Abstract
Complete loss or deletion of the long arm of chromosome 5 is frequent in myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML). The putative gene(s) deleted and responsible for the pathogenesis of these poor prognosis hematological disorders remain controversial. This study is a comprehensive analysis of previously implicated and novel genes for epigenetic inactivation in AML and MDS. In 146 AML cases, methylation of CTNNA1 was frequent, and more common in AML patients with 5q deletion (31%) than those without 5q deletion (14%), while no methylation of other 5q genes was observed. In 31 MDS cases, CTNNA1 methylation was only found in high risk MDS (≥RAEB2), but not in low risk MDS (<RAEB2), indicating CTNNA1 methylation may be important in the transformation of MDS to AML. CTNNA1 expression was lowest in AML/MDS patients with CTNNA1 methylation, although reduced expression was found in some patients without promoter methylation. Repressive chromatin marks (H3K27me3) at the promoter were identified in CTNNA1 repressed AML cell lines and primary leukemias, with the most repressive state correlating with DNA methylation. These results suggest progressive, acquired epigenetic inactivation at CTNNA1, including histone modifications and promoter CpG methylation, as a component of leukemia progression in patients with both 5q-and non 5q- myeloid malignancies.
Keywords: del(5q), monosomy 5, CTNNA1, methylation, myelodysplastic syndrome, acute myelogenous leukemia, methylation, Progressive silencing, AML transformation
Introduction
Myelodysplastic syndrome (MDS) represents a heterogeneous group of clonal bone marrow (BM) failure disorders with significant clinical morbidity and mortality. MDS typically progresses in severity over time with increased BM and peripheral blood (PB) blasts. Cases with greater than 20% blasts are considered acute myeloid leukemia with tri-lineage dysplasia (AML-TLD). The molecular events associated with progression from MDS to AML-TLD transformation are unknown (1, 2).
Loss of all (−5) or interstitial loss of the long arm of chromosome (Ch)5 [del(5q)], either as the sole karyotypic abnormality or part of more complex karyotypes, is frequent and has distinct implications for MDS and AML. The 5q– syndrome has unique clinical features, isolated 5q deletion and <5% BM blasts. With a low probability of leukemic transformation, it has a good prognosis compared to other −5/del(5q) disorders (3, 4). In contrast, del (5q) occurring with additional chromosomal abnormalities, or complete loss of Ch 5, has a median survival of 45 months compared to 146 months with isolated del(5q) (5).
The recurrent nature of chromosomal deletion suggests that 5q contains tumor suppressor gene(s) important to hematologic transformation. Detailed cytogenetic and molecular analyses have shed light on this complex genomic region (6,7). Boultwood et al. (8) narrowed the common deleted region (CDR) for good prognosis 5q-syndrome to an approximately 1.5-megabase interval at 5q33.1, flanked by D5S413 and GLRA1. This region is distinct from the proximal 5q deletion(s)at 5q31 in advanced MDS or AML (9–11). Despite efforts over the past 30 years, no biallelic deletions or point mutations within CDRs have been found in either 5q- syndrome or complex del (5q), suggesting alternative mechanisms of gene alteration.
One proposed mechanism is haploinsufficency. Loss of a single allele of EGR1 on 5q31 cooperates with mutations induced by alkylating agents in mouse models of malignant lymphoid and myeloid diseases (12). Distinct from this CDR, the ribosomal subunit protein RPS14 on 5q33 was identified as a candidate 5q-syndrome gene using RNA interference screening (13), with partial loss of RPS14 phenocopying components of human disease in normal hematopoietic progenitor cells, and forced expression of RPS14 rescuing the disease phenotype in patient-derived BM cells.
Epigenetic changes, including promoter hypermethylation and post-translational histone modifications, may inactivate tumor suppressor genes. Genes, including p15INK4b/CDKN2B, CDH1/E-cadherin, HIC1, and ER are inactivated by DNA methylation in hematopoietic malignancies (14,15). The activity of two DNA methyltransferase inhibitors, 5-azacitidine and 2′-deoxy-5-azacytidine (5-aza-dC) in patients with MDS provides an additional rational for the study of 5q epigenetic changes.
Using a large cohort of hematological malignancies and a multimodal gene discovery approach, we examine implicated 5q genes, including Catenin, alpha-1(CTNNA1). CTNNA1, identified as a putative −5/del(5q) hematopoietic tumor suppressor gene (16), is expressed at lower levels in the leukemia-initiating stem cells in del(5q) ML or MDS (17), but previous studies have supported (17) or not detected (18) DNA methylation. Our studies identify CTNNA1 as a specific epigenetically inactivated tumor suppressor gene on 5q through multiple repressive mechanisms.
Materials and Methods
Patient Samples
Peripheral blood (PB) or bone marrow (BM) samples from 146 patients with de novo or secondary AML, 31 MDS, 19 acute lymphocytic leukemia (ALL), 14 chronic myelogenous leukemia (CML), and 15 normal controls were obtained with informed consent as part of IRB approved protocols at Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, University Hospital of Aachen Germany, or the Cleveland Clinic Taussig Cancer Center. BM and PB mononuclear cells (MNCs) were Ficoll-Hypaque purified (Sigma).
Cell Culture
HL-60, HNT34, KG1a, KG1, ML-1, and U937 (ATCC) were maintained in 90% RPMI 1640 medium (Invitrogen) with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were treated with either 5-aza-dC (Sigma) at a concentration of 1 μM for three days with replacement of the medium and 5-aza-dC every 24 hours or Suberoylanilide Hydroxamic Acid (SAHA, Upstate Biotechnology) at 2.5μM for 24 hours.
DNA Preparation
Genomic DNA from BM or PB-MNCs and from AML cell lines were prepared using the previously described proteinase-K method.(19)
RNA Isolation and Semi-quantitative RT-PCR
Total RNA was isolated using Trizol (Life Technologies). First strand cDNA was synthesized from 5μg total RNA using random hexamers with the Superscript™ First-Strand Synthesis System (Invitrogen). Completed cDNA was diluted to 100μl with ddH2O and 2.5 μl diluted cDNA used in a 25μl PCR reaction. Primer sequences (Supplementary Table 1) spanned intronic sequences between adjacent exons. GAPDH was amplified with 25 cycles. Amplified products were analyzed on 2% agarose gels.
Methylation-Specific PCR (MSP)
Genomic DNA from primary leukemia and cell lines was bisulfite modified by EZ DNA Methylation Kit (Zymo Research). Primer sequences and PCR conditions (Supplementary Table 1) for each MSP reaction included approximately 100ng of bisulfite-treated DNA, 25pmoles of each primer, 100pmoles dNTPs, 10X PCR buffer, and 1 unit of JumpStart Red Taq Polymerase (Sigma) in a final 25μl volume. MSP products were analyzed on 6% polyacrylamide gels.
