Abstract
String vessels are thin connective tissue strands, remnants of capillaries, with no endothelial cells; they do not carry blood flow. They occur in numerous species, particularly in the central nervous system, but can occur in any tissue where capillaries have died. String vessels are often associated with pathologies such as Alzheimer’s disease, ischemia, and irradiation, but are also found in normal human brains from preterm babies to the aged. They provide a record of the original blood vessel location, but gradually disappear after months or years. There have been numerous studies of string vessels (acellular capillaries) in the retina, because retinal vessels can be seen in great detail in whole mounts after trypsin digestion. Capillary regression occurs by apoptosis, synchronously along capillary segments, with macrophages engulfing apoptotic endothelial cells. Macrophages may cause the apoptosis, or the regression may be triggered by loss of the endothelial cell survival factor VEGF. VEGF expression is induced by hypoxia and promotes capillary growth. Cessation of blood flow eliminates the shear stress that helps maintain endothelial cell survival. Capillaries can re-grow by proliferation and migration of endothelial cells into empty basement membrane tubes, which provide a structural scaffold, replete with signaling molecules. This is a problem in tumor control, but useful for recovery from capillary loss. There is an age-related waning of VEGF expression in response to hypoxia. This causes an age-related decline in cerebral angiogenesis and results in neuronal loss. It may also contribute to the proposed age-related loss of brain reserve.
Keywords: Alzheimer’s disease, angiogenesis, basement membranes, capillary loss, intervascular strand, leukoaraiosis, retinal blood vessels, string vessels, vascular dementia
THE SCOPE OF THIS REVIEW
After an extensive literature search, only one review of this subject was found, and that was published in 1960 [1]. That review by Cammermeyer is a good connection to the early research, with 135 references, but it has been rarely cited and it is fading into history. There have, of course, been many advances in the half-century since the last review. However, the literature lacks cross-referencing, with separate pockets of research in neuropathology of aging, vascular development, irradiation, diabetic retinopathy, tumor biology, and others. One problem is that these structures have been described with a variety of names. The term “string vessels” arose in reports in the Alzheimer’s disease literature in the past two decades [2–10]. Besides string vessels, other names have included empty basement membrane tubes, acellular capillary strands, intercapillary bridges, and intervascular connective tissue strands. Ramon y Cajal [11] referred to them as connective tissue bridges between capillaries. This review will cover all articles since the last review that are involved with research on string vessels. These reports include many that investigate the causes and mechanisms of capillary loss. This review is organized under concepts, with an attempt to present them in order of historical development, where possible or appropriate. Many of the names and dates are given to highlight the historical progression.
