Abstract
Since most breast cancers occur in post-menopausal women and are hormone dependent, we developed a model system that mimics this situation. In this model, tumors of human estrogen receptor ER positive breast cancer cells stably transfected with aromatase (Ac-1) are grown in immune compromised mice. Using this model we have explored a number of therapeutic strategies to maximize the antitumor efficacy of antiestrogens (AEs) and aromatase inhibitors (AIs). This intratumoral aromatase xenograft model has proved accurate in predicting the outcome of several clinical trials. In this current study we compared the effect of an AE toremifene and steroidal AI atamestane, alone or in combination, on growth of hormone dependent human breast cancer. We have also compared toremifene plus atamestane combination with tamoxifen in this study.
The growth of Ac-1 cells was inhibited by tamoxifen, toremifene and atamestane in vitro with IC50 values of 1.8±1.3μM, 1±0.3μM and 60.4±17.2μM, respectively. The combination of toremifene plus atamestane was found to be better than toremifene or atamestane alone in vitro. The effect of this combination was then studied in vivo using Ac-1 xenografts grown in ovariectomized female SCID mice. The mice were injected with toremifene (1000μg/day), atamestane (1000μg/day), tamoxifen (100μg/day), or the combination of toremifene plus atamestane. In this study, our results indicate that the combination of toremifene plus atamestane was as effective as toremifene or tamoxifen alone but may not provide any additional benefit over toremifene alone or tamoxifen alone.
Keywords: Ac-1 cells, aromatase inhibitors, antiestrogens
Introduction
Toremifene is a relatively new selective estrogen receptor modulator (SERM) with properties and side effects similar to those of tamoxifen [1,2]. Unlike tamoxifen, toremifene does not seem to increase the risk of endometrial cancer. Based on research carried out so far, the U.S. Food and Drug Administration has approved toremifene only for post-menopausal women with advanced (metastatic) breast cancer. Atamestane (1-methyl-1,4-androstadiene-3,17-dione) is a competitive, irreversible inhibitor of estrogen biosynthesis. It is an orally-administered, steroidal aromatase inhibitor that reduces the production of estrogen [3,4]. However, to date, clinical studies with atamestane have only been carried out in men with BPH (Benign Prostatic Hyperplasia) [3,5-7]. Both compounds have potential in treating hormone responsive breast cancer either in sequence or in combination. To investigate the activities of these compounds, we have utilized a xenograft model, which we previously reported to be responsive to both antiestrogens (AEs) and aromatase inhibitors (AIs) [8]. Previous clinical trials (BIG, ATAC, MA-17) have confirmed prior results of our xenograft model [9-13]. As found in these studies, AIs were significantly better than AEs in controlling tumor growth. These AIs are now FDA approved for the treatment of postmenopausal breast cancer. Despite the availability of successful agents, the search for improved AIs and SERMs continues.
Studies in model systems have proved valuable in understanding the development and progression of breast cancer and also in our ability to treat the disease. Both cell lines and rodent models have been used to study the treatment of various forms of cancers. The original rat models with mammary tumors induced with carcinogens have been used in the past for studying the effects of estrogens, AEs and AIs on tumor growth. However, the development of immune deficient mice enabled tumors of human cancerous cell lines to be grown in vivo and the effect of treatment on growth of human breast cancer could be examined. Athymic mice with tumors of MCF-7 cells were used to compare AEs. However, for the development of AIs, this model was of limited utility, since tumors of MCF-7 cells need estrogen supplement for growth. A number of studies have shown that in postmenopausal breast cancer patients, estrogens are produced locally by aromatase within the breast and tumors. In order to mimic this situation in a model we have utilized estrogen receptor positive human breast cancer cell line (MCF-7) stably transfected with aromatase (Ac-1). When inoculated into the mice these cells form tumors and provide a local source of estrogens by conversion of androstenedione (Δ4A) by aromatase in the cells to estrogen, which stimulates tumor growth. Thus, both AEs and AIs can be compared in the same model system [14,15]. In the current study, we have investigated effects of a steroidal AI, atamestane and a SERM, toremifene alone and in combination on the growth of Ac-1 xenografts in SCID mice.
