Abstract
Background
Diastolic dysfunction associated with high blood pressure (BP) leads to cardiac remodeling and fibrosis and progression to congestive heart failure. B-type natriuretic peptide (BNP) has BP lowering, anti-fibrotic and anti-hypertrophic properties, which makes BNP an attractive agent for attenuating the adverse cardiac remodeling associated with hypertension. In the current study, we tested the effects of sustained cardiac proBNP gene delivery on BP, cardiac function and remodeling in spontaneously hypertensive rats (SHR).
Methods and Results
We used the myocardium-tropic adeno-associated virus serotype 9 (AAV9) vector to achieve continuously enhanced cardiac rat proBNP expression. In SHR, a single systemic administration of AAV9 vector allowed long-term, cardiac BNP overexpression, resulting in reductions in systolic and diastolic BP for nine months after injection. Left ventricular (LV) thickness, LV end-systolic dimensions and LV mass were reduced, while ejection fraction was significantly increased in BNP-treated compared to untreated SHR. Circumferential systolic strain and strain rate of the early phase of diastole were improved in BNP-treated compared to untreated SHR. Non-cardiac overexpression of BNP via AAV2 vector was not associated with changes in BP and plasma BNP in SHR. Furthermore, normal Wistar rats injected with AAV9 proBNP vector showed significantly reduced heart weights four weeks after injection without BP reduction.
Conclusions
AAV9 vector facilitates sustained cardiac proBNP overexpression improves LV function in hypertensive heart disease. Long-term proBNP delivery improved both systolic and diastolic function. The effects on cardiac structure and function occurred independently of BP lowering effects in normal Wistar rats.
Keywords: natriuretic peptide, BNP, hypertension, diastolic function, AAV9
INTRODUCTION
Hypertension is a highly common condition that, if not controlled, progresses toward more severe cardiovascular and renal morbidity. Its major clinical phenotype is hypertensive heart disease (HHD), which is characterized by diastolic dysfunction, cardiac remodeling and fibrosis. Over time, diastolic dysfunction evolves into systolic impairment, which leads to the worsening of overall cardiac function and to increased morbidity and mortality 1, 2. New evidence indicates that levels of the circulating cardiac B-type natriuretic peptide (BNP) are reduced in early stages of hypertension 3. Importantly, BNP plays a critical role in cardiorenal homeostasis and, through binding to theguanylyl cyclase-A receptor (GC-A), it increases sodium excretion, lowers blood pressure (BP), suppresses the renin-angiotensin-aldosterone system (RAAS), inhibits cardiomyocyte hypertrophy, proliferation of cardiac fibroblasts, and has potent pro-lusitropic properties 4–6. Therefore, a reduced production and/or release of this cardiovascular and renal protective hormone may expose to higher risk of worsening HHD.
Importantly, several experimental models, as well as genetic studies in humans, have also demonstrated a key role of BNP in the control of normal cardiac function and structure of BP. Indeed, the genetic murine BNP knockout model is characterized primarily by cardiac fibrosis 4, while genetic disruption of NPR-A results in murine models of hypertension, impaired sodium excretion, cardiac hypertrophy and fibrosis, and increased mortality 5, underscoring the cardioprotective roles of BNP. In line with these observations, the recent seminal study by Newton-Cheh et al. recently established in a cohort of 48,939 subjects that basal BP and risk for hypertension is associated with common genetic variants of the atrial natriuretic peptide (ANP) and BNP genes that affect their circulating levels 6. Reduced ANP and BNP levels were characterized by elevated BP and increased risk for HTN. In contrast, the presence of the single nucleotide polymorphism (SNP) rs5068, characterized by a significantly higher level of circulating ANP and BNP, was associated with a 15% reduction in odds of hypertension. Therefore, the inherent biological properties of BNP with its proven role in the control of cardiac structure and function, BP lowering and RAAS inhibiting properties, make this hormone an attractive therapeutic tool for disease states such as hypertension and HHD, which are characterized by elevated BP, cardiovascular remodeling and reduced levels of circulating BNP. The recent reports of an impaired endogenous production of this cardiovascular and renal protective hormone, in early stages of hypertension, make sustained BNP delivery strategies highly attractive and rational 3, 7.
The goal of the current study was to investigate the potential benefit of prolonged BNP production by innovative technologies in preventing the worsening of HHD in a model of spontaneous progressive hypertension. Here, in order to develop a continuous BNP-based therapy for HHD, we employed the myocardium-tropic adeno-associated virus (AAV) 9-based vector and examined the influence of long-term (up to nine months) rat proBNP expression in spontaneously hypertensive rats (SHR) after a single intravenous injection. We hypothesized that long-term cardiac proBNP delivery would improve global cardiac structure and performance in SHR. We further hypothesized that the beneficial cardiac effects are, at least in part, independent of BP reduction in normal Wistar rats.
METHODS
SHR and Wistar rats
Four week-old SHR and five week-old Wistar rats were purchased from Charles River. SHR served as a model of progressive HHD. Strains of rats, number of animals, treatment and duration of treatment in each experiment was summarized in Table 1. All animal studies were approved by the Institutional Animal Care and Use Committee.
Table 1.
Summary of the rats used in this study.
| Study | Strain | Treatment | Number | Duration (weeks) |
|---|---|---|---|---|
| Cardiac delivery | SHR | AAV9-Luc | 2 | 3 |
| Toxicology | SHR | Untreated | 3 | 3 |
| SHR | AAV9-Luc | 3 | 3 | |
| SHR | AAV9-BNP | 3 | 3 | |
| Pharmacokinetics/dynamics | SHR | Untreated | 8 | 40 |
| SHR | AAV9-BNP | 8 | 40 | |
| Non-cardiac delivery | SHR | Untreated | 5 | 8 |
| SHR | AAV2-BNP | 5 | 8 | |
| SHR | AAV9-BNP | 3 | 8 | |
| Normotensive rat study | Wistar | AAV9-GFP | 6 | 4 |
| Wistar | AAV9-BNP | 6 | 4 |
SHR, spontaneously hypertensive rats; Luc, luciferase; GFP, green fluorescent protein.
Plasmids
The codon-optimized rat pre-proBNP was synthesized by GenScript, and cloned into a lentiviral vector, pSIN-CSGWdlNotI. The BamHI-XhoI short fragment, which contains rat pre-proBNP and WPRE post-transcriptional regulatory element, was then cloned into the mammalian expression plasmid, pAAV-MCS (Stratagene), resulting in pAAV-rat-pre-proBNP.