WHAT, WHERE, AND WHEN OF STRING VESSELS
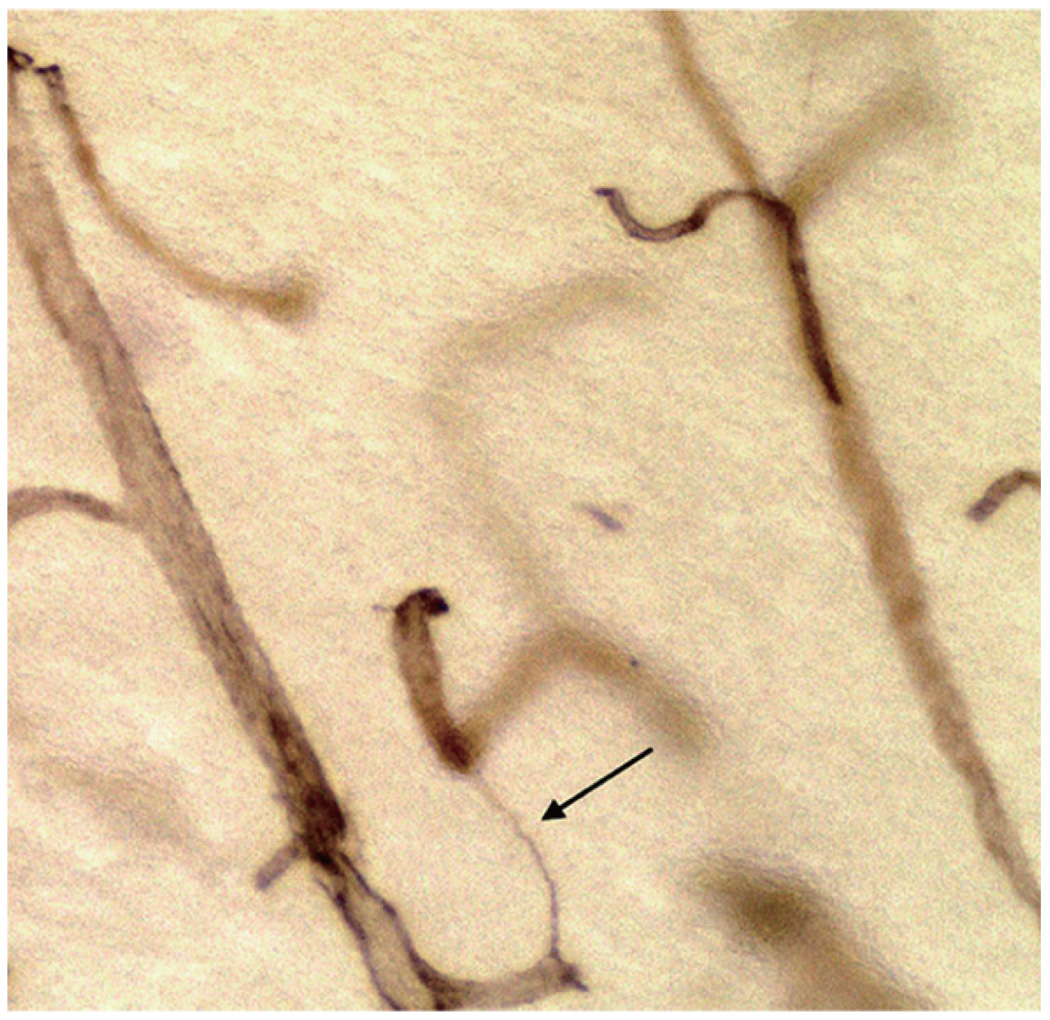
String vessels have been identified as thin connective tissue strands within the capillary network (Fig. 1). They have no endothelial cells and are essentially composed of basement membrane (BM) connective tissue components such as collagen IV, laminin, and heparin sulfate. Reports of thin connective tissue strands within the capillary network go back to Henle in 1838 [12], who performed early studies of blood vessels within the eye. In an excellent early (1899) study of the brains of humans, dogs, and sheep, Robertson [13] reported that fibers may be seen to pass over from one capillary to another. He concluded that they were connective tissue fibers.
Fig. 1.
String vessel (arrow) in the brain of a 5 year-old.
The list of species shown to have string vessels includes: monkey, dog, cat, rat, mouse, guinea pig, hamster, chinchilla, rabbit, raccoon, squirrel, and pigeon [1]. They have often been found in the capillary bed of normal brain, spinal cord, and eye of humans and other animals [1]. Although most studies of string vessels have been in tissues of the central nervous system (CNS), string vessels have also been found outside the CNS. Cammermeyer concluded that they are “rather universal.” In principle, they should be found in any tissue where the endothelial cells of a capillary have died and left behind the empty BM tube. Studies using electron microscopy have shown denuded BM tubes in the skeletal muscles of diabetic patients [14,15]. String vessels have also been found in the thymus [16], glomeruli of the kidney [17], connective tissue of the tadpole fin [18], connective tissue of the rabbit ear [19], pancreatic islets [20], thyroid [20], intestine [20], epididymal adipose tissue [20], and tumors [21].
We found string vessels in normal human brains at all ages from preterm babies to the very old [3], with especially large numbers of string vessels near birth. In 1917, Binswanger and Schaxel [22] found large numbers of them in newborns, and in Biggart’s 1936 book, Pathology of the Nervous System, they were reported to occur from childhood to old age in man [23]. Because the numbers of string vessels are fewer at older ages, they seem to gradually disappear after some months or years. String vessels in the brain have been shown to be increased in such pathologies as Alzheimer’s disease [2,4,6–10], ischemia [24,25], irradiation [26,27], and thiamine deficiency [28].
