Summary
The p53 tumor suppressor protein is a key transcription factor that regulates several signaling pathways involved in the cell’s response to stress. Through stress-induced activation, p53 accumulates and triggers the expression of target genes that protect the genetic integrity of all cells including hematopoietic stem cells (HSCs). These protective mechanisms include cell-cycle arrest, DNA repair, induction of apoptosis, or initiation of senescence. In addition to its function under stress conditions, p53 has important functions during steady-state hematopoiesis, regulating HSC quiescence and self-renewal. In addition, it appears that p53 levels affect HSC competition for the hematopoietic niche, with the less p53 activated HSCs preferentially surviving. The specific genes and precise mechanisms underlying p53’s effects on normal HSCs are slowly being clarified. p53 also plays an important role in leukemia stem cell (LSC) behavior, with p53 loss affecting drug resistance and disease progression. Pharmacologic activation of p53 function could overcome the adverse impact of p53 inactivation in LSCs. Thus, understanding the p53 regulatory mechanisms active in HSCs and LSCs may promote the development of new therapeutic strategies that could eliminate the population of largely quiescent LSCs.
Keywords: p53, hematopoietic stem cell, quiescence, self-renewal, leukemia stem cell
Introduction
p53 was originally isolated as a cellular partner of simian virus 40 (SV-40)-derived tumor antigens (Lane and Crawford, 1979; Linzer and Levine, 1979), and a decade later shown to be an important tumor suppressor (Baker et al., 1989; Donehower et al., 1992). p53 functions as a transcription factor (Bargonetti et al., 1991; el-Deiry et al., 1992; Farmer et al., 1992; Kern et al., 1991), mediating DNA damage responses to a variety of cellular stresses and inducing cell-cycle arrest (Mercer et al., 1990; Scheffner et al., 1990), senescence (Serrano et al., 1997), and apoptosis (Shaw et al., 1992; Yonish-Rouach et al., 1991) in order to maintain genomic instability (Liu et al., 2004). These imply that p53 serves as a guardian of the genome under stress conditions.
In the steady state, p53 activity is strictly restrained through its ubiquitylation and proteasome mediated degradation, involving several E3 ubiquitin ligases, primarily MDM2 (Jones et al., 1995; Montes de Oca Luna et al., 1995; Ringshausen et al., 2006). p53 is stabilized and activated in response to stresses, such as acute genotoxic stress or oncogenic activation (Meek, 2009; Toledo and Wahl, 2006; Vousden and Lane, 2007). However, questions remain as to whether p53 is kept inactive until its expression is induced by cellular stress. Recent studies have revealed an important role for p53 under conditions of apparently normal growth and development, by promoting the survival of slightly damaged cells, through effects on DNA repair (Lassus et al., 1996), or by lowering reactive oxygen species (ROS) levels and reducing DNA damage (Sablina et al., 2005). Moreover, p53 has been shown to play a homeostatic function regulating of hematopoietic stem cell quiescence and self-renewal. We will focus on the role of p53 in HSCs in this review (Figure 1).
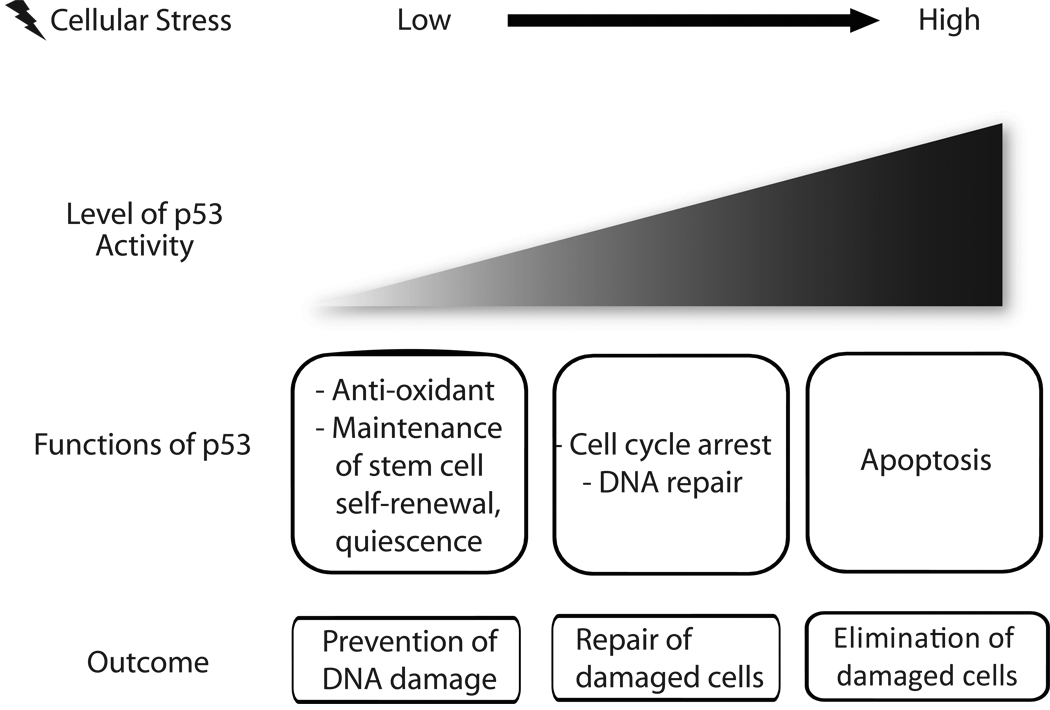
Fig 1. Multiple functions of p53 at physiological and stress conditions.
p53 can induce a variety of cellular responses depending on the nature, level and duration of stress signals. When expressed at low level under physiological condition, p53 plays homeostatic roles such as anti-oxidant function and maintenance of stem cell functions. Increasing level of stress augments the level of p53 expression which induces apoptosis, thereby eliminate damaged cells.
Hematopoietic stem cells (HSCs) have the ability to differentiate into all blood cell lineages and to self-renew. Under steady state conditions, most HSCs are quiescent, while a fraction enter the cell cycle, giving rise to progenitors of each lineage, in order to adequately replenish the circulating blood cells each day (Attar and Scadden, 2004). Under stress conditions, HSCs will enter the cell cycle and maintain their cell pool by self-renewal. Cell division will promote the repair of double strand breaks (DSBs) in HSCs via homologous recombination (Passegue et al., 2005). In contrast, quiescent HSCs appear to utilize nonhomologous end joining (NHEJ)-mediated DNA repair, which can be associated with acquisition of genomic rearrangements (Mohrin et al., 2010).
Although p53-null mice show almost normal hematopoiesis (Lotem and Sachs, 1993), many studies have identified roles for p53 in the proliferation, differentiation, apoptosis, and aging of HSCs (Dumble et al., 2007; Kastan et al., 1991; Park et al., 2003; Shounan et al., 1996). Furthermore, recent more detailed analyses of p53-null mice have gradually revealed the important function of p53 in HSCs.
Function of p53 in Steady-state Hematopoiesis
HSC quiescence is critical for preserving a lifelong pool of HSCs that can sustain a highly regenerative hematopoietic system. The functions and dynamics of HSCs are strictly controlled by both HSC-intrinsic and bone marrow microenvironmental mechanisms (Hock et al., 2004; Krosl et al., 2003; Lacorazza et al., 2006; Ling et al., 2004; Wilson and Trumpp, 2006; Zeng et al., 2004) and many proteins and signaling pathways have been implicated in regulating HSC quiescence, including cell-intrinsic transcription factors, such as MEF/ELF4 (Lacorazza et al., 2006), MLL (Jude et al., 2007), GATA-2 (Tipping et al., 2009), Pbx1 (Ficara et al., 2008), FoxO (Miyamoto et al., 2007), and cell cycle regulators, such as p21cip1/waf1 (Cheng et al., 2000), and Cyclin C (Miyata et al., 2010). Interactions between the bone marrow microenvironment and HSCs, such as Tie2/angiopoietin-1 signaling (Arai et al., 2004), Wnt/Frizzled signaling (Fleming et al., 2008), Thrombopoietin/MPL signaling (Yoshihara et al., 2007), and CXCR4/CXCL12 signaling (Nie et al., 2008) regulate HSC quiescence as do several tumor suppressor genes, including PML, Fbxw7, and PTEN (Ito et al., 2008; Perry and Li, 2008; Thompson et al., 2008; Yilmaz et al., 2006; Zhang et al., 2006).