Bisulfite Sequencing
Bisulfite-treated DNA was amplified with sequencing primers in the CTNNA1 promoter: CTNNA1-BTS-forward, 5′-TAGGGGTTATTTTYGGTTTAAGTTTTTATTAGGGG-3′; CTNNA1-BTS-reverse, 5′-TACTTTATCTCCCTCCAATCCRACTAAAAA. PCR products were gel purified and cloned into pCR2.1-TOPO vector (Invitrogen). Plasmids from single colonies were purified using QIAprep Spin Miniprep Kit (Qiagen) and sequenced with M13 reverse primers (Johns Hopkins Sequencing Facility).
Real time PCR
Real-time RT-PCR used the QuantiTecti™ SYBR Green PCR kit (Qiagen) in an iCycler Optical Module (Bio-Rad), with 2.5μL cDNA per reaction in a volume of 25 μL. Experiments were performed in triplicate with primers forCTNNA1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). CTNNA1 expression was normalized to GAPDH: relative expression = 2−(Sample ΔCt−Control ΔCt), where ΔCt= average Ct (CTNNA1) − average Ct (GAPDH).
Western Blot
Whole cell lysates were prepared used RIPA lysis buffer (Sigma-Aldrich) with complete protease inhibitors (Roche Diagnostics GmbH). Protein concentrations were determined by BIO-RAD assay (Bio-Rad), 40μg protein electrophoresed on 4–12% Bid-Tris Gels (NuPAGE Novex), electrotransferred onto Immobilon-P membrane (Millipore) and blocked with 5% milk/TSA buffer. Anti-CTNNA1 c-7894 (Santa Cruz Biotechnology) 1:100 was followed by 1:5000 secondary antibody-horseradish peroxidase conjugate, and visualized using Supersignal West Femto Maximum Sensitivity Substrate (Pierce).
Human CTNNA1 promoter and luciferase reporter assays
The human CTNNA1 promoter, −692 to +394 bp (pGL3-P1.0) from transcription initiation, was amplified with primers CTNNA1forward 2: 5′-CTGGGGTACCGGTGTTTCCATCTGTGGAGTGA-3′; CTNNA1reverse: 5′-CTGAAGATCTCGCTGGGCCTATAGTTTCTCC-3′, gel purified, subcloned into the pGL3-Basic vector (Promega) via KpnI and BglII sites, and sequence verified. Promoter activity was assessed as described (20), with 5 × 104 HEK293T cells seeded in 24-well plates 24h before transfection. Human CTNNA1 promoter constructs or empty vector were transfected using FuGENE 6 (Roche Applied Science) at 100 ng/well, and pRL-TK vector (Promega) was cotransfected at 8 ng/well as an internal reporter. For in vitro methylated assays, methylated and mock-methylated constructs were transfected at 200 ng/well. 72 hours post-transfection, cells were washed and lysed in Passive Lysis Buffer (Promega). Luciferase activity was measured and transfection efficiency normalized to Renilla luciferase activity using the Dual Luciferase Reporter Assay (Promega).
In vitro DNA Methylation
Luciferase reporter plasmid DNA (20 μg) was digested with KpnI and BglII, and CTNNA1 promoter fragments gel purified. KpnI-BglII fragments were treated with M. SssI methylase in the presence (methylated) or absence (mock-methylated) of S-adenosylmethionine (New England Biolabs). DNA was phenol-chloroform extracted and ethanol precipitated, and complete DNA methylation confirmed by digestion with the methylation sensitive restriction endonuclease HpaII. Methylated and mock-methylated promoter fragments were ligated into pGL3-Basic vector and used directly for transfection.
Chromatin Immunoprecipitation (ChIP)
Cultured cells or MNCs from BM or PB by Ficoll-Hypaque (Sigma) were washed with PBS, resuspended in PBS and crosslinked in 1% formaldehyde for 10 min at room temperature (RT). Glycine to a final concentration of 0.125M for 5 min at RT quenched crosslinking. Cells were washed with 1x PBS and processed with ChIP Assay kit (Upstate). One hundred to 130 μg of sonicated DNA were used for each immunoprecipiration, using anti-H3 dimethyl-K4 (07–030), anti-acetyl-Histone H3 (Lys 9) (07–352), anti-H3 dimethyl-K9 (07–441), anti-H3 trimethyl-K27 (07–449) (Upstate), anti-DNMT1(IMG-261A, IMGENEX) and anti-DNMT3b (IMG-184A, IMGENEX), with normal rabbit IgG (2 μg/IP; Upstate) as a control. Fifty μl of sonicated, pre-IP DNA was used as input control. Real Time PCR conditions and primers used are available upon request. 20 μL PCR reactions used 2 μL of either immunoprecipitated (bound) DNA or 1:100 dilution of non-immunoprecipitated (input) DNA. Enrichment compared to input is the average from at least two independent ChIP experiments and multiple PCR analyses (three PCR reactions per independent ChIP).
Statistical analysis
Comparisons were made with Fisher’s exact and χ2 tests using STATA statistical software. Results are reported as odds ratios (OR) with corresponding 95% confidence intervals (CIs).
Results
CTNNA1 is silenced by hypermethylation and can be induced by 5-aza-dC in myeloid cell lines
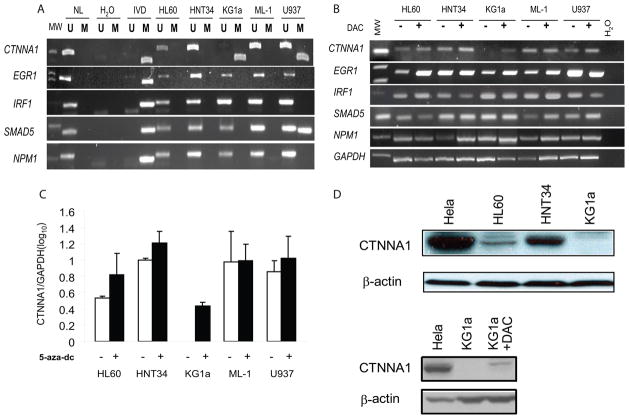
Methylation of the promoter regions of five published candidate pathogenesis genes IRF1 (21), SMAD5 (22, 23), EGR1 (12), CTNNA1 (16), and NPM1 (24) on chromosome 5q31-5 was examined using MSP. CTNNA1 was completely methylated in KG1a (Figure 1A). CTNNA1 was partially methylated in U937 cells, while unmethylated in HL60, HNT34, and ML-1 cell lines. There was partial methylation of SMAD5 in U937, but the remaining genes, EGR1, IRF1 and NPM1, were unmethylated in all cell lines. The NPM1 results are consistent with Oki et al. (24).