AAV9 and AAV2 vectors
The AAV9 vector stocks were produced in human 293T cells using the helper-free transfection method according to the manufacturer’s protocol (Stratagene). For AAV9 vector production, we used AAV9 capsid-expressing plasmid pRep2Cap9 8 (kindly provided by Dr. James M. Wilson), while AAV2 vector was made with the AAV2 capsid-expressing plasmid, pAAV-RC (Stratagene). Firefly luciferase-, humanized recombinant green fluorescent protein (GFP)-, or rat proBNP-encoding AAV genome constructs were packaged. Three days after transfection, AAV9 vector-producing 293T cells were harvested for vector purification. The cells were lysed by freeze and thaw cycling, followed by ultracentrifuge concentration (62,500 rpm for 2 hr) through Optiprep Density Gradient Medium (Sigma). The resulting AAV9 vectors were desalted and further concentrated using Amicon Ultra-15 100k filtration (Amicon). The titers (genomic copy numbers/ml) of concentrated AAV9 vector stocks were determined by quantitative PCR using plasmid DNA standards and AAV genomic sequence-specific primers and fluorescent probe.
Non-invasive tail blood pressure measurement
BP of conscious rats were measured by the CODA High-Throughput Non-Invasive Tail BP System (Kent Scientific).
Echocardiography (ECHO) for non-invasive assessment of ventricular function and structure
To evaluate cardiac function and structure we performed both standard ECHO and Two-Dimensional Speckle-Derived Strain ECHO (2DSE) examinations at four and nine months post injections in the BNP-treated and the untreated SHR. We also performed standard ECHO and 2DSE in normal Wistar rats at 4 weeks after AAV9 injections. All ECHO examinations were performed by skilled sonographer (E.A.O.) blinded to the treatment. Detailed protocols for ECHO examinations were described in Supplemental Materials.
Acute experiment procedure
Rats for the acute protocol were anesthetized with isoflurane (1.5% in oxygen). Placement of PE-50 tubing into the carotid artery for BP monitoring and blood sampling were performed. A portion of the neck skin was removed, and the carotid artery were isolated and cleared. A cut was made with micro-scissors and a PE-50 tubing was introduced into the vessel for direct BP monitoring. Blood was drawn to evaluate toxicological reactions in AAV9-BNP transduced rats, to measure BNP and cGMP. At the end of the experiments, we harvested rat organs for further analysis.
Masson’s Trichrome staining
The sections of frozen cardiac samples were assessed by Trichrome staining for collagen contents. Percent blue signals were analyzed by KS400 Image Analysis Software (version 3.0, Zeiss).
Sample size and statistical analysis
Groups were compared by unpaired t-test, changes within-groups were assessed by paired t-test. Comparisons of BP values between groups were performed by two-way ANOVA for repeated measurements. Data were expressed as mean ± SD. Significance was accepted for p<0.05.
Detailed protocols for cell culture, immunostaining, immunoblotting, IVIS Luciferase imaging, and toxicological testings were described in Supplemental Materials.
RESULTS
In vitro expression and localization of proBNP
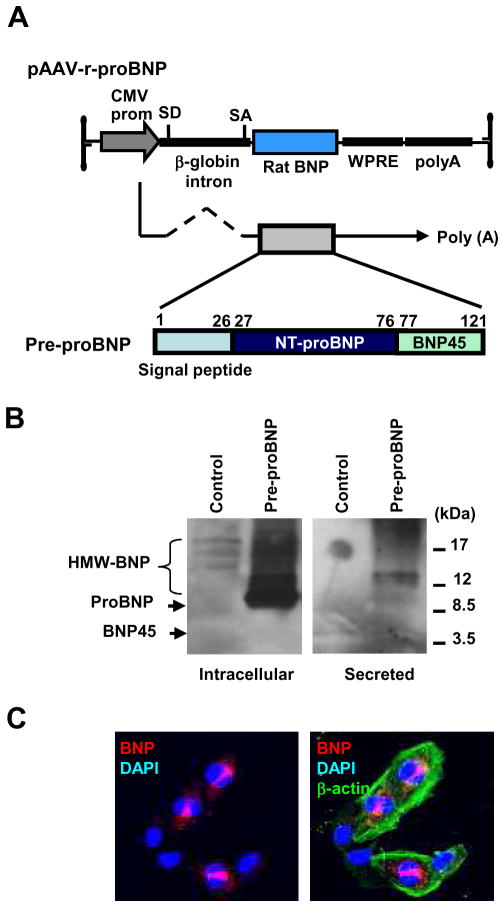
After successfully engineering AAV9 encoding for the rat pre-proBNP, which comprises the signal peptide (SP), NT-proBNP and BNP1-45 domains (Figure 1A), we verified the protein expression of BNP in 293T cells. Non-glycosylated proBNP (10 kDa) and high molecular weight (HMW, 12–24 kDa) glycosylated proBNP were detected in the cell lysates by Western blotting analysis. Of note, HMW forms of BNP were predominantly secreted (Figure 1B). When the pre-proBNP was expressed in mouse cardiomyocytes (HL-1 cells 9, kindly provided by Dr. William C. Claycomb) and analyzed by immunostaining with an anti-rat BNP1-45 antibody, clear supranuclear localization of immunoreactive BNP (red) as well as discrete cytoplasmic body signals (red) were detected (Figure 1C).
FIGURE 1. Generation of pre-proBNP-expressing AAV9 vector.
(A) Schematic representation of the AAV9 vector encoding rat pre-proBNP. SD; splice donor, SA; splice acceptor, WPRE; woodchuck hepatitis virus post-transcriptional regulatory elements. CMV, Cytomegalovirus; SD, splice donor; SA, splice acceptor; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element. (B) Verification of proBNP expression in 293T cells transfected with pAAV-r-proBNP. Immunoreactive BNPs in cell lysates and culture supernatants were detected by anti-rat BNP45 antibody. HMW, high molecular weight. (C) Immunostaining of rat BNP in pAAV-r-proBNP-transfected mouse cardiomyocytes (HL1 cells). When the plasmid encoding for the full length of pre-proBNP was introduced in HL1 cells, immunoreactive BNP signals were detected supra-nuclearly and in cytoplasmic secretory vesicles.
