Summary
The present report provides evidence that axons in the medial part of the posterior column at T10 convey ascending nociceptive signals from pelvic visceral organs. This evidence was obtained from human surgical case studies and histological verification of the lesion in one of these cases, along with neuroanatomical and neurophysiological findings in animal experiments. A restricted lesion in this area can virtually eliminate pelvic pain due to cancer. The results remain excellent even in cases in which somatic structures of the pelvic body wall are involved. Following this procedure, neurological testing reveals no additional neurological deficit. There is no analgesia to pinprick stimuli applied to the body surface, despite the relief of the visceral pain. Since it is reasonable to attribute the favorable results of limited midline myelotomies to the interruption of axons of visceral nociceptive projection neurons in the posterior column, we have performed experiments in rats to test this hypothesis. The results in rats indicate that the dorsal column does indeed include a nociceptive component that signals pelvic visceral pain. The pathway includes neurons of the postsynaptic dorsal column pathway at the L6-S1 segmental level, axons of these neurons in the fasciculus gracilis, and neurons of the nucleus gracilis and the ventral posterolateral nucleus of the thalamus.
Keywords: Colorectal distention, Fasciculus gracilis, Limited midline myelotomy, Nucleus gracilis, Postsynaptic dorsal column path, Ventral posterior lateral nucleus
Introduction
The spinothalamic and accompanying ascending tracts in the anterolateral quadrant of the human spinal cord have been considered during most of this century to be the most important tracts for the transmission of signals to the brain that lead to the perception of pain (White and Sweet 1969; Vierck et al. 1986; Gybels and Sweet 1989). The earliest effective surgical procedure introduced for pain relief was anterolateral cordotomy (Spiller and Martin 1912). The best results from unilateral anterolateral cordotomy are when the pain is unilateral, especially when somatic structures are involved. A variety of surgical procedures have been used for the treatment of intractable pain due to cancer involving the pelvic viscera. Especially vexing is the pain associated with bilateral neoplastic involvement. Bilateral anterolateral cordotomies have been successfully performed for the relief of diffuse pelvic visceral cancer pain (Armour 1927; Mansuy et al. 1976; see reviews by White and Sweet 1969; Gybels and Sweet 1989). However, significant complications can follow cordotomy, especially bilateral, including extremity paresis, bowel, bladder and sexual dysfunction, respiratory difficulty, hypotension, and occasionally dysesthesias due to development of a central pain state; furthermore, the mortality rate of the procedure is not negligible because of the typically debilitated state of the patients (Gybels and Sweet 1989). Commissural myelotomy was designed as a surgical technique to produce bilateral analgesia by interrupting the decussating axons of the spinothalamic tract by means of a longitudinal incision extending over several spinal segments (Armour 1927; Putnam 1934; Leriche 1936). Complications of commissural myelotomy have been regarded as less severe than those of bilateral anterolateral cordotomy, but include a decrease in proprioception, incapacitating dysesthesias, transient paresis, bowel and bladder dysfunction, and occasionally death (Gybels and Sweet 1989). Interestingly, the incisions used in the earliest operations were only about 2–3 mm deep and therefore might not always have reached the commissures (Mansuy et al. 1944; see Gybels and Sweet 1989). Limited midline myelotomies were introduced by Hitchcock (1970, 1972a,b), who made a small midline stereotactic lesion at the C1 level that was followed by an unexpectedly widespread pain relief, despite the location of the lesion rostral to the decussation of almost all of the spinothalamic tract. Others had comparable results from limited midline myelotomies at C1 (Papo and Luongo 1976; Schvarcz 1976, 1978) or at no (Gildenberg and Hirshberg 1984). The finding that a limited midline myelotomy can result in pain relief far beyond that predicted from the extent of interruption of spinothalamic axons led Gybels and Sweet (1989) to state that ‘it compels a major revision in our thinking anent the pathways for pain in the spinal cord of man’.
Although it is known from experimental work that primate spinothalamic tract neurons can be activated by visceral as well as by somatic afferents (Foreman et al. 1981; Milne et al. 1981; Ammons et al. 1985), a composite view of available clinical and experimental evidence reveals that visceral pain is more effectively relieved by spinal cord lesions that affect the central part of the spinal cord (Davis et al. 1929; Hitchcock 1970, 1974; Vierck et al. 1971; Schvarcz 1976, 1978; Vierck and Luck 1979; Gildenberg and Hirshberg 1984; reviewed by Vierck et al. 1986).
Evidence is presented here from clinical and experimental findings that an important visceral nociceptive pathway ascends in the fasciculus gracilis. The findings have been reported in abstract form (Hirshberg et al. 1995).
Methods
Method for T10 midline myelotomy
Limited midline myelotomy is perfonned at the T10 segmental level of the spinal cord under general anesthesia with the patient in the prone position. This segmental level is just rostral to the pelvic visceral input conveyed through the hypogastric plexus and well rostral to that reaching the spinal cord through the pelvic nerves. Therefore, a lesion at this level should interrupt ascending pathways that signal pelvic pain. The operation is done using a surgical microscope at high magnification. The posterior median sulcus and septum are easily identified by observing small arteries ‘diving down’ into the sulcus. The posterior spinal vein is serpiginous and does not designate the midline (Fig. 1A). An avascular area between midline arteries dipping into the posterior median sulcus must be identified, but these arteries need not be disrupted. Bleeding is not evident with this procedure. A sharp micro-hook can be used to cut a 1–2 mm longitudinal segment of the pia mater that may be thickened over the posterior median sulcus. A flat, thin, 2 mm wide spatula may then be used to enter the posterior median sulcus in the midline to about 5 mm in depth. Resistance is felt when the instrument reaches either the ependyma of the central canal or the infolding of the pia of the anterior median fissure. A small straight ball-ended searcher can also be used for this procedure. Either instrument should be marked at 5 mm. Penetration to more than 5–6 mm should be avoided because of vascular structures, including branches of the anterior spinal artery, that are within the anterior median fissure. The lesion is extended rostrocaudally by carefully moving the probe in the sagittal plane. The length of the lesion may vary from 3–5 mm, but the lesion must be kept immediately adjacent to the midline. When the lesion is made, small fragments of grayish material float to the surface in cerebrospinal fluid. The patient is given intravenous steroids at this time and is usually placed on prophylactic intravenous antibiotics. Percutaneous stereotactic lesions should be avoided because of the variability in size and mobility of the cord and the difficulty in producing the discrete midline lesions that can be done with an open surgical procedure.
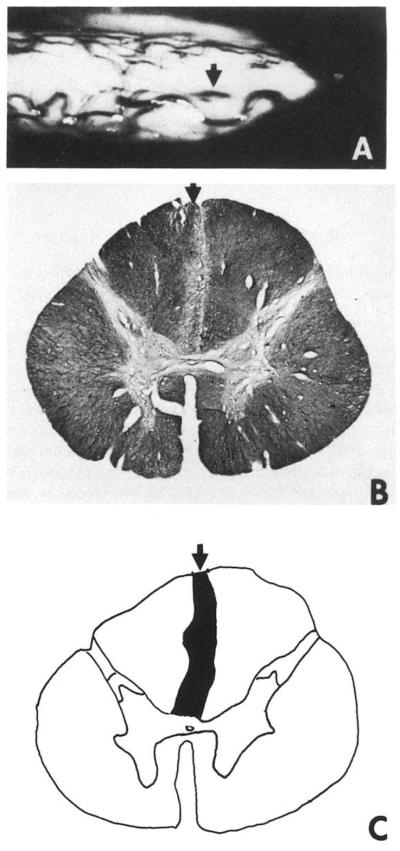
Fig. 1.
(A) Photograph of the spinal cord of Case 1 as viewed through the operating microscope just after a limited posterior midline myelotomy was done at T10. The entry line of the mechanical microdissector is indicated by the arrow. The spinal cord of the patient was removed postmortem and fixed in 10% neutral fonnalin. (B) Photomicrograph of a histological section stained for myelin through the spinal cord of Case 1 rostral to the lesion. The vertical, linear area of demyelination indicated by the arrow just to the left of the midline defines the part of the fasciculus gracilis that was interrupted by the lesion. The tissue section was stained with luxol fast blue, hematoxylin and eosin. (C) Composite drawing of the lesion made by projecting a series of sections through the lesioned area. The diameter of the human spinal cord at T10 is 8 mm.
Experimental methods
Morphological experiments
Retrograde tracing studies
Three male Sprague-Dawley rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). The upper cervical spinal cord was exposed by laminectomy. The dura in each case was opened to allow the insertion of a glass micropipette attached to the needle of a Hamilton microsyringe to inject the retrograde tracer, wheatgerm agglutinin conjugated to horseradish peroxidase (WGA-HRP; 10% in saline; Sigma). A total volume of 0.2 μ1 of WGA-HRP was injected into the medial fasciculus gracilis at the C5-C6 segments of the spinal cord at a depth of 0.2 mm from the dorsal pial surface for identification of retrogradely labeled neurons in the L6-S2 segments. After a survival time of 7 days, the animals were perfused with phosphate-buffered 1% paraformaldehyde, 1% glutaraldehyde. The tissue was cryoprotected by overnight incubation in 20% sucrose buffer prior to frozen sectioning at 30 μm. The tracer complex was visualized with nickel-enhanced diaminobenzidine (Hancock 1984; Brandt and Apkarian 1992). Rinsed sections were mounted on gelatin-coated glass slides and coverslipped.
