Abstract
Visceral adipose tissue (VAT) is a key pathogenic fat depot in the metabolic syndrome (MetS), but liver fat (LF) may also play an important role. We evaluated associations of VAT and LF with MetS in normal weight, overweight, and obese men and women (BMI <25, 25-29.9, and ≥30 kg/m2, respectively). This analysis included 2495 participants from the AGES-Reykjavik Study with computed tomography measurements for VAT and LF. MetS was defined by ≥3 of the following: larger abdominal circumference, hypertension, elevated TG, low HDL, impaired fasting glucose, and microalbuminuria. We estimated the odds of MetS per 1-SD increase in VAT and LF, adjusting for key covariates. VAT was associated with an increased odds of MetS in normal weight, overweight, and obese women (OR=2.78, 1.63, and 1.43, respectively; all P<0.01) that diminished in magnitude with increasing BMI (VAT*BMI class interaction P<0.001). In men, VAT was related to MetS only among the overweight (OR=1.69, P<0.01). LF was associated with MetS in the overweight and obese groups in women (OR=1.38 and 1.45; both P<0.001) and in men (OR=1.38, P=0.01; and OR=1.27, P=0.10), but not in the normal weight groups. These BMI-specific relationships persisted when both fat depots were included in the model. VAT and LF were associated with MetS independently of each other, and these relationships were modified by BMI class such that, VAT was the more important depot at lower levels of obesity and LF at higher levels. Importantly, fatty liver may be a novel metabolic risk factor in overweight and obese individuals.
INTRODUCTION
The metabolic syndrome (MetS) represents a cluster of cardiovascular and endocrine risk factors including abdominal obesity, a characteristic dyslipidemia of elevated triglyceride (TG) and low high-density lipoprotein (HDL) cholesterol levels, hyperglycemia, and hypertension (1), as well as microalbuminuria (2). Most, if not all, of these metabolic features have their basis in insulin resistance and confer an increased risk of cardiovascular disease (CVD) (3, 4) and diabetes mellitus (DM) (3). Prevalence of MetS in the US is approximately 22% among adults (5) and markedly higher in the obese population, with an estimated 50-60% of obese men and women affected (6).
Although obesity is a strong risk factor for MetS, not all obese persons develop the syndrome and even normal weight individuals can be at risk (7, 8). Specific fat compartments have been shown to be more strongly associated with metabolic abnormalities. In particular, visceral adipose tissue (VAT) has been called a key pathogenic fat depot such that, for any given level of adiposity, individuals with excess VAT have a substantially greater risk of insulin resistance (9), impaired glucose tolerance (7, 10), MetS (11-13), and DM (7, 14). Strong and consistent relationships between visceral adiposity and metabolic risk are the basis for including larger waist circumference as part of the NCEP ATP-III criteria for MetS (1). There is also a growing body of evidence demonstrating that accumulation of fat in the liver is related to metabolic abnormalities. Liver fat (LF) content is reportedly 4-fold higher in individuals with MetS compared to those without the syndrome (15), and highly correlated with each constituent metabolic feature even after adjusting for BMI (15, 16). Furthermore, LF may be a better marker than VAT for metabolic complications of obesity (17). Based on such evidence, fatty liver has been called the hepatic manifestation of MetS (18).
Since viscerally obese individuals tend to have greater fatty deposition in the liver (15), it is important to delineate a relationship between LF and MetS that is independent of VAT and vice versa. However, few studies have evaluated both LF and VAT and examined these relationships adjusting for overall adiposity and thigh subcutaneous adipose tissue (SAT), which has been shown to be protective against metabolic abnormalities (19). Data are especially lacking from population-based studies, particularly among older adults who bear a significant burden of MetS (5) and its consequences (20). Evaluation of the independent contributions of VAT and LF to metabolic risk may provide insights into the role of these fat depots in the pathogenesis of MetS. Furthermore, examination of these relationships within clinically-defined BMI categories may help clarify the contributions of overall and regional fat distribution in metabolic dysfunction.
Therefore, we evaluated the independent associations of visceral and liver fat with MetS in older adults across a spectrum of BMI, and examined whether these relationships differed between normal weight, overweight, and obese groups.
METHODS
Study Population
We evaluated participants from the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study, which is a continuation of the previously described Reykjavik Study (21). In brief, the Reykjavik Study began recruiting in 1967 a population-based sample of over 30,000 residents of Reykjavik, Iceland from the 1907-1935 birth cohort, and in 2002, the AGES-Reykjavik Study began recruiting 5764 of the surviving members. The AGES-Reykjavik Study was approved by the institutional review boards of the National Institute on Aging and the Icelandic National Bioethics Committee (VSN: 00-063), and written informed consent was obtained from all participants.
