Abstract
Background
Lymph node (LN) metastases are a major negative prognostic factor for peri-hilar cholangiocarcinoma (PCC). Prognostic significance of the extent of LN dissection, number of metastatic LN and the lymph node ratio (LNR) are still under debate.
Aims
The aims of the present study were to evaluate the prognostic value of the LN status, the total number of LNs evaluated and LNR in PCC.
Methods
Between 1990 and 2008, 62 patients with PCC submitted to surgical resection with curative intent were retrospectively evaluated. Number and status of harvested LN were recorded.
Results
In 53 patients (85.4%) regional lymphadenectomy was performed. Median number of LNs examined was 7 (range 1–25). Median survival was 41.9 months in patients with N0 compared with 22.7 months in 21 patients (39.6%) with N+ (P = 0.03). Median survival was 3, 18.5 and 29 months for patients with 0, 1–3 and >3 LN retrieved, respectively (P < 0.01). Five-year survival for patients above and below the LNR cut-off value of 0.25 was 0% and 22.5%, respectively (P = 0.03).
Conclusions
LN metastases are a major prognostic factor for survival after surgical resection of PCC. The number of LN harvested and LNR showed high prognostic value.
Keywords: cholangiocarcinoma, surgery, prognostic factors
Introduction
Cholangiocarcinoma is a rare but increasing worldwide tumour, originating from either intra- or extrahepatic bile.1 Accordingly it is classified by its origin into intrahepatic (ICC), peri-hilar (PCC) or extrahepatic (EHC) cholangiocarcinoma.2 Surgical treatment with radical intent is the only therapeutic option which has been shown to prolong survival.3 Several prognostic factors have been evaluated for cholangiocarcinoma. Among these, lymph node metastases is one of the most important prognostic factors. Several clinical studies described shorter survival in patients with positive lymph nodes, with a 5-year survival of 0–20%.4–8
The extent of lymph node dissection and its prognostic value is still a matter of debate in the literature. Moreover the lymph node ratio (LNR) has been evaluated for different gastrointestinal tumours and has been shown to give greater prognostic stratification for patients with positive lymph nodes.9–11 Currently, within the literature there is only one study that evaluated LNR in cholangiocarcinoma.12 This was limited to a cohort of patients with intrahepatic cholangiocarcinoma and hence no studies exist for peri-hilar cholangiocarcinoma.
The aims of the present study were to evaluate, in a cohort of patients submitted to surgical resection with curative intent for perihilar cholangiocarcinoma, the relationship between lymph nodes status and survival, the prognostic significance of the total number of resected lymph nodes and to evaluate the prognostic significance of the LNR.
Methods
Retrospective analysis of sixty-two patients with PCC who underwent surgery with curative intent between 1990 and 2008 in a single Divisions of Surgery at University of Verona were included in the study. Written informed consent was obtained from each patient.
Pre-operative evaluation included blood chemistry tests with AST, ALT, ALP, GGT, direct and total bilirubin, blood count, PT, aPTT, albumin, CEA, CA19.9, alpha-fetoprotein and serology for hepatitis viruses (HBV and HCV). The tumour extension was evaluated with ultrasonography (US), colour Doppler ultrasonography, computed tomography (CT) or magnetic resonance imaging (MRI). The differential diagnosis between cholangiocarcinoma and gastro-intestinal tumours liver metastases was made with tumour histology and by exclusion of other primary tumours using esophagogastroduodenoscopy and colonoscopy.
In patients with obstructive jaundice, the extension of the tumour was assessed using different diagnostic methods during the study period. Between 1990 and 1997, all of the patients underwent percutaneous trans-hepatic cholangiography (PTC) with the placement of single or multiple biliary drains; subsequently, mainly non-invasive diagnostic methods such as colangio-pancreatography MRI were used. All of the patients with obstructive jaundice (serum bilirubin level higher than 3 mg/dl) underwent percutaneous biliary drainage in order to define more precisely the longitudinal extension of the tumour and to resolve obstructive jaundice. In patients with segmental cholangitis multiple hepatic drainages of the excluded biliary segments were performed. More recently the use of CT-PET and diagnostic laparoscopy was introduced in selected patients.