Figure 1. CTNNA1 is hypermethylated and repressed in AML cell lines.
(A) Methylation of 5q31-5 genes in AML cell lines using MSP. (U), unmethylated allele, (M), methylated allele. Normal human lymphocytes (NL) and in vitro methylated DNA (IVD) are negative and positive methylated controls. (B) Expression of 5q31-5 genes in AML cell lines before (−) and after (+) treatment with 1 μmol/L 5-aza-dc for 72 hours by semi-quantitative RT-PCR, with GAPDH expression for control. (C) Quantitative real-time expression of CTNNA1 in AML cell lines, normalized to GAPDH and calculated relative to HNT34. Data is the mean±SD from at least two independent PCR in triplicate. (D) Western blot of CTNNA1 protein in AML cell lines. Hela is the positive control for CTNNA1 protein expression, and β-actin is a loading control.
mRNA expression of these genes at baseline demonstrates complete loss of CTNNA1 expression only in KG1a, consistent with DNA methylation patterns (Figure 1B). CTNNA1 mRNA was re-expressed after 5-aza-dC treatment of KG1a. The other 4 candidate genes: EGR1, IRF1, SMAD5, and NPM1, were all expressed at baseline in all cell lines, and no major changes in expression were observed following 5-aza-dC treatment (Figure 1B). By real-time RT-PCR, KG1a indeed has complete loss of CTNNA1 expression, while CTNNA1 mRNA is expressed at varying levels in leukemia cell lines without DNA methylation (Figure 1C). CTNNA1 was re-expressed after 5-aza-dC treatment of KG1a, but other cell lines also increased CTNNA1 expression after 5-aza-dC treatment. Western blot confirmed that CTNNA1 mRNA correlated with CTNNA1 protein (Figure 1D). Expression was highest in HNT34, decreased in HL60 and absent in KG1a, consistent with quantitative mRNA analysis. CTNNA1 protein was detected in KG1a after 5-aza-dC treatment.
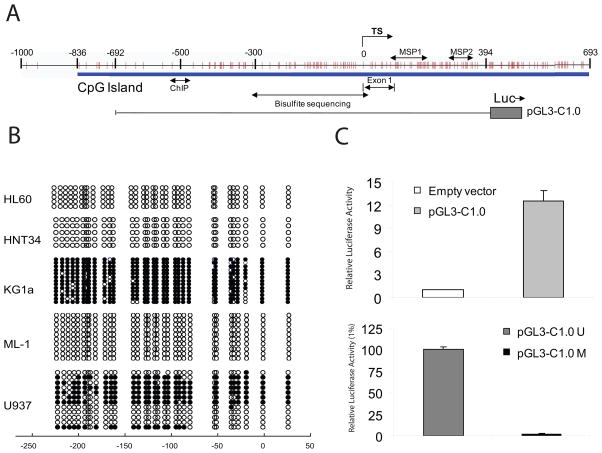
The promoter region of CTNNA1 was characterized to determine the mechanisms underlying loss of expression (Figure 2A). Genomic bisulfite sequencing was used to comprehensively examine DNA methylation in leukemia cell lines (Figure 2B). Cell lines with detectable CTNNA1 expression by RT-PCR (HL60, HNT34, and ML-1) demonstrated no CTNNA1 allelic methylation by MSP, and bisulfite sequencing of 5–13 individual clones of PCR products revealed methylation of only rare CpGs within the promoter region. In contrast, KG1a had nearly complete 5′ CpG island methylation. Bisulfite sequencing of U937 alleles revealed a hemi-methylated status, confirming MSP results. To determine whether methylation of this region was functionally resulting in gene repression, we created a CTNNA1 promoter luciferase reporter transfected into HEK293T cells. Transient transfection of −692/+394 of the human CTNNA1 gene inserted in the pGL3-Basic vector (pGL3-C1.0) demonstrated strong promoter activity (12 fold higher than vector, Figure 2C). However, in vitro methylation of this construct (pGL3-C1.0M) almost completely abolished promoter activity (Figure 2C, similar results with −836/+394 construct), suggesting that extensive CpG methylation within this region represses CTNNA1 promoter activity.
Figure 2. Bisulfite sequencing of CTNNA1 and direct repression by DNA methylation.
(A) Schematic of the CTNNA1 promoter CpG island. Vertical lines, individual CpG sites. Transcriptional start site (TS) and double-headed arrow show amplification for MSP, bisulfite sequencing (BSS), and location for ChIP primers. The region of the human CTNNA1 promoter for luciferase assays (−692 to +394 bp) is shown. (B) BSS of CTNNA1 in AML cell lines. Filled circles represent methylated CpG sites, and open circles denote unmethylated CpG sites. CpG sites are numbered relative to the transcription start site. (C) Inhibition of CTNNA1 promoter by CpG methylation. (Top) Luciferase activity of wild-type human CTNNA1 promoter, with empty pGL3 as negative control. Fold increase was calculated relative to control cells. (Bottom), Luciferase activity of the in vitro methylated construct, pGL3-C1.0M compared to mock-methylated, pGL3-C1.0U (percentage relative to mock). Data represent the average ± SD of two independent experiments performed in triplicate.
CTNNA1 methylation is common in AML patients with del(5q)
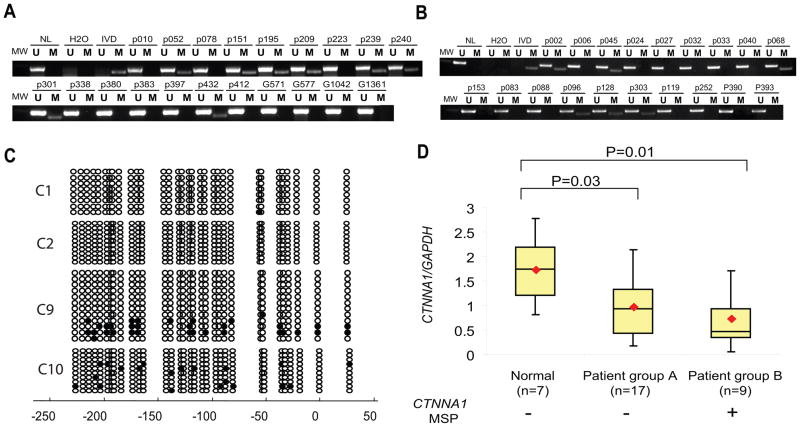
We examined CTNNA1 methylation in primary myeloid leukemia, where del(5q) is most common. In 146 individual AML cases, including 26 patients with del(5q) and 120 without del(5q), CTNNA1 methylation was more frequent in del(5q) AML patients (Figure 3A), compared to those without del(5q) (Figure 3B), (31% vs. 14%, p=0.047, Table 1). No CTNNA1 methylation was present in 15 normal controls. Bisulfite sequencing of primary AML samples (Figure 3C) is consistent with MSP, showing no CpG methylation in MSP negative patients (C1 and C2) and variegated hypermethylation in MSP positive patients (C9 and C10). The degree and heterogeneity of methylation in primary leukemias suggests that repression of CTNNA1 progresses through transformation. Although CTNNA1 methylation was more frequent in AML patients with preexisting MDS, unfavorable karyotypes, and secondary AML, none of these associations reached independent statistical significance. In multivariate analysis, only 5q deletion was associated with CTNNA1 methylation (Table 1), with an adjusted odds ratio of 3.13, (95% CI 1.07–9.13, p=0.037). No methylation of CTNNA1 was found in either CML or ALL.
