Abstract
Exercise has been linked to a reduced cancer risk in animal models. However, the underlying mechanisms are unclear. This study assessed the impact of exercise with dietary consideration on the phospholipid profile in TPA-induced mouse skin tissues. CD-1 mice were randomly assigned to one of the three groups: ad libitum-fed sedentary control, ad libitum-fed treadmill exercise at 13.4 m/min for 60 min/d, 5 d/wk (Ex+AL), and treadmill exercised but pair-fed with the same amount as the control (Ex+PF). After 14 wks, Ex+PF but not Ex+AL mice demonstrated ~25% decrease in both body weight and body fat when compared to the controls. Of the total 338 phospholipids determined by electrospray ionization tandem mass spectrometry, 57 were significantly changed, and 25 species could distinguish effects of exercise and diet treatments in a stepwise discriminant analysis. A 36–75% decrease of phosphatidylinositol (PI) levels in Ex+PF mice occurred along with a significant reduction of PI3K in TPA-induced skin epidermis, as measured by both western blotting and immunohistochemistry. In addition, near 2-fold increase of the long chain polyunsaturated fatty acids, docosahexaenoic and docosapentaenoic acids, in phosphatidylcholines, phosphatidylethanolamines, and lysophosphatidylethanolamines wasobserved in the Ex+PF group. Microarray analysis indicated that the expression of fatty acid elongase-1 increased. Taken together, these data indicate that exercise with controlled dietary intake but not exercise alone significantly reduced body weight and body fat as well as modified the phospholipid profile, which may contribute to cancer prevention by reducing TPA-induced PI3K and by enhancing ω-3 fatty acid elongation.
Keywords: Weight control, exercise, phospholipids, skin tissues, mice
Introduction
Overweight or obesity, which may be due to a life style of over consumption or less expenditure of energy, has become a major public health problem associated with increased risk of many chronic diseases including cancer (1). Physical activity as a major factor in energy expenditure has been suggested for cancer prevention by both human and animal studies. A large cohort study including 42,672 US postmenopausal women recently reported that light and moderate physical activities were associated with a reduced risk of endometrial cancer (2). Another large cohort study including 79,771 Japanese men and women found that a decreased cancer risk was related to increased daily physical activity (3). A population-based study from the 2005 Canadian Community Health Survey showed physical activity was associated with a high survival rate in the skin cancer patients (4).
The cancer-inhibitory activity of weight loss by calorie restriction and/or exercise has been well documented in many animal models, including the skin carcinogenic model (5). A relationship between reduced dietary energy intake and decreased tumor rates has been established in rodents (6). The molecular targets in response to energy balance for prevention of skin carcinogenesis has also been suggested (7). Michna et al. demonstrated that voluntary wheel running exercise inhibited UVB-induced skin tumorigenesis in mice (8). The exercise-induced skin cancer inhibition was further associated with enhanced apoptosis in the epidermis via a p53-independent mechanism (9). Most studies from animal models suggest physical activity inhibits carcinogenesis; however, the exercise-induced inhibition appears less consistent or less potent than calorie restriction (10). Research by the Hursting group suggested it is negative energy balance rather than exercise alone that inhibited the development of intestinal polyps in APCMin mice (11). By means of dietary regimens in both of high fat and calorie restricted diet, the groups of DiGiovanni and Hursting recently concluded that the dietary energy balance might modulate IGF-1 and IGF-1 receptor signaling (12). This reduction is due to both reduced total levels of IGF-1 and a reduction in bioavailability due to an increase in levels of IGF-1 binding proteins. Similarly, our previous studies found that exercise with pair feeding, but not exercise alone, was effective in controlling body weight and selectively abrogating the tumor promoter-induced PI3K-Akt pathway in the skin epidermis, resulting in enhanced apoptosis and reduced proliferation (13).
Many studies have investigated the molecular mechanism of cancer prevention by physical activity. However, the underlying mechanisms of this phenomenon are not clear. Physical activity has been proposed to prevent cancer through deletion of reactive oxygen species and an increase in antioxidant status (14), reduction of sex hormone levels (15), reduction of the metabolic hormones insulin, IGF-1 and/or leptin (16–18), and improvement in immune function (19). Hormone-related signaling alterations, especially of IGF-1, seem to be an important factor in weight-control for cancer prevention. Phosphatidylinostiol 3-kinase (PI3K) is one of the targets activated by IGF-1. Activated PI3K usually phosphorylates phosphatidylinositols (PIs)1 in the cell membrane to produce PI phosphates or phosphotinositides. PI(3, 4, 5)P3, a major product of PI3K, is able to bind and activate Akt, therefore activating many downstream signaling proteins that regulate cell survival and cell cycle progress. Elevated levels of PI(3, 4, 5)P3 and upregulation of Akt are found to be oncogenic and promote the transition to malignancy (20–22). Therefore, in addition to being membrane structural building blocks, PIs and their derivatives phosphoinositides, have been found to play an important role in cellular signaling and intracellular trafficking as well as in the cancer disease process (23).
Among the phospholipids, some lysophosphatidylcholines (LysoPC) may activate phospholipase C, leading to the production of diacyglycerols and inositol triphosphate, activation of protein kinase C, release of and intracellular Ca2+, and activation of MAP kinase signaling (24). In addition to the signaling, processes involving specific lipid head group classes, alterations in the membrane lipid fatty acid composition may also be involved in cancer progression. For example, breast cancer patients were found to have a higher risk of early occurrence of visceral metastasis when they had a lower level of polyunsaturated fatty acids in phosphatidylethanolamines (PE) (25). Decreased levels of stearic acid in phosphatidylcholine (PC) were found in breast cancer patients with metastasis compared to those who remained metastasis-free (26). Recent studies by the Pougnoux group showed that a lipid profile, rather than a single fatty acid, could be important; they showed that decreased linoleic acid, increased cis-monounsaturated fatty acids, and a low ω6/ω3 fatty acid ratio, was associated with lower risk of breast cancer (27). Very long chain ω-3 fatty acids, such as docosahexaenoic acid (DHA), are well known to have a preventive effect on cardiovascular disease and cancer and may be involved in alterations of membrane structure and function, eicosanoid metabolism, gene expression, and inhibition of lipid peroxidation (28).
Physical activity has been found to affect membrane phospholipids. For example, it is reported that particular types of exercise increase sphinganine and sphingosine in rat skeletal muscle (29) and reduce total content of ceramide in rat heart muscle (30). Regular exercise was also found to significantly increase oleic acid 18:1 (ω-9) and DHA 22:6 (ω-3) in human muscle (31). However, previous lipid composition studies related to cancer were usually focused on total cholesterol, lipoproteins, and triglyceride (32–33). The overview of membrane phospholipid profile and the response to exercise-induced weight control has not been studied yet.
With the development of recent lipid analysis, phospholipid compositional profiles can be determined by electrospray ionization tandem mass spectrometry (ESI/MS-MS). The technique is highly sensitive, accurate, and reproducible (34–36).
In this study, we measured 338 membrane phospholipid species in the skin tissues of mice whose body weights were controlled via moderate treadmill exercise. The impact of exercise-induced weight loss upon the phospholipid profile was further evaluated for potential cancer prevention mechanisms. The significant changes in certain species of phospholipids, especially PI3K-related PIs and ω-3 fatty acid-containing PCs, PEs and lysoPE, might suggest novel cancer preventive mechanisms by exercise-induced weight control.
Materials and Methods
Animals and animal treatments
Female CD-1 mice (Charles River Lab, Wilmington, MA) at eight week old were housed individually at 24 ± 1 °C and 80% relative humidity with 12-hr light/12-hr dark cycle. They were randomly assigned into one of three groups: ad libitum feeding sedentary control, exercise and ad libitum feeding (Ex+AL), and exercise but pair feeding (Ex+PF). Ad libitum feeding groups were allowed to freely access the basal diet (AIN-93), while the pair-fed group was fed daily the same amount as the sedentary control.
A zero-grade adjustable-speed rodent treadmill (Boston Gears, Boston, MA) was used to exercise the mice. After a two-week training period, mice performed treadmill exercise at 13.4 m/min for 60 min/d, 5 d/week for 14 weeks. This exercise was recognized as moderate, based upon the intensity at 65–70% of the maximal oxygen uptake calculated by the predicting regression equation of Fernando et al (37). To take into account the biological clocks of nocturnal rodents, the light cycle was adjusted for mice to exercise at nigh. The mice were fed until the last day, but exercise was stopped 24 hrs after the last bout to measure the effect of exercise training rather than acute exercise. At the end of experiment, the dorsal skin of the mice was shaved and topically treated once with TPA at 3.2 nmol in 200 µL of acetone. Mice were sacrificed two hrs after TPA treatment. The dorsal skin samples were snap-frozen in liquid nitrogen and kept at −70 °C until further analyses.