Anterograde tracing studies
The spinal cords of three anesthetized male rats were exposed by laminectomy at the level of the L6-S1 segments. The anterograde tracer, biotin dextran (0.2 μ1, 10% in saline; Molecular Probes), was injected into the dorsal commissural region near the midline at a depth of 0.8 mm through a glass micropipette attached to the needle of a Hamilton microsyringe. After an 18–22 day survival period the animals were reanesthetized with sodium pentobarbital, then transcardially perfused with warm saline followed by mixed aldehydes (11 of 4% paraformaldehyde, 0.05% glutaraldehyde in 0.1 M acetate buffer, pH 4.5, 4°C) followed by 10% sucrose (11 in 0.1 M phosphate buffer, pH 7.6, 4°C). The lumbosacral, thoracic and C1-C2 segments of the spinal cord and the lower brainstem were dissected so that both the injection site and the target region could be analyzed. The tissue was cryoprotected by overnight incubation in 20% sucrose prior to frozen sectioning at 30 μm. Anterogradely labeled axons with cells of origin in the dorsal commissural region at L6-S1 were identified immunohistochemically with ExtrAvidin (Vector; 1:200 in 1% normal goat serum in 0.1 M phosphate buffer with 0.05% Triton X-100 for 60 min). The tracer complex was visualized with nickel-enhanced diaminobenzidine (Hancock 1984; Brandt and Apkarian 1992). Rinsed sections were mounted on gelatin-coated glass slides and coverslipped.
Electrophysiological experiments
Experiments were done on 14 male Sprague-Dawley rats weighing 280–350 g. The animals were anesthetized with sodium pentobarbital (40 mg/kg, i.p., followed by an infusion of 5 mg/kg per h, i.v., through a catheter in a jugular vein). When necessary, the rats were paralyzed (gallamine triethiodide, 10 mg initially and then 5 mg/kg per h) and artificially ventilated through a cannula placed in the trachea. End-tidal CO2 concentration was measured and regulated at 3.5–4.5%. Body temperature was monitored and kept near 37°C with a regulated heating blanket. Different experiments involved one or more of the following surgical procedures: a laminectomy to expose either the L6-S1 or the T10 segments of the spinal cord; removal of part of the occipital bone and an upper cervical laminectomy to reveal the nucleus gracilis and rostral fasciculus gracilis; and a craniectomy to allow insertion of an electrode into the thalamus.
In five experiments, extracellular single unit recordings were made from 16 neurons in the dorsal commissural region, lamina X and deep dorsal hom of the L6-S1 segments of the spinal cord. Five of these cells were shown to project in the upper cervical dorsal column by antidromic activation from the fasciculus gracilis at a level just caudal to the nucleus gracilis. In other experiments, recordings were made from three neurons of the nucleus gracilis and from 13 neurons in the ventral posterior lateral (VPL) nucleus of the thalamus. The recording electrodes used in the spinal cord and nucleus gracilis were carbon fiber microelectrodes made by pulling glass micro pipettes containing a carbon fiber with a diameter of 7 μm and etching the tip so that the impedance of the microelectrodes was 3–5 MΩ. Electrodes used in the VPL nucleus of the thalamus were tungsten microelectrodes having impedances of 1–2 MΩ. Action potentials were displayed on oscilloscopes after suitable amplification and filtering. They were also led to a window discriminator that produced stanxdard output pulses used by a data analysis system (CED 1401+) and digital computer (486 personal computer) to compile rate histograms.
All of the units examined had convergent cutaneous and visceral receptive fields. Stimuli that were employed included (1) mechanical stimuli applied to the cutaneous receptive fields of the neurons; (2) a visceral mechanical stimulus, colorectal distention (Ness and Gebhart 1988) and (3) a chemical visceral stimulus, injection of mustard oil into the colon. The following standard sequence of cutaneous mechanical stimuli was applied to the cutaneous receptive field: (1) brushing with a camel hair brush, (2) compressing the skin with an arterial clip that is only marginally painful to human subjects and (3) compressing the skin with an arterial clip that is distinctly painful. Receptive fields were mapped using a cotton Q-tip. The fields were in the perineal region and were unilateral. The colon was distended by inflating a balloon attached to a length of plastic tubing. The balloon was made from the finger of a surgical glove and was prestretched overnight so that pressure readings made through the plastic tubing reflected bowel wall tension and not tension in the wall of the balloon. According to Ness and Gebhart (1988), when the balloon is inserted 7 cm into the colon and inflated, pressures of 0–30 mmHg are innocuous and pressures of 40–100 mmHg are noxious. Bowel distentions used in the present study were restricted to levels of 80 mmHg or less. The pressure was monitored both with a pressure gauge and through the data analysis system. Distentions consisted of rapid increases in pressure to a stable level held for 20 sec, followed by a return to the original level. Four minutes were allowed to pass before visceral stimuli were repeated. Usually, colon distention produced no observable movement of the base of the tail or body wall. In unusual experiments when movements were observed, deliberate movements made by the investigator failed to activate the neuron under observation. Thus, neuronal activity was not related to movements produced by visceral stimulation. In some experiments, the activity of neurons responding to colon distention was recorded before and after injection of 0.5 ml of a solution of 2.5% mustard oil in mineral oil to inflame the colon. These experiments provided further evidence that postsynaptic dorsal column neurons and neurons of the nucleus gracilis and the VPL thalamic nucleus do indeed receive visceral nociceptive input that is independent of movements that might be produced by mechanical stimuli.
For the data analysis, the background discharge rates of neurons were subtracted from response rates recorded during stimulation to give the responses above background to each stimulus. In some of the experiments in which recordings were made from VPL neurons, a lesion was made in the fasciculus gracilis at the T10 segmental level after control responses to colon distention and cutaneous stimulation were recorded. The responses to the same stimuli were again recorded to determine if the lesion had any effect.
The positions of the recording electrodes were marked at the end of each expenment. The animals were either perfused or the nervous tissue fixed by immersion, so that the electrode positions could be localized and the extent of the lesion examined histologically.
Results
Clinical cases
Eight cases of limited midline myelotomy for the relief of pelvic cancer pain are reviewed here. The myelotomies in all eight cases were performed by Hirshberg in the same manner. The first two cases described were performed in 1993 and 1992, respectively, and a full clinical report is given here. The remaining six cases were performed prior to 1984, and these were reported briefly in Gildenberg and Hirshberg (1984). All eight cases are summarized in Table I. The cases were all open surgical procedures done under high magnification. In each case, a detailed neurological examination was done pre- and post-myelotomy. Neurological testing included cremasteric and anal reflexes, as well as a detailed motor and sensory examination. The latter included an evaluation of two point discrimination, proprioception, pinprick sensation, light touch, pressure, and vibratory sense. Pain was evaluated by (1) the level of mobility and activity of the patient; (2) the amount and type of medication required to manage the pain; and (3) the patient’s description ofthe pain (Table I).
TABLE I.
SUMMARY OF THE EIGHT CASES OF PELVIC VISCERAL PAIN THAT WERE TREATED WITH LIMITED MIDLINE MYELOTOMY IN HIRSHBERG SERIES
| Case | Age/Sex | Diagnosis | Pre-myelotomy |
Post-myelotomy |
Post-op survival (months |
Cause of death |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pain Rx | Neurol. exam | Activity | Pain report | Pain Rx | Neurol. exam | Activity | Pain report | |||||
| 1 | 44/M | Colon cancer | i. v. morphine sulfate with PCA pump |
Normal | Bedridden | ‘Intolerable’ | None | Normal | Ambulatory; fishing |
‘Pain gone’ until death |
3 | Cancer- related causes |
| 2 | 40/F | Ovarian cancer |
i.v. morphine sulfate with PCA pump |
Right leg limp due to plexus involvement |
Bedridden | ‘Severe’ | Oral hydro- codone bitar- trate 5 mg + acetaminophen 33 mg |
Normal | Ambulatory | ‘No pelvic pain; only pain due to rib and thor- acic vertebral metastases, until death |
6 | Cancer- related causes |
| 3 | 48/F | Disseminated squamous cell cancer of the anus |
Meperidine HCI 100 mg i.m. every 3 h |
Right leg weakness due to plexus invasion by cancer |
Bedridden | ‘Unbearable’ | Over the counter analgesic |
Right leg limp |
Ambulatory | ‘Minimal burning sen- sation’ in perineum, until death |
5 | Cancer- related causes |
| 4 | 51/M | Adenocarci- noma of rectum; metastasis to liver and lungs; cancer fixed to pelvic wall |
Meperidine HCI 100 mg i.m. every 3 h |
Normal | Minimal ambulation |
‘Unbearable’ | Over the counter analgesic |
Right leg limp |
Ambulatory | ‘Minimal burning sen- sation’ in perineum, until death |
5 | Perirectal abscess and sepsis |
| 5 | 37/F | Malignant lymphoma with node involvement ment and gangrenous bowel |
Hydromor- phone HCI 2 mg i.m. |
Normal except sympathetic dystrophy of right leg due to sciatic injection |
Bedridden | ‘Extreme’ | No opioid Rx at 4 mo. |
Normal except for sympathetic dystrophy right leg; limp of right leg |
Ambulatory | ‘Occasional burning pain in right leg |
At least 4 | Lost to follow-up |
| 6 | 74/F | Adenocarci- noma of rectum |
Meperidine HCI i.m. every 4 h |
Normal | Bedridde | ‘Unbearable’ | Acetamino- phen codeine sulfate 30 mg at night |
Normal | Ambulatory | ‘Excellent’ ; pain controlled until death |
4 | Cancer-related causes; developed metastases to pelvis and pathological fracture of left hip |
| 7 | 67/M | Adenocarci- noma of rectum |
Hydromor- phone HCI 4 mg i.m. or p.o. every 3 h |
Norma | Minimal ambulation with help, due to pain |
‘Excruciating’ especially hip and leg |
Initially hydrocodone (4.5 mg) + as- pirin (325 mg) 1–2 tab/day; changed to 1 grain codeine phosphate 30–60 mg before bed |
Normal | Ambulatory | ‘Excellent,’ until death |
5 | Cancer- related causes |
| 8 | 76/F | Cancer of vulva |
Hydromor- phone HCI 2-4 mg i.m. every 3 h |
Normal | Barely ambulatory |
‘Awful’ | Acetamino- phen 300 mg, 1–2 tab/day |
Normal | Ambulatory | ‘Significant relief’; mild ‘burning pain’ in perineum, until death |
11 | Cancer- related causes |
Case 1: male, aged 44
The most recent case was unusual in that it was possible to do a histological examination of the spinal cord postmortem to determine the extent of the neurosurgical lesion placed at the T10 segmental level.