During surgery, intra-operative US was routinely used in order to confirm pre-operative diagnosis, to evaluate the relationship between tumour and blood vessel and to evaluate the presence of intrahepatic metastases. The extent of liver resection was defined according to the Brisbane classification.13 Peri-operative death was defined as death within 30 days of the operation or at any point during the index hospitalization.
Lymphadenectomy of the regional lymph nodes was classified according to the classification of the Japanese Society of Biliary Surgery.14 Lymph nodes of the hepatoduodenal ligament (12h, 12a, 12p, 12b), the proper hepatic artery (8) and of the posterior surface of the head of the pancreas (13) were routinely dissected and retrieved; interaortocaval lymph nodes were retrived when macroscopically abnormal. The surgical technique included complete dissection of the hilar structures, all the fatty and lymph node tissue sorrounding the common hepatic artery, the main portal vein and the bile buct.
For the purposes of the present study, PCC was defined as tumours with involvement of structures of the hepatic hilus that often had required resection of the biliary confluence associated with liver and caudate lobe resection.7,15
Microscopical invasion of the bile duct wall, the portal pedicle and the neural tissue was evaluated in each surgical specimen.
Vascular invasion was defined as neoplastic invasion of the arterial, portal or hepatic vessels confirmed by pathological examination.
After surgery patients were regularly followed up with blood tests, tumour markers (CEA and CA19.9) and an abdominal CT scan every 6 months.
From 2000 after surgery patients with lymph node metastases (N+) received adjuvant chemotherapy; adjuvant radiotherapy was reserved for patients with positive resection margins (R+).
Statistical analysis
The study data were prospectively collected and retrospectively analysed using SPSS statistical software (version 16.0; SPSS, Inc., Chicago, IL, USA). The hypothesis about the lack of an association between categorical variables was tested using Pearson's chi-squared test. Survival was modelled by means of a multivariate Cox's regression model. Factors that enter the model were selected using the univariate log-rank test. An association between selected factors and survival was graphically analysed using Kaplan–Meier curves. P-values < 0.05 were considered statistically significant.
Results
Sixty-two patients submitted to surgical resection for PCC with curative intent, from 1990 to 2008, were included in the present study. Clinical characteristics are summarized in Tables 1 and 2.
Table 1.
Patients characteristics
| Variable (n = 62) | n (%) |
|---|---|
| Median age (range) | 66 (30–84) |
| Median CA19.9 U/ml (range) | 132 (9–6835) |
| Bile duct resection | 62 (100) |
| Associated liver resection | 54 (87) |
| Major hepatectomy | 51 (82) |
| Caudate lobe resection | 47 (76) |
| Portal vein resection/reconstruction | 10 (16) |
| Hepatic artery resection/recontruction | 2 (3) |
| Regional lymph node dissection | 53 (85) |
| Perineural invasion | 50 (81) |
| Vascular invasion | 35 (56) |
Table 2.
Type of surgical intervention in patients included in the study
| Surgical procedure | N | % |
|---|---|---|
| Common bile duct resection | 7 | 11.3 |
| Common bile duct with caudate lobe resection | 1 | 1.6 |
| Bisegmentectomy | 2 | 3.2 |
| Bisegmentectomy with caudate lobe resection | 1 | 1.6 |
| Mesohepatectomy and caudate lobe resection (S1 + S4 + S5 + S8) | 1 | 1.6 |
| Left hepatectomy (S2 + S3 + S4) | 4 | 6.5 |
| Left hepatectomy and caudate lobe resection (S1 + S2 + S3 + S4) | 23 | 37.1 |
| Left trisectionectomy (S1 + S2 + S3 + S4 + S5 + S8) | 2 | 3.2 |
| Right hepatectomy (S5 + S6 + S7 + S8) | 2 | 3.2 |
| Right hepatectomy and caudate lobe resection (S1 + S5 + S6 + S7 + S8) | 10 | 16.1 |
| Right trisectionectomy and caudate lobe resection (S1 + S4 + S5 + S6 + S7 + S8) | 9 | 14.6 |
| Total | 62 | 100.0 |
Radical resection (R0 resection) was obtained in 46 patients (76.7%). In 16 patients with positive margin resection (R1) tumor involved the proximal bile duct in 12, the distal bile duct in 2 and the liver parenchyma in 2.
