Abstract
With a projected population of 10 billion by 2050, an immediate priority for agriculture is to achieve increased crop yields in a sustainable and cost-effective way. The concept of using a transgenic approach was realized in the mid-1990s with the commercial introduction of genetically modified (GM) crops. By 2010, the global value of the seed alone was US $11.2 billion, with commercial biotech maize, soya bean grain and cotton valued at approximately US $150 billion. In recent years, it has become evident that insect-resistant crops expressing δ-endotoxin genes from Bacillus thuringiensis have made a significant beneficial impact on global agriculture, not least in terms of pest reduction and improved quality. However, because of the potential for pest populations to evolve resistance, and owing to lack of effective control of homopteran pests, alternative strategies are being developed. Some of these are based on Bacillus spp. or other insect pathogens, while others are based on the use of plant- and animal-derived genes. However, if such approaches are to play a useful role in crop protection, it is desirable that they do not have a negative impact on beneficial organisms at higher trophic levels thus affecting the functioning of the agro-ecosystem. This widely held concern over the ecological impacts of GM crops has led to the extensive examination of the potential effects of a range of transgene proteins on non-target and beneficial insects. The findings to date with respect to both commercial and experimental GM crops expressing anti-insect genes are discussed here, with particular emphasis on insect predators and parasitoids.
Keywords: food security; genetically modified/biotech crops; insecticidal proteins; biological control; non-target effects; natural enemies (predators, parasitoids)
1. Introduction—food security
Food security features highly on both the political and social agenda [1]. This is not surprising given that the global population increased fourfold during the past century, with current estimates placing it around 9.2 billion by 2050 [2]. However, this increase has primarily been seen in the developing regions of the world, with population remaining relatively static in the developed world. Thus, in global terms, we are now facing a situation where food supply is beginning to be outstripped by demand, as predicted by the economist and demographer Robert Malthus some two centuries ago. In 2008, soaring food prices around the world demonstrated both the importance and interdependence of global systems of food production. The Director General of the FAO, Jaques Diouf, recently stated that [3]:
The silent hunger crisis—affecting one sixth of all of humanity—poses a serious risk for world peace and security. We urgently need to forge a broad consensus on the total and rapid eradication of hunger in the world.
Further, the FAO also stated that by 2050 food productivity must increase by 70 per cent to feed an additional 2.3 billion people, although the Royal Society in its recent report ‘Reaping the benefits—science and the sustainable intensification of global agriculture’ [4] was more conservative, suggesting an increase of about 50 per cent to meet the requirements of an estimated global population of 9 billion people. As expected, it is those countries least able that need to significantly increase food production, with an estimated increase of 300 per cent for Africa and 80 per cent for Latin America; even so, it is estimated that North America will need to increase productivity by some 30 per cent [5]. Without an increase in ‘farm productivity’, this equates to an additional 1.6 billion ha of arable land by 2050, a scenario that is becoming increasingly less sustainable or, indeed, achievable.
The challenge society is currently faced with is to increase productivity, particularly crop production, which represents primary productivity, in a manner that embraces the principles of agricultural sustainability, namely that we must meet the needs of the present without compromising the ability of future generations to meet their own needs, acknowledging the need for environmental health, profitability and social and economic equity. In order to take steps to achieving this goal it is important to identify, and then address, the current major constraints on productivity. In the context of this paper, we will focus on the sustainable production of crops and specifically on strategies to protect crops from insect pest damage, estimated globally to be in the region of 35–100% [6]. Since their introduction in 1947, synthetic insecticides have made a major contribution to food production. Despite an annual pesticide budget of US $30 billion, losses owing to insects, weeds and disease for eight of the world's major crops are estimated to be in the region of US $244 billion per annum, representing 43 per cent of world production [7,8], with post-harvest losses contributing to a further 10 per cent. Paoletti & Pimentel [9] estimated that in the absence of these synthetic pesticides losses might well increase by a further 30 per cent. Reports emerging from China quote officials saying that pesticide use saves China millions of tonnes of food and fibre every year [10–12]. However, despite their contribution, it has long been recognized that such chemicals pose both environmental and health concerns. In her book Silent Spring, published nearly half a century ago, Rachel Carson [13] cautions society about an over reliance on neurotoxic insecticides and states:
…and that [the insect control] methods employed must be such that they do not destroy us along with the insects.
This theme is further developed in the more recent Curry report [14] published in 2002, where it clearly states:
…in the interests of safety we recommend that older broad spectrum chemistry is replaced by newer, more selective, less persistent chemistry as soon as practicably possible.
And herein lies a major challenge, compounded by the fact that we may be at the limit of the existing genetic resources available in our major crops [15]. Thus, it is clear that new genetic resources must be found that are more tolerant and/or resistant to insect pests, and only new technologies will enable this. As so aptly stated in 2005, by the then President of the Royal Society, Lord Robert May [16]:
We could not feed today's world with yesterday's agriculture and we won't be able to feed tomorrow's world with today's.
Recombinant DNA technology to produce transgenic (genetically modified (GM) and/or engineered, biotech) crops with enhanced tolerance to stress, whether it be biotic or abiotic, can and does make a significant contribution to achieving greater food security, although it is important to recognize that sustainable solutions must embrace different, but complementary, approaches and cannot rely on any one technology. Although the vast majority of transgenic crops grown commercially today are tolerant to herbicides, this review will focus on insect-resistant transgenic crops and their impact on non-target organisms, specifically those beneficial insects that play a valuable role in biological control of pest populations. For detailed information on the current status of herbicide-tolerant (HT) crops, the reader is thus referred to a recent and comprehensive review by Owen [17].
2. Insect-resistant biotech crops
(a). Currently commercialized
Insect-resistant transgenic crops were first commercialized in the mid-1990s with the introduction of GM corn (maize), potato and cotton plants expressing genes encoding the entomocidal δ-endotoxin from Bacillus thuringiensis (Bt; also known as Cry proteins). In 2010, 148 million ha of biotech crops were grown in 29 countries, representing 10% of all 1.5 billion hectares of cropland in the world. The global value of this seed alone was valued at US $11.2 billion in 2010, with commercial biotech maize, soybean grain and cotton valued at approximately US $150 billion per year [18]. The concept of using genes encoding Cry proteins was not novel as Bt formulations (Dipel, Foil) have been used commercially for approximately four decades to control insect pests, and in particular Lepidoptera [19]. Early commercial varieties of insect-resistant transgenic crops expressed single Cry proteins with specific activity against lepidopteran pests, as illustrated by Bollgard cotton expressing Cry1Ac developed by Monsanto and attribute maize expressing Cry1Ab developed by Syngenta. Subsequently, other lepidopteran-active Bt toxins, such as Cry1F and Cry2Ab2, were introduced and often presented as pyramided genes in a single variety (Widestrike cotton expressing both Cry1F + Cry1Ac developed by Dow Agrosciences and Bollgard II cotton expressing Cry1Ac + Cry2Ab2 developed by Monsanto). Cry3 toxins with activity against coleopteran pests are also being used in commercial transgenic crops, particularly maize, to protect against chrysomelid rootworms (e.g. Monsanto's Yieldgard Rootworm maize expressing Cry3Bb1, Dow Agrosciences' Herculex RW maize expressing Cry34Ab1 and Cry35Ab1, stacked with a HT gene, and Syngenta's Agrisure RW maize expressing a modified version of Cry3A). More recently released transgenic maize varieties express genes encoding Cry proteins active against Lepidoptera and Coleoptera which, in some cases, are stacked with HT genes. Furthermore, Syngenta has recently launched Agrisure Viptera trait-stacked maize, the first commercially available variety to exploit a non-Cry Bt protein (Vip3) for the provision of multiple pest resistance. In China, Bt cotton cultivars expressing Cry1Ac together with a modified cowpea trypsin inhibitor (CpTI) were commercially released in 2000 [20] and in 2005 accounted for approximately 15 per cent of the cotton crop [21]. Although, like other protease inhibitors, CpTI does have insecticidal properties, the major reason for co-expressing Bt and CpTI in cotton was to reduce the likelihood of the insects becoming resistant to this cultivar, so extending its effective life.
Detailed knowledge on the mode of action of these Cry proteins is not only essential to optimize their efficacy against targets, but also to predict the potential for non-target effects. On ingestion, Bt toxins are solubilized in the midgut where they are proteolytically cleaved at the N-terminal to a 65–70 kDa truncated (active) form. The active molecules then exert their pathological effects by binding to a specific receptor(s) in the midgut epithelial cells and insert into the membrane where they form pores; this results in cell death by colloid osmotic lysis, followed by death of the insect [22]. In most commercial crop varieties, these Cry proteins are expressed in the active form and as such differ from those used in biopesticide formulations where the Cry proteins are present as protoxins.
(b). Research into alternative strategies
In recent years, it has become evident that Bt-expressing crops have made a significant beneficial impact on global agriculture, not least in terms of pest reduction and improved quality. However, because of the potential for pest populations to evolve resistance, and owing to lack of effective control of homopteran pests, alternative strategies are being developed. Some of these are based on Bacillus spp., e.g. vegetative insecticidal proteins (VIPs) or other insect pathogens. Other strategies are based on the use of plant-derived or animal-derived genes, including those from insects, such as those encoding immunosuppressive proteins. More recently, the potential to identify and exploit endogenous resistance genes using functional genomics and the use of RNAi are actively being investigated. (For recent reviews on alternative strategies to Bt that are currently under development, the reader is referred to the following: Gatehouse [23,24], Christou et al. [25], Malone et al. [26] and Price & Gatehouse [27].
However, if such approaches are to play a useful role in crop protection, it is desirable that they do not have a negative impact on beneficial organisms at higher trophic levels, which would inevitably result in affecting the functioning of the agro-ecosystem; this is also true for Bt-expressing crops. With this in mind, the impact of insect-resistant transgenic crops on natural enemies such as predators and parasitoids will be addressed.
(c). Impact on non-target insects
For any technology to be acceptable to the public at large, the perceived benefits have to outweigh any potential risk—this is equally true for biotech crops [28]. Furthermore, when evaluating potential risks of a given technology it is important to use relevant comparators. Few studies have actually been designed to directly compare GM technology with conventional pest control strategies, although recent studies by Mulligan et al. [29,30] directly compared the non-target effects of cypermethrin with oilseed rape plants expressing the cysteine protease inhibitor OC-1 with two predators, the carabid Pterostichus melanarius and the lacewing Chrysoperla carnea. While the former predator was not negatively affected by either form of pest control treatment, the effects of GM crops on the lacewing were significantly lower than with the commonly used pesticide. With current insect-resistant transgenic crops, there are clear benefits to both the grower and consumer, not least in the significant increases in productivity accompanied by significant reductions in chemical pesticide usage [31–33]. However, it must be recognized that alongside these benefits, there are concerns over the wide-scale growing of such crops, including potential effects on human health, and the environment at large (horizontal gene transfer, geneflow, potential invasiveness). While these are important issues, they are outside the scope of this paper, which focuses on the impact of such crops on beneficial non-target organisms and in particular parasitoids and predators; the reader is thus referred elsewhere for information on these broader issues [34].