Body fat analysis
In the last week, the body composition of the mice was determined by a dual-energy X-ray absorptiometer (DXA) using the small animal software (v5.6, Prodigy GE, Lunar-General Electric, Milwaukee, WI).
Phospholipid measurments and profiling
Each skin sample was ground with liquid nitrogen. After 2 mL of solvent (chloroform: methanol 1:2 + 0.01% butylated hydroxytoluene) were mixed with 1 g of tissue, 1 mL of chloroform and 1 mL of water were added; the mixture was centrifuged at 1,000 rpm for 15 min, and the lower layer was collected. Then, 1 mL of chloroform was added to the tissue, the mixture was centrifuged and the lower layer was removed and combined with the previously removed lower layer. The combined extract was analyzed for phospholipids using an automated electrospray ionization-tandem mass spectrometry approach. Data acquisition and analysis for acyl group identification were carried out as described previously (35–36). Twelve phospholipid classes or subclasses, including phosphatidic acid (PA), PI, PC, lysoPC, alk(en)yl/acyl phosphocholine (ePC), PE, lysoPE, alk(en)yl/acyl phosphoethanolamine (ePE), phosphatidylserine (PS), alk(en)yl/acyl phosphoserine (ePS), sphingomyelin (SM), and ceramide phosphoethanolamine (PE-cer) were determined. Identification of phospholipid molecular species was based on total mass/charge and the presence of a fragment of mass/charge consistent with the head group class. For 40:5-PC or PE and 40:6-PC or PE acyl composition analysis, four samples chosen randomly from each group were further analyzed. Acyl ions of PE species were identified after collision induced dissociation of the [M−H]− ions, and acyl ions of PC species were identified following collision induced dissociation of the [M + OAc]− ions.
PI3K expression detected by western blotting
As described in our previous publication (13), skin tissue was homogenized in Mg2+ lysis/wash buffer and the lysate was collected after centrifugation at 12,000 g at 4 °C for 15 min. Protein concentration was measured by the Bio-Rad protein assay (Bio-Rad, Hercules, CA). After running on 12% SDS-PAGE gel, the proteins were transferred to a nitrocellulose membrane. PI3K at 110 kDa and the internal loading control β-actin at 43 kDa were bound to their monoclonal antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA). After incubation with HRP-conjugated secondary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA), the blot was visualized by the FluorChem™ 8800 Advanced Imaging System (Alpha Innotech, San Leandro, CA). The relative density of the target PI3K band was normalized to the loading control β-actin and then expressed as a percentage of the controls.
PI3K expression in situ detected by immunohistochemistry
As described in our previous publication (13), the frozen dorsal skin tissues of mice 2-hr after TPA treatment were fixed in −70 °C absolute ethanol and rinsed with PBS before adding 10% formaldehyde. The skin tissues were sectioned and the slides were exposed to steam in the target retrieval solution (Dakocytomation, Carpinteria, CA). The monoclonal antibody against mouse PI3K (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a primary antibody, and the secondary antibody was BioGenex QP900 SS multilink HRP kit (BioGenex, San Ramon, CA). Slides were counterstained with Gills hematoxylin followed by dehydration in alcohol and xylene. Staining was developed with diaminobenzidine chromogen and the density of the stain for each section was scored by a pathologist. Ten to 15 sections for each group were blindly graded using computer standards. The standards of staining intensity were established at 400× by grading up to 40 cells in 5 unit increments from 3–5 mice per group. Data were statistically analyzed and group scores with p ≤ 0.05 were considered significantly different.
Microarray and data analyses
As described in our previous publications (13, 38), the skin samples from 4 mice in each group were analyzed by a GeneChip Mouse Genome 430 2.0 Array (Affymetrix, Santa Clara, CA). The intensities of probe sets were quantified by GeneChip operating software 1.0 (GCOS 1.0; Affymetrix). One–way ANOVA and False Discovery Rate (FDR, Benjamini, p < 0.1) were applied to identify the gene expression difference between the treatment and control group. Then the data were filtered using 1.5 fold change as a cutoff. The intensities of various probe sets for each gene expression of six fatty acid elongases (Elovl) were averaged and reported in this study.
Statistical analysis
Phospholipid data outliers were detected using a Q test and statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC). Results of phospholipid profiling between mice to which acetone-vehicle and to which TPA was applied were found not significantly different, and the data for these groups were thus pooled. Phospholipid levels among the three treatment groups were compared using a one-way ANOVA and an F-test for significance, followed by pairwise comparisons by LSD method. To determine which variables that may discriminate the three treatment groups and generate linear combinations, allowing the classification of unknown samples, an automatic backward stepwise discriminant analysis was performed using 57 phospholipid species that were significantly different among three treatment groups. Thus these discriminating variables could be considered putative biomarkers for the effects of diet and exercise on skin polar lipidome.
Results
Body weight and body fat change
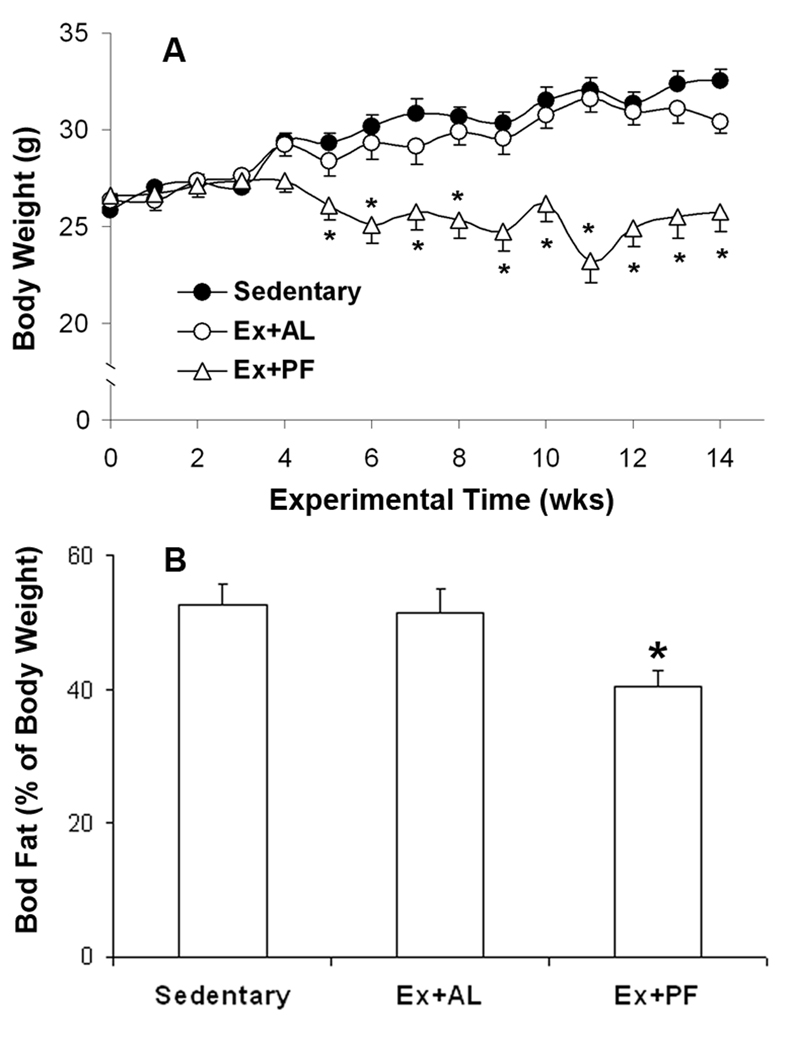
Body weights of mice during the total 14 wks of experimentation are shown in Fig. 1A. Adult CD-1 mice in the sedentary control group gained weight gradually. No significant difference was found between Ex+AL mice and sedentary controls. However, the body weight of Ex+PF mice was significantly lower when compared with either sedentary or Ex+AL mice, beginning in the fifth week of the experiment. As shown in Fig. 1B, when compared to the sedentary controls, the percentage of body fat was significantly lower in Ex+PF mice down to ~25% of the control but not in Ex+AL mice. The average food intake for sedentary control and Ex+AL group are 3.43±0.28 g/day and 3.67±0.18 g/day, respectively. The food intake for Ex+PF group is same as the control. Although no significant difference of food intake is observed between sedentary control and Ex+AL, exercise alone did not prevent weight gain, as compared to sedentary controls, which may be due to a slight increase in food consumption.
Figure 1. Effects of exercise with or without controlled dietary intake on body weight and body fat.
