Abstract
Background
The aim of the present study was to analyse the outcome after hepatic resection for non-colorectal, non-neuroendocrine, non-sarcomatous (NCNNNS) metastatic tumours and to identify the factors predicting survival.
Methods
All patients who underwent hepatic resection for NCNNNS metastatic tumours between September 1996 and June 2009 were included. Patients' demographics, clinical and histopathological parameters, overall survival and the factors predicting survival were analysed.
Results
In all, 65 patients underwent hepatic resection for metastasis. The most common site of a primary tumour was the kidney (24 patients). Fifteen patients had synchronous tumours. Fifty patients had major liver resections and 22 patients had bilobar disease. The median number of liver lesions resected was 1 and the median maximum diameter of the metastasis was 6 cm. A R0 resection was performed in 51 patients. The 1-, 3- and 5-year overall survival from the time of metastasectomy was 72.9%, 47.9% and 25.6%, respectively, with a median survival of 19 months. The presence of a tumour of greater than 6 cm (P = 0.048) and a positive resection margin (P = 0.04) were associated with poor survival.
Conclusion
Hepatic resection for metastasis from NCNNNS tumours can offer acceptable long-term survival in selected patients. To offer a chance of a cure a R0 resection must be performed.
Keywords: hepatic resection, metastasis, non-colorectal, non-neuroendocrine
Introduction
Hepatic resection is an accepted form of treatment for patients with liver metastasis from colorectal cancer. The reported 5-year survival rates range from 16% to 51% depending on patient selection.1–4 Recent studies have shown that hepatic metastasectomy for colorectal cancer metastasis is associated with a good chance of long-term survival.5,6
Similarly the role of liver resection is widely accepted for metastasis from neuroendocrine tumours, both for symptomatic control and improvement in survival.7–10 Liver resection is appealing in this group of patients because these cancers typically metastasize through the portal venous system similar to colorectal cancers and have a protracted natural history compared with other gastrointestinal cancers and solid tumours. A 5-year survival rate of up to 75% has been reported after hepatic resection.11,12
More recently, liver resection is being increasingly performed for metastasis from soft tissue sarcomas. Several studies have shown a promising 5-year survival rate of 27% to 49% after hepatic resection for metastatic sarcoma.13–15
However, the role of liver resection remains ill defined and controversial for patients with metastasis from non-colorectal, non-neuroendocrine, non-sarcomatous (NCNNNS) tumours. Although several studies have been published recently, the results are inconsistent as a result of the heterogeneity of the study group.16–19 Most of the studies included patients with metastasis from soft tissue sarcomas and this may significantly influence the results of these studies. Moreover, limited data are available from centres in the United Kingdom (UK).20 Hence, the present study was performed to analyse the outcome after hepatic resection for NCNNNS metastasis and to define the factors predicting survival to facilitate appropriate patient selection.
Patients and methods
All patients who underwent hepatic resection for NCNNNS metastasis between September 1996 and June 2009 at the University Hospital of Birmingham NHS Foundation Trust were included in the present study. The data were obtained from the prospectively maintained hospital-based liver unit and pathology database.
Selection criteria
Liver resection was considered if all known disease was technically resectable with an adequate hepatic functional reserve and if the general condition of the patient permitted liver resection. Pre-operatively all patients underwent a computerized tomographic (CT) scan of the thorax, abdomen and pelvis with contrast and/or magnetic resonance imaging (MRI) of the liver. Radiological images were discussed in a multi-disciplinary meeting (MDM) involving surgeons, oncologists, radiologists and pathologists. Further imaging, in the form of a positron emission tomography (PET)-CT, was performed based on the outcome of the MDM. Patients with a primary tumour infiltrating into the liver were excluded from the study. The presence of resectable extrahepatic disease did not preclude liver resection.
Follow-up
Post-operatively, all patients were followed up radiologically, clinically and biochemically up to the fifth post-operative year. Clinical and biochemical surveillance was performed every 3 months for the first year, every 4 months for the second year, every 6 months for the third year and yearly thereafter until the fifth post-operative year. A CT scan of the chest, abdomen and pelvis was done annually up to 5 years after surgery. Tumour markers were measured as appropriate based on the type of primary tumour.