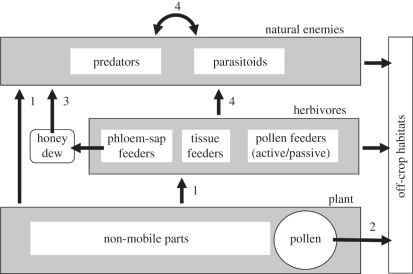
Assessing the environmental consequences of transgenic crops is an important prerequisite to their commercialization. The expression of transgenes that confer enhanced levels of resistance to insect pests is of particular relevance since they are aimed at manipulating the biology of organisms in a different trophic level to that of the plant. Numerous studies have been carried out since the early 1990s to investigate potential non-target effects at both the bitrophic and tritophic levels. The nature of these investigations have been very varied, ranging from detailed studies at the molecular/biochemical level under controlled environment conditions, to glasshouse trials using deliberately infested plants and released predators/natural enemies, to effects at the population level in the field. Further, they have been involved in delivering the transgene product both in artificial diet and in planta and either directly (at the bitrophic level) or via a tritrophic (plant–pest–natural enemy) interaction. Irrespective of the type of investigation carried out, or the experimental system used, one of the important considerations to be addressed is the likelihood of exposure of the transgene product and indeed the different routes of exposure. The most obvious exposure route for non-target herbivores is through direct ingestion of plant material, although this will be influenced by the mode of feeding and spatial expression patterns of the transgene product. In terms of exposure to natural enemies, these routes are more diverse since many predators and parasitoids, particularly in the adult stage, are facultative herbivores. They can thus be exposed to transgene products directly from consuming plant tissues (pollen, nectar) or, more usually, from consuming insects that have themselves fed on plant tissues where the transgene product has been expressed and accumulated. These different routes of exposure are presented in figure 1. It is pertinent to point out that in some cases, particularly when the host is an aphid, the predator/parasitoid is rarely exposed to the transgene product; however, the scenario is quite different when the host is a chewing insect (Lepidoptera, Coleoptera), when the likelihood of the natural enemy being exposed is very high.
Figure 1.
Exposure pathways. Routes through which non-target arthropods can be exposed to transgene products expressed in insect-resistant transgenic plants (after [35]).
Although one of the major concerns of GM technology is its impact on non-target organisms, and thus on biodiversity, these fears have not, in the main, been realized, although there have been some well-publicized cases to the contrary. For example, laboratory-based studies suggested that Bt-expressing maize pollen would have significant detrimental affects on the monarch butterfly [36], although subsequent large-scale field trials demonstrated this not to be the case, one factor being that when the maize was in flower the monarchs were not present [37–42]. Thus in this instance, while the potential hazard was high, exposure was negligible resulting in effectively zero risk. This work emphasizes the need to place such studies within an ecological context and the importance of demonstrating ‘cause and effect’. The latter point is well illustrated by initial studies concerning toxicity of Bt-maize fed hosts towards the predator Chrysoperla carnae (the green lacewing) via a tritrophic interaction [43]. Subsequent studies demonstrated that Bt Cry1Ab was not toxic to the larvae but that the effects reported were mediated by prey quality [44]. As mentioned above, numerous studies have now been carried out to evaluate the impact of GM crops on natural enemies that play an important role in biological control (tables 1 and 2). Interestingly, in the majority of cases studied to date, it is apparent that the predator/parasitoid is able to avoid the toxic effects of the different insecticidal proteins being expressed, despite exposure at physiologically relevant levels.
Table 1.
Impacts of transgenic crops and transgene products of parasitoids.
| protein | transgenic plant or diet | pest | natural enemy | effects on natural enemy | reference |
|---|---|---|---|---|---|
| Bt (Cry1Ab) | corn (Zea mays) | Chilo partellus (Lep: Crambidae) | Diaraetiella rapae (Hym: Braconidae) | reduced survival owing to host mortality, smaller cocoons and adults | Prutz & Dettner [45] |
| Bt (Cry1Ab) | corn (Z. mays) | Ostrinia nubilalis (Lep: Crambidae) | Macrocentrus cingulum (Hym: Braconidae) | reductions of 29–60% is numbers of wasps found in the field | Pilcher et al. [46] |
| Bt (Cry1Ab) | corn (Z. mays) | Spodoptera frugiperda (Lep: Noctuidae) | Campoletis sonorensis (Hym: Ichneumonidae) | wasps were significantly smaller when developing in Bt-fed hosts | Sanders et al. [47] |
| Bt(Cry1Ab) | corn (Z. mays) | Eoreuma loftini (Lep: Pyralidae) | Parallorhogas pyralophagus (Hym: Braconidae) | various aspects of parasitoid biology (not all) negatively affected | Bernal et al. [48] |
| Bt (Cry1Ac) | cotton (Gossypium hirsutum) | Helicoverpa armigera (Lep: Noctuidae) | Microplitis mediator (Hym: Braconidae) | wasp survival and development negatively affected | Liu et al. [49,50] |
| Bt (Cry1Ac) | cotton (G. hirsutum) | Pseudoplusia includens (Lep: Noctuidae) | Cotesia marginiventris (Hym: Braconidae), Copidosoma floridanum (Hym: Encyrtidae) | development times for both wasp species negatively affected. Adult longevity for C. marginiventris reduced | Baur & Boethel [51] |
| Bt (Cry1Ac) | cotton (G. hirsutum) | hemipteran pests | aphelinid parasitoids | small reduction of parasitoid population density relative to non-Bt cotton | Naranjo et al. [52] |
| Bt (Cry1Ac) | diet | Spodoptera litura/H. armigera (Lep: Noctuidae) | Meteorus pulchricornis (Hym: braconidae), Cotesia kazak (Hym: Braconidae) | survival of both unaffected in Bt-fed S. litura, M. pulchricornis negatively affected by Bt-fed H. armigera | Walker et al. [53] |
| Bt (Cry1Ac) | oilseed rape (Brassica napus) | Plutella xylostella (Lep: Plutellidae) | Cotesia plutellae (Hym: Braconidae) | no effect when Bt-resistant hosts were parasitized | Schuler et al. [54] |
| Bt (Cry1Ac) | Broccoli (Brassica oleracea) | P. xylostella | Diadegma insulare (Hym: Ichneumonidae) | no effect on parasitoid when exposed by Bt-resistant hosts | Chen et al. [55] |
| Bt (Cry1Ac) | pine (Pinus radiata) | Pseudocoremia suavis (Lep: Geometridae) | M. pulchricornis | no effect on parasitoid developmental parameters | Barraclough et al. [56] |
| Bt (Cry9Aa) | diet | Galleria mellonella (Lep: Gelechiidae) | Exorista larvarum (Dip: Tachinidae) | no effect on fly parasitoid when exposed to facticious Bt-fed hosts | Marchetti et al. [57] |
| Bt (Cry1Ab) | tobacco (Nicotiana tabacum) | Heliothis viresecens (Lep. Noctuidae) | C. sonorensis | rates of parasitism increased | Johnson & Gould [58] |
| CpTI | potato (Solanum tuberosum)/diet | Lacanobia oleracea (Lep: Noctuidae) | Eulophus pennicornis (Hym: Eulophidae) | fewer hosts parasitized, no effects on parasitoids | Bell et al. [59,60] |
| CpTI | diet | direct feeding (adult wasps) | E. pennicornis | no effects | Bell et al. [61] |
| CpTI and Bt | cotton (G. hirsutum) | H. armigera | M. mediator (Hym: Braconidae) | no greater effects than with Bt-cotton | Liu et al. [49] |
| CpTI and Bt | cotton (G. hirsutum) pollen | direct feeding | Trichogramma chilonis (Hym: Trichogrammatidae) | no adverse effects owing to CpTI | Geng et al. [62] |
| OC-1 | diet | Macrosiphum euphorbiae (Hom: Aphididae) | Aphelinus abdominalis (Hym: Braconidae) | parasitoid fitness impaired | Azzouz et al. [63] |
| OC-1 | potato (S. tuberosum) | M. euphorbiae | Aphidius nigripes (Hym: Braconidae) | wasp size and fecundity increased on OC1 line | Ashouri et al. [64] |
| OC-1 | oilseed rape (B. napus) | Myzus persicae (Hom: Aphididae) | Diaeretiella rapae (Hym: Braconidae) | no consistent effects on adult wasp emergence and sex ratio; no effects on control of aphids | Schuler et al. [65] |
| OC1 IΔD86 (for nematode control) | potato (S. tuberosum) | M. euphorbiae, M. persicae | Aphidius ervi (Hym: Braconidae) | no effects on per cent parasitism, adult wasp emergence; parasitoid communities more diverse on GM plants | Cowgill et al. [66] |
| Con A | diet | direct feeding | E. pennicornis | higher doses reduced adult longevity and reproductive fitness | Bell et al. [61], Wakefield et al. [67] |
| GNAa | diet | M. euphorbiae | A. abdominalis | no effect via host feeding on aphids, reduced size and longevity of adults when exposed via host | Couty et al. [68,69], Couty & Poppy [70] |
| GNA | diet | direct feeding (adult wasps) | Aphidius colemani (Hym: Braconidae) | higher doses reduced adult longevity | Romies et al. [71] |
| GNA | honeydew | direct feeding (adult wasps) | A. ervi | indirect negative affect potentially owing to altered honeydew composition | Hogervorst et al. [72] |
| GNA | potato (S. tuberosum)/diet | M. persicae | A. ervi | no effects via potato, dose-dependent effects on development via diet | Couty et al. [68,69] |
| GNA | sucrose diet | direct feeding | Cotesia glomerata (Hym: Braconidae) | Higher doses reduced longevity | Romies et al. [71] |
| GNA | diet/sugarcane (Saccharum officinarum) | Diatraea saccharalis (sugarcane borer) | Cotesia flavipes (Hym: Braconidae) | small negative effects on parasitism, no effects on host location of prey or parasitism | Setamou et al. [73,74] |
| GNA | potato (S. tuberosum)/diet | L. oleracea | E. pennicornis | no effect on parasitism success | Bell et al. [75] |
| GNA | diet | direct feeding | E. pennicornis | reduced adult longevity and reproductive fitness | Bell et al. [61], Wakefield et al. [67] |
| GNA | host diet/host injection | L. oleracea | E. pennicornis | negative affects on the survival of parasitoid larvae | Wakefield et al. [67] |
| GNA | sugarcane (S. officinarum) | E. loftini | P. pyralophagus | reduced size and longevity of adult wasps | Tomov et al. [76] |
| GNA | tomato (Solanum lycopersicum)/potato (S. tuberosum)/diet | L. oleracea | Meteorus gyrator (Hym: Braconidae) | no effects | Wakefield et al. [77] |
| GNA | sucrose diet | direct feeding (adult wasps) | Trichogramma brassicae (Hym: Trichogrammatidae) | anti-feedant; high dose reduced longevity | Romeis et al. [71] |
aGalanthus nivalis agglutinin.