Between 2002 and 2006, study participants underwent a comprehensive evaluation that included a detailed medical history, physical examination, laboratory and screening tests, and questionnaires on health-related behaviors such as alcohol consumption, smoking history, and physical activity. Blood pressure was measured using a Mercury sphygmomanometer, BMI was calculated as weight (kg) divided by height (in meters) squared, and total percentage of body fat was estimated by bioelectrical impedance (Xitron Hydra ECF/ICF Bio-Impedance Spectrum Analyzer). TG, HDL cholesterol, and plasma glucose levels were measured on fasting blood samples. TG was measured using enzymatic colorimetry (Roche Triglyceride Assay Kit), HDL with an enzymatic in vitro assay (Roche Direct HDL Cholesterol Assay Kit), and glucose was measured using photometry (Roche Hitachi 717 Photometric Analysis System). Urine albumin concentration was measured using an immunoturbidimetric assay (Roche Tina-quant Albumin Assay Kit).
Definition of the Metabolic Syndrome
The primary analytic endpoint was MetS based mainly on ATP-III criteria (1) but also included microalbuminuria, which has been strongly linked with cardiovascular mortality in European populations and is part of the WHO definition (2). Thus, MetS was defined in our study by the presence of 3 or more of the following features: 1) larger waist circumference (WC) defined by WC >102 cm in men and >88 cm in women; 2) TG ≥150 mg/dL; 3) HDL cholesterol <40 mg/dL in men and <50 mg/dL in women; 4) impaired fasting glucose (IFG) defined by fasting plasma glucose ≥110 mg/dL; 5) hypertension defined by blood pressure ≥130/85 mm Hg; and 6) microalbuminuria defined by albumin/creatinine ratio ≥30 mg/g.
CT Measures of Fat Depots
Study participants underwent computed tomography (CT) imaging for assessment of fat deposition (Siemens Sensation 4, Four Detector Scanner). VAT area was estimated from a single 10 mm thick trans-axial section in the abdomen at the level of the L4/L5 vertebras and calculating all pixels in the abdominal cavity within the range of −50 to −200 Hounsfield Units (HU). Liver fat content was estimated from a CT scan (1mm thick) at the level of the L1/L2 vertebras by calculating the average density (in HU) in a region of interest with a diameter of 1 cm and 10% of the distance between the rib and mid anterior aspect of the spinal canal. Liver density is a surrogate measure of liver fat, with lower HU values indicating greater fatty infiltration. To make our results more easily interpretable, we created a variable for LF by multiplying liver density values by negative one. Thus, higher LF values indicate greater fatty infiltration in the liver. Lastly, thigh SAT area was estimated from a single 10 mm thick axial image at the femoral midpoint, by manually drawing a line along the deep fascial plane surrounding the thigh muscles and calculating the area. All CT measurements were calculated relative to the water cell of the CT phantom.
Excluded Study Participants
Since coronary artery disease (CAD) and diabetes are thought to occur secondary to MetS rather than as part of the syndrome, we excluded participants with a history of CAD [ECG-confirmed MI; prior coronary revascularization; or angina treated with nitrates (n=1034)] and DM [based on self-report; use of insulin or hypoglycemic agents; or fasting glucose ≥126 mg/dL (n=749)]. Other exclusion criteria included self-reported alcohol intake of more than 20 grams per day (n=53), known history of liver disease (n=75), and BMI < 18.5 kg/m2 (n=86). Of the remaining 3993 participants, an additional 1498 were excluded from our analysis due to missing adiposity data, including those with missing values for VAT (n=313), LF (n=631), total body fat (n=1158), and BMI (n=48). Compared to the analytic sample, individuals with missing fat measures were generally older, more frail, and had a more severe cardiometabolic profile (data not presented).
Statistical Analyses
All analyses were stratified by sex due to the differential patterns of fat deposition and metabolic risk factors between men and women, and based on our study purpose, data are reported separately for normal weight, overweight, and obese groups defined by BMI of 18.5-24.9, 25-29.9, and ≥30 kg/m2, respectively. Distribution of covariates across BMI class was compared using the Cochran-Armitage trend test for categorical variables and ANOVA for continuous variables.
For the primary analysis, multivariate logistic regression was used to estimate the odds of MetS per 1-standard deviation (SD) increase in VAT and LF, independent of each other as well as other measures of adiposity. We applied a series of staged analyses, starting with a base model that included variables for age, height, estrogen use (in women only), smoking history, and alcohol use (grams/day). Then, we additionally adjusted for total percentage of body fat, thigh SAT, and lastly, VAT or LF. Standardized beta coefficients from the full multivariate model were compared between VAT and LF to assess which is the more important fat depot in MetS. Two-way interactions between VAT, LF, and BMI class were tested and interaction terms with P<0.05 were retained. Collinearity between fat measures was assessed and all statistics for VIF were <2 and Tolerance >0.6. Estimates for odds ratios (OR), 95% confidence intervals (CI), and p-values are presented, and 2-tailed P<0.05 was considered statistically significant. All statistical analyses were performed using SAS v9.1 (Cary, NC).