In vivo cardiac specific tropism of AAV9 vector-mediated gene transfer
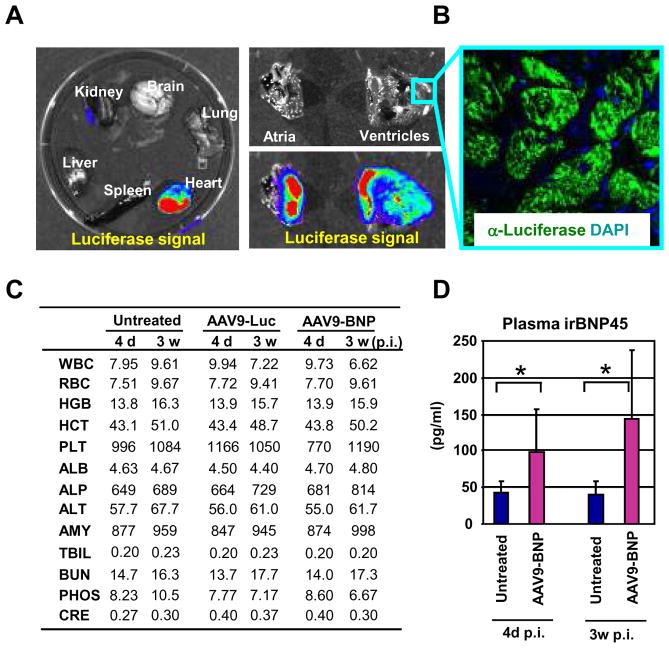
AAV9-based vectors have been shown to be cardiotropic. We therefore packaged the rat pre-proBNP- or firefly luciferase-expressing vectors in AAV9 capsid and examined the influence of AAV9 vector-mediated gene delivery in SHR. Four week-old SHR (n=2) were used for this study. Three weeks after tail intravenous injection of AAV9 carrying luciferase (1012 genome copy/animal), we determined the tissue specificity of the AAV9 vector by luciferase expression in the SHR, which demonstrated high levels of luciferase expression in myocardium (Figure 2A–B). To confirm luciferase expression in the heart, we stained the heart section with anti-luciferase antibody and the signals were detected predominately in the cardiomyocytes (Figure 2B). When we injected AAV9-luciferase (n=3) and AAV9-pre-proBNP (n=3) and compared acute (4 days) and chronic (3 weeks) toxicological responses to those of the untreated SHR (n=3), no notable toxicity was observed among these three groups of rats (Figure 2B). However, plasma BNP, by rat BNP1-45 ELISA, was significantly higher in the AAV9-pre-proBNP-treated SHR compared with untreated SHR both at four days and three weeks after injections (Figure 2D), thus confirming the sustained BNP expression upon AAV9 vector-mediated gene delivery.
FIGURE 2. AAV9 vector facilitates efficient cardiac gene delivery in SHR.
(A) Distribution of luciferase activities in firefly luciferase-expressing AAV9 vector-administered SHR organs was monitored by Xenogen IVIS Living Image. Strong luciferase expression in heart demonstrated efficient cardiac gene delivery by AAV9 in SHR (n=2) (left panel). Higher magnifications of Luciferase signals were found in both atria and ventricles (right panels). (B) Detection of luciferase by immunostaining. Luciferase in the sections of heart ventricles were detected by anti-firefly luciferase antibody, confirming the efficient cardiac luciferase gene expression upon AAV9 vector-mediated gene transfer. (C) No apparent toxicity observed in AAV9 vector-administered SHR. Toxicological and pharmacological parameters in vector-injected SHR (n=3) were measured at 4 days and 3 weeks after vector administration. Averages of three rats were shown. *P<0.05 vs respective untreated controls. WBC, white blood cells; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; PLT, platelets; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transferase; AMY, amylase; TBIL, total bilirubin; BUN, blood urea nitrogen; PHOS, phosphorus; CRE, creatinine. (D) Sustained BNP expression in the proBNP-expressing vector-administered rats (n=3). The levels of plasma immunoreactive BNP were measured at 4 days and 3 weeks after vector administration by the rat BNP45 ELISA. Error bars indicate ± SD. *P<0.05 vs respective untreated controls.
Effects of sustained proBNP expression in SHR
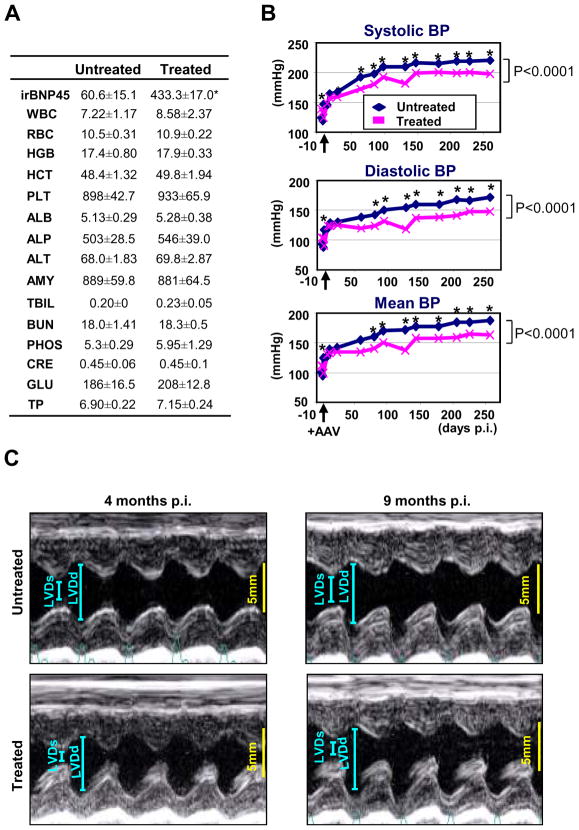
Next, we monitored the effects of sustained proBNP expression in SHR through cardiac proBNP delivery by AAV9 vector. Four months after AAV9-pre-proBNP injections in SHR, there was no toxicological reaction compared to untreated SHR. Importantly, plasma immune reactive BNP was significantly higher in the AAV9-pre-proBNP-treated group compared with the untreated SHR (Figure 3A). Tail cuff BP measurements indicate significant reduction in SBP, DBP and MAP in the AAV9-pre-proBNP-treated SHR as compared with untreated SHR. Indeed, in the AAV9-pre-proBNP, SBP was significantly reduced one month after injection and followed by a reduction in both DBP and MAP at two months post-injection as compared with the untreated SHR. These reductions in SBP, DBP and MAP in conscious rats throughout the nine month study (Figure 3B).
FIGURE 3. Effects of long-term BNP overexpression in SHR.