Table 2.
Impacts of transgenic crops and transgene products of predators.
| protein | transgenic plant or diet | pest | natural enemy | effects on natural enemy | reference |
|---|---|---|---|---|---|
| Bt (Cry1Ab) | maize (Zea mays) | direct feeding (pollen) | several (Coleoptera, Heteoptera, Neuroptera) | no effects on predators in both laboratory and field experiments | Pilcher et al. [78] |
| Bt (Cry3Bb1) | maize (Z. mays) | Diabrotica spp. (Col: Chrysomelidae) | several (Araneae, Carabidae, Staphylinidae) | no consistent negative effect | Bhatti et al. [79] |
| Bt (Cry1Ab) | maize (Z. mays) | O. nubilalis; Spodoptera littoralis (Lep: Noctuidae) | C. carnea | Bt-fed prey increased predator mortality and development times | Hilbeck et al. [43] |
| Bt (Cry1Ab) | maize (Z. mays) | S. littoralis | C. carnea | negative effects, as observed previously, determined to be due to reduced prey quality | Dutton et al. [80] |
| Bt (Cry1Ab) | maize (Z. mays) | Tetranychus urticae (Acari) | Stenothus punctillum (Col: Cocinellidae) | no effects in both laboratory and field experiments | Alvarez-Alfageme et al. [81] |
| Bt (Cry1Ab) | maize (Z. mays) | direct feeding (pollen) | spiders (Araneae) | no effects in both laboratory and field experiments | Ludy & Lang [82,83] |
| Bt (Cry3Bb1) | maize (Z. mays) | Rhopalosiphum maidis (Hom: Aphididae) | Coleomegilla maculate (Col: Coccinellidae) | no effects when fed non-target aphid prey | Lundgren & Wiedenmann [84] |
| Bt (VIP3A + Cry1Ab) | maize (Z. mays) | lepidopterous pests | several (13 arthropod orders) | large-scale study showed no negative effects of stacked traits over conventional corn | Dively [85] |
| Bt (Cy1Ac) | cotton (G. hirsutum) | Aphis gossypii (Hem. Aphididae) | Chrysopa pallens (Neu: Chrysopidae) | no effect | Guo et al. [86] |
| Bt (Cry1Ac) | cotton (G. hirsutum) | several | predators (several) | minor reductions in predator density in the field | Naranjo [52,87] |
| Bt (Cry1Ac/Cry2Ab) | cotton (G. hirsutum) | lepidopterous pests | predators (several) | predator numbers similar or higher in Bt cotton field plots | Hagerty et al. [88], Head et al. [89] |
| Bt (Cry1Ac) | cotton (G. hirsutum) | Spodoptera exigua, Helicoverpa zea (Lep: Noctuidae) | Geocoris punctipes (Het: Lygaeidae) | no effects in field experiments | Torres & Ruberson [90] |
| Bt (Cry1Ac) | cotton (G. hirsutum) | S. exigua | P. maculiventris | no effects | Torres & Ruberson [91] |
| Bt (Cry1Ac) | cotton (G. hirsutum) | lepidopterous pests | C. carnea; Orius tristicolor (Het: Anthocoridae) | no effects in field experiments | Sisterson et al. [92] |
| Bt (Cry3Aa) | potato (S. tuberosum) | L. decemlineata | several heteropteran predators and spiders | no effect of predator densities in the field | Reed et al. [93] |
| Bt (Cry3Aa) | potato (S. tuberosum) | L. decemlineata | Coleoptera, Araneae | no effects on field pitfall trap capture numbers | Duan et al. [94] |
| Bt (Cry3) | potato (S. tuberosum) | M. persicae | Hippodamia convergens (Col: Coccinellidae) | no effect | Dogan et al. [95] |
| Bt (Cry3A) | potato (S. tuberosum) | Lep: Hem. | several (Heteroptera) | no effects on development time | Armer et al. [96] |
| Bt (Cry3 A) | potato (S. tuberosum)/pollen | L. oleracea | H. axyridis, N. brevicollis | no effects | Ferry et al. [97] |
| CpTI | injected prey | L. oleracea | Podisus maculiventris (Het: Pentatomidae) | reduced growth of predators | Bell et al. [98] |
| CpTI | potato (S. tuberosum) | L. oleracea | P. maculiventris | no effects | Bell et al. [98] |
| CpTI | strawberry (Fragaria sp.) | Otiorhynchus sulcatus (Col: Curculionidae) | carabids and others | field abundance not affected | Graham et al. [99] |
| HvCPI-1 C68 → G | potato (S. tuberosum) | L. decemlineata | P. maculiventris | no effect | Alvarez-Alfageme et al. [100] |
| HvCPI-1 C68 → G | potato (S. tuberosum) | S. littoralis | P. maculiventris | no effect | Alvarez-Alfageme et al. [100] |
| MTI-2 | oilseed rape (B. napus) | P. xylostella | Pterostichus madidus (Col: Carabidae) | no effects on reproductive fitness; female weight gain reduced at first but compensated for later | Ferry et al. [101] |
| aprotinin (bovine pancreatic or bovine spleen trypsin inhibitor) (BPTI/BSTI) | diet | H. armigera | Harpalus affinis (Col: Carabidae) | beetles consumed less prey after 24 h of exposure to inhibitor-fed prey | Jorgensen & Lovei [102] |
| BPTI/BSTI | diet | H. armigera | Nebria brevicollis (Col: Carabidae) | transient minor changes in adult beetle weights | Burgess et al. [103] |
| OCI | diet | direct feeding | C. carnea | no effect via contaminated pollen | Mulligan et al. [30] |
| OC1 | oilseed rape (B. napus) | Deroceras reticulatum (Mollusca) | Pterostichus melanarius (Col. Carabidae) | no effects on beetle mortality, weight gain or food consumption | Mulligan et al. [29] |
| OC1 | oilseed rape (B. napus) | P. xylostella (Lep: Plutellidae) | H. axyridis | no effect on survival or development of ladybird | Ferry et al. [104] |
| OC1 | potato (S. tuberosum) | Leptinotarsa decemlineata (Colorado potato beetle) | Perillus bioculatus (Het: Pentatomidae) | no effects | Bouchard et al. [105] |
| Soya bean trypsin inhibitor (SBTI) | diet | direct feeding | C. carnea | no effects | Lawo & Romeis [106] |
Four cases studies are presented in §3 that demonstrate how the natural enemy is able to circumvent the effects of exposure to transgene products, highlighting the necessity of understanding the modes of activity of the insecticidal proteins being expressed. One such study investigates the non-target effects of Bt Cry proteins that are known to be specific to one or two insect orders, while the other three case studies presented investigate the non-target effects of plant-derived insecticidal proteins; these are known to have a broader spectrum of activity and hence are thought more likely to exert deleterious effects on natural enemies.
3. Case studies
(a). Tritrophic effects of Bt Cry3A transgenic potato on the beneficial carabid beetle Nebria brevicollis
Carabid beetles are routinely used for safety testing of pesticides since they are sensitive to environmental perturbation; they are thus considered an appropriate model to use in risk assessment studies for GM crops. However, in the case of Cry proteins, it is important to use those that target the insect order in question. For this reason, the effects of potato expressing the coleopteran-specific B. thuringiensis δ-endotoxin Cry3A on the carabid beetle Nebria brevicollis was investigated via the tritrophic interaction of the carabid consuming a non-target potato pest [97]. Despite N. brevicollis belonging to the targeted insect order, no significant effects upon survival or overall body mass change were observed. Furthermore, Bt Cry3A had no detrimental effects on reproductive fitness, either in terms of fecundity or subsequent egg viability. Surprisingly, ligand blots demonstrated the absence of Bt-binding sites in the brush border membrane vesicles (BBMVs) isolated from the predator. For Bt Cry proteins to exert their insecticidal effects, the protein must first bind to specific receptors on the midgut epithelial cells. The results presented in this case study suggest that the observed lack of toxicity, despite exposure to a coleopteran-specific Bt, was due to the lack of Bt receptors in the carabid [97]. These results were unexpected, and this particular study once again emphasizes the importance of understanding modes of action of insecticidal compounds and, in turn, the response of the insect to such molecules.
Results of this case study can be compared with the extensive work on Bt non-target impacts conducted against a range of beneficial insects, both in the laboratory and at the field scale (tables 1 and 2). While some negative effects for the predator C. carnea have been reported in laboratory studies [43,80], several large-scale field studies have indicated negative impact towards beneficial insects to be either minor [52,87] or absent [88,89]. Although recent meta-analysis of the field-scale ecological impacts of Bt cotton and maize by Marvier et al. [107] quite clearly indicates that non-target biodiversity can be negatively impacted upon by the expression of Cry proteins in these crops, these negative impacts were generally markedly lower when comparisons were made with conventionally managed crops. These findings suggest that the use of insect-resistant biotech crops constitute a major advance over the use of broad-spectrum synthetic insecticides for control of insect pests since they are environmentally more benign.
(b). Impact of oilseed rape expressing the insecticidal cysteine protease inhibitor oryzacystatin on the beneficial predator Harmonia axyridis (multicoloured Asian ladybeetle)
The cysteine protease inhibitor oryzacystatin-1 (OC-1), derived from rice seed [108], has been widely demonstrated to exert deleterious effects on a number of economically important insects, including coleopteran [109,110] and homopteran pests [63,111]. Protection against plant-pathogenic nematodes has also been demonstrated [112]. The potential of this inhibitor as a novel trait for expression in crop plants has led to the examination of its impacts on several beneficial/non-target insect species (tables 1 and 2). There is currently no evidence of any mammalian toxicity or allergenicity associated with this protein, as might be expected for a protein that is already present in the diets of many millions of people.
The second case study we present here provides an example of the examination of the non-target impacts of OC-1 at the third trophic level. Here, the effects of oilseed rape expressing OC-1 on the predatory ladybird Harmonia axyridis were investigated using the diamondback moth Plutella xylostella as the pest species. As expected, expression of OC-1 had no effects on either development or survival of the pest, since it is known that the host species uses serine, not cysteine, digestive proteases. However, since the ladybird was shown to use digestive cysteine proteases and since immunoassays confirmed the presence of the transgene product in pest larval tissues it was considered that expression of this particular protease inhibitor in oilseed rape may cause undesirable effects at the third trophic level. However, the results obtained were contrary to expectation since the inhibitor initially stimulated development, with a shortening of the developmental period of second instar larvae by 27 per cent, accompanied by a 36 per cent increase in weight; there was also a significant increase in consumption of OC-1 dosed prey. Critically, OC-1 had no detrimental effects on reproductive fitness of adult H. axyridis. Thus, the results clearly demonstrated that prey reared on transgenic plants expressing a protein that inhibited ladybird digestive enzymes in vitro had no effects in vivo. Subsequent studies revealed that the ladybird was able to upregulate digestive proteases in response to the inhibitor. Although it was known that some phytophagous insects are able to modulate their digestive proteases in response to diet, particularly to the presence of dietary protease inhibitors, either by a general up-regulation or the synthesis of novel enzymes [113], that predators could respond in a similar way had not previously been shown [104]. The examinations of OC-1 in other tritrophic systems largely corroborate the findings of the case study presented here in that most reports indicate little or no negative responses to the inhibitor in predators or parasitoids [29,63–65] (tables 1 and 2).
