Abstract
Hydrogen/deuterium exchange (HDX) mass spectrometry has been widely applied to the characterization of protein dynamics. More recently, differential HDX has been shown to be effective for the characterization of ligand binding. Previously we have described a fully automated HDX system for use as a ligand screening platform. Here we describe and validate the required data analysis workflow to facilitate the use of HDX as a robust approach for ligand screening. Following acquisition of HDX data at a single on-exchange time point (n ≥ 3), one way analysis of variance in conjunction with the Tukey multiple comparison procedure is used to establish the significance of any measured difference. Analysis results are graphed with respect to a single peptide, ligand or group of ligands, or displayed as an overview within a heat map. For the heat map display, only Δ%D values with a Tukey-adjusted P value less than 0.05 are colored. Hierarchical clustering is used to bin compounds with highly similar HDX signatures. The workflow is evaluated with a small data set showing the ligand binding domain (LDB) of the nuclear receptor peroxisome proliferator-activated receptor gamma (PPARγ) screened against 10 functionally selective ligands. More significantly, data for the vitamin D receptor (VDR) in complex with 87 ligands are presented. To highlight the robustness and precision of our automated HDX platform we analyzed the data from 4191 replicate HDX measurements acquired over an eight month timeframe. Ninety six percent of these measurements were within 10 percent of the mean value. Work has begun to integrate these analysis and graphing components within our HDX software suite.
Keywords: HDX, MS, ligand, Screening, nuclear receptor
1. Introduction
Hydrogen/deuterium exchange (HDX) in combination with mass spectrometry (MS)[1–5] is a powerful method for the characterization of protein dynamics.[6–10] The technique has been applied to determine changes in dynamics (differential HDX) between a protein and its binding partners.[11] These binding partners have included proteins, peptides, DNA, RNA, small molecules[12–22], or combinations of all five.[23] HDX has found use as an approach to guide construct design in x-ray crystallography methods development[24, 25] and has been applied to challenging molecules such as antibodies[10] and membrane proteins.[26–30]
Many of these experiments report HDX results from differential analysis between two states of a protein, for example HDX of a protein ± ligand. Our group has advocated the use of automation[13, 31] to reduce random, gross and systematic errors during data acquisition.[14] Following acquisition, the percent deuterium values must be extracted from the MS spectra, and the correlated data volume can be considerable. For example, a single differential HDX experiment may contain 100 peptides. Four replicate analyses of seven on-exchange time points will yield 5600 datapoints. Screening just 50 ligands will expand that dataset to 280,000 values. The data generated from a single research group over just a few years can quickly top a million data points, all of which require tools for visualization and protocols for quality control, archiving and data backup. More importantly, approaches to provide significance of changes on large datasets are needed.
Significant effort has been dedicated to software tools for the analysis of HDX data[31–40]; however the majority of published software are focused upon the generation of percent deuterium values and not downstream data analysis. Some applications contain no graphing components and others contain useful, but somewhat limited, display elements such as the ability to compare two datasets. The need therefore remains for a software solution that is capable of the cross comparison of multiple (>50) datasets acquired over a significant time period by multiple users. Such a software package will complete our goal of producing a HDX platform that is comprehensive, rapid, and robust enough for use in screening applications. In this context we are interested in developing methods for screening functionally selective nuclear receptor modulators; however, the approach can be applied to other target proteins such as kinases or G-protein coupled receptors (GPCRs).
Our approach to HDX software development has been to store all the relevant MS spectral data and HDX results in a relational database and is unique in this regard.[35] Unlike a standalone application, this structure is ideal for HDX screening applications that require the cross comparison of multiple datasets. The database also provides a single storage point for all data generated within the laboratory and allows for easy sharing of data between members of a laboratory. Critically, the database provides a single target for data archiving. Quality control for processed data is also possible. For example, any project can be queried to provide the standard deviation of all measurements. At the time of writing there were 1,334,978 HDX values and associated MS spectra stored within our database (active since April 2008, not including values in the external user database). All of these data points and spectra may be accessed directly from any web browser. This amount of data would impossible to manage across multiple users with the current generation of standalone HDX software applications.
We have previously published the differential HDX data for PPARγ-LBD in complex with 10 ligands of interest at single time point.[14] During the evaluation of this dataset we determined statistical significance between apo (ligand free) receptor and ligand bound receptor with a two tailed t-test. Although this approach allowed for the determination of peptides that are different from apo receptor, we did not address a crucial question; what is the statistical significance of the changes observed between those ten ligands? We understand that both ligand “A” and “B” differ from “apo” for a particular peptide, however is “A” different from “B”? To answer these questions we have chosen to re-evaluate the aforementioned dataset. In doing so, we seek to establish a validated workflow for HDX screening data that will subsequently be integrated into our HDX software. This workflow was then applied to a large VDR HDX dataset (>20,000 %D values) obtained for analysis of the receptor in complex with 87 ligands, including small molecules, cofactor proteins and DNA.