Hospital mortality and morbidity were observed in 9.6% (6/62 patients) and 55% (34/62 patients), respectively; mortality was to 2/41 patients for time period 2000–08.
Regional lymphadenectomy was performed in 53 patients (85.4%), with a median number of harvested LN in this group of 7 (range 1–25).
LN metastases were present in 21 patients (39.6%). According to Japanese Society of Biliary Surgery14 classification LN metastases involved the N1 station in 21/53 patients (39.6%), the N2 station in 6/16 patients and the N3 station in 3/14.
Median follow-up for 56 (90.4%) patients who survived surgery was of 18.5 months (range 6–67 months).
Median survival was 21.9 months [95% confidence interval (CI) 18–26], with actuarial 3- and 5-year survival of 30% and 15%, respectively.
Survival analysis by LN status, number of LNs retrieved, number of positive LNs and LNR are shown in Table 3 and Figures 1–3.
Table 3.
Survival analysis for different subgroups of patients included into the study: number of patients, median survival with 95% confidence interval (CI), actuarial 3- and 5-year survival and P-value of the log rank test
| Variable | n | Median survival (Months) | 95% CI | 3-y surv (%) | 5-y surv (%) | P |
|---|---|---|---|---|---|---|
| Overall | 62 | 21.9 | 18.2–25.5 | 30 | 15 | – |
| LN | 0.032 | |||||
| N0 | 32 | 41.9 | 13.3–70.4 | 50 | 25 | |
| N+ | 21 | 22.7 | 18.2–27.2 | 0 | 0 | |
| LN harvested | 0.001 | |||||
| 0 LN | 9 | 3 | 1.0–5.9 | 0 | 0 | |
| 1–3 LN | 18 | 18.5 | 12.7–24.2 | 25 | 16 | |
| >3 LN | 35 | 28.8 | 17.0–46.6 | 41 | 16 | |
| Positive LN | 0.721 | |||||
| 1–3 N+ | 14 | 22.7 | 10.1–35.3 | 0 | 0 | |
| >3 N+ | 7 | 25 | – | 0 | 0 | |
| Negative LN | 0.152 | |||||
| 1–3 N0 examined | 14 | 18.5 | 13.1–23.8 | 31 | 20 | |
| >3 N0 examined | 18 | 43.1 | 25.6–60.5 | 68 | 27 | |
| LNR | 0.031 | |||||
| ≤0.25 | 42 | 26 | 16.2–35.8 | 44 | 22.5 | |
| >0.25 | 9 | 22.7 | 1.0–45.0 | 0 | 0 | |
LN, lymph node; LNR, lymph node ratio.
Figure 1.
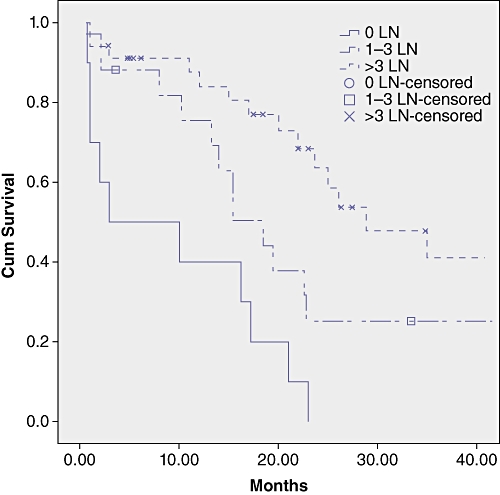
Survival curves in the subgroups by total number of harvested lymph nodes (LN). Survival was significantly related to the number of harvested LN (P-value of the log-rank test = 0.01)
Figure 3.