The patient was diagnosed with carcinoma of the colon and had a colon resection. A diverting colostomy was done when recurrence was discovered. A right ureteral stent was placed because of involvement of the right distal ureter, and later a left ureteral stent was necessary because of obstruction of the left ureter. After further abdominal exploration, a jejuno-transverse colostomy was performed and the patient was given a complete course of radiotherapy and chemotherapy. The malignancy was observed to extend into the penile urethra and scrotum. On neurological examination prior to the midline myelotomy, the patient was found to have no neurological deficit, either sensory or motor. Cremasteric reflexes were present bilaterally, the anal reflex was normal, and there was no perineal sensory deficit (including on the penis and scrotum). The patient was in extreme pain and required frequent dosage of i.v. morphine sulfate through a Portacath (Diavol) in the subclavian vein delivered by a patient-controlled analgesia pump (Baxter).
Because of the progression of the perineal and pelvic pain, a limited myelotomy at the T10 segmental level was performed 4 years after the initial diagnosis of colon cancer was made (the extent of the degeneration produced by the lesion is shown in Fig. 1A). When it was presumed that the tip of the instrument was at the level of the commissural gray, the instrument tip was tilted rostrally in order to extend the lesion a few mm rostrocaudally. Following surgery, the patient’s pain was completely relieved. The intravenous morphine dose was reduced over the period of 3 days and then discontinued. The postoperative neurological examination was unchanged. The patient became ambulatory and required no pain medication until his death 3 months later. However, the patient developed a subcutaneous abscess in the lower abdomen below the umbilicus during the postoperative interval. This abscess was quite painful until it was drained.
Histological examination of the spinal cord in this case revealed that the midline lesion extended from the posterior surface of the spinal cord to the level of the posterior commissure. The arrow in Fig. 1A shows the entry line of the mechanical probe used to produce the lesion. The arrows in Fig. 1B,C indicate an area of demyelination in the medial part of the posterior columns at T10 in a section taken just rostral to the level of the lesion. Intact axons of the posterior and anterior white commissures at this level were seen under the microscope at high power. The lesion was narrow, but interrupted axons of the fasciculus gracilis on both sides of the midline, although the main area of damage was on one side. The central gray matter did not appear to be affected.
Case 2: woman, aged 40
The patient was found to have a large pelvic mass extending from the midline to the right side. The mass was associated with intractable pain in the pelvis and right lower extremity. A diagnosis of ovarian squamous cell carcinoma with partial bowel obstruction was made. The patient underwent a total abdominal hysterectomy, bilateral salpingo-oophorectomy and intestinal bypass procedure. This was followed by radiation therapy and chemotherapy. The patient’s pain was not controlled by i.v. morphine sulfate administered through a Portacath and a patient-controlled analgesia pump. It was evident that the pain in the right hip and leg and the pelvic pain were due in part to involvement of the lumbosacral plexus by the malignancy.
Six months after diagnosis, a limited midline myelotomy was performed at T10 without incident. There was immediate complete resolution of the pain. The patient did not have bowel or bladder incontinence, and there was no evidence of peri-anal or vaginal hypalgesia. The anal reflex was intact. At the time of her last admission, the patient had developed a rectovaginal fistula. The patient continued to have no pelvic or lower extremity pain. Pain present was above the T10 segmental level, in the area of the upper abdomen and costovertebral areas bilaterally and was secondary to hepatic, rib and vertebral metastases. This pain was treated with oral medication with hydrocodone bitartrate (Knoll). She remained ambulatory with a right lower extremity limp until she expired 12 months after the diagnosis was made and 6 months postoperatively.
Experimental results
Morphological experiments
Morphological studies were initiated to determine the origins ofaxons projecting in the medial dorsal column that might mediate the transmission of visceral nociceptive information, as well as the sites of termination of such projections in the brainstem.
Retrograde study
The retrograde tracer, WGA-HRP, was injected into the dorsal column in three rats at a cervical level. The retrograde tracer was taken up by aXOns of the dorsal columns and transported caudally to label cells in the central regions of the spinal cord. In the sacral spinal cord, numerous labeled neurons were observed in the dorsal commissural region above the central canal, in the gray matter surrounding the central canal (lamina X), as well as more laterally in lamina VII (Fig. 2). Labeled neurons were later mapped in the L6-S2 segments of the spinal cord, as shown in the schematic summary diagram in Fig. 4. These neurons are therefore likely to be the source of the axons traveling to the brains tern in the dorsal columns.
Fig. 2.
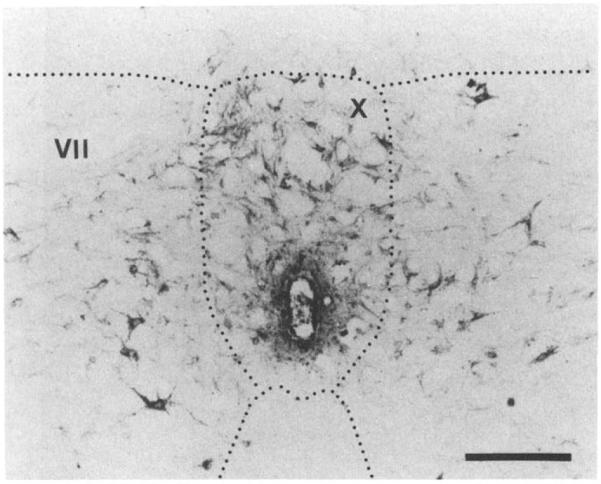
The photomicrograph shows the central portion of a rat spinal cord section taken through the S2 segment that had had a bilateral injection of WGA-HRP into the dorsal columns at the C5-6 level 7 days prior to sacrificing the animal. Note the numerous retrogradely labeled neurons around the central canal (lamina X), especially dorsally, and in the adjacent gray matter (lamina VII). The calibration bar indicates a length of 130 μm.
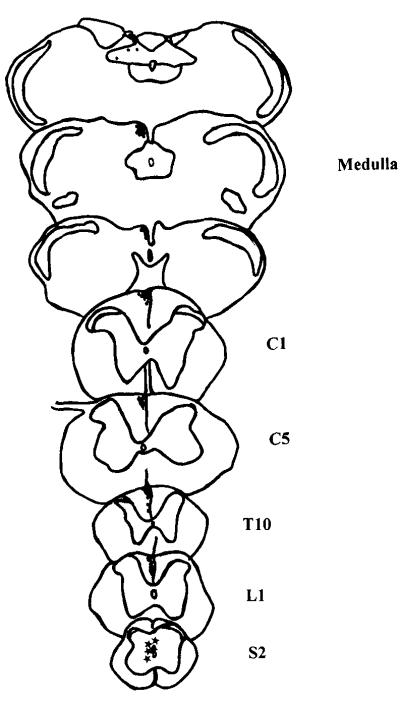
Fig. 4.
Schematic summary showing, on drawings of spinal cord and brainstem sections, the location of retrogradely labeled neurons and the course of the axons of these postsynaptic dorsal column neurons as they ascend in the dorsal column. The axons in this animal were labeled by a unilateral injection of biotin dextran into the central gray matter at S2. The location of the axons is indicated by fine stipple in drawings of transverse sections of the spinal cord at L1, T10, C5 and C1, as well as at the level of the caudal medulla.
Anterograde study
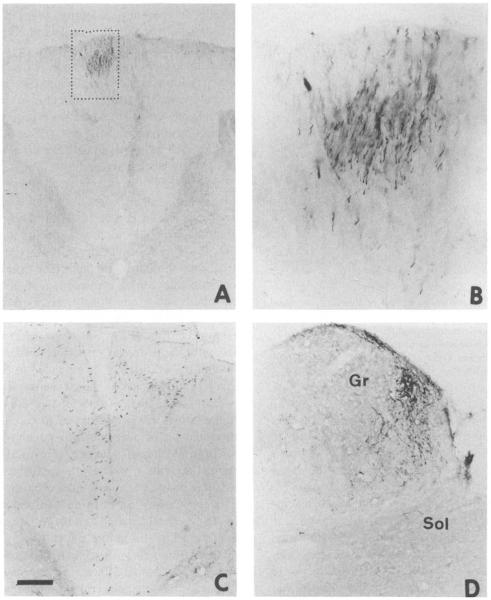
In three other animals, an anterograde tracer, biotin dextran, was injected into the midline gray matter at L6-S1, and labeled axons were followed rostrally in the fasciculus gracilis to the brainstem. Depending on the extent of the injection, labeled axons were found either unilaterally or bilaterally in the most medial portion of the dorsal column from the lumbosacral cord through the upper lumbar and lower thoracic cord (Figs. 3C and 4). In the upper thoracic and cervical cord, the axons traveled in the superficial part of the medial fasciculus gracilis (Figs. 3A,B and 4). Terminals were found in the medial part of the nucleus gracilis (Figs. 3D and 4) and the nucleus of the solitary tract, including the commissural region.
Fig. 3.
(A–D) Photomicrographs showing axons that were anterogradely labeled following an injection of biotin dextran into the medial gray matter of the S2 segment of the spinal cord 18 days prior to sacrifice in two of the animals. The axons in the smaller box in (A), and shown at higher magnification in (B) are from an animal with a unilateral injection. The ascending axons were in the medial fasciculus gracilis on one side at C1. The labeled axons in (C) were in the medial fasciculus gracilis bilaterally at the L1 level in a different animal. The labeled axons and terminal boutons shown in (D) were in the medial part of the nucleus gracilis (Gr) and the solitary nucleus (Sol) in the same animal as that illustrated in (A,B). The calibration bar indicates a length of 190 μm for (A), 65 μm for (B) 112 μm for (C), and 73 μm for (D).
