Abstract
Marked reductions in the densities of the fast, transient voltage-dependent K+ (Kv) current, Ito,f, and of the inwardly rectifying (Kir) K+ current, IK1, are routinely observed in the hypertrophied and failing human heart and in experimental models of pathological cardiac hypertrophy. Attenuation of these prominent repolarizing K+ currents results in action potential prolongation and increased dispersion of repolarization, both of which are arrhythmogenic. Cardiac hypertrophy and failure are also associated with increased expression and activity of the multifunctional calcium (Ca2+) calmodulin (CaM) dependent protein kinase II (CaMKII) and several lines of evidence suggest that CaMKII activation can (directly or indirectly) lead to changes in the functional cell surface expression and the biophysical properties of cardiac Ito,f and IK1 channels.
Keywords: Kv Channels, Kir Channels, Kv4.3, Macromolecular Kv Channel Complexes, Post Translational Regulation/Modulation
Myocardial potassium (K+) selective channels function to control resting membrane potentials, action potential waveforms, refractory periods and automaticity and, in most cardiac cells, multiple types of voltage-gated K+ (Kv) and non-voltage-gated, inwardly rectifying, K+ (Kir) channels, are co-expressed (1). The densities and the properties of these channels change during normal development and in response to altered cardiac output. In contrast with this ‘physiological’ remodeling, changes in Kv and Kir channel functioning associated with inherited or acquired cardiac disease can have dramatic effects on action potential waveforms, synchronization and propagation, predisposing individuals to potentially life threatening arrhythmias (2). Considerable evidence suggests that myocardial Kv and Kir channels function as components of macromolecular protein complexes, comprising pore-forming (α) and accessory (β) subunits, and linked to cytosolic, cytoskeletal and membrane-associated regulatory proteins (1,3). In addition, these channels are potential targets for the actions of a variety of transmitters, hormones and intracellular signaling pathways, suggesting that multiple, transcriptional, translational and posttranslational, mechanisms contribute to the physiological regulation of myocardial Kv and Kir channels and to the derangements in channel expression and functioning evident in the diseased myocardium.
Cardiac action potential amplitudes and durations are largely determined by Kv channels and, in most cells, two types of Kv currents, transient outward (Ito) and delayed rectifier (IK), are distinguished. These are broad classifications, however, and there are multiple components of Ito and IK, several of which are differentially expressed, contributing to regional heterogeneities in action potential waveforms and in the responses to transmitters and intracellular signaling pathways (1). Outward K+ currents through Kir, particularly IK1, channels also contribute to repolarization and to regional differences in action potential waveforms and response properties. Changes in cardiac K+ channel densities, particularly changes in the densities of the K+ channels that are differentially distributed, are expected to have profound effects on the waveforms of action potentials, the normal propagation of activity in the heart, and the propensity to develop and to sustain arrhythmias (1,2).
In the hypertrophied and failing human heart and in experimental models of (pathological) cardiac hypertrophy, membrane excitability and excitation-contraction coupling are altered (2). Although the molecular mechanisms underlying “electrical remodeling” in the hypertrophied and failing heart are poorly understood, marked reductions in the densities of the fast, transient Kv current, Ito,f, and of the Kir current, IK1, are consistently reported (2). Downregulation of repolarizing Ito,f and IK1 channels will lead to action potential prolongation and increased dispersion of repolarization, both of which are arrhythmogenic, motivating interest in defining the molecular determinants of Ito,f and IK1 channel expression and remodeling (1,2). Cardiac hypertrophy and failure are also associated with increased expression and activity of the multifunctional calcium (Ca2+) calmodulin (CaM) dependent protein kinase II (CaMKII), a serine-threonine kinase activated by binding of Ca2+-bound calmodulin (Ca2+/CaM) and autophosphorylation (4). These observations and studies in experimental models of hypertrophy and failure have led to suggestions that CaMKII might be a potential therapeutic target for the failing heart (4) and direct support for this hypothesis was provided recently with the demonstration that inhibition of CaMKII improves contractility in falling human heart (5).