(c). Impact of oilseed rape expressing the insecticidal serine protease inhibitor mustard trypsin inhibitor-2 on the beneficial predator Pterostichus madidus
The third case study presented here investigates the effects of the serine protease inhibitor, mustard trypsin inhibitor-2 (MTI-2), on the predatory ground beetle Pterostichus madidus with the diamondback moth P. xylostella as the intermediary pest species. While this transgene protein has only received limited attention, it has been demonstrated that expression of MTI-2 in tobacco, oilseed rape and Arabidopsis can elicit significant deleterious effects on larval growth in several lepidopteran pest species [114].
A major difference between this study and that presented above (§3(b)) is that in this particular case both the insect host (pest) and the predator predominantly use serine proteases for protein digestion. Thus, as expected, oilseed rape expressing MTI-2 had a deleterious effect on the development and survival of the pest [101]. However, incomplete pest mortality resulted in survivors being available to predators at the next trophic level, and inhibition studies confirmed the presence of biologically active transgene products in pest larvae. Although digestive proteolytic activity of the predator was completely inhibited by MTI-2 in vitro, when P. madidus consumed prey reared on MTI-2-expressing plants over the reproductive period in their life cycle, no significant effects upon survival were observed as a result of exposure to the inhibitor. However, there was a short-term significant inhibition of weight gain in female beetles fed unlimited prey containing MTI-2, with a concomitant reduction of prey consumption. Biochemical analyses revealed that the predator was able to circumvent the effects of the PI by expression of novel insensitive chymotrypsin-like enzymes. Perhaps of greater ecological significance was the finding that consumption of MTI-2 dosed prey had no detrimental effects on reproductive fitness of the predator [101]. The results for MTI-2 presented here can be contrasted with tritrophic studies obtained for a second serine protease inhibitor, CpTI, where deleterious effects have been recorded for both predatory insects and parasitoids [59–61]. In these instances, the much poorer quality of the prey/hosts was thought to be the primary reason for the observed effects as opposed to any direct toxicity at the third trophic level.
(d). Transgenic Galanthus nivalis agglutinin expressing potato plants augment the beneficial biocontrol of Lacanobia oleracea by the parasitoid Eulophus pennicornis
The expression of plant lectins has been widely evaluated as a means of conferring a degree of resistance to phytophagous insects in a range of crops. Of the many lectins that have been evaluated, snowdrop lectin (Galanthus nivalis agglutinin (GNA)) has shown promise against several lepidopteran and homopteran pests when delivered via their diets. Such findings have led to the expression of the lectin in a number of crops of varying economic importance, including tobacco [115], potato [116], papaya [117], wheat [118], sugarcane [73,74] and maize [119]. In most, but not all, cases expression of the lectin afforded some measure of control against the pest insects that were examined.
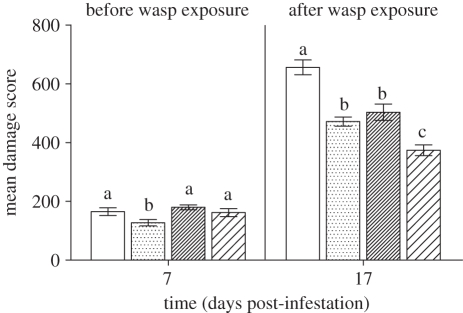
The potential impacts of GNA have been extensively evaluated for over 10 years, in several tritrophic systems (tables 1 and 2) and in a number of simpler scenarios whereby the lectin has been delivered directly to the non-target insect (e.g. via an artificial diet). In the final case study presented here, the effects of a GNA-expressing insect-resistant transgenic crop on a parasitoid, rather than on a predator, is discussed. This study is particularly noteworthy since it was the first demonstration that two different pest control strategies, namely recombinant DNA technology and biological control, could be compatible with one another as opposed to the predicted incompatibility of these two very different approaches [60]. These studies were carried out in large-scale glasshouse trials and demonstrated that while expression of GNA was able to significantly reduce the levels of pest damage caused by Lacanobia oleracea, the presence of the parasitoid resulted in further reductions (ca 21%) in the level of damage (figure 2). The ability of the wasp to parasitize and subsequently develop on the pest/host larvae was not altered by the presence of GNA. The progeny of Eulophus pennicornis that developed on L. oleracea reared on GNA-expressing plants showed no significant alteration in fecundity when compared with wasps that had developed on hosts fed control potato plants, although mean size and longevity of female parasitoids was significantly reduced. Furthermore, the number of F2 progeny produced by parasitoids derived from hosts fed on GNA-expressing plants was not significantly different to those produced by parasitoids from hosts fed control plants.
Figure 2.
GNA-mediated crop protection demonstrating that transgene expression does not deleteriously affect parasitoid performance but leads to enhanced biocontrol [60]. Bars indicate the levels of foliar damage scored in potatoes 7 days after infestation with L. oleracea larvae (15 per plant) and at 17 days, one week after the release of E. pennicornis into two of the glasshouses at a rate of two wasps per host. Plain bars, control (−parasitoid); dotted bars, GNA (−parasitoid); thin striped bars, control (+parasitoid); thick striped bars, GNA (+parasitoid).
Recent studies have since shown that GNA, when present at high levels (microgram quantities) in host haemolymph, via injection of the lectin, does exert a highly negative effect on E. pennicornis in terms of developmental success. The concentrations required to elicit this response, however, are almost certainly far above what might be expected to occur when the pest consumes GNA-expressing plant material [67]. Interestingly, it has also been observed that lower GNA concentrations in host larvae actually facilitate significantly greater survival of E. pennicornis (H. Bell 2009, unpublished data), a fact that may explain the benign or moderately beneficial effects that this lectin has been observed to elicit in this and other tritrophic systems that have been investigated.
Parasitoids, owing to their importance in regulating populations of phytophagous pests, have been popular insects for use in the assessment of the potential adverse effects of GNA and other transgene proteins. In the case of GNA, when examined in highly controlled laboratory experiments, a number of detrimental effects have been elicited. However, the rapid acute toxicity that is frequently observed when parasitoids are exposed to most conventional neurotoxic insecticides has not been reported (table 1). Instead, a number of sub-lethal impacts on parasitoids have been recorded, such as reduced longevity, reduced fecundity and extended development times [67,71,76]. Notably, directly feeding parasitoids with diets containing the lectin generally elicits the most pronounced effects, indicating that contamination of nectar and other sugar sources would constitute the greatest risk to parasitic Hymenoptera [61,72]. However, the generally minor impacts on parasitoids that have been reported to date, mostly in worse-case scenarios, are unlikely to significantly inhibit their effectiveness as biological control agents in more realistic settings.
4. Conclusion
The challenges that face twenty-first century agriculture are to increase crop yield while limiting environmental impact. This will necessitate not only a reduction in pesticide usage, but also improvement in stress tolerance in crops along with improvement in nutritional content. Since, at least for some major crops, we may be at the limit of the existing genetic resources, new genetic resources and new technologies will be required to meet these challenges. One such approach has been to use recombinant DNA technology. However, with new technologies come new concerns, and in the case of GM crops this includes non-target effects. Thus, assessing the consequences of this technology on non-target organisms is an important precursor to it becoming adopted in mainstream agriculture. There is a growing body of scientific literature on the impacts of transgenic crops on non-target organisms and in particular on predators and parasitoids, since these insects are known to make an important contribution to crop protection. What is clear from these studies is that not only is it important to understand the mode of activity of the expressed proteins on both target and non-target insects and how, if at all, they are able to respond to circumvent potential toxic effects, but to ensure that such studies are of ecological relevance. Lovei et al. [120] recently carried out a meta-analysis in an attempt to summarize the published literature on the impact of GM plants on arthropod natural enemies from laboratory-based experiments. However, their findings were considered by many to be misleading since important methodological limitations relative to risk assessment led the authors to reach conclusions that were in conflict with those of several recent comprehensive reviews and meta-analyses concerning the effects of Cry proteins on natural enemies [121].
Importantly, there must be an attempt to assess potential negative effects on beneficial insects within the context of current pest control practices for a given crop, a factor neglected by much of the research and several of the meta-analyses, conducted to date. Here, we consider the intensively managed, pesticide-reliant agriculture that prevails across much of the world as the benchmark against which the impacts of anti-insect GM technology should be assessed as opposed to organic and low-input systems that, while common, are unlikely to supplant the former as the predominant global production model. While we recognize that it can also be argued that the default comparator should be the untreated and/or organic scenario, this constitutes a much less useful comparison, particularly as one of the major claims made for GM technology is the reduced ecological impact when viewed against insecticide-reliant intensive agriculture. Therefore, although it is clear that some negative effects do occur in predatory arthropods and parasitoids following exposure to GM crops and/or the insecticidal proteins they express, one must also take into account the impacts of the pest control measures that a given biotech crop seeks to replace. When this is taken into consideration, the relatively few negative effects that have been recorded are invariably substantially less than would have occurred under traditional pesticide-reliant regimes. Therefore, despite there being some reports of negative effects on beneficial arthropods, insecticidal biotech crops, for the most part, would appear to have the potential to be much more environmentally benign in this respect than insecticide-based pest management approaches. However, it is only now that we are beginning to see data on the responses of beneficial insects to GM crops being generated concurrently with that for the insecticides that would otherwise be used. The onus on future research, therefore, is to examine whether crops engineered to express insecticidal proteins constitute an appreciable improvement, in terms of environmental impact, over the frequently highly damaging strategies that have gone before.
Arthropod pests have proven incredibly successful in exploiting the artificial and highly simplified ecosystems that modern agricultural creates. Pesticides have provided short-term solutions to this problem but insect-resistant biotech crops now give us an unparalleled opportunity for the development of strategies that move broadacre crops away from a reliance on synthetic chemistry. This shift will increase beneficial insect activity which, in turn, will enhance the control provided by the engineered resistance and allow for the realization of truly integrated and environmentally sustainable pest management in the crops that are required to feed the world's burgeoning population in the twenty-first century.
Footnotes
One contribution of 13 to a Theme Issue ‘Community genetics: at the crossroads of ecology and evolutionary genetics’.
References
- 1.Anon 2010. Food 2030. London, UK: Department for Food and Rural Affairs. [Google Scholar]
- 2.United Nations 2009. World population prospects: the 2008 revision. Popul. Newsl. 87, 1–20 [Google Scholar]
- 3.Diouf J. 2011 See http://www.fao.org/news/story/0/item/20568/icode/en/ (accessed 18 February 2011) [Google Scholar]
- 4.Royal Society. Reaping the benefits—science and the sustainable intensification of global agriculture. 2009. RS Policy Document 11/09. London, UK: The Royal Society.