(A) No apparent toxicity observed in SHR at 4 months after proBNP-expressing AAV9 vector-administration. Toxicological and pharmacological parameters of the treated and untreated SHR were shown (n=4). No toxicity was observed in the rats, while plasma immunoreactive BNP45 was significantly elevated in the AAV9 vector-treated group. WBC, white blood cells; RBC, red blood cell; HGB, hemoglobin; HCT, hematocrit; PLT, platelets; ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transferase; AMY, amylase; TBIL, total bilirubin; BUN, blood urea nitrogen; PHOS, phosphorus; CRE, creatinine; GLU, glucose; TP, total protein. (B) BP measurements of proBNP-expressing AAV9 vector-administered SHR. BP in treated and untreated SHR were measured by tail-cuff method (n=8). *P<0.05 vs respective untreated controls. (C) M-mode echocardiography of untreated and AAV9-proBNP treated SHR at four and nine months post AAV9 vector injection. AAV9 vector-treated SHR had significantly improved diastolic functions at four months and both diastolic and systolic functions at nine months as compared with untreated SHR. LVDs, left ventricular end-systolic dimension; LVDd, left ventricular end-diastolic dimension.
Echocardiographic parameters in untreated SHR and in AAV9 pre-proBNP treated SHR are summarized in Table 2. While no difference was detected in HR between AAV9 pre-proBNP treated and untreated SHR both at four and nine months post injection, echo analysis indicated a significant improvement of diastolic function at four and nine months as well as systolic function at nine months post injection in AAV9 pre-proBNP treated SHR as compared with untreated SHR (Figure 3C). Of note, EF, PWTd, LVDd, and dSR-E circumferential were improved and LVMi was lower at nine months in the AAV9 pre-proBNP treated SHR even when compared to four months untreated SHR (Table 2).
Table 2.
Echocardiographic parameters in untreated (n=8) and BNP treated SHR (n=8).
| 4 Month p.i. | 9 Months p.i. | |||
|---|---|---|---|---|
| Untreated | BNP Treated | Untreated | BNP Treated | |
| HR | 403±27.3 | 392±22.3 | 381±25.2 | 393±13 |
| SWTd | 2.09±0.1 | 1.86±0.1* | 2.71±0.1† | 2.17±0.2*† |
| PWTd | 2.03±0.1 | 1.86±0.2* | 2.16±0.3 | 1.87±0.1*‡ |
| LVDd | 6.77±0.2 | 6.71±0.1 | 7.56±0.6† | 7.57±0.4†‡ |
| LVDs | 3.83±0.4 | 3.47±0.1* | 4.66±0.6† | 3.96±0.3*† |
| Ejection Fraction | 80±4.1 | 85±1.8* | 74±4.5† | 83±2.1*†‡ |
| LV Mass Index | 0.44±0.01 | 0.4±0.02* | 0.49±0.01 | 0.4±0.01*†‡ |
| sSR Circumferential | −4.6±0.7 | −4.75±0.6 | −3.74±0.4† | −5.04±0.4* |
| dSR-E Circumferential | 2.41±0.8 | 4.07±1.5* | 2.09±0.8 | 3.25±0.9*‡ |
| sSR-Radial | 7.17±1.1 | 6.77±0.8 | 6.34±1.6 | 8.13±1.9*† |
| dSR-E Radial | −3.29±1.2 | −5.72±2.5* | −2.41±1.3 | −4.57±2.1*† |
P<0.05 vs respective Untreated;
P<0.05 vs four months within group;
P<0.05 between nine months BNP-treated and four months Untreated.
HR, heart rate; SWTd, septal wall thickness at end diastole; PWTd, posterior wall thickness at end diastole; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; sSR, systolic strain rate; dSR, diastolic strain rate.
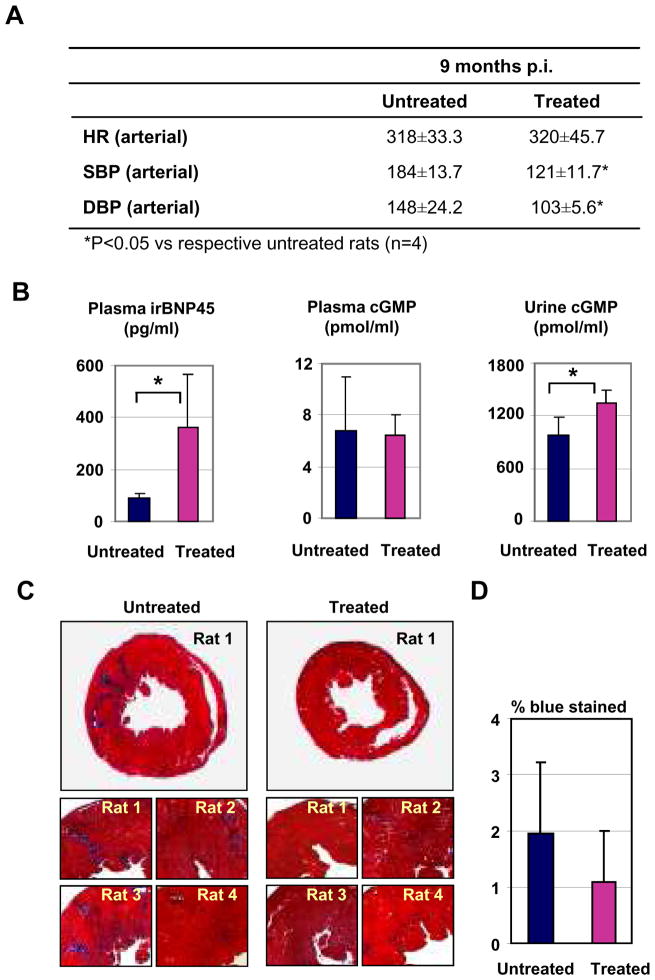
At nine months post-injection, four rats per group were sacrificed for acute experiments. Direct intra-carotid systolic and diastolic blood pressure was reduced in the AAV9 pre-proBNP treated anesthetized SHR (Figure 4A), while no significant differences were found in weights and heart rates between treated and untreated rats. The heart weight corrected for the body weight was significantly reduced in the BNP-treated as compared with the control SHR (0.37±0.01 vs 0.43±0.02, respectively, p<0.05). Plasma BNP was higher in the BNP-treated as compared with the control SHR (Figure 4B). Although plasma cGMP was not different, urinary cGMP was greater in the BNP-treated as compared with the control SHR (Figure 4B). Connective tissue (assessed by Mason’s trichrome staining) tended to increase in heart sections of untreated SHR as compared with AAV9-preproBNP treated SHR (Figure 4C–D).