Clearly, these new findings further demonstrate that understanding the functional role(s) of CaMKII in the regulation of cardiac contractility and cardiac excitability is an important, and a timely, problem. Interestingly, transgenic mice overexpressing CaMKIIδ, the predominant cardiac CaMKII isoform, develop hypertrophy and display increased susceptibility to ventricular arrhythmias (6,7). Action potentials are prolonged in CaMKIIδ-overexpressing ventricular myocytes reflecting, at least in part, reductions in the densities of Ito,f and IK1 (6). Chronic overexpression of CaMKIIδ also results in reduced expression of the transcripts (Kcnd2 and Kcnj2) encoding the main pore-forming α subunits underlying the generation of mouse ventricular Ito,f (Kv4.2) and IK1 (Kir2.1) channels (8), suggesting a potential role for CaMKII-mediated transcriptional regulation of these channels, at least under conditions of chronic upregulation of CaMKIIδ expression/activity. It has also been reported, however, that increased CaMKII activity results in down-regulation of KCND3 (Kv4.3) transcript expression and reduced Ito,f densities in canine ventricular myocytes (9).
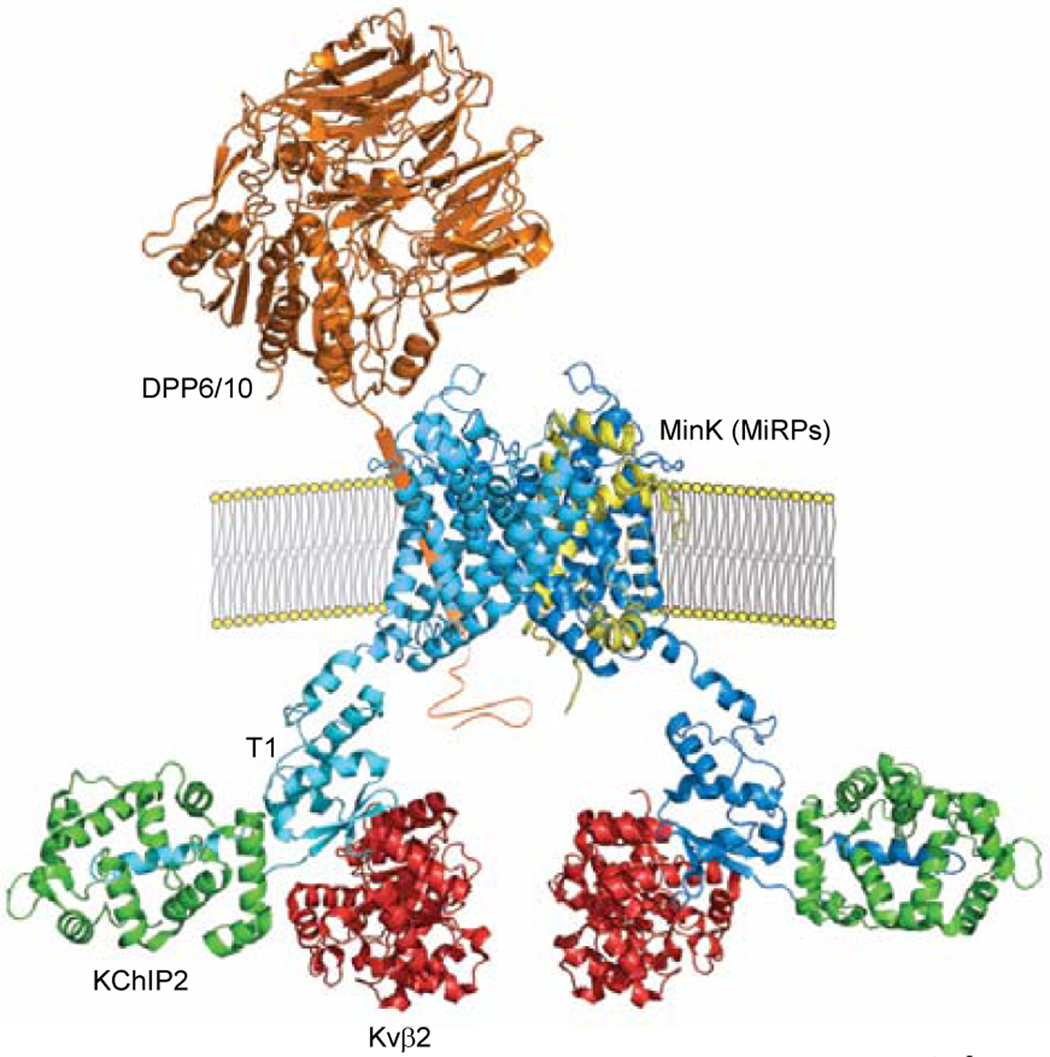
In contrast, chronic inhibition of CaMKII in the mouse heart results in increased Ito,f and IK1 densities, although, in this case, no measurable differences in Kcnd2 and Kcnj2 transcript expression, or in Kv4.2 or Kir2.1 protein expression, were observed (10). The results of several previous studies have also suggested potential roles for CaMKII in the acute regulation of cardiac Ito,f channels. It has been reported, for example, that CaMKII co-immunoprecipitates from adult rat heart lysates with both Kv4.2 and Kv4.3 (11), suggesting that CaMKII may be a component of native cardiac Ito,f channel complexes. Interestingly, a number of cytosolic (KChIP/Kvβ) and transmembrane (DPP6/10, MinK/MiRPs) Kv channel accessory subunits have been shown to associate with Kv4 α subunits in heterologous expression systems and to modify the cell surface expression and/or the properties of Kv4-encoded channels (12), observations interpreted as suggesting that native cardiac Ito,f channels function in macromolecular complexes (Figure 1) and, in addition, that each of the protein components of these Ito,f channel complexes might well be a target for CaMKII-mediated regulation.