Variables
Patient demographics, tumour characteristics, clinical and operative parameters were analysed. The following variables were assessed as prognostic factors: age (≤59 vs. >59 years, the median age being 59 years), gender (male vs. female), site of the primary tumour (renal cell carcinoma vs. others, genital tumours vs. others, genitourinary tumours vs. others), time of occurrence of metastases (synchronous vs. metachronous), interval between surgery for a primary tumour and liver resection (<30 months vs. ≥30 months), number of metastases (1 vs. >1), maximum diameter of the metastasis (≤6 cm vs. >6 cm), presence of resectable extrahepatic disease (yes vs. no), type of liver resection (<3 segments vs. ≥3 segments), site of metastases (bilobar vs. unilobar) and status of the resection margin (negative margin vs. involved margin). As the median maximum tumour diameter was 6 cm and the median time interval between the primary and liver surgery was 30 months, these were used as a cut-off for analysis as binary variables. As a result of variation in the type, timing and duration of chemotherapy, it was not analysed as a variable in this study.
Synchronous liver lesions were defined as the simultaneous development of a primary tumour and liver metastasis or the occurrence of liver metastasis within 3 months of resection of the primary tumour. A R0 resection was defined as the presence of a negative microscopic resection margin and R1 as a positive microscopic resection margin.
The overall actuarial 1-, 3- and 5-year survival was calculated from the time of hepatic metastasectomy. Mortality data were described as 30-, 60- and 90-day mortality.
Statistics
The overall survival was calculated with survival curve analysis using the Kaplan–Meier method. Survival comparison between the high-risk groups was compared with a log-rank test and the significance was assigned at 0.05. The statistical programme for social statistics (SPSS version 17.0; SPSS Inc., Chicago, IL, USA) was used for statistical analysis.
Results
Clinical and pathological features
In all, 65 patients underwent liver resection for metastasis from NCNNNS tumours, between September 1996 and June 2009. At the time of hepatic resection, the median age was 59 years (range: 23–75 years). There were 25 men and 40 women.
The most common site of a primary tumour was the kidney, seen in 24 patients (36.9%). The sites of other primary tumours are shown in Table 1. The histology of the testicular cancer was choriocarcinoma, that of thyroid cancer was follicular carcinoma and the cutaneous and eye tumours were melanomas. In 15 patients (23.1%), a synchronous liver lesion was identified, whereas the remaining 50 patients (76.9%) had metachronous liver metastasis. The median interval between resection of a primary tumour and liver resection was 30 months (range: 0–463 months) for the entire cohort and 48 months for those with metachronous lesions.
Table 1.
Sites of primary tumours in patients who underwent liver resections for metastases
| Primary tumour site | n (%) |
|---|---|
| Kidney | 24 (36.9) |
| Ovary | 16 (24.6) |
| Uterus | 3 (4.6) |
| Adrenal | 2 (3.1) |
| Testis | 1 (1.5) |
| Bile duct | 1 (1.5) |
| Jejunum | 1 (1.5) |
| Oropharynx | 1 (1.5) |
| Breast | 6 (9.2) |
| Thyroid | 3 (4.6) |
| Eye | 1 (1.5) |
| Skin | 4 (6.2) |
| Lungs | 2 (3.1) |
The type of surgical resection was based on the need of each individual patient, as discussed at the MDM. The different hepatic resections performed were a right hemihepatectomy (n = 26, 40%), an extended right hepatectomy (n = 11, 16.9%), a right hepatectomy with non-anatomical resection (NAR) (n = 6, 9.2%), a left hepatectomy (n = 2, 3.1%), an extended left hepatectomy (n = 1, 1.5%), a left lateral segmentectomy (n = 3, 4.6%), a left lateral segmentectomy with NAR (n = 2, 3.1%) and NAR alone (n = 14, 21.5%). Major liver resection, defined as resection of more than three Couinaud's segments, was undertaken in 50 patients (76.9%). Twenty-two patients (33.8%) were found to have bilobar disease.
Significant morbidity after liver resection was seen in 15 patients. One patient had intra-abdominal bleeding requiring re-laparotomy. One patient developed a bile leak. This was managed by biliary stenting and percutaneous drainage. Two patients had a wound infection requiring drainage. Two patients developed intra-abdominal collection, necessitating percutaneous radiological guided drainage. A chest infection was seen in five patients. Small-for-size syndrome and renal failure were seen in two patients each. Both the patients with renal failure needed renal replacement therapy.
