Abstract
Objectives
This study examines changes in the expression of growth factors following thermal ablation (TA) of selected colorectal cancer (CRC) liver metastases.
Methods
Using mice with established CRC liver metastases, two tumours in each animal were thermally ablated. Liver and tumour tissues were collected at various time-points (days 0, 1, 2, 3, 5 and 7) following TA treatment from the ablation site and from sites distant from ablated tumour. Changes in growth factor expression (epidermal growth factor [EGF], vascular endothelial growth factor [VEGF], hepatocyte growth factor [HGF] and transforming growth factor-β[TGF-β]) in comparison with baseline levels (non-ablated) were assessed by enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry.
Results
Baseline TGF-β and VEGF levels in the liver parenchyma of tumour-bearing mice were significantly higher than levels in naive liver parenchyma. Levels of VEGF and HGF decreased after TA treatment in all tissues. Levels of EGF decreased in ablated and distant tumour tissues, but displayed a tendency to increase in liver tissue. Levels of TGF-β also decreased during the first 2 days following TA, but later increased in liver and tumour tissues distant from the ablation site to a level that reached significance in tumour tissue at day 7 (P < 0.001). Decreases in growth factor levels were also observed in animals that underwent laparotomy without TA treatment, which indicates that these decreases were caused by the experimental procedure.
Conclusions
Tumour induces upregulation of TGF-β and VEGF in liver parenchyma. Growth factors decreased after TA, but this appears to be the result of the experimental procedure rather than the TA itself. However, TA resulted in increased levels of TGF-β, which may contribute to tumour recurrence.
Keywords: colorectal tumour, metastasis, thermal ablation, growth factors, hepatectomy
Introduction
Colorectal cancer (CRC) is the most common solid organ cancer across both genders and the third most common cause of cancer-related deaths.1 More than 50% of patients with CRC will subsequently develop liver metastases (CRCLM), which represent the leading cause of death in this population. Surgical resection is the only potential curative option. The spatial distribution of metastases, presence of extrahepatic disease, potential residual liver volume and function, as well as the general health of the patient, are the main factors that limit the surgical option to approximately 10–25% of patients.2,3 Advances in systemic therapies have progressively increased the potential for surgical intervention by downstaging hepatic metastases in a small subset of patients.4 Despite successful surgery, the majority of patients experience disease recurrence, most frequently in the liver. Residual micrometastases are thought to be the main aetiology for such recurrences. Several studies indicate that recurrence may be significantly stimulated by the process of liver regeneration.5 The mechanism of this stimulation remains unclear. However, a number of growth factors (GFs) associated with liver regeneration have been implicated as they have also been demonstrated to be upregulated in aggressive tumours and to be able to promote tumour growth in experimental in vitro and in vivo studies. These GFs include transforming growth factor-β (TGF-β), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF).5
Local thermal ablation (TA) was developed to increase the therapeutic options for patients with liver metastases.6,7 This involves the application of laser, radiofrequency or microwave energy inside the tumour. The conversion of such energy to heat leads to the destruction of the tumour by coagulative necrosis, which extends to a rim of normal liver surrounding the tumour. When applied as a minimally invasive technique, TA has a number of potential advantages, including significantly lower morbidity and minimal destruction of normal liver tissue, leading to lesser regenerative response and the facility of repeated application.8,9 Experimental studies have also strongly suggested a positive effect on host immune response following TA of tumours in which the ablated tumour acts as a tumour vaccine.10,11 These studies have also demonstrated the suppression of subsequent tumour challenge, as well as reduced systemic and intraperitoneal metastases. Apart from the potential immunological responses, the smaller volume of normal liver destroyed and the lower regenerative effort may play a part in these outcomes. By contrast with experimental studies, TA in clinical practice is associated with significant levels of locally recurrent disease.6,12 The limitation of real-time imaging of tumour destruction during TA may be partly responsible for incomplete tumour destruction and local recurrence.13 However, the effect of TA on the surrounding normal liver, its impact on proinflammatory and proangiogenic cytokine release and their effects on liver parenchyma and on any residual micrometastases remain poorly defined. This study investigates changes in the local expression (liver parenchyma and residual tumour) of the angiogenic growth factors TGF-β, VEGF, HGF and EGF following TA of selected tumours. We hypothesized that this scenario would reflect changes occurring in the clinic after TA when residual micrometastases or tumour at the margins of an ablation site remain.
Materials and methods
Animals
Male CBA mice aged 6–8 weeks (Laboratory Animal Services, University of Adelaide, Adelaide, SA, Australia) were maintained in standard cages with access to irradiated food and water ad libitum, and exposed to a 12:12-h light : dark cycle. All procedures were implemented in accordance with the guidelines of the Austin Health Animal Ethics Committee.
Experimental design
Three study groups were used: the first study aimed to establish baseline GF expression in tumour and tumour-bearing liver tissues and involved two groups of mice. The experimental group was induced with metastatic tumour cells 21 days prior to tissue collection. Controls consisted of a group of mice from the same cohort that were not induced with tumour. The second study investigated temporal changes in levels of GFs in liver and metastases following TA (at days 0, 1, 2, 3, 5 and 7) compared with baseline levels (day 21 post-tumour induction and day 0 post-TA treatment). The third study was undertaken in response to unexpected findings in the second study and investigated GF changes in groups of animals that were sham-ablated in order to establish whether a number of results reflected experimental procedures rather than the TA.
Experimental model of CRC liver metastases
The primary cell line MoCR was derived from a dimethyl hydrazine (DMH)-induced primary colon carcinoma in the CBA mouse and maintained in vivo by serial passage in the flanks of CBA mice.14 Liver metastases were induced by an intrasplenic injection of 5 × 104 tumour cells prior to splenectomy, as reported previously.14 In this model, liver metastases are fully established by 21 days following tumour induction. For passage and experimentation, tumours grown subcutaneously were teased and washed in phosphate-buffered saline (PBS) to make a single-cell suspension.