RESULTS
Study Population Characteristics
This analysis included 1616 women and 879 men from the AGES-Reykjavik Study with mean age of 75.7±5.5 and 76.4±5.4 years, respectively. Among women, 37% were normal weight, 41% overweight, and 22% obese, and men followed a similar distribution with 40%, 45%, and 15% in the 3 BMI groups. As presented in Table 1, characteristics that were significantly associated with higher BMI included: younger age (P<0.001 in both sexes); lower education (P<0.001) and less alcohol use (P<0.01) in women; and smoking history (P<0.01) and greater alcohol intake (P=0.02) in men. In both sexes, the occurrence of MetS increased markedly across the normal weight, overweight, and obese groups (14%, 32%, and 48% in women and 6%, 25%, and 59% in men; both P<0.001), as did each individual MetS feature with the exception of microalbuminuria which had a low overall prevalence of 4% in women and 10% in men. Mean levels of VAT and LF also increased across the normal weight, overweight, and obese groups (all P<0.001).
TABLE 1.
Characteristics of Normal Weight, Overweight, and Obese Women and Men in the AGES-Reykjavik Study
| Characteristics | Women (n=1616) | Men (n=879) | ||||||
|---|---|---|---|---|---|---|---|---|
| Normal (n=592) |
Overweight (n=670) |
Obese (n=354) |
Trend P |
Normal (n=349) |
Overweight (n=396) |
Obese (n=134) |
Trend P |
|
| Age, in years | 76.4 (5.8) | 75.7 (5.3) | 74.5 (5.4) | <0.001 | 77.3 (5.5) | 76.0 (5.2) | 75.3 (5.3) | <0.001 |
| < College education, % | 73 | 77 | 83 | <0.001 | 69 | 70 | 76 | 0.18 |
| Low physical activity, % a | 61 | 61 | 66 | 0.10 | 55 | 54 | 60 | 0.45 |
| Ever smoker, % | 48 | 46 | 47 | 0.65 | 66 | 71 | 81 | <0.01 |
| Alcohol use, g/day | 0.2 (0, 1.9) | 0.2 (0, 1.8) | 0.2 (0, 0.9) | <0.01 | 0.9 (0, 3.4) | 1.4 (0, 3.8) | 1.1 (0, 3.8) | 0.07 |
| BMI, kg/m2 | 22.7 (1.7) | 27.3 (1.4) | 33.7 (3.4) | <0.001 | 22.8 (1.5) | 27.2 (1.4) | 32.4 (2.1) | <0.001 |
| Total body fat, % | 30 (4) | 35 (3) | 39 (3) | <0.001 | 17 (4) | 23 (4) | 28 (4) | <0.001 |
| Thigh SAT area, cm2 | 66 (22) | 97 (29) | 140 (43) | <0.001 | 28 (11) | 40 (16) | 60 (24) | <0.001 |
| VAT area, cm2 | 102 (40) | 153 (49) | 208 (66) | <0.001 | 135 (55) | 208 (61) | 300 (91) | <0.001 |
| Liver fat, HU | -61.9 (5) | -59.9 (8) | -57.1 (11) | <0.001 | -61.0 (6) | -59.5 (7) | -57.6 (10) | <0.001 |
| MetS, % | 14 | 32 | 48 | <0.001 | 6 | 25 | 59 | <0.001 |
| Individual MetS criteria, % | ||||||||
| Large waist circumference | 52 | 93 | 100 | <0.001 | 5 | 54 | 100 | <0.001 |
| Hypertension | 83 | 86 | 90 | 0.01 | 80 | 84 | 95 | <0.001 |
| Elevated TG | 8 | 14 | 28 | <0.001 | 6 | 12 | 29 | <0.001 |
| Low HDL | 8 | 13 | 25 | <0.001 | 7 | 14 | 21 | <0.001 |
| Impaired fasting glucose | 6 | 13 | 23 | <0.001 | 9 | 18 | 31 | <0.001 |
| Microalbuminuria | 2 | 5 | 3 | 0.22 | 9 | 10 | 10 | 0.68 |
Categorical variables are presented as column percentages and continuous variables as mean (standard deviation), except for alcohol use which is presented as median (interquartile range). Normal weight, overweight, and obese groups are defined by BMI <25, 25-29.9, and ≥30 kg/m2, respectively.
Low physical activity indicates never or rarely engaging in moderate or vigorous physical activity based on self-report.