Materials and Methods
Cell Culture
Dulbecco's Minimum Essential medium (DMEM), Improved Minimum Essential Medium (IMEM), penicillin/streptomycin solution (10,000IU each), 0.25% trypsin–1 mM EDTA solution, Dulbecco's Phosphate-Buffered Saline (DPBS), and were obtained from Invitrogen Life Technologies (Grand Island, NY). Fetal bovine serum was obtained from Hyclone (Logan, UT), and androstenedione, tamoxifen, geneticin (G418) and Matrigel were obtained from Sigma Chemical Company (St. Louis, MO). Atamestane, toremifene were supplied by Peter Langeker, Biomedicine, CA. MCF-7 human breast cancer cells stably transfected with the human aromatase gene (Ac-1) were developed as described earlier [16]. Ac-1 cells were routinely maintained DMEM with 10% fetal bovine serum, 1% penicillin/streptomycin solution, and 600μg/ml G418. Growth studies were performed by plating 103 cells/well into 96-well plates as described earlier [16]. The results were expressed as a percentage of the cell number in the Δ4A-treated control wells. IC50 values for inhibitors were calculated from the linear regression line of the plot of percentage inhibition versus log inhibitor concentration.
Tumor Growth in Ovariectomized Female SCID Mice
All animal studies were performed according to the guidelines and approval of the Animal Care Committee of the University of Maryland School of Medicine. Female ovariectomized SCID mice 4–6 weeks of age were obtained from the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed in a pathogen-free environment under controlled conditions of light and humidity and received food and water ad libitum.
The tumor xenografts were grown in the mice as previously described [17-19]. Ac-1 cells were routinely maintained DMEM with 10% fetal bovine serum, 1% penicillin/streptomycin solution, and 600μg/ml G418. Sub-confluent cells were scraped into DPBS, collected by centrifugation, and re-suspended in Matrigel (10 mg/mL) at 2.5 X 107 cells/flank. Each mouse received subcutaneous inoculations in one site per flank with 100μL of cell suspension. All mice were then injected daily with Δ4A (100μg/day) in the vehicle (0.3% HydroxyPropylCellulose (HPC) in 0.9% NaCl) for the duration of the experiment. Measurements and treatments began when the tumors reached a measurable size (~300 mm3), approximately 4-6 weeks after cell inoculation. Mice were assigned to groups for treatment so that there was no statistically significant difference in tumor volume among the groups at the beginning of the treatment. The tumors were measured weekly with calipers and the tumor volumes were calculated using the formula (4/3) π r12 r2 (r1 ≤ r2). Mice were then injected subcutaneously daily with the indicated drugs: 100μg/day (5X weekly) of Δ4A plus 1000μg/day (5X weekly) of atamestane; Δ4A plus 100μg/day (5X weekly) of tamoxifen; Δ4A plus 1000μg/day (5X weekly) of toremifene; Δ4A plus 1000μg/day (5X weekly) of toremifene plus 1000μg/day of atamestane. The doses of Δ4A, tamoxifen used are the same as previously reported. For the dose of toremifene and atamestane a dose response study was performed and the dose which caused a marked response in tumor growth was selected for further study. At the end of the study, mice were sacrificed by decapitation and the trunk blood was collected. Tumors and uteri were excised, cleaned, weighed, and stored at −80°C.
Statistics
Mixed-effects models were used, and analyzed with S-PLUS (7.0, Insightful Corp.) to estimate and compare an exponential parameter (βi) controlling the tumor growth rate for each of the five treatment groups. Model of exponential growth was appropriate for the tumor volume data. We used random-effects for both intercept and slope at the animal level, and a single random effect for the intercept at the flank within mouse level. The ‘day zero’ tumor volume was used as a covariate in the regression model. The normality of random effects and errors were assessed. Random effects seem to be also independent. The tumor weights and uterine weights are expressed as mean ± standard error. All statistical tests were two-sided, and performed at 0.05 level of significance. For in vitro studies, all comparisons were tested with One Way ANOVA and all pair wise multiple comparison procedure were performed using Tukey Test.