Final histological examination of the resected specimen confirmed the number of liver lesions to range from 1 to 7, the median being 1. The majority of patients had a solitary metastasis (n = 47, 72.3%). Further data on the number of liver metastases are shown in Table 2. The median maximum diameter of the largest lesion was 6 cm, ranging from 1.5 to 16 cm.
Table 2.
The number of liver lesions in patients who underwent metastasectomy for non-colorectal, non-neuroendocrine, non-sarcoma metastases
| Number of liver metastases | Number of patients (%) |
|---|---|
| 1 | 47 (72.3) |
| 2 | 6 (9.2) |
| 3 | 6 (9.2) |
| 4 | 2 (3.1) |
| 5 | 1 (1.5) |
| 6 | 1 (1.5) |
| 7 | 2 (3.1) |
A R0 resection was successfully achieved in 51 patients (78.5%), while the remaining 14 patients (21.5%) had a R1 resection. There was no difference between the R0 and R1 resections with respect to the number of liver metastases (median of 1 vs. 1.5), size of metastases (median of 6 cm vs. 7 cm) and type of liver resection (37 major, 14 minor vs. 13 major, 1 minor). In no patients was a gross tumour left behind (R2 resection).
Extrahepatic disease
Resectable extrahepatic disease at the time of liver resection was seen in 19 patients (29.2%). The sites of extrahepatic disease were the diaphragm (6 patients), peritoneal nodule (4 patients), diaphragm, omentum and peritoneum (1 patient), omentum (1 patient), diaphragm and adrenal (2 patients), adrenal (1 patient), pancreas (1 patient), right lower lobe of the lung (1 patient), lymph node (1 patient) and pelvic recurrence (1 patient). Twelve patients had R0 resection and the remaining seven patients had R1 resection.
Recurrence
The disease-free interval before liver resection for the 50 patients with metachronous liver metastases ranged from 4 to 463 months, with a median of 48 months. The median disease-free interval after liver resection of the entire cohort was 19 months.
Five patients had extrahepatic disease recurrence before liver resection. One patient developed axillary nodal recurrence, requiring axillary clearance, two and a half years after excision of a primary cutaneous melanoma. This was followed by liver resection for metastasis 6 months later. The second patient had en bloc resection of a distal pancreas, spleen and lymph node for extensive retroperitoneal recurrence, 5 years after resection of testicular cancer. This patient subsequently had a NAR of a segment IV liver metastasis a year later. A third patient had resection of abdominal wall recurrence 10 years after excision of primary ovarian cancer, followed by groin lymphadenectomy 9 years later and a subsequent liver resection 2 years later. Another patient developed renal bed recurrence, requiring excision, 8 years after nephrectomy. This was followed by liver resection for metastasis a year later. Axillary nodal recurrence was noticed in another patient 2 years after a mastectomy for primary breast cancer. This patient subsequently developed a liver metastasis 7 years later, requiring resection.
One patient had repeat liver resection for recurrent disease within the liver. This patient had a right hemihepatectomy and NAR of a segment 2 lesion for a synchronous metastasis from a primary renal cell carcinoma. This patient developed recurrence in segment 3 of the liver 10 months later, requiring another NAR.
Survival
The median overall survival, calculated from the time of hepatic metastasectomy, was 19 months, ranging from 0 to 125 months (Fig. 1). The 1-, 3- and 5-year survival rates were 72.9%, 47.9% and 25.6%, respectively. There was no peri-operative mortality. Six patients (9.2%) died within 30 days of liver resection. All of them died of multi-organ failure. The 60- and 90-day mortalities were eight patients (12.3%) each. Comparison of cancer-specific survival revealed a median survival of 22.5 months for renal cell carcinoma, 23 months for genital tumours and 22.5 months for genitourinary tumours.
Figure 1.