- 5.Anon 2000. The State of Food and Agriculture 2000. Agricultural and Development Economics Working Papers-32. Rome, Italy: FAO [Google Scholar]
- 6.Ferry N., Gatehouse A. M. R. 2010. Transgenic crop plants for resistance to biotic stress. In Transgenic crop plants: utilization and biosafety, vol. 2 (eds Kole C., Michler C. H., Abbott A. G., Hall T. C.), pp. 1–66 Germany: Springer-Verlag [Google Scholar]
- 7.Oerke E. C. 1999. The importance of disease control in modern plant production. Mod. Fungicides Antifungal Compd. II, 11–17 [Google Scholar]
- 8.Oerke E. C. 2006. Crop losses to pests. J. Agric. Sci. 144, 31–43 10.1017/S0021859605005708 (doi:10.1017/S0021859605005708) [DOI] [Google Scholar]
- 9.Paoletti M. G., Pimentel D. 2000. Environmental risks of pesticides versus genetic engineering for agricultural pest control. J. Agric. Environ. Ethics 12, 279–303 10.1023/A:1009571131089 (doi:10.1023/A:1009571131089) [DOI] [Google Scholar]
- 10.Pray C., Ma D. M., Huang J. K., Qiao F. B. 2001. Impact of Bt cotton in China. World Dev. 29, 813–825 10.1016/S0305-750X(01)00010-9 (doi:10.1016/S0305-750X(01)00010-9) [DOI] [Google Scholar]
- 11.Pray C. E., Huang J. K., Hu R. F., Rozelle S. 2002. Five years of Bt cotton in China—the benefits continue. Plant J. 31, 423–430 10.1046/j.1365-313X.2002.01401.x (doi:10.1046/j.1365-313X.2002.01401.x) [DOI] [PubMed] [Google Scholar]
- 12.Huang J. K., Hu R. F., Pray C., Qiao F. B., Rozelle S. 2003. Biotechnology as an alternative to chemical pesticides: a case study of Bt cotton in China. Agric. Econ. 29, 55–67 10.1111/j.1574-0862.2003.tb00147.x (doi:10.1111/j.1574-0862.2003.tb00147.x) [DOI] [Google Scholar]
- 13.Carson R. 1962. Silent spring, p. 9 Greenwich, CT: Fawcett [Google Scholar]
- 14.Curry D. 2002. Farming and food: a sustainable future. Report of the Policy Commission on the Future of Farming and Food London, UK: Her Majesty's Stationery Office [Google Scholar]
- 15.Gressel J. 2008. Genetic glass ceilings. Transgenics for crop biodiversity. Baltimore, MD: The John Hopkins University Press [Google Scholar]
- 16.May R. 2005. Threats to tomorrow's world. Anniversary Address. The Royal Society. See http://royalsociety.org/uploadedFiles/Royal_Society_Content/about-us/history/Anniversary_Address_2005.pdf [Google Scholar]
- 17.Owen M. D. K. 2009. Herbicide-tolerant genetically modified crops: resistance management. In Environmental impact of genetically modified crops (eds Ferry N., Gatehouse A. M. R.). Wallingford, UK: CAB International [Google Scholar]
- 18.James C. 2010. Global status of commercialized biotech/GM crops: 2010. ISAAA brief no. 42 Ithaca, NY: ISAAA [Google Scholar]
- 19.Cannon R. J. C. 1996. Bacillus thuringiensis use in agriculture: a molecular perspective. Biol. Rev. 71, 561–636 10.1111/j.1469-185X.1996.tb01285.x (doi:10.1111/j.1469-185X.1996.tb01285.x) [DOI] [Google Scholar]
- 20.Song X. X., Wang S. M. 2000. Status and evaluation on the expression of cotton varieties in the production in China in the past 20 years. Cotton Sci. 13, 315–320 [Google Scholar]
- 21.He K. L., Wang Z. Y., Zhang Y. J. 2009. Monitoring Bt resistance in the field: China as a case study. In Environmental impact of genetically modified crops, ch. 16 (eds Ferry N., Gatehouse A. M. R.), pp. 344–359 Wallingford, UK: CAB International [Google Scholar]
- 22.de Maagd R. A., Bravo A., Crickmore N. 2001. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 17, 193–199 10.1016/S0168-9525(01)02237-5 (doi:10.1016/S0168-9525(01)02237-5) [DOI] [PubMed] [Google Scholar]
- 23.Gatehouse J. A. 2002. Plant resistance towards insect herbivores: a dynamic interaction. New Phytol. 156, 145–169 10.1046/j.1469-8137.2002.00519.x (doi:10.1046/j.1469-8137.2002.00519.x) [DOI] [PubMed] [Google Scholar]
- 24.Gatehouse J. A. 2008. Biotechnological prospects for engineering insect-resistant plants. Plant Physiol. 146, 881–887 10.1104/pp.107.111096 (doi:10.1104/pp.107.111096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christou P., Capell T., Kohli A., Gatehouse J. A., Gatehouse A. M. R. 2006. Recent developments and future prospects in insect pest control in transgenic crops. Trends Plant Sci. 11, 302–308 10.1016/j.tplants.2006.04.001 (doi:10.1016/j.tplants.2006.04.001) [DOI] [PubMed] [Google Scholar]
- 26.Malone L. A., Gatehouse A. M. R., Barratt B. I. P. 2008. Beyond Bt: alternative strategies for insect-resistant crops. In Integration of insect-resistant genetically modified crops within integrated pest management programs (eds Romeis J., Shelton T., Kennedy G.). Springer Series on Progress in Biological Control Berlin, Germany: Springer [Google Scholar]
- 27.Price D. R., Gatehouse J. A. 2008. RNAi-mediated crop protection against insects. Trends Biotechnol. 26, 393–400 10.1016/j.tibtech.2008.04.004 (doi:10.1016/j.tibtech.2008.04.004) [DOI] [PubMed] [Google Scholar]
- 28.Waltz E. 2009. Battlefield. Nature 461, 27–32 10.1038/461027a (doi:10.1038/461027a) [DOI] [PubMed] [Google Scholar]
- 29.Mulligan E. A., Ferry N., Jouanin L., Walters K., Port G. R., Gatehouse A. M. R. 2006. Comparing the impact of conventional pesticide and use of a transgenic pest-resistant crop on the beneficial carabid beetle Pterostichus melanarius. Pest Manag. Sci. 62, 999–1012 10.1002/ps.1276 (doi:10.1002/ps.1276) [DOI] [PubMed] [Google Scholar]
- 30.Mulligan E. A., Ferry N., Jouanin L., Romeis J., Gatehouse A. M. R. 2010. Characterisation of adult green lacewing (Chrysoperla carnea) digestive physiology: comparison of the impact of genetically modified crops and conventional pest control. Pest Manag. Sci. 66, 325–336 10.1002/ps.1879 (doi:10.1002/ps.1879) [DOI] [PubMed] [Google Scholar]
- 31.Betz F. S., Hammond B. G., Fuchs R. L. 2000. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharmacol. 32, 156–173 10.1006/rtph.2000.1426 (doi:10.1006/rtph.2000.1426) [DOI] [PubMed] [Google Scholar]
- 32.Cattaneo M. G., et al. 2006. Farm-scale evaluation of the impacts of transgenic cotton on biodiversity, pesticide use, and yield. Proc. Natl Acad. Sci. USA 103, 7571–7576 10.1073/pnas.0508312103 (doi:10.1073/pnas.0508312103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thies J. E., Devare M. H. 2007. An ecological assessment of transgenic crops. J. Dev. Stud. 43, 97–129 10.1080/00220380601055593 (doi:10.1080/00220380601055593) [DOI] [Google Scholar]
- 34.Ferry N., Gatehouse A. M. R. () 2009. Environmental impact of genetically modified crops. Wallingford, UK: CAB International [Google Scholar]
- 35.Romeis J., Meissle M., Raybould A., Hellmich R. L. 2009. Impact of insect-resistant transgenic crops on above-ground non-target arthropods. In Environmental impact of genetically modified crops (eds Ferry N., Gatehouse A. M. R.). Wallingford, UK: CAB International [Google Scholar]
- 36.Losey J. E., Rayor L. S., Carter M. E. 1999. Transgenic pollen harms monarch larvae. Nature 399, 214. 10.1038/20338 (doi:10.1038/20338) [DOI] [PubMed] [Google Scholar]
- 37.Gatehouse A. M. R., Ferry N., Raemaekers R. J. M. 2002. The case of the monarch butterfly: a verdict is returned. Trends Genet. 18, 249–251 10.1016/S0168-9525(02)02664-1 (doi:10.1016/S0168-9525(02)02664-1) [DOI] [PubMed] [Google Scholar]
- 38.Oberhauser K. S., et al. 2001. Temporal and spatial overlap between monarch larvae and corn pollen. Proc. Natl Acad. Sci. USA 98, 11 913–11 918 10.1073/pnas.211234298 (doi:10.1073/pnas.211234298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pleasants J. M., Hellmich R. L., Dively G. P., Sears M. K., Stanley-Horn D. E., Mattila H. R., Foster J. E., Clark P., Jones G. D. 2001. Corn pollen deposition on milkweeds in and near cornfields. Proc. Natl Acad. Sci. USA 98, 11 919–11 924 10.1073/pnas.211287498 (doi:10.1073/pnas.211287498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sears M. K., Hellmich R. L., Stanley-Horn D. E., Oberhauser K. S., Pleasants J. M., Mattila H. R., Siegfried B. D., Dively G. P. 2001. Impact of Bt corn pollen on monarch butterfly populations: a risk assessment. Proc. Natl Acad. Sci. USA 98, 11 937–11 942 10.1073/pnas.211329998 (doi:10.1073/pnas.211329998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanley-Horn D. E., et al. 2001. Assessing the impact of Cry1Ab-expressing corn pollen on monarch butterfly larvae in field studies. Proc. Natl Acad. Sci. USA 98, 11 931–11 936 10.1073/pnas.211277798 (doi:10.1073/pnas.211277798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zangerl A. R., McKenna D., Wraight C. L., Carroll M., Ficarello P., Warner R., Berenbaum M. R. 2001. Effects of exposure to event 176 Bacillus thuringiensis corn pollen on monarch and black swallowtail caterpillars under field conditions. Proc. Natl Acad. Sci. USA 98, 11 908–11 912 10.1073/pnas.171315698 (doi:10.1073/pnas.171315698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilbeck A., Baumgartner M., Fried P. M., Bigler F. 1998. Effects of transgenic Bacillus thuringiensis corn-fed prey on mortality and development time of immature Chrysoperla carnea (Neuroptera: Chrysopidae). Environ. Entomol. 27, 480–487 [Google Scholar]