Electrophysiological studies
Electrophysiological experiments were initiated to determine if postsynaptic dorsal column neurons in the L6-S1 segments of the spinal cord, neurons in the nucleus gracilis, and neurons in the VPL nucleus of the thalamus can be found that respond to noxious visceral stimuli.
Responses of interneurons and dorsal column projection neurons at L6-S1
Recordings were made from 16 neurons in the L6-S1 segments of the spinal cord in the region that contained retrogradely labeled neurons in our morphological study. A total of 11 unidentified neurons near the midline in the L6-S1 segments of the rat spinal cord responded to noxious colorectal distention. Of these, six were activated by noxious colorectal distention and five were inhibited. Five other neurons in the same location were activated antidromically from the rostral fasciculus gracilis, just caudal to the nucleus gracilis, and so were postsynaptic dorsal column projection neurons. The antidromic stimulus at C1 was applied at approximately the same position as the anterogradely labeled axons shown in Figs. 3A,B and 4.
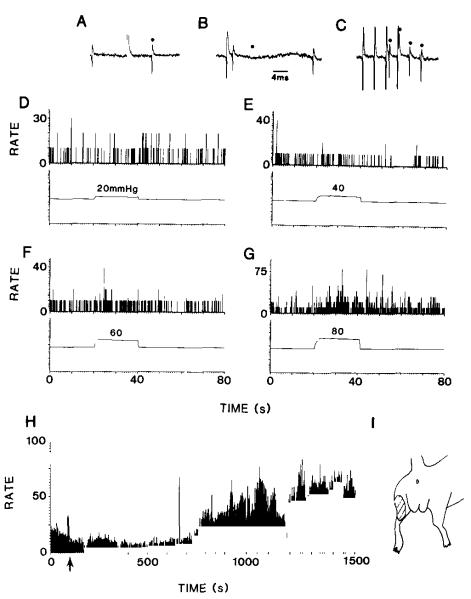
Examples of the responses of dorsal column projection neurons to graded colorectal distentions and to mustard oil are illustrated in Fig. 5. Tests for antidromic activation by stimulation in the fasciculus gracilis at C1 are shown in Fig. 5A–C. These include collision (Fig. 5A,B) and high frequency following (Fig. 5C). The neuron did not have an obvious response to a distention of 20 mmHg (Fig. 5D), and there was a questionable response to 40 mmHg (Fig. 5E). However, there were distinct responses to 60 and 80 mmHg (Fig. 5F,G). The same neuron had a cutaneous receptive field on the posterior surface of the ipsilateral hindlimb (Fig. 5I), and it responded to brush, pressure and pinch stimuli applied to the cutaneous receptive field.
Fig. 5.
The action potentials shown in (A–C) were recorded from a neuron located near the midline of the L6-S1 spinal cord. The neuron was shown to belong to the postsynaptic dorsal column pathway by antidromic activation following stimulation of the fasciculus gracilis at C1. In (A) are shown a spontaneously occurring action potential, followed by the shock artifact and the antidromic action potential (marked by a dot). In (B), a spontaneous action potential collided with the antidromic action potential, which should have occurred at the time indicated by the dot. In (C), the antidromic action potential is shown to follow stimulation at a frequency of 300 Hz. One of the four action potentials is partly obscured by a shock artifact. The rate histograms in (D–G) represent the discharge rate of the neuron before, during and after graded colorectal distentions over the range of 20–80 mmHg. The timing and intensity of the stimuli are indicated by the monitor trace in each panel. There was no response to 20 mrnHg (A), but increasingly greater responses followed stimuli of 40 (B), 60 (C) and 80 (D) mmHg. The histogram in (H) shows the background firing of a different postsynaptic dorsal column neuron located in the gray matter near the midline at L6-S1. At the time indicated by the arrow, 0.5 ml of a 2.5% solution of mustard oil in mineral oil was injected into the colon 7 mm proximal to the anus. Note the increase in the discharge rate after about 15 min. The drawing in (I) illustrates the receptive field of the neuron.
The background activity of two dorsal column projection neurons was demonstrated to be enhanced during the development of an acute inflammation of the colon following injection of mustard oil. Fig. 5H shows the background activity of one of these neurons. The mustard oil was injected at the time indicated by the arrow. There was a steep increase in the firing rate about 15 min after the injection. The increased discharge rate continued for several more minutes before the cell was lost. Thus, we can infer that the neuron had input from chemically sensitive visceral nociceptive afferent fibers that innervated the colon, as well as from visceral mechanical nociceptors.
Four of the five postsynaptic dorsal column neurons studied responded to graded colorectal distention in a reproducible manner (Fig. 6), and the two tested following injection of mustard oil into the colon showed an increased background activity. Fig. 6 shows a plot of the mean responses of four postsynaptic dorsal column neurons to graded colorectal distension. Superimposed over the plot is a best fit curve that intersects the abscissa axis at 10 mmHg. The inset shows the recording sites for these four neurons. All were in the dorsal commissural region in the sacral spinal cord.
Fig. 6.
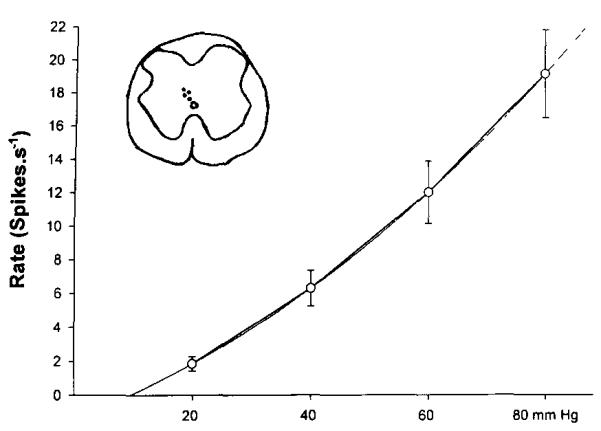
The graph illustrates the mean responses ± SEM of four postsynaptic dorsal column cells to graded colorectal distention. A best fit curve, superimposed over the plot, intersects the abscissa axis at around 10 mmHg, indicating no response at this distention pressure. The insert shows a drawing of a cross section of the spinal cord at S1 with the locations of the recording electrode tips marked as dots.
Responses of neurons in the nucleus gracilis
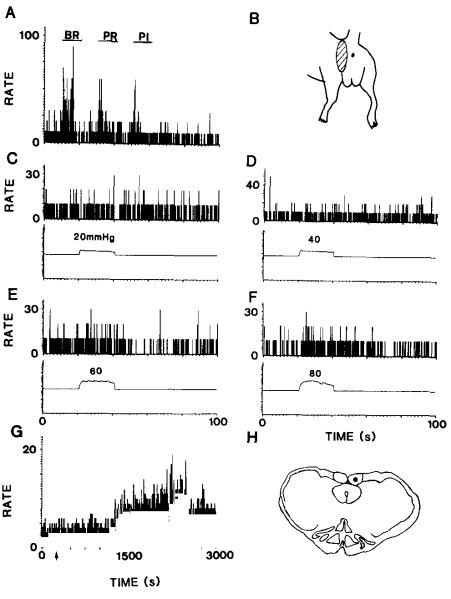
Presuming that visceral nociceptive information is transmitted by the axons of postsynaptic dorsal column neurons that ascend in the dorsal column to the nucleus gracilis, similar responses should be obtained from neurons of the nucleus gracilis. In three experiments, recordings from the nucleus gracilis demonstrated responses of three gracile neurons to noxious colorectal distention. The responses of one of these neurons to 60 and 80 mmHg stimuli are shown in Fig. 7E,F. Weaker colorectal distentions had no clearcut effect (Fig. 7C,D). The same neuron responded to mechanical stimulation of the skin (Fig. 7A) and to injection of mustard oil into the colon (Fig. 7G). The cutaneous receptive field is shown in Fig. 7B and the recording site in Fig. 7H.
Fig. 7.
The rate histogram in (A) shows the responses of a neuron in the nucleus gracilis to graded intensities of mechanical stimulation of the skin in the perineal region. The stimuli included brush (BR), pressure (PR) and pinch (PI) and were applied at the times indicated by the horizontal bars above the histogram. The receptive field is illustrated in (B). The histograms in (C–F) show the responses of the neuron to graded colorectal distentions. There was no response to distentions of 20 and 40 mmHg (C,D), but clear responses were seen to distentions of 60 and 80 mmHg (E,F). Injection of mustard oil into the colon provoked an increase in discharge rate within 20–30 min (G). The injection was at the time indicated by the arrow. The location of the tip of the recording electrode in the nucleus gracilis is indicated in (H).
Responses of neurons in the VPL nucleus of the thalamus
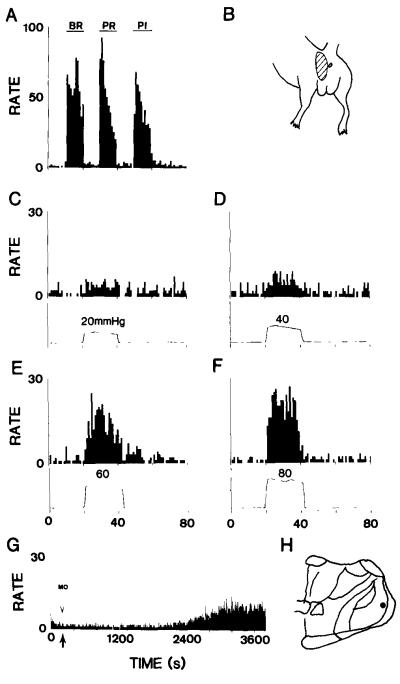
The main thalamic target of the nucleus gracilis is the VPL nucleus, and so that nucleus was explored to see if neurons that respond to visceral noxious stimuli could be found. Thirteen VPL neurons were found that responded to noxious colorectal distention in six animals. VPL neurons were also shown to respond to injection of mustard oil into the colon. Figs. 8 and 9 show examples of the responses of VPL neurons to graded mechanical stimulation of the skin and to colorectal distentions of 20, 40, 60 and 80 mmHg, as well as to mustard oil. The responses of one VPL neuron to BR, PR and PI stimuli are shown in Fig. 8A, and the responses of the same neuron to graded colorectal distention in Fig. 8C–F. The cutaneous receptive field of this neuron is shown in Fig. 8B and its location is indicated on the drawing in Fig. 8H. The response of another VPL neuron to mustard oil is shown in Fig. 8G.