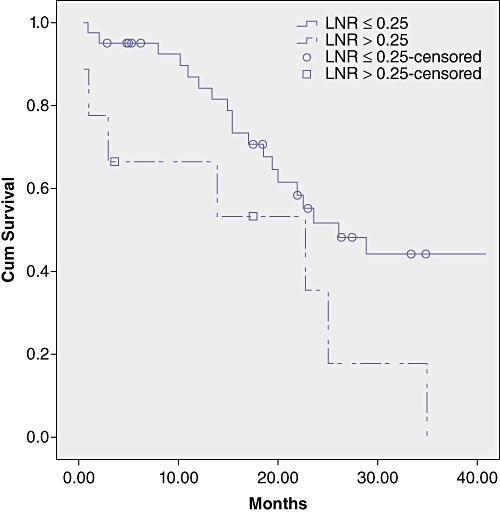
Survival curves in the subgroups by lymph node ratio (LNR). Survival was significantly longer in patients with LNR lower or equal to 0.25 (P = 0.03)
Figure 2.
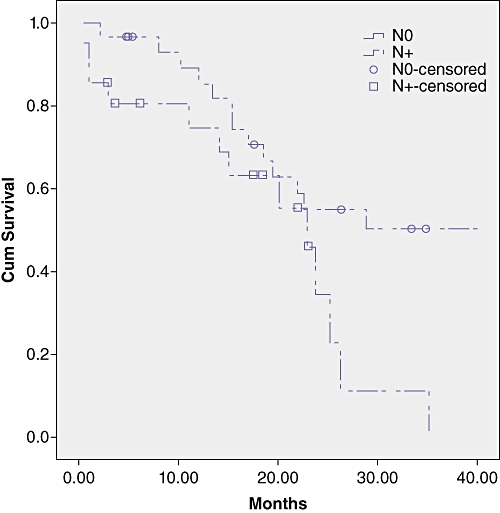
Survival curves in the subgroups by lymph node (LN) status. Survival was significantly shorter in N+ patients (P = 0.03)
Survival was not significantly different in patients with LN metastases in the N1, N2 and N3 stations with a median survival of 20, 22 and 25 months, respectively (P = 0.66).
In the group with no LN metastases patients with 1–3 LN harvested showed a median survival of 18.5 compared with 43.1 months for patients with more than 3 LN examinated, the difference did not reach statistical significance (P = 0.152) (Table 3).
In order to evaluate the prognostic significance of LN metastases and its relationship with the total number of LN harvested, we evaluated the LNR with a cut-off value of 0.25.
Multivariate survival analysis using Cox's proportional hazards model (Table 4) showed that factors significantly realted with survival were surgical radicality and LNR with hazard ratios of 4.40 (95% CI 1.19–16.24) and 3.16 (1.16–8.62), respectively.
Table 4.
Multivariate survival analysis using Cox's proportional hazards model: estimated hazard ratios, 95% confidence interval (CI) and P-values of the hypothesis test about independence between factors and survival
| Variable | HR | 95% CI | P |
|---|---|---|---|
| LNR >0.25 vs. ≤0.25 | 3.16 | 1.16–8.62 | 0.024 |
| R1 vs. R0 | 4.40 | 1.19–16.24 | 0.026 |
| TNM stage | 1.52 | 0.78–3.01 | 0.229 |
| Macroscopic vascular invasion | 0.83 | 0.27–2.47 | 0.741 |
| Perineural invasion | 0.31 | 0.79–1.27 | 0.105 |
LNR, lymph node ratio.