CD-1 mice at 8 weeks old were fed ad libitum or pair-fed the same amount as the sedentary control. They performed treadmill exercise at 13.4 m/min, 60 min/day, 5 days/week, for 14 weeks. A: Body weight (A) and body fat (B) were significantly lower in exercised and pair-fed mice in comparison with either sedentary control mice or exercised mice with ad libitum-feeding. Results are means ± SE, n = 10–15, *p ≤ 0.05 vs. sedentary control or exercised but ad libitum-fed mice.
Impact of exercise with or without controlled diet intake on phospholipid profile
A total of 338 phospholipid species were measured and 57 of them were found to be significantly different among the treatment groups.
Among the phospholipids, PC was the most abundant head group class in mouse skin, and it represented 45.5% of the total phospholipids analyzed (Table 1). Compared to other classes, PC has the largest amount of shorter chain species, with species with 28, 30 and 32 acyl carbons in the two chains making up 9.1% of diacyl PCs, while species with 38 or more acyl carbons made up about 26.7% of diacyl PC. Some PCs with short chain fatty acids, including 30:0-PC, 32:0-PC, and 32:1-PC, were significantly lower in Ex+PF mice than in either group of ad libitum-fed or control mice. However, 40:4-PC, 40:5-PC and 40:6-PC were significantly higher in Ex+PF mice up to 2-fold when compared to sedentary control as well as Ex+AL mice.
Table 1.
Effects of exercise with or without controlled dietary intake on the profiles of phospholipids in mouse skin tissues1
| Phospholipid Group |
Species2 | M/Z | Mol% of Total Polar Lipids3 | ||
|---|---|---|---|---|---|
| Sedentary | Ex+AL | Ex+PF | |||
| Phospho-coline | 30:0 | 706.5 | 0.30±0.03a | 0.28±0.03a | 0.19±0.03b |
| 32:0 | 734.6 | 2.21±0.34a | 2.10±0.27a | 1.02±0.13b | |
| 32:1 | 732.6 | 1.49±0.10a | 1.18±0.12a | 0.91±0.08b | |
| 40:4 | 838.6 | 0.16±0.01b | 0.20±0.01ab | 0.21±0.02a | |
| 40:5 | 836.6 | 0.39±0.00b | 0.43±0.09b | 0.81±0.13a | |
| 40:6 | 834.6 | 0.64±0.14ab | 0.51±0.10b | 1.03±0.17a | |
| Phosphatidylinositol | 36:1 | 863.6 | 0.02±0.01ab | 0.07±0.04a | 0.00±0.00b |
| 36:2 | 861.5 | 0.04±0.02a | 0.02±0.02a | 0.01±0.01b | |
| 36:4 | 857.5 | 0.07±0.04a | 0.03±0.02ab | 0.00±0.00b | |
| 38:4 | 885.5 | 4.17±0.61a | 3.10±0.41ab | 2.66±0.32b | |
| 38:5 | 883.5 | 0.14±0.08ab | 0.20±0.07b | 0.02±0.01b | |
|
Ether phosphatidyl- choline |
32:0 | 720.6 | 0.26±0.06a | 0.27±0.04a | 0.12±0.03b |
| 32:1 | 718.6 | 0.11±0.02a | 0.11±0.02a | 0.06±0.01b | |
| 32:2 | 716.6 | 0.03±0.01a | 0.03±0.01a | 0.01±0.00b | |
| 34:0 | 748.6 | 0.05±0.02a | 0.04±0.01a | 0.01±0.00b | |
| 34:1 | 746.6 | 0.40±0.07a | 0.42±0.06a | 0.21±0.03b | |
| 34:2 | 744.6 | 0.21±0.04ab | 0.25±0.04a | 0.13±0.02b | |
| 36:1 | 774.6 | 0.23±0.05a | 0.33±0.05a | 0.18±0.04b | |
| 36:2 | 772.6 | 0.22±0.05ab | 0.34±0.06a | 0.16±0.02b | |
| 36:3 | 770.6 | 0.08±0.02ab | 0.10±0.02a | 0.06±0.01b | |
| 36:4 | 768.6 | 0.17±0.03ab | 0.16±0.02a | 0.10±0.02b | |
| 36:5 | 766.6 | 0.15±0.02a | 0.16±0.01a | 0.11±0.01b | |
| 38:1 | 802.7 | 0.06±0.01ab | 0.08±0.01a | 0.04±0.01b | |
| 38:2 | 800.7 | 0.15±0.05b | 0.36±0.07a | 0.14±0.04b | |
| 38:3 | 798.6 | 0.03±0.01ab | 0.06±0.01a | 0.03±0.01b | |
| 38:4 | 796.6 | 0.14±0.01a | 0.13±0.01a | 0.09±0.01b | |
| 38:5 | 794.6 | 0.17±0.03ab | 0.21±0.03a | 0.12±0.02b | |
| 40:2 | 828.7 | 0.02±0.00b | 0.04±0.01a | 0.03±0.01b | |
|
Ether phosphor- etherine |
36:2 | 730.6 | 0.21±0.05b | 0.44±0.08a | 0.23±0.03b |
| 38:2 | 758.6 | 0.05±0.01b | 0.24±0.05a | 0.09±0.02b | |
| 38:5 | 752.6 | 0.22±0.03ab | 0.26±0.03a | 0.19±0.02b | |
|
Lysophosphatidly- choline |
16:0 | 496.3 | 0.43±0.10a | 0.36±0.03a | 0.23±0.05b |
| 18:1 | 522.4 | 0.16±0.03ab | 0.20±0.04a | 0.10±0.02b | |
| 18:2 | 520.3 | 0.17±0.05ab | 0.22±0.04a | 0.12±0.02b | |
| 20:4 | 544.3 | 0.08±0.02a | 0.08±0.02a | 0.04±0.01b | |
|
Lysophopho- etherine |
22:5 | 528.3 | 0.03±0.01ab | 0.03±0.01b | 0.07±0.01a |
| 22:6 | 526.3 | 0.05±0.01b | 0.05±0.01b | 0.11±0.02a | |
| Sphingomylin | 16:0 | 703.6 | 2.36±0.51a | 2.72±0.45a | 1.14±0.25b |
| 24:1 | 813.7 | 2.31±0.41ab | 2.62±0.35a | 1.73±0.26b | |
CD-1 mice were exercised with or without controlled dietary intake for 14 weeks. Quantitative profiling of detectable phospholipids in the mouse skin tissues was performed by electrospray ionization tandem mass spectrometry (ESI-MS/MS); Only the significantly changed phospholipids are shown.
Total acyl carbons:total double bonds;
Results are means ±SE, n=8–15 per group. Means with different letters differ significantly, p ≤ 0.05.
The second most abundant head group of phospholipids in mouse skin samples is PE, which accounts 31.5% of the total phospholipids. PE has less short chain species, with only 0.5% of diacyl PEs having 28, 30 or 32 total acyl carbons and over 68% of diacyl PE having 38 or more carbons. The levels of 40:5-PE, 40:6-PE, and 40:7-PE of Ex+PF mice tended to be higher than that of sedentary mice, although they were not significantly different (4.99±0.63 vs. 3.13±0.51, 5.94±0.82 vs. 4.17±0.90, 1.48±0.22 vs. 1.10±0.15, respectively).
PS diacyl species are 4.6% of the total phospholipids and only 40:6-PS was significantly higher in Ex+PF mice compared with Ex+AL mice (40.71±0.09 vs. 0.58±0.11).
