: 10.1093/cercor/bhp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong KM, Chang MH, Moore T. Selection and maintenance of spatial information by frontal eye field neurons. J Neurosci. 2009;29(50):15621–15629. doi: 10.1523/JNEUROSCI.4465-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong KM, Moore T. Rapid enhancement of visual cortical response discriminability by microstimulation of the frontal eye field. Proc Natl Acad Sci U S A. 2007;104(22):9499–9504. doi: 10.1073/pnas.0701104104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbeck BB, Seo M. The statistical neuroanatomy of frontal networks in the macaque. PLoS Comput Biol. 2008;4(4):e1000050. doi: 10.1371/journal.pcbi.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Armstrong KM, Moore T. Visual and oculomotor selection: links, causes and implications for spatial attention. Trends Cogn Sci. 2006;10(3):124–130. doi: 10.1016/j.tics.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Reuter-Lorenz PA. Rehearsal in spatial working memory. J Exp Psychol Hum Percept Perform. 1998;24(3):780–790. doi: 10.1037//0096-1523.24.3.780. [DOI] [PubMed] [Google Scholar]
- Awh E, Jonides J, Smith EE, Buxton RB, Frank LR, Love T, et al. Rehearsal in Spatial Working Memory: Evidence From Neuroimaging. Psychological Science. 1999;10(5):433–437. [Google Scholar]
- Awh E, Smith EE, Jonides J. Human rehearsal processes and the frontal lobes: PET evidence. Ann N Y Acad Sci. 1995;769:97–117. doi: 10.1111/j.1749-6632.1995.tb38134.x. [DOI] [PubMed] [Google Scholar]
- Barbas H. Architecture and cortical connections of the prefrontal cortex in the rhesus monkey. Adv Neurol. 1992;57:91–115. [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299(5603):81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53(3):603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Carrasco M. Covert attention increases contrast sensitivity: Psychophysical, neurophysiological and neuroimaging studies. Prog Brain Res. 2006;154:33–70. doi: 10.1016/S0079-6123(06)54003-8. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J Comp Neurol. 1989a;287(4):393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 1989b;287(4):422–445. doi: 10.1002/cne.902870403. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ. The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci. 1999;2(7):671–676. doi: 10.1038/10230. [DOI] [PubMed] [Google Scholar]
- Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10(9):910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- Conway AR, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user's guide. Psychon Bull Rev. 2005;12(5):769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. J Cogn Neurosci. 2002;14(3):508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Connolly JD. Saccade preparation signals in the human frontal and parietal cortices. J Neurophysiol. 2008;99(1):133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends Cogn Sci. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D'Esposito M. Selection and maintenance of saccade goals in the human frontal eye fields. J Neurophysiol. 2006;95(6):3923–3927. doi: 10.1152/jn.01120.2005. [DOI] [PubMed] [Google Scholar]
- Curtis CE, Lee D. Beyond working memory: the role of persistent activity in decision making. Trends Cogn Sci. 2010;14(5):216–222. doi: 10.1016/j.tics.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol. 1989;61(2):331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex--an update: time is of the essence. Neuron. 2001;30(2):319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173(997):652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gaymard B, Ploner CJ, Rivaud S, Vermersch AI, Pierrot-Deseilligny C. Cortical control of saccades. Exp Brain Res. 1998;123(1-2):159–163. doi: 10.1007/s002210050557. [DOI] [PubMed] [Google Scholar]