Figure 3. CTNNA1 methylation in AML and diminished expression.
(A) Representative MSP results of CTNNA1 in primary AML patients with del(5q). (B) Representative MSP results of CTNNA1 in primary AML patients without del(5q). (C) BSS of CTNNA1 in AML patients. C1 and C2 were AML patients without CTNNA1 methylation detected by MSP, C9 and C10 were AML patients with CTNNA1 methylation. (D) Quantitative analysis of CTNNA1 expression levels in MNCs from normal and individuals with or without CTNNA1 methylation. Real Time PCR was performed as described in materials and methods. CTNNA1 expression levels were normalized to GAPDH and calculated relative to HNT34. The results were presented by Box-and whisker plot. The plots show CTNNA1 expression levels in two patient groups with different CTNNA1 methylation status and one healthy control group. In these plots, lines within boxes represent median values and the diamonds indicate the mean; the upper and lower lines of the boxes represent the 25th and 75th percentiles, respectively; and the upper and lower bars outside the boxes represent the maximum and minimum value, respectively.
Table 1.
CTNNA1 Methylation
| Methylated | Unmethylated | |||||||
| Normal Lymphocytes (n=15) | 0 (0%) | 15 (100%) | ||||||
| Hematological malignancies | ||||||||
| MDS(n=31) | ||||||||
| 5q deletion (n=18) | 2 (11%) | 16 (89%) | ||||||
| <10% blasts(n=12) | 0 | 12 | ||||||
| 10%<blasts<19%(n=3) | 1 | 2 | ||||||
| >20%blasts (n=3) | 1 1 | 2 | ||||||
| non 5q deletion (n=13) | 1 (8%) | 12 (92%) | ||||||
| <10% blasts(n=9) | 0 | 9 | ||||||
| 10%<blasts<19%(n=2) | 0 | 2 | ||||||
| >20%blasts (n=2) | 1 1 | 1 | Crude | Adjusted | ||||
| AML (n=146) | OR | 95% CI | p value | OR4 | 95% CI | p value | ||
| non 5q deletion (n=114) | 16 (14%) | 98(86%) | 1.00 | referent | 1.00 | referent | ||
| 5q deletion (n=26) | 8 (31%) | 18 (69%) | 2.72 | 1.01 – 7.30 | 0.047 | 3.13 | 1.07 – 9.13 | 0.037 |
| AML w/o preexisting MDS (n=87) | 13 (15%) | 74 (85%) | 1.00 | referent | 1.00 | referent | ||
| MDS/AML (n=59) | 11 (19%) | 48 (81%) | 1.30 | 0.54 – 3.15 | 0.55 | 0.72 | 0.20 – 2.67 | 0.63 |
| De novo (n=65) | 8 (12%) | 57 (82%) | 1.00 | referent | 1.00 | referent | ||
| Secondary AML (n=81) | 16 (20%) | 65 (80%) | 1.75 | 0.70 – 4.40 | 0.23 | 1.61 | 0.43 – 6.01 | 0.48 |
| <602 (n=54) | 9 (17%) | 45 (83%) | 1.00 | referent | 1.00 | referent | ||
| ≥60 (n=78) | 13 (17%) | 65 (83%) | 1.04 | 0.41 – 2.63 | 0.94 | 1.09 | 0.39 – 3.00 | 0.87 |
| Favorable Karyotype3 (n=13) | 0 (0%) | 13 (100%) | ||||||
| Intermediate Karyotype(n=61) | 9 (15%) | 52 (85%) | ||||||
| Adverse Karyotype(n=65) | 15 (23%) | 50 (77%) | ||||||
| CML (n=14) | 0 (0%) | 14 (100%) | ||||||
| ALL (n=19) | 0 (0%) | 19 (100%) | ||||||
|
Non-Hematological malignancies | ||||||||
| Colon cell lines (n=8) | 0 (0%) | 8 (100%) | ||||||
| Lung cell lines (n=9) | 0 (0%) | 9 (100%) | ||||||
| Liver cell lines (n=6) | 0 (0%) | 6 (100%) | ||||||
| Cervix cell lines (n=6) | 0 (0%) | 6 (100%) | ||||||
| Breast cell lines (n=7) | 0 (0%) | 7 (100%) | ||||||
| Gastric cancer (n=51) | 0 (0%) | 51 (100%) | ||||||
| Esophageal cancer (n=48) | 0 (0%) | 48 (100%) | ||||||
Those two MDS cases are RAEB-t which were reclassified by WHO as AML.
There are no age information available for 14 patients.
Karyotypic features: Favorable, t(8;21); inv(16)/t(16;16):t(15;17); Intermediate, normal karyotype and less than 3 chromosomal abnormalities; Adverse, Complex karyotype, −7, −5, del(5q), and MLL gene rearrangements (11q23). There are 7 patients lacking karyotypic data.
Model adjusted by MDS status, 5q deletion, Age, and AML subtype
To determine whether CTNNA1 methylation was related to del(5q), or alternatively related to higher methylation frequencies in del(5q) disease, we examined CDKN2B and CDH1 methylation. CDKN2B was methylated in 38% (10/26) of AML patients with del(5q) and 55% (62/113) without del(5q); CDH1 was methylated in 0% (0/26) in AML patients with del(5q) and 16% (18/113) in non-del(5q) group, suggesting that del(5q) are less frequently methylated at other loci. In fact, the lack of methylation of CDH1 in patients with genetic and epigenetic alterations of CTNNA1 is of interest, since both genes are part of the same adhesion complex.