Figure 1. Schematic of putative Kv4.3-encoded channel Ito,f macromolecular complex.
Cross section of Kv4.3 channel in the membrane showing two Kv4.3 α subunits (blue), each interacting with a KChIP2 (green) and Kvβ2 (red) cytosolic accessory subunit (1:1:1: stoichiometry) through distinct, non-overlapping N-terminal domains (12). The transmembrane accessory subunits, DPP6/10 and MinK/MiRPs, have also been proposed to interact with Kv4.3 α subunits in a 1:2 stoichiometry and contribute to the formation of functional Ito,f channels (12). All protein structures were generated based on published structural data (13–17) using PyMOL.
Chronic inhibition of CaMKII also reportedly accelerates Ito,f inactivation in human atrial and in rat ventricular myocytes (11,18). In HEK-293 and CHO cells, inhibition of CaMKII also accelerates the rate of Kv4.3-encoded current inactivation (11,19). The opposite effect is produced by autophosphorylated (activated) CaMKII and has been suggested to be mediated by phosphorylation of a CaMKII consensus motif in the C-terminus (Figure 2) of Kv4.3 (19). The association of CaMKII with Kv4.3 reportedly occurs in the absence of increased intracellular free Ca2+ concentration ([Ca2+]i), and the Kv4.3 protein is phosphorylated at low [Ca2+]i (11), again suggesting that CaMKII may be a component of, or closely associated with, Kv-encoded cardiac Ito,f channel complexes. In marked contrast to these results, it has also been reported that chronic and acute overexpression of CaMKIIδ in mouse (chronic) and in rabbit (acute) ventricular myocytes accelerates Ito,f inactivation (8). Some of the differences in experimental observations may reflect the fact that different Kv4 α subunits underlie Ito,f channels in rodents (Kv4.3) and in larger mammals (Kv4.3), including humans (1). It is certainly possible that some of the apparent differences reflect the molecular complexities of endogenous Ito,f channels (Figure 1), which are likely not recapitulated in heterologous expression systems (12). Each of the various Kv4-accessory and regulatory proteins might also be a potential target for direct regulation, or indirect modulation, by CaMKII dependent phosphorylation.