Thermal ablation treatment
Thermal ablation of liver tumours was performed using a diode laser with a 400-µm bare tip optical quartz fibre (D-6100-BF; Dornier MedTech Laser GmbH, Wessling, Germany), applying 40 J of power per tumour (20 s at 2 Watt). The treatment parameters were chosen based on our previous extensive studies examining the nature and extent of injury, including temperature profiles.15–18 Average tissue temperatures reach 65 °C adjacent to the fibre site without causing tissue charring. Higher power settings in this animal model generally produce charring. The setting used produces incomplete tumour necrosis extending to the tumour margins. Animals were divided into groups (n = 6) and were anaesthetized. A bilateral sub-costal incision was performed to expose the liver. Similarly located intraparenchymal tumours, 7 mm in diameter, were chosen for sub-total laser ablation. A marking tissue dye (Davidson Tissue Marking System; Bradley Products, Grale Scientific Pty Ltd, Melbourne, Vic, Australia) was applied to each treated tumour for identification. For the day 0 time-point, the whole liver was removed immediately after TA and samples collected. For other time-points, the abdomen was closed with sutures and the animals were allowed to recover until they were killed at specific time-points (days 1, 2, 3, 5 and 7) following TA treatment. In the sham ablation study, the probe was inserted into the selected tumour, but not activated. Animals were killed at time-points matched to those used in the TA groups.
Tissue sample collection
At each endpoint after TA treatment, mice were anaesthetized and the liver excised. The two ablated or sham-treated tumours were identified and immediately dissected from the liver, together with surrounding liver tissue. Each tumour was cut into halves with a sharp blade. One half was fixed in 10% formalin; the other half, cleared from liver tissue, was snap-frozen in liquid nitrogen. Samples of liver tissue and untreated tumours distant from the ablation site were also collected from sites as shown in Fig. 1 and processed as above. All frozen specimens were stored at −80 °C for future lysate preparation. Formalin-fixed specimens were processed for immunohistochemistry.
Figure 1.
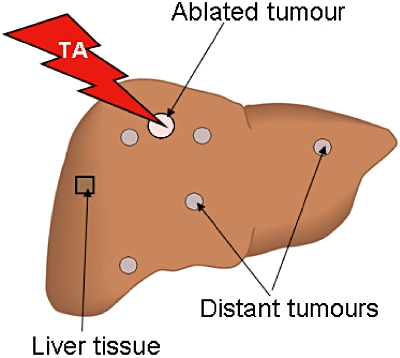
Schematic representation of thermal ablation (TA) treatment and areas of tissue collection for growth factor analysis. Two tumours were selected and ablation was performed using a 400-µm bare tip optical quartz fibre diode laser, applying 40 J of power per tumour (20 s at 2 Watt). In the sham-ablated groups, the probe was inserted into two selected tumours, but was not activated. Treated tumours were marked with tissue dye for future identification. Tissues from treated tumours, distant tumours and distant liver parenchyma were collected from the areas shown and used for immunochemistry and lysate preparations
Immunohistochemistry
Formalin-fixed, paraffin-embedded, 4-µm thick sections of the tissues were deparaffinized and rehydrated using standard techniques. Endogenous peroxidases were blocked by incubation in 3% peroxide in methanol for 10 min. Antigen retrieval was performed by treating sections with ethylene diamine tetra-acetic acid (EDTA) pH 8 for 20 min at 99 °C. For HGF staining, enzymatic digestion using 0.1% trypsin solution at 37 °C for 1 h was used for antigen retrieval. Normal goat serum (20%) was used to block non-specific binding. Commercially available rabbit primary antibodies raised against the mouse GFs (or cross-reactive to them) were used for staining (EGF: E2635 [Sigma-Aldrich Pty Ltd, Castle Hill, NSW, Australia] at 1 : 1500 dilution; HGF: sc7949 [Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA] at 20 µg/ml; TGF-β: sc7892 [Santa Cruz Biotechnology, Inc.] at 2 µg/ml; VEGF: Ab-4 PC315 [EMD Biosciences, San Diego, CA, USA] at 1.5 µg/ml). For negative controls, sections were incubated with non-immune rabbit immunoglobulin G (IgG) only (DakoCytomation Norden A/S, Glostrup, Denmark) at the same concentration as the primary antibody. Sections were incubated with primary antibodies overnight at 4 °C. A polymer-based detection kit containing goat anti-rabbit immunoglobulins (IgG) complexed with horseradish peroxidase (HRP) (EnVision Plus™; DakoCytomation Pty Ltd, Botany, NSW, Australia) was then used. Each incubation step was followed by two 5-min washes with PBS plus 0.05% Tween 20. The presence of GFs was visualized using diaminobenzidine (DAB) as a substrate.
Preparation of protein extracts from liver or tumour tissues
Collected tissues that were snap-frozen in liquid nitrogen as previously described were used for protein preparations. The tissues were weighed into microcentrifuge tubes and kept on ice. Cold lysis buffer (50 mM HEPES, 150 mM NaCl, 10 mM EDTA, 10 mM Na4P2O7, 10 mM NaF, 2 mM Na3VO4, 0.5 mM PMSF, 20 µM leupeptine, 10 µg/ml aprotinine and 1% triton X-100) was added to each tissue at a ratio of 5 volumes/mg tissue weight. The tissues were homogenized using an Ultra Turrax T25 homogenizer (John Morris Scientific Pty Ltd, Sydney, NSW, Australia) in three 10-s bursts while the sample was kept on ice. The homogenates were then sonicated in three 10-s bursts using a Branson Sonifier 250 (Branson Ultrasonics Corp., Danbury, CT, USA) at a constant output of 30 Hz while the sample was kept on ice. The sonicates were centrifuged at 17 000 g at 4 °C for 15 min and the supernatant (lysate) was collected, aliquoted and kept frozen at −80 °C until used.