2. Material and Methods
PPARγ LBD was expressed and purified as described previously.12, 14 His-hVDR ligand binding domain (residues 118–425, Δ[165–215]) was expressed in E. coli BL21 cells as inclusion bodies. Inclusion bodies were solubilized in a guanidine-HCL buffer, captured by Ni-NTA, refolded by dialysis and purified on Q Sepharose FastFlow (QFF) chromatography. The final protein buffer was 20 mM Hepes (pH 7.5), 150mM NaCl, 10 mM Methionine, 10% glycerol, 5mM DTT. Full length WT His-hVDR and WT Flag-hRXRα were expressed in Baculovirus system and purified by Ni-NTA/SEC or Flag/SEC, respectively, as described previously.[41]
HDX mass spectrometry was performed with a fully automated system as described previously.[13, 14, 31] Briefly, a CTC Twin PAL liquid handling robot (LEAP technologies, Carborro, NC) was interfaced with either a linear ion trap mass or orbitrap mass spectrometer (LTQ & Exactive respectively, Thermo Electron, San Jose, CA). 4μL of protein solution was diluted to 20μL with D2O buffer. Following on exchange, the reaction was quenched with a cold 3M urea solution containing 1%TFA. The sample was then passed over an immobilized pepsin column (prepared in house), desalted with a C8 sample trap (1mm × 10mm; Thermo Fisher Hypersil gold) and eluted across a C18 HPLC (1mm × 50mm; Thermo Fisher Hypersil gold) column into the ESI source of the mass spectrometer. All HDX values are the average of three or four individual on-exchange experiments acquired in a random order.
HDX data analysis was performed with an updated version of our HDX software platform[35, 42]. Statistical tests were performed with Prism v5.0 (Graphpad Software, CA) and in custom software with the Apache Commons Mathematics Library. Hierarchical clustering was performed with SpotFire Decisionsite v 9.1 for functional genomics (TIBCO, Palo Alto, CA). For HCA analysis data were clustered according to Wards method.[43]
3. Results and Discussion
In this work we establish a workflow for the analysis of differential HDX data that is compatible with large datasets. The complete experimental workflow is shown in Figure 1 and the individual components of the scheme are described and evaluated in detail below.
Figure 1.
Schematic representation of our differential HDX data analysis workflow.
3.1 HDX Screening data set
We have previously shown that single time point differential HDX data can provide rapid discriminatory information between multiple synthetic and endogenous PPARγ ligands.[14] To illustrate this approach, Figure 2(A) shows that a reduction in exchange was observed for PPARγ-LBD residues 240–252 ([M+3H]3+) following binding of the partial agonist MRL-24, but not full agonist rosiglitazone. The results from a two tailed t-test of the 30s data show that rosiglitazone is not significantly different from apo receptor while the p-value for the MRL-24 data was < 0.0001 (n=4). For this region of the receptor, the distinction between rosiglitazone and MRL24 can be made from the 30s exchange data, and therefore a complete differential HDX data set is not required to distinguish between these two functionally selective ligands. We have demonstrated that ligand discrimination at the 30s time point is possible for all regions of PPARγ contained within the dataset and as such acquisition and analysis of a complete HDX dataset is not required to differentiate functionally selective PPARγ modulators. For additional details and supporting data pertaining to the single time point HDX method we direct the reader to our recent publication.[14] We do acknowledge the possibility that certain ligands may exhibit significant changes in HDX behavior which are not reflected to statistical significance in the 30s data. Regardless, this does not diminish the validity of information obtained from the 30s HDX dataset.
Figure 2.
(A). HDX data for PPARγ LBD in complex with full agonist rosiglitazone and the partial agonist MRL-24. A two tailed t-test of the 30s data showed no significant difference between ligand free PPAR (apo) and the rosliglitazone bound receptor. In contrast, a significant reduction in exchange was observed following binding of MRL-24. It should be noted that each differential HDX experiment contains its own “apo” internal control. For clarity, only the apo data associated with the rosiglitazone are displayed. There was complete overlap between the two apo samples (see text for a discussion on the repeatability of our automated platform for data acquisition). Figure 1(B). Differential HDX data for the PPARγ beta sheet region 159–169 ([M+2H]2+ ion) following 30s of exchange. Data are shown for 10 ligands of interest. The chart is annotated with the results from the Tukey multiple comparison test. The letters above each bar represent those ligands that exhibit a significant difference with a p-value <0.05. For example, A(rosi) is significantly different from B, C, D and E.
To establish a robust data analysis workflow for future HDX screening efforts we have revisited a differential HDX dataset obtained for PPARγ LBD in complex with ten ligands of interest.[14] The complete data set is shown in Table 1. The mean percent change (n=4) in HDX kinetics (and standard deviation) for each region (peptide start-end (z)) of the receptor is shown for all ligands. A negative number indicates that the measured %D (30 s on-exchange) for the ligand bound receptor was less than the measured %D for the unliganded apo receptor.
Table 1.
HDX Screenting data for PPARγLBD with synthetic, and endogenous, ligands.