Our studies of quiescence evolved from characterizing the biological function of an Ets transcription factor, MEF/ELF4, which we initially identified based on its ability to regulate cytokine gene expression in hematopoietic cells (Miyazaki et al., 1996). MEF/ELF4 generally promotes cell growth and functions as an oncogene: MEF/ELF4 can transform NIH3T3 cells and is overexpressed in some human ovarian cancer tumor samples (Yao et al., 2007). We generated MEF/ELF4-null mice and subsequently identified its role in regulating both HSC quiescence and self-renewal (Lacorazza et al., 2006). After discovering that MEF/ELF4 can directly regulate MDM2 expression (Sashida et al., 2009), we showed that MEF/ELF4-null long-term reconstituting HSCs (LT-HSCs) and HSC-enriched Lineage−Sca-1+ c-kit+ (LSK) cells express high levels of p53 (Liu et al., 2009), that could play an important role in HSC physiology.
p53-null mice have a two- to threefold increase in LSK cells and in LT-HSC-enriched SLAM+ (CD150+ CD48−) LSK cells (Akala et al., 2008; Chen et al., 2008; Liu et al., 2009; TeKippe et al., 2003). p53 promotes HSC quiescence and in its absence, HSCs more easily to enter the cell cycle (Liu et al., 2009). However, lack of p53 returned the enhanced stem cell quiescence of MEF/ELF4-null HSCs to normal, indicating that p53 functions to block cell-cycle entry of HSCs and maintain their quiescence status. This does not involve the standard p21-mediated pathway (el-Deiry et al., 1993; Liu et al., 2009).
p53 plays a cytoprotective function within the hematopoietic compartment, mediated by its effects on HSC quiescence, helping to protect HSCs from DNA damage. Furthermore, elevated ROS levels have been shown to limit the life span of HSCs in vivo (Ito et al., 2006) and p53 has been shown to lower ROS levels, thereby reducing DNA damage and the mutational rate (Sablina et al., 2005).
In vivo bone marrow transplantation experiments (Zon, 2008) have shown that p53 negatively regulates HSC self-renewal (Akala et al., 2008; Chen et al., 2008; Liu et al., 2009; TeKippe et al., 2003). p53-null bone marrow cells outcompete wild type bone marrow cells in competitive repopulation assays (Liu et al., 2009; TeKippe et al., 2003), and although multipotent progenitor cells generally lack the ability to self-renew, p16Ink4a and p19Arf null multipotent progenitor cells gain the ability to reconstitute long-term hematopoiesis when p53 is absent (Akala et al., 2008). The pathways controlled by these proteins are commonly repressed in the course of oncogenesis, which would allow hematopoietic progenitor cells to gain the ability to self-renew, and become more susceptible to transformation by oncogenic mutations.
Based on these findings, inhibition of p53 activity has been suggested to represent a therapeutic strategy capable of amplifying the HSC pool. Small molecule inhibitors of p53, the pififthrins (PFTs) have been identified, that can suppress genotoxic stress-induced p53-dependent apoptosis, thereby protecting mice from otherwise lethal doses of irradiation or chemotherapy (Komarov et al., 1999). A recent study showed that PFTs can stimulate HSC proliferation in vitro and in vivo; the amplified HSCs are functional in bone marrow transplantation experiments, without promoting tumor development (Marion et al., 2009), which suggests that transient exposure to p53 inhibitors could be used to stimulate HSC self-renewal or survival.
As p53 has been shown to negatively regulate neural stem cell self-renewal (Meletis et al., 2006) and mammary stem cell self-renewal (Cicalese et al., 2009), such strategies may not be limited to the hematopoietic compartment. In addition, disruption of the p53 network enhances the generation of induced pluripotent stem (iPS) cells, which are capable of self-renewal and of giving rise to multiple types of differentiated cells (Hong et al., 2009; Kawamura et al., 2009; Li et al., 2009; Marion et al., 2009; Utikal et al., 2009). Thus, p53 impedes somatic cell reprogramming, yet another p53 function in addition to its role as a regulator of cellular stress responses.
What are the p53 target genes relevant to the steady-state behavior of HSCs? While the cyclin-dependent kinase inhibitor p21 is a major target of p53, p21 has been shown to play a modest role in regulating HSC quiescence, in a mixed mouse strain background (Cheng et al., 2000), but not in a pure C57BL/6 background (van Os et al., 2007). In our study of p53 function, p21 did not appear to be involved in regulating the quiescence of either wild type or MEF/ELF4-null HSCs. By performing transcript profiling of LSK cells isolated from p53-null and p53, MEF/ELF4-double null mice, we identified Gfi-1 and Necdin as two direct p53 target genes that could regulate HSC quiescence (Liu et al., 2009).
Gfi-1 (Growth factor independent-1) is a SNAG-domain-containing zinc-finger transcriptional repressor that promotes the proliferation of T cells and sometimes functions as a cooperating oncogene in lymphoid cells (Gilks et al., 1993; Zhu et al., 2002). Gfi-1 has been shown to restrict HSC proliferation and preserve HSC functional integrity. Gfi-1 null HSCs demonstrate excessive cycling status and impaired self renewal, shown by in vivo competitive repopulation assays (Hock et al., 2004; Zeng et al., 2004).
Necdin is a growth-suppressing protein first identified in post-mitotic neurons (Maruyama et al., 1991). The gene encoding Necdin is one of the several genes which are deleted in individuals with Prader-Willi syndrome (Jay et al., 1997; MacDonald and Wevrick, 1997), a disorder associated with a mildly increased risk of myeloid leukemia (Davies et al., 2003). Like the retinoblastoma protein, Necdin interacts with multiple cell cycle promoting proteins, such as simian virus 40 large T antigen, adenovirus E1A, and transcription factor E2F1 (Hu et al., 2003; Taniura et al., 2005; Taniura et al., 1999). Necdin is highly expressed in LT-HSCs (Forsberg et al., 2005; Liu et al., 2009). Downregulation of Necdin diminished HSC quiescence, whereas its upregulation increased HSC quiescence, identifying its role as a rheostat controlling HSC quiescence (Liu et al., 2009). Necdin and p53 has been shown to inhibit cell growth in an additive manner (Taniura et al., 1999), suggesting the presence of a positive feedback loop that may control quiescent HSCs. Recently our analysis using Necdin-null HSCs shows that Necdin protects HSCs from genotoxic stress (Liu and Asai et al., in submission) (Figure 2).