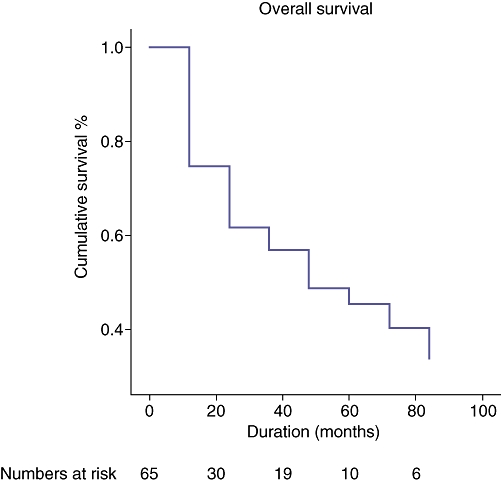
A cumulative survival curve for patients who underwent a liver resection for metastases, calculated from the time of hepatic metastasectomy
Prognostic factors
Among all the factors which were analysed to predict survival on univariate analysis, two variables, namely metastatic tumour size of >6 cm (P = 0.048) and R1 resection (P = 0.04), were found to be associated with a significantly poor outcome (Figs 2,3, Table 3). The negative predictive factors were further individually analysed to define their impact on 5-year survival. The 5-year survival dropped from 44.4% for a tumour of ≤6 cm to 13.6% for a tumour of >6 cm. Similarly, the 5-year survival was 32.1% for a R0 resection, in contrast to 16.7% for a R1 resection. However on multivariate analysis, none of the variables were found to significantly affect outcome.
Figure 2.
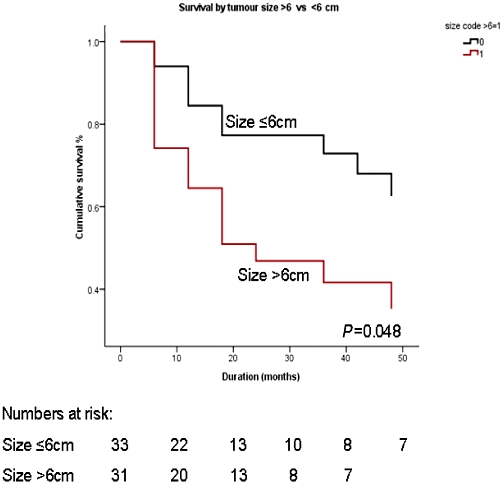
Cumulative survival curves comparing metastases size of ≤6 and >6 cm
Figure 3.
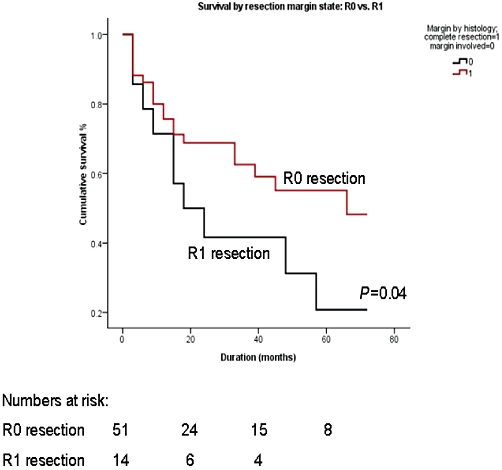
Cumulative survival curves comparing R0 and R1 resections
Table 3.
Univariate analysis of variables predicting outcome after hepatic metastasectomy
| Variables | P-value | Hazard ratio |
|---|---|---|
| Age, ≤59 vs. >59 years | 0.79 | 0.024 |
| Gender, male vs. female | 0.09 | 0.515 |
| Primary tumour, renal vs. others | 0.294 | 0.000 |
| Synchronous vs. metachronous | 1.0 | 0.000 |
| Interval between primary and liver resection, <30 vs. ≥30 months | 0.413 | 0.000 |
| Number of metastases, 1 vs. >1 | 0.201 | 0.334 |
| Size of metastases, ≤6 vs. >6 cm | 0.048a | 1.175 |
| Extrahepatic disease, present vs. absent | 0.778 | 0.020 |
| Type of resection, major vs. minor | 0.08 | 0.713 |
| Bilobar vs. unilobar metastases | 0.204 | 0.330 |
| Resected margin, positive vs. negative | 0.04a | 1.538 |
Statistically significant.
Interestingly, the presence of resectable extra-hepatic disease did not influence outcome (Table 3). The median survival for those with extra-hepatic disease was 15 months (range: 0 to 125 months) compared with 21 months for those without (range: 0 to 120 months).