- Geng JJ, Ruff CC, Driver J. Saccades to a remembered location elicit spatially specific activation in human retinotopic visual cortex. J Cogn Neurosci. 2009;21(2):230–245. doi: 10.1162/jocn.2008.21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnadt JW, Andersen RA. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70(1):216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bisley J, Powell KD, Gottlieb J, Kusunoki M. The role of the lateral intraparietal area of the monkey in the generation of saccades and visuospatial attention. Ann N Y Acad Sci. 2002;956:205–215. doi: 10.1111/j.1749-6632.2002.tb02820.x. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field: effects on visual perception and attention. J Cogn Neurosci. 2002;14(7):1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Ikkai A, Curtis CE. Cortical activity time locked to the shift and maintenance of spatial attention. Cereb Cortex. 2008;18(6):1384–1394. doi: 10.1093/cercor/bhm171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipata AE, Gee AL, Bisley JW, Goldberg ME. Neurons in the lateral intraparietal area create a priority map by the combination of disparate signals. Exp Brain Res. 2009;192(3):479–488. doi: 10.1007/s00221-008-1557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. Computational modelling of visual attention. Nat Rev Neurosci. 2001;2(3):194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- Jack AI, Patel GH, Astafiev SV, Snyder AZ, Akbudak E, Shulman GL, et al. Changing human visual field organization from early visual to extra-occipital cortex. PLoS One. 2007;2(5):e452. doi: 10.1371/journal.pone.0000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalla R, Muggleton NG, Cowey A, Walsh V. Human dorsolateral prefrontal cortex is involved in visual search for conjunctions but not features: a theta TMS study. Cortex. 2009;45(9):1085–1090. doi: 10.1016/j.cortex.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22(4):751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kubota K, Niki H. Prefrontal cortical unit activity and delayed alternation performance in monkeys. J Neurophysiol. 1971;34(3):337–347. doi: 10.1152/jn.1971.34.3.337. [DOI] [PubMed] [Google Scholar]
- Li CS, Mazzoni P, Andersen RA. Effect of reversible inactivation of macaque lateral intraparietal area on visual and memory saccades. J Neurophysiol. 1999;81(4):1827–1838. doi: 10.1152/jn.1999.81.4.1827. [DOI] [PubMed] [Google Scholar]
- Liu T, Abrams J, Carrasco M. Voluntary attention enhances contrast appearance. Psychol Sci. 2009;20(3):354–362. doi: 10.1111/j.1467-9280.2009.02300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77(1):24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Graybiel AM, Lobeck LJ. The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol. 1985;235(2):241–254. doi: 10.1002/cne.902350207. [DOI] [PubMed] [Google Scholar]
- McPeek RM. Incomplete suppression of distractor-related activity in the frontal eye field results in curved saccades. J Neurophysiol. 2006;96(5):2699–2711. doi: 10.1152/jn.00564.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1325–1346. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421(6921):370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91(1):152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70(3):909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Juan CH, Cowey A, Walsh V. Human frontal eye fields and visual search. J Neurophysiol. 2003;89(6):3340–3343. doi: 10.1152/jn.01086.2002. [DOI] [PubMed] [Google Scholar]
- Munneke J, Heslenfeld DJ, Theeuwes J. Spatial working memory effects in early visual cortex. Brain Cogn. 2010;72(3):368–377. doi: 10.1016/j.bandc.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Muri RM, Vermersch AI, Rivaud S, Gaymard B, Pierrot-Deseilligny C. Effects of single-pulse transcranial magnetic stimulation over the prefrontal and posterior parietal cortices during memory-guided saccades in humans. J Neurophysiol. 1996;76(3):2102–2106. doi: 10.1152/jn.1996.76.3.2102. [DOI] [PubMed] [Google Scholar]
- O'Shea J, Muggleton NG, Cowey A, Walsh V. Timing of target discrimination in human frontal eye fields. J Cogn Neurosci. 2004;16(6):1060–1067. doi: 10.1162/0898929041502634. [DOI] [PubMed] [Google Scholar]
- Offen S, Schluppeck D, Heeger DJ. The role of early visual cortex in visual short-term memory and visual attention. Vision Res. 2009;49(10):1352–1362. doi: 10.1016/j.visres.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opris I, Barborica A, Ferrera VP. Microstimulation of the dorsolateral prefrontal cortex biases saccade target selection. J Cogn Neurosci. 2005;17(6):893–904. doi: 10.1162/0898929054021120. [DOI] [PubMed] [Google Scholar]