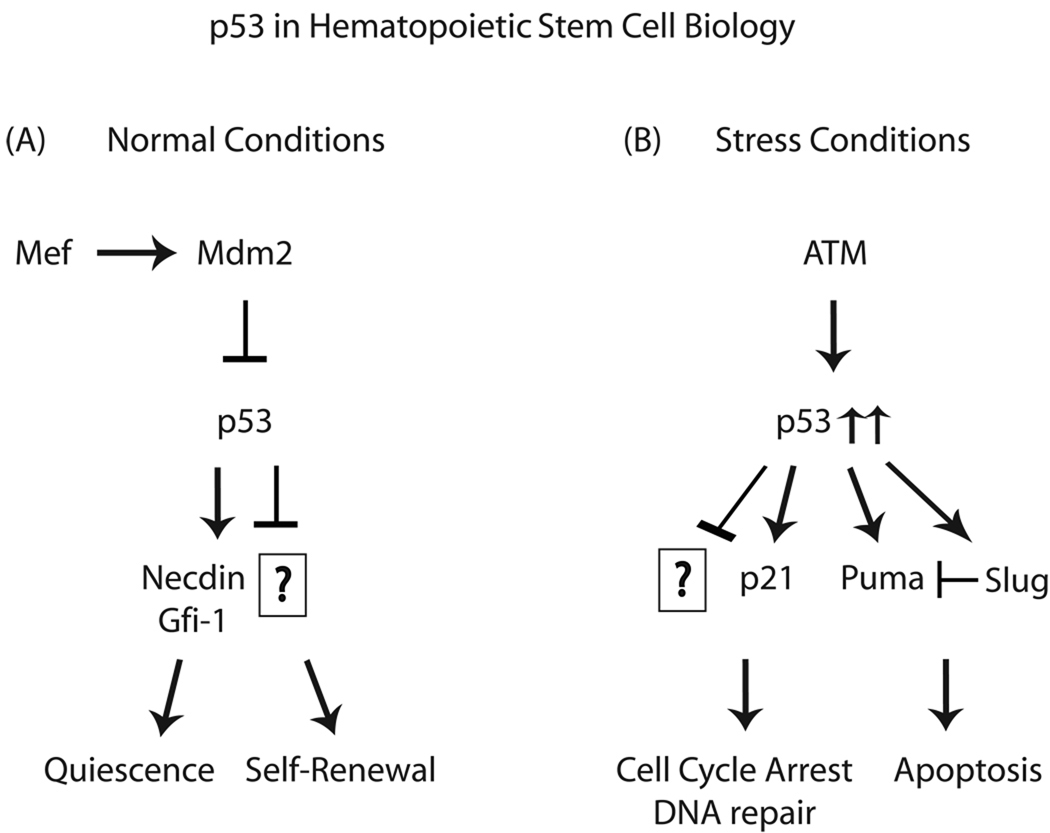
Fig 2. Role of p53 in hematopoietic stem cells.
(A) Under normal condition, p53 has been shown to regulate hematopoietic stem cell quiescence mediated by Gfi-1 and Necdin. It is likely to maintain self-renewal by yet unknown mechanism. (B) Increasing level of stress triggers ATM activation which will increase p53 activation. By downstream mediators such as p21, Puma and Slug, p53 triggers cell cycle arrest, DNA repair or apoptosis.
Function of p53 in Stress Hematopoiesis
Hematopoiesis is perturbed by genotoxic stresses such as γ-irradiation or chemotherapy and in response to high levels of genotoxic stress, HSCs and progenitor cells undergo apoptosis, leading to severe anemia, bleeding, and infections. Some sensor proteins (Mre11, Rad50, Nbs1, Rad9, Rad1, Rad17, Hus1) bind damaged DNA and relay the DNA damage signal to transducer proteins such as ATM and ATR, which phosphorylate several effector kinases including Chk1 and Chk2. These effector kinases, together with ATM, phosphorylate p53 and other target proteins (Zhou and Elledge, 2000). Once activated, p53 induces growth arrest and DNA repair, or apoptosis depending on many variables (Balint and Vousden, 2001).
p53 is a critical regulator of apoptosis in HSCs. Lack of Bmi-1 in the hematopoietic compartment leads to impaired self-renewal capacity and bone marrow failure due to p19Arf accumulation, which triggers p53-dependent cell death (Park et al., 2003). Similarly, inactivation of Fbxw7, an SCF-type ubiquitin ligase complex subunit, causes premature loss of HSCs due to active cell cycling and p53-dependent apoptosis (Matsuoka et al., 2008).
While p53 initiates apoptosis within HSCs, inhibition of p53 function leads to resistance to the apoptosis, promoting effects of ionizing irradiation (Komarov et al., 1999; Komarova et al., 2004; Leonova et al., 2010; Westphal et al., 1997) or DNA-damaging chemotherapeutic agents (Lotem and Sachs, 1993). The transcription-independent pro-apoptotic effects of p53 are triggered by the movement of p53 to the mitochondria (Erster et al., 2004), where it can react with Bcl-xL and Bcl-2 and antagonize their mitochondrial membrane stabilizing effects (Mihara et al., 2003). Mitochondrial p53 also increases permeabilization of the outer mitochondrial membrane, leading to the release of cytochrome c (Moll et al., 2005), and it directly promotes the pro-apoptotic functions of Bak (Leu et al., 2004).
The precise p53 role in HSCs under genotoxic stress is not fully understood; using γ-H2AX as an indicator of DNA damage, we demonstrated that p53, MEF/ELF4 double-null HSCs have more foci than MEF-null HSCs 3 hours following ionizing irradiation (Liu et al., 2009), which suggests that p53 facilitates DNA damage repair in HSCs. The transcriptional regulatory functions of p53 control the expression of its pro-apoptotic target genes, such as Puma (Han et al., 2001; Nakano and Vousden, 2001; Yu et al., 2001), Noxa (Oda et al., 2000), Bid (Mandal et al., 2008), and Bax (Miyashita and Reed, 1995) (Table 1). Puma (p53 upregulated mediator of apoptosis) is one of the most potent p53 target genes that induce apoptosis in HSCs under conditions of genotoxic stress. Puma has been reported to be essential for hematopoietic cell death triggered by ionizing radiation and cytokine withdrawal among others (Jeffers et al., 2003; Villunger et al., 2003). The function of Puma in determining the sensitivity of HSCs to high-dose ionizing irradiation has been characterized (Shao et al., 2010; Yu et al., 2010); in the absence of Puma, HSCs are highly resistant to ionizing irradiation in a cell autonomous manner. Puma null HSCs also show enhanced quiescence and more efficient DNA repair than wild type HSCs (Yu et al., 2010), as do Puma-null hematopoietic progenitor cells (Shao et al., 2010). In contrast to Puma, activation of Slug, a transcriptional repressor induced by p53 upon irradiation, protects hematopoietic progenitors from apoptosis by repressing the transcription of Puma (Wu et al., 2005). Thus, promoting Slug function or blocking pro-apoptotic p53 targets, such as Puma, may be potential strategies to protect HSCs from the myelosuppressive effects of intensive radiotherapy or chemotherapy.
Table 1.
List of p53 target genes that mediate p53 activities.
| Cellular Effect | Target genes | References |
|---|---|---|
| Cell cycle arrest | p21 | (el-Deiry et al., 1993) |
| 14-3-3-sigma | (Chan et al., 1999; Weber et al., 2002) | |
| Cdc25 | (Resnick-Silverman et al., 1998) | |
| DNA repair | DDIT4 | (Ellisen et al., 2002) |
| Gadd45 | (Canman et al., 1995) | |
| Apoptosis | Puma | (Han et al., 2001; Nakano and Vousden, 2001; Yu et al., 2001) |
| Noxa | (Oda et al., 2000) | |
| Slug | (Inoue et al., 2002) | |
| Bid | (Mandal et al., 2008) | |
| Bax | (Miyashita and Reed, 1995) | |
| Stem cell quiescence | Gfi1 | (Liu et al., 2009) |
| Necdin | (Liu et al., 2009) |
In response to moderate levels of genotoxic stress, p53 can initiate the repair of damaged HSCs by triggering cell cycle arrest and activating the DNA repair machinery. Among the p53 target genes implicated in these processes are DDB2 (Takimoto et al., 2002), DDIT4 (Ellisen et al., 2002), and Gadd45 (Canman et al., 1995), which are involved in DNA damage repair, and p21 (CDKN1A) (el-Deiry et al., 1993), 14-3-3-sigma (SFN) (Chan et al., 1999; Weber et al., 2002), and Cdc25 (Resnick-Silverman et al., 1998), which are involved in inducing cell cycle arrest (Table 1).