Enzyme linked immunosorbent assay
An enzyme-linked immunosorbent assay (ELISA) was used to determine tissue levels of TGF-β1, VEGF, HGF and EGF. Growth factor concentrations in tissue extracts were determined with a sandwich ELISA using commercially available kits (mouse DuoSets®: EGF, DY2028; HGF, DY2207; VEGF, DY493; TGF-β1, DY1679; R&D Systems, Inc., Minneapolis, MN, USA). The assay was performed according to the manufacturer's instructions with minor modifications. In brief, the capture antibody was suitably diluted in PBS and then used to coat 96-well microplates (Nunc Immuno Plate Maxisorb; In Vitro Technologies Pty Ltd, Noble Park, Vic, Australia) at 50 µl per well. The plates were incubated overnight at 4 °C. Free binding spaces were blocked with the appropriate reagent diluent and test samples and standards were applied at suitable concentrations. Samples of TGF-β were activated using 2.5 N acetic acid/10 M and neutralized by 2.7 N NaOH/1.0 M HEPES as recommended by the kit manufacturer before being applied to the ELISA plate. After a 2-h incubation, biotinylated detection antibody was added, followed by Streptavidin-HRP. The plate was washed before each successive step with 0.05% Tween 20 in PBS. Substrate (H2O2 and tetramethylbenzidine, DY994; R&D Systems, Inc.) was added and developed for 20 min at room temperature. The colour development was terminated by the addition of 25 µl of stop solution (2 N H2SO4). The optical density was determined immediately using a microplate reader (Benchmark Plus Microplate Spectrophotometer System; Bio-Rad Laboratories, Inc., Hercules, CA, USA) set at dual wavelengths of 450 nm and 540 nm. The 540-nm reading was subtracted from the 450-nm reading. Using a computer-generated four-parameter logistic (4-PL) curve fit from the standard absorbance values and the corresponding concentrations, the unknown sample concentrations were directly calculated by the spectrophotometer software.
Statistical analysis
All data are expressed as the mean ± standard error of the mean unless otherwise stated. Data were tested for normality. Pairwise comparisons of group means for parametric data were performed using either Student's t-test or analysis of variance (anova) with post-hoc analysis (Scheffé for groups with equal variances and the Games–Howell method for groups with unequal variances, as appropriate). All statistical analyses were performed using spss Version 16.0 (SPSS, Inc., Chicago, IL, USA). P-values of <0.05 were considered statistically significant.
Results
Effect of liver metastases on the expression of angiogenic growth factors
The expression of angiogenic GFs in the tumour and liver was evaluated by ELISA at 21 days after tumour induction when CRCLM are fully established. Animals not induced with tumour were used as controls to assess baseline expression. Liver parenchyma of tumour-bearing animals expressed higher levels of TGF-β, VEGF and HGF compared with normal liver. Significant increases were found in the expression of TGF-β (62.4 ± 19.2 pg/mg vs. 8.5 ± 0.7 pg/mg; P = 0.043) and VEGF (1938 ± 136 pg/mg vs. 453 ± 243 pg/mg; P < 0.001), but the increase in HGF did not reach significance (Fig. 2). There was no significant difference in EGF expression between the two groups.
Figure 2.
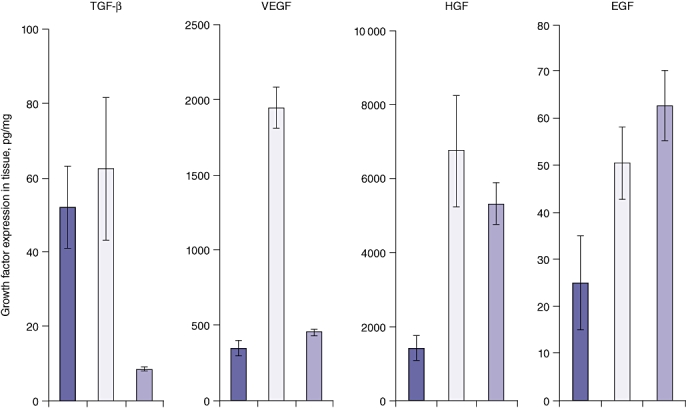
Baseline growth factor (GF) expression in tumour-bearing mice. Growth factor levels in tumour tissue ( ; day 21 post-tumour induction, n = 5), liver tissue of tumour-bearing mice (
; day 21 post-tumour induction, n = 5), liver tissue of tumour-bearing mice ( ; day 21 post-tumour induction, n = 5) and liver tissue of control mice (
; day 21 post-tumour induction, n = 5) and liver tissue of control mice ( ; non-induced with tumour, n = 4) was determined by enzyme-linked immunosorbent assay (ELISA) using specific GF ELISA kits supplied with recombinant GF protein for the standard curve. Levels of transforming GF-β (TGF-β) and vascular endothelial GF (VEGF) are significantly higher in tumour-bearing liver tissue compared with controls (P < 0.0001 for both). Levels of hepatocyte GF (HGF) and epidermal GF (EGF) in tumour-bearing mice did not differ significantly from those in control mice (P = 0.443 and P = 0.305, respectively)
; non-induced with tumour, n = 4) was determined by enzyme-linked immunosorbent assay (ELISA) using specific GF ELISA kits supplied with recombinant GF protein for the standard curve. Levels of transforming GF-β (TGF-β) and vascular endothelial GF (VEGF) are significantly higher in tumour-bearing liver tissue compared with controls (P < 0.0001 for both). Levels of hepatocyte GF (HGF) and epidermal GF (EGF) in tumour-bearing mice did not differ significantly from those in control mice (P = 0.443 and P = 0.305, respectively)
In animals with CRCLM, expression of VEGF, HGF and EGF was lower in tumour tissues compared with surrounding liver parenchyma (Fig. 2), but there was no difference in expression of TGF-β. These differences in the expression of GFs between tumour tissue and the surrounding liver parenchyma were also seen with immunohistochemistry (Fig. 3A–D, row 1), in which TGF-β stained strongly in both tumour and liver parenchyma. By contrast, HGF and, in particular, VEGF displayed strong staining in liver parenchyma while exhibiting only weak expression in tumour tissue. Expression of VEGF in tumour tissue was heterogeneous, with groups of tumour cells expressing VEGF and others demonstrating none (Fig. 3B, row 1). The growth factors TGF-β, VEGF and EGF were expressed in the cytoplasm of both tumour cells and hepatocytes, whereas HGF was also strongly localized within the nuclear region of some hepatocytes and tumour cells (Fig. 3C, row 1).