Results
Effect of atamestane and toremifene on growth of Ac-1 cells in vitro
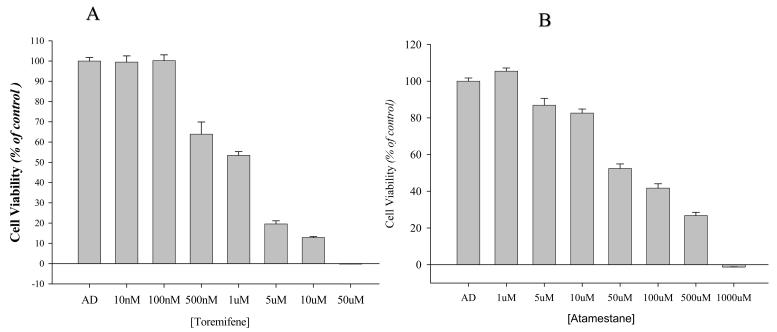
Ac-1 cells were examined for growth inhibitory effects of toremifene (Fareston®), and atamestane alone or in combination. Growth studies were performed as described in “Materials and Methods”. The growth of Ac-1 cells was inhibited by both toremifene and atamestane in a dose dependent manner, with IC50 values of 1±0.3μM and 60.4±17.2μM respectively (Figure. 1 A, B). To examine the effect of combining an AI with AE on breast cancer, we treated Ac-1 cells with Δ4A 1nM plus toremifene 1μM or Δ4A 1nM plus atamestane 1μM or the combination of the two. As shown in Figure. 2, atamestane alone at 1μM resulted in 99.55±2.32% cell viability compared to treated control, whereas toremifene 1μM treatment resulted in 53.43±1.86% cell viability. However, the combination of toremifene and atamestane resulted in 26.67±1.57% cell viability. The combination of atamestane plus toremifene was significantly better than either drug alone or the control. (p<0.001). At the same time toremifene alone was also significantly better than control (p<0.001). This synergistic inhibition of proliferation of Ac-1 cells prompted us to examine the effect of the combination on the growth of Ac-1 xenografts in vivo. Ac-1 cells also showed dose dependent growth inhibition in response to tamoxifen in a dose dependent manner with an IC50 of 1.8±1.3μM (data not shown). We compared the combination of toremifene plus atamestane with tamoxifen in vivo, since tamoxifen is the most widely used antiestrogen in the United States.
Figure 1. Effect of (A) toremifene and (B) atamestane on proliferation of Ac-1 cells in vitro.
Cells were cultured in IMEM steroid-reduced medium without phenol red for 3 days before platting in 96-well plates and, 24 hours later, were exposed to the specified treatments for 6 days. Cell growth was measured using the MTT assay. Cell proliferation is expressed as the percentage of the cells compared with the control wells (1 nM Δ4A-treated cells). Columns, mean of two to six experiments, each with six replicates; bars, SE.
Figure 2. Effect of combination of toremifene and atamestane on proliferation of Ac-1 cells in vitro.
Cells were cultured in IMEM steroid-reduced medium without phenol red for 3 days before platting in 96-well plates and, 24 hours later, were exposed to the specified agents (1μM) for 6 days. Cell growth was measured using the MTT assay. Cell proliferation is expressed as the percentage of the cells compared with the control wells (1 nM Δ4A-treated cells). Columns, mean of two to six experiments, each with six replicates; bars, SE. * p<0.001 One Way ANOVA, all pairwise comparisons using Tukey's test; combination better than either drug alone or control.
Dose response effects of toremifene and atamestane in vivo
Ac-1 xenografts were grown as described in “Materials and Methods”. The dose response study with atamestane was performed with following doses of atamestane; 50μg/day, 100μg/day, 250μg/day, 500μg/day, 1000μg/day and 2500μg/day. The dose response study of toremifene was performed with following doses: 50μg/day, 100μg/day, 250μg/day, 500μg/day and 1000μg/day. The mice in the different groups were treated for eight weeks. After which, they were sacrificed by decapitation and the trunk blood, tumors and uteri were collected. The mean tumor weights and uterine weights are mentioned in Table 1. Both toremifene and atamestane caused dose dependent reductions in mean tumor weights. The doses of toremifene and atamestane which caused maximum reduction in tumor growth were selected for the combination study.