| Peptide | Rosi | MRL20 | MRL24 | GW1929 | BVT13 | MCC555 | 15PGJ2 | 9SHODE | 13SHODE | 15SHETE |
|---|---|---|---|---|---|---|---|---|---|---|
| 30–40 (+2) | −1 (2) | −9 (0) | −3 (3) | −16 (1) | −22 (1) | 3 (1) | −4 (4) | 4 (2) | 1 (1) | −2 (2) |
| 97–105 (+2) | −30 (19) | −66 (3) | −51 (2) | −42 (3) | −63 (0) | −3 (2) | NaN | −7 (4) | −6 (3) | −11 (1) |
| 117–127 (+2) | −1 (2) | −7 (3) | 0 (5) | −17 (3) | −29 (1) | 4 (1) | −6 (4) | 5 (2) | −1 (2) | −8 (5) |
| 136–145 (+2) | −2 (2) | −6 (0) | 0 (2) | −9 (1) | −14 (0) | 2 (2) | −4 (2) | 1 (2) | −2 (1) | −4 (3) |
| 136–142 (+2) | −3 (5) | −7 (2) | 7 (5) | −14 (1) | −26 (1) | −1 (1) | −8 (4) | −2 (2) | −3 (1) | −9 (3) |
| 159–169 (+2) | −10 (7) | −26 (4) | −29 (8) | −28 (5) | −44 (2) | −1 (1) | −21 (4) | 0 (1) | −6 (2) | −13 (3) |
| 170–188 (+3) | −10 (6) | −25 (3) | −14 (5) | −26 (3) | −34 (1) | −5 (1) | −13 (3) | 1 (3) | −6 (2) | −11 (5) |
| 170–181 (+3) | −10 (5) | −19 (1) | −18 (7) | −18 (6) | −26 (2) | −2 (0) | −16 (2) | −1 (2) | −4 (2) | −10 (2) |
| 182–188 (+2) | −8 (14) | −37 (4) | −29 (2) | −34 (4) | −46 (0) | 2 (2) | −19 (6) | 1 (4) | −7 (3) | −16 (8) |
| 189–196 (+2) | −6 (9) | −31 (1) | −9 (3) | −22 (1) | −36 (1) | 1 (1) | −16 (4) | 0 (2) | −5 (2) | −13 (6) |
| 189–195 (+2) | −7 (9) | −26 (1) | −17 (2) | −24 (2) | −40 (1) | 2 (2) | −18 (4) | 2 (3) | −6 (3) | −14 (8) |
| 208–219 (+2) | −1 (1) | −4 (2) | −2 (3) | −8 (4) | −16 (1) | 1 (0) | −5 (2) | 1 (2) | −1 (1) | −5 (3) |
| 209–219 (+2) | −3 (2) | −5 (3) | −3 (4) | −11 (5) | −20 (1) | 1(1) | −6 (2) | 4 (2) | −1 (1) | −6 (4) |
| 220–226 (+1) | 1 (1) | −6 (1) | 0 (8) | −17 (7) | −25 (2) | 4 (1) | −5 (5) | 6 (2) | 0 (2) | −6 (4) |
| 238–249 (+2) | −4 (3) | −6 (3) | −4 (9) | −11 (7) | −14 (3) | 0 (2) | −3 (4) | 2 (1) | −1 (2) | −4 (1) |
| 239–249 (+2) | −3 (3) | −2 (2) | −1 (7) | −7 (7) | −2 (3) | 1 (2) | −1 (3) | 2 (1) | 3 (2) | −2 (1) |
| 240–249 (+2) | −5 (5) | −6 (3) | −4 (9) | −6 (9) | −6 (4) | −1 (3) | −2 (3) | 0 (1) | 0 (3) | −2 (2) |
| 250–260 (+2) | −5 (3) | −13 (3) | −9 (7) | −19 (6) | −28 (2) | 2 (1) | −6 (2) | 0 (2) | −2 (1) | −6 (2) |
| 250–260 (+3) | −5 (3) | −10 (3) | −7 (5) | −8 (4) | −25 (2) | 2 (1) | −6 (2) | 0 (1) | −2 (1) | −7 (3) |
| 261–270 (+2) | −18 (15) | −32 (2) | −13 (3) | −28 (4) | −33 (2) | −9 (1) | −20 (3) | −3 (2) | −5 (2) | −13 (4) |
| 261–266 (+2) | −13 (12) | −33 (3) | −9 (5) | −31 (2) | −36 (2) | −3 (1) | −18 (4) | −3 (1) | −5 (1) | −14 (5) |
| 271–287 (+2) | −12 (6) | −13 (3) | −7 (8) | −9 (5) | −11 (4) | −5 (3) | −7 (3) | −3 (2) | −2 (3) | −4 (2) |
| 271–287 (+3) | −12 (6) | −13 (3) | −7 (7) | −9 (5) | −11 (4) | −5 (3) | −6 (3) | −3 (2) | −2 (3) | −4 (2) |
| 271–295 (+3) | −16 (8) | −14 (3) | −7 (7) | −8 (4) | −8 (5) | −5 (3) | −5 (3) | −3 (3) | −2 (3) | −3 (3) |
| 271–295 (+4) | −16 (8) | −15 (3) | −6 (8) | −9 (4) | −7 (5) | −5 (3) | −5 (3) | −3 (3) | −2 (3) | −3 (3) |
| 282–295 (+2) | −25 (13) | −18 (2) | −7 (9) | −8 (4) | −5 (7) | −9 (7) | −2 (3) | −8 (4) | −1 (6) | −2 (4) |
| 288–295 (+1) | −28 (14) | −19 (2) | −5 (11) | −7 (5) | −5 (6) | −4 (3) | −2 (3) | −3 (4) | −1 (4) | −2 (3) |
| 288–295 (+2) | −29 (15) | −18 (2) | −5 (12) | −10 (5) | −5 (7) | −5 (3) | −2 (3) | −4 (5) | −1 (5) | −2 (4) |
A bar chart representing the 30s HDX data for the 159–169 [M+2H]2+ peptide is shown in Figure 2(B). These data are plotted as the difference in HDX rate from the apo receptor for 10 PPARγ ligands. It should be noted that the HDX data for each ligand was acquired in a random order with its own internal “apo” or receptor only plus DMSO control.[13, 14] Although the internal control requires an increase in data acquisition and analysis time, the use of a control ensures that the precision of the experiment is minimally impacted by changes in environmental or experimental conditions such as pH, Temperature, and chromatographic retention time. The acquisition of data in this fashion allows data acquired over many days, or even months to years to be compared (see section 3.4 for more details).