- 44.Obrist L. B., Dutton A., Romeis J., Bigler F. 2006. Biological activity of Cry1Ab toxin expressed by Bt maize following ingestion by herbivorous arthropods and exposure of the predator Chrysoperla carnea. Biocontrol 51, 31–48 10.1007/s10526-005-2936-8 (doi:10.1007/s10526-005-2936-8) [DOI] [Google Scholar]
- 45.Prutz G., Dettner K. 2004. Effect of Bt corn leaf suspension on food consumption by Chilo partellus and life history parameters of its parasitoid Cotesia flavipes under laboratory conditions. Entomol. Exp. Appl. 111, 179–187 10.1111/j.0013-8703.2004.00166.x (doi:10.1111/j.0013-8703.2004.00166.x) [DOI] [Google Scholar]
- 46.Pilcher C. D., Rice M. E., Obrycki J. J. 2005. Impact of transgenic Bacillus thuringiensis corn and crop phenology on five nontarget arthropods. Environ. Entomol. 34, 1302–1316 10.1603/0046-225X(2005)034[1302:IOTBTC]2.0.CO;2 (doi:10.1603/0046-225X(2005)034[1302:IOTBTC]2.0.CO;2) [DOI] [Google Scholar]
- 47.Sanders C. J., Pell J. K., Poppy G. M., Raybould A., Garcia-Alonso M., Schuler T. H. 2007. Host-plant mediated effects of transgenic maize on the insect parasitoid Campoletis sonorensis (Hymenoptera: Ichneumonidae). Biol. Control 40, 362–369 10.1016/j.biocontrol.2006.12.010 (doi:10.1016/j.biocontrol.2006.12.010) [DOI] [Google Scholar]
- 48.Bernal J. S., Griset J. G., Gillogly P. O. 2002. Impacts of developing on Bt maize-intoxicated hosts on fitness parameters of a stem borer parasitoid. J. Entomol. Sci. 37, 27–40 [Google Scholar]
- 49.Liu X. X., Zhang Q. W., Zhao J. Z., Cai Q. N., Xu H. L., Li J. C. 2005. Effects of the Cry1Ac toxin of Bacillus thuringiensis on Microplitis mediator, a parasitoid of the cotton bollworm, Helicoverpa armigera. Entomol. Exp. Appl. 114, 205–213 10.1111/j.1570-7458.2005.00248.x (doi:10.1111/j.1570-7458.2005.00248.x) [DOI] [Google Scholar]
- 50.Liu X. X., Zhang Q. W., Zhao J. Z., Li H. C., Xu B. L., Ma X. M. 2005. Effects of Bt transgenic cotton lines on the cotton bollworm parasitoid Microplitis mediator in the laboratory. Biol. Control 35, 134–141 10.1016/j.biocontrol.2005.08.006 (doi:10.1016/j.biocontrol.2005.08.006) [DOI] [Google Scholar]
- 51.Baur M. E., Boethel D. J. 2003. Effect of Bt-cotton expressing Cry1A(c) on the survival and fecundity of two hymenopteran parasitoids (Braconidae, Encyrtidae) in the laboratory. Biol. Control 26, 325–332 10.1016/S1049-9644(02)00160-3 (doi:10.1016/S1049-9644(02)00160-3) [DOI] [Google Scholar]
- 52.Naranjo S. E. 2005. Long-term assessment of the effects of transgenic Bt cotton on the abundance of nontarget arthropod natural enemies. Environ. Entomol. 34, 1193–1210 10.1603/0046-225X(2005)034[1193:LAOTEO]2.0.CO;2 (doi:10.1603/0046-225X(2005)034[1193:LAOTEO]2.0.CO;2) [DOI] [Google Scholar]
- 53.Walker G. P., Cameron P. J., MacDonald F. M., Madhusudhan V. V., Wallace A. R. 2007. Impacts of Bacillus thuringiensis toxins on parasitoids (Hymenoptera: Braconidae) of Spodoptera litura and Helicoverpa armigera (Lepidoptera: Noctuidae). Biol. Control 40, 142–151 10.1016/j.biocontrol.2006.09.008 (doi:10.1016/j.biocontrol.2006.09.008) [DOI] [Google Scholar]
- 54.Schuler T. H., Denholm I., Clark S. J., Stewart C. N., Poppy G. M. 2004. Effects of Bt plants on the development and survival of the parasitoid Cotesia plutellae (Hymenoptera: Braconidae) in susceptible and Bt-resistant larvae of the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). J. Insect Physiol. 50, 435–443 10.1016/j.jinsphys.2004.03.001 (doi:10.1016/j.jinsphys.2004.03.001) [DOI] [PubMed] [Google Scholar]
- 55.Chen M., Zhao J. Z., Collins H. L., Earle E. D., Cao J., Shelton A. M. 2008. A critical assessment of the effects of Bt transgenic plants on parasitoids. PLoS ONE 3, e2284. 10.1371/journal.pone.0002284 (doi:10.1371/journal.pone.0002284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barraclough E. I., Burgess E. P. J., Philip B. A., Wohlers M. W., Malone L. A. 2009. Tritrophic impacts of Bt-expressing transgenic pine on the parasitoid Meteorus pulchricornis (Hymenoptera: Braconidae) via its host Pseudocoremia suavis (Lepidoptera: Geometridae). Biol. Control 49, 192–199 10.1016/j.biocontrol.2008.12.013 (doi:10.1016/j.biocontrol.2008.12.013) [DOI] [Google Scholar]
- 57.Marchetti E., Alberghini S., Battisti A., Squartini A., Baronio P., Dindo M. L. 2009. Effects of conventional and transgenic Bacillus thuringiensis galleriae toxin on Exorista larvarum (Diptera: Tachinidae), a parasitoid of forest defoliating Lepidoptera. Biocontrol Sci. Technol. 19, 463–473 10.1080/09583150902807535 (doi:10.1080/09583150902807535) [DOI] [Google Scholar]
- 58.Johnson M. T., Gould F. 1992. Interaction of genetically engineered host plant resistance and natural enemies of Heliothis virescens (Lepidoptera: Noctuidae) in tobacco. Environ. Entomol. 21, 586–597 [Google Scholar]
- 59.Bell H. A., Fitches E. C., Marris G. C., Bell J., Edwards J. P., Gatehouse J. A., Gatehouse A. M. R. 2001. Transgenic crop enhances beneficial biocontrol agent performance. Transgenic Res. 10, 35–42 10.1023/A:1008923103515 (doi:10.1023/A:1008923103515) [DOI] [PubMed] [Google Scholar]
- 60.Bell H. A., Fitches E. C., Down R. E., Ford L., Marris G. C., Edwards J. P., Gatehouse J. A., Gatehouse A. M. R. 2001. Effect of dietary cowpea trypsin inhibitor (CpTI) on the growth and development of the tomato moth Lacanobia oleracea (Lepidoptera: Noctuidae) and on the success of the gregarious ectoparasitoid Eulophus pennicornis (Hymenoptera: Eulophidae). Pest Manag. Sci. 57, 57–65 (doi:10.1002/1526-4998(200101)57:1<57::AID-PS273>3.0.CO;2-4) [DOI] [PubMed] [Google Scholar]
- 61.Bell H. A., Kirkbride-Smith A. E., Marris G. C., Edwards J. P., Gatehouse A. M. R. 2004. Oral toxicity and impact on fecundity of three insecticidal proteins on the gregarious ectoparasitoid Eulophus pennicornis (Hymenoptera: Eulophidae). Agric. For. Entomol. 6, 215–222 10.1111/j.1461-9555.2004.00225.x (doi:10.1111/j.1461-9555.2004.00225.x) [DOI] [Google Scholar]
- 62.Geng J. H., Shen Z. R., Song K., Zheng L. 2006. Effect of pollen of regular cotton and transgenic Bt plus CpTI cotton on the survival and reproduction of the parasitoid wasp Trichogramma chilonis (Hymenoptera: Trichogrammatidae) in the laboratory. Environ. Entomol. 35, 1661–1668 10.1603/0046-225X(2006)35[1661:EOPORC]2.0.CO;2 (doi:10.1603/0046-225X(2006)35[1661:EOPORC]2.0.CO;2) [DOI] [Google Scholar]
- 63.Azzouz H., Cherqui A., Campan E. D. M., Rahbe Y., Duport G., Jouanin L., Kaiser L., Giordanengo P. 2005. Effects of plant protease inhibitors, oryzacystatin I and soybean Bowman-Birk inhibitor, on the aphid Macrosiphum euphorbiae (Homoptera, Aphididae) and its parasitoid Aphelinus abdominalis (Hymenoptera, Aphelinidae). J. Insect Physiol. 51, 75–86 10.1016/j.jinsphys.2004.11.010 (doi:10.1016/j.jinsphys.2004.11.010) [DOI] [PubMed] [Google Scholar]
- 64.Ashouri A., Michaud D., Cloutier C. 2001. Recombinant and classically selected factors of potato plant resistance to the Colorado potato beetle, Leptinotarsa decemlineata, variously affect the potato aphid parasitoid Aphidius nigripes. Biocontrol 46, 401–418 10.1023/A:1014123712776 (doi:10.1023/A:1014123712776) [DOI] [Google Scholar]
- 65.Schuler T. H., Denholm I., Jouanin L., Clark S. J., Clark A. J., Poppy G. M. 2001. Population-scale laboratory studies of the effect of transgenic plants on nontarget insects. Mol. Ecol. 10, 1845–1853 10.1046/j.0962-10183.2001.01309.x (doi:10.1046/j.0962-10183.2001.01309.x) [DOI] [PubMed] [Google Scholar]
- 66.Cowgill S. E., Danks C., Atkinson H. J. 2004. Multitrophic interactions involving genetically modified potatoes, nontarget aphids, natural enemies and hyperparasitoids. Mol. Ecol. 13, 639–647 10.1046/j.1365-294X.2004.02078.x (doi:10.1046/j.1365-294X.2004.02078.x) [DOI] [PubMed] [Google Scholar]
- 67.Wakefield M. E., Bell H. A., Gatehouse A. M. R. 2010. Longevity and fecundity of Eulophus pennicornis, an ectoparasitoid of the tomato moth Lacanobia oleracea, is affected by nutritional state and diet quality. Agric. For. Entomol. 12, 19–27 10.1111/j.1461.9563.2009.00441.x (doi:10.1111/j.1461.9563.2009.00441.x) [DOI] [Google Scholar]