Function of p53 in HSC aging
Aging can be defined as a progressive functional decline associated with an increasing risk of mortality over time (Sharpless and DePinho, 2007). Advancing age is accompanied by a number of pathophysiological changes in the hematopoietic system, possibly reflecting loss of homeostatic control of hematopoietic stem and progenitor cell (HSPC) behavior (Rossi et al., 2008). Accumulation of DNA damage is thought to be a physiological consequence of HSC aging, which may contribute to the diminished capacity of older HSCs to return to their basal state after exposure to acute stress or injury (Rossi et al., 2007)
The relationship between stem cell aging and tumor suppressor gene (TSG) expression has received much attention recently. Inactivation of tumor suppressors contributes to the development of cancer, while TSG activation contributes to HSC aging. Increased expression of p16INK4a has been proposed to be one of the principal biomarkers of aging (Krishnamurthy et al., 2004). p16INK4a is elevated in HSCs isolated from older mice, and the HSC repopulating defects and apoptosis of older HSCs are less apparent in older p16INK4a-null mice (Janzen et al., 2006).
p53 has also been implicated in HSC aging (Dumble et al., 2007; Tyner et al., 2002). Knock-in mice expressing a truncated but active form of p53 (p53+/m) exhibit an early aging phenotype with fewer proliferating HSCs compared with older wild-type mice (Maier et al., 2004; Tyner et al., 2002). p53+/− mice, that have slightly reduced p53 levels, show the opposite phenotype. In addition, p53+/m HSCs have reduced engraftment capacity, compared to wild-type or p53-null HSCs (Dumble et al., 2007). In contrast, mice carrying three functional copies of the p53 gene (super-p53) have a normal lifespan but some evidence of early aging (Garcia-Cao et al., 2006). It is possible that the constitutively high activity of p53 in p53+/m mice accelerates aging, while the extra copy of p53 in the super-p53 mice is subject to normal regulation, and therefore p53 functional activity is unchanged.
p53-mediated apoptosis may be involved in the physiological regulation of HSC population size and function with aging. Mice carrying a hypermorphic form of the Rad50 DNA repair protein exhibit precipitous bone marrow failure due to enhanced signaling through an ATM-Chk2-p53-dependent apoptotic pathway (Bender et al., 2002; Morales et al., 2005). On the other hand, mice that overexpress BCL-2 within the hematopoietic compartment show an expanded HSC pool and improved HSC repopulating capacity (Domen et al., 2000), similar to the p53-null mice (TeKippe et al., 2003). These studies illustrate the tenuous relationship between tumor suppression and HSC aging (Gatza et al., 2007). Although manipulating the p53 pathway could delay aging, this could occur at the expanse of a marked increased risk of developing cancer.
p53-mediated HSC competition
Cell competition is an important aspect of many homeostatic processes, and two groups recently identified a role for p53 in the competition among HSPCs (Bondar and Medzhitov, 2010; Marusyk et al., 2010). In competitive repopulation experiments, unirradiated HSCs were shown to outcompete HSCs that were treated with a low dose of ionizing irradiation (1 Gy), dependent on p53 (Bondar and Medzhitov, 2010). Similarly, wild type HSCs outcompete MDM2-null HSCs in the absence of external stress, and low- or middle-dose irradiation enhances this competitive advantage, suggesting that the p53 level itself is critical for HSC competition within the stem cell niche. This p53-dependent HSC competition is related to cell proliferation, and not to a higher rate of apoptosis in the outcompeted cells. It is also mediated by the expression of growth arrest and senescence-related genes in the outcompeted cells, such as p16 (Bondar and Medzhitov, 2010; Janzen et al., 2006).
It is problematic that p53-mutated HSCs could potentially dominate the stem cell niche and outcompete normal HSCs. As p53-null HSCs are more proliferative and less quiescent than normal HSCs (Liu et al., 2009), it is possible that mechanisms exist to detect p53-mutated HSCs and limit their inherent advantage (Green, 2010; Marusyk et al., 2010). The presence of p53 may help cells tolerate stress via metabolic processes (Vousden and Ryan, 2009), or overall patterns of gene expression may be optimized during p53 regulated G0 or G1 phases of the cell cycle. Defining how wild-type HSCs sustain the advantage over p53-mutated HSCs under various conditions will help us understand leukemogenesis and how regulating p53 activity could affect disease initiation and progression.
Function of p53 in Leukemia Stem Cells
p53 is the most common gene targeted for inactivation by deletion and/or mutation in human tumors (Harris and Hollstein, 1993). p53 mutations are much less frequent in leukemia than in other solid tumors (Peller and Rotter, 2003), occurring in less than 10% of de novo acute myeloid leukemia (AML) (Fenaux et al., 1992; Greenblatt et al., 1994; Imamura et al., 1994; Slingerland et al., 1991). Patients with p53 mutations are generally resistant to chemotherapy and have relatively short survival (Haferlach et al., 2008; Nahi et al., 2008; Wattel et al., 1994). p53 mutations or deletions are thought to be an independent prognostic factor for survival and they are more common in elder patients with complex karyotypes (Nakano et al., 2000; Stirewalt et al., 2001). Similar observations have been made in patients with myelodysplastic syndrome (MDS) (Padua et al., 1998). However, in patients who develop AML or MDS following exposure to alkylating agents, the incidence of p53 mutation increases to 30%, and is associated with increased resistance to chemotherapy and shorter overall survival (Christiansen et al., 2001). In addition, p53 mutations are found in 25% of blast phase chronic myelogenous leukemia (CML) (Feinstein et al., 1991; Kelman et al., 1989), generally accompanying disease progression (Ahuja et al., 1989). Thus, p53 mutations occur late in the course of these diseases and promote drug resistance.
Leukemia stem cells (LSCs) are thought to be resistant to various types of therapy, because they are in a relatively quiescent state (Komarova and Wodarz, 2007). Leukemia relapse may occur because most therapies eliminate proliferating cells, but not the quiescent cells, such as the LSCs, that can reinitiate the disease after some latency period (Holtz et al., 2007). New therapeutic approaches that can target LSCs will help eradicate acute leukemia and given the effect of p53 on HSC quiescence, understanding how p53 promotes quiescence may lead to therapeutic strategies that could eliminate the largely quiescent LSCs (Liu et al., 2009).
p53 mutations are found in a minority of human leukemias, and if p53 signaling is intact, then disrupting the p53-MDM2 interact could lead to the induction of apoptosis in the leukemic cells (Saha et al., 2010; Shangary and Wang, 2008; Tovar et al., 2006). One of the most promising p53 activating agents is nutlin (and its active form, nutlin-3a or nutlin-3), which is a small-molecule inhibitor of MDM2 (Vassilev et al., 2004). Recent studies demonstrated that nutlin-3, used alone or in combination with other drugs, effectively induces apoptosis in AML (Kojima et al., 2005; Kojima et al., 2008; Zhang et al., 2010). While its precise function on LSCs has not been clarified, gene expression analyses suggest that the p53-regulated genes, Bax, Gadd45, and possibly p21cip1/waf1, are involved in triggering LSC apoptosis (Guzman et al., 2002). Other approaches to target LSCs include the combination of a proteasome inhibitor (MG-132) with the anthracycline idarubicin, which induces p53-dependent apoptosis of LSCs while leaving normal HSCs viable (Guzman et al., 2002; Tergaonkar et al., 2002) In addition, parthenolide, a naturally occurring small molecule, induces robust apoptosis in LSCs, through similar mechanisms (Guzman et al., 2005; Guzman et al., 2007). Therefore, p53 may play a different role in inducing apoptosis of LSCs, compared to HSCs.
p53 has been shown to be involved in some mouse models of leukemia. Loss of p53 dramatically accelerates AML1/ETO9a-driven, but not MLL/ENL-driven leukemogenesis (Zuber et al., 2009). Loss of p53 also cooperates with overexpression of sPRDM16, the short isoform of PR domain containing 16, in leukemogenesis promoting LSC self renewal (Shing et al., 2007), and it accelerates the development of acute lymphoblastic leukemia (ALL) in Fbxw7 null mice (Matsuoka et al., 2008).
Recently, a mouse model of the human 5q- syndrome was reported (Barlow et al., 2010). These mice have macrocytic anemia and dysplastic bone marrow features, which are also observed in human 5q- MDS; the mice show an increase in p53-positive cells in the bone marrow with elevated apoptosis and defective hematopoietic progenitor development. Loss of p53 rescues the defect in hematopoietic progenitor development, suggesting that p53-dependent mechanisms underlie the pathogenesis of the 5q- syndrome (Barlow et al., 2010).