Effect of combination of Toremifene and Atamestane on growth of Ac-1 xenografts
For combination study, 1000μg/day of toremifene and atamestane were used. The mice were treated for 12 weeks (except the control group receiving Δ4A 100μg/day were sacrificed on week 8 due to large tumors). During these 12 weeks, the tumor volumes were measured weekly as explained in “Materials and Methods”. The growth rates of all treated tumors were significantly slower than the control tumors. Atamestane, however, was found to have a higher p value than the other comparisons. We therefore compared group treated with Atamestane to the following treatment groups: Tamoxifen, Toremifene, Toremifene plus Atamestane. Tumor growth in the group treated with Atamestane was higher than in any of these groups, p-values <0.0001. However, no significant difference was found in growth rate across the following three treatment groups: Tamoxifen, Toremifene, Toremifene plus Atamestane (p-value=0.12).
At the end of the 12 weeks, the mice were sacrificed by decapitation and the trunk blood, tumors and uteri were collected. The mean tumor weights and uterine weights were measured. The mean tumor weight was found to be 1178.36±58.92 mg for Δ4A group. This group was sacrificed on week 8 due to large tumor size. The mean tumor weights were 355.14± 17.76 mg for atamestane group, 74.83±3.04 mg toremifene group and 59.5±2.98 mg for toremifene plus atamestane group. All treatments resulted in significantly lower mean tumor weights (Figure 4A) compared to that of the control group (p<0.001). However, toremifene alone was found to cause significantly lower mean tumor weight than atamestane alone and there was no significant difference between mean tumor weights after treatment with toremifene plus atamestane or toremifene alone (p=0.996). This suggests that no increase in efficacy may be achieved by addition of atamestane to the toremifene regimen on the growth of Ac-1 xenografts (Figure. 3,4A).
Figure. 4. Effect of toremifene, atamestane alone or in combination and tamoxifen, on the growth of Ac-1 xenografts.
Each mouse received s.c. injections at one site on each flank with 100μL of suspension of Ac-1 cells (2.5 ×107 cells/mL). Mice were then injected subcutaneously daily with the indicated drugs along with 100μg/mouse/day (5X weekly) of Δ4A. Measurements and treatments began when the tumors reached a measurable size (~ 300 mm3). Tumor volumes were measured weekly and were expressed as the percent change relative to the initial tumor volume. The animals that were injected with vehicle alone were then treated for the indicated times. Mice were assigned to groups for treatment so that there was no statistically significant difference in tumor volume among the groups at the beginning of the treatment. The mice in control group received 100μg/mouse/day (5X weekly) of Δ4A alone.
Figure. 3. The effect of (A) toremifene and (B) atamestane at different doses on growth of Ac-1 xenografts grown in female ovariectomized SCID mice.
Each mouse received s.c. injections at one site on each flank with 100μL of suspension of Ac-1 cells (2.5 ×107 cells/mL). Mice were then injected subcutaneously daily with the indicated drugs along with 100μg/mouse/day (5X weekly) of Δ4A. Measurements and treatments began when the tumors reached a measurable size (approximately 300 mm3). Tumor volumes were measured weekly and were expressed as the percent change relative to the initial tumor volume. The animals that were injected with vehicle alone were then treated for the indicated times. Mice were assigned to groups for treatment so that there was no statistically significant difference in tumor volume among the groups at the beginning of the treatment. The mice in control group received 100μg/mouse/day (5X weekly) of Δ4A alone. The dose of each drug that causes maximum reduction in tumor volume, tumor weight (not shown) without causing any significant increase in the uterine weight (not shown) was selected for the combination study.