THE FORMATION OF STRING VESSELS
Regarding how they form, Ramon y Cajal [29] suggested in 1925 that string vessels may form by narrowing of the lumen, lack of blood flow, and atrophy of the vessel, leaving connective tissue fibers. Cammermeyer [1] concurred with this view, describing the process as vascular atresia. Insight into the steps leading to the formation of string vessels have been gained from early studies of direct observation of developing capillary networks and later studies of irradiated and ischemic retinal capillaries. In 1918, Clark [18] observed that the formation of new capillaries occurs with the growth of endothelial cell sprouts from existing capillaries. The sprout begins as a solid process which may have more than one leading thread. If a process encounters another sprout or endothelial cell, a union will form. A lumen will then extend through the new sprout and widen. This process of lumen formation has recently been clearly described by Kamei et al [30], who reported that lumenization occurs via vacuole formation and intercellular fusion of large vacuoles. Clark found that endothelial cell sprouts from capillaries sometimes grew out a short distance and then retracted. Other sprouts formed a lumen, but never developed a circulation, and then regressed and disappeared. He reported retraction of endothelial cells into a parent vessel after no circulation for a day or more, and wrote that “the last that can be seen is a thread so minute that it is barely visible with high power lenses.” Modern studies have made similar findings. For example, McDonald and colleagues described thin strands of BM interconnecting with the surviving vessels [21]. They considered them to be a record of the original blood vessel location [20].
Sandison [19] also described the retraction of endothelial cells into a parent vessel after no circulation for a day or more. In later studies in retinal capillaries, one of the first changes seen was the appearance of acellular capillaries [25,31], capillaries in the early stages of strand formation that have lost endothelial cells, but have not yet collapsed into a thin strand. They are slightly smaller in diameter than normal capillaries and they do not appear to carry blood. Acellular capillaries may be considered an early string vessel or a transitional form between a capillary and a string vessel. Kuwabara et al. [24] concluded that acellular capillaries underwent a metamorphosis into fibrous strands. Archer et al. [27] described acellular capillaries in the retina, as well as residual BM tubes that were fused, shrunken, or collapsed. Archer also reported that pericytes typically survived within the wall of the collapsed BM tube for some time after the loss of the endothelium [31].
STRING VESSELS CANNOT CARRY BLOOD FLOW
In his review, Cammermeyer [1] seemed to accept that string vessels were simply remnants of destroyed capillaries, but he later argued that a small subset of string vessels might be able to transport minute granules released from mast cells [32]. This seems very unlikely. More recent studies have vigorously investigated whether or not string vessels could transport blood or plasma. The evidence is that they cannot. Perhaps the best such studies have been done in acellular retinal capillaries. Nonperfusion of acellular capillaries was demonstrated with combined studies of fluorescein angiograms and retinal digest preparations in human patients by Kohner and Henkind in 1970 [33] and by de Venecia and collaborators in 1976 [34].
CAPILLARIES CANNOT REGULATE BLOOD FLOWBY OPENING AND CLOSING
The question of whether capillaries can remain closed for a period of time is important in the study of string vessels because modern studies suggest that the shutdown of blood flow would result in capillary destruction and the formation of a string vessel. The closing and then reopening (recruitment) of capillaries to regulate blood flow was first proposed by Krogh [35] in 1919. Support for this concept continued into the 1980s [36]. However, in 1994 Villringer et al. [37], using confocal laser-scanning microscopy in a closed cranial window in rat brain, found that no capillaries appeared or disappeared, and all capillaries showed perfusion within 20 seconds of fluoresein injection. Several other studies also found no reserve of non-perfused brain capillaries [38–43]. These modern findings confirm Clark’s 1918 descriptions. He found that the capillaries disappear if the circulation ceases or never starts. He concluded that a vessel retrogresses and disappears if the circulation within it ceases for a day or more.
CAPILLARY TONE
Another issue related to capillary closure is whether capillaries have tone or can constrict. In 1961, Zweifach [44] concluded that endothelial cells exhibit tone. Tilton et al. [45] found that pericytes in skeletal muscle capillaries demonstrated contraction and reduced the capillary lumens. In vitro studies by Kelley et al. [46] support the theory that pericytes are contractile and endothelial cells may also contribute to microvascular tonus. Das et al. [47] found that ATP causes retinal pericytes to contract in vitro. Tilton [48] also concluded that pericytes are contractile. However, none of these studies suggest that capillary tone could cause complete closure of the capillaries.