Fig. 8.
The rate histograms show the activity of a neuron in the VPL nucleus that responded to graded mechanical stimulation of the skin (A) and to graded colorectal distentions (C–F). The receptive field of the neuron is indicated in (B) and the location of the neuron in (H). The histogram in (G) shows the response of a different VPL neuron to injection of mustard oil into the colon at the time indicated by the arrow.
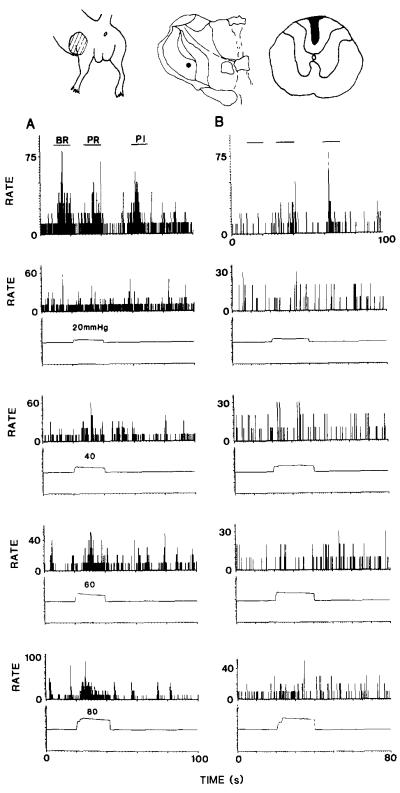
Fig. 9.
At the top of the figure are shown the receptive field of the neuron, its location in the VPL nucleus and the maximum extent of the dorsal column lesion. The rate histograms show the responses of a neuron in the VPL nucleus to graded mechanical stimulation of the skin (upper row) and to graded colorectal distentions (lower four rows) before (A) and after (B) a lesion of the fasciculus gracilis. The lesions reduced or eliminated most of the responses, except the response to pinch.
In three experiments, the fasciculus gracilis was interrupted at the T10 level to determine if the responses to visceral and cutaneous stimuli were mediated by axons that ascend in the dorsal column. The responses to noxious colorectal stimulation were either eliminated or dramatically reduced. The responses to innocuous mechanical stimulation of the skin were also eliminated or markedly reduced, but the responses to noxious mechanical stimulation of the skin were only slightly affected. Fig. 9 illustrates one such experiment. The cutaneous receptive field, location of the neuron in the VPL nucleus and the lesion of the dorsal column are shown on top of Fig. 9. (It should be noted that the lesion does not extend into the gray matter and in fact leaves largely intact the corticospinal tract, which in rats is located just ventral to the dorsal column pathway.) The responses of this neuron to graded colorectal distention are shown before (left column) and after (right column) a lesion of the fasciculus gracilis. The lesion eliminated the responses of the neuron to brush and pressure stimuli and to all intensities of colorectal distention. The response to pinch was reduced but not eliminated.
Discussion
The present report includes eight case histories of patients with pelvic cancer pain who were successfully relieved of their pain following a midline lesion of the spinal cord at the T10 level. In one of these cases, it was possible to remove the spinal cord postmortem and to examine the lesion histologically. The results of the limited midline myelotomies were generally excellent for the period during which follow-up was possible. Pain was relieved either completely or at least sufficiently so that strong pain medications could be discontinued or replaced by milder drugs. In the case in which the spinal cord was examined postmortem, morphine was completely discontinued for the remaining 3 months of the patient’s life, and the patient enjoyed a pain-free terminal period, except for a period of time when there was cutaneous pain from an abscess below the umbilicus. Presumably the cutaneous pain was mediated by the intact spinothalamic tract and was therefore unaffected by the limited midline myelotomy. The surgical lesion interrupted axons ascending in the fasciculus gracilis just adjacent to the midline. There were no sensory or motor deficits on postoperative clinical testing. The clinical effectiveness of the limited midline myelotomy in reducing pelvic visceral pain suggests that there is a visceral nociceptive pathway in the posterior columns.
A peculiar discrepancy in the distribution of pain relief and in the findings obtained with sensory testing is commonly noted following either commissural myelotomy or limited midline myelotomy (Mansuy et al. 1944; Schvarcz 1976; King 1977; Cook et al. 1984; Gildenberg and Hirshberg 1984; see review by Gybels and Sweet 1989). The distribution of the pain relief is generally far greater than the area of sensory deficit. Therefore, interruption of just the decussating axons of the spinothalamic tract cannot account for more than part of the pain relief. Explanations offered for this discrepancy are that commissural myelotomies interrupt not only the decussating axons of spinothalamic neurons involved in pain sensation, but also a polysynaptic extralemniscal pain pathway ascending in the central gray matter or a pathway in the immediately adjacent white matter; limited midline myelotomies have been presumed to interrupt chiefly one of the latter (Hitchcock 1970; Schvarcz 1976, 1978; King 1977; see discussion in Noordenbos 1959; Cook et al. 1984; Gybels and Sweet 1989). Evidence that supports this idea comes from experiments by Karplus and Kreidl (1914) and Basbaum (1973), who showed that hemisections at different levels and on different sides of the spinal cords of cats or rats fail to prevent supraspinal responses to noxious stimuli. However, when this experiment was done in monkeys, Karplus and Kreidl (1914) found no nocifensive responses after the two hemisections, suggesting that nociception in monkeys depends on long pathways that ascend in the white matter. A further argument against the proposal of an extralemniscal pathway is that myelotomies are unlikely to interrupt completely a polysynaptic pathway ascending in the gray matter (Cook et al. 1984).
On the other hand, several experimental studies have demonstrated the presence of visceroreceptive neurons in the vicinity of the central canal, many of which project to the brainstem (Nahin et al. 1983, 1986; Honda and Perl 1985). Furthermore, Vierck et al. (1986) have found that anterolateral cordotomies in monkeys that produce the longest lasting analgesia damage the gray matter around the central canal. They suggest that a combination of an anterolateral cordotomy with a lesion in the central core region of the spinal cord would give the best analgesia, especially for deep and midline pain. We suggest from our results that a lesion directed toward the central canal may interrupt axons of the postsynaptic dorsal column pathway involved in signalling visceral pain.
Evidence for a nociceptive pathway in the posterior column is that the number of ‘pain spots’ decreases after a lesion of the posterior column in man (Kroll 1930). Furthermore, the reactivity of monkeys to noxious stimuli decreases after a dorsal column lesion, either alone or in combination with a contralateral ventrolateral cordotomy (Vierck et al. 1971; Vierck and Luck 1979). Davis et al. (1929) report that visceral pain in dogs depends on conduction in the central core region of the spinal cord. Nociceptive responses have been recorded from neurons of the nucleus gracilis in cats and monkeys (Angaut-Petit 1975b; Ferrington et al. 1988; Cliffer et al. 1992; Berkley and Hubscher 1995), and many of these nociceptive neurons were shown by antidromic activation to project to the ventral posterior lateral nucleus of the thalamus (Ferrington et al. 1988; Cliffer et al. 1992). In monkeys, the responses of at least some VPL neurons to noxious cutaneous stimuli are eliminated by a lesion that interrupts pathways ascending in the dorsal quadrant of the spinal cord on the same side as the stimulus (Chung et al. 1986), although usually a contralateral lesion of the lateral funiculus is required (Kenshalo et al. 1980; Chung et al. 1986).
One way in which nociceptive information could be carried in the dorsal column is by the fine, peptide-containing primary afferent fibers that have recently been described in the dorsal column (Patterson et al. 1989, 1990; Conti et al. 1990). Although the dorsal column has traditionally been viewed as composed exclusively of myelinated axons, electron microscopic observations indicate that there are numerous unmyelinated axons in the fasciculus gracilis at the lumbosacral level (Langford and Coggeshall 1981). Counts show that these represent 29% of the axonal population at the sacral level in rats (Chung and Coggeshall 1985) and 25% in humans (Briner et al. 1988). When the fasciculus gracilis was examined at the C3 level in rats, 29% of the axons were found to be unmyelinated, and 80% of these degenerated following an extensive dorsal rhizotomy (Patterson et al. 1989). Many of the unmyelinated axons at C3 contained immunoreactivity for calcitonin gene-related peptide (CGRP), a marker of primary afferent fibers (Patterson et al. 1990). Similarly, a large proportion of the unmyelinated dorsal column axons in the fasciculus gracilis of the human sacral cord contain CGRP-immunoreactivity (Briner et al. 1988). In addition to CGRP-immunoreactive fibers, there are also numerous substance P-immunoreactive axons in the upper cervical dorsal columns, and double labeling experiments show that many of these axons originate from dorsal root ganglion cells (Tamatani et al. 1989; Fabri and Conti 1990; Hoeflinger et al. 1993) and terminate in the dorsal column nuclei (Kawai et al. 1985; Kruger et al. 1988a,b; however, cf. Giufridda and Rustioni 1992). The SP-containing endings in the dorsal column nuclei are derived partly from neurons of dorsal root ganglia and partly from postsynaptic dorsal column neurons (Conti et al. 1990).