Comparison of Mean VAT and LF by MetS Status
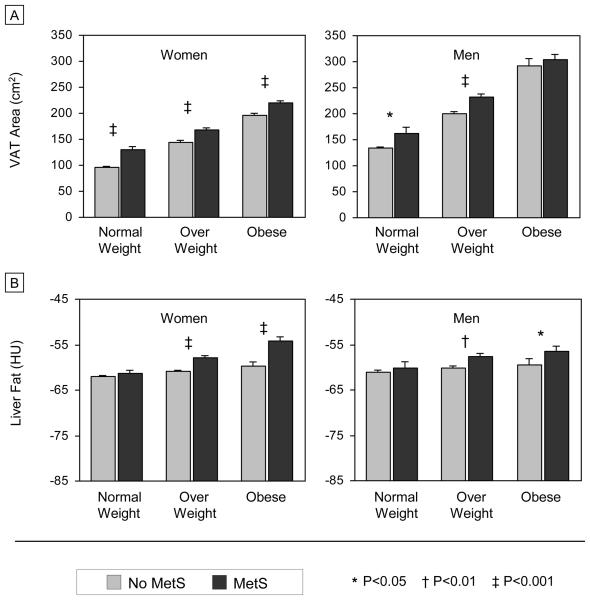
Age-adjusted mean levels of VAT and LF in those with and without MetS are presented in Figures 1A and 1B, respectively. In every BMI class, women with the metabolic syndrome had a higher mean VAT area compared to those without MetS (all P<0.001). Similar trends were observed in men, but the relationship between higher VAT and MetS was significant only in the normal weight and overweight groups (P<0.05 and P<0.001, respectively). Results for liver fat were more consistent between sexes in that, MetS was associated with a higher mean level of LF in the overweight and obese groups (women: both P<0.001; and men: P<0.01 and P=0.02, respectively). Among normal weight individuals, mean LF did not differ significantly by MetS status.
FIGURE 1.
Age-Adjusted Mean Levels of Visceral Adipose Tissue (A) and Liver Fat (B) by MetS Status
Independent Associations of VAT and LF with MetS
Results from the multivariate analyses demonstrated relationships between visceral adiposity and MetS that differed across levels of obesity and by sex (Table 2A). In women, VAT was associated with an increased odds of MetS after adjusting for age, height, estrogen use, history of smoking, and alcohol consumption. This relationship was significant in every BMI class but decreased in magnitude across the higher BMI groups. Estimates were attenuated after further adjusting for total body fat, thigh SAT, and lastly, LF, but VAT remained associated with an increased likelihood of MetS in normal weight, overweight, and obese women [adjusted OR (95% CI): 2.77 (1.78-4.30); 1.56 (1.21-2.01); and 1.28 (0.99-1.64), respectively]. There was a statistical interaction between VAT and BMI class, indicating that the relationship between visceral fat and MetS differed significantly across the 3 BMI groups (interaction P<0.001). In contrast to our results in women, VAT was related to MetS in normal weight and overweight men, but after further adjusting for measures of overall and regional adiposity including LF, this relationship remained significant only in overweight men [OR=1.62 (1.12-2.34)].
TABLE 2.
Odds Ratios for MetS Associated with 1-Standard Deviation Increase in Visceral Adipose Tissue (A) and Liver Fat (B), Adjusting for Measures of Adiposity
| (A). Estimates for VAT | ||||||
|---|---|---|---|---|---|---|
| Covariates in Model | Women |
Men |
||||
| Normal Weight | Overweight | Obese | Normal Weight | Overweight | Obese | |
| MV a | 3.47 (2.32–5.18) | 1.93 (1.54-2.43) | 1.50 (1.20-1.87) | 2.10 (1.09-4.03) | 2.06 (1.48-2.88) | 1.12 (0.80-1.57) |
| MV + Total Fat | 3.21 (2.09-4.92) | 1.89 (1.49-2.39) | 1.50 (1.19-1.89) | 1.65 (0.76-3.56) | 1.74 (1.21-2.49) | 1.09 (0.77-1.53) |
| MV + Total Fat + Thigh SAT | 2.78 (1.79-4.32) | 1.63 (1.27-2.10) | 1.43 (1.12-1.82) | 1.50 (0.67-3.38) | 1.69 (1.17-2.44) | 1.05 (0.74-1.49) |
| MV + Total Fat + Thigh SAT + LF b | 2.77 (1.78-4.30) | 1.56 (1.21-2.01) | 1.28 (0.99-1.64) | 1.49 (0.65-3.39) | 1.62 (1.12-2.34) | 1.03 (0.72-1.46) |
|
| ||||||
| (B). Estimates for LF | ||||||
|
| ||||||
| Covariates in Model | Women |
Men |
||||
| Normal Weight | Overweight | Obese | Normal Weight | Overweight | Obese | |
|
| ||||||
| MV a | 1.21 (0.84-1.74) | 1.49 (1.25-1.78) | 1.49 (1.25-1.78) | 1.21 (0.72-2.04) | 1.39 (1.11-1.76) | 1.28 (0.98-1.68) |
| MV + Total Fat | 1.22 (0.85-1.74) | 1.49 (1.24-1.78) | 1.49 (1.25-1.78) | 1.14 (0.70-1.87) | 1.39 (1.10-1.76) | 1.30 (0.99-1.71) |
| MV + Total Fat + Thigh SAT | 1.16 (0.81-1.67) | 1.38 (1.15-1.65) | 1.45 (1.21-1.74) | 1.07 (0.67-1.72) | 1.38 (1.09-1.74) | 1.27 (0.96-1.69) |
| MV+ Total Fat + Thigh SAT + VAT | 1.13 (0.79-1.61) | 1.33 (1.10-1.59) | 1.39 (1.16-1.67) | 1.03 (0.64-1.68) | 1.33 (1.05-1.69) | 1.27 (0.96-1.68) |
Adjusted OR (95% CI) are presented.