Because the uterus is very sensitive to estrogens, change in uterine weight in these mice serves as a bioassay of endogenous estrogen levels. The uterine weights among the groups were found not to be statistically different. The mean uterine weight was 64± 3.2mg for Δ4A group, 31±1.45mg toremifene group, 29±12.55mg for atamestane group and 44.8±2.24mg for toremifene plus atamestane group. The mean uterine weights were all significantly lower (p<0.001) than that of the mice treated with Δ4A, suggesting that estrogen synthesis or its action is inhibited by all treatments. (Figure. 4B) The combination of toremifene plus atamestane, however, resulted in a higher uterine weight compared to toremifene alone (p=0.003) and atamestane (p=0.002). The mean uterine weights observed in toremifene and atamestane groups were not significantly different (p=0.932). These findings also lead to the conclusion that addition of atamestane to the toremifene regimen may not result in any additional suppression of estrogenic effects. The mean uterine weight of the mice receiving tamoxifen was not measured in this study, as those mice were not sacrificed at this time point. The mean uterine weight of tamoxifen has however, been measured and published in several reports and is usually found to be as high as Δ4A supplemented controls, reflecting the agonistic activity of tamoxifen [20].
Discussion
Tamoxifen has proved to be effective in metastatic breast cancer and in preventing breast cancer in high-risk women. However, its duration of efficacy for adjuvant therapy is 5 years. Therefore, there is a need for alternative treatments. Treatment with aromatase inhibitors is now proving to be an effective strategy for sequential therapy after 5 years of tamoxifen [21-24]. More significantly, ATAC and BIG trials have demonstrated superior efficacy of aromatase inhibitors compared to tamoxifen [13,21,25,26]. Several aromatase inhibitors have become available and now FDA approved. Although, tamoxifen is well tolerated by patients, it is associated with weak estrogenic effects. Thus, the increased risk of stroke and endometrial hyperplasia have been reported [27]. The development of antiestrogens without any estrogenic side effects has therefore been of interest. Toremifene although, not significantly more effective than tamoxifen appears to be less estrogenic. Our results indicate that neither atamestane nor toremifene were as effective as tamoxifen in inhibiting the growth of Ac-1 cells in vitro. The IC50 values were about 10 to 500 fold higher than that of that of tamoxifen. Although, the combination of toremifene plus atamestane was found to be better than either drug alone in vitro, this combination did not prove to be any more beneficial in vivo. This finding may be due to drug interactions and/or changes in the clearance rates of the compounds in vivo. Consistent with this hypothesis, the significantly higher uterine weight of the combination than either drug alone suggests that aromatase may be inhibited less effectively by the combination. The tumor growth rates of tamoxifen, toremifene and toremifene plus atamestane were found to be similar. The uterine and tumor weights of mice treated with toremifene, or toremifene plus atamestane were also not significantly different from one another. The only advantage of toremifene over tamoxifen was absence of estrogenic effects on uterus.
In summary, we have used a hormone-responsive xenograft model and shown that the newer SERM toremifene is as effective as tamoxifen on tumor growth but has no estrogenic effects on the uterus. In addition, our results indicate that although toremifene and atamestane were effective in inhibiting tumor growth, the combination of AI atamestane with AE toremifene may not provide any additional advantage over single agent toremifene or tamoxifen.
Figure 5. The effect of tamoxifen and toremifene-atamestane alone or in combination on (A) tumor weight and (B) uterine weight in female ovariectomized SCID mice bearing Ac-1 tumors.
After the tumors reached a measurable size, animals were assigned to four groups with similar tumor volumes and injected s.c. daily with Δ4A (control; n = 5), or indicated treatments along with Δ4A (n = 5 in each group). The plot shows (A) tumor weights and (B) uterine weights of mice treated with indicated treatment group. The mice were sacrificed at different time-points and the total days they were treated are indicated with the following symbols.
Acknowledgments
Supported by grant CA-62483 to Dr. Brodie from the National Cancer Institute, National Institute of Health.