FIGURE 4. Effects of long-term BNP overexpression on cardiac remodeling.
(A) Intra-arterial measurements of heart rate (HR), Systolic blood pressure (SBP) and diastolic blood pressure (DBP) in anesthetized, treated and untreated, SHR are indicated ± SD. (B) Plasma immunoreactive BNP45, plasma cGMP, and urinary cGMP are shown (n=4). Error bars indicate ± SD. *P<0.05 vs respective untreated controls. (C) Cross sections were stained by Mason’s trichrome staining for muscle fibers (red) and collagen/fibrosis (blue). Representative images of whole section of treated and untreated SHR (upper panels) and higher magnifications of heart images of rats (n=4, lower panels) are shown. (D) Connective tissue deposition was evaluated as percent blue signals/red signals by KS400 Image Analysis software. The averages of four heart samples in treated and untreated SHR groups are shown.
Non cardiac BNP-transduction by AAV2 vector
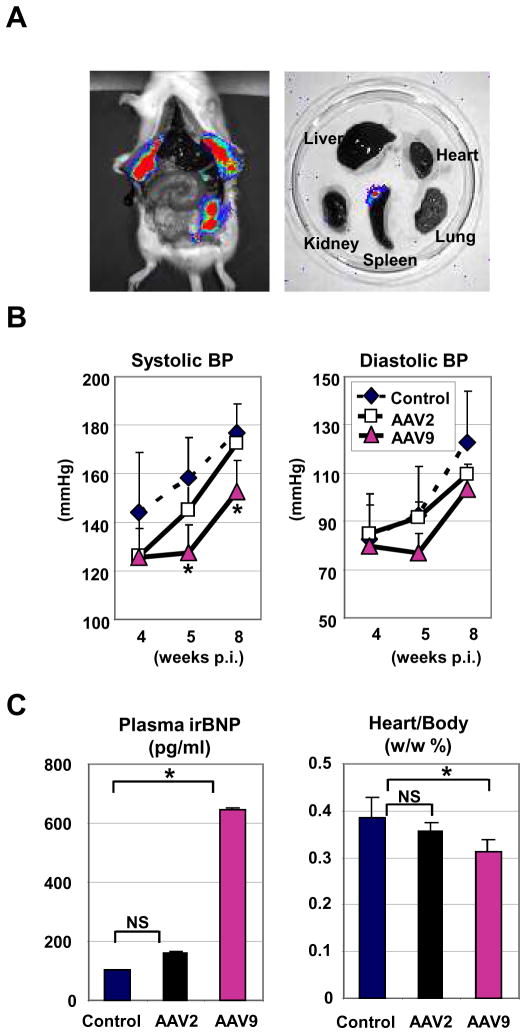
Next, we assessed the effects of non-cardiac proBNP gene delivery on BP, plasma BNP levels, and heart weight in SHR. For non-cardiac gene delivery, we administered conventional AAV2 vectors through intra-peritoneal injection. One month after administration of AAV2 vector carrying luciferase, we found high levels of luciferase expression in peritoneum, but not in heart (Figure 5A), confirming efficient, but non-cardiac, gene delivery by AAV2 vector. To assess the influence of non-cardiac proBNP gene delivery, SHR were injected with pre-proBNP-carrying AAV2 vector and compared with untreated (n=5) and AAV9-pre-proBNP vector-administered rats (n=3). Tail cuff BP measurements indicate that AAV2-pre-proBNP administration had no significant effects on SBP and DBP. In contrast, SBP was significantly reduced five and eight weeks after injection of the AAV9-pre-proBNP vector (Figure 5B). The pre-proBNP gene delivery by AAV9, but not AAV2, showed significantly higher plasma level of BNP (Figure 5C) and significant reduction in the heart weight/body weight ratio (Figure 5C), suggesting the requirement of cardiac pre-proBNP delivery for efficient BNP release and the anti-hypertrophic effects of BNP in SHR.
FIGURE 5. Effects of non-cardiac BNP overexpression in SHR.
(A) Distribution of luciferase activities in firefly luciferase-expressing AAV2 vector-administered SHR organs was monitored by Xenogen Living Image. Strong luciferase expression was evident in peritoneum by AAV2 in SHR (left panel), while no detectable luciferase expression was observed in heart (right panels). (B) BP measurements of proBNP-expressing AAV2 and AAV9 vector-administered SHR. BP in AAV2-treated (n=5), AAV9-treated (n=3) and untreated SHR (n=5) were measured by tail-cuff method. Error bars indicate ± SD. *P<0.05 vs respective untreated controls. (C) Plasma immunoreactive BNP45 and the heart weight/body weight ratios are shown. Error bars indicate ± SD. *P<0.05 vs respective untreated controls.
Effects of sustained proBNP expression in normotensive rats
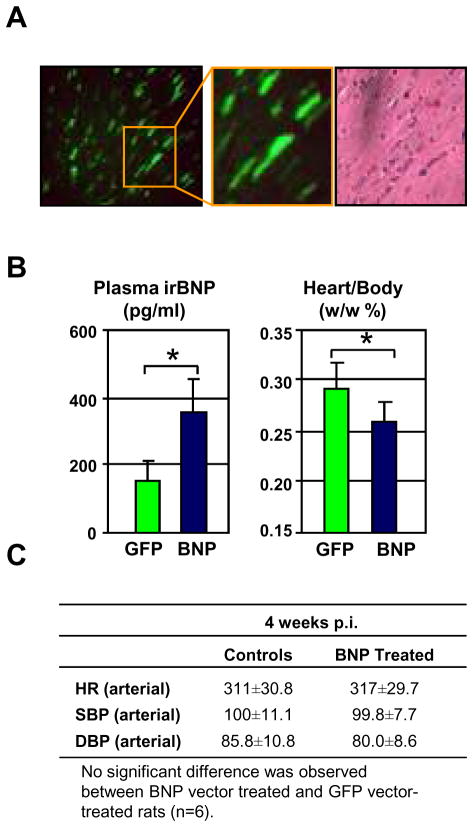
To investigate whether the beneficial effects on both cardiac structure and function observed in the BNP-treated SHR were mainly due to the sustained BP effects, we injected AAV9 encoding for GFP or pre-proBNP in normal Wistar rats (n=12). Six rats per group underwent echocardiographic examination 4 weeks after injections, and sacrificed for acute experiments thereafter. Normal rats treated with AAV9 carrying GFP (n=6) showed wide spread GFP expression in cardiomyocytes 4 weeks after injection, further confirming the cardiac transduction of the AAV9 vector (Figure 6A). Echocardiographic examination by strain analysis demonstrated that at 4 weeks post injection, AAV9-pre-proBNP treated normal rats (n=6) had a significantly improved systolic function compared with AAV9-GFP as indicated by a thinner SWTd, and higher sSR circumferential, while LVMi was only slightly reduced (p=0.07, NS)(Table 3). AAV9-pre-proBNP treated normal rats had significantly higher plasma level of BNP compared to the GFP-control rats (Figure 6B). Although direct intra-carotid BP measurement found similar SBP, DBP and MAP between the two groups, the heart weight corrected for the body weight was significantly reduced in the BNP-treated as compared with the GFP-control rats. (Figure 6B–C).