3.2 Tukey multiple comparison procedure
The β-sheet region of PPARγ LBD has been implicated in a novel helix 12-independent mode of activation of the receptor.[12] Therefore the changes in HDX kinetics for this region upon ligand interaction are of interest. To establish the statistical significance of changes in HDX in this region for each ligand we employed a one way analysis of variance (optional) in conjunction with the Tukey multiple comparison procedure (Prism v5.0) in a fashion similar to that described by Hsu et al.[11] Results from this analysis are given in Table 2. In a pair wise fashion, ligands are compared and a P-value obtained. Annotation of Figure 2(B) illustrates ligands that have significant differences in differential HDX kinetics (P < 0.05) as compared to each other from those pairs that do not demonstrate significance. For example ligand “A” (rosiglitazone) was significantly different from ligands B, C, D, E but not F, G, H, I, J. It should be noted that although the Tukey comparison provides a cross comparison of all 10 ligands, a much larger dataset is generated. From this data set comprised of 10 ligands there are 45 comparisons (9+8+7+6+5+4+3+2+1) that must be performed (see Table 2). The results from the Tukey comparison for all peptides in the dataset are presented in Supplemental Table 1. This table illustrates the complexity of the expansion of the dataset (10 ligands and 28 peptides generate 1260 datapoints). It should also be noted that by presenting only the P-values from the cross comparison test crucial information on the direction of the change in HDX is lost. It may be shown that two ligands of interest are different, but is that change an increase or decrease in HDX kinetics? For the reasons outlined above, we choose to represent the results of the Tukey comparison data in combination with a histogram of the HDX data (Figures 2(B) and 3).
Table 2.
Tukey’s multiple comparison test for PPARγ peptide [159–169 + 2H]2+ (ISEGQFMTRE).
| Comparison | Mean Difference | P<0.05 ? | Summary |
|---|---|---|---|
| Rosi vs MRL20 | 15.41 | Yes | ** |
| Rosi vs MRL24 | 18.86 | Yes | *** |
| Rosi vs GW1929 | 17.80 | Yes | *** |
| Rosi vs BVT13 | 33.54 | Yes | *** |
| Rosi vs MCC555 | −9.080 | No | ns |
| Rosi vs 15PGJ2 | 10.24 | No | ns |
| Rosi vs 9SHODE | −10.31 | No | ns |
| Rosi vs 13SHODE | −4.197 | No | ns |
| Rosi vs 15SHETE | 2.512 | No | ns |
| MRL20 vs MRL24 | 3.453 | No | ns |
| MRL20 vs GW1929 | 2.393 | No | ns |
| MRL20 vs BVT13 | 18.13 | Yes | *** |
| MRL20 vs MCC555 | −24.49 | Yes | *** |
| MRL20 vs 15PGJ2 | −5.165 | No | ns |
| MRL20 vs 9SHODE | −25.72 | Yes | *** |
| MRL20 vs 13SHODE | −19.61 | Yes | *** |
| MRL20 vs 15SHETE | −12.90 | Yes | * |
| MRL24 vs GW1929 | −1.060 | No | ns |
| MRL24 vs BVT13 | 14.68 | Yes | ** |
| MRL24 vs MCC555 | −27.94 | Yes | *** |
| MRL24 vs 15PGJ2 | −8.618 | No | ns |
| MRL24 vs 9SHODE | −29.17 | Yes | *** |
| MRL24 vs 13SHODE | −23.06 | Yes | *** |
| MRL24 vs 15SHETE | −16.35 | Yes | ** |
| GW1929 vs BVT13 | 15.74 | Yes | ** |
| GW1929 vs MCC555 | −26.88 | Yes | *** |
| GW1929 vs 15PGJ2 | −7.558 | No | ns |
| GW1929 vs 9SHODE | −28.11 | Yes | *** |
| GW1929 vs 13SHODE | −22.00 | Yes | *** |
| GW1929 vs 15SHETE | −15.29 | Yes | ** |
| BVT13 vs MCC555 | −42.62 | Yes | *** |
| BVT13 vs 15PGJ2 | −23.30 | Yes | *** |
| BVT13 vs 9SHODE | −43.85 | Yes | *** |
| BVT13 vs 13SHODE | −37.74 | Yes | *** |
| BVT13 vs 15SHETE | −31.03 | Yes | *** |
| MCC555 vs 15PGJ2 | 19.32 | Yes | *** |
| MCC555 vs 9SHODE | −1.226 | No | ns |
| MCC555 vs 13SHODE | 4.883 | No | ns |
| MCC555 vs 15SHETE | 11.59 | No | ns |
| 15PGJ2 vs 9SHODE | −20.55 | Yes | *** |
| 15PGJ2 vs 13SHODE | −14.44 | Yes | * |
| 15PGJ2 vs 15SHETE | −7.733 | No | ns |
| 9SHODE vs 13SHODE | 6.109 | No | ns |
| 9SHODE vs 15SHETE | 12.82 | Yes | * |
| 13SHODE vs 15SHETE | 6.708 | No | ns |
P<0.001,
P<0.01,
P<0.05.
ns = not significant.
Figure 3.
Differential HDX data for the H3 (A) and H12 (B) regions of the PPARγ LBD. Annotations to the bar chart correspond to the results from a Tukey multiple comparison test (P<0.05). Rosi, MRL20, MRL24, GW1929, BVT13 and MCC555 are synthetic PPARγ modulators. 15PGJ2, 9SHODE, 13SHODE, 15SHETE are putative endogenous ligands.