Discussion
The present study analysed the long-term results of patients undergoing hepatic resection for NCNNNS metastasis over a period of 13 years from a high-volume single centre. Patients with metastatic sarcoma were excluded from the present study for two reasons. First, sarcomas are a heterogenous group of tumours of mesenchymal origin with a completely different tumour biology. Second, studies have shown promising 5-year survival rates for patients undergoing liver resection for metastatic sarcomas and have concluded that surgery should be considered if the hepatic metastasis is functionally and technically resectable.13–15
The results from the present study show that an aggressive surgical approach was associated with a significant long-term survival, resulting in one-quarter of all patients surviving up to 5 years. The 5-year survival reported in the literature ranges from 17% to 38%,17,19–27 although most of the studies included metastasis from endocrine tumours and sarcomas (Table 4). Hence direct comparison of this cohort with the published data cannot be made as a result of the heterogeneity of the study population. However, in order to have a meaningful comparison, survival for the entire population of patients who had liver resection for non-colorectal and non-neuroendocrine metastases in the study centre was calculated. This group had a median survival of 22 months and a 5-year survival of 29.03%.
Table 4.
Studies showing factors predicting poor survival after hepatic resection for non-colorectal non-neuroendocrine metastatic tumours
| Authors | Year | No. of patients | 5-year survival | Poor prognostic factors |
|---|---|---|---|---|
| Schmelzle M21 | 2010 | 44 | 20% | Lack of chemotherapy Adenocarcinoma DFI < 18 months |
| Ercolani G22 | 2009 | 134 | 40% | Tumour > 5 cm DFI < 12 months Gastrointestinal primary |
| Lehner F23 | 2009 | 242 | 28% | R0 resection Synchronous tumours |
| O'Rourke TR20 | 2007 | 102 | 38% | Tumour > 5 cm Extrahepatic nodal disease |
| Lendoire J24 | 2007 | 106 | 19% | Non-genitourinary primary Non-breast primary R1 resection Synchronous tumours |
| Adam R19 | 2006 | 1452 | 36% | Age > 60 years Non-breast primary Melanoma/squamous tumours DFI < 12 months Extrahepatic disease R2 resection Major hepatectomy |
| Reddy SK25 | 2006 | 82 | 37% | No adjuvant chemoradiotherapy Diagnosis to resection < 6 months |
| Earle26 | 2006 | 69 | 30.5% | Tumour type DFISynchronous tumours |
| Weitz J17 | 2005 | 141 | 17% | Non-genital primary R1 resection DFI < 2 years |
| Laurent C27 | 2001 | 39 | 35% | DFI < 24 months |
DFI, disease-free interval.
Patient selection is vital to any treatment and it continues to pose a challenge with respect to liver resection for NCNNNS metastasis. A number of previous studies have reported different prognostic variables associated with survival and are summarized in Table 4. The present study found a significant correlation between tumour size, resection margin status and survival on univariate analysis. A maximum diameter of a metastatic lesion of >6 cm (P = 0.048) and a positive microscopic resection margin (P = 0.04) were associated with poor survival. However, on multivariate analysis these variables were not found to be significant. This could be attributed to the small sample size. O'Rourke et al. and Ercolani et al. confirmed that a metastatic tumour size of >5 cm had a negative influence on survival.20,22 Patients with larger tumours should be considered for downsizing chemotherapy before liver resection. Unfortunately, the precise role of chemotherapy could not be ascertained in the present study as a result of the heterogenous nature of the tumour type and the different types and timing of chemotherapy. Schmelzle et al. studied the role of liver resection in 44 patients with NCNNNS metastasis and found adjuvant chemotherapy to be an independent prognostic variable associated with better survival.21 Fifty-five per cent of patients in their study received chemotherapy. However, the precise nature of the chemotherapy was not mentioned. In the study by Reddy et al., 46% of patients received pre-operative chemoradiotherapy and 51% received post-operative chemoradiotherapy. It was found that chemoradiotherapy significantly improved survival on univariate analysis.25 However, this did not reach significance on multivariate analysis.
A number of studies have found the site of the primary tumour to significantly affect survival.17,19,24 In general, metastases from genitourinary and breast tumours have a better prognosis than other primary tumours. Gastrointestinal primary tumours specifically are associated with a worse outcome. This highlights the significance of individual tumour biology. In the present study genitourinary tumours were compared with other primary tumours, renal cell carcinomas alone with other tumours and genital tumours alone with other tumours and did not find any significant correlation with survival. This could possibly be as a result of the small size of the study group or because of the confounding effect of adjuvant therapy.