To further investigate CTNNA1 as a primary target of inactivation on 5q, published microarray expression data from primary AML cases was examined (25). An individual patient’s expression level for genes is represented by a single data point. For CDH1, a biphasic pattern of expression is present consistent with known repression of CDH1 in AML (26) (Supplemental Figure 1). A similar pattern is seen for CTNNA1, although with fewer cases (10%) with low expression. This is consistent with the frequency of promoter methylation in AML without -5/del(5q) of 14%, since only 4 of 287 cases in this data set have 5q deletions. Among other genes examined for methylation (EGR1, SMAD5, IF1, NPM1) or the 5q syndrome candidate gene RPS14, only EGR1 demonstrated differential expression among the samples, but was not the biphasic pattern seen with CDH1 or CTNNA1. For eight other 5q genes in the deleted region, there was no evidence of gene repression in AML (Supplemental Figure 2).
CTNNA1 methylation was examined in 31 primary MDS samples. In these 31 samples, while 17 have −5/del(5q), methylation of CTNNA1 was detected in only 3 of 31 MDS patients (Table 1). Of note, all three cases of MDS with CTNNA1 methylation had advanced MDS (14% to 20% blasts), with the difference in CTNNA1 methylation between high risk MDS (≥RAEB2, 3 of 10, 30%) and low risk MDS (<RAEB2, 0 of 21, 0%) reaching statistical significance (p=0.03). This suggests that CTNNA1 promoter methylation may be associated with progression within MDS and from MDS to AML. To determine whether these samples were suitable for detection of CTNNA1 methylation, p15/CDKN2B methylation was examined, since it is frequent in MDS and AML (27–29). Seventeen of 31 (55%) MDS samples had CDKN2B methylation, with a trend towards a higher frequency of methylation in high risk MDS (≥RAEB2, 8 of 10 MDS, 80%), than low risk MDS (<RAEB2, 9 of 21 MDS, 43%), consistent with previous reports.
To further investigate the specificity of CTNNA1 methylation among 5q genes, we examined primary AML samples for other 5q genes. No EGR1 methylation was detected in 24 primary MDS cases (8 with 5q loss) nor in 86 primary AML (15 with 5q loss) cases. In addition, methylation was not seen at the promoter regions for IRF1, SMAD5, and NPM1 in 34 AML samples (15 with 5q loss). Although loss of 5q is of particular clinical significance for MDS and AML, recurrent loss of the long arm of chromosome 5 is also found in other malignancies (30–32) and diminished expression of CTNNA1 has been reported in gastric and esophageal cancer (33–35). We examined whether CTNNA1 was hypermethylated in other cancer phenotypes. In 41 solid tumor cancer cell lines and in 99 primary gastric and esophageal cancer samples (Table 1), no methylation of CTNNA1 was detected, suggesting that methylation of CTNNA1 is specific to myeloid malignancies.
Reduced CTNNA1 expression associated with DNA methylation and chromatin changes
The observed correlation of promoter methylation to repression of CTNNA1 mRNA in KG1a, led us to examine CTNNA1 mRNA expression in a subset of AML cases with sufficient RNA. Normal MNCs express high levels of CTNNA1 (Figure 3D). In contrast, 9 AML patient samples with CTNNA1 methylation had much lower expression. Unexpectedly, primary AML without CTNNA1 methylation had a mean expression lower than normal MNCs, and a number of these AML have repressed CTNNA1.
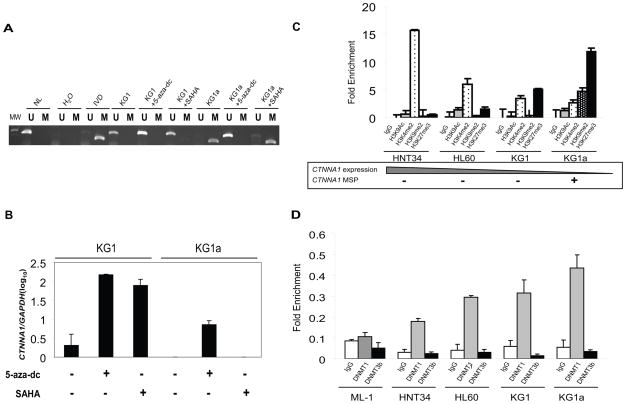
These results suggest multiple genetic (deletion) and epigenetic alterations leading to CTNNA1 inactivation in hematological malignancies. One model to integrate these observations would be progressive inactivation of the CTNNA1 locus, with CpG promoter methylation as the final and most definitive inactivation. To test this hypothesis, we investigated the KG1/KG1a system, since the more well differentiated KG1 is the origin of KG1a (36), and there is progressive inactivation of CDKN2B (37). Both cell lines have identical karyotypic abnormalities including monosomy 5 (38), but KG1a cells are morphologically and functionally less differentiated than KG1. KG1 has no DNA methylation, but nearly undetectable CTNNA1expression (Figure 4A and 4B). However, unlike KG1a, expression could be restored either by 5-aza-dC or the HDAC inhibitor SAHA, an observation consistent with a repressed but unmethylated gene (39). In contrast, in KG1a, CTNNA1 is completely methylated and silenced, and can be induced only by 5-aza-dC. This suggests that transcriptional repression in KG1 “progressed” to complete silencing associated with DNA methylation in KG1a.
Figure 4. Progressive repression and silencing of CTNNA1.
(A) MSP of CTNNA1 in KG1 and KG1a before and after treatment with 5-aza-dc or SAHA. (B) Quantitative real-time expression of CTNNA1 in KG1 and KG1a before and after treatment with 5-aza-dc or SAHA. CTNNA1 expression was normalized to GAPDH and calculated relative to HNT34 (mean±SD from at least two independent PCR in triplicate). CTNNA1 is induced by 5-aza-dc or SAHA in KG1, but only by 5-aza-dc in KG1a. (C) ChIP of histone modifications at the CTNNA1 promoter in AML cell lines. Cell lines are displayed in order of descending expression of CTNNA1, with DNA methylation shown on the bottom. The CTNNA1 promoter has enrichment of repressive chromatin in a DNA hypermethylated and silent state (KG1a) compared to when unmethylated and expressed (HNT34), with reciprocal enrichment of active marks. H3K27me3 was also enriched in KG1, in which CTNNA1 is not methylated but expression is low. Enrichment was normalized to total input (mean±SD from at least two independent ChIP experiments and multiple independent PCR analyses). (D) ChIP of DNMT1 and DNMT3b occupancy at the CTNNA1 promoter in AML cell lines. DNMT1 shows greatest enrichment at the promoter of CTNNA1 in KG1a, lower enrichment in HNT34, HL60 and KG1 cell line, and is not present in ML-1. DNMT3b was not present at the CTNNA1 promoter. Data are the mean±SD from at least two independent ChIP experiments and multiple independent PCR analyses.