3.3 Hierarchical cluster analysis
We have demonstrated how heat maps in combination with hierarchical clustering can be used to classify and display differential HDX data obtained from the analysis of the estrogen receptor alpha (ERα) in complex with various SERMS.[16] The advantage of the heat map approach is it provides a condensed view that can be expanded to many hundreds of ligands. This is in contrast to a multiple comparison table (similar to that shown in Supplemental Table 1) which would expand to contain many thousands of comparisons. For example, the comparison of 100 ligands would yield 4950 results per peptide, or 138,600 data points for the PPARγ peptides shown in Table 1. The complete PPARγ HDX dataset has therefore been plotted as a heat map and is shown in Figure 4. For this visualization we represent a reduction in HDX kinetics according to a blue color gradient, and an increase in HDX kinetics according to a red colored gradient. The grey color represents no statistical significance (p>0.05) in a two tailed t-test between the apo receptor DMSO only 30s HDX data and the receptor/ligand complex 30s HDX data. The inclusion of this t-test allows us to color only those regions that show a significant change and the blue/red color scheme maintains consistency across our HDX visualization components. Data were clustered according to Ward’s method as described in the Materials and Methods section.
Figure 4.
Heat map of differential HDX data for PPARγ LBD. Changes with Tukey-adjusted P-values <0.05 are colored according to the key. P-values > 0.05 are colored grey.
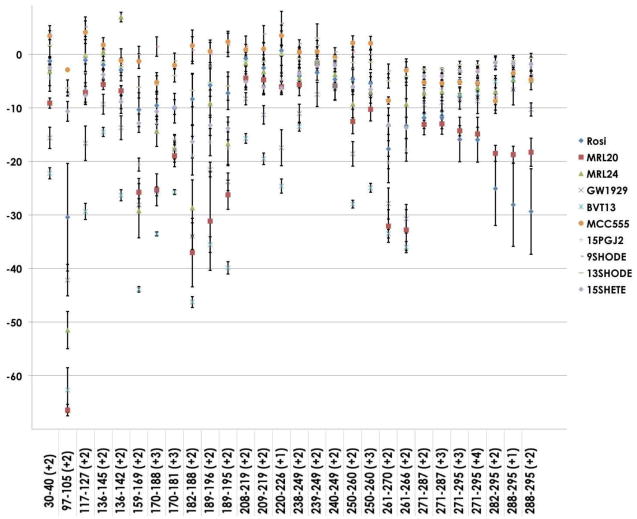
Clearly the heat map provides a detailed overview of the screening dataset. When more detailed information is required for a specific region of the protein, the Tukey comparison annotated histogram of the peptide of interest should be consulted. When information is required for a specific ligand, a plot showing the %D change for all ligands across all peptides would provide additional information. Figure 5 shows the HDX data plotted against all peptides in the dataset. The inclusion of error bars (standard errors, based on n=4) provides for a visual representation of the precision of the measurements, a key feature of the data that cannot be represented on a heat map. As such we will incorporate all three of these graphical components into future versions of our HDX data analysis software.
Figure 5.
Differential HDX data for PPARγ LBD with 10 ligands.
3.4 HDX data provides new information about the PPARγ LBD
Figure 3 shows the HDX data and Tukey comparison for two regions of the receptor implicated in the regulation of its activity. Helix three (Figure 3(A)) is located in the center of the ligand binding pocket and interacts with many of the synthetic ligands. For example MRL20 is positioned closer to H3 than MRL24 (PDB:2Q59 and PDB:2Q5P, Bruning et al Figure 8.[12]) and is within contact distance of Ile281, Gly284, Cys285, Arg288 and Ala292 (numbering scheme according to full length PPARγ). The proximity of the ligand to H3 results in a marked decrease in HDX kinetics (a reduction of 66% ±3 from the apo value (87% ± 4)). MRL24 which is located further from H3 in the x-ray structure, reduced the dynamics of the receptor by 51% (±2). This change between the two ligands is significant in the Tukey comparison (<0.05). These data highlight the ability of the HDX data to reflect changes in ligand binding within the receptor. It is apparent from Figure 3(A) that none of the putative endogenous agonists of the receptor perturb the dynamics of H3 to the same extent as the synthetic ligands. No data could be obtained for 15PGJ2 which is a known to form a covalent bond to cysteine residue 103 of the receptor (Cys307 in full length PPARγ).
Helix 12 (H12) has been described as the “master switch” of nuclear receptors including PPARγ.[44] H12 along with H3–H4 loop comprise the so-called AF-2 (activation function-2) coactivator binding site. Deletions of H12 or the point mutation Y473A (position in the full length PPARγ sequence) renders the PPARγ silent to full agonists. Previous studies we have shown that full agonists reduced HDX kinetics of the AF-2 surface. Thus, HDX analysis is sensitive not only to binding, but to reading out dynamics that can have a direct impact on receptor function. The HDX data for H12 are shown in Figure 3(B). As expected, the most significant reduction in HDX was observed for the synthetic agonist rosiglitazone.
Although the visualization of the data in Supplemental Table 1 are somewhat difficult due to the large number of data points displayed, results for the peptide spanning residues 239–249 (LKLNHPESSQL [M+2H]2+) stand out because there are no statistically significant changes in this region (H9–H10 link). Therefore, for this set of ligands, the dynamics of this region of the receptor can be determined to be totally insensitive to ligand binding. This is the only region of the receptor that exhibits this behavior.