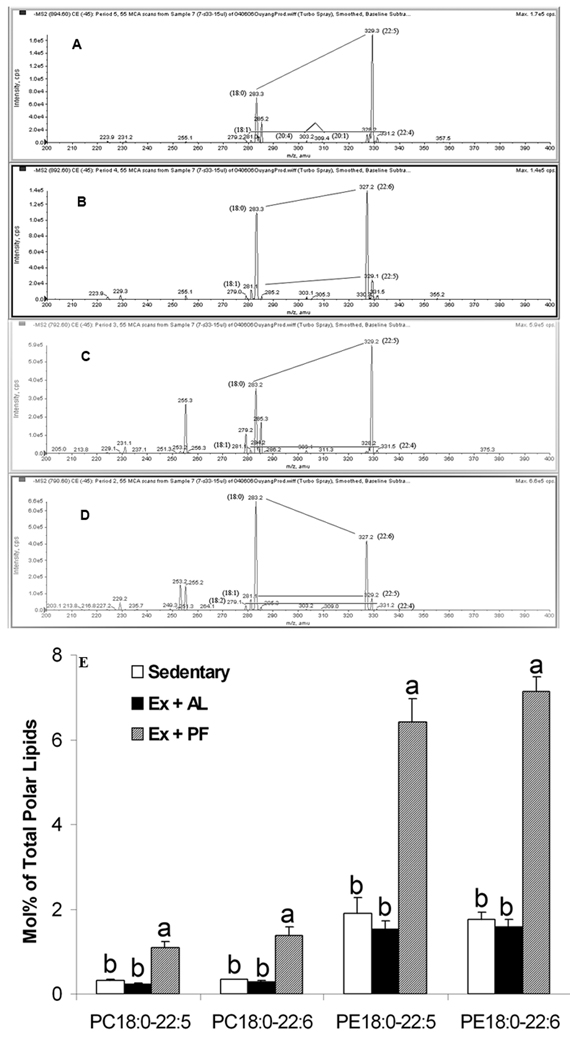
Acyl composition analysis was conducted to identify the fatty acid species in 40:5-PC and PE and 40:6-PC and PE. The data in Figure 2A suggest that 40:5-PC is primarily 18:0–22:5 with small amounts of 18:1–22:4 and 20:1–20:4. The 40:6-PC includes primarily 18:0–22:6 and also 18:1–22:5 species (Fig. 2B). For 40:5-PE, the acyl composition was mainly 18:0–22:5 and also 18:1–22:4 (Fig. 2C), while for 40:6-PE, the acyl composition was mainly 18:0–22:6 with small amounts of 18:1–22:5 and 18:2–22:4 (Fig. 2D). The 18:0–22:5 and 18:0–22:6 pair species of PC and PE were similar between Ex+AL and control groups, but significantly higher in Ex+PF mice (p < 0.001) (Fig.2E).
Figure 2. Quantification of 22:5 and 22:6-containing acyl species in PC/PE 40:5 and PC/PE 40:6 phospholipids via production spectra for acyl group identification.
CD-1 mice were exercised with controlled dietary intake for 14 weeks. The acyl groups of PC/PE 40:5 and PC/PE 40:6 in a lipid extract were identified as acyl anions from the appropriate negative ion precursors by ESI-MS/MS. PC and PE were analyzed as [M + OAc]− and [M−H]− ions, respectively. The lines show pairs of acyl species consistent with the observed PC or PE m/z. These product ion analyses were performed on selected molecular ions, indicating three major pairs of acyl composition (18:0–22:5 > 18:1–22:4 and 20:1–20:4) for PC40:5 (A), two major pairs of acyl composition (18:0–22:6 > 18:1–22:5) for PC 40:6 (B), two major pairs of acyl composition (18:0–22:5 > 18:1–22:4) for PE 40:5 (C), and three major pairs of acyl composition (18:0–22:6 > 18:1–22:5 and 18:2–22:4) for PE 40:6 (D). The fractions of the 40:5 and 40:6 species (determined from the lipid profile) that corresponded to the indicated 22:5 and 22:6-containing species were determined from the production spectra analysis (E). The PC/PE 18:0–22:5 and PC/PE 18:0–22:6 were significantly higher in exercise and pair fed mice, but not in exercise and ad libitum-fed mice when compared with the sedentary control. Results are means ± SE, n = 4. Means with different letters differ significantly, p ≤ 0.001.
For PIs, only seven species were detected and they comprise about 4.6% of the total phospholipids. The major molecular specie is 38:4 PI, which represents 91.4% of the total PI. Most PI species (five out of seven) showed a significant ~ 36–75% decrease in the Ex+PF mice compared to sedentary control or Ex+AL mice (Table 1).
Table 1 also shows the profile of ePC and ePE, which represent 3.0% and 2.3% of total phospholipids, respectively. Most of the ePCs in the Ex+PF mice significantly decreased compared to the two ad libitum-treated groups. Two ePEs (36:2 and 38:2) significantly increased in Ex+AL mice, but not in Ex+PF mice. The 38:5-ePE was significantly lower in Ex+PF mice compared to the Ex+AL mice.
LysoPC, lysoPE and SM compromise only a small percentage of total phopholipids, with 1.2%, 0.4%, and 3%, respectively. The data on lysoPC and lysoPE are shown in Table 1. For lyso PC, 16 and 18 carbon species are the predominant species. The lysoPC in Ex+AL mice were not significantly different compared to the sedentary control. In Ex+PF mice, however, 16:0-lysoPC, 18:1-lysoPC, 18:2-lysoPC, and 20:4-lysoPC were decreased significantly when compared to the control or the Ex+AL group. Near half (51%) of lysoPE was species with 20 or 22 carbons. The 22:6 and 22:5 lysoPE significantly increased in Ex+PF mice. Two major specie that make up 70% of total SM, 16:0 and 24:1 SM, were reduced in Ex+PF (Table 1).
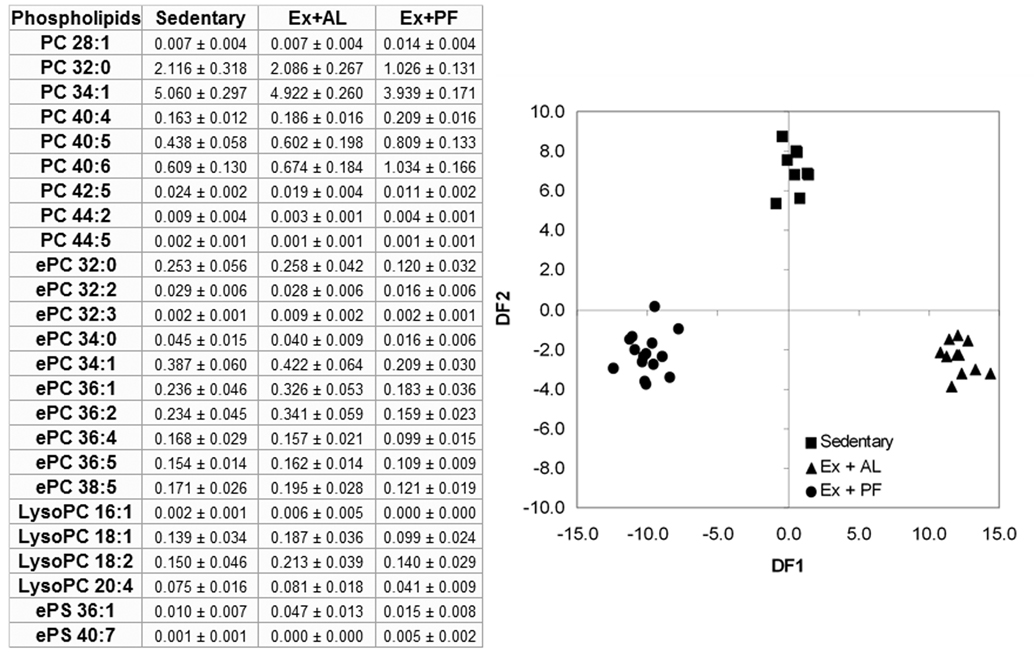
Discriminant analysis
An automatic backward stepwise discriminant analysis generated two discriminant functions using 25 phospholipid variables. The variables selected are listed in Figure 3. The three treatments (sedentary, Ex+AL, Ex+PF) were significantly distinguishable (Wilks' lambda = 0.001 at p<0.00001) using the two discriminant functions. DF1 represented the effect of diet and DF2 the effect of exercise.
Figure 3. Stepwise discriminant analysis of 25 phospholipid variables.
CD-1 mice were exercised with or without controlled dietary intake for 14 weeks, and then quantitative profiling of phospholipids was performed by ESI-MS/MS in mouse skin tissues. Total 57 phospholipids were significantly changed among three treatment groups and 25 of them are indicated in the inserted table with mol% of total polar lipid content. Automatic backward stepwise variable selection in discriminant analysis identified 25 phospholipids as indicated in the inserted table that could successfully predict a treatment group with 92% correct classification (Wilks' lambda = 0.001 at p ≤ 0.00001) based upon the first two principal discriminant functions.