ChIP was used to examine the CTNNA1 promoter, using two active, H3K9Ac and H3K4me2, and two inactive, H3K9me2 and H3K27me3, histone marks, in leukemia cell lines (Figure 4C). The unmethylated cell line HNT34, with high CTNNA1 expression, has enrichment of the active mark H3K4me2, and to a lesser degree, H3K9Ac, while inactive marks were absent. In contrast, CTNNA1 methylation in KG1a was accompanied by enrichment of inactive marks, H3K9me2 and H3K27me3, and depletion of active marks. Of greatest interest were HL60 and KG1, where CTNNA1 is unmethylated and decreased expression was accompanied by a mix of active and inactive marks. This mixed chromatin phenotype has recently been termed bivalent chromatin (40, 41), which is characteristic of cancer genes predisposed to aberrant DNA methylation (42). Our results are consistent with histone deacetylation and/or methylation establishing condensed chromatin and transcriptional repression of CTNNA1 (HL60, KG1), which may result in promoter DNA methylation and complete gene inactivation (KG1a).
To explore the mechanism of CTNNA1 activation by 5-aza-dC in unmethylated cell lines, we performed ChIP for DNA methyltransferases 1(DNMT1) and DNMT3b (Figure 4D). While as expected, DNMT1 is present at the CTNNA1 promoter in DNA methylated KG1a, it was also present, but with less enrichment, in unmethylated HNT34, HL60, and KG1. DNMT1 enrichment directly correlates with CTNNA1 repression. In contrast, ML-1, with no increase in CTNNA1 expression with 5-aza-dC treatment, DNMT1 is absent. DNMT3b was not detected at the CTNNA1 promoter in any cell line. This suggest that DNMT1 plays a critical role in initiating and/or maintaining DNA repression of CTNNA1 in AML cell lines, which is relieved by 5-aza-dC. This occupancy may position DNMT1 to initiate promoter DNA methylation, leading to complete gene silencing.
In vivo chromatin at the CTNNA1 promoter in primary AML
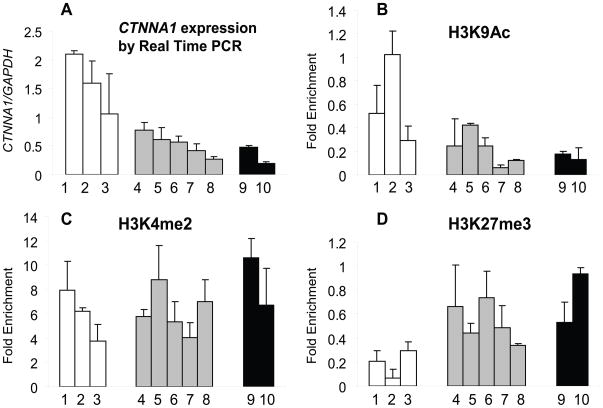
We examined chromatin at the CTNNA1 promoter in primary AML using ChIP. Ten freshly collected samples with the initial diagnosis of AML had sufficient number and purity of blasts, including cases with del(5q) (n=3) and with CTNNA1 methylation (n=2) (Supplemental Table 2). High CTNNA1 expression cases lack del(5q), and lack CTNNA1 DNA methylation (Figure 5). Consistent with high CTNNA1 expression, H3K9Ac was enriched (active chromatin mark) and H3K27me3 depleted (repressive mark). In contrast, seven AML cases with lower CTNNA1 expression (samples 4–10) had lower levels of H3K9Ac and greater enrichment of H3K27me3. The greatest enrichment of H3K27me3 was found in sample 10, which also had CTNNA1 promoter methylation (Figure 5B–D). Thus, repressive chromatin is present at the CTNNA1 promoter in some primary AML, and in the most repressed transcriptional state, associated with promoter DNA methylation.
Figure 5. ChIP assay of histone marks at the CTNNA1 promoter in primary AML patients.
(A) Quantitative expression of CTNNA1 in AML patients. One, 2, and 3 are patients with normal CTNNA1 expression and without methylation; 4,5,6,7, and 8 are patients with decreased CTNNA1 expression and without methylation; 9 and 10 have low CTNNA1 expression and methylation. CTNNA1 expression levels were normalized to GAPDH and calculated relative to HNT34. (B) Enrichment of active mark H3K9Ac on the CTNNA1 promoter (500bp upstream transcriptional start site) was greatest in patients with normal CTNNA1 expression and without DNA methylation and least in patients with decreased CTNNA1 expression and with methylation. (C) Enrichment of H3K4me2 on the CTNNA1 promoter, revealed no significant difference in enrichment. (D) Enrichment of the repressive mark (H3K27me3) was seen in patients with diminished expression, with or without CTNNA1 DNA methylation.
Discussion
The search for the gene(s) associated with deletions of −5/del(5q) and responsible for the pathogenesis of MDS and AML has been long, and has not reached consensus. Complicating this search are clinical differences between 5q loss in “the 5q syndrome” and del(5q) associated with complex karyotypes. Through examination of epigenetic alterations, our data point to CTNNA1 as a critical target for loss of function in 5q deletions associated with MDS/AML.
CTNNA1 is a cytoplasmic adhesion protein forming a trimolecular complex with CDH1 and β-Catenin. CTNNA1 was proposed as a candidate 5q tumor suppressor gene (16), but additional evidence has been challenging. Reduced expression of CTNNA1 in MDS and AML patients with del(5q) compared to those without del(5q) has been reported but without a molecular explanation (43). Studies associating reduced CTNNA1 expression with DNA methylation (17) or without any evidence of DNA methylation (18) have been recently published. These studies were relatively small in sample size, with 12 patients with del(5q) and ten without del(5q) analyzed in the former study, and only 6 del(5q) samples examined for methylation in the latter study.
Our comprehensive analysis of CTNNA1 for copy number, DNA methylation, chromatin, and gene expression in a large cohort of 31 MDS and 146 AML patients resolve this controversy and provide a molecular explanation. CTNNA1 methylation occurs more frequently in patients with del(5q) AML, but is not exclusive to del(5q). Reduced expression of CTNNA1 is more frequent than DNA methylation and is associated with repressive chromatin marks. This unique picture of progressive silencing is supported by leukemia cell lines, and for the first time, primary leukemia ChIP analysis. CTNNA1 methylation exclusively in high, but not low risk MDS, provides an unusual insight into progression of this disease, since more often, loci are methylated in both MDS and AML (29,44,45).