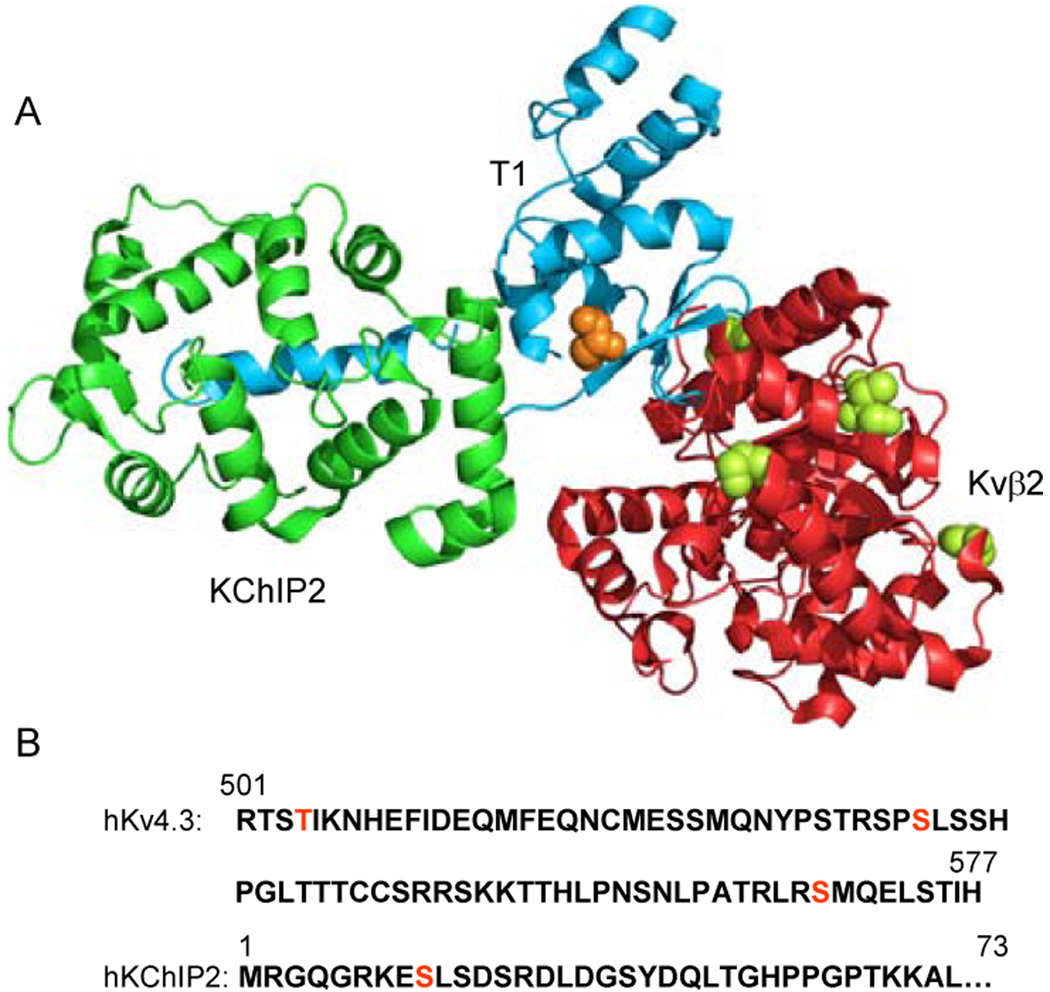
Figure 2. Putative CaMKII phosphorylation sites in Kv4.3, Kvβ2, and KChIP2.