Summary and Consideration for Future Investigation
The p53 tumor suppressor protein is a key transcription factor that regulates signaling pathways controlling the cellular stress response. Through stress-induced activation, p53 accumulates and mediates the expression of genes that protect the genetic integrity of HSCs. During steady-state hematopoiesis, basal-level p53 activity regulates HSC quiescence and self-renewal. Inhibition of p53 may be clinically applicable to amplify the HSC pool, and recent studies show that the level of p53 is critical for HSC competition within the hematological niche, allowing the least damaged HSCs to survive. The target genes and precise mechanisms underlying basal p53 functional activity will be clarified in the future. On the other hand, p53 loss is related to decreased apoptosis, increased drug resistance, and disease progression of leukemic cells. Activation of p53 function represents a positive strategy to overcome the adverse impact of p53 inactivation in LSCs. Understanding the p53 regulatory mechanisms active in HSCs vs LSCs will shed light on new therapeutic strategies that could eliminate the largely quiescent LSCs.
Acknowledgement
This work was founded by an NIH RO1 grant (DK52208 to S.D.N) and an LLS SCOR grant (S.D.N.). We would like to thank Ms. Erica Chuang for helping in the preparation of this manuscript.
Contract grant sponsor: NIH; Contract grant number: DK52208. Contract grant sponsor: LLS SCOR; Contract grant number: 7415-07.
Reference
- Ahuja H, Bar-Eli M, Advani SH, Benchimol S, Cline MJ. Alterations in the p53 gene and the clonal evolution of the blast crisis of chronic myelocytic leukemia. Proc Natl Acad Sci U S A. 1989;86(17):6783–6787. doi: 10.1073/pnas.86.17.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akala OO, Park IK, Qian D, Pihalja M, Becker MW, Clarke MF. Long-term haematopoietic reconstitution by Trp53−/−p16Ink4a−/−p19Arf−/− multipotent progenitors. Nature. 2008;453(7192):228–232. doi: 10.1038/nature06869. [DOI] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118(2):149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Attar EC, Scadden DT. Regulation of hematopoietic stem cell growth. Leukemia. 2004;18(11):1760–1768. doi: 10.1038/sj.leu.2403515. [DOI] [PubMed] [Google Scholar]
- Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, vanTuinen P, Ledbetter DH, Barker DF, Nakamura Y, White R, Vogelstein B. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Balint EE, Vousden KH. Activation and activities of the p53 tumour suppressor protein. Br J Cancer. 2001;85(12):1813–1823. doi: 10.1054/bjoc.2001.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargonetti J, Friedman PN, Kern SE, Vogelstein B, Prives C. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell. 1991;65(6):1083–1091. doi: 10.1016/0092-8674(91)90560-l. [DOI] [PubMed] [Google Scholar]
- Barlow JL, Drynan LF, Hewett DR, Holmes LR, Lorenzo-Abalde S, Lane AL, Jolin HE, Pannell R, Middleton AJ, Wong SH, Warren AJ, Wainscoat JS, Boultwood J, McKenzie AN. A p53-dependent mechanism underlies macrocytic anemia in a mouse model of human 5q- syndrome. Nat Med. 2010;16(1):59–66. doi: 10.1038/nm.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender CF, Sikes ML, Sullivan R, Huye LE, Le Beau MM, Roth DB, Mirzoeva OK, Oltz EM, Petrini JH. Cancer predisposition and hematopoietic failure in Rad50(S/S) mice. Genes Dev. 2002;16(17):2237–2251. doi: 10.1101/gad.1007902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondar T, Medzhitov R. p53-mediated hematopoietic stem and progenitor cell competition. Cell Stem Cell. 2010;6(4):309–322. doi: 10.1016/j.stem.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canman CE, Gilmer TM, Coutts SB, Kastan MB. Growth factor modulation of p53-mediated growth arrest versus apoptosis. Genes Dev. 1995;9(5):600–611. doi: 10.1101/gad.9.5.600. [DOI] [PubMed] [Google Scholar]
- Chan TA, Hermeking H, Lengauer C, Kinzler KW, Vogelstein B. 14-3-3Sigma is required to prevent mitotic catastrophe after DNA damage. Nature. 1999;401(6753):616–620. doi: 10.1038/44188. [DOI] [PubMed] [Google Scholar]
- Chen J, Ellison FM, Keyvanfar K, Omokaro SO, Desierto MJ, Eckhaus MA, Young NS. Enrichment of hematopoietic stem cells with SLAM and LSK markers for the detection of hematopoietic stem cell function in normal and Trp53 null mice. Exp Hematol. 2008;36(10):1236–1243. doi: 10.1016/j.exphem.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287(5459):1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- Christiansen DH, Andersen MK, Pedersen-Bjergaard J. Mutations with loss of heterozygosity of p53 are common in therapy-related myelodysplasia and acute myeloid leukemia after exposure to alkylating agents and significantly associated with deletion or loss of 5q, a complex karyotype, and a poor prognosis. J Clin Oncol. 2001;19(5):1405–1413. doi: 10.1200/JCO.2001.19.5.1405. [DOI] [PubMed] [Google Scholar]
- Cicalese A, Bonizzi G, Pasi CE, Faretta M, Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP, Pelicci PG. The tumor suppressor p53 regulates polarity of self-renewing divisions in mammary stem cells. Cell. 2009;138(6):1083–1095. doi: 10.1016/j.cell.2009.06.048. [DOI] [PubMed] [Google Scholar]
- Davies HD, Leusink GL, McConnell A, Deyell M, Cassidy SB, Fick GH, Coppes MJ. Myeloid leukemia in Prader-Willi syndrome. J Pediatr. 2003;142(2):174–178. doi: 10.1067/mpd.2003.81. [DOI] [PubMed] [Google Scholar]
- Domen J, Cheshier SH, Weissman IL. The role of apoptosis in the regulation of hematopoietic stem cells: Overexpression of Bcl-2 increases both their number and repopulation potential. J Exp Med. 2000;191(2):253–264. doi: 10.1084/jem.191.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356(6366):215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Dumble M, Moore L, Chambers SM, Geiger H, Van Zant G, Goodell MA, Donehower LA. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood. 2007;109(4):1736–1742. doi: 10.1182/blood-2006-03-010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry WS, Kern SE, Pietenpol JA, Kinzler KW, Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992;1(1):45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]
- el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, Oliner JD, McKeon F, Haber DA. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol Cell. 2002;10(5):995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- Erster S, Mihara M, Kim RH, Petrenko O, Moll UM. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol. 2004;24(15):6728–6741. doi: 10.1128/MCB.24.15.6728-6741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer G, Bargonetti J, Zhu H, Friedman P, Prywes R, Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992;358(6381):83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Feinstein E, Cimino G, Gale RP, Alimena G, Berthier R, Kishi K, Goldman J, Zaccaria A, Berrebi A, Canaani E. p53 in chronic myelogenous leukemia in acute phase. Proc Natl Acad Sci U S A. 1991;88(14):6293–6297. doi: 10.1073/pnas.88.14.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenaux P, Preudhomme C, Quiquandon I, Jonveaux P, Lai JL, Vanrumbeke M, Loucheux-Lefebvre MH, Bauters F, Berger R, Kerckaert JP. Mutations of the P53 gene in acute myeloid leukaemia. Br J Haematol. 1992;80(2):178–183. doi: 10.1111/j.1365-2141.1992.tb08897.x. [DOI] [PubMed] [Google Scholar]
- Ficara F, Murphy MJ, Lin M, Cleary ML. Pbx1 regulates self-renewal of long-term hematopoietic stem cells by maintaining their quiescence. Cell Stem Cell. 2008;2(5):484–496. doi: 10.1016/j.stem.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming HE, Janzen V, Lo Celso C, Guo J, Leahy KM, Kronenberg HM, Scadden DT. Wnt signaling in the niche enforces hematopoietic stem cell quiescence and is necessary to preserve self-renewal in vivo. Cell Stem Cell. 2008;2(3):274–283. doi: 10.1016/j.stem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1(3):e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cao I, Garcia-Cao M, Tomas-Loba A, Martin-Caballero J, Flores JM, Klatt P, Blasco MA, Serrano M. Increased p53 activity does not accelerate telomere-driven ageing. EMBO Rep. 2006;7(5):546–552. doi: 10.1038/sj.embor.7400667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatza C, Moore L, Dumble M, Donehower LA. Tumor suppressor dosage regulates stem cell dynamics during aging. Cell Cycle. 2007;6(1):52–55. doi: 10.4161/cc.6.1.3667. [DOI] [PubMed] [Google Scholar]