Figure 3.
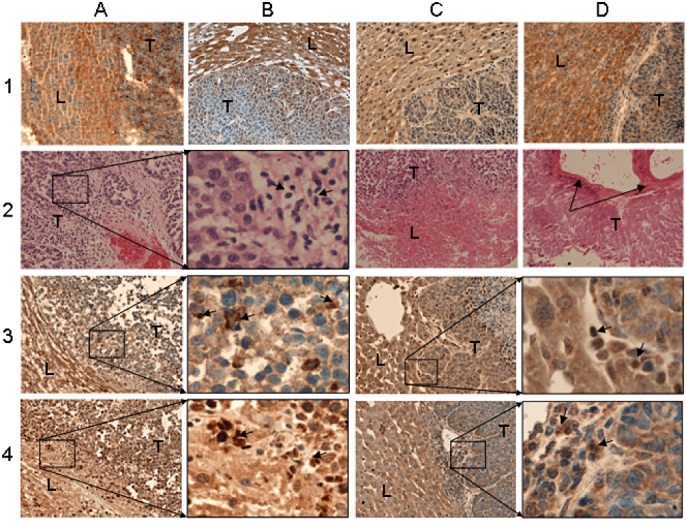
Haematoxylin and eosin (H&E) and immunostaining of control and thermally ablated tissues. Original magnification ×400. Row 1 (A–D): control tumour tissues stained for transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF) and epidermal growth factor (EGF), respectively. Row 2: H&E staining of ablated tumour section (A) immediately after ablation, (B) magnified inset of (A) showing inflammatory cell infiltration, (C) at day 1 after ablation, and (D) at day 7 after ablation. Arrows in (D) indicate prominent fibrotic deposition. Row 3: (A, C) ablated and distant tumour sections, respectively, stained for TGF-β; (B) and (D) are magnified insets of (A) and (C), respectively, showing inflammatory cell infiltration with positive staining. Row 4: (A, C) ablated and distant tumour sections, respectively, stained for EGF; (B) and (D) are magnified insets of (A) and (C), respectively, showing inflammatory cell infiltration with positive staining. L, liver parenchyma, T, tumour tissue. Small arrows indicate leukocyte infiltration
Temporal changes in histopathology and growth factor immunostaining in liver and tumour tissues following thermal ablation
Two liver metastases underwent TA in each animal with CRCLM. Tissues were collected at days 0, 1, 2, 3, 5 and 7 after ablation. Immediately following ablation, tumour cells within the ablated region appeared disorganized histologically, with loss of cell contact on histology (Fig. 3A, row 2). This was accompanied by a significant influx of leukocytes, as seen in the magnified inset (Fig. 3B, row 2). At day 1, the ablative changes had progressed to involve the adjacent liver parenchyma, with resultant alterations in cellular morphology and loss of nuclei (Fig. 3C, row 2). By day 7, the ablated tumours exhibited substantial necrosis of tumour cells extending to the tumour margins, tissue cavitation and prominent fibrosis in the treated region (Fig. 3D, row 2). This is consistent with findings in our previous studies, as we will describe in the Discussion. Immunohistochemical staining of ablated tumour tissues reflected the tissue injury pattern observed using haematoxylin and eosin (H&E) staining. The specific staining appeared to diminish at each successive time-point compared with that in untreated controls, as demonstrated in Fig. 3(A) (rows 3 and 4) in treated tissues stained for TGF-β and EGF, respectively, at day 2. Similar reductions in GF staining after treatment occurred in the distant untreated tumour tissues (Fig. 3C, rows 3 and 4). As the H&E staining shows, infiltrating cells are present in the immunostained sections. A proportion of the infiltrating cells stained strongly positive for TGF-β and EGF, particularly in ablated tumours, in which they are more prominent at the injury front (Fig. 3B, rows 3 and 4), although they are also apparent in distant untreated tumours (Fig. 3D, rows 3 and 4). In addition, the treated tissues generally displayed higher background non-specific staining compared with untreated controls or tissues distant from the ablation site.
Temporal changes in the expression of angiogenic growth factors after thermal ablation
Expression of TGF-β, VEGF, HGF and EGF was assessed by ELISA. The temporal changes in GF expression were compared with their respective baseline values (day 0). Baseline values for each GF did not differ significantly from those in the untreated tumour-bearing control group (Figs 2, 4).
Figure 4.
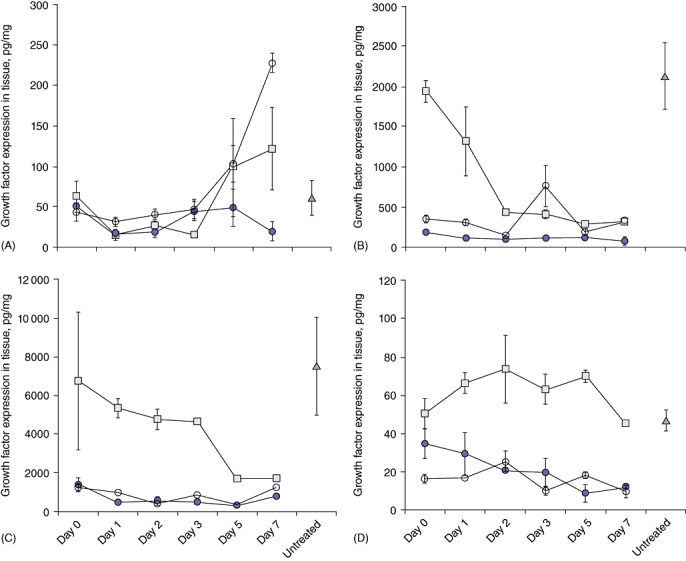
Temporal changes in growth factor (GF) expression in liver and tumour tissues following thermal ablation. Six groups of tumour-induced mice (for days 0, 1, 2 and 3 [n = 6] and days 5 and 7 [n = 4]) were used. Two selected tumours were ablated and tissues collected post-treatment as shown in Fig. 1 at the time-points indicated. Growth factor levels in tissue lysates were determined by enzyme-linked immunosorbent assay (ELISA).  , ablated tumour;
, ablated tumour;  , liver tissue;
, liver tissue;  , distant tumour.