An alternative route for nociceptive information to reach the dorsal column nuclei is through the postsynaptic dorsal column path (Gybels and Sweet 1989; Willis and Coggeshall 1991). This pathway was first described by Uddenberg (1968), who recorded the responses of postsynaptic axons in the dorsal funiculus of cats and found that many of them discharged in response to noxious stimuli. Similar results have been reported by a number of investigators (Angaut-Petit 1975a,b; Brown and Fyffe 1981; Brown et al. 1983; Lu et al. 1983; Bennett et al. 1984; Kamogawa and Bennett 1986; Noble and Riddell 1988; however, cf. Giesler and Cliffer 1985). The cells of origin of these axons have been mapped in rats, cats and monkeys (Rustioni and Kaufman 1977; Rustioni et al. 1979; Brown and Fyffe 1981; Bennett et al. 1983; de Pommery et al. 1984; Giesler et al. 1984; Enevoldson and Gordon 1989). The axons of this pathway terminate in the nucleus gracilis or the nucleus cuneatus, depending on whether the cells of origin are in the caudal or rostral segments of the spinal cord (Rustioni 1973, 1974; Rustioni et al. 1979; Cliffer and Giesler 1989; Pierce et al. 1990; Cliffer and Willis 1994). As mentioned above, at least some of the postsynaptic dorsal column neurons contain SP immunoreactivity (Conti et al. 1990).
Previous studies of postsynaptic dorsal column neurons have not sought to demonstrate responses to visceral stimuli, including pelvic visceral stimuli. Stimulation of visceral afferent fibers has been shown to activate axons of the dorsal column (Yamamoto et al. 1956) and neurons in the dorsal column nuclei (Rigamonti and Hancock 1974, 1978; Berkley and Hubscher 1995). However, it is unclear if these responses depended on primary afferent or postsynaptic dorsal column projections. It is also unclear whether or not visceral nociceptive neurons in the dorsal commissural region, lamina X and the deep dorsal horn at the sacral level (Honda 1985) belong to the postsynaptic dorsal column pathway. Few neurons in these areas seem to have been found in previous retrograde labeling studies (Rustioni and Kaufman 1977; Rustioni et al. 1979; Bennett et al. 1983; Giesler et al. 1984), in contrast to the large number of retrogradely labeled neurons observed in this study. Others have recorded responses to visceral and somatic stimuli from cells near the central canal in the sacral cord of rats and cats (Honda 1985; Ness and Gebhart 1987). Some of these cells are antidromic ally activated from the rostral ventrolateral cord (Ness and Gebhart 1987) and might possibly be components of the spinothalamic tract. Spinothalamic tract cells in the monkey have been shown to respond to visceral stimuli (Foreman et al. 1981; Milne et al. 1981; Ammons 1989).
The experimental findings reported here lend support to the view that there is a visceral nociceptive pathway in the dorsal column of rats that serves as an alternative nociceptive pathway to the spinothalamic tract. If humans have a visceral nociceptive pathway similar to that in rats, its interruption could explain why a lesion of the medial fasciculus gracilis in humans by a limited midline myelotomy at T10 could provide the pain relief that was observed in the series of pelvic cancer pain cases reviewed here. The same explanation may also apply to the favorable results of the stereotactic midline lesions of Hitchcock (1970, 1972a,b) and Schvarcz (1976, 1978), as well as those of commissural myelotomies. These procedures relieve pain over a much greater part of the body than would be expected from the interruption of the decussating axons of spinothalamic neurons over a limited length of spinal cord (Wertheimer and Lecuire 1953; Sourek 1969; Mansuy et al. 1976; Cook and Kawakami 1977; Cook et al. 1984).
The experiments in rats support the view that the postsynaptic dorsal column pathway plays an important role in visceral nociception. Neurons located near the midline in the dorsal commissural region and in lamina X, as well as neurons in the deep dorsal horn of the rat spinal cord, were labeled retrogradely by injections of WGA-HRP into the dorsal columns at C5-6 and, therefore, are the cells of origin of axons coursing rostrally in the dorsal columns. Projections from neurons at L6-S1 were also traced anterogradely in the dorsal columns. At various spinal levels, these fibers in rats (Figs. 3C and 4) were aligned adjacent to the midline of the dorsal columns in a position similar to that of the surgical lesion in Case 1 (Fig. 1B,C). These appeared to end in the nucleus gracilis and the nucleus of the solitary tract (Fig. 3D). Neurons in the medial part of the gray matter at L6-S1 that project up the dorsal columns as far as Cl, as shown by antidromic activation from the rostralmost fasciculus gracilis, can be found that respond to noxious colorectal distention and to acute inflammation of the colon by mustard oil. The same neurons have cutaneous receptive fields in the L6-S1 dermatomes. It is as yet uncertain if these projection neurons actually terminate in the nucleus gracilis. This will need to be proved by mapping the terminal zones with an antidromic stimulating electrode (cf. Applebaum et al. 1979; Dado et al. 1994). The termination zones of some of these neurons may well prove to be in other brainstem nuclei, such as the nucleus of the solitary tract (Menetrey and Basbaum 1987) or the reticular formation (Lima 1990). The fact that visceral nociceptive responses could also be recorded from neurons in the nucleus gracilis suggests that at least some of the dorsal column projection neurons at L6-S1 belong to the postsynaptic dorsal column pathway. This observation is consistent with the findings of Berkley and Hubscher (1995) that there are neurons in the nucleus gracilis of rats that respond to noxious stimulation of female reproductive organs.
In the present report, visceroceptive neurons were also observed in the VPL nucleus of the thalamus in rats. Similar observations were made by others, including Chandler et al. (1992), Berkley et al. (1993) and Brüggemann et al. (1994). In this study, a lesion of the fasciculus gracilis at a lower thoracic segmental level greatly diminished or eliminated the responses of VPL neurons to noxious colorectal distention, as well as the responses to innocuous mechanical stimulation of the skin. However, responses to noxious cutaneous stimulation were only slightly reduced. The observation that interruption of the fasciculus gracilis reduces visceral more than cutaneous nociceptive transmission to the VPL nucleus of the thalamus is consistent with a role for the dorsal column-medial lemniscus system in visceral nociception.
Although the spinothalamic tract also receives nociceptive visceral input (Foreman et al. 1981; Milne et al. 1981; Ammons et al. 1985), it may be that visceral pain depends more on the visceral nociceptive pathway in the dorsal column proposed above (see Dargent et al. 1963). The responses of VPL neurons to colorectal distention that remain after a lesion of the fasciculus gracilis are presumably mediated by the spinothalamic tract. Thus, the full expression of visceral nociceptive responses of VPL neurons may depend on convergence of activity in both the postsynaptic dorsal column and spinothalamic paths at the thalamic level. Referred pain (Head 1893) may depend on both pathways, but a more dominant cutaneous input to the thalamus by way of the spinothalamic tract could be an important factor (cf. Brüggemann et al. 1994).
Acknowledgements
We are grateful to Drs. John S. Stehlin, P. DeIpolyi and T. Trezona for patient referrals, and to Griselda Gonzales for help with the histology and the illustrations. This work was supported, in part, by NIH grants NS 09743 and NS 11255.
References
- Ammons WS. Primate spinothalamic cell responses to ureteral occlusion. Brain Res. 1989;496:124–130. doi: 10.1016/0006-8993(89)91058-5. [DOI] [PubMed] [Google Scholar]
- Ammons WS, Giradot MN, Foreman RD. T2-T5 spinothalamic neuron projection to medial thalamus with viscerosomatic input. J. Neurophysiol. 1985;54:73–89. doi: 10.1152/jn.1985.54.1.73. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system, I: existence of long ascending postsynaptic fibres in the cat’s fasciculus gracilis. Exp. Brain Res. 1975a;22:457–470. doi: 10.1007/BF00237348. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system, II: functional properties and bulbar relay of the postsynaptic fibres of the cat’s fasciculus gracilis. Exp. Brain Res. 1975b;22:471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Applebaum AE, Leonard RB, Kenshalo DR, Martin RF, Willis WD. Nuclei in which functionally identified spinothalamic neurons terminate. J. Comp. Neurol. 1979;188:575–586. doi: 10.1002/cne.901880405. [DOI] [PubMed] [Google Scholar]
- Armour D. On the surgery of the spinal cord and its membranes. Lancet. 1927;2:691–697. [Google Scholar]