Repression and silencing of CTNNA1 results from an interplay between DNA methylation and changes in histone marks, notably enrichment of H3K27me3. Our results demonstrate the functional role of DNA methylation in this region in silencing CTNNA1 expression. However, repression with low levels of methylation and in leukemias without DNA methylation suggest progressive epigenetic silencing does not initiate with DNA methylation. Methylated H3K27 serves as an anchorage point for the recruitment of EZH2-containing Polycomb group (PcG) proteins (46), whose binding contributes to formation of repressive chromatin. CTNNA1, but not other 5q genes (IRF1, SMAD5, EGR, and NPM1) without DNA methylation in AML, is Polycomb marked (47), providing further support for repressive marks promoting epigenetic silencing (48). Interaction of PcG complexes with DNA methyltransferases could facilitate CpG methylation (46). Demonstration of DNMT1 occupancy of the CTNNA1 promoter in cell lines with repressed, but unmethylated CTNNA1, support this association. The discovery of intermediate stages of decreased CTNNA1 expression and repressed chromatin marks without DNA methylation has important implications for epigenetic therapy, particularly those combining histone deacetylase and DNA methylation inhibitors.
Supplementary Material
Acknowledgments
This work was supported by the Commonwealth Fund (J.G.H.), DOD MPO48018 (M.A.M.), and a charitable donation from Marian and Robert E. Fischell (M.A.M., J.E.K.).
References
- 1.Brunning RD. MDS--new classification, new problem? Leukemia Research. 2003;27:567–9. doi: 10.1016/s0145-2126(02)00302-8. [DOI] [PubMed] [Google Scholar]
- 2.Nosslinger T, Reisner R, Koller E, et al. Myelodysplastic syndromes, from French-American-British to World Health Organization: comparison of classifications on 431 unselected patients from a single institution. Blood. 2001;98:2935–41. doi: 10.1182/blood.v98.10.2935. [DOI] [PubMed] [Google Scholar]
- 3.Boultwood J, Lewis S, Wainscoat JS. The 5q-syndrome. Blood. 1994;84:3253–60. [PubMed] [Google Scholar]
- 4.Nimer SD, Golde DW. The 5q- abnormality. Blood. 1987;70:1705–12. [PubMed] [Google Scholar]
- 5.Giagounidis AAN, Germing U, Haase S, et al. Clinical, morphological, cytogenetic, and prognostic features of patients with myelodysplastic syndromes and del(5q) including band q31. Leukemia. 2003;18:113–9. doi: 10.1038/sj.leu.2403189. [DOI] [PubMed] [Google Scholar]
- 6.Kelaidi C, Eclache V, Fenaux P. The role of lenalidomide in the management of myelodysplasia with del 5q. British Journal of Haematology. 2008;140:267–78. doi: 10.1111/j.1365-2141.2007.06910.x. [DOI] [PubMed] [Google Scholar]
- 7.Nimer SD. Clinical Management of Myelodysplastic Syndromes With Interstitial Deletion of Chromosome 5q. J Clin Oncol. 2006;24:2576–82. doi: 10.1200/JCO.2005.03.6715. [DOI] [PubMed] [Google Scholar]
- 8.Boultwood J, Fidler C, Strickson AJ, et al. Narrowing and genomic annotation of the commonly deleted region of the 5q- syndrome. Blood. 2002;99:4638–41. doi: 10.1182/blood.v99.12.4638. [DOI] [PubMed] [Google Scholar]
- 9.Fairman J, Chumakov I, Chinault AC, Nowell PC, Nagarajan L. Physical Mapping of the Minimal Region of Loss in 5q- Chromosome. Proceedings of the National Academy of Sciences. 1995;92:7406–10. doi: 10.1073/pnas.92.16.7406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horrigan SK, Westbrook CA, Kim AH, Banerjee M, Stock W, Larson RA. Polymerase chain reaction-based diagnosis of del (5q) in acute myeloid leukemia and myelodysplastic syndrome identifies a minimal deletion interval. Blood. 1996;88:2665–70. [PubMed] [Google Scholar]
- 11.Zhao N, Stoffel A, Wang PW, et al. Molecular delineation of the smallest commonly deleted region of chromosome 5 in malignant myeloid diseases to 1–1.5 Mb and preparation of a PAC-based physical map. Proc Natl Acad Sci U S A. 1997;94:6948–53. doi: 10.1073/pnas.94.13.6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joslin JM, Fernald AA, Tennant TR, et al. Haploinsufficiency of EGR1, a candidate gene in the del(5q), leads to the development of myeloid disorders. Blood. 2007;110:719–26. doi: 10.1182/blood-2007-01-068809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebert BL, Pretz J, Bosco J, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature. 2008;451:335–9. doi: 10.1038/nature06494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chim CS, Liang R, Kwong YL. Hypermethylation of gene promoters in hematological neoplasia. Hematological Oncology. 2002;20:167–76. doi: 10.1002/hon.694. [DOI] [PubMed] [Google Scholar]
- 15.Rush LJ, Plass C. Alterations of DNA methylation in hematologic malignancies. Cancer Letters. 2002;185:1–12. doi: 10.1016/s0304-3835(02)00288-4. [DOI] [PubMed] [Google Scholar]
- 16.Horrigan SK, Arbieva ZH, Xie HY, et al. Delineation of a minimal interval and identification of 9 candidates for a tumor suppressor gene in malignant myeloid disorders on 5q31. Blood. 2000;95:2372–7. [PubMed] [Google Scholar]
- 17.Liu TX, Becker MW, Jelinek J, et al. Chromosome 5q deletion and epigenetic suppression of the gene encoding alpha-catenin (CTNNA1) in myeloid cell transformation. Nat Med. 2007;13:78–83. doi: 10.1038/nm1512. [DOI] [PubMed] [Google Scholar]
- 18.Desmond JC, Raynaud S, Tung E, Hofmann WK, Haferlach T, Koeffler HP. Discovery of epigenetically silenced genes in acute myeloid leukemias. Leukemia. 2007;21:1026–34. doi: 10.1038/sj.leu.2404611. [DOI] [PubMed] [Google Scholar]
- 19.Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9821–6. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang W, Glockner SC, Guo M, et al. Epigenetic Inactivation of the Canonical Wnt Antagonist SRY-Box Containing Gene 17 in Colorectal Cancer. Cancer Res. 2008;68:2764–72. doi: 10.1158/0008-5472.CAN-07-6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boultwood J, Fidler C, Lewis S, et al. Allelic loss of IRF1 in myelodysplasia and acute myeloid leukemia: retention of IRF1 on the 5q- chromosome in some patients with the 5q- syndrome. Blood. 1993;82:2611–6. [PubMed] [Google Scholar]