The disease-free interval was another commonly described prognostic indicator in many reported series,17,19,21,22,26,27 although the duration of the interval varied in different studies. In essence, the longer the disease-free interval, the better was the prognosis. This correlates with the assertion that the tumour which metastasizes later is likely to be less aggressive than those that spread early. In the present study, analysis was performed to identify any association with survival of the time interval between primary surgery and liver resection and failed to show any correlation. As the median time interval was 30 months, this was used as the cut-off for the purpose of analysis.
An important factor that has to be taken into consideration during liver resection for metastasis is the chance of achieving microscopic negative resection margins. In the present study, a R0 resection was associated with a significantly better outcome. This is in agreement with the results reported in other series.19,23,24
The presence of resectable extrahepatic disease did not influence the outcome in the present study. This is possibly because all the patients had either a R0 or R1 resection of the extra-hepatic disease and no gross tumour was left behind. Moreover, all but one patient had extrahepatic disease within the abdominal cavity. Ng et al. reported that the presence of a resectable extrahepatic intra-abdominal metastasis carries a better prognosis compared with extra-abdominal metastasis.28 It is vital that all extrahepatic disease is sought pre-operatively in order to make a decision about resectability.
The limitations of the present study include the small patient population size and the retrospective nature of the study, although the data were collected prospectively. Also, the inclusion of a highly selected cohort of patients, who are likely to have favourable tumour biology and are unlikely to represent the general population of patients with metastasis from NCNNNS tumours. Despite the limitations, this study does provide a reasonable conclusion in this group of patients. Although many reports have been published on this subject recently, there are only a few from the centres in the UK
In conclusion, hepatic resection for metastasis from NCNNNS tumours can offer acceptable long-term survival in selected patients. To offer a chance of a cure a R0 resection must be performed. However, caution should be exercised in patients with large metastatic tumours, as it is not associated with significant long-term survival. The presence of extrahepatic disease should not be considered a contraindication for liver resection, provided the extrahepatic disease is resectable.
Conflicts of interest
None declared.
References
- 1.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 2.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 3.Schlag P, Hohenberger P, Herfarth C. Resection of liver metastases in colorectal cancer- competitive analysis of treatment results in synchronous versus metachronous metastases. Eur J Surg Oncol. 1990;16:360–365. [PubMed] [Google Scholar]
- 4.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 6.Vigano L, Ferrero A, Lo Tesoriere R, Capusotti L. Liver surgery for colorectal metastases: results after 10 years of follow-up. Long-term survivors, late recurrences and prognostic role of morbidity. Ann Surg Oncol. 2008;15:2458–2464. doi: 10.1245/s10434-008-9935-9. [DOI] [PubMed] [Google Scholar]
- 7.Sutcliffe R, Maguire D, Ramage J, Rela M, Heaton N. Management of neuroendocrine liver metastases. Am J Surg. 2004;187:39–46. doi: 10.1016/j.amjsurg.2003.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Touzios JG, Kiely JM, Pitt SC, Rilling WS, Quebbeman EJ, Wilson ST, et al. Neuroendocrine hepatic metastases: Does aggressive management improve survival? Ann Surg. 2005;241:776–783. doi: 10.1097/01.sla.0000161981.58631.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musunuru S, Chen H, Rajpal S, Stephani N, Mcdermott JC, Holen K, et al. Metastatic neuroendocrine hepatic tumours: resection improves survival. Arch Surg. 2006;141:1000–1004. doi: 10.1001/archsurg.141.10.1000. [DOI] [PubMed] [Google Scholar]