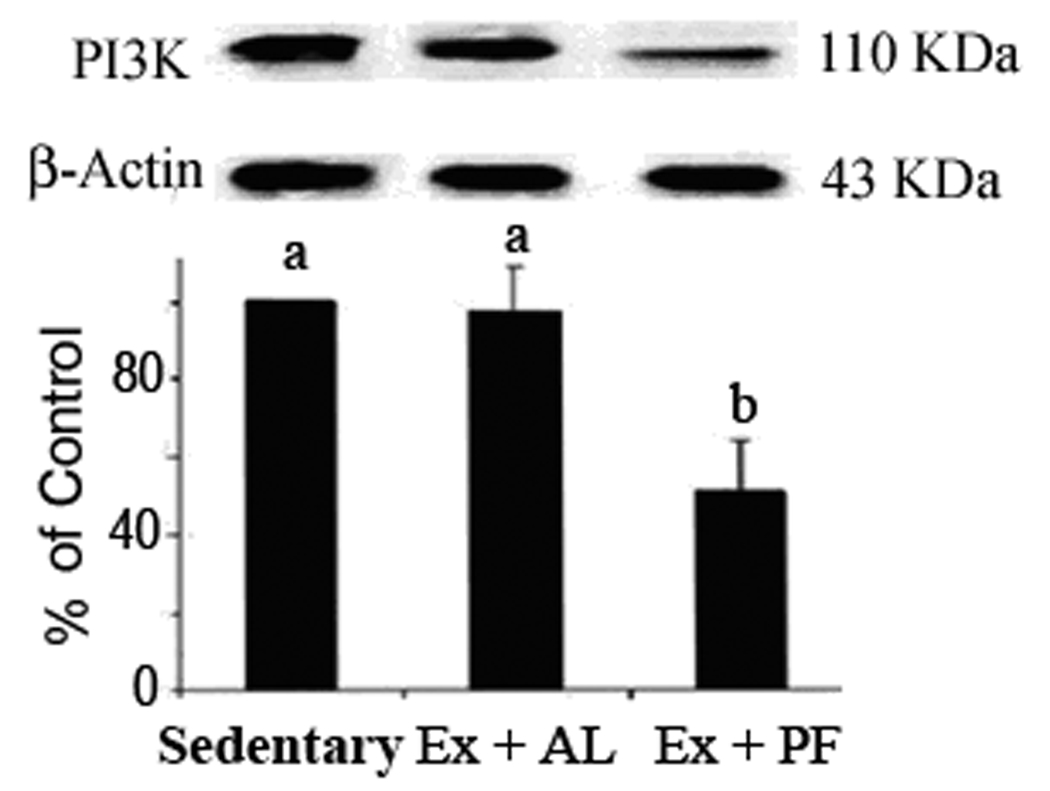
PI3K protein expression detected by western blotting and immunohistochemistry
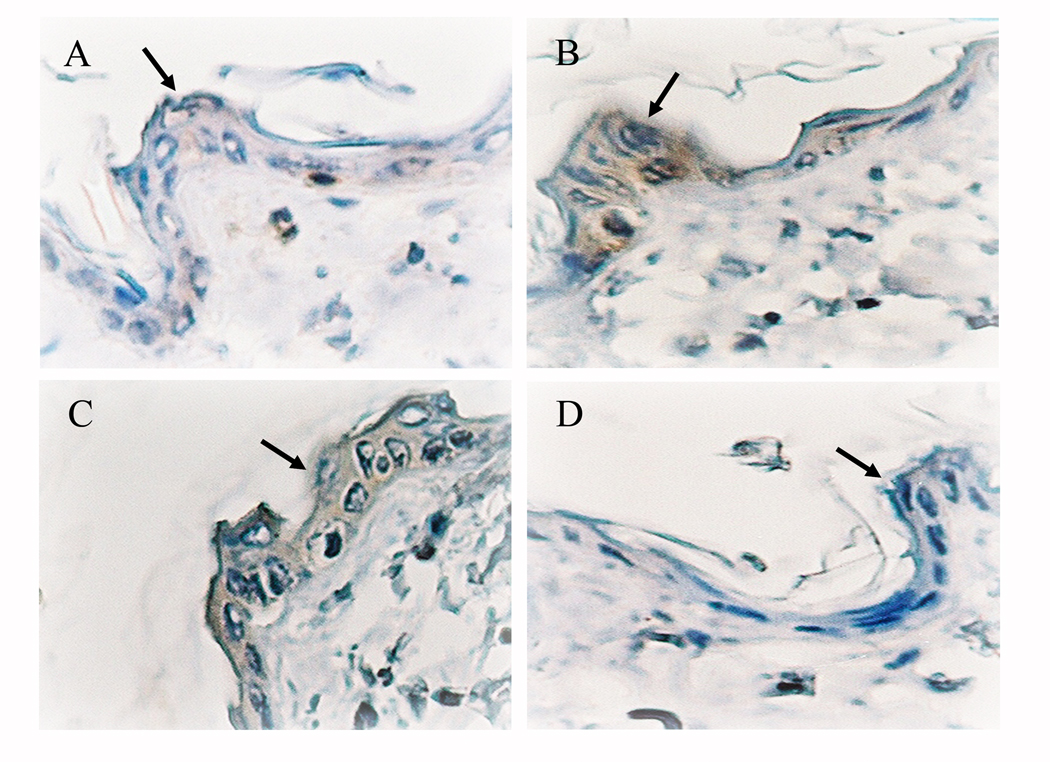
The western blot showed that class I PI3K expression was significantly decreased in Ex+PF but not Ex+AL mice (Fig. 4). The image in Figure 5 depicts the median expression score of class I PI3K as measured by immunohistochemistry. In sedentary control mice, the expression of PI3K in epidermal cells (arrows) was higher in TPA-treated mice (Fig. 5B, scored as 10) than in acetone-vehicle treated mice (Fig. 5A, scored as 5). As the PI3K expression increased in Ex+AL mice (Fig. 5C, scored as 15), the expression level of PI3K in Ex+PF mice decreased to the same level as that of the acetone-vehicle sedentary mice (Fig. 5D, scored as 5).
Figure 4. Effects of exercise with or without controlled dietary intake on PI3K protein expression in mouse skin tissues.
CD-1 mice were exercised with or without controlled diet intake for 14 weeks. The level of PI3K protein in skin tissues was determined by western blotting and quantified by the FluorChem™ 8800 Advanced Imaging System. Results are means ± SE, n = 10–15. Means with different letters differ significantly, p ≤ 0.01.
Figure 5. Effects of exercise with or without controlled dietary intake on PI3K protein expression in skin epidermis.
CD-1 mice were exercised with or without controlled dietary intake for 14 weeks. Representative histological skin sections with immunohistochemical staining for p110-PI3K in epidermis in acetone-treated control (A) and TPA-treated control (B), exercise with ad libitum-feeding (C), and exercise with pair-feeding (D) are shown. The arrows indicate representative staining of the target protein in epidermis (n = 3–5).
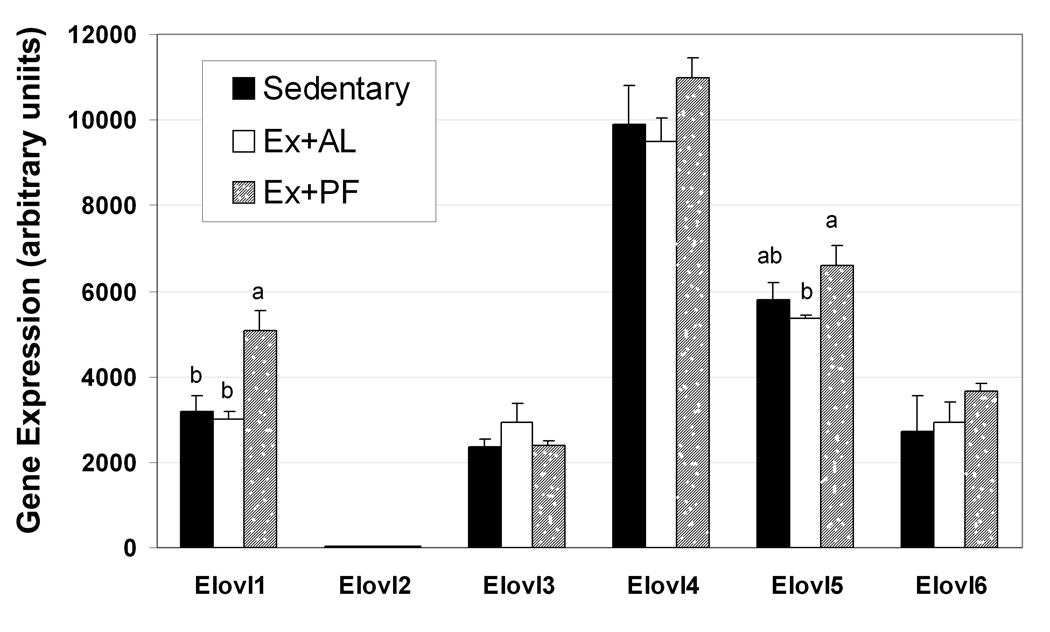
Gene expression of the Elovl family
All six members of Elovl gene family are detectable in mouse skin tissues by microarray analysis with various probe sets (up to 11 for each Elovl gene). As the Elovl2 is specifically expressed in the liver, it is not unexpected that the expression level was lowest in skin, as shown in Figure 6. Among the other 5 Elovl members, only Elov11 expresssion increased significantly in Ex+PF mice when compared to either the sedentary control or Ex+AL group. Elov15 expression appeared to be enhanced in Ex+PF mice with comparison to the EX+AL group only but not the sedentary controls. One of four probe sets for Elov16 indicated over-expression; this was confirmed by a real-time PCR analysis. However, the average of Elov16 expression as measured by all the four probe sets was not significantly different among the groups.