Discussion
LN metastases are a major prognostic factor in the treatment of perhilar cholangiocarcinoma; 30–50% of patients who undergo surgical resection will have nodal involvement.2,16–19
Within the literature 5-year survival for positive node patients does not reach 20%,4–8 and similar findings have been shown in the present study. The prevalence of non-regional lymphnode metastases, defined as M1 according to the UICC TNM, is not well known in hilar cholangiocarcinoma as systematic dissection of non-regional lymphnodes is not routinely performed in the majority of centres. Kitagawa et al. identified a prevalence of positive para-aortic lymph nodes of 17% and reported a 5-year survival of 14% in this group of patients.17 Moreover, 5-year survival decreased to 0% in patients with macroscopically and microscopically positive para-aortic lymph nodes.17
The extent of LN dissection is still a matter of debate in literature. According to 7th edn the AJCC/UICC Guidelines at least three LN should be harvested for adequate staging of perhilar cholangiocarcinoma.20 In a recent study, Ito et al. demonstrated the relationship between the total number of LN examined and survival in patients without LN metastases.21 Ito et al. identified an optimum cut-off value for hilar cholangiocarcinoma of seven LN for a higher disease-specific survival for N0 patients.21 In the current study the median number of examined lymphnodes was grater than 7, a result that is similar to other data in literature.12
The multi-istitutional US study based upon the Surveillance, Epidemiology and End Results (SEER) database identified a significant relationship between the number of examined lymphnodes in N0 patients submitted for surgical resection for extrahepatic bile duct cancer, gallbladder cancer or ampullary carcinoma.22 The authors reported a median survival of 21 and 34 months for patients with 1 to 2 and more than 10 lymph nodes examined, respectively.22 Moreover, the authors identified a relationship between the number of positive LN and survival.22 However, the major limitation of this study was that the authors included different types of tumours with known different biological behaviour.22
This issue had not been clearly demonstrated in patient with peri-hilar cholangiocarcinoma.
In the present study the value of systematic regional lymphadenectomy has been confirmed. Patients with more than three LN harvested showed longer survival. Within the literature no data are avaible regarding the therapeutic value of lymphadenectomy in the bile duct and liver cancers but a systematic dissection of regional LN improves staging of the disease, statification of patients and could increase survival in selected groups of patients.
In order to investigate the prognostic value of the number of positive LN and its relationship with the total number of LN harvested the present study also evaluated the LNR.
LNR has demonstrated its prognostic value in oesophageal, gastric, colorectal and pancreatic cancer.9–11 Recently the prognostic value of LNR was also demonstred in intrahepatic cholangiocarcinoma.12 To the authors knowledge no studies have analysed this issue in perihilar cholangiocarcinoma. The present study has identified a relationship between survival and LNR demonstrating the prognostic value of this factor. Moreover in our sample a cut-off value of 0.25 demonstrated its prognostic value in univariate and multivariate analysis. The LNR can be applied in clinical practice to stratify more accurately patients after surgical resection. The current analysis also demonstrated that patients with LN metastases can have long-term survival if LNR is below 0.25.
Conclusions
LN metastases is a major prognostic factors for survival after surgical resection of PCC. The number of LN harvested and LNR showed a prognostic value. Likewise of other gastrointestinal tumours, the present study identifies the prognostic role of LNR also in PCC.