- 10.Gurusamy KS, Ramamoorthy R, Sharma D, Davidson BR. Liver resection versus other treatments for neuroendocrine tumours in patients with resectable liver metastases. Cochrane Database Syst Rev. 2009;15:CD007060. doi: 10.1002/14651858.CD007060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumours. Surg Oncol Clin North Am. 2003;12:231–242. doi: 10.1016/s1055-3207(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 12.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 13.Rehders A, Peiper M, Stoecklein NH, Alexander A, Boelke E, Knoefel WT, et al. Hepatic metastasectomy for soft tissue sarcomas: is it justified? World J Surg. 2009;33:111–117. doi: 10.1007/s00268-008-9777-4. [DOI] [PubMed] [Google Scholar]
- 14.DeMatteo RP, Shah A, Fong Y, Jarnagin WR, Blumgart LH, Brennan MF. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;234:540–547. doi: 10.1097/00000658-200110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawlik TM, Vauthey JN, Abdalla EK, Pollock RE, Ellis LM, Curley SA. Results of a single-center experience with resection and ablation for sarcoma metastatic to the liver. Arch Surg. 2006;141:537–543. doi: 10.1001/archsurg.141.6.537. [DOI] [PubMed] [Google Scholar]
- 16.Ercolani G, Grazi GL, Ravaioli M, Ramacciato G, Cescon M, Varotti G, et al. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol. 2005;12:459–456. doi: 10.1245/ASO.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Weitz J, Blumgart LH, Fong Y, Jarnagin WR, D'Angelica M, Harrison LE, et al. Partial hepatectomy for metastases from noncolorectal nonneuroendocrine carcinoma. Ann Surg. 2005;241:269–276. doi: 10.1097/01.sla.0000150244.72285.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yedibela S, Gohl J, Graz V, Pfaffenberger MK, Merkel S, Hohenberger W, et al. Changes in indication and results after resection of hepatic metastases from noncolorectal primary tumours: a single-institutional review. Ann Surg Oncol. 2005;12:778–785. doi: 10.1245/ASO.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Adam R, Chiche L, Aloia T, Elias D, Salmon R, Rivoire M, et al. Hepatic resection for noncolorectal nonneuroendocrine liver metastases: analysis of 1452 patients and development of a prognostic model. Ann Surg. 2006;244:524–535. doi: 10.1097/01.sla.0000239036.46827.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Rourke TR, Tekkis P, Yeung S, Fawcett J, Lynch S, Strong R, et al. Long-term results of liver resection for non-colorectal, non-neuroendocrine metastases. Ann surg Oncol. 2008;15:207–218. doi: 10.1245/s10434-007-9649-4. [DOI] [PubMed] [Google Scholar]
- 21.Schmelzle M, Eisenberger CF, Schulte am Esch J, Matthaei H, Krausch M, Knoefel WT. Non-colorectal, non-neuroendocrine, and non-sarcoma metastases of the liver: resection as a promising tool in the palliative management. Langenbecks Arch Surg. 2010;395:227–234. doi: 10.1007/s00423-009-0580-y. [DOI] [PubMed] [Google Scholar]
- 22.Ercolani G, Vetrone G, Grazi GL, Cescon M, Di Gioia P, Ravaioli M, et al. The role of liver surgery in the treatment of non-colorectal non-neuroendocrine metastases. Analysis of 134 resected patients. Minerva Chir. 2009;64:551–558. [PubMed] [Google Scholar]
- 23.Lehner F, Ramackers W, Bektas H, Becker T, Klempnauer J. Liver resection for non-colorectal, non-neuroendocrine liver metastases- is hepatic resection justified as part of the oncological treatment? Zentralbl Chir. 2009;134:430–436. doi: 10.1055/s-0029-1224601. [DOI] [PubMed] [Google Scholar]
- 24.Lendoire J, Moro M, Andriani O, Grondona J, Gil O, Raffin G, et al. Liver resection for non-colorectal, non-neuroendocrine metastases: analysis of a multicenter study from Argentina. HPB. 2007;9:435–439. doi: 10.1080/13651820701769701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy SK, Barbas AS, Marroquin CE, Morse MA, Kuo PC, Clary BM. Resection of noncolorectal, nonneuroendocrine liver metastases: a comparative analysis. J Am Coll Surg. 2007;204:372–382. doi: 10.1016/j.jamcollsurg.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Earle SA, Perez EA, Gutierrez JC, Sleeman D, Livingstone AS, Franceschi D, et al. Hepatectomy enables prolonged survival in select patients with isolated noncolorectal liver metastasis. J Am Coll Surg. 2006;203:436–446. doi: 10.1016/j.jamcollsurg.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 27.Laurent C, Rullier E, Feyler A, Masson B, Saric J. Resection of noncolorectal and nonneuroendocrine liver metastases: late metastases are the only chance of cure. World J Surg. 2001;25:1532–1536. doi: 10.1007/s00268-001-0164-7. [DOI] [PubMed] [Google Scholar]
- 28.Ng EH, Pollock RE, Romsdahl MM. Prognostic implications of patterns of failure for gastrointestinal leomyosarcomas. Cancer. 1992;69:1334–1341. doi: 10.1002/1097-0142(19920315)69:6<1334::aid-cncr2820690606>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]