MV includes: age, height, estrogen use (women only), smoking history, and alcohol use (g/day).
VAT * BMI class interaction in women (test of interaction P<0.001)
The association of liver fat with MetS also differed by level of obesity but similarly across sex (Table 2B). In both women and men, LF was associated with an increased odds of MetS in the overweight and obese, but not normal weight, groups after initially controlling for key covariates. After further adjusting for adiposity measures including VAT, BMI-specific associations of LF with MetS were slightly attenuated but persisted in the overweight and obese groups [OR=1.33 (95% CI 1.10-1.59) and 1.39 (1.16-1.67) in women; and 1.33 (1.05-1.69) and 1.27 (0.96-1.68) in men, respectively]. Although this relationship was not statistically significant in obese men, there was a consistent trend of an association between LF and MetS across the multivariate models, with odds ratios ranging from 1.27 to 1.30 (all P≥0.10). In both sexes, OR estimates in the overweight and obese groups were similar to each other (test of equality: P>0.20), indicating a similar effect of LF on MetS in these BMI groups. Although an independent association between LF and MetS was consistently present in those with BMI ≥25 kg/m2 and absent in those with BMI <25 kg/m2, interaction between LF and BMI class (≥25 vs. <25) was not statistically significant (test of interaction P>0.20 in both sexes).
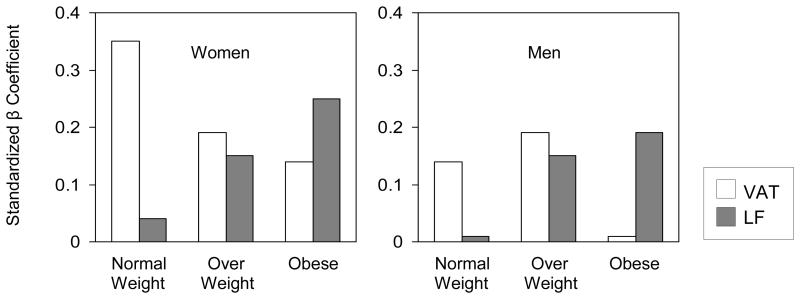
To evaluate which is the more important fat depot with respect to MetS at different levels of obesity, we compared standardized beta coefficients for VAT and LF within and across BMI categories (Figure 2). Standardized betas for VAT generally decreased across the normal weight, overweight, and obese groups, while estimates for LF increased. Although these trends were more robust in women than in men, it was clear that in both sexes, the stronger correlate of MetS was visceral fat in normal weight and liver fat in the obese.
FIGURE 2.
Comparison of Standardized β Coefficients for Visceral Adipose Tissue and Liver Fat in Normal Weight, Overweight, and Obese Women and Men
Standardized β coefficients were estimated from logistic regression model for MetS adjusting for age, height, estrogen use (women only), smoking history, alcohol intake, total body fat, and thigh subcutaneous fat.
Distribution of Metabolic Risk Factors across LF Tertiles
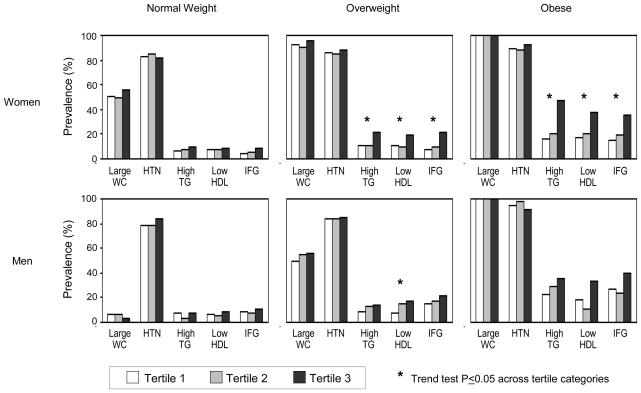
We examined the prevalence of individual MetS features across tertiles of LF (Figure 3). In overweight and obese women, the occurrence of elevated TG, low HDL, and IFG rose significantly across increasing levels of LF and was markedly greater in the highest LF tertile compared to the lower 2 tertiles. An increasing trend in the prevalence of high TG, low HDL, and IFG was also observed across LF tertiles in overweight and obese men; however, the relationship was statistically significant only for low HDL in overweight men. Among normal weight men and women, increasing LF levels was not significantly associated with any of the metabolic risk factors. Similar examination across tertiles of VAT did not reveal any consistent trends for individual MetS risk factors (data not presented).