3.5 Appraising the quality of the HDX MS platform
To evaluate the workflow described above with a larger dataset and to assess the precision of our system we have focused on a set of experiments performed with the vitamin D receptor (VDR). We have a significant ongoing effort to profile the HDX fingerprint of VDR ligands, including small molecules, fragments, cofactor proteins and DNAs. Because all the data are readily accessible from the relational database, we extracted the differential HDX data for 40 VDR ligands acquired over an eight month time period. Each VDR experiment contained data for 34 different peptides and total 4191 discrete %D values. In order to maintain the quality of our data, each experiment contained a DMSO internal control and all were acquired with the same orbitrap mass spectrometer. Subsequent to acquisition all mass spectral data were visually inspected to assess the quality of the measured isotopic distribution. The manual inspection is time-consuming, however we believe this is an important part of the QC process for HDX data analysis (at least until the reliability of fully automated approaches are demonstrated on a manually verified dataset of a similar complexity). From these data we can assess the performance of our HDX platform over a significant period of time.
The acquisition of the DMSO control with each experiment provides us with many replicate analyses of the same protein acquired under the same HDX and MS conditions. To evaluate the quality of our HDX data we determined the mean %D value for 34 peptides across the 40 HDX experiments (127 total %D measurements for each peptide) and the results are given in Table 3. The inset to Figure 6 shows the mean and standard deviation for five of these peptides following 30s of on-exchange (n=127). The peptide spanning residues 134–150 ([M+2H]2+ ion) gave a mean % HDX value of 51 and a standard deviation of only 3.7 percent. An alternate way to display the precision of the data is to plot the distance of these measured values from the mean values. Figure 6 shows all 4191 measurements (measured %D − mean %D for all 34 peptides (4191 values)) ordered from largest to smallest values. The data illustrate the precision of these replicate HDX experiments. It was determined that 4039 values were within 10% of the measured mean (>96%) and 3452 were within 5% of the mean (82%). These data show our ability to acquire HDX MS data with high precision over a significant period of time, and therefore validates our large scale cross comparison of multiple HDX datasets.
Table 3.
Data from 40 replicate HDX experiments acquired over an eight month time period.
| Peptide | Mean | Standard Deviation |
|---|---|---|
| 134–150(+2) | 51 | 3.7 |
| 134–150(+3) | 53 | 3.2 |
| 219–224(+1) | 106 | 7.3 |
| 225–233(+1) | 74 | 6.7 |
| 225–233(+2) | 92 | 5.8 |
| 234–244(+2) | 62 | 4.4 |
| 244–258(+3) | 23 | 6.9 |
| 244–259(+2) | 27 | 2.2 |
| 245–258(+3) | 27 | 6.0 |
| 245–259(+2) | 30 | 2.5 |
| 273–279(+2) | 88 | 5.9 |
| 274–285(+2) | 85 | 6.2 |
| 286–308(+3) | 87 | 4.7 |
| 300–308(+2) | 87 | 5.6 |
| 309–316(+1) | 37 | 4.0 |
| 309–316(+2) | 40 | 3.6 |
| 309–325(+3) | 43 | 4.8 |
| 309–329(+3) | 37 | 3.9 |
| 309–333(+3) | 27 | 3.3 |
| 317–325(+2) | 50 | 6.0 |
| 317–329(+2) | 40 | 4.1 |
| 336–351(+2) | 34 | 2.4 |
| 338–351(+2) | 43 | 2.9 |
| 352–365(+2) | 3 | 0.5 |
| 354–365(+2) | 3 | 1.1 |
| 366–379(+2) | 39 | 2.7 |
| 366–379(+3) | 39 | 2.6 |
| 366–383(+3) | 33 | 2.3 |
| 366–383(+4) | 34 | 2.5 |
| 384–389(+2) | 3 | 1.3 |
| 384–390(+2) | 3 | 0.8 |
| 384–403(+3) | 53 | 3.8 |
| 384–403(+4) | 54 | 3.9 |
| 390–403(+2) | 72 | 5.3 |
Values provided are the mean and standard deviation values obtained from 127 replicates.
Figure 6.
Precision of the VDR LBD 30s HDX experiment over an eight month time period. The inset to the figure shows the mean and standard deviation for five peptides calculated from 127 replicate analysis. The peptide spanning residues 134–150 was measured to have a mean %D value of 51%D and a standard deviation of 3.7. The main panel to the figure plots the difference of each replicate from the mean (33 peptides × 127 replicates = 4191 values). Data are ordered from high to low values. 96 percent of all values are within 10% of the mean.
Having established that our HDX system can operate with high precision over significant periods of time, we performed the cross comparison of 87 HDX datasets acquired over a two year period. The data were treated as described in sections 3.2 and 3.3. The heat map and cluster analysis are shown in Figure 7. This figure summarizes by far the largest single HDX experiment published to date and the acquisition and analysis of the data are ongoing.
Figure 7.
HDX heat map obtained from the analysis of VDR LBD in complex with 87 ligands of interest. Data are clustered and ordered according the the methods table. Changes with Tukey-adjusted P-values <0.05 are colored according to the key. P-values > 0.05 are colored grey.