- 68.Couty A., Clark S. J., Poppy G. M. 2001. Are fecundity and longevity of female Aphelinus abdominalis affected by development in GNA-dosed Macrosiphum euphorbiae? Physiol. Entomol. 26, 287–293 10.1046/j.0307-6962.2001.00248.x (doi:10.1046/j.0307-6962.2001.00248.x) [DOI] [Google Scholar]
- 69.Couty A., de la Vina G., Clark S. J., Kaiser L., Pham-Delegue M. H., Poppy G. M. 2001. Direct and indirect sublethal effects of Galanthus nivalis agglutinin (GNA) on the development of a potato-aphid parasitoid, Aphelinus abdominalis (Hymenoptera: Aphelinidae). J. Insect Physiol. 47, 553–561 10.1016/S0022-1910(00)00148-7 (doi:10.1016/S0022-1910(00)00148-7) [DOI] [PubMed] [Google Scholar]
- 70.Couty A., Poppy G. M. 2001. Does host-feeding on GNA-intoxicated aphids by Aphelinus abdominalis affect their longevity and/or fecundity? Entomol. Exp. Appl. 100, 331–337 10.1046/j.1570-7458.2001.00880.x (doi:10.1046/j.1570-7458.2001.00880.x) [DOI] [Google Scholar]
- 71.Romeis J., Babendreier D., Wackers F. L. 2003. Consumption of snowdrop lectin (Galanthus nivalis agglutinin) causes direct effects on adult parasitic wasps. Oecologia 134, 528–536 10.1007/s00442-002-1144-9 (doi:10.1007/s00442-002-1144-9) [DOI] [PubMed] [Google Scholar]
- 72.Hogervorst P. A. M., Wackers F. L., Woodring J., Romeis J. 2009. Snowdrop lectin (Galanthus nivalis agglutinin) in aphid honeydew negatively affects survival of a honeydew-consuming parasitoid. Agric. For. Entomol. 11, 161–173 10.1111/j.1461-9563.2008.00412.x (doi:10.1111/j.1461-9563.2008.00412.x) [DOI] [Google Scholar]
- 73.Setamou M., Bernal J. S., Legaspi J. C., Mirkov T. E. 2002. Effects of snowdrop lectin (Galanthus nivalis agglutinin) expressed in transgenic sugarcane on fitness of Cotesia flavipes (Hymenoptera: Braconidae), a parasitoid of the nontarget pest Diatraea saccharalis (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 95, 75–83 10.1603/0013-8746(2002)095[0075:EOSLGN]2.0.CO;2 (doi:10.1603/0013-8746(2002)095[0075:EOSLGN]2.0.CO;2) [DOI] [Google Scholar]
- 74.Setamou M., Bernal J. S., Legaspi J. C., Mirkov T. E., Legaspi B. C. 2002. Evaluation of lectin-expressing transgenic sugarcane against stalkborers (Lepidoptera: Pyralidae): effects on life history parameters. J. Econ. Entomol. 95, 469–477 10.1603/0022-0493-95.2.469 (doi:10.1603/0022-0493-95.2.469) [DOI] [PubMed] [Google Scholar]
- 75.Bell H. A., Fitches E. C., Down R. E., Marris G. C., Edwards J. P., Gatehouse J. A., Gatehouse A. M. R. 1999. The effect of snowdrop lectin (GNA) delivered via artificial diet and transgenic plants on Eulophus pennicornis (Hymenoptera: Eulophidae), a parasitoid of the tomato moth Lacanobia oleracea (Lepidoptera: Noctuidae). J. Insect Physiol. 45, 983–991 10.1016/S0022-1910(99)00077-3 (doi:10.1016/S0022-1910(99)00077-3) [DOI] [PubMed] [Google Scholar]
- 76.Tomov B. W., Bernal J. S., Vinson S. B. 2003. Impacts of transgenic sugarcane expressing GNA lectin on parasitism of Mexican rice borer by Parallorhogas pyralophagus (Marsh) (Hymenoptera: Braconidae). Environ. Entomol. 32, 866–872 10.1603/0046-225X-32.4.866 (doi:10.1603/0046-225X-32.4.866) [DOI] [PubMed] [Google Scholar]
- 77.Wakefield M. E., Bell H. A., Fitches E. C., Edwards J. P., Gatehouse A. M. R. 2006. Effects of Galanthus nivalis agglutinin (GNA) expressed in tomato leaves on larvae of the tomato moth Lacanobia oleracea (Lepidoptera: Noctuidae) and the effect of GNA on the development of the endoparasitoid Meteorus gyrator (Hymenoptera: Braconidae). Bull. Entomol. Res. 96, 43–52 10.1079/BER2005396 (doi:10.1079/BER2005396) [DOI] [PubMed] [Google Scholar]
- 78.Pilcher C. D., Obrycki J. J., Rice M. E., Lewis L. C. 1997. Preimaginal development, survival, and field abundance of insect predators on transgenic Bacillus thuringiensis corn. Environ. Entomol. 26, 446–454 [Google Scholar]
- 79.Bhatti M. A., Duan J., Head G., Jiang C. J., Mckee M. J., Nickson T. E., Pilcher C. L., Pilcher C. D. 2005. Field evaluation of the impact of corn rootworm (Coleoptera: Chrysomelidae)-protected Bt corn on ground-dwelling invertebrates. Environ. Entomol. 34, 1325–1335 10.1603/0046-225X(2005)034[1325:FEOTIO]2.0.CO;2 (doi:10.1603/0046-225X(2005)034[1325:FEOTIO]2.0.CO;2) [DOI] [Google Scholar]
- 80.Dutton A., Klein H., Romeis J., Bigler F. 2002. Uptake of Bt-toxin by herbivores feeding on transgenic maize and consequences for the predator Chrysoperla carnea. Ecol. Entomol. 27, 441–447 10.1046/j.1365-2311.2002.00436.x (doi:10.1046/j.1365-2311.2002.00436.x) [DOI] [Google Scholar]
- 81.Alvarez-Alfageme F., Ferry N., Castanera P., Ortego F., Gatehouse A. M. R. 2008. Prey mediated effects of Bt maize on fitness and digestive physiology of the red spider mite predator Stethorus punctillum Weise (Coleoptera: Coccinellidae). Transgenic Res. 17, 943–954 10.1007/s11248-008-9177-4 (doi:10.1007/s11248-008-9177-4) [DOI] [PubMed] [Google Scholar]
- 82.Ludy C., Lang A. 2006. A 3-year field-scale monitoring of foliage-dwelling spiders (Araneae) in transgenic Bt maize fields and adjacent field margins. Biol. Control 38, 314–324 10.1016/j.biocontrol.2006.05.010 (doi:10.1016/j.biocontrol.2006.05.010) [DOI] [Google Scholar]
- 83.Ludy C., Lang A. 2006. Bt maize pollen exposure and impact on the garden spider, Araneus diadematus. Entomol. Exp. Appl. 118, 145–156 10.1111/j.1570-7458.2006.00375.x (doi:10.1111/j.1570-7458.2006.00375.x) [DOI] [Google Scholar]
- 84.Lundgren J. G., Wiedenmann R. N. 2005. Tritrophic interactions among Bt (CryMb1) corn, aphid prey, and the predator Coleomegilla maculata (Coleoptera: Coccinellidae). Environ. Entomol. 34, 1621–1625 10.1603/0046-225X-34.6.1621 (doi:10.1603/0046-225X-34.6.1621) [DOI] [Google Scholar]
- 85.Dively G. P. 2005. Impact of transgenic VIP3A × Cry1Ab lepidopteran-resistant field corn on the nontarget arthropod community. Environ. Entomol. 34, 1267–1291 10.1603/0046-225X(2005)034[1267:IOTVCL]2.0.CO;2 (doi:10.1603/0046-225X(2005)034[1267:IOTVCL]2.0.CO;2) [DOI] [Google Scholar]
- 86.Guo J. Y., Wan F. H., Dong L., Lovei G. L., Han Z. J. 2008. Tri-trophic interactions between Bt cotton, the herbivore Aphis gossypii Glover (Homoptera: Aphididae), and the predator Chrysopa pallens (Rambur) (Neuroptera: Chrysopidae). Environ. Entomol. 37, 263–270 10.1603/0046-225X(2008)37[263:TIBBCT]2.0.CO;2 (doi:10.1603/0046-225X(2008)37[263:TIBBCT]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 87.Naranjo S. E. 2005. Long-term assessment of the effects of transgenic Bt cotton on the function of the natural enemy community. Environ. Entomol. 34, 1211–1223 10.1603/0046-225X(2005)034[1211:LAOTEO]2.0.CO;2 (doi:10.1603/0046-225X(2005)034[1211:LAOTEO]2.0.CO;2) [DOI] [Google Scholar]
- 88.Hagerty A. M., Kilpatrick A. L., Turnipseed S. G., Sullivan M. J., Bridges W. C. 2005. Predaceous arthropods and lepidopteran pests on conventional, Bollgard, and Bollgard II cotton under untreated and disrupted conditions. Environ. Entomol. 34, 105–114 10.1603/0046-225X-34.1.105 (doi:10.1603/0046-225X-34.1.105) [DOI] [Google Scholar]
- 89.Head G., Moar M., Eubanks M., Freeman B., Ruberson J., Hagerty A., Turnipseed S. 2005. A multiyear, large-scale comparison of arthropod populations on commercially managed Bt and non-Bt cotton fields. Environ. Entomol. 34, 1257–1266 10.1603/0046-225X(2005)034[1257:AMLCOA]2.0.CO;2 (doi:10.1603/0046-225X(2005)034[1257:AMLCOA]2.0.CO;2) [DOI] [Google Scholar]
- 90.Torres J. B., Ruberson J. R. 2006. Interactions of Bt-cotton and the omnivorous big-eyed bug Geocoris punctipes (Say), a key predator in cotton fields. Biol. Control 39, 47–57 10.1016/j.biocontrol.2006.03.006 (doi:10.1016/j.biocontrol.2006.03.006) [DOI] [Google Scholar]
- 91.Torres J. B., Ruberson J. R. 2008. Interactions of Bacillus thuringiensis Cry1Ac toxin in genetically engineered cotton with predatory heteropterans. Transgenic Res. 17, 345–354 10.1007/s11248-007-9109-8 (doi:10.1007/s11248-007-9109-8) [DOI] [PubMed] [Google Scholar]
- 92.Sisterson M., Biggs R. W., Manhardt N. M., Carriere Y., Dennehy T. J., Tabashnik B. E. 2007. Effects of transgenic Bt cotton on insecticide use and abundance of two generalist predators. Entomol. Exp. Appl. 124, 305–311 10.1111/j.1570-7458.2007.00584.x (doi:10.1111/j.1570-7458.2007.00584.x) [DOI] [Google Scholar]
- 93.Reed G. L., Jensen A. S., Riebe J., Head G., Duan J. J. 2001. Transgenic Bt potato and conventional insecticides for Colorado potato beetle management: comparative efficacy and non-target impacts. Entomol. Exp. Appl. 100, 89–100 10.1046/j.1570-7458.2001.00851.x (doi:10.1046/j.1570-7458.2001.00851.x) [DOI] [Google Scholar]