FIGURE 3.
Prevalence of MetS Components across Tertiles of Liver Fat in Normal Weight, Overweight, and Obese Women and Men
WC indicates waist circumference; HTN hypertension; TG triglyceride; HDL high density lipoprotein; and IFG impaired fasting glucose. Data are not presented for microalbuminuria due to low overall prevalence of 4% in women and 10% in men.
DISCUSSION
Summary of Findings
Among older adults in the AGES-Reykjavik Study, visceral adiposity was associated with an increased likelihood of MetS in normal weight, overweight, and obese women and in overweight men. Notably in women, the magnitude of the association between VAT and MetS decreased across higher BMI categories, indicating that the importance of VAT diminishes with increasing obesity. Liver fat, on the other hand, was associated with MetS in overweight and obese, but not normal weight, women and men. Associations of VAT and LF with MetS were attenuated but persisted even after adjusting for each other. Although these relationships were stronger and more consistent in women, particularly for VAT, standardized beta estimates indicated that regardless of gender, the more important fat depot with respect to MetS was visceral fat in the lower BMI range and liver fat at higher levels of obesity. This may have important clinical implications, as the better target for monitoring metabolic risk may be waist circumference (a surrogate measure of VAT) in normal weight and overweight adults, and signs of fatty liver in overweight and obese individuals.
Comparison with Previous Studies
Our findings regarding VAT are consistent with previous reports from Framingham (11) and Health ABC (7, 12) cohorts demonstrating significant associations with metabolic traits in normal weight, overweight, and obese men and women that diminished in magnitude with increasing obesity. But in contrast to these studies, we did not observe an independent relationship between VAT and MetS in normal weight men, which may be explained by the fact that normal weight men in our study were especially lean, with a mean waist circumference of 92 cm and only 5% meeting the MetS criteria for abdominal obesity. It is notable that in the Framingham and Health ABC studies, BMI-stratified results were presented as part of secondary analyses and, as such, the relationship between VAT and MetS was not rigorously evaluated within and across levels of obesity. Moreover, it is unclear how the BMI-specific association of VAT with MetS is affected by the extent of fatty infiltration in the liver since LF was not evaluated.
Emerging evidence suggests that, among adipose tissue compartments, liver fat is the most important determinant of the metabolic complications of obesity. (17, 22) Stefan et al. reported that, of all the adiposity measures that were examined including VAT, LF emerged as the strongest correlate of insulin sensitivity among the obese, with 54% less LF in insulin-sensitive versus insulin-resistant individuals. (22) Liver fat also appears to be a better marker than VAT in determining categories of prediabetes, with an increasing trend in LF across groups characterized by normal glucose tolerance, isolated IFG, impaired glucose tolerance (IGT), and combined IFG and IGT. (23) While these and most other fatty liver studies have been limited to smaller samples selected for metabolic and obesity-related traits, corroborating epidemiological data are emerging. In a recent report from the Framingham Heart Study that included 2,589 participants, fatty liver was significantly associated with MetS and, more specifically, dyslipidemia and dysglycemia independently of VAT. (24)
We extend these findings in older adults by first, demonstrating that the risk of MetS associated with liver fat occurs mainly at higher levels of obesity. Furthermore, the positive relationship of LF with elevated TG, low HDL, and IFG among the overweight and obese in our study, which was significant in women and suggested in men, indicates that our main finding of an association between fatty liver and MetS in the higher BMI range may be driven by dyslipidemia and dysglycemia, which is also consistent with the Framingham report. (24) In addition, prevalence of elevated TG, low HDL, and IFG was nearly two-fold higher among overweight and obese women in the highest LF tertile compared to the lower 2 tertiles, suggesting that metabolic risk is greatest in those with the most extensive fatty infiltration in the liver. This may have important clinical implications for identifying those at greatest risk for metabolic disease and intervening to reduce this risk. Lastly, it will be of great interest to know whether higher levels of LF leads to an increased risk for developing diabetes and cardiovascular disease, and we look to future prospective studies for these answers.
The metabolic influence of fatty liver in obesity has been previously described, but few studies have examined this relationship in lean individuals. Among normal weight men and women in our study, LF was not associated with MetS or any of its constituent features. Since hepatic fat content is correlated with adiposity, it is possible that leaner men and women have too little LF to exert metabolic effects. Alternatively, this finding may reflect the limited ability of CT to detect mild steatosis, particularly in the liver. While CT has high sensitivity and specificity for detecting moderate to severe levels of fatty infiltration in the liver (25, 26), sensitivity is poor at lower levels. (27) Magnetic resonance spectroscopy is superior to CT for qualitative and quantitative assessment of the liver, particularly for detecting mild steatosis and small changes in fat content. (28) Therefore, by using CT to estimate LF, we may be underestimating the role of fatty liver in MetS, particularly in normal weight individuals.