ENDOTHELIAL TURNOVER TIME
Endothelial turnover time is an issue related to the maintenance of capillaries. The data suggest that endothelial turnover takes a long time. In 1967, Engerman and coworkers [49] found that the retinal endothelial cell turnover time was more than 3 years; Tannock and Hayashi [50] estimated endothelial cell turnover to be in excess of 2 months; Hirst et al. [51] estimated it to be in excess of 2 years; Stewart et al. [52] estimated it to be between 0.3 and 2.8 years; Hirst and colleagues [53] estimated it to be more than 2 years. Hobson and Denekamp [54] found endothelial cell turnover to vary in different tissues, but it usually took years; skin 64, jejunum27, brain16, muscle 2.5, lung 0.9, kidney 0.8, and heart 0.7. Sharma et al. [55], using 8 days of continuous labeling, confirmed earlier studies showing very low labeling indices for capillary endothelial cells and pericytes. Archambeau and collaborators [26] concluded that the endothelial doubling time was about 6 years.
STRING VESSELS IN THE RETINAL VASCULAR NETWORK
Studies in the retinal vascular network have formed the mainstream of string vessel research (although they did not use the term “string vessel”). The retinal vascular network is particularly suited to such studies because the vessels can be seen in great detail in whole mounts of retinas after mild digestion by trypsin. This was first demonstrated by Kuwabara and Cogan in 1960 [56]. This method reveals the endothelial cells and pericytes enclosed by the BM. Large areas can be examined and the view of the capillary network is not obscured by parenchymal cells. Because the retina has the highest metabolic rate and oxygen consumption of any human tissue [57], the vascular supply is critical in the retina. Consequently, there are many opportunities to study disease conditions involving ischemia and vascular pathology.
The mainstream of string vessel research could be considered to begin with Ashton’s [58] 1953 studies of diabetic retinopathy in human subjects. He reported that the capillary bed, starved of blood, atrophies and disappears. The remnants of these degenerative vessels stained faintly, and in the final stages of obliteration there was little trace of them. In later studies [59, 60], he found that in the developing retina only a delicate strand remained after capillary loss, whereas in the mature retina the collapsed BM was more substantial. He suggested that during the development of acellular capillaries in the retina, endothelial cells could detach from their BM and slide along the capillary lumen to adjacent capillaries. As will be discussed later, this seems incorrect. Another early contributor, before the retinal digest technique, was Wolter [61], who in 1957, described bridge-like connections between blood vessels by connective tissue strands in retina and brain. In 1961 [62], he reported bridge-like intervascular fibrous strands in retinoblastoma and medulloblastoma. In 1962 [63], he suggested that they might be degenerated remnants of occluded capillaries.
When Kuwabara and Cogan [56] introduced the retinal digest technique, they reported that the intercapillary bridges had no lumina, but they thought they were not obliterated vessels. Soon after, they described acellular capillaries, both singly and in clusters, that they believed were occluded [24,64]. In further studies [25], they found that ischemia caused string vessels to begin to form at 3 to 5 days, with the loss of endothelial cells and a progressive increase in acellular capillaries. By 8 days, some acellular capillaries were reduced to thin strands, and at 40 days the strands were still there [25]. They noted [65] that the BM of the capillary is particularly thick in retinal capillaries. Cogan [66] commented that with age the capillary BM becomes progressively thicker, and when capillaries first lose their endothelial cells the residual BM often shows accordionlike pleating. They noted that during regression many vessels were reduced to threadlike remnants and then disappeared [67]. In their final article [68], they noted that cerebral capillaries also have a thick BM.
From 1964 to 1968, Kornzweig et al. [69–71] also published studies using Kuwabara’s retinal digest technique. They identified intercapillary strands in human retina, and found them to be increased in macular degeneration, diabetes, and chronic glaucoma. Henkind and DeOliveira [72] found that during retinal vascular development, the capillary-free zone around arterioles develops by atrophy and loss of capillaries, leaving vascular strands. Glatt and Henkind [73] noted an age-related increase in thick acellular capillaries, although they were always much rarer than thin strands. In 1978, Folkman’s group [74] found that during the regression of vessels in neovascularized rabbit cornea, there is endothelial cell damage, platelet adhesion, blood stasis, and removal of vascular debris by macrophages. Similarly, Wang and colleagues [75] found capillary remnants with macrophages attached to them during regression of a temporary vascular network of the developing lens.