- Basbaum AI. Conduction of the effects of noxious stimulation by short-fiber multisynaptic systems of the spinal cord in the rat. Exp. Neurol. 1973;40:699–716. doi: 10.1016/0014-4886(73)90105-2. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Seltzer Z, Lu GW, Nishikawa N, Dubner R. The cells of origin of the dorsal column postsynaptic projection in the lumbosacral enlargements of cats and monkeys. Somatosensory Res. 1983;1:131–149. doi: 10.3109/07367228309144545. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Nishikawa N, Lu GW, Hoffert MJ, Dubner R. The morphology of dorsal column postsynaptic (DCPS) spinomedullary neurons in the cat. J. Comp. Neurol. 1984;224:568–578. doi: 10.1002/cne.902240406. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH. Are there separate central nervous system pathways for touch and pain? Nature Med. 1995;1:766–773. doi: 10.1038/nm0895-766. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Guilbaud G, Benoist JM, Gautron M. Responses of neurons in and near the thalamic ventrobasal complex of the rat to stimulation of uterus, cervix, vagina, colon, and skin. J. Neurophysiol. 1993;69:557–568. doi: 10.1152/jn.1993.69.2.557. [DOI] [PubMed] [Google Scholar]
- Brandt HM, Apkarian AV. Biotin-dextran: a sensitive anterograde tracer for neuroanatomic studies in rat and monkey. J. Neurosci. Methods. 1992;45:35–40. doi: 10.1016/0165-0270(92)90041-b. [DOI] [PubMed] [Google Scholar]
- Briner RP, Carlton SM, Coggeshall RE, Chung K. Evidence for unmyelinated sensory fibres in the posterior columns in man. Brain. 1988;111:999–1007. doi: 10.1093/brain/111.5.999. [DOI] [PubMed] [Google Scholar]
- Brown AG, Fyffe REW. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J. Physiol. 1981;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AG, Brown PB, Fyffe REW, Pubols LM. Receptive field organization and response properties of spinal neurones with axons ascending the dorsal columns in the cat. J. Physiol. 1983;337:575–588. doi: 10.1113/jphysiol.1983.sp014643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann J, Shi T, Apkarian AV. Squirrel monkey lateral thalamus, II: viscerosomatic convergent representation of urinary bladder, colon, and esophagus. J. Neurosci. 1994;14:6796–6814. doi: 10.1523/JNEUROSCI.14-11-06796.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler MJ, Hobbs SF, Fu QG, Kenshalo DR, Jr., Blair RW, Foreman RD. Responses of neurons in ventroposterolateral nucleus of primate thalamus to urinary bladder distention. Brain Res. 1992;571:26–34. doi: 10.1016/0006-8993(92)90506-5. [DOI] [PubMed] [Google Scholar]
- Chung K, Coggeshall RE. Unmyelinated primary afferent fibers in dorsal funiculi of cat sacral spinal cord. J. Comp. Neurol. 1985;238:365–369. doi: 10.1002/cne.902380310. [DOI] [PubMed] [Google Scholar]
- Chung JM, Lee KH, Surmeier DJ, Sorkin LS, Kim J, Willis WD. Response characteristics of neurons in the ventral posterior lateral nucleus of the monkey thalamus. J. Neurophysiol. 1986;6:370–390. doi: 10.1152/jn.1986.56.2.370. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Giesler GJ. Postsynaptic dorsal column pathway of the rat, III: distribution of ascending afferent fibers. J. Neurosci. 1989;9:3146–3168. doi: 10.1523/JNEUROSCI.09-09-03146.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffer KD, Willis WD. Distribution of the postsynaptic dorsal column projection in the cuneate nucleus of monkeys. J. Comp. Neurol. 1994;345:84–93. doi: 10.1002/cne.903450106. [DOI] [PubMed] [Google Scholar]
- Cliffer KD, Hasegawa T, Willis WD. Responses of neurons in the gracile nucleus of cats to innocuous and noxious stimuli: basic characterization and antidromic activation from the thalamus. J. Neurophysiol. 1992;68:818–832. doi: 10.1152/jn.1992.68.3.818. [DOI] [PubMed] [Google Scholar]
- Conti F, De Biasi S, Giuffrida R, Rustioni A. Substance P-containing projections in the dorsal columns of rats and cats. Neuroscience. 1990;34:607–621. doi: 10.1016/0306-4522(90)90168-4. [DOI] [PubMed] [Google Scholar]
- Cook AW, Kawakami Y. Commissural myelotomy. J. Neurosurg. 1977;47:1–6. doi: 10.3171/jns.1977.47.1.0001. [DOI] [PubMed] [Google Scholar]
- Cook AW, Nathan PW, Smith MC. Sensory consequences of commissural myelotomy: a challenge to traditional anatomical concepts. Brain. 1984;107:547–568. doi: 10.1093/brain/107.2.547. [DOI] [PubMed] [Google Scholar]
- Dado RJ, Katter JT, Giesler GJ. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats, I: locations of antidromically identified axons in the thalamus and hypothalamus. J. Neurophysiol. 1994;71:959–980. doi: 10.1152/jn.1994.71.3.959. [DOI] [PubMed] [Google Scholar]
- Dargent M, Mansuy L, Colon J, De Rougement J. Les problèmes poses par la douleur dans I’évolution des cancer gynécologiques. Lyon Chir. 1963;59:62–83. [PubMed] [Google Scholar]
- Davis LE, Hart JT, Crain RC. The pathway for visceral afferent impulses within the spinal cord, II: experimental dilatation of the biliary ducts. Surg. Gynecol. Obstet. 1929;48:647–651. [Google Scholar]
- De Pommery J, Roudier F, Menétrey D. Postsynaptic fibers reaching the dorsal column nuclei in the rat. Neurosci. Lett. 1984;50:319–323. doi: 10.1016/0304-3940(84)90506-8. [DOI] [PubMed] [Google Scholar]
- Dougherty PM, Willis WD. Enhanced responses of spinothalamic tract neurons to excitatory amino acids accompany capsaicin-induced sensitization in the monkey. J. Neurosci. 1992;12:883–894. doi: 10.1523/JNEUROSCI.12-03-00883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enevoldson TP, Gordon G. Postsynaptic dorsal column neurons in the cat: a study with retrograde transport of horseradish peroxidase. Exp. Brain Res. 1989;75:611–620. doi: 10.1007/BF00249912. [DOI] [PubMed] [Google Scholar]
- Fabri M, Conti F. Calcitonin gene-related peptide-positive neurons and fibers in the cat dorsal column nuclei. Neuroscience. 1990;35:167–174. doi: 10.1016/0306-4522(90)90131-m. [DOI] [PubMed] [Google Scholar]
- Ferrington DG, Dowme JW, Willis WD. Primate nucleus gracilis neurons: responses to innocuous and noxious stimuli. J. Neurophysiol. 1988;59:886–907. doi: 10.1152/jn.1988.59.3.886. [DOI] [PubMed] [Google Scholar]
- Foreman RD, Hancock MB, Willis WD. Responses of spinothalamic tract cells in the thoracic spinal cord of the monkey to cutaneous and visceral inputs. Pain. 1981;11:149–162. doi: 10.1016/0304-3959(81)90002-6. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Cliffer KD. Postsynaptic dorsal column pathway of the rat, II: evidence against an important role in nociception. Brain Res. 1985;326:347–356. doi: 10.1016/0006-8993(85)90044-7. [DOI] [PubMed] [Google Scholar]
- Giesler GJ, Nahin RL, Madsen AM. Postsynaptic dorsal column pathway of the rat, I: anatomical studies. J. Neurophysiol. 1984;51:260–275. doi: 10.1152/jn.1984.51.2.260. [DOI] [PubMed] [Google Scholar]
- Gildenberg PL, Hirshberg RM. Limited myelotomy for the treatment of intractable cancer pain. J. Neurol. Neurosurg. Psychiatry. 1984;47:94–96. doi: 10.1136/jnnp.47.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida R, Rustioni A. Dorsal root ganglion neurons projecting to the dorsal column nuclei of rats. J. Comp. Neurol. 1992;316:206–220. doi: 10.1002/cne.903160206. [DOI] [PubMed] [Google Scholar]
- Gybels JM, Sweet WH. Neurosurgical Treatment of Persistent Pain, Physiological and Pathological Mechanisms of Human Pain. Karger; Basel: 1989. [PubMed] [Google Scholar]
- Hancock MB. Visualization of peptide-immunoreactive processes on serotonin-immunoreactive cells using two-color immuno-peroxidase staining. J. Histochem. Cytochem. 1984;32:311–314. doi: 10.1177/32.3.6198359. [DOI] [PubMed] [Google Scholar]
- Head H. On disturbance of sensation with especial reference to the pain of visceral disease. Brain. 1893;16:1–132. [Google Scholar]
- Hirshberg RM, AI-Chaer ED, Lawand NB, Westlund KN, Willis WD. Is there a pathway in the dorsal funiculus that signals visceral pain?. 10th European Congress of Neurosurgery; 1995. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock ER. Stereotactic cervical myelotomy. J. Neurol. Neurosurg. Psychiatry. 1970;33:224–230. doi: 10.1136/jnnp.33.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock ER. Eiectrophysiological exploration of the cervicomedullary region. In: Somjen G, editor. Neurophysiology Studied in Man. Excerpta Medica; Amsterdam: 1972a. pp. 237–245. [Google Scholar]
- Hitchcock ER. Stereotaxis of the spinal cord. Confin. Neurol. 1972b;34:229–310. doi: 10.1159/000103073. [DOI] [PubMed] [Google Scholar]
- Hitchcock ER. Stereotactic myelotomy. Proc. R. Soc. Med. 1974;67:771–772. doi: 10.1177/003591577406700840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflinger BF, Bennett-Clarke CA, Chiaia NL, Killackey HP, Wall JT, Rhoades RW. Lesion-induced changes in the central terminal distribution of galanin-immunoreactive axons in the dorsal column nuclei. J. Compo Neurol. 1993;332:378389. doi: 10.1002/cne.903320309. [DOI] [PubMed] [Google Scholar]
- Honda CN. Visceral and somatic afferent convergence onto neurons near the central canal in the sacral spinal cord of the cat. J. Neurophysiol. 1985;53:1059–1078. doi: 10.1152/jn.1985.53.4.1059. [DOI] [PubMed] [Google Scholar]
- Honda CN, Perl ER. Functional and morphological features of neurons in the midline region of the caudal spinal cord of the cat. Brain Res. 1985;340:285–295. doi: 10.1016/0006-8993(85)90925-4. [DOI] [PubMed] [Google Scholar]
- Kamogawa H, Bennett GJ. Dorsal column postsynaptic neurons in the cat are excited by myelinated nociceptors. Brain Res. 1986;364:386–390. doi: 10.1016/0006-8993(86)90853-x. [DOI] [PubMed] [Google Scholar]
- Karplus JP, Kreidl A. Ein Beitrag zur Kenntnis der Schmerzleitung im Rückenmark. Pflügers Arch. Physiol. 1914;158:275–287. [Google Scholar]