(A) The T1 domain of one Kv4.3 α subunit associated with Kvβ2 and KChIP2 accessory subunits. Putative CaMKII phosphorylation sites are shown in orange for Kv4.3 (residue T53) and green for Kvβ2 (residues S112, S132, T174, and S192). (B) Linear sequences of the hKv4.3C terminus and the hKChIP2N terminus with additional putative CaMKII phosphorylation sites in red
In addition to effects on current kinetics, it has also been reported that co-transfection (in COS-7 cells) of activated CaMKII with Kv4.2 and the accessory subunit, KChIP3, results in increased total and cell surface Kv4.2 expression and that these effects reflect phosphorylation of two CaMKII consensus motifs in the C-terminus of Kv4.2, resulting in increased current amplitudes without measurable changes in gating (20). In HEK-293 cells, increasing [Ca2+]i reportedly resulted in Kv4.2 phosphorylation and a slowing of inactivation (11). The latter effect is prevented by inhibition of CaMKII, an observation interpreted as suggesting that increased [Ca2+]i results in the direct modulation of Kv4.2 channels by endogenous CaMKII (11). Given the likely critical roles of the various Kv4 channel accessory subunits (Figure 1) in determining the time-and voltage-dependent properties and the functional cell surface expression of native cardiac Ito,f channels (12), however, the possibility that accessory subunits are also important targets of CaMKII-mediated effects on Ito,f cannot be excluded. There are multiple putative consensus sites for CaMKII-mediated phosphorylation of KChIP2, as well as Kvβ (Figure 2), any of which might affect functional Ito,f channel densities and/or properties. More importantly, this is a possibility that needs to be explored experimentally using Mass spectrometry-based proteomic approaches (21) that will allow basal and stimulated phosphorylation sites on each of the components of native Ito,f channel complexes to be identified directly.
Considerable evidence suggests a role for CaMKII in the functional regulation of cardiac IK1 channels. In ventricular myocytes from mice overexpressing CaMKIIδ, IK1 is attenuated and expression of Kcnj2 (which encodes Kir2.1) is markedly reduced (8). In contrast, acute overexpression of CaMKIIδ in rabbit ventricular myocytes results in increased IK1 densities and this enhancement is blocked by inhibitors of CaMKII (8). It has also been reported that Kir2.× subunits are regulated by a variety of protein kinases (22) and examination of the C terminus of Kir2.1 reveals three putative CaMKII phosphorylation sites. Experiments focused on isolating endogenous cardiac IK1 channels complexes and identification of basal and stimulated CaMKII-dependent phosphorylation sites (23) on Kir2.1, as well as of other components of IK1 channel complexes, are needed to delineate the mechanisms involved in the acute and the chronic CaMKII-mediated regulation of native cardiac IK1 channel expression and functioning.
Summary and Future Directions
The discussion here was focused on Ito,f and IK1 channels, primarily because the expression/properties of these channels are affected in cardiac hypertrophy and in the failing myocardium and the expression of Kv4-encoded Ito,f and Kv3-encoded IK1 channels are regulated by CaMKII activity. Nevertheless, it is clear that there are multiple other K+ channels expressed in cardiac cells and that many of these are also potential targets for CaMKII-mediated regulation. Sequence scanning, for example, reveals a number of putative CaMKII phosphorylation sites in several other repolarizing K+ channel pore-forming α subunits, including Kv1.5, Kv7.1 (KvLQT1) and Kv12.1 (HERG), which encode Kv channels that underlie other critical repolarizing cardiac K+ currents, IKur, IKr and IKs, in atrial and ventricular myocytes. In Kv1.5, for example, one (T133) putative CaMKII phosphorylation site in the N-terminus and three (T490, S557 and S580) in the C terminus. The number of potential CaMKII sites in Kv12.1 (HERG) is even larger, totaling 20 (9T and 11S) in the cytosolic N- and C-termini, although at least two of these may be mutually exclusive. Similar to cardiac Ito,f and IK1 channels, proteomic approaches (23) hold the promise of identifying CaMKII-mediated phosphorylation sites on native Kv1.5 , Kv7.1 and Kv12.1 α subunits and/or on the accessory/regulatory proteins that contribute to the functioning of the repolarizing K+ channels encoded by these α subunits in the mammalian myocardium.