, distant tumour.  , represents baseline expression of GF in untreated tumour-bearing liver tissue. (A) Temporal changes in transforming GF-β (TGF-β) expression. Significantly lower levels of expression were observed at days 1 and 2 in the ablated tumour tissues (P = 0.022 and P = 0.029, respectively) compared with at baseline. Significantly higher levels of expression were seen in the distant tumour tissue of ablated animals at day 7 (P < 0.001) compared with baseline. (B) Temporal changes in vascular endothelial GF (VEGF) expression. Significant decreases in expression from baseline were observed at all time-points after day 1 in liver tissues (P < 0.001) and at day 2 in tumour tissues (P = 0.038 for ablated tumour and P = 0.003 for distant tumour). (C) Temporal changes in hepatocyte GF (HGF) expression. Significant decreases in expression from baseline were observed at days 5 (P = 0.049) and 7 (P = 0.046) in liver tissue and at all time-points except day 7 in ablated tumour tissues (P = 0.008 at day 1, P = 0.007 at day 2, P = 0.003 at day 3, P = 0.005 at day 5, P = 0.105 at day 7). Levels of HGF in distant tumour tissues differed significantly from baseline only at day 5 (P = 0.008). (D) Temporal changes in epidermal GF (EGF) expression. In the TA-treated animals significant decreases in expression from baseline were observed in ablated tumour tissues at days 5 and 7 (P = 0.031 and P = 0.041, respectively)
, represents baseline expression of GF in untreated tumour-bearing liver tissue. (A) Temporal changes in transforming GF-β (TGF-β) expression. Significantly lower levels of expression were observed at days 1 and 2 in the ablated tumour tissues (P = 0.022 and P = 0.029, respectively) compared with at baseline. Significantly higher levels of expression were seen in the distant tumour tissue of ablated animals at day 7 (P < 0.001) compared with baseline. (B) Temporal changes in vascular endothelial GF (VEGF) expression. Significant decreases in expression from baseline were observed at all time-points after day 1 in liver tissues (P < 0.001) and at day 2 in tumour tissues (P = 0.038 for ablated tumour and P = 0.003 for distant tumour). (C) Temporal changes in hepatocyte GF (HGF) expression. Significant decreases in expression from baseline were observed at days 5 (P = 0.049) and 7 (P = 0.046) in liver tissue and at all time-points except day 7 in ablated tumour tissues (P = 0.008 at day 1, P = 0.007 at day 2, P = 0.003 at day 3, P = 0.005 at day 5, P = 0.105 at day 7). Levels of HGF in distant tumour tissues differed significantly from baseline only at day 5 (P = 0.008). (D) Temporal changes in epidermal GF (EGF) expression. In the TA-treated animals significant decreases in expression from baseline were observed in ablated tumour tissues at days 5 and 7 (P = 0.031 and P = 0.041, respectively)
Following TA, all four GF levels decreased in the ablated tumour tissues at all time-points. At particular time-points, this decrease differed significantly from baseline (Fig. 4). With the exception of TGF-β, a similar decreasing trend in GF levels was also seen in tumour tissues distant from the ablated sites. As in the ablated tumours, this decrease resulted in levels significantly lower than baseline values at certain time-points (Fig. 4). A significant reduction in the expression of TGF-β was also seen in distant untreated tumours on days 1 (P < 0.02) and 2 (P < 0.03) compared with baseline levels. This was followed by a rapid increase in expression at day 5, which resulted in a statistically significant difference compared with baseline by day 7 (P < 0.001) (Fig. 4A).
Growth factor changes also occurred within the liver tissues following tumour ablation. Levels of TGF-β, VEGF and HGF significantly decreased after treatment. The time-point at which expression fell to a level significantly lower than baseline differed for each factor. Levels of TGF-β decreased significantly at days 1 and 2 following treatment and then increased above baseline levels at days 5 and 7 in a pattern similar to that seen in distant tumour tissues (Fig. 4A). The increase, however, did not reach significance. Expression of VEGF was significantly lower at all time-points from day 1 after treatment onwards (P < 0.001) (Fig. 4B). Expression of HGF showed a decreasing trend from day 1 post-treatment and became significantly lower than at baseline at days 5 and 7 (Fig. 4C). Levels of EGF, unlike those of the other GFs, increased in the liver tissue; however, this increase was not found to be statistically significant at early time-points after treatment. Expression of EGF then returned to baseline levels by day 7 (Fig. 4D).
Temporal changes in the expression of angiogenic GFs after sham ablation
Published studies report increases in GF levels following tumour resection.5 We expected to find some increase in GF levels following TA in this study, but, on the contrary, with the exception of TGF-β, GF levels decreased after treatment. To investigate whether any of these changes were caused by the experimental procedures (laparotomy), groups of mice were treated as shams. These mice underwent laparotomy in which the liver was exposed and the laser probe was inserted into two tumours but was not activated (sham ablation). Tissues were collected at time-points equivalent to those used in the TA study. Changes in levels of GF expression in these tissues were assessed and were found to be similar to those seen in the tissues in the TA treatment study, with one exception: levels of TGF-β remained below baseline levels at all time-points tested (results not shown).