- 94.Duan J. J., Head G., Jensen A., Reed G. 2004. Effects of transgenic Bacillus thuringiensis potato and conventional insecticides for Colorado potato beetle (Coleoptera: Chrysomelidae) management on the abundance of ground-dwelling arthropods in Oregon potato ecosystems. Environ. Entomol. 33, 275–281 10.1603/0046-225X-33.2.275 (doi:10.1603/0046-225X-33.2.275) [DOI] [Google Scholar]
- 95.Dogan E. B., Berry R. E., Reed G. L., Rossignol P. A. 1996. Biological parameters of convergent lady beetle (Coleoptera: Coccinellidae) feeding on aphids (Homoptera: Aphididae) on transgenic potato. J. Econ. Entomol. 89, 1105–1108 [Google Scholar]
- 96.Armer C. A., Berry R. E., Kogan M. 2000. Longevity of phytophagous heteropteran predators feeding on transgenic Bt-potato plants. Entomol. Exp. Appl. 95, 329–333 10.1046/j.1570-7458.2000.00672.x (doi:10.1046/j.1570-7458.2000.00672.x) [DOI] [Google Scholar]
- 97.Ferry N., Mulligan E. A., Majerus M. E. N., Gatehouse A. M. R. 2007. Bitrophic and tritrophic effects of Bt Cry3A transgenic potato on beneficial, non-target, beetles. Transgenic Res. 16, 795–812 10.1007/s11248-007-9088-9 (doi:10.1007/s11248-007-9088-9) [DOI] [PubMed] [Google Scholar]
- 98.Bell H. A., Down R. E., Fitches E. C., Edwards J. P., Gatehouse A. M. R. 2003. Impact of genetically modified potato expressing plant-derived insect resistance genes on the predatory bug Podisus maculiventris (Heteroptera: Pentatomidae). Biocontrol Sci. Technol. 13, 729–741 10.1080/09583150310001606543 (doi:10.1080/09583150310001606543) [DOI] [Google Scholar]
- 99.Graham J., Gordon S. C., Smith K., McNicol R. J., McNicol J. W. 2002. The effect of the cowpea trypspin inhibitor in strawberry on damage by vine weevil under field conditions. J. Hortic. Sci. Biotechnol. 77, 33–40 [Google Scholar]
- 100.Alvarez-Alfageme F., Martinez M., Pascual-Ruiz S., Castanera P., Diaz I., Ortego F. 2007. Effects of potato plants expressing a barley cystatin on the predatory bug Podisus maculiventris via herbivorous prey feeding on the plant. Transgenic Res. 16, 1–13 10.1007/s11248-006-9022-6 (doi:10.1007/s11248-006-9022-6) [DOI] [PubMed] [Google Scholar]
- 101.Ferry N., Jouanin L., Ceci L. R., Mulligan E. A., Emami K., Gatehouse J. A., Gatehouse A. M. R. 2005. Impact of oilseed rape expressing the insecticidal serine protease inhibitor, mustard trypsin inhibitor-2 on the beneficial predator Pterostichus madidus. Mol. Ecol. 14, 337–349 10.1111/j.1365-294X.2004.02381.x (doi:10.1111/j.1365-294X.2004.02381.x) [DOI] [PubMed] [Google Scholar]
- 102.Jorgensen H. B., Lovei G. L. 1999. Tri-trophic effect on predator feeding: consumption by the carabid Harpalus affinis of Heliothis armigera caterpillars fed on proteinase inhibitor-containing diet. Entomol. Exp. Appl. 93, 113–116 10.1046/j.1570-7458.1999.00568.x (doi:10.1046/j.1570-7458.1999.00568.x) [DOI] [Google Scholar]
- 103.Burgess E. P. J., Malone L. A., Christeller J. T., Lester M. T., Murray C., Philip B. A., Phung M. M., Tregidga E. L. 2002. Avidin expressed in transgenic tobacco leaves confers resistance to two noctuid pests, Helicoverpa armigera and Spodoptera litura. Transgenic Res. 11, 185–198 10.1023/A:1015297302990 (doi:10.1023/A:1015297302990) [DOI] [PubMed] [Google Scholar]
- 104.Ferry N., Raemaekers R. J. M., Majerus M. E. N., Jouanin L., Port G., Gatehouse J. A., Gatehouse A. M. R. 2003. Impact of oilseed rape expressing the insecticidal cysteine protease inhibitor oryzacystatin on the beneficial predator Harmonia axyridis (multicolored Asian ladybeetle). Mol. Ecol. 12, 493–504 10.1046/j.1365-294X.2003.01736.x (doi:10.1046/j.1365-294X.2003.01736.x) [DOI] [PubMed] [Google Scholar]
- 105.Bouchard E., Cloutier C., Michaud D. 2003. Oryzacystatin I expressed in transgenic potato induces digestive compensation in an insect natural predator via its herbivorous prey feeding on the plant. Mol. Ecol. 12, 2439–2446 10.1046/j.1365-294X.2003.01919.x (doi:10.1046/j.1365-294X.2003.01919.x) [DOI] [PubMed] [Google Scholar]
- 106.Lawo N. C., Romeis J. 2008. Assessing the utilization of a carbohydrate food source and the impact of insecticidal proteins on larvae of the green lacewing, Chrysoperla carnea. Biol. Control 44, 389–398 10.1016/j.biocontrol.2007.12.002 (doi:10.1016/j.biocontrol.2007.12.002) [DOI] [Google Scholar]
- 107.Marvier M., McCreedy C., Regetz J., Kareiva P. 2007. A meta-analysis of effects of Bt cotton and maize on nontarget invertebrates. Science 316, 1475–1477 10.1126/science.1139208 (doi:10.1126/science.1139208) [DOI] [PubMed] [Google Scholar]
- 108.Abe K., Kondo H., Watanabe H., Emori Y., Arai S. 1991. Oryzacystatins as the 1st well-defined cystatins of plant-origin and their target proteinases in rice seeds. Biomed. Biochim. Acta 50, 637–641 [PubMed] [Google Scholar]
- 109.Kuroda M., Ishimoto M., Suzuki K., Kondo H., Abe K., Kitamura K., Arai S. 1996. Oryzacystatins exhibit growth-inhibitory and lethal effects on different species of bean insect pests, Callosobruchus chinensis (Coleoptera) and Riptortus clavatus (Hemiptera). Biosci. Biotechnol. Biochem. 60, 209–212 10.1271/bbb.60.209 (doi:10.1271/bbb.60.209) [DOI] [PubMed] [Google Scholar]
- 110.Leple J. C., Bonade Bottino M., Augustin S., Pilate G., Letan V. D., Delplanque A., Cornu D., Jouanin L. 1995. Toxicity to Chrysomela tremulae (Coleoptera, Chrysomelidae) of transgenic poplars expressing a cysteine proteinase-inhibitor. Mol. Breed. 1, 319–328 10.1007/BF01248409 (doi:10.1007/BF01248409) [DOI] [Google Scholar]
- 111.Ribeiro A. P. O., Pereira E. J. G., Galvan T. L., Picanco M. C., Picoli E. A. T., da Silva D. J. H., Fari M. G., Otoni W. C. 2006. Effect of eggplant transformed with oryzacystatin gene on Myzus persicae and Macrosiphum euphorbiae. J. Appl. Entomol. 130, 84–90 10.1111/j.1439-0418.2005.01021.x (doi:10.1111/j.1439-0418.2005.01021.x) [DOI] [Google Scholar]
- 112.Samac D. A., Smigocki A. C. 2003. Expression of oryzacystatin I and II in alfalfa increases resistance to the root-lesion nematode. Phytopathology 93, 799–804 10.1094/PHYTO.2003.93.7.799 (doi:10.1094/PHYTO.2003.93.7.799) [DOI] [PubMed] [Google Scholar]
- 113.Bown D. P., Wilkinson H. S., Gatehouse J. A. 1997. Differentially regulated inhibitor-sensitive and insensitive protease genes from the phytophagous insect pest, Helicoverpa armigera, are members of complex multigene families. Insect Biochem. Mol. Biol. 27, 625–638 10.1016/S0965-1748(97)00043-X (doi:10.1016/S0965-1748(97)00043-X) [DOI] [PubMed] [Google Scholar]
- 114.de Leo F., Bonade-Bottino M., Ceci L. R., Gallerani R., Jouanin L. 2001. Effects of a mustard trypsin inhibitor expressed in different plants on three lepidopteran pests. Insect Biochem. Mol. Biol. 31, 593–602 10.1016/S0965-1748(00)00164-8 (doi:10.1016/S0965-1748(00)00164-8) [DOI] [PubMed] [Google Scholar]
- 115.Zhang H. Y., Liu X. Z., Wei L., Zhou L. Y., Yang Y. M. 2007. Transgenic tobacco plants containing Bt and GNA genes. Biol. Plant. 51, 746–748 10.1007/s10535-007-0152-3 (doi:10.1007/s10535-007-0152-3) [DOI] [Google Scholar]
- 116.Fitches E., Gatehouse A. M. R., Gatehouse J. A. 1997. Effects of snowdrop lectin (GNA) delivered via artificial diet and transgenic plants on the development of tomato moth (Lacanobia oleracea) larvae in laboratory and glasshouse trials. J. Insect Physiol. 43, 727–739 10.1016/S0022-1910(97)00042-5 (doi:10.1016/S0022-1910(97)00042-5) [DOI] [PubMed] [Google Scholar]
- 117.McCafferty H. R. K., Moore P. H., Zhu Y. J. 2008. Papaya transformed with the Galanthus nivalis GNA gene produces a biologically active lectin with spider mite control activity. Plant Sci. 175, 385–393 10.1016/j.plantsci.2008.05.007 (doi:10.1016/j.plantsci.2008.05.007) [DOI] [Google Scholar]
- 118.Stoger E., Williams S., Christou P., Down R. E., Gatehouse J. A. 1999. Expression of the insecticidal lectin from snowdrop (Galanthus nivalis agglutinin; GNA) in transgenic wheat plants: effects on predation by the grain aphid Sitobion avenae. Mol. Breed. 5, 65–73 10.1023/A:1009616413886 (doi:10.1023/A:1009616413886) [DOI] [Google Scholar]
- 119.Wang Z. Y., Zhang K. W., Sun X. F., Tang K. X., Zhang J. R. 2005. Enhancement of resistance to aphids by introducing the snowdrop lectin gene GNA into maize plants. J. Biosci. 30, 627–638 10.1007/BF02703563 (doi:10.1007/BF02703563) [DOI] [PubMed] [Google Scholar]
- 120.Lovei G. L., Andow D. A., Arpaia S. 2009. Transgenic insecticidal crops and natural enemies: a detailed review of laboratory studies. Environ. Entomol. 38, 293–306 10.1603/022.038.0201 (doi:10.1603/022.038.0201) [DOI] [PubMed] [Google Scholar]
- 121.Shelton A. M., et al. 2009. Appropriate analytical methods are necessary to assess non-target effects of insecticidal proteins in GM crops through meta-analysis. Environ. Entomol. 38, 1533–1538 10.1603/022.038.0603 (doi:10.1603/022.038.0603) [DOI] [PubMed] [Google Scholar]