Abbreviations used
- ER
Estrogen Receptor
- Δ4A
Androstenedione
- E2
Estradiol
- AIs
aromatase inhibitors
- AEs
antiestrogens
- Tor
Toremifene
- Ata
Atamestane
- Tam
Tamoxifen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerken P. Toremifene citrate (Fareston) Clin J Oncol Nurs. 2004;8:529–530. doi: 10.1188/04.CJON.529-530. [DOI] [PubMed] [Google Scholar]
- 2.Kusama M, Miyauchi K, Aoyama H, Sano M, Kimura M, Mitsuyama S, Komaki K, Doihara H. Effects of toremifene (TOR) and tamoxifen (TAM) on serum lipids in postmenopausal patients with breast cancer. Breast Cancer Res Treat. 2004;88:1–8. doi: 10.1007/s10549-004-4384-z. [DOI] [PubMed] [Google Scholar]
- 3.el Etreby MF. Atamestane: an aromatase inhibitor for the treatment of benign prostatic hyperplasia. A short review. J Steroid Biochem Mol Biol. 1993;44:565–572. doi: 10.1016/0960-0760(93)90260-4. [DOI] [PubMed] [Google Scholar]
- 4.Lombardo ME, Hakky SI, Hudson PB. In vitro studies on the inhibition of testosterone synthesis in the human testis by atamestane. J Steroid Biochem Mol Biol. 1993;44:287–290. doi: 10.1016/0960-0760(93)90089-f. [DOI] [PubMed] [Google Scholar]
- 5.Muller M, van den Beld AW, van der Schouw YT, Grobbee DE, Lamberts SW. Effects of Dehydroepiandrosterone and Atamestane Supplementation on Frailty in Elderly Men. J Clin Endocrinol Metab. 2006 doi: 10.1210/jc.2005-2433. [DOI] [PubMed] [Google Scholar]
- 6.Gingell JC, Knonagel H, Kurth KH, Tunn UW. Placebo controlled double-blind study to test the efficacy of the aromatase inhibitor atamestane in patients with benign prostatic hyperplasia not requiring operation. The Schering 90.062 Study Group. J Urol. 1995;154:399–401. doi: 10.1097/00005392-199508000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Radlmaier A, Eickenberg HU, Fletcher MS, Fourcade RO, Reis Santos JM, van Aubel OG, Bono AV. Estrogen reduction by aromatase inhibition for benign prostatic hyperplasia: results of a double-blind, placebo-controlled, randomized clinical trial using two doses of the aromatase-inhibitor atamestane. Atamestane Study Group. Prostate. 1996;29:199–208. doi: 10.1002/(SICI)1097-0045(199610)29:4<199::AID-PROS1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Yue W, Brodie A. MCF-7 human breast carcinomas in nude mice as a model for evaluating aromatase inhibitors. J Steroid Biochem Mol Biol. 1993;44:671–673. doi: 10.1016/0960-0760(93)90278-5. [DOI] [PubMed] [Google Scholar]
- 9.Buzdar A, Douma J, Davidson N, Elledge R, Morgan M, Smith R, Porter L, Nabholtz J, Xiang X, Brady C. Phase III, multicenter, double-blind, randomized study of letrozole, an aromatase inhibitor, for advanced breast cancer versus megestrol acetate. J Clin Oncol. 2001;19:3357–3366. doi: 10.1200/JCO.2001.19.14.3357. [DOI] [PubMed] [Google Scholar]
- 10.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Jaenicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Chaudri-Ross H, Lang R, Wyld P, Bhatnagar A. Phase III study of letrozole versus tamoxifen as first-line therapy of advanced breast cancer in postmenopausal women: analysis of survival and update of efficacy from the International Letrozole Breast Cancer Group. J Clin Oncol. 2003;21:2101–2109. doi: 10.1200/JCO.2003.04.194. [DOI] [PubMed] [Google Scholar]
- 11.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Janicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Lassus M, Verbeek JA, Staffler B, Chaudri-Ross HA, Dugan M. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 12.Thurlimann B, Hess D, Koberle D, Senn I, Ballabeni P, Pagani O, Perey L, Aebi S, Rochlitz C, Goldhirsch A. Anastrozole (‘Arimidex’) versus tamoxifen as first-line therapy in postmenopausal women with advanced breast cancer: results of the double-blind cross-over SAKK trial 21/95--a sub-study of the TARGET (Tamoxifen or ‘Arimidex’ Randomized Group Efficacy and Tolerability) trial. Breast Cancer Res Treat. 2004;85:247–254. doi: 10.1023/B:BREA.0000025420.78346.f9. [DOI] [PubMed] [Google Scholar]