Figure 6. Effects of exercise with or without controlled dietary intake on Elovl gene expression in skin tissues.
CD-1 mice were exercised with or without controlled dietary intake for 14 weeks. The expression of six Elovl family genes was measured by microarray analysis as described in the Materials and Methods. The accession number for these six genes are as follows, including both NM_001039176 (elongation of very long chain fatty acids-like 1 isoform 1) and NM_001039175 ( elongation of very long chain fatty acids-like 1 isoform 2 for Elovl1, NM_019423 (elongation of very long chain fatty acids-like 2) for Elovl2, NM_007703 (elongation of very long chain fatty acids-like 3) for Elovl3, NM_001145974 (elongation of very long chain fatty acids-like 4) for Elovl4, NM_134255 (elongation of very long chain fatty acids-like 5) for Elovl5, and NM_130450 (elongation of very long chain fatty acids-like 6) for Elovl6. Results are means ± SE (n = 4). Means with different letters differ significantly, p ≤ 0.05.
Discussion
This study showed that exercise with controlled dietary intake successfully prevented body weight gain and reduced body fat in the CD-1 mice. It also modified the phospholipid profile significantly in the skin tissues, by decreasing some lysoPCs, most PIs and ePCs as well as decreasing PI3K protein expression in the skin epidermis, and increasing long chain polyunsaturated PC, PE, and lysoPE species containing 22:6 and 22:5 that are likely to be ω-3 fatty acids.
The results demonstrated that exercise with ad libitum feeding did not effectively decrease body weight and body fat. Similar results are also observed by Mehl et al. in APCMin mice and Michna et al., in SKH mice (39, 8). A moderate increase of food intake from 3.43±0.28 g/day for the control to 3.67±0.18 g/day for Ex+AL mice may compensate, at least in part, the exercise-induced energy expenditure. Furthermore, the dietary energy increase in Ex+AL might not necessarily match the treadmill exercise-induced energy expenditure, since the energy expenditure could have been altered due to the changed spontaneous activity in the cage and/or resting energy metabolism. In addition to physical activity, Badman and Flier (40) have suggested that the total energy expenditure should also consist of adaptive thermogenesis and obligatory energy expenditure. It is interesting to compare our results with Huffman’s study (41). The exercise training protocol appears a little bit different between two studies. In our experiment, the mice run treadmill at zero grade for 13 weeks, but Huffman’s mice were exercised at 8% grade for 24 weeks. The most significant difference is due to strain- and diet-related phenomena. We used a lean mouse model that fed a normal AIN-93 diet (5% of calories from fat), but Huffman et al. (41) used C57BL/6 strain, a classic high-fat diet-induced obese model. To induce weight gain, Huffman’s mice were fed a moderately high-fat diet (35% of calories from fat). Therefore, the average of body weights for the control mice in Haffman’s study was about 30 g in the beginning of experiments and 46 g in Week 14. In contrast, the average of body weight for our control mice is ~26 g in the beginning and 32 g in Week 14. It is no doubt that the moderately high-fat diet-induced overweight C57BL/6 strain in Haffman’s study should be much more susceptible to exercise-induced weight loss than our lean CD-1 strain. Actually, our data from the lean CD-1 strain are consistent with what we observed in another lean SENCAR strain (Xie et al., 2007). When compared with Haffman’s study, our contradictory data may provide a diverse phenomenon for a lean strain model with normal diet treatment.
When we adjusted the food consumption of the exercised mice to that of sedentary control mice, significant effects on body weight and body fat were observed. Studies in the Hursting lab found that voluntary wheel running mice with restricted food consumption, a pair-feeding strategy similar to ours, had a significantly lower body weight and less intestinal polyp development (12). Overall, this study indicates that a negative calorie balance via both increasing energy expenditure and limiting calorie intake seems most effective in preventing body weight gain. It should be noted that we may not be able to differentiate body weight control from caloric balance. Although exercise alone with ad libitum feeding was not sufficient to decrease body weight due to, at least in part, the corresponding increase in dietary intake, the lack of an exercise effect in AL+Exe mice on the body weight/fat and various phospholipids might be in part due to the insufficient magnitude of the calorie deficit. Since the average food intake for three treatment groups are comparable, we did not find any significant correlation of calorie intake with specific phospholipids, and the caloric deficit via exercise was not matched with the diet intake, and thus the results should be interpreted accordingly.
Although it wonders whether the turnover rate of skin would make it a better indicator of subtle changes than other tissues, previous studies by ours and others have demonstrated a cancer-inhibitory activity of weight loss by dietary calorie restriction and/or exercise in animal skin cancer model (5–8). Furthermore, exercise-induced skin cancer inhibition has been linked to apoptosis induction and anti- proliferation in the skin epidermis (9, 13). To further evaluate the impact of weight control, lipidomics analysis for all the phospholipids in skin tissues 2 hours after TPA treatment was performed. First of all, we did not find any significant differences of phospholipids between TPA and acetone-vehicle control. The reason related to a lack of a significant impact of TPA on the phospholipid profile may be due to a short time exposure to TPA treatment in vivo. The selection of 2-hr period for TPA treatment is based on the previous observation that was adequate for a significant activation of Ras and ERK activities in skin epidermis (13).
Furthermore, the finding that the major molecular specie of PI was 38:4 is consistent with the typical pattern of PIs found in mammals, and in particular in mouse tissues where this major specie has demonstrated to be 1–18:0, 2–20:4 PI (42). The observed decrease of the most PI species in the Ex+PF mice led us to measure expression of PI3K, a key kinase required for various signaling cascades for cancer-related cellular function including cell growth, proliferation, differentiation, motility, survival, and intracellular trafficking (13). As expected, we found the decreased PI levels corresponded to lowered levels of PI3K protein in the skin epidermis of Ex+PF mice. Skin cancer development is usually associated with uncontrolled proliferation of epidermal cells (43), so the lower protein expression of class I PI3K in epidermal cells as measured by immunohistochemistry may result in less proliferation as found in our previous report (13). Furthermore, the increased levels of PI3K staining observed in TPA-treated sedentary control when compared with acetone-vehicle control. When compared with TPA-treated sedentary control (Fig 5B) but not acetone-vehicle sedentary control (Fig 5A), no significant difference was found in Ex+AL group. However, TPA-induced increase of PI3K protein expression was significantly suppressed by exercise with pair-feeding, suggesting the direct product of PI3K, the second messenger PI(3, 4, 5)P3, might be reduced. It is well recognized that PI(3, 4, 5)P3 recruits some signaling enzymes with pleckstrin homology domains, such as protein serine-threonine kinases, including Akt and adaptor proteins, to the membrane. The activation of these enzymes impacts protein synthesis, cell cycle entry, and cell survival function, etc. (20). Decreased PIs and down regulation of PI3K expression observed in this study may imply that body weight control through exercise with controlled dietary intake may prevent against TPA-induced cancer risk. In addition, many studies by us and others have found that weight control was associated with reduced levels of circulating growth hormones or factors such as IGF-1 (44). Considering the requirement of PI3K activation by IGF-1-dependent signaling, the down-regulation of PI3K protein expression, and the reduced PI3K-related PI substrates in the exercised but pair-fed mice might be caused by a decrease in plasma IGF-1 levels. In our studies, IGF-1 was restored in the exercised and pair-fed mice by either intraperitoneal injection at 10 µg/g body weight twice per wk (13) or via osmotic minipumps (unpublished data). We have found the reduction of PI3K protein expression and the PI species were partially reversed by IGF-1 restoration.
In addition to PIs, we also found most of the ePCs and LysoPCs were significantly reduced in the exercised and pair-fed mice, while 22:6 lysoPE was increased in Ex+PF group. The lower levels of ePCs and lysoPCs may prevent cancer by reducing cellular damage and proliferation, since ePCs are required for the formation of platelet-activating-factor and lysoPCs are produced during LDL oxidation within atherosclerotic plaques for atherosclerotic lesion development (45–48).