Discussion
Because of its low morbidity rates, limited hepatic parenchymal damage and applicability to a wider cross-section of the target population, local TA has the potential to be used as an alternative to surgery. It is available for patients who are not suitable for surgical intervention as a result of the spatial distribution of the tumour, limited hepatic reserve or lack of general fitness. In addition, there is a sound theoretical basis and accumulating experimental evidence to indicate that TA would reduce the incidence of recurrent disease. The major disadvantage of using TA in the clinic is that patients who undergo this treatment are subject to a high rate of local recurrence in the liver, mainly as a result of incomplete tumour destruction stemming from problems with tumour imaging in the percutaneous procedure. In a study by Elias et al., in which radiofrequency ablation (RFA) was performed with laparotomy, survival rates were equivalent to those in anatomical or wedge resections.19
We have extensive experience in both clinical20 and experimental application of laser TA for CRCLM. We have previously characterized the effects of TA at the cellular level on temperature profiles,15–18 temporal injury progression,21,22 tumour microvasculature,16,18 blood flow occlusion23 and in combination with thermal sensitizers.24 We have demonstrated no additional tumour stimulation by TA.25 The current study continues our published work characterizing the various aspects of TA and, in particular, focuses on the effect of TA on angiogenic GFs.
In establishing the baseline expression of the four GFs in liver and tumour tissue, we have demonstrated that VEGF, HGF and EGF expression in the surrounding liver parenchyma is higher than that found within the tumour. More importantly, we found that liver parenchyma in tumour-bearing mice expresses significantly increased levels of TGF-β and VEGF in comparison with normal liver. In another study, Meredith et al. demonstrated significant increases in mRNA levels of HGF and basic fibroblast GF (bFGF) between tumour-bearing liver and normal liver in a different CRCLM model.26 In our study, HGF expression demonstrated an increasing trend, but this did not reach statistical significance. These findings suggest that the presence of tumour influences the liver parenchyma, leading to increased expression of proangiogenic GFs which may support and sustain tumour growth. As this was an unexpected finding further studies using more appropriate controls (carrier-injected and splenectomized mice) are required to verify these findings and to investigate additional changes induced by the presence of tumour.
Following TA treatment, tumour cells show progressive injury and death. By day 7 no normal tumour structure can be seen, although dissociated live tumour cells remain at the tumour margin. Limited injury at this stage has also spread into the adjacent liver parenchyma. These effects on the tumour seen in this study are in agreement with our earlier findings.17 All GFs displayed progressively decreasing levels within the ablated tumours following treatment. This is as expected, given that the tissue is becoming progressively necrotic and the proteins denatured. However, with the exception of TGF-β, a progressive decrease in GF expression after TA was also seen in tumours distant from the ablation site, which was unlikely to be a result of local injury. Additionally, similar GF decreases were seen in the tumours in the control groups. Similarly, expression of HGF and VEGF progressively decreased in the liver parenchyma in both the ablated and control groups, whereas that of EGF did not change significantly in the liver parenchyma in either group. These findings strongly suggest that, with the exception of TGF-β, the GF changes reflect a systemic effect in the animal in response to laparotomy and manipulation. Meredith et al.26 reported decreases in liver bFGF and HGF mRNA levels at days 2 and 3, respectively, following TA in a mouse model of a single implanted tumour in the liver. The decrease was attributed to the complete eradication of the single tumour and therefore the removal of the stimulus. This explanation cannot account for our findings as the decrease in GFs in our study occurred after the ablation of two specific tumours in the presence of multiple distant untreated tumours. In addition, we found similar decreases in GFs in the sham-ablated animals, in which no tumour destruction occurred. In light of this, it is important to note that no sham group was included in the study reported by Meredith et al.26 In another study, Isbert et al.27 compared the effects of TA or resection on HGF mRNA levels using an implanted CRC rat tumour model. In this study,27 two tumours were implanted, but only one was ablated, resembling the design of our experiment. Isbert et al.27 reported that HGF mRNA levels at 48 h were significantly lower in distant tumours in animals undergoing TA compared with those undergoing resection. By contrast, no difference emerged between TA-treated animals and sham-ablated controls at this time-point, which is in agreement with our findings. Isbert et al.27 did not present baseline GF levels (day 0) that may also have shown a temporal decrease, as in our study. In agreement with our study, these two studies26,27 reported decreased expression of particular GFs following TA, with an increase in HGF common to all three studies. We demonstrated a similar decrease in GF expression in the sham-ablated controls, suggesting that the decrease most likely reflects a general effect of laparotomy rather than TA. One possible explanation for this reduction is that the major injury caused by laparotomy mobilized local tissue stores of GFs towards the site of the injury (dissected peritoneal muscles), thus leading to local depletion in organs such as the liver. If this hypothesis is correct, a concomitant systemic GF increase would be expected. A recent human study by Evrard et al. reported systemic increases in HGF and VEGF following laparotomy and RFA treatment,28 thus supporting our hypothesis. However, further studies are necessary to unequivocally demonstrate that laparotomy reduces the GFs in the liver and possibly in other organs. By contrast with the findings that laparotomy and local tumour ablation result in a local decrease in GF expression, laparotomy and liver resection resulted in significant local increases in several GFs.26,27,29 This difference may be a measure of the extent of liver injury in the two procedures. Liver resection requires the prompt replacement of liver mass by regeneration as liver function is of the utmost importance for survival. By contrast, TA causes minor localized liver injury, which does not lead to significant regeneration, and the wound healing of the laparotomy may represent a more urgent demand on the animal's healing resources.