- Gilks CB, Bear SE, Grimes HL, Tsichlis PN. Progression of interleukin-2 (IL-2)-dependent rat T cell lymphoma lines to IL-2-independent growth following activation of a gene (Gfi-1) encoding a novel zinc finger protein. Mol Cell Biol. 1993;13(3):1759–1768. doi: 10.1128/mcb.13.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR. Cell competition: pirates on the tangled bank. Cell Stem Cell. 2010;6(4):287–288. doi: 10.1016/j.stem.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54(18):4855–4878. [PubMed] [Google Scholar]
- Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, Jordan CT. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105(11):4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Rossi RM, Neelakantan S, Li X, Corbett CA, Hassane DC, Becker MW, Bennett JM, Sullivan E, Lachowicz JL, Vaughan A, Sweeney CJ, Matthews W, Carroll M, Liesveld JL, Crooks PA, Jordan CT. An orally bioavailable parthenolide analog selectively eradicates acute myelogenous leukemia stem and progenitor cells. Blood. 2007;110(13):4427–4435. doi: 10.1182/blood-2007-05-090621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman ML, Swiderski CF, Howard DS, Grimes BA, Rossi RM, Szilvassy SJ, Jordan CT. Preferential induction of apoptosis for primary human leukemic stem cells. Proc Natl Acad Sci U S A. 2002;99(25):16220–16225. doi: 10.1073/pnas.252462599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haferlach C, Dicker F, Herholz H, Schnittger S, Kern W, Haferlach T. Mutations of the TP53 gene in acute myeloid leukemia are strongly associated with a complex aberrant karyotype. Leukemia. 2008;22(8):1539–1541. doi: 10.1038/leu.2008.143. [DOI] [PubMed] [Google Scholar]
- Han J, Flemington C, Houghton AB, Gu Z, Zambetti GP, Lutz RJ, Zhu L, Chittenden T. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci U S A. 2001;98(20):11318–11323. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CC, Hollstein M. Clinical implications of the p53 tumor-suppressor gene. N Engl J Med. 1993;329(18):1318–1327. doi: 10.1056/NEJM199310283291807. [DOI] [PubMed] [Google Scholar]
- Hock H, Hamblen MJ, Rooke HM, Schindler JW, Saleque S, Fujiwara Y, Orkin SH. Gfi-1 restricts proliferation and preserves functional integrity of haematopoietic stem cells. Nature. 2004;431(7011):1002–1007. doi: 10.1038/nature02994. [DOI] [PubMed] [Google Scholar]
- Holtz M, Forman SJ, Bhatia R. Growth factor stimulation reduces residual quiescent chronic myelogenous leukemia progenitors remaining after imatinib treatment. Cancer Res. 2007;67(3):1113–1120. doi: 10.1158/0008-5472.CAN-06-2014. [DOI] [PubMed] [Google Scholar]
- Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460(7259):1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Wang S, Zhang Y, Feghali CA, Dingman JR, Wright TM. A nuclear target for interleukin-1alpha: interaction with the growth suppressor necdin modulates proliferation and collagen expression. Proc Natl Acad Sci U S A. 2003;100(17):10008–10013. doi: 10.1073/pnas.1737765100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura J, Miyoshi I, Koeffler HP. p53 in hematologic malignancies. Blood. 1994;84(8):2412–2421. [PubMed] [Google Scholar]
- Inoue A, Seidel MG, Wu W, Kamizono S, Ferrando AA, Bronson RT, Iwasaki H, Akashi K, Morimoto A, Hitzler JK, Pestina TI, Jackson CW, Tanaka R, Chong MJ, McKinnon PJ, Inukai T, Grosveld GC, Look AT. Slug, a highly conserved zinc finger transcriptional repressor, protects hematopoietic progenitor cells from radiation-induced apoptosis in vivo. Cancer Cell. 2002;2(4):279–288. doi: 10.1016/s1535-6108(02)00155-1. [DOI] [PubMed] [Google Scholar]
- Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, Pandolfi PP. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453(7198):1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443(7110):421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Jay P, Rougeulle C, Massacrier A, Moncla A, Mattei MG, Malzac P, Roeckel N, Taviaux S, Lefranc JL, Cau P, Berta P, Lalande M, Muscatelli F. The human necdin gene, NDN, is maternally imprinted and located in the Prader-Willi syndrome chromosomal region. Nat Genet. 1997;17(3):357–361. doi: 10.1038/ng1197-357. [DOI] [PubMed] [Google Scholar]
- Jeffers JR, Parganas E, Lee Y, Yang C, Wang J, Brennan J, MacLean KH, Han J, Chittenden T, Ihle JN, McKinnon PJ, Cleveland JL, Zambetti GP. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4(4):321–328. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378(6553):206–208. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- Jude CD, Climer L, Xu D, Artinger E, Fisher JK, Ernst P. Unique and independent roles for MLL in adult hematopoietic stem cells and progenitors. Cell Stem Cell. 2007;1(3):324–337. doi: 10.1016/j.stem.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan MB, Radin AI, Kuerbitz SJ, Onyekwere O, Wolkow CA, Civin CI, Stone KD, Woo T, Ravindranath Y, Craig RW. Levels of p53 protein increase with maturation in human hematopoietic cells. Cancer Res. 1991;51(16):4279–4286. [PubMed] [Google Scholar]
- Kawamura T, Suzuki J, Wang YV, Menendez S, Morera LB, Raya A, Wahl GM, Belmonte JC. Linking the p53 tumour suppressor pathway to somatic cell reprogramming. Nature. 2009;460(7259):1140–1144. doi: 10.1038/nature08311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelman Z, Prokocimer M, Peller S, Kahn Y, Rechavi G, Manor Y, Cohen A, Rotter V. Rearrangements in the p53 gene in Philadelphia chromosome positive chronic myelogenous leukemia. Blood. 1989;74(7):2318–2324. [PubMed] [Google Scholar]
- Kern SE, Kinzler KW, Bruskin A, Jarosz D, Friedman P, Prives C, Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, Ruvolo V, Tsao T, Zeng Z, Vassilev LT, Andreeff M. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106(9):3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima K, Konopleva M, Tsao T, Nakakuma H, Andreeff M. Concomitant inhibition of Mdm2-p53 interaction and Aurora kinases activates the p53-dependent postmitotic checkpoints and synergistically induces p53-mediated mitochondrial apoptosis along with reduced endoreduplication in acute myelogenous leukemia. Blood. 2008;112(7):2886–2895. doi: 10.1182/blood-2008-01-128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285(5434):1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- Komarova EA, Kondratov RV, Wang K, Christov K, Golovkina TV, Goldblum JR, Gudkov AV. Dual effect of p53 on radiation sensitivity in vivo: p53 promotes hematopoietic injury, but protects from gastro-intestinal syndrome in mice. Oncogene. 2004;23(19):3265–3271. doi: 10.1038/sj.onc.1207494. [DOI] [PubMed] [Google Scholar]
- Komarova NL, Wodarz D. Effect of cellular quiescence on the success of targeted CML therapy. PLoS One. 2007;2(10):e990. doi: 10.1371/journal.pone.0000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krosl J, Austin P, Beslu N, Kroon E, Humphries RK, Sauvageau G. In vitro expansion of hematopoietic stem cells by recombinant TAT-HOXB4 protein. Nat Med. 2003;9(11):1428–1432. doi: 10.1038/nm951. [DOI] [PubMed] [Google Scholar]
- Lacorazza HD, Yamada T, Liu Y, Miyata Y, Sivina M, Nunes J, Nimer SD. The transcription factor MEF/ELF4 regulates the quiescence of primitive hematopoietic cells. Cancer Cell. 2006;9(3):175–187. doi: 10.1016/j.ccr.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Lane DP, Crawford LV. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lassus P, Ferlin M, Piette J, Hibner U. Anti-apoptotic activity of low levels of wild-type p53. EMBO J. 1996;15(17):4566–4573. [PMC free article] [PubMed] [Google Scholar]