- Peers PV, Ludwig CJ, Rorden C, Cusack R, Bonfiglioli C, Bundesen C, et al. Attentional functions of parietal and frontal cortex. Cereb Cortex. 2005;15(10):1469–1484. doi: 10.1093/cercor/bhi029. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35(5):975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Efferent association pathways originating in the caudal prefrontal cortex in the macaque monkey. J Comp Neurol. 2006;498(2):227–251. doi: 10.1002/cne.21048. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci. 1984;4(7):1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Awh E, Jonides J, Smith EE, D'Esposito M. The where and how of attention-based rehearsal in spatial working memory. Brain Res Cogn Brain Res. 2004;20(2):194–205. doi: 10.1016/j.cogbrainres.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Ress D, Backus BT, Heeger DJ. Activity in primary visual cortex predicts performance in a visual detection task. Nat Neurosci. 2000;3(9):940–945. doi: 10.1038/78856. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, et al. Concurrent TMS-fMRI and psychophysics reveal frontal influences on human retinotopic visual cortex. Curr Biol. 2006;16(15):1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Schall JD. The neural selection and control of saccades by the frontal eye field. Philos Trans R Soc Lond B Biol Sci. 2002;357(1424):1073–1082. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. J Neurosci. 2006;26(19):5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Selective visual attention and perceptual coherence. Trends Cogn Sci. 2006;10(1):38–45. doi: 10.1016/j.tics.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Serences JT, Yantis S. Spatially selective representations of voluntary and stimulus-driven attentional priority in human occipital, parietal, and frontal cortex. Cereb Cortex. 2007;17(2):284–293. doi: 10.1093/cercor/bhj146. [DOI] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294(5545):1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Silver MA, Kastner S. Topographic maps in human frontal and parietal cortex. Trends Cogn Sci. 2009;13(11):488–495. doi: 10.1016/j.tics.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol. 2005;94(2):1358–1371. doi: 10.1152/jn.01316.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MA, Ress D, Heeger DJ. Neural correlates of sustained spatial attention in human early visual cortex. J Neurophysiol. 2007;97(1):229–237. doi: 10.1152/jn.00677.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DT, Jackson SR, Rorden C. Transcranial magnetic stimulation of the left human frontal eye fields eliminates the cost of invalid endogenous cues. Neuropsychologia. 2005;43(9):1288–1296. doi: 10.1016/j.neuropsychologia.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Smyth MM. Interference with rehearsal in spatial working memory in the absence of eye movements. Q J Exp Psychol A. 1996;49(4):940–949. doi: 10.1080/713755669. [DOI] [PubMed] [Google Scholar]
- Smyth MM, Scholey KA. Interference in immediate spatial memory. Mem Cognit. 1994;22(1):1–13. doi: 10.3758/bf03202756. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Batista AP, Andersen RA. Coding of intention in the posterior parietal cortex. Nature. 1997;386(6621):167–170. doi: 10.1038/386167a0. [DOI] [PubMed] [Google Scholar]
- Srimal R, Curtis CE. Persistent neural activity during the maintenance of spatial position in working memory. Neuroimage. 2008;39(1):455–468. doi: 10.1016/j.neuroimage.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes M, Thompson R, Nobre AC, Duncan J. Shape-specific preparatory activity mediates attention to targets in human visual cortex. Proc Natl Acad Sci U S A. 2009;106(46):19569–19574. doi: 10.1073/pnas.0905306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VA. A neural correlate of working memory in the monkey primary visual cortex. Science. 2001;293(5527):120–124. doi: 10.1126/science.1060496. [DOI] [PubMed] [Google Scholar]
- Taylor PC, Nobre AC, Rushworth MF. FEF TMS affects visual cortical activity. Cereb Cortex. 2007;17(2):391–399. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76(6):4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci. 2006;26(16):4228–4235. doi: 10.1523/JNEUROSCI.3336-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womelsdorf T, Anton-Erxleben K, Treue S. Receptive field shift and shrinkage in macaque middle temporal area through attentional gain modulation. J Neurosci. 2008;28(36):8934–8944. doi: 10.1523/JNEUROSCI.4030-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]