The exception to these findings concerns the expression of TGF-β in the aftermath of TA. After an initial reduction at days 1 and 2, there was a significant increase above baseline levels in the treated groups. This is likely to represent a specific response to TA because it was not seen in the sham-ablated groups. This finding may have clinical significance as high serum levels of this GF following liver tumour resection were shown by Tsushima et al. to be a negative predictor for survival in cancer patients.30 Increases in TGF-β levels were also reported by Ohno et al. following tumour microwave coagulation therapy.31 Additionally, an increase in connective tissue GF (CTGF) expression, a downstream effector of the TGF-β signalling pathway, was seen in distant tumours after TA by Isbert et al.27 Over-expression of CTGF in liver has been associated with hepatic fibrosis.32 Although we did not investigate changes in CTGF in our study, we did observe what appears to be fibrotic tissue accumulation in the TA-treated areas. TGF-β is a multifunctional GF and plays important roles in injury and healing, liver regeneration, tumour growth and metastasis.5,33–35 The upregulation of TGF-β may well represent a healing response to the injury caused by TA. However, this upregulation may also have an impact on the growth of residual dormant micrometastases or tumour remnant at a site of incomplete TA and thus contribute to disease recurrence.30 The large infiltration of leukocytes seen in the ablated area and in the distant tumours following TA may contribute to tumour inhibition by the induction of anti-tumour immune responses.11,36 We have evidence of macrophage and T cell accumulation and interferon-γ (IFN-γ) upregulation within the ablated sites and in distant tumours after TA treatment (present authors, unpublished results, 2010). However, a proportion of infiltrating cells stained strongly for GFs, particularly for TGF-β and EGF, within the ablated viable/necrotic tissue interface, which supports our earlier observation.22 These GF-positive infiltrating cells may contribute to tumour regrowth. Thus, tumour recurrence may depend on the net balance between proinflammatory and proangiogenic factors in the residual tumour microenvironment.
In conclusion, whereas tumour removal by liver resection results in increased expression of GFs shown to play key roles in the stimulation of tumour growth and recurrence, TA treatment of tumours results in the local reduction of GF expression. The only exception to this finding concerned expression of TGF-β, which significantly increased after TA. The observed reduction in GF expression is in agreement with two other published studies on TA treatment.26,27 Our study, however, also shows similar reductions in GFs in the control groups, prompting us to suggest that the observed changes are caused by laparotomy rather than TA treatment. The two other studies26,27 did not include sufficient controls to indicate the same conclusion.
Our study demonstrated significant increases in local TGF-β expression in response to TA. As this GF is closely associated with tumour progression and poor prognosis, we believe further studies should explore the inhibition of this GF in combination with TA for more effective anti-tumour treatment.
Acknowledgments
This work was supported by the Cancer Council of Victoria and Austin Health Medical Research Foundation. The authors wish to thank Liyana Ahmad Zamri for her excellent technical assistance.
Conflicts of interest
None declared.
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Cromheecke M, de Jong KP, Hoekstra HJ. Current treatment for colorectal cancer metastatic to the liver. Eur J Surg Oncol. 1999;25:451–463. doi: 10.1053/ejso.1999.0679. [DOI] [PubMed] [Google Scholar]
- 3.Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, et al. Five-year survival following hepatic resection after neoadjuvant therapy for non-resectable colorectal. Ann Surg Oncol. 2001;8:347–353. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 4.Ruers T, Bleichrodt RP. Treatment of liver metastases, an update on the possibilities and results. Eur J Cancer. 2002;38:1023–1033. doi: 10.1016/s0959-8049(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 5.Christophi C, Harun N, Fifis T. Liver regeneration and tumour stimulation – a review of cytokine and angiogenic factors. J Gastrointest Surg. 2008;12:966–980. doi: 10.1007/s11605-007-0459-6. [DOI] [PubMed] [Google Scholar]
- 6.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. discussion 825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iannitti DA, Martin RC, Simon CJ, Hope WW, Newcomb WL, McMasters KM, et al. Hepatic tumour ablation with clustered microwave antennae: the US Phase II Trial. HPB (Oxford) 2007;9:120–124. doi: 10.1080/13651820701222677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solbiati L, Ierace T, Tonolini M, Osti V, Cova L. Radiofrequency thermal ablation of hepatic metastases. Eur J Ultrasound. 2001;13:149–158. doi: 10.1016/s0929-8266(01)00127-6. [DOI] [PubMed] [Google Scholar]
- 9.Vogl TJ, Straub R, Eichler K, Sollner O, Mack MG. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy – local tumour control rate and survival data. Radiology. 2004;230:450–458. doi: 10.1148/radiol.2302020646. [DOI] [PubMed] [Google Scholar]
- 10.Isbert C, Ritz JP, Roggan A, Schuppan D, Ruhl M, Buhr HJ, et al. Enhancement of the immune response to residual intrahepatic tumour tissue by laser-induced thermotherapy (LITT) compared to hepatic resection. Lasers Surg Med. 2004;35:284–292. doi: 10.1002/lsm.20097. [DOI] [PubMed] [Google Scholar]
- 11.Hu Z, Yang XY, Liu Y, Sankin GN, Pua EC, Morse MA, et al. Investigation of HIFU-induced anti-tumour immunity in a murine tumour model. J Transl Med. 2007;5:34. doi: 10.1186/1479-5876-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. 2009;197:728–736. doi: 10.1016/j.amjsurg.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Nicholl MB, Bilchik AJ. Thermal ablation of hepatic malignancy: useful but still not optimal. Eur J Surg Oncol. 2008;34:318–323. doi: 10.1016/j.ejso.2007.07.203. [DOI] [PubMed] [Google Scholar]