The impact of weight control via exercise on the PCs and PEs is interesting. When compared with the sedentary control, exercise with ad libitum-fed did not change the profile of PCs and PEs. However, there are significant changes observed in the exercised and pair-fed mice. As some short chain fatty acids of PCs were decreased, the long chain polyunsaturated fatty acids, i.e., 40:5 and 40:6, increased significantly in Ex+PF mice in comparison with either control or Ex+AL group. A similar impact was found on PEs (data not shown). By means of product ion analysis, the increased polyunsaturated 40:5 and 40:6 fatty acids in PCs and PEs were further discovered to contain a combination of either 18:0–22:5 or 18:0–22:6. The 22:6 fatty acid is undoubtedly DHA. The 22:5 fatty acid, i.e., docosapentaenoic acid (DPA), could be either ω-6 adrenic acid or ω-3 clupanodonic acid. As one of the three major ω-3 long chain polyunsaturated fatty acids, clupanodonic acid could be intermediary between eicosapentaenoic acid (EPA) and DHA (49). It should be noted that ω-3 22:6 DHA was found to be elevated significantly in the exercised and pair-fed mice not only for PCs and PEs, but also for lysoPEs. It is well known that the mammals can make DHA and EPA through desaturation and elongation of essential ω3-linolenic acid (50–51). Our microarray data further confirmed that elongation of (very) long chain fatty acid-like elongase gene 1 (Elvol1) was expressed significantly more in Ex+PF as compared to the Ex+AL group. Elevation of DHA by exercise has been reported by others in human studies (31, 52). Considering the general health benefits of ω-3 fatty acids (53–56) and a specific inhibitory role in TPA-induced signaling activation (28, 57), the increase of ω-3 fatty acids found in this study may provide a novel approach to understand the mechanisms by which exercise with controlled calorie intake may protect against cancer.
To overview the effects of exercise with or without consideration of diet intake upon the phospholipid profiling, we applied a discriminant function analysis to the 57 significantly changed phospholipids. Twenty-five of the total 57 phospholipids were able to distinguish the treatment groups with 92% classification efficiency. These 25 phospholipids are possible candidates for biomarkers to distinguish the effects of diet regiments and exercise in mice. It should be noted that the most 25 phospholipids selected are PCs, ePC, or lysiPCs. The functional impact of these PC-related species changes and how such changes might afford protection from cancer warrant further studies.
Taken together, these data indicate, for the first time, that exercise with controlled diet interventions, but not exercise alone, significantly reduced body weight and body fat as well as modified the phospholipid profile. This modified profile might provide potential cancer prevention benefits, perhaps via reducing TPA-induced PIs and PI-related PI3K expression and by enhancing ω-3 PC, PẸ and/or lysoPE elongation mechanisms.
Acknowledgments
The authors thank Ms. Mary Roth for assistance in lipidomics analysis and Dr. Mark Haub for assistance in body fat analysis by DXA.
Grant support: National Cancer Institute grants R01 CA106397 and P20 RR15563 (W. Wang), a graduate student summer stipend from Terry Johnson Center for Basic Cancer Research, Kansas State University (P. Ouyang), and National Science Foundation Major Research Instrumentation Grant DBI 0521587 and EPSCoR Grant EPS-0236913 with matching support from the State of Kansas, National Institute of Health P20 RR016475 for the Kansas Lipidomics Research Center Analytical Laboratory (R. Welti), and KSU Functional Genomics Consortium (KSU Targeted Excellence Program). This is journal contribution #06-200-J of the Agricultural Experiment Station, Kansas State University.
Footnotes
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
Abbreviations used: DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; DXA, dual-energy X-ray absorptiometer; EPA, eicosapentaenoic acid; ePC, ether phosphatidylcholine, i.e., alk(en)yl,acyl phosphocholine; ePE, ether phosphatidylethanolamine, i.e., alk(en)yl,acyl phosphoethanolamine); ePS, ether phosphatidylserine, i.e., alk(en)yl,acyl phosphoserine; Ex+AL, exercised and ad libitum fed group; Ex+PF, exercised and pair-fed group; HRP, horseradish peroxidase; lysoPC, lysophosphatidylcholine; lysoPE, lysophosphatidylethanolamine; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PE-cer, ceramide phosphoethanolamine; PI, phosphatidylinositol; PI(3, 4, 5)P3, phosphatidylinositol-3,4,5-trisphosphate; PI3K, phosphatidylinositol 3-kinase; SM, sphingomyelin; PS, phosphatidylserine; TPA, 12-O-tetradecanoyl phorbol-13-acetate.
References
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.Patel AV, Feigelson HS, Talbot JT, et al. The role of body weight in the relationship between physical activity and endometrial cancer: results from a large cohort of US women. Int J Cancer. 2008;123:1877–1882. doi: 10.1002/ijc.23716. [DOI] [PubMed] [Google Scholar]
- 3.Inoue M, Yamamoto S, Kurahashi N, et al. Daily total physical activity level and total cancer risk in men and women: results from a large-scale population-based cohort study in Japan. Am J Epidemiol. 2008;168:391–403. doi: 10.1093/aje/kwn146. [DOI] [PubMed] [Google Scholar]
- 4.Courneya KS, Katzmarzyk PT, Bacon E. Physical activity and obesity in Canadian cancer survivors: population-based estimates from the 2005 Canadian Community Health Survey. Cancer. 2008;112:2475–2482. doi: 10.1002/cncr.23455. [DOI] [PubMed] [Google Scholar]
- 5.Kritchevsky D, Klurfeld DM. Influence of caloric intake on experimental carcinogenesis: a review. Adv Exp Med Biol. 1986;206:55–68. doi: 10.1007/978-1-4613-1835-4_7. [DOI] [PubMed] [Google Scholar]
- 6.Kritchevsky D. Caloric restriction and experimental carcinogenesis. Adv Exp Med Biol. 1992;322:131–141. doi: 10.1007/978-1-4684-7953-9_12. [DOI] [PubMed] [Google Scholar]
- 7.Birt DF, Przybyszewski J, Wang W, Stewart J, Liu Y. Identification of molecular targets for dietary energy restriction prevention of skin carcinogenesis: an idea cultivated by Edward Bresnick. J Cell Biochem. 2004;91:258–264. doi: 10.1002/jcb.10741. [DOI] [PubMed] [Google Scholar]
- 8.Michna L, Wagner GC, Lou YR, et al. Inhibitory effects of voluntary running wheel exercise on UVB-induced skin carcinogenesis in SKH-1 mice. Carcinogenesis. 2006;27:2108–2115. doi: 10.1093/carcin/bgl057. [DOI] [PubMed] [Google Scholar]
- 9.Lu YP, Lou YR, Nolan B, et al. Stimulatory effect of voluntary exercise or fat removal (partial lipectomy) on apoptosis in the skin of UVB light-irradiated mice. Proc Natl Acad Sci USA. 2006;103:16301–16306. doi: 10.1073/pnas.0607789103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers CJ, Colbert LH, Greiner JW, Perkins SN, Hursting SD. Physical activity and cancer prevention: pathways and targets for intervention. Sports Med. 2006;38:271–296. doi: 10.2165/00007256-200838040-00002. [DOI] [PubMed] [Google Scholar]
- 11.Colbert LH, Mai V, Tooze JA, Perkins SN, Berrigan D, Hursting SD. Negative energy balance induced by voluntary wheel running inhibits polyp development in APCMin mice. Carcinogenesis. 2006;27:2103–2107. doi: 10.1093/carcin/bgl056. [DOI] [PubMed] [Google Scholar]
- 12.Moore T, Beltran L, Carbajal S, et al. Cancer Prev Res. 2008;1:65–76. doi: 10.1158/1940-6207.CAPR-08-0022. [DOI] [PubMed] [Google Scholar]
- 13.Xie L, Jiang Y, Ouyang P, et al. Effects of dietary calorie restriction or exercise on the PI3K and Ras signaling pathways in the skin of mice. J Biol Chem. 2007;282:28025–28035. doi: 10.1074/jbc.M604857200. [DOI] [PubMed] [Google Scholar]
- 14.Ji LL. Exercise-induced modulation of antioxidant defense. Ann NY Acad Sci. 2002;959:82–92. doi: 10.1111/j.1749-6632.2002.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 15.Stoll BA. Adiposity as a risk determinant for postmenopausal breast cancer. Int J Obes Relat Metab Disord. 2000;24:527–533. doi: 10.1038/sj.ijo.0801247. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–3120S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 17.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 18.Polak J, Klimcakova E, Moro C, et al. Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism. 2006;55:1375–1381. doi: 10.1016/j.metabol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Karacabey K. Effect of regular exercise on health and disease. Neuro Endocrinol Lett. 2005;26:617–623. [PubMed] [Google Scholar]