FIGURE 6. Effects of AAV9 vector-mediated long-term BNP expression in normal Wistar rats.
(A) Efficient cardiac transgene expression upon systemic AAV9 vector-administration. Normal rats were injected by GFP-carrying AAV9 vector. Four weeks after injection, heart sections were analyzed for GFP expression. (B) Plasma immunoreactive BNP and the heart weight/body weight ratios are shown. Error bars indicate ± SD. *P<0.05 vs respective untreated controls. (C) Intra-arterial measurements of heart rate (HR), Systolic blood pressure (SBP) and diastolic blood pressure (DBP) in anesthetized, treated and untreated, SHR are indicated ± SD.
Table 3.
Echocardiographic parameters in control (n=6) and BNP treated (n=6) normal rats.
| 4 Weeks p.i. | ||
|---|---|---|
| Controls | BNP Treated | |
| HR | 407±27.2 | 408±24.5 |
| SWTd | 2.03±0.15 | 1.80±0.11* |
| PWTd | 1.66±0.09 | 1.77±0.19 |
| LVDd | 7.0+0.67 | 7.1±0.53 |
| LVDs | 3.84±0.67 | 3.94±0.37 |
| Ejection Fraction | 83±1.72 | 83±4.17 |
| LV Mass Index | 0.35±0.01 | 0.33±0.01 |
| sSR Circumferential | −5.37±0.5 | −6.54±0.8* |
| dSR-E Circumferential | 5.09±0.7 | 5.98±1.0 |
| sSR-Radial | 7.63±1.5 | 9.08±1.7 |
| dSR-E Radial | −5.59±1.3 | −7.84±2.7 |
P<0.05 vs respective Controls.
HR, heart rate; SWTd, septal wall thickness at end diastole; PWTd, posterior wall thickness at end diastole; LVDd, left ventricular end-diastolic dimension; LVDs, left ventricular end-systolic dimension; sSR, systolic strain rate; dSR, diastolic strain rate.
DISCUSSION
Due to the low toxicity and efficient and long-term transduction in vivo, AAV vectors are currently evaluated in many clinical studies 10–12. Intriguingly, AAV vector genome packaged with the capsid protein from AAV9 was shown to efficiently deliver marker genes into cardiac tissue in neonatal and adult mice, as well as newborn rhesus monkeys 13, 14. The current study demonstrates successful in vivo cardiomyocyte transduction via AAV9 vector which facilitated sustained cardiac proBNP overexpression. Long-term proBNP delivery led to reduced BP and improved LV function and structure in an HHD rat model without any short- or long-term toxicological adverse effects or development of tolerance. Although long-term proBNP delivery improved both systolic and diastolic function, the effect on diastolic performance was more remarkable and preceded the improvement in systolic function in this HHD model. Importantly, the effects on cardiac structure and function occurred independently of BP lowering effects in normal Wistar rats.
HMW proBNP secretion
We and others have shown that pro-BNP glycosylation process is necessary for the release of proBNP and glycosylated proBNP is circulating in humans 15. We speculate that once glycosylated proBNP is released into the blood stream, a progressive deglycosylation occurs and pro-BNP is processed to mature BNP and NT-proBNP at the tissue level. In the current study, we further demonstrated that rat BNP is released from pre-proBNP-expressing 293T cells as a HMW form.
Effects on BP, plasma BNP, cGMP and urinary cGMP
Rat proBNP overexpression was associated with significant and sustained BP reduction in SHR. Indeed, SBP, DBP and MAP were lower in the BNP-treated as compared with the control SHR from two months up to nine months post-AAV9 injection. This reduction of BP was rather modest and occurred without changes in HR. Of note, BP was reduced at the vector dose used in the current study, while it did not completely normalized BP, which remained elevated throughout the period of observation. It is possible that a higher vector dose would result in a more profound BP reduction. Although the use of telemetry would have helped in better assessing BP changes throughout the study, we confirmed a significant BP lowering effect of BNP in unconscious BNP-treated SHR compared to the untreated SHR via direct intra-arterial BP measurements at the time of the acute experiments (9 months).
Plasma immunoreactive rat BNP45 was elevated in the BNP-treated as compared with the control SHR at four days, three weeks, and four and nine months post-injection, confirming a sustained overexpression of BNP in the heart. At nine months post-injection, plasma cGMP was not different between the BNP-treated and the control SHR. In contrast, urinary cGMP was increased in the BNP-treated as compared with the control SHR. Thus, the lack of elevation of plasma cGMP may be explained by the increased urinary cGMP excretion.
Effects on cardiac function and structure
Chronic overexpression of proBNP prevented the development of HHD which began at four weeks of age in the SHR. Indeed, AAV9 induced proBNP production resulted in a sustained and significant reduction (up to nine months) of SBP and DBP. Thorough echo analysis demonstrated a significant improvement of diastolic function at four months post transfection in the BNP-treated group as compared with the untreated SHR. Importantly, at nine months untreated SHR also developed signs of impaired systolic function which was prevented in the BNP-treated SHR. Of note, global cardiac function and remodeling were not only improved in the BNP-treated SHR compared to untreated SHR of the same age but also, BNP-treated SHR at nine months showed improved diastolic function and reduced cardiac hypertrophy even when compared with the untreated SHR at four months of age. This finding further supports the beneficial role of BNP in preventing cardiac dysfunction and remodeling. Of note, all these favorable actions occurred without signs of any short- or long-term toxicological side effects and BNP maintained its biological actions up to nine months post injection without developing tolerance. It should be noted that, although sustained, the BP reduction was minimal, thus further studies are required to address the pathogenic role of BNP in hypertension.