- Leonova KI, Shneyder J, Antoch MP, Toshkov IA, Novototskaya LR, Komarov PG, Komarova EA, Gudkov AV. A small molecule inhibitor of p53 stimulates amplification of hematopoietic stem cells but does not promote tumor development in mice. Cell Cycle. 2010;9(7) doi: 10.4161/cc.9.7.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6(5):443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M, Blasco MA, Serrano M. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature. 2009;460(7259):1136–1139. doi: 10.1038/nature08290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling KW, Ottersbach K, van Hamburg JP, Oziemlak A, Tsai FY, Orkin SH, Ploemacher R, Hendriks RW, Dzierzak E. GATA-2 plays two functionally distinct roles during the ontogeny of hematopoietic stem cells. J Exp Med. 2004;200(7):871–882. doi: 10.1084/jem.20031556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer DI, Levine AJ. Characterization of a 54K dalton cellular SV40 tumor antigen present in SV40-transformed cells and uninfected embryonal carcinoma cells. Cell. 1979;17(1):43–52. doi: 10.1016/0092-8674(79)90293-9. [DOI] [PubMed] [Google Scholar]
- Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK, Multani A, Chang S, Lozano G. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet. 2004;36(1):63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- Liu Y, Elf SE, Miyata Y, Sashida G, Huang G, Di Giandomenico S, Lee JM, Deblasio A, Menendez S, Antipin J, Reva B, Koff A, Nimer SD. p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell. 2009;4(1):37–48. doi: 10.1016/j.stem.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J, Sachs L. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood. 1993;82(4):1092–1096. [PubMed] [Google Scholar]
- MacDonald HR, Wevrick R. The necdin gene is deleted in Prader-Willi syndrome and is imprinted in human and mouse. Hum Mol Genet. 1997;6(11):1873–1878. doi: 10.1093/hmg/6.11.1873. [DOI] [PubMed] [Google Scholar]
- Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18(3):306–319. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Crusio KM, Meng F, Liu S, Kinsella M, Clark MR, Takeuchi O, Aifantis I. Regulation of lymphocyte progenitor survival by the proapoptotic activities of Bim and Bid. Proc Natl Acad Sci U S A. 2008;105(52):20840–20845. doi: 10.1073/pnas.0807557106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion RM, Strati K, Li H, Murga M, Blanco R, Ortega S, Fernandez-Capetillo O, Serrano M, Blasco MA. A p53-mediated DNA damage response limits reprogramming to ensure iPS cell genomic integrity. Nature. 2009;460(7259):1149–1153. doi: 10.1038/nature08287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusyk A, Porter CC, Zaberezhnyy V, DeGregori J. Irradiation selects for p53-deficient hematopoietic progenitors. PLoS Biol. 2010;8(3):e1000324. doi: 10.1371/journal.pbio.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Usami M, Aizawa T, Yoshikawa K. A novel brain-specific mRNA encoding nuclear protein (necdin) expressed in neurally differentiated embryonal carcinoma cells. Biochem Biophys Res Commun. 1991;178(1):291–296. doi: 10.1016/0006-291x(91)91812-q. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Oike Y, Onoyama I, Iwama A, Arai F, Takubo K, Mashimo Y, Oguro H, Nitta E, Ito K, Miyamoto K, Yoshiwara H, Hosokawa K, Nakamura Y, Gomei Y, Iwasaki H, Hayashi Y, Matsuzaki Y, Nakayama K, Ikeda Y, Hata A, Chiba S, Nakayama KI, Suda T. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 2008;22(8):986–991. doi: 10.1101/gad.1621808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek DW. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9(10):714–723. doi: 10.1038/nrc2716. [DOI] [PubMed] [Google Scholar]
- Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133(2):363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- Mercer WE, Shields MT, Amin M, Sauve GJ, Appella E, Romano JW, Ullrich SJ. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci U S A. 1990;87(16):6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11(3):577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1(1):101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80(2):293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Liu Y, Jankovic V, Sashida G, Lee JM, Shieh JH, Naoe T, Moore M, Nimer SD. Cyclin C regulates human hematopoietic stem/progenitor cell quiescence. Stem Cells. 2010;28(2):308–317. doi: 10.1002/stem.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y, Sun X, Uchida H, Zhang J, Nimer S. MEF, a novel transcription factor with an Elf-1 like DNA binding domain but distinct transcriptional activating properties. Oncogene. 1996;13(8):1721–1729. [PubMed] [Google Scholar]
- Mohrin M, Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, Morrison CG, Passegue E. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. 2010;7(2):174–185. doi: 10.1016/j.stem.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll UM, Wolff S, Speidel D, Deppert W. Transcription-independent pro-apoptotic functions of p53. Curr Opin Cell Biol. 2005;17(6):631–636. doi: 10.1016/j.ceb.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378(6553):203–206. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- Morales M, Theunissen JW, Kim CF, Kitagawa R, Kastan MB, Petrini JH. The Rad50S allele promotes ATM-dependent DNA damage responses and suppresses ATM deficiency: implications for the Mre11 complex as a DNA damage sensor. Genes Dev. 2005;19(24):3043–3054. doi: 10.1101/gad.1373705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahi H, Lehmann S, Bengtzen S, Jansson M, Mollgard L, Paul C, Merup M. Chromosomal aberrations in 17p predict in vitro drug resistance and short overall survival in acute myeloid leukemia. Leuk Lymphoma. 2008;49(3):508–516. doi: 10.1080/10428190701861645. [DOI] [PubMed] [Google Scholar]
- Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Naoe T, Kiyoi H, Kitamura K, Minami S, Miyawaki S, Asou N, Kuriyama K, Kusumoto S, Shimazaki C, Akiyama H, Saito K, Nishimura M, Motoji T, Shinagawa K, Saito H, Ohno R. Prognostic value of p53 gene mutations and the product expression in de novo acute myeloid leukemia. Eur J Haematol. 2000;65(1):23–31. doi: 10.1034/j.1600-0609.2000.90138.x. [DOI] [PubMed] [Google Scholar]
- Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205(4):777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288(5468):1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- Padua RA, Guinn BA, Al-Sabah AI, Smith M, Taylor C, Pettersson T, Ridge S, Carter G, White D, Oscier D, Chevret S, West R. RAS, FMS and p53 mutations and poor clinical outcome in myelodysplasias: a 10-year follow-up. Leukemia. 1998;12(6):887–892. doi: 10.1038/sj.leu.2401044. [DOI] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423(6937):302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Passegue E, Wagers AJ, Giuriato S, Anderson WC, Weissman IL. Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J Exp Med. 2005;202(11):1599–1611. doi: 10.1084/jem.20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peller S, Rotter V. TP53 in hematological cancer: low incidence of mutations with significant clinical relevance. Hum Mutat. 2003;21(3):277–284. doi: 10.1002/humu.10190. [DOI] [PubMed] [Google Scholar]
- Perry JM, Li L. Self-renewal versus transformation: Fbxw7 deletion leads to stem cell activation and leukemogenesis. Genes Dev. 2008;22(9):1107–1109. doi: 10.1101/gad.1670708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick-Silverman L, St Clair S, Maurer M, Zhao K, Manfredi JJ. Identification of a novel class of genomic DNA-binding sites suggests a mechanism for selectivity in target gene activation by the tumor suppressor protein p53. Genes Dev. 1998;12(14):2102–2107. doi: 10.1101/gad.12.14.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringshausen I, O'Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell. 2006;10(6):501–514. doi: 10.1016/j.ccr.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447(7145):725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132(4):681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11(12):1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha MN, Micallef J, Qiu L, Chang H. Pharmacological activation of the p53 pathway in haematological malignancies. J Clin Pathol. 2010;63(3):204–209. doi: 10.1136/jcp.2009.070961. [DOI] [PubMed] [Google Scholar]