- 20.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 21.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 22.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krauss M, Haucke V. Phosphoinositide-metabolizing enzymes at the interface between membrane traffic and cell signalling. EMBO Rep. 2007;8:241–246. doi: 10.1038/sj.embor.7400919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y. Sphingosylphosphorylcholine and lysophosphatidylcholine: G protein-coupled receptors and receptor-mediated signal transduction. Biochim Biophys Acta. 2002;1582:81–88. doi: 10.1016/s1388-1981(02)00140-3. [DOI] [PubMed] [Google Scholar]
- 25.Chajès V, Lanson M, Fetissof F, Lhuillery C, Bougnoux P. Membrane fatty acids of breast carcinoma: contribution of host fatty acids and tumor properties. Int J Cancer. 1999;63:169–175. doi: 10.1002/ijc.2910630204. [DOI] [PubMed] [Google Scholar]
- 26.Bougnoux P, Chajes V, Lanson M, et al. Prognostic significance of tumor phosphatidylcholine stearic acid level in breast carcinoma. Breast Cancer Res Treat. 1992;20:185–194. doi: 10.1007/BF01834624. [DOI] [PubMed] [Google Scholar]
- 27.Bougnoux P, Giraudeau B, Couet C. Diet, cancer, and the lipidome. Cancer Epidemiol Biomarkers Prev. 2006;5:416–421. doi: 10.1158/1055-9965.EPI-05-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapkin RS, Seo J, McMurray DN, Lupton JR. Mechanisms by which docosahexaenoic acid and related fatty acids reduce colon cancer risk and inflammatory disorders of the intestine. Chem. Phys. Lipids. 2008;153:14–23. doi: 10.1016/j.chemphyslip.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dobrzyń A, Górski J. Effect of acute exercise on the content of free sphinganine and sphingosine in different skeletal muscle types of the rat. Horm Metab Res. 2002;34:523–529. doi: 10.1055/s-2002-34793. [DOI] [PubMed] [Google Scholar]
- 30.Dobrzyń A, Knapp M, Górski J. Effect of acute exercise and training on metabolism of ceramide in the heart muscle of the rat. Acta Physiol Scand. 2004;181:313–319. doi: 10.1111/j.1365-201X.2004.01295.x. [DOI] [PubMed] [Google Scholar]
- 31.Helge JW, Wu BJ, Willer M, Daugaard JR, Storlien LH, Kiens B. Training affects muscle phospholipid fatty acid composition in humans. J Appl Physiol. 2001;90:670–677. doi: 10.1152/jappl.2001.90.2.670. [DOI] [PubMed] [Google Scholar]
- 32.Markopoulos C, Polychronis A, Zobolas V, et al. The effect of exemestane on the lipidemic profile of postmenopausal early breast cancer patients: preliminary results of the TEAM Greek sub-study. Breast Cancer Res Treat. 2005;93:61–66. doi: 10.1007/s10549-005-3783-0. [DOI] [PubMed] [Google Scholar]
- 33.Veena K, Shanthi P, Sachdanandam P. The biochemical alterations following administration of Kalpaamruthaa and Semecarpus anacardium in mammary carcinoma. Chem Biol Interact. 2006;161:69–78. doi: 10.1016/j.cbi.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Forrester JS, Milne SB, Ivanova PT, Brown HA. Computational lipidomics: a multiplexed analysis of dynamic changes in membrane lipid composition during signal transduction. Mol Pharmacol. 2004;65:813–821. doi: 10.1124/mol.65.4.813. [DOI] [PubMed] [Google Scholar]
- 35.Welti R, Li W, Li M, et al. Profiling membrane lipids in plant stress responses. Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J Biol Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 36.Bartz R, Li WH, Venables B, et al. Lipidomics reveals that adiposomes store ether lipids and mediate phospholipid traffic. J Lipid Res. 2007;48:837–847. doi: 10.1194/jlr.M600413-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Fernando P, Bonen A, Hoffman-Goetz L. Predicting submaximal oxygen consumption during treadmill running in mice. Can. J Physiol Pharmacol. 1993;71:854–857. doi: 10.1139/y93-128. [DOI] [PubMed] [Google Scholar]
- 38.Lu J, Xie L, Sylvester J, et al. Different gene expression of skin tissues between mice with weight controlled by either calorie restriction or physical exercise. Exp Biol Med. 2007;232:473–480. [PubMed] [Google Scholar]
- 39.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol. 2005;98:2219–2225. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 40.Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science. 2005;307:1909–1914. doi: 10.1126/science.1109951. [DOI] [PubMed] [Google Scholar]
- 41.Huffman DM, Moellering DR, Grizzle WE, Stockard CR, Johnson MS, Nagy TR. Effect of exercise and calorie restriction on biomarkers of aging in mice. Am J Physiol Regul Integr comp Physiol. 2008;294:R1618–R1627. doi: 10.1152/ajpregu.00890.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postle AD, Dombrowsky H, Clarke H, Pynn CJ, Koster G, Hunt AN. Mass spectroscopic analysis of phosphatidylinositol synthesis using 6-deuteriated-myo-inositol: comparison of the molecular specificities and acyl remodelling mechanisms in mouse tissues and cultured cells. Biochem Soc Trans. 2004;32:1057–1059. doi: 10.1042/BST0321057. [DOI] [PubMed] [Google Scholar]
- 43.Dwivedi C, Maydew ER, Hora JJ, Ramaeker DM, Guan X. Chemopreventive effects of various concentrations of alpha-santalol on skin cancer development in CD-1 mice. Eur J Cancer Prev. 2005;14:473–476. doi: 10.1097/01.cej.0000178075.20124.2a. [DOI] [PubMed] [Google Scholar]
- 44.Jiang Y, Wang W. Potential mechanisms of weight control for cancer prevention. Biophys Rev Lett. 2008:421–437. [Google Scholar]
- 45.Quinn MT, Parthasarathy S, Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci USA. 1998;85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chai YC, Howe PH, DiCorleto PE, Chisolm GM. Oxidized low density lipoprotein and lysophosphatidylcholine stimulate cell cycle entry in vascular smooth muscle cells. Evidence for release of fibroblast growth factor-2. J Biol Chem. 1996;271:17791–17797. doi: 10.1074/jbc.271.30.17791. [DOI] [PubMed] [Google Scholar]
- 47.Kugiyama K, Kerns SA, Morrisett JD, Roberts R, Henry PD. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990;344:160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- 48.Sidik K, Smerdon MJ. Bleomycin-induced DNA damage and repair in human cells permeabilized with lysophosphatidylcholine. Cancer Res. 1990;50:1613–1619. [PubMed] [Google Scholar]
- 49.Beare-Rogers J, Dieffenbacher A, Holm JV. Lexicon of lipid nutrition. IUPAC Pure Appl Chem. 2001;73:685–744. [Google Scholar]
- 50.Vance DE, Vance JE. In: Biochemistry of lipids, Lipoproteins and Membranes. 4th Edition. Vance JE, Vance D, editors. Burlington: Elsevier; 2002. pp. 192–197. [Google Scholar]
- 51.Burdge GC, Jones AE, Wootton SA. Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr. 2002;88:355–363. doi: 10.1079/BJN2002662. [DOI] [PubMed] [Google Scholar]
- 52.Gudbjarnason S. Dynamics of n-3 and n-6 fatty acids in phospholipids of heart muscle. J Intern Med. 1989;731:117–128. doi: 10.1111/j.1365-2796.1989.tb01445.x. [DOI] [PubMed] [Google Scholar]
- 53.Mori TA, Beilin LJ. Omega-3 fatty acids and inflammation. Curr Atheroscler Rep. 2004;6:461–467. doi: 10.1007/s11883-004-0087-5. [DOI] [PubMed] [Google Scholar]
- 54.Marchioli R. Omega-3 polyunsaturated fatty acids and cardiovascular diseases. Minerva. Cardioangiol. 2003;51:561–576. [PubMed] [Google Scholar]
- 55.Jho DH, Cole SM, Lee EM, Espat NJ. Role of omega-3 fatty acid supplementation in inflammation and malignancy. Integr Cancer Ther. 2004;3:98–111. doi: 10.1177/1534735404264736. [DOI] [PubMed] [Google Scholar]
- 56.Hardman WE. (n-3) fatty acids and cancer therapy. J Nutr. 2004;134:3427S–3430S. doi: 10.1093/jn/134.12.3427S. [DOI] [PubMed] [Google Scholar]
- 57.Fan YY, Ly LH, Barhoumi R, McMurray DN, Chapkin RS. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J Immunol. 2004;173:6151–6160. doi: 10.4049/jimmunol.173.10.6151. [DOI] [PubMed] [Google Scholar]