In SHR transduced with non-cardiac AAV2 vector, no increase in plasma immunoreactive BNP was observed. This could be due to a less efficient intracellular processing and/or release of rat BNP in non cardiac cells. Furthermore, in these AAV2-transduced SHR we did not observe changes in heart weight compared to untreated controls. Of note, however, a transgenic mouse model by Ogawa et al. with liver specific BNP overexpression, characterized by 10 fold increases in BNP mRNA, showed 10–100 fold increases in circulating BNP with concomitant BP reduction compared to their non-transgenic littermates 16. This perhaps indicates that also non cardiac cells are able to release BNP but only if higher vector dose is used. Indeed, in this study we used a comparable vector dose for both AAV2 and AAV9 studies.
Effects in normal rats
We also extended our studies to normal rats to investigate the anti-hypertrophic actions of BNP overexpression in the absence of hypertension. In this model, age induced systolic impairment was significantly ameliorated in the BNP-treated rats by echo strain analysis at 4 weeks. Also cardiac mass was reduced in the BNP-treated rats as compared with the controls and heart weight/body weight was significantly lower in the BNP-treated rats as compare to the controls. Importantly, the improved cardiac function and the reduced cardiac mass were observed after 4 weeks post injections of the AAV9 vector and occurred despite any difference in BP (measured directly intra-carotid) between the BNP-treated and the control group.
Chronic BNP delivery
Based upon the current studies, the possible use of chronic supplementation of the cardiorenal protective hormone BNP could be employed in hypertension, to prevent the progression toward more severe stages of HHD and the onset of heart and renal failure. Today, the use of chronic peptide delivery is far from being implemented in clinical practice, although recent studies in experimental hypertension have reported the successful conjugation of BNP, which elicited one week delivery of oral BNP in normal dogs and resulted in sustained BP reduction, suppression of the RAAS, increased natriuresis and diuresis in a canine hypertensive model 17, 18. Instead of oral delivery, in the current study we employed a gene transfection strategy which facilitated a nine month delivery of bioactive BNP with single intravenous injection of the AAV9 vector. Clearly, chronic overexpression of BNP in SHR reduced BP, decreased LVH, tended to reduce fibrosis, and improved systolic and diastolic function. To date, there is a large unmet need for novel therapies for diastolic dysfunction. Studies have reported the positive lusitropic actions of BNP in animal models of heart failure complementing studies in isolated cardiomyocytes. Currently the mechanism(s) for the anti-hypertrophic and positive lusitropic actions of BNP overexpression in the SHR are not clear and may be a direct effect on the cardiomyocyte and the extracellular matrix to enhance cardiomyocyte relaxation and/or reduce cardiac fibrosis and to the BP lowering effect observed throughout the study.
Discordant results have been reported to the use of this hormone in CHF patients treated with intravenous BNP and severe renal dysfunction. Moreover, an increased risk of mortality has also been reported secondary to the intravenous administration of BNP in acutely decompensated heart failure 19–21. In depth analysis of these data suggests that high doses of BNP are responsible for the manifestation of these side effects, as BP reduction leads, in turn, to decreased renal perfusion and function. Therefore, these studies suggest using great caution when BNP is used in humans. The latest results from the ASCEND-HF trial, however, indicate that the use of BNP in patients with acute heart failure is safe, but showed only a modest but not significant improvement of both symptoms and mortality 22. All together, these results may suggest that the optimal therapeutic approach for BNP could be achieved through a long-term low-dose delivery strategy. Here, we do not suggest the use of genetic therapy for the treatment of human HHD. However, the use of regulatory promoters that can activate BNP production only under increased need, such as elevated BP, can be used to minimize possible side effects of BNP on BP. We are currently evaluating the possible use of BNP promoter as a stress-inducible promoter to drive BNP expression in models of CHF and impaired renal perfusion characterized by low BP.
In conclusion, the current findings demonstrate the successful cardiac delivery of the AAV9 vector which mediated sustained cardiac proBNP overexpression without any short- or long-term toxicological effects and any signs of tolerance. Importantly, sustained cardiac BNP overexpression reduced BP and improved LV function in a model of progressive HHD after a single intravenous injection. Although long-term proBNP delivery improved both systolic and diastolic function, the effect on diastolic performance was more remarkable and appeared earlier during the development of HHD. Ultimately, sustained overexpression of BNP in SHR prevented the development of HHD as nine month old BNP-treated SHR had a significantly improved cardiac function and structure even when compared with four month old untreated SHR. Non cardiac BNP-overexpression was not associated with increase in plasma BNP, changes in BP, and reduced heart weight. The direct cardiac effects of overexpressed BNP seem to be, at least in part, independent of BP lowering action as indicated by the improved systolic function and reduced heart weight in the normotensives rats despite no changes in BP.
Supplementary Material
Acknowledgments
Funding Source: This work was supported by NIH RO1 HL098502-01A1 (to A. Cataliotti and Y. Ikeda), Mayo Foundation, Marriott Individualized Medicine Award, Bernard and Edith Waterman Pilot Grant (to Y. Ikeda) and grants NIH RO1 HL36634 and PO1 HL76611 (to J. C. Burnett) and by the M.I.U.R. (Ministero Istruzione Università e Ricerca) Italy, Progetto Rientro dei Cervelli (to A. Cataliotti).
Footnotes
Disclosures: None.