4. Conclusions
We have outlined a data analysis workflow for integration into our HDX software and a schematic representation of this workflow is shown in Figure 1. We validated the approach with a small dataset of 10 PPARγ ligands. Data are compared with a Tukey cross comparison test and statistically significant changes are plotted in a heat map. The performance of our HDX system was then evaluated with a dataset comprised of 40 differential HDX experiments. The precision of the data exceeded our expectations. For example, over an eight month period 127 replicate measurements of the 134–150 [M+2H]2+ peptide yielded an mean of 51%D with a standard deviation of only 3.7%. Having established the precision of the system for an additional 36 peptides we determined that over 96 percent of our measured %D values were within 10 percent of their mean values. Finally we show the differential HDX data for 87 ligands acquired over a two-year period. Together we show how subtle, but statistically significant changes in HDX data can be used to probe the mechanism of action for synthetic and endogenous ligands of interest.
Supplementary Material
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health National Institute of Mental Health [Grant U54-MH074404] and by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM084041].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engen JR. Analysis of Protein Conformation and Dynamics by Hydrogen/Deuterium Exchange MS. Analytical Chemistry. 2009;81(19):7870–7875. doi: 10.1021/ac901154s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engen JR, Smith DL. Investigating protein structure and dynamics by hydrogen exchange MS. Analytical Chemistry. 2001;73(9):256a–265a. doi: 10.1021/ac012452f. [DOI] [PubMed] [Google Scholar]
- 3.Englander SW. Hydrogen exchange and mass spectrometry: A historical perspective. J Am Soc Mass Spectrom. 2006;17(11):1481–9. doi: 10.1016/j.jasms.2006.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan X, Maier CS. Hydrogen/deuterium exchange mass spectrometry. Methods Mol Biol. 2009;492:255–71. doi: 10.1007/978-1-59745-493-3_15. [DOI] [PubMed] [Google Scholar]
- 5.Kaltashov IA, Bobst CE, Abzalimov RR. H/D exchange and mass spectrometry in the studies of protein conformation and dynamics: is there a need for a top-down approach? Anal Chem. 2009;81(19):7892–9. doi: 10.1021/ac901366n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chetty PS, et al. Helical structure and stability in human apolipoprotein A-I by hydrogen exchange and mass spectrometry. Proc Natl Acad Sci U S A. 2009;106(45):19005–10. doi: 10.1073/pnas.0909708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gajiwala KS, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A. 2009;106(5):1542–7. doi: 10.1073/pnas.0812413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn JR, et al. The role of protein dynamics in increasing binding affinity for an engineered protein-protein interaction established by H/D exchange mass spectrometry. Biochemistry. 2006;45(28):8488–98. doi: 10.1021/bi0604328. [DOI] [PubMed] [Google Scholar]
- 9.Gertsman I, Komives EA, Johnson JE. HK97 maturation studied by crystallography and H/2H exchange reveals the structural basis for exothermic particle transitions. J Mol Biol. 2010;397(2):560–74. doi: 10.1016/j.jmb.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houde D, et al. Characterization of IgG1 Conformation and Conformational Dynamics by Hydrogen/Deuterium Exchange Mass Spectrometry. Analytical Chemistry. 2009;81(7):2644–2651. doi: 10.1021/ac802575y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu YH, Johnson DA, Traugh JA. Analysis of conformational changes during activation of protein kinase Pak2 by amide hydrogen/deuterium exchange. J Biol Chem. 2008;283(52):36397–405. doi: 10.1074/jbc.M805581200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruning JB, et al. Partial agonists activate PPAR gamma using a helix 12 independent mechanism. Structure. 2007;15(10):1258–1271. doi: 10.1016/j.str.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers MJ, et al. Probing protein ligand interactions by automated hydrogen/deuterium exchange mass spectrometry. Analytical Chemistry. 2006;78(4):1005–1014. doi: 10.1021/ac051294f. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers MJ, et al. A two-stage differential hydrogen deuterium exchange method for the rapid characterization of protein/ligand interactions. J Biomol Tech. 2007;18(4):194–204. [PMC free article] [PubMed] [Google Scholar]
- 15.Dai SY, et al. Unique Ligand Binding Patterns between Estrogen Receptor alpha and beta Revealed by Hydrogen-Deuterium Exchange. Biochemistry. 2009;48(40):9668–9676. doi: 10.1021/bi901149t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai SY, et al. Prediction of the tissue-specificity of selective estrogen receptor modulators by using a single biochemical method. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(20):7171–7176. doi: 10.1073/pnas.0710802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamuro Y, et al. Hydrogen/deuterium-exchange (H/D-Ex) of PPARgamma LBD in the presence of various modulators. Protein Sci. 2006;15(8):1883–92. doi: 10.1110/ps.062103006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan X, et al. Dynamics and ligand-induced solvent accessibility changes in human retinoid X receptor homodimer determined by hydrogen deuterium exchange and mass spectrometry. Biochemistry. 2004;43(4):909–17. doi: 10.1021/bi030183c. [DOI] [PubMed] [Google Scholar]