Mechanisms underlying the relationships of visceral and liver fat with metabolic dysfunction are not entirely understood, but free fatty acids (FFA) are thought to play an important role. During lipolysis, FFAs are secreted from VAT into the portal circulation and carried to the liver where they are oxidized. (29) In the presence of excess visceral fat, more FFAs are released from VAT, flooding the liver and accumulating in the hepatocytes over time, (30) and subsequently leading to alterations in energy and lipid metabolism. (31, 32) Our data are consistent with this known pathway in that, overweight and obese individuals with greater LF had a higher prevalence of IFG and lipid abnormalities, likely resulting from abnormal liver cell function. While direct hepatic exposure to FFAs in the portal circulation suggests a primary role of visceral fat in the relationship between LF and MetS, liver fat may be involved in metabolic disease even in the absence of VAT. For example, fatty liver has been shown to cause insulin resistance in mice lacking visceral and subcutaneous fat (33) and has been observed in insulin-resistant humans with lipodystrophies. (34) Consistent with this, we also found that BMI-specific associations of VAT and LF with MetS persisted independently of each other. The relationship between liver fat and insulin sensitivity is thought to be mediated, at least in part, by the secretion of hepatokines. In particular, fetuin-A is a humoral product of the liver that binds the insulin receptor tyrosine kinase in muscle and fat, leading to insulin resistance in target tissues. (35, 36) Importantly, fetuin-A is a predictor of incident diabetes (37, 38) and, given that it is secreted almost exclusively from the liver, (39) fetuin-A may be a mediator in fatty liver-induced diabetes. Other mechanisms underlying relationships of LF and VAT with metabolic abnormalities may involve oxidative stress, adipokines, and inflammation.
To our knowledge, ours is the first population-based study to concurrently evaluate relationships of visceral and liver fat with MetS by BMI class, and our findings extend the current literature in important ways. First, examination of both VAT and LF allowed us to investigate their shared and independent associations with MetS. The likelihood of MetS associated with VAT and LF was attenuated after adjusting for each other, indicating some (albeit small) common effect of these fat depots. More importantly, these relationships persisted after adjusting for each other, suggesting that VAT and LF provide complementary information on metabolic risk. Second, our BMI class-stratified results demonstrated that both fat depots contribute to metabolic risk but at opposite ends of the BMI spectrum, suggesting that mechanisms underlying the relationships of VAT and LF with metabolic traits differs across levels of obesity. In light of the extensive literature demonstrating that VAT is pathogenic in MetS, it is unclear why the risk associated with VAT is the lowest among those with the most visceral adiposity. Furthermore, this leads to the question of what other factors are driving the increased occurrence and severity of metabolic complications in obesity. Our findings suggest that the liver is a pathogenic fat depot at higher levels of obesity, with a greater impact on MetS than visceral fat.
Limitations
There were several limitations to our study. First, we cannot establish a temporal association between visceral and liver fat depots and the development of MetS due to the cross-sectional nature of our data. Second, VAT and LF were estimated using CT, which has low sensitivity in detecting mild steatosis. (27) Nonetheless, CT has been widely used in epidemiological evaluations of body composition, including the Framingham and Health ABC studies. Third, we excluded a large number of participants with missing fat measures who were older and in poorer overall health compared to the analytic sample. However, it is unlikely that inclusion of these individuals would have changed our main results in a meaningful way. Lastly, our study only included individuals of European ancestry and our findings may not be generalizeable to other ethnic groups.
CONCLUSION
In older adults, relationships of visceral and liver fat depots with the metabolic syndrome were modified by BMI class such that, VAT was more strongly associated at lower levels of obesity and LF at higher levels, independently of each other and of overall adiposity. Our findings suggest that even normal weight individuals may be at risk for MetS, as even a small degree of visceral adiposity can substantially increase their risk. In addition, fatty liver is a novel metabolic risk factor in overweight and obesity that may be useful for distinguishing individuals who are likely to develop MetS from those who are not. Further BMI-stratified analyses are needed to clarify the roles of visceral and liver fat depots on metabolic dysfunction and how mechanisms involved in these relationships differ across levels of obesity.