References
- 1.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 2.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part II: causal mechanisms and treatment. Circulation. 2002;105:1503–1508. doi: 10.1161/hc1202.105290. [DOI] [PubMed] [Google Scholar]
- 3.Cataliotti A, Macheret F, McKie PM, Rodeheffer RJ, Bailey KR, Malatino LS, Burnett JC. Deficiency of the cardiorenal protective hormone BNP in early stages of hypertension. Journal of Hypertension. 2010;28:E21–E21. [Google Scholar]
- 4.Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, Kasahara M, Hashimoto R, Katsuura G, Mukoyama M, Itoh H, Saito Y, Tanaka I, Otani H, Katsuki M. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kishimoto I, Rossi K, Garbers DL. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:2703–2706. doi: 10.1073/pnas.051625598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newton-Cheh C, Johnson T, Gateva V, Tobin MD, Bochud M, Coin L, Najjar SS, Zhao JH, Heath SC, Eyheramendy S, Papadakis K, Voight BF, Scott LJ, Zhang F, Farrall M, Tanaka T, Wallace C, Chambers JC, Khaw KT, Nilsson P, van der Harst P, Polidoro S, Grobbee DE, Onland-Moret NC, Bots ML, Wain LV, Elliott KS, Teumer A, Luan J, Lucas G, Kuusisto J, Burton PR, Hadley D, McArdle WL, Brown M, Dominiczak A, Newhouse SJ, Samani NJ, Webster J, Zeggini E, Beckmann JS, Bergmann S, Lim N, Song K, Vollenweider P, Waeber G, Waterworth DM, Yuan X, Groop L, Orho-Melander M, Allione A, Di Gregorio A, Guarrera S, Panico S, Ricceri F, Romanazzi V, Sacerdote C, Vineis P, Barroso I, Sandhu MS, Luben RN, Crawford GJ, Jousilahti P, Perola M, Boehnke M, Bonnycastle LL, Collins FS, Jackson AU, Mohlke KL, Stringham HM, Valle TT, Willer CJ, Bergman RN, Morken MA, Doring A, Gieger C, Illig T, Meitinger T, Org E, Pfeufer A, Wichmann HE, Kathiresan S, Marrugat J, O’Donnell CJ, Schwartz SM, Siscovick DS, Subirana I, Freimer NB, Hartikainen AL, McCarthy MI, O’Reilly PF, Peltonen L, Pouta A, de Jong PE, Snieder H, van Gilst WH, Clarke R, Goel A, Hamsten A, Peden JF, Seedorf U, Syvanen AC, Tognoni G, Lakatta EG, Sanna S, Scheet P, Schlessinger D, Scuteri A, Dorr M, Ernst F, Felix SB, Homuth G, Lorbeer R, Reffelmann T, Rettig R, Volker U, Galan P, Gut IG, Hercberg S, Lathrop GM, Zelenika D, Deloukas P, Soranzo N, Williams FM, Zhai G, Salomaa V, Laakso M, Elosua R, Forouhi NG, Volzke H, Uiterwaal CS, van der Schouw YT, Numans ME, Matullo G, Navis G, Berglund G, Bingham SA, Kooner JS, Connell JM, Bandinelli S, Ferrucci L, Watkins H, Spector TD, Tuomilehto J, Altshuler D, Strachan DP, Laan M, Meneton P, Wareham NJ, Uda M, Jarvelin MR, Mooser V, Melander O, Loos RJ, Elliott P, Abecasis GR, Caulfield M, Munroe PB. Genome-wide association study identifies eight loci associated with blood pressure. Nat Genet. 2009;41:666–676. doi: 10.1038/ng.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belluardo P, Cataliotti A, Bonaiuto L, Giuffre E, Maugeri E, Noto P, Orlando G, Raspa G, Piazza B, Babuin L, Chen HH, Martin FL, McKie PM, Heublein DM, Burnett JC, Jr, Malatino LS. Lack of activation of molecular forms of the BNP system in human grade 1 hypertension and relationship to cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2006;291:H1529–1535. doi: 10.1152/ajpheart.00107.2006. [DOI] [PubMed] [Google Scholar]
- 8.Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12993–12998. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 11.Fiandaca M, Forsayeth J, Bankiewicz K. Current status of gene therapy trials for Parkinson’s disease. Exp Neurol. 2008;209:51–57. doi: 10.1016/j.expneurol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Warrington KH, Jr, Herzog RW. Treatment of human disease by adeno-associated viral gene transfer. Hum Genet. 2006;119:571–603. doi: 10.1007/s00439-006-0165-6. [DOI] [PubMed] [Google Scholar]
- 13.Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, Zolotukhin I, Tarantal AF, Byrne BJ. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006;99:e3–9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 14.Bostick B, Ghosh A, Yue Y, Long C, Duan D. Systemic AAV-9 transduction in mice is influenced by animal age but not by the route of administration. Gene Ther. 2007;14:1605–1609. doi: 10.1038/sj.gt.3303029. [DOI] [PubMed] [Google Scholar]
- 15.Giuliani I, Rieunier F, Larue C, Delagneau JF, Granier C, Pau B, Ferriere M, Saussine M, Cristol JP, Dupuy AM, Merigeon E, Merle D, Villard S. Assay for measurement of intact B-type natriuretic peptide prohormone in blood. Clinical chemistry. 2006;52:1054–1061. doi: 10.1373/clinchem.2005.061770. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa Y, Itoh H, Tamura N, Suga S, Yoshimasa T, Uehira M, Matsuda S, Shiono S, Nishimoto H, Nakao K. Molecular cloning of the complementary DNA and gene that encode mouse brain natriuretic peptide and generation of transgenic mice that overexpress the brain natriuretic peptide gene. The Journal of clinical investigation. 1994;93:1911–1921. doi: 10.1172/JCI117182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cataliotti A, Chen HH, Schirger JA, Martin FL, Boerrigter G, Costello-Boerrigter LC, James KD, Polowy K, Miller MA, Malkar NB, Bailey KR, Burnett JC., Jr Chronic actions of a novel oral B-type natriuretic peptide conjugate in normal dogs and acute actions in angiotensin II-mediated hypertension. Circulation. 2008;118:1729–1736. doi: 10.1161/CIRCULATIONAHA.107.759241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cataliotti A, Schirger JA, Martin FL, Chen HH, McKie PM, Boerrigter G, Costello-Boerrigter LC, Harty G, Heublein DM, Sandberg SM, James KD, Miller MA, Malkar NB, Polowy K, Burnett JC., Jr Oral human brain natriuretic peptide activates cyclic guanosine 3′,5′-monophosphate and decreases mean arterial pressure. Circulation. 2005;112:836–840. doi: 10.1161/CIRCULATIONAHA.105.538520. [DOI] [PubMed] [Google Scholar]
- 19.Sackner-Bernstein JD, Kowalski M, Fox M, Aaronson K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. Jama. 2005;293:1900–1905. doi: 10.1001/jama.293.15.1900. [DOI] [PubMed] [Google Scholar]
- 20.Sackner-Bernstein JD, Skopicki HA, Aaronson KD. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation. 2005;111:1487–1491. doi: 10.1161/01.CIR.0000159340.93220.E4. [DOI] [PubMed] [Google Scholar]
- 21.Shah SJ, Teerlink JR. Nesiritide: a reappraisal of efficacy and safety. Expert Opin Pharmacother. 2007;8:361–369. doi: 10.1517/14656566.8.3.361. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez AF, O’Connor CM, Starling RC, Armstrong PW, Dickstein K, Gennivois D, Hasselblad V, Heizer GM, Komajda M, Massie B, McMurray JJ, Nieminen M, Reist C, Rouleau JL, Swdberg K, Califf RM. Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF) Circulation. 2010;122:2217. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.