- 19.Yan X, et al. Investigation of ligand interactions with human RXRalpha by hydrogen/deuterium exchange and mass spectrometry. J Am Soc Mass Spectrom. 2006;17(11):1510–7. doi: 10.1016/j.jasms.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Yan X, et al. Deuterium exchange and mass spectrometry reveal the interaction differences of two synthetic modulators of RXRalpha LBD. Protein Sci. 2007;16(11):2491–501. doi: 10.1110/ps.073019707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan XG, et al. Dynamics and ligand-induced solvent accessibility changes in human retinoid X receptor homodimer determined by hydrogen deuterium exchange and mass spectrometry. Biochemistry. 2004;43(4):909–917. doi: 10.1021/bi030183c. [DOI] [PubMed] [Google Scholar]
- 22.Edwards AA, et al. Conformational states of human purine nucleoside phosphorylase at rest, at work, and with transition state analogues. Biochemistry. 49(9):2058–67. doi: 10.1021/bi902041j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandra V, et al. Structure of the intact PPAR-gamma-RXR-alpha nuclear receptor complex on DNA. Nature. 2008:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spraggon G, et al. On the use of DXMS to produce more crystallizable proteins: structures of the T. maritima proteins TM0160 and TM1171. Protein Sci. 2004;13(12):3187–99. doi: 10.1110/ps.04939904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pantazatos D, et al. Rapid refinement of crystallographic protein construct definition employing enhanced hydrogen/deuterium exchange MS. Proc Natl Acad Sci U S A. 2004;101(3):751–6. doi: 10.1073/pnas.0307204101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Dynamics of the beta(2)-Adrenergic G-Protein Coupled Receptor Revealed by Hydrogen-Deuterium Exchange. Analytical Chemistry. 2010;82(3):1100–1108. doi: 10.1021/ac902484p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joh NH, et al. Modest stabilization by most hydrogen-bonded side-chain interactions in membrane proteins. Nature. 2008;453(7199):1266–70. doi: 10.1038/nature06977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busenlehner LS, et al. Location of substrate binding sites within the integral membrane protein microsomal glutathione transferase-1. Biochemistry. 2007;46(10):2812–22. doi: 10.1021/bi6023385. [DOI] [PubMed] [Google Scholar]
- 29.Busenlehner LS, et al. Structural elements involved in proton translocation by cytochrome c oxidase as revealed by backbone amide hydrogen-deuterium exchange of the E286H mutant. Biochemistry. 2008;47(1):73–83. doi: 10.1021/bi701643a. [DOI] [PubMed] [Google Scholar]
- 30.Busenlehner LS, et al. Mapping protein dynamics in catalytic intermediates of the redox-driven proton pump cytochrome c oxidase. Proc Natl Acad Sci U S A. 2006;103(42):15398–403. doi: 10.1073/pnas.0601451103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamuro Y, et al. Rapid analysis of protein structure and dynamics by hydrogen/deuterium exchange mass spectrometry. J Biomol Tech. 2003;14(3):171–82. [PMC free article] [PubMed] [Google Scholar]
- 32.Hotchko M, et al. Automated extraction of backbone deuteration levels from amide H/2H mass spectrometry experiments. Protein Sci. 2006;15(3):583–601. doi: 10.1110/ps.051774906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmblad M, Buijs J, Hakansson P. Automatic Analysis of Hydrogen/Deuterium Exchange Mass Spectra of Peptides and Proteins Using Calculations of Isotopic Distributions. Journal of the American Society for Mass Spectrometry. 2001;12:1153–1162. doi: 10.1016/S1044-0305(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 34.Weis DD, Engen JR, Kass IJ. Semi-Automated Data Processing of Hydrogen Exchange Mass Spectra Using HX-Express. Journal of the American Society for Mass Spectrometry. 2006;17(12):1700–1703. doi: 10.1016/j.jasms.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Pascal BD, et al. HD Desktop: An Integrated Platform for the Analysis and Visualization of H/D Exchange Data. Journal of the American Society for Mass Spectrometry. 2009;20(4):601–610. doi: 10.1016/j.jasms.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascal BD, et al. The Deuterator: software for the determination of backbone amide deuterium levels from H/D exchange MS data. Bmc Bioinformatics. 2007 doi: 10.1186/1471-2105-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazazic S, et al. Automated data reduction for hydrogen/deuterium exchange experiments, enabled by high-resolution Fourier transform ion cyclotron resonance mass spectrometry. J Am Soc Mass Spectrom. 2010;21(4):550–8. doi: 10.1016/j.jasms.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slysz GW, et al. Hydra: software for tailored processing of H/D exchange data from MS or tandem MS analyses. Bmc Bioinformatics. 2009;10 doi: 10.1186/1471-2105-10-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikamanon P, et al. “TOF2H”: A precision toolbox for rapid, high density/high coverage hydrogen-deuterium exchange mass spectrometry via an LC-MALDI approach, covering the data pipeline from spectral acquisition to HDX rate analysis. Bmc Bioinformatics. 2008;9 doi: 10.1186/1471-2105-9-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lou X, et al. Deuterium Distribution Estimation with Improved Sequence Coverage for HX/MS Experiments. Bioinformatics. 2010 doi: 10.1093/bioinformatics/btq165. online. [DOI] [PubMed] [Google Scholar]
- 41.Juntunen K, et al. Large-scale expression and purification of the human vitamin D receptor and its ligand-binding domain for structural studies. Biochem J. 1999;344(Pt 2):297–303. [PMC free article] [PubMed] [Google Scholar]
- 42.Pascal B, et al. Software for the calculation of backbone amide deuterium levels from H/D exchange MS data. Molecular & Cellular Proteomics. 2006;5(10):S90–S90. [Google Scholar]
- 43.Ward JH. Hierarchical Grouping to optimize an objective function. Journal of American Statistical Association. 1963;58(301):236–244. [Google Scholar]
- 44.Nettles KW, Greene GL. Ligand control of coregulator recruitment to nuclear receptors. Annual Review of Physiology. 2005;67:309–333. doi: 10.1146/annurev.physiol.66.032802.154710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.