- 22.Zavadil J, Brezinová J, Svoboda P, Zemanová Z, Michalová K. Smad5, a tumor suppressor candidate at 5q31.1, is hemizygously lost and not mutated in the retained allele in human leukemia cell line HL60. Leukemia. 1997;11:1187–92. doi: 10.1038/sj.leu.2400750. [DOI] [PubMed] [Google Scholar]
- 23.Hejlik DP, Kottickal LV, Liang H, et al. Localization of SMAD5 and its evaluation as a candidate myeloid tumor suppressor. Cancer Research. 1997;57:3779–83. [PubMed] [Google Scholar]
- 24.Oki Y, Jelinek J, Beran M, Verstovsek S, Kantarjian HM, Issa JP. Mutations and promoter methylation status of NPM1 in myeloproliferative disorders. Haematologica. 2006;91:1147–8. [PubMed] [Google Scholar]
- 25.Valk PJM, Verhaak RGW, Beijen MA, et al. Prognostically Useful Gene-Expression Profiles in Acute Myeloid Leukemia. New England Journal of Medicine. 2004;350:1617–28. doi: 10.1056/NEJMoa040465. [DOI] [PubMed] [Google Scholar]
- 26.Corn PG, Smith BD, Ruckdeschel ES, Douglas D, Baylin SB, Herman JG. E-Cadherin Expression Is Silenced by 5′ CpG Island Methylation in Acute Leukemia. Clinical Cancer Research. 2000;6:4243–8. [PubMed] [Google Scholar]
- 27.Cameron EE, Baylin SB, Herman JG. p15INK4B CpG Island Methylation in Primary Acute Leukemia Is Heterogeneous and Suggests Density as a Critical Factor for Transcriptional Silencing. Blood. 1999;94:2445–51. [PubMed] [Google Scholar]
- 28.Herman JG, Civin CI, Issa J-PJ, Collector MI, Sharkis SJ, Baylin SB. Distinct Patterns of Inactivation of p15INK4B and p16INK4A Characterize the Major Types of Hematological Malignancies. Cancer Res. 1997;57:837–41. [PubMed] [Google Scholar]
- 29.Quesnel B, Guillerm G, Vereecque R, et al. Methylation of the p15INK4b Gene in Myelodysplastic Syndromes Is Frequent and Acquired During Disease Progression. Blood. 1998;91:2985–90. [PubMed] [Google Scholar]
- 30.Mendes-da-Silva P, Moreira A, Duro-da-Costa J, Matias D, Monteiro C. Frequent loss of heterozygosity on chromosome 5 in non-small cell lung carcinoma. Mol Pathol. 2000;53:184–7. doi: 10.1136/mp.53.4.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogasawara S, Tamura G, Maesawa C, et al. Common deleted region on the long arm of chromosome 5 in esophageal carcinoma. Gastroenterology. 1996;110:52–7. doi: 10.1053/gast.1996.v110.pm8536888. [DOI] [PubMed] [Google Scholar]
- 32.Tamura G, Ogasawara S, Nishizuka S, et al. Two Distinct Regions of Deletion on the Long Arm of Chromosome 5 in Differentiated Adenocarcinomas of the Stomach. Cancer Res. 1996;56:612–5. [PubMed] [Google Scholar]
- 33.Kadowaki T, Shiozaki H, Inoue M, et al. E-Cadherin and {alpha}-Catenin Expression in Human Esophageal Cancer. Cancer Res. 1994;54:291–6. [PubMed] [Google Scholar]
- 34.Setoyama T, Natsugoe S, Okumura H, et al. Alpha-catenin is a significant prognostic factor than E-cadherin in esophageal squamous cell carcinoma. Journal of Surgical Oncology. 2007;95:148–55. doi: 10.1002/jso.20610. [DOI] [PubMed] [Google Scholar]
- 35.Zhou YN, Xu CP, Chen Y, Han B, Yang SM, Fang DC. Alpha-catenin expression is decreased in patients with gastric carcinoma. World J Gastroenterol. 2005;11:3468–72. doi: 10.3748/wjg.v11.i22.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furley AJ, Reeves BR, Mizutani S, et al. Divergent molecular phenotypes of KG1 and KG1a myeloid cell lines. Blood. 1986;68:1101–7. [PubMed] [Google Scholar]
- 37.Dodge JE, Munson C, List AF. KG-1 and KG-1a model the p15 CpG island methylation observed in acute myeloid leukemia patients. Leukemia Research. 2001;25:917–25. doi: 10.1016/s0145-2126(01)00053-4. [DOI] [PubMed] [Google Scholar]
- 38.Mrózek K, Tanner SM, Heinonen K, Bloomfield CD. Molecular cytogenetic characterization of the KG-1 and KG-1a acute myeloid leukemia cell lines by use of spectral karyotyping and fluorescence in situ hybridization. Genes, Chromosomes and Cancer. 2003;38:249–52. doi: 10.1002/gcc.10274. [DOI] [PubMed] [Google Scholar]
- 39.Soengas MS, Capodieci P, Polsky D, et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–11. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 40.Azuara V, Perry P, Sauer S, et al. Chromatin signatures of pluripotent cell lines. Nature Cell Biology. 2006;8:532–8. doi: 10.1038/ncb1403. [DOI] [PubMed] [Google Scholar]
- 41.Bernstein BE, Mikkelsen TS, Xie X, et al. A Bivalent Chromatin Structure Marks Key Developmental Genes in Embryonic Stem Cells. Cell. 2006;125:315–26. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 42.McGarvey KM, Fahrner JA, Greene E, Martens J, Jenuwein T, Baylin SB. Silenced Tumor Suppressor Genes Reactivated by DNA Demethylation Do Not Return to a Fully Euchromatic Chromatin State. Cancer Res. 2006;66:3541–9. doi: 10.1158/0008-5472.CAN-05-2481. [DOI] [PubMed] [Google Scholar]
- 43.Schoch C, Kohlmann A, Dugas M, et al. Genomic gains and losses influence expression levels of genes located within the affected regions: a study on acute myeloid leukemias with trisomy 8, 11, or 13, monosomy 7, or deletion 5q. Leukemia. 2005;19:1224–8. doi: 10.1038/sj.leu.2403810. [DOI] [PubMed] [Google Scholar]
- 44.Aggerholm A, Holm MS, Guldberg P, Olesen LH, Hokland P. Promoter hypermethylation of p15INK4B, HIC1, CDH1, and ER is frequent in myelodysplastic syndrome and predicts poor prognosis in early-stage patients. European Journal Of Haematology. 2006;76:23–32. doi: 10.1111/j.1600-0609.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- 45.Galm O, Wilop S, Lüders C, et al. Clinical implications of aberrant DNA methylation patterns in acute myelogenous leukemia. Annals of Hematology. 2005;84:39–46. doi: 10.1007/s00277-005-0005-0. [DOI] [PubMed] [Google Scholar]
- 46.Vire E, Brenner C, Deplus R, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–4. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 47.Lee TI, Jenner RG, Boyer LA, et al. Control of Developmental Regulators by Polycomb in Human Embryonic Stem Cells. Cell. 2006;125:301–13. doi: 10.1016/j.cell.2006.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohm JE, McGarvey KM, Yu X, et al. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nature Genetics. 2007;39:237–42. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.