- 13.Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG, Sahmoud T. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 14.Brodie A, Jelovac D, Long BJ. Predictions from a preclinical model: studies of aromatase inhibitors and antiestrogens. Clin Cancer Res. 2003;9:455S–459S. [PubMed] [Google Scholar]
- 15.Brodie A, Lu Q, Liu Y, Long B, Wang JP, Yue W. Preclinical studies using the intratumoral aromatase model for postmenopausal breast cancer. Oncology (Huntingt) 1998;12:36–40. [PubMed] [Google Scholar]
- 16.Macedo LF, Guo Z, Tilghman SL, Sabnis GJ, Qiu Y, Brodie A. Role of androgens on MCF-7 breast cancer cell growth and on the inhibitory effect of letrozole. Cancer Res. 2006;66:7775–7782. doi: 10.1158/0008-5472.CAN-05-3984. [DOI] [PubMed] [Google Scholar]
- 17.Yue W, Zhou D, Chen S, Brodie A. A new nude mouse model for postmenopausal breast cancer using MCF-7 cells transfected with the human aromatase gene. Cancer Res. 1994;54:5092–5095. [PubMed] [Google Scholar]
- 18.Long BJ, Jelovac D, Thiantanawat A, Brodie AM. The effect of second-line antiestrogen therapy on breast tumor growth after first-line treatment with the aromatase inhibitor letrozole: long-term studies using the intratumoral aromatase postmenopausal breast cancer model. Clin Cancer Res. 2002;8:2378–2388. [PubMed] [Google Scholar]
- 19.Long BJ, Jelovac D, Handratta V, Thiantanawat A, MacPherson N, Ragaz J, Goloubeva OG, Brodie AM. Therapeutic strategies using the aromatase inhibitor letrozole and tamoxifen in a breast cancer model. J Natl Cancer Inst. 2004;96:456–465. doi: 10.1093/jnci/djh076. [DOI] [PubMed] [Google Scholar]
- 20.Jelovac D, Macedo L, Handratta V, Long BJ, Goloubeva OG, Ingle JN, Brodie AM. Effects of exemestane and tamoxifen in a postmenopausal breast cancer model. Clin Cancer Res. 2004;10:7375–7381. doi: 10.1158/1078-0432.CCR-04-0565. [DOI] [PubMed] [Google Scholar]
- 21.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Cameron DA, Palmer MJ, Pater JL. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 22.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, Castiglione M, Tu D, Shepherd LE, Pritchard KI, Livingston RB, Davidson NE, Norton L, Perez EA, Abrams JS, Therasse P, Palmer MJ, Pater JL. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N Engl J Med. 2003;349:1793–1802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 23.Goss PE, Strasser K. Tamoxifen resistant and refractory breast cancer: the value of aromatase inhibitors. Drugs. 2002;62:957–966. doi: 10.2165/00003495-200262060-00007. [DOI] [PubMed] [Google Scholar]
- 24.Goss PE, Strasser-Weippl K. Prevention strategies with aromatase inhibitors. Clin Cancer Res. 2004;10:372S–379S. doi: 10.1158/1078-0432.ccr-031210. [DOI] [PubMed] [Google Scholar]
- 25.Baum M. The ATAC (Arimidex, Tamoxifen, Alone or in Combination) adjuvant breast cancer trial in postmenopausal patients: factors influencing the success of patient recruitment. Eur J Cancer. 2002;38:1984–1986. doi: 10.1016/s0959-8049(02)00154-5. [DOI] [PubMed] [Google Scholar]
- 26.Goss PE. Preventing relapse beyond 5 years: the MA.17 extended adjuvant trial. Semin Oncol. 2006;33:S8–12. doi: 10.1053/j.seminoncol.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Dignam JJ, Fisher B. Occurrence of stroke with tamoxifen in NSABP B-24. Lancet. 2000;355:848–849. doi: 10.1016/S0140-6736(05)72466-1. [DOI] [PubMed] [Google Scholar]