- 14.Kuruppu D, Christophi C, Bertram JF, O'Brien PE. Characterization of an animal model of hepatic metastasis. J Gastroenterol Hepatol. 1996;11:26–32. doi: 10.1111/j.1440-1746.1996.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 15.Nikfarjam M, Muralidharan V, Su K, Malcontenti-Wilson C, Christophi C. Patterns of heat shock protein (HSP70) expression and Kupffer cell activity following thermal ablation of liver and colorectal liver metastases. Int J Hyperthermia. 2005;21:319–332. doi: 10.1080/02656730500133736. [DOI] [PubMed] [Google Scholar]
- 16.Muralidharan V, Nikfarjam M, Malcontenti-Wilson C, Christophi C. Effect of interstitial laser hyperthermia in a murine model of colorectal liver metastases: scanning electron microscopic study. World J Surg. 2004;28:33–37. doi: 10.1007/s00268-003-6973-0. [DOI] [PubMed] [Google Scholar]
- 17.Nikfarjam M, Malcontenti-Wilson C, Christophi C. Focal hyperthermia produces progressive tumour necrosis independent of the initial thermal effects. J Gastrointest Surg. 2005;9:410–417. doi: 10.1016/j.gassur.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Nikfarjam M, Muralidharan V, Malcontenti-Wilson C, Christophi C. Progressive microvascular injury in liver and colorectal liver metastases following laser-induced focal hyperthermia therapy. Lasers Surg Med. 2005;37:64–73. doi: 10.1002/lsm.20194. [DOI] [PubMed] [Google Scholar]
- 19.Elias D, Baton O, Sideris L, Matsuhisa T, Pocard M, Lasser P. Local recurrences after intraoperative radiofrequency ablation of liver metastases: a comparative study with anatomic and wedge resections. Ann Surg Oncol. 2004;11:500–505. doi: 10.1245/ASO.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Christophi C, Nikfarjam M, Malcontenti-Wilson C, Muralidharan V. Longterm survival of patients with unresectable colorectal liver metastases treated by percutaneous interstitial laser thermotherapy. World J Surg. 2004;28:987–994. doi: 10.1007/s00268-004-7202-1. [DOI] [PubMed] [Google Scholar]
- 21.Muralidharan V, Christophi C. Interstitial laser thermotherapy in the treatment of colorectal liver metastases. J Surg Oncol. 2001;76:73–81. doi: 10.1002/1096-9098(200101)76:1<73::aid-jso1014>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Nikfarjam M, Muralidharan V, Christophi C. Altered growth patterns of colorectal liver metastases after thermal ablation. Surgery. 2006;139:73–81. doi: 10.1016/j.surg.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 23.Muralidharan V, Malcontenti-Wilson C, Christophi C. Effect of blood flow occlusion on laser hyperthermia for liver metastases. J Surg Res. 2002;103:165–174. doi: 10.1006/jsre.2002.6365. [DOI] [PubMed] [Google Scholar]
- 24.Muralidharan V, Malcontenti-Wilson C, Christophi C. Interstitial laser hyperthermia for colorectal liver metastases: the effect of thermal sensitization and the use of a cylindrical diffuser tip on tumour necrosis. J Clin Laser Med Surg. 2002;20:189–196. doi: 10.1089/104454702760230500. [DOI] [PubMed] [Google Scholar]
- 25.Muralidharan V, Nikfarjam M, Malcontenti-Wilson C, Christophi C. Interstitial laser hyperthermia and the biological characteristics of tumour: study in a murine model of colorectal liver metastases. J Clin Laser Med Surg. 2003;21:75–83. doi: 10.1089/104454703765035493. [DOI] [PubMed] [Google Scholar]
- 26.Meredith K, Haemmerich D, Qi C, Mahvi D. Hepatic resection but not radiofrequency ablation results in tumour growth and increased growth factor expression. Ann Surg. 2007;245:771–776. doi: 10.1097/01.sla.0000261319.51744.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isbert C, Ritz JP, Roggan A, Schuppan D, Ajubi N, Buhr HJ, et al. Laser-induced thermotherapy (LITT) elevates mRNA expression of connective tissue growth factor (CTGF) associated with reduced tumour growth of liver metastases compared to hepatic resection. Lasers Surg Med. 2007;39:42–50. doi: 10.1002/lsm.20448. [DOI] [PubMed] [Google Scholar]
- 28.Evrard S, Menetrier-Caux C, Biota C, Neaud V, Mathoulin-Pelissier S, Blay JY, et al. Cytokines pattern after surgical radiofrequency ablation of liver colorectal metastases. Gastroenterol Clin Biol. 2007;31:141–145. doi: 10.1016/s0399-8320(07)89344-4. [DOI] [PubMed] [Google Scholar]
- 29.Mullhaupt B, Feren A, Fodor E, Jones A. Liver expression of epidermal growth factor RNA. Rapid increases in immediate-early phase of liver regeneration. J Biol Chem. 1994;269:19667–19670. [PubMed] [Google Scholar]
- 30.Tsushima H, Ito N, Tamura S, Matsuda Y, Inada M, Yabuuchi I, et al. Circulating transforming growth factor-beta 1 as a predictor of liver metastasis after resection in colorectal cancer. Clin Cancer Res. 2001;7:1258–1262. [PubMed] [Google Scholar]
- 31.Ohno T, Kawano K, Yokoyama H, Tahara K, Sasaki A, Aramaki M, et al. Microwave coagulation therapy accelerates growth of cancer in rat liver. J Hepatol. 2002;36:774–779. doi: 10.1016/s0168-8278(02)00058-2. [DOI] [PubMed] [Google Scholar]
- 32.Abou-Shady M, Friess H, Zimmermann A, di Mola FF, Guo XZ, Baer HU, et al. Connective tissue growth factor in human liver cirrhosis. Liver. 2000;20:296–304. doi: 10.1034/j.1600-0676.2000.020004296.x. [DOI] [PubMed] [Google Scholar]
- 33.Massague J. TGF-beta in cancer. Cell. 2008;134:215–230. [Google Scholar]
- 34.Lin SW, Lee MT, Ke FC, Lee PP, Huang CJ, Ip MM, et al. TGFbeta1 stimulates the secretion of matrix metalloproteinase 2 (MMP2) and the invasive behaviour in human ovarian cancer cells, which is suppressed by MMP inhibitor BB3103. Clin Exp Metastasis. 2000;18:493–499. doi: 10.1023/a:1011888126865. [DOI] [PubMed] [Google Scholar]
- 35.Dumont N, Bakin AV, Arteaga CL. Autocrine transforming growth factor-beta signalling mediates Smad-independent motility in human cancer cells. J Biol Chem. 2003;278:3275–3285. doi: 10.1074/jbc.M204623200. [DOI] [PubMed] [Google Scholar]
- 36.Isbert C, Boerner A, Ritz JP, Schuppan D, Buhr HJ, Germer CT. In situ ablation of experimental liver metastases delays and reduces residual intrahepatic tumour growth and peritoneal tumour spread compared with hepatic resection. Br J Surg. 2002;89:1252–1259. doi: 10.1046/j.1365-2168.2002.02205.x. [DOI] [PubMed] [Google Scholar]