References
- 1.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakeeb A, Pitt HA, Sohn TA, Coleman J, Abrams RA, Piantadosi S, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. discussion 473–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Capelli P, et al. Does intrahepatic cholangiocarcinoma have better prognosis compared to perihilar cholangiocarcinoma? J Surg Oncol. 2010;101:111–115. doi: 10.1002/jso.21452. [DOI] [PubMed] [Google Scholar]
- 4.Launois B, Terblanche J, Lakehal M, Catheline JM, Bardaxoglou E, Landen S, et al. Proximal bile duct cancer: high resectability rate and 5-year survival. Ann Surg. 1999;230:266–275. doi: 10.1097/00000658-199908000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagakawa T, Kayahara M, Ikeda S, Futakawa S, Kakita A, Kawarada H, et al. Biliary tract cancer treatment: results from the Biliary Tract Cancer Statistics Registry in Japan. J Hepatobiliary Pancreat Surg. 2002;9:569–575. doi: 10.1007/s005340200076. [DOI] [PubMed] [Google Scholar]
- 6.Lai EC, Lau WY. Aggressive surgical resection for hilar cholangiocarcinoma. ANZ J Surg. 2005;75:981–985. doi: 10.1111/j.1445-2197.2005.03595.x. [DOI] [PubMed] [Google Scholar]
- 7.Nishio H, Nagino M, Oda K, Ebata T, Arai T, Nimura Y. TNM classification for perihilar cholangiocarcinoma: comparison between 5th and 6th editions of the AJCC/UICC staging system. Langenbecks Arch Surg. 2005;390:319–327. doi: 10.1007/s00423-005-0561-8. [DOI] [PubMed] [Google Scholar]
- 8.Young AL, Prasad KR, Toogood GJ, Lodge JP. Surgical treatment of hilar cholangiocarcinoma in a new era: comparison among leading Eastern and Western centers, Leeds. J Hepatobiliary Pancreat Surg. 2009 doi: 10.1007/s00534-009-0203-6. [DOI] [PubMed] [Google Scholar]
- 9.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–784. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 10.Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Lee HY, Choi HJ, Park KJ, Shin JS, Kwon HC, Roh MS, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol. 2007;14:1712–1717. doi: 10.1245/s10434-006-9322-3. [DOI] [PubMed] [Google Scholar]
- 12.Tamandl D, Kaczirek K, Gruenberger B, Koelblinger C, Maresch J, Jakesz R, et al. Lymph node ratio after curative surgery for intrahepatic cholangiocarcinoma. Br J Surg. 2009;96:919–925. doi: 10.1002/bjs.6654. [DOI] [PubMed] [Google Scholar]
- 13.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 14.Liver Cancer Study Group of Japan. General Rules for Clinical and Pathological Study of Primary Liver Cancer. 2nd English edn. Tokyo: Kanehara; 2003. [Google Scholar]
- 15.Igami T, Nishio H, Ebata T, et al. Surgical treatment of hilar cholangiocarcinoma in the ‘new era’: the Nagoya University experience. J Hepatobiliary Pancreat Surg. 2009;17:449–454. doi: 10.1007/s00534-009-0209-0. [DOI] [PubMed] [Google Scholar]
- 16.Neuhaus P, Jonas S, Bechstein WO, Lohmann R, Radke C, Kling N, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. doi: 10.1097/00000658-199912000-00010. discussion 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitagawa Y, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–392. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwatsuki S, Todo S, Marsh JW, Madariaga JR, Lee RG, Dvorchik I, et al. Treatment of hilar cholangiocarcinoma (Klatskin tumors) with hepatic resection or transplantation. J Am Coll Surg. 1998;187:358–364. doi: 10.1016/s1072-7515(98)00207-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogura Y, Kawarada Y. Surgical strategies for carcinoma of the hepatic duct confluence. Br J Surg. 1998;85:20–24. doi: 10.1046/j.1365-2168.1998.00532.x. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Handbook. New York, NY: Springer; 2010. [Google Scholar]
- 21.Ito K, Ito H, Allen PJ, Gonen M, Klimstra D, D'Angelica MI, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251:675–681. doi: 10.1097/SLA.0b013e3181d3d2b2. [DOI] [PubMed] [Google Scholar]
- 22.Schwarz RE, Smith DD. Lymph node dissection impact on staging and survival of extrahepatic cholangiocarcinomas, based on U.S. population data. J Gastrointest Surg. 2007;11:158–165. doi: 10.1007/s11605-006-0018-6. [DOI] [PubMed] [Google Scholar]