- Kawai Y, Takami K, Shiosaka S, Emson PC, Hillyard CJ, Girgis S, Macintyre I, Tohyama M. Topographical localization of calcitonin gene-related peptide in the rat brain: an immunohistochemical analysis. Neuroscience. 1985;15:747–763. doi: 10.1016/0306-4522(85)90076-4. [DOI] [PubMed] [Google Scholar]
- Kenshalo DR, Giesler GJ, Leonard RB, Willis WD. Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J. Neurophysiol. 1980;43:1594–1614. doi: 10.1152/jn.1980.43.6.1594. [DOI] [PubMed] [Google Scholar]
- King RB. Anterior commissurotomy for intractable pain. J. Neurosurg. 1977;47:7–11. doi: 10.3171/jns.1977.47.1.0007. [DOI] [PubMed] [Google Scholar]
- Kroll FW. Schwellenuntersuchungen bei Läsionen der afferenten Leitungsbahnen. Zeitschrift für die gesamte Neurologie und Psychiatrie. 1930;128:751–776. [Google Scholar]
- Kruger L, Mantyh PW, Sternini C, Brecha NC, Mantyh CR. Calcitonin gene-related peptide (CGRP) in the rat central nervous system: patterns of immunoreactivity and receptor binding sites. Brain Res. 1988a;463:223–244. doi: 10.1016/0006-8993(88)90395-2. [DOI] [PubMed] [Google Scholar]
- Kruger L, Sternini C, Brecha NC, Mantyh PW. Distribution of calcitonin gene-related peptide immunoreactivity in relation to the rat central somatosensory projection. J. Comp. Neurol. 1988b;273:149–162. doi: 10.1002/cne.902730203. [DOI] [PubMed] [Google Scholar]
- Langford LA, Coggeshall RE. Unmyelinated axons in the posterior funiculi. Science. 1981;211:176–177. doi: 10.1126/science.7444459. [DOI] [PubMed] [Google Scholar]
- Leriche R. Du traitment de la douleur dans les cancers abdominaux et pelviens inopérables ou récidivés. Gaz. Hop. Paris. 1936;109:917–922. [Google Scholar]
- Lima D. A spinomedullary projection terminating in the dorsal reticular nucleus of the rat. Neuroscience. 1990;34:577–589. doi: 10.1016/0306-4522(90)90166-2. [DOI] [PubMed] [Google Scholar]
- Lu GW, Bennett GJ, Nishikawa N, Hoffert MJ, Dubner R. Extra- and intracellular recordings from dorsal column postsynaptic spinomedullary neurons in the cat. Exp. Neurol. 1983;82:456–477. doi: 10.1016/0014-4886(83)90417-x. [DOI] [PubMed] [Google Scholar]
- Mansuy L, Lecuire J, Acassat L. Technique de la myelotomie commissurale posterieur. J. Chir. 1944;60:206–213. [Google Scholar]
- Mansuy L, Sindou M, Fischer G, Brunon J. La cordotomie spino-thalamique dans les douleurs cancéreuses. Résultats d’une série de 124 malades opérés par abord direct posterieure. Neuro-Chir. 1976;22:437–444. [PubMed] [Google Scholar]
- Menétrey D, Basbaum AI. Spinal and trigeminal projections to the nucleus of the solitary tract: a possible substrate for somatovisceral and viscerovisceral reflex activation. J. Comp. Neurol. 1987;255:439–450. doi: 10.1002/cne.902550310. [DOI] [PubMed] [Google Scholar]
- Milne RJ, Foreman RJ, Giesler GJ, Willis WD. Convergence of cutaneous and pelvic visceral nociceptive inputs onto primate spinothalamic neurons. Pain. 1981;11:163–183. doi: 10.1016/0304-3959(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Madsen AM, Giesler GJ. Anatomical and physiological studies of the gray matter surrounding the spinal cord central canal. J. Comp. Neurol. 1983;220:321–335. doi: 10.1002/cne.902200306. [DOI] [PubMed] [Google Scholar]
- Nahin RL, Madsen AM, Giesler GJ. Funicular location of the ascending axons of neurons adjacent to the spinal cord central canal in the rat. Brain Res. 1986;384:367–372. doi: 10.1016/0006-8993(86)91174-1. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. J. Neurophysiol. 1987;57:1867–1892. doi: 10.1152/jn.1987.57.6.1867. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distention as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudoaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Noble R, Riddell JS. Cutaneous excitatory and inhibitory input to neurones of the postsynaptic dorsal column system in the cat. J. Physiol. (London) 1988;396:497–513. doi: 10.1113/jphysiol.1988.sp016974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordenbos W. Pain: Problems Pertaining to the Transmission of Nerve Impulses which Give Rise to Pain. Elsevier; Amsterdam: 1959. [Google Scholar]
- Owens CM, Zhang D, Willis WD. Changes in the response states of primate spinothalamic tract cells caused by mechanical damage of the skin or activation of descending controls. J. Neurophysiol. 1992;67:1509–1527. doi: 10.1152/jn.1992.67.6.1509. [DOI] [PubMed] [Google Scholar]
- Papo I, Luongo A. High cervical commissural myelotomy in the treatment of pain. J. Neurol. Neurosurg. Psychiatry. 1976;39:705–710. doi: 10.1136/jnnp.39.7.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JT, Head PA, McNeill DL, Chung K, Coggeshall RE. Ascending unmyelinated primary afferent fibers in the dorsal funiculus. J. Comp. Neurol. 1989;290:384–390. doi: 10.1002/cne.902900307. [DOI] [PubMed] [Google Scholar]
- Patterson JT, Coggeshall RE, Lee WT, Chung K. Long ascending unmyelinated primary afferent axons in the rat dorsal column: immunohistochemical localizations. Neurosci. Lett. 1990;108:6–10. doi: 10.1016/0304-3940(90)90697-8. [DOI] [PubMed] [Google Scholar]
- Pierce JP, Weinberg RJ, Rustioni A. Single fiber studies of ascending input to the cuneate nucleus of cats, II: postsynaptic afferents. J. Comp. Neurol. 1990;300:134–152. doi: 10.1002/cne.903000109. [DOI] [PubMed] [Google Scholar]
- Putnam TJ. Myelotomy of the commissure: a new method of treatment for pain in the upper extremities. Arch. Neurol. Psychiatry. 1934;32:1189–1193. [Google Scholar]
- Rigamonti DD, Hancock MB. Analysis of field potentials elicited in the dorsal column nuclei by splanchnic nerve A-beta afferents. Brain Res. 1974;77:326–329. doi: 10.1016/0006-8993(74)90796-3. [DOI] [PubMed] [Google Scholar]
- Rigamonti DD, Hancock MB. Viscerosomatic convergence in the dorsal column nuclei of the cat. Exp. Neurol. 1978;61:337–348. doi: 10.1016/0014-4886(78)90251-0. [DOI] [PubMed] [Google Scholar]
- Rustioni A. Non-primary afferents to the nucleus gracilis from the lumbar cord of the cat. Brain Res. 1973;51:81–95. doi: 10.1016/0006-8993(73)90366-1. [DOI] [PubMed] [Google Scholar]
- Rustioni A. Non-primary afferents to the cuneate nucleus in the brachial dorsal funiculus of the cat. Brain Res. 1974;75:247–259. doi: 10.1016/0006-8993(74)90745-8. [DOI] [PubMed] [Google Scholar]
- Rustioni A, Kaufman AB. Identification of cells of origin of non-primary afferents to the dorsal column nuclei of the cat. Exp. Brain Res. 1977;27:1–14. doi: 10.1007/BF00234821. [DOI] [PubMed] [Google Scholar]
- Rustioni A, Hayes NL, O’Neill S. Dorsal column nuclei and ascending spinal afferents in neurons in the dorsal column of the cat. Brain. 1979;102:95–125. doi: 10.1093/brain/102.1.95. [DOI] [PubMed] [Google Scholar]
- Schvarcz JR. Stereotactic extralemniscal myelotomy. J. Neurol. Neurosurg. Psychiatry. 1976;39:53–57. doi: 10.1136/jnnp.39.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvarcz JR. Spinal cord stereotactic techniques, trigeminal nucleotomy and extralemniscal myelotomy. Appl. Neurophysiol. 1978;41:99–112. doi: 10.1159/000102406. [DOI] [PubMed] [Google Scholar]
- Simone DA, Sorkin LS, Oh U, Chung JM, Owens C, LaMotte RH, Willis WD. Neurogenic hyperalgesia: central neural correlates in responses of spinothalamic tract neurons. J. Neurophysiol. 1991;66:228–246. doi: 10.1152/jn.1991.66.1.228. [DOI] [PubMed] [Google Scholar]
- Sourek K. Commissural myelotomy. J. Neurosurg. 1969;31:524–527. doi: 10.3171/jns.1969.31.5.0524. [DOI] [PubMed] [Google Scholar]
- Spiller WG, Martin E. The treatment of persistent pain of organic origin in the lower part of the body by division of the anterolateral column of the spinal cord. J. Am. Med. Assoc. 1912;58:1489–1490. [Google Scholar]
- Tamatani M, Senba E, Tohyama M. Calcitonin gene-related peptide- and substance P-containing primary afferent fibers in the dorsal column of the rat. Brain Res. 1989;495:122–130. doi: 10.1016/0006-8993(89)91225-0. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp. Brain Res. 1968;4:377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Luck MM. Loss and recovery of reactivity to noxious stimuli in monkeys with primary spinothalamic chordotomies, followed by secondary and tertiary lesions of other cord sectors. Brain. 1979;102:233–248. doi: 10.1093/brain/102.2.233. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Hamilton DM, Thomby JI. Pain reactivity of monkeys after lesions to the dorsal and lateral columns of the spinal cord. Exp. Brain Res. 1971;13:140–158. doi: 10.1007/BF00234083. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Greenspan JD, Ritz LA, Yeomans DC. The spinal pathways contributing to the ascending conduction and the descending modulation of pain sensations and reactions. In: Yaksh TL, editor. Spinal Afferent Processing. Plenum Press; New York: 1986. pp. 275–329. [Google Scholar]
- Wertheimer P, Lecuire J. La myélotomie commissurale postérieure. À propos de 107 observations. Acta Chir. Belg. 1953;52:568–574. [PubMed] [Google Scholar]
- White JC, Sweet WH. Pain and the Neurosurgeon: a Forty-Year Experience. Charles C Thomas; Springfield, IL: 1969. [Google Scholar]
- Willis WD, Coggeshall RE. Sensory Mechanisms of the Spinal Cord. 2nd edn Plenum Press; New York: 1991. [Google Scholar]
- Yamamoto S, Sugihara S, Kuru M. Microelectrode studies on sensory afferents in the posterior funiculus of cat. Jpn. J. Physiol. 1956;6:68–85. doi: 10.2170/jjphysiol.6.68. [DOI] [PubMed] [Google Scholar]