- Sashida G, Liu Y, Elf S, Miyata Y, Ohyashiki K, Izumi M, Menendez S, Nimer SD. ELF4/MEF activates MDM2 expression and blocks oncogene-induced p16 activation to promote transformation. Mol Cell Biol. 2009;29(13):3687–3699. doi: 10.1128/MCB.01551-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14(17):5318–5324. doi: 10.1158/1078-0432.CCR-07-5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao L, Sun Y, Zhang Z, Feng W, Gao Y, Cai Z, Wang ZZ, Look AT, Wu WS. Deletion of proapoptotic Puma selectively protects hematopoietic stem and progenitor cells against high-dose radiation. Blood. 2010 doi: 10.1182/blood-2009-10-248872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8(9):703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Shaw P, Bovey R, Tardy S, Sahli R, Sordat B, Costa J. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci U S A. 1992;89(10):4495–4499. doi: 10.1073/pnas.89.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shing DC, Trubia M, Marchesi F, Radaelli E, Belloni E, Tapinassi C, Scanziani E, Mecucci C, Crescenzi B, Lahortiga I, Odero MD, Zardo G, Gruszka A, Minucci S, Di Fiore PP, Pelicci PG. Overexpression of sPRDM16 coupled with loss of p53 induces myeloid leukemias in mice. J Clin Invest. 2007;117(12):3696–3707. doi: 10.1172/JCI32390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shounan Y, Dolnikov A, MacKenzie KL, Miller M, Chan YY, Symonds G. Retroviral transduction of hematopoietic progenitor cells with mutant p53 promotes survival and proliferation, modifies differentiation potential and inhibits apoptosis. Leukemia. 1996;10(10):1619–1628. [PubMed] [Google Scholar]
- Slingerland JM, Minden MD, Benchimol S. Mutation of the p53 gene in human acute myelogenous leukemia. Blood. 1991;77(7):1500–1507. [PubMed] [Google Scholar]
- Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, Radich JP. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97(11):3589–3595. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]
- Takimoto R, MacLachlan TK, Dicker DT, Niitsu Y, Mori T, el-Deiry WS. BRCA1 transcriptionally regulates damaged DNA binding protein (DDB2) in the DNA repair response following UV-irradiation. Cancer Biol Ther. 2002;1(2):177–186. doi: 10.4161/cbt.65. [DOI] [PubMed] [Google Scholar]
- Taniura H, Kobayashi M, Yoshikawa K. Functional domains of necdin for protein-protein interaction, nuclear matrix targeting, and cell growth suppression. J Cell Biochem. 2005;94(4):804–815. doi: 10.1002/jcb.20345. [DOI] [PubMed] [Google Scholar]
- Taniura H, Matsumoto K, Yoshikawa K. Physical and functional interactions of neuronal growth suppressor necdin with p53. J Biol Chem. 1999;274(23):16242–16248. doi: 10.1074/jbc.274.23.16242. [DOI] [PubMed] [Google Scholar]
- TeKippe M, Harrison DE, Chen J. Expansion of hematopoietic stem cell phenotype and activity in Trp53-null mice. Exp Hematol. 2003;31(6):521–527. doi: 10.1016/s0301-472x(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Tergaonkar V, Pando M, Vafa O, Wahl G, Verma I. p53 stabilization is decreased upon NFkappaB activation: a role for NFkappaB in acquisition of resistance to chemotherapy. Cancer Cell. 2002;1(5):493–503. doi: 10.1016/s1535-6108(02)00068-5. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Jankovic V, Gao J, Buonamici S, Vest A, Lee JM, Zavadil J, Nimer SD, Aifantis I. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J Exp Med. 2008;205(6):1395–1408. doi: 10.1084/jem.20080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipping AJ, Pina C, Castor A, Hong D, Rodrigues NP, Lazzari L, May GE, Jacobsen SE, Enver T. High GATA-2 expression inhibits human hematopoietic stem and progenitor cell function by effects on cell cycle. Blood. 2009;113(12):2661–2672. doi: 10.1182/blood-2008-06-161117. [DOI] [PubMed] [Google Scholar]
- Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6(12):909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A. 2006;103(6):1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415(6867):45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- Utikal J, Polo JM, Stadtfeld M, Maherali N, Kulalert W, Walsh RM, Khalil A, Rheinwald JG, Hochedlinger K. Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature. 2009;460(7259):1145–1148. doi: 10.1038/nature08285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os R, Kamminga LM, Ausema A, Bystrykh LV, Draijer DP, van Pelt K, Dontje B, de Haan G. A Limited role for p21Cip1/Waf1 in maintaining normal hematopoietic stem cell functioning. Stem Cells. 2007;25(4):836–843. doi: 10.1634/stemcells.2006-0631. [DOI] [PubMed] [Google Scholar]
- Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303(5659):844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302(5647):1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8(4):275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9(10):691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- Wattel E, Preudhomme C, Hecquet B, Vanrumbeke M, Quesnel B, Dervite I, Morel P, Fenaux P. p53 mutations are associated with resistance to chemotherapy and short survival in hematologic malignancies. Blood. 1994;84(9):3148–3157. [PubMed] [Google Scholar]
- Weber HO, Samuel T, Rauch P, Funk JO. Human p14(ARF)-mediated cell cycle arrest strictly depends on intact p53 signaling pathways. Oncogene. 2002;21(20):3207–3212. doi: 10.1038/sj.onc.1205429. [DOI] [PubMed] [Google Scholar]
- Westphal CH, Rowan S, Schmaltz C, Elson A, Fisher DE, Leder P. atm and p53 cooperate in apoptosis and suppression of tumorigenesis, but not in resistance to acute radiation toxicity. Nat Genet. 1997;16(4):397–401. doi: 10.1038/ng0897-397. [DOI] [PubMed] [Google Scholar]
- Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6(2):93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell. 2005;123(4):641–653. doi: 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Yao JJ, Liu Y, Lacorazza HD, Soslow RA, Scandura JM, Nimer SD, Hedvat CV. Tumor promoting properties of the ETS protein MEF in ovarian cancer. Oncogene. 2007;26(27):4032–4037. doi: 10.1038/sj.onc.1210170. [DOI] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, Miyazaki H, Takahashi T, Suda T. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1(6):685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Yu H, Shen H, Yuan Y, XuFeng R, Hu X, Garrison SP, Zhang L, Yu J, Zambetti GP, Cheng T. Deletion of Puma protects hematopoietic stem cells and confers long-term survival in response to high-dose gamma-irradiation. Blood. 2010;115(17):3472–3480. doi: 10.1182/blood-2009-10-248278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7(3):673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Zeng H, Yucel R, Kosan C, Klein-Hitpass L, Moroy T. Transcription factor Gfi1 regulates self-renewal and engraftment of hematopoietic stem cells. EMBO J. 2004;23(20):4116–4125. doi: 10.1038/sj.emboj.7600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, Haug JS, Rupp D, Porter-Westpfahl KS, Wiedemann LM, Wu H, Li L. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441(7092):518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- Zhang W, Konopleva M, Burks JK, Dywer KC, Schober WD, Yang JY, McQueen TJ, Hung MC, Andreeff M. Blockade of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase and murine double minute synergistically induces Apoptosis in acute myeloid leukemia via BH3-only proteins Puma and Bim. Cancer Res. 2010;70(6):2424–2434. doi: 10.1158/0008-5472.CAN-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408(6811):433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
- Zhu J, Guo L, Min B, Watson CJ, Hu-Li J, Young HA, Tsichlis PN, Paul WE. Growth factor independent-1 induced by IL-4 regulates Th2 cell proliferation. Immunity. 2002;16(5):733–744. doi: 10.1016/s1074-7613(02)00317-5. [DOI] [PubMed] [Google Scholar]
- Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453(7193):306–313. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
- Zuber J, Radtke I, Pardee TS, Zhao Z, Rappaport AR, Luo W, McCurrach ME, Yang MM, Dolan ME, Kogan SC, Downing JR, Lowe SW. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. 2009;23(7):877–889. doi: 10.1101/gad.1771409. [DOI] [PMC free article] [PubMed] [Google Scholar]