ACKNOWLEDGEMENTS
This study was funded by the National Institutes of Health contract N01-AG-12100, Hjartavernd (the Icelandic Heart Association), and the Althingi (Icelandic Parliament). This research was also supported in part by the Intramural Research Program of the NIH, National Institute on Aging. Lastly, we are indebted to the study participants for their willingness to take part in this study.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 2.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 3.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes care. 2002;25:1790–4. doi: 10.2337/diacare.25.10.1790. [DOI] [PubMed] [Google Scholar]
- 4.Lakka HM, Laaksonen DE, Lakka TA, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. Jama. 2002;288:2709–16. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 6.Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988-1994. Archives of internal medicine. 2003;163:427–36. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes care. 2003;26:372–9. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 8.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 9.Wagenknecht LE, Langefeld CD, Scherzinger AL, et al. Insulin sensitivity, insulin secretion, and abdominal fat: the Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes. 2003;52:2490–6. doi: 10.2337/diabetes.52.10.2490. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, Boyko EJ, Leonetti DL, et al. Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes care. 2003;26:650–5. doi: 10.2337/diacare.26.3.650. [DOI] [PubMed] [Google Scholar]
- 11.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Archives of internal medicine. 2005;165:777–83. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 13.Pou KM, Massaro JM, Hoffmann U, et al. Patterns of abdominal fat distribution: the Framingham Heart Study. Diabetes care. 2009;32:481–5. doi: 10.2337/dc08-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyko EJ, Fujimoto WY, Leonetti DL, Newell-Morris L. Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes care. 2000;23:465–71. doi: 10.2337/diacare.23.4.465. [DOI] [PubMed] [Google Scholar]
- 15.Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H. Liver fat in the metabolic syndrome. The Journal of clinical endocrinology and metabolism. 2007;92:3490–7. doi: 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen-Duy T-B, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat and liver fat are independent predictors of metabolic risk factors in men. Am J Physiol Endocrinol Metab. 2003;284:E1065–71. doi: 10.1152/ajpendo.00442.2002. [DOI] [PubMed] [Google Scholar]
- 17.Fabbrini E, Magkos F, Mohammed BS, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–23. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 19.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–8. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 20.Resnick HE, Harris MI, Brock DB, Harris TB. American Diabetes Association diabetes diagnostic criteria, advancing age, and cardiovascular disease risk profiles: results from the Third National Health and Nutrition Examination Survey. Diabetes care. 2000;23:176–80. doi: 10.2337/diacare.23.2.176. [DOI] [PubMed] [Google Scholar]
- 21.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. American journal of epidemiology. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Archives of internal medicine. 2008;168:1609–16. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 23.Kantartzis K, Machann J, Schick F, Fritsche A, Haring HU, Stefan N. The impact of liver fat vs visceral fat in determining categories of prediabetes. Diabetologia. 2010;53:882–9. doi: 10.1007/s00125-010-1663-6. [DOI] [PubMed] [Google Scholar]
- 24.Speliotes EK, Massaro JM, Hoffmann U, et al. Fatty liver is associated with dyslipidemia and dysglycemia independent of visceral fat: the Framingham Heart Study. Hepatology. 2010;51:1979–87. doi: 10.1002/hep.23593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clinics in liver disease. 2007;11:37–54. doi: 10.1016/j.cld.2007.02.014. viii. [DOI] [PubMed] [Google Scholar]
- 26.Saadeh S, Younossi ZM, Remer EM, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 27.Park SH, Kim PN, Kim KW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–12. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 28.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–8. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 29.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–7. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 30.Fong DG, Nehra V, Lindor KD, Buchman AL. Metabolic and nutritional considerations in nonalcoholic fatty liver. Hepatology. 2000;32:3–10. doi: 10.1053/jhep.2000.8978. [DOI] [PubMed] [Google Scholar]
- 31.Adiels M, Taskinen MR, Packard C, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 2006;49:755–65. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 32.Malmstrom R, Packard CJ, Caslake M, et al. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;40:454–62. doi: 10.1007/s001250050700. [DOI] [PubMed] [Google Scholar]
- 33.Moitra J, Mason MM, Olive M, et al. Life without white fat: a transgenic mouse. Genes & development. 1998;12:3168–81. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal AK, Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends in endocrinology and metabolism: TEM. 2003;14:214–21. doi: 10.1016/s1043-2760(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 35.Auberger P, Falquerho L, Contreres JO, et al. Characterization of a natural inhibitor of the insulin receptor tyrosine kinase: cDNA cloning, purification, and anti-mitogenic activity. Cell. 1989;58:631–40. doi: 10.1016/0092-8674(89)90098-6. [DOI] [PubMed] [Google Scholar]
- 36.Rauth G, Poschke O, Fink E, et al. The nucleotide and partial amino acid sequences of rat fetuin. Identity with the natural tyrosine kinase inhibitor of the rat insulin receptor. European journal of biochemistry / FEBS. 1992;204:523–9. doi: 10.1111/j.1432-1033.1992.tb16663.x. [DOI] [PubMed] [Google Scholar]
- 37.Stefan N, Fritsche A, Weikert C, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57:2762–7. doi: 10.2337/db08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ix JH, Wassel CL, Kanaya AM, et al. Fetuin-A and incident diabetes mellitus in older persons. Jama. 2008;300:182–8. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denecke B, Graber S, Schafer C, Heiss A, Woltje M, Jahnen-Dechent W. Tissue distribution and activity testing suggest a similar but not identical function of fetuin-B and fetuin-A. The Biochemical journal. 2003;376:135–45. doi: 10.1042/BJ20030676. [DOI] [PMC free article] [PubMed] [Google Scholar]