Acknowledgments
Funding Sources: Work in the author’s laboratory is supported by grants from the National Heart, Lung and Blood Institute (HL034161, HL066388 and HL098781) of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None
Abbreviations: CaMKII, Ito,f; IKr; IKs; IK1
References
- 1.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 2.Aiba T, Tomaselli GF. Electrical remodeling in the failing heart. Curr Opin Cardiol. 2010;25:29–36. doi: 10.1097/HCO.0b013e328333d3d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology. 2008;23:151–159. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 5.Sossalla S, Fluschnik N, Schotola H, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 6.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Maier LS, Dalton ND, et al. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 8.Wagner S, Hacker E, Grandi E, et al. Ca/calmodulin kinase II differentially modulates potassium currents. Circ Arrhythm Electrophysiol. 2009;2:285–294. doi: 10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao L, Coutu P, Villeneuve LR, et al. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res. 2008;103:733–742. doi: 10.1161/CIRCRESAHA.108.171157. [DOI] [PubMed] [Google Scholar]
- 10.Li J, Marionneau C, Zhang R, et al. Calmodulin kinase II inhibition shortens action potential duration by upregulation of K+ currents. Circ Res. 2006;99:1092–1099. doi: 10.1161/01.RES.0000249369.71709.5c. [DOI] [PubMed] [Google Scholar]
- 11.Colinas O, Gallego M, Setien R, Lopez-Lopez JR, Perez-Garcia MT, Casis O. Differential modulation of Kv4.2 and Kv4.3 channels by calmodulin-dependent protein kinase II in rat cardiac myocytes. Am J Physiol Heart Circ Physiol. 2006;291:H1978–H1987. doi: 10.1152/ajpheart.01373.2005. [DOI] [PubMed] [Google Scholar]
- 12.Niwa N, Nerbonne JM. Molecular determinants of cardiac transient outward K+ current (Ito): Expression and regulation. J Mol Cell Cardiol. 2010;48:12–25. doi: 10.1016/j.yjmcc.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pioletti M, Findeisen F, Hura GL, Minor DL., Jr Three-dimensional structure of the KChIP1-Kv4.3 T1 complex reveals a cross-shaped octamer. Nat Struct Mol Biol. 2006;13:987–995. doi: 10.1038/nsmb1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long SB, Tao X, Campbell, MacKinnon R. Atomic structure of a voltage-depednet K+ channels in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 15.Kang C, Tian C, Sonnichsen FD, et al. Structure of KCNE1 and implications for how it modulates the KCNQ1 potassium channel. Biochemistry. 2008;47:7999–8006. doi: 10.1021/bi800875q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Wang Q, Ni F, Ma J. Structure of the full-length Shaker potassium channel Kv1.2 by normal-mode-based X-ray crystallographic refinement. Proc Natl Acad Sci USA. 2010;107:11352–11357. doi: 10.1073/pnas.1000142107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strop P, Bankovich AJ, Hansen KC, Garcia KC, Brunger AT. Structure of a human A-type potassium channel interacting protein DPPX, a member of the dipeptidyl aminopeptidase family. J Mol Biol. 2004;343:1055–1065. doi: 10.1016/j.jmb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Tessier S, Karczewski P, Krause EG, et al. Regulation of the transient outward K+ current by Ca2+/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res. 1999;85:810–819. doi: 10.1161/01.res.85.9.810. [DOI] [PubMed] [Google Scholar]
- 19.Sergeant GP, Ohya S, Reihill JA, et al. Regulation of Kv4.3 currents by Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Cell Physiol. 2005;288:C304–C313. doi: 10.1152/ajpcell.00293.2004. [DOI] [PubMed] [Google Scholar]
- 20.Varga AW, Yuan LL, Anderson AE, et al. Calcium-calmodulin-dependent kinase II modulates Kv4.2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci. 2004;24:3643–3654. doi: 10.1523/JNEUROSCI.0154-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marionneau C, Townsend RR, Nerbonne JM. Proteomic analysis highlights the molecular complexities of native Kv4 channel macromolecular complexes. Sem Cell Devel Biol. 2010 October 17; doi: 10.1016/j.semcdb.2010.10.004. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruppersberg JP. Intracellular regulation of inward rectifier K+ channels. Pflügers Archiv. 2000;441:1–11. doi: 10.1007/s004240000380. [DOI] [PubMed] [Google Scholar]
- 23.Cerda O, Baek JH, Trimmer JS. Mining recent brain proteomic databases for ion channel phosphosite nuggets. J Gen Physiol. 2010 December 13; doi: 10.1085/jgp.201010555. ePub. [DOI] [PMC free article] [PubMed] [Google Scholar]