In the retinas of diabetic rats, Schroder et al. [76] found increased numbers of activated monocytes and granulocytes that adhered to the endothelium, mainly in the venules. This resulted in vascular occlusions with subsequent endothelial cell vacuolation and capillary loss distal to the occlusions. In 1994, Lang and collaborators [77] reported that during developmental regression of the pupillary membrane, capillary regression occurred by apoptosis, synchronously along capillary segments from one vascular junction to the next. Macrophage engulfment of apoptotic endothelial cells was a prominent feature of this regression, and the macrophages were suspected of eliciting the apoptotic cell death. Alternatively, the regression may have been triggered by downregulation of vascular endothelial growth factor (VEGF). They suggested that the synchronous endothelial cell death in a capillary segment is triggered by cessation of blood flow in that segment and the blockage of blood flow may be caused by macrophage-induced endothelial cell death and consequent lumen closure. Los [78] observed vascular remnants and ghost vessels in the vitreous of the eye. This was one of the few articles to reference Cammermeyer’s review.
In a series of articles from 1995 to 2007, Kern, Adamis, and colleagues investigated vascular pathology in diabetic retinopathy. They noted many acellular capillaries [79] and commented that endothelial cell death occurs early in diabetic retinopathy and may prematurely exhaust the replicative potential [80]. They saw an increase in adherent leukocytes in retinal vessels, particularly in the venules and capillaries, and this was associated with endothelial cell damage and death [81]. In mice deficient in CD18 and ICAM-1 genes, leukocyte adhesion in retinal vessels was inhibited. This resulted in decreased endothelial cell injury, blood–retinal barrier breakdown, and fewer acellular capillaries [82]. In diabetic retinopathy in rats, there was an increase in adherent leukocytes in retinal vessels [83]. In experimental retinal ischemia, retinal neuron loss preceded capillary loss [84]. At 2 days there was neuronal loss and an increase in apoptotic capillary endothelial cells, but no increase in acellular capillaries. At 7 days, there was a significant increase in acellular capillaries. There was also upregulation of TNF-α and ICAM-1 at 2 days and 7 days.
Blocking the elevated leukocyte adhesion in capillaries in diabetic retinopathy reduced the numbers of acellular capillaries [85]. This approach might possibly be effective in reducing capillary loss in vascular dementia in any cases that might have a component of inflammation that leads to elevated leukocyte adhesion in brain capillaries. They reported that CD34+ endothelial precursor cells (EPCs) integrate into damaged vessels [86]. EPCs from diabetics were found to be impaired in this function. Perhaps EPCs are defective in other conditions such as aging or AD and this defective condition might be amenable to modulation or improvement. The aims of such therapy would be to increase mobilization of EPCs from the bone marrow, increase circulating EPCs, increase migration of EPCs into areas of ischemia, increase incorporation of EPCs into capillaries, and increase differentiation of EPCs into endothelial cells.
In 2002, Friedlander’s group reported that bone marrow-derived EPCs rescued and maintained normal retinal vasculature in a mouse mutant (rd/rd) whose vessels ordinarily degenerate with age [87]. The EPCs migrated to activated astrocytes which formed the preexisting astrocytic template for the radial pattern of retinal vessels, spreading from the optic disc. In further studies [88], they found that blocking R-cadherin, a cell adhesion molecule involved in the targeting of endothelial cells to astrocytes, prevented targeting of stem cells to the developing retinal vasculature.
In 2009, Ye et al. [89] studied capillary loss in genetic diseases, such as Norrie disease, which causes retinal hypovascularization and vision loss. The rods and cones (neurons) remain largely intact but unable to transmit signals in their hypoxic milieu. However, their function can be revived when adequate oxygenation is provided. This illustration of revival of neuronal function after chronic hypoxia in the retina is the first solid evidence that some degree of rescue of neuronal function may be possible in the brain after extended periods of hypoperfusion. In Norrie disease, hemorrhages are common, affecting new vessels, but not established vessels. There is incomplete pericyte coverage of capillaries and veins, but arterial coverage with smooth muscle cells is minimally affected.
STRING VESSELS IN THE BRAIN
Studies of string vessels in the brain have had a long but patchy history going back to Henle [12], Robertson [13], and Ramon y Cajal [11]. In the two decades after Cammermeyer’s review, in 1960 [1], there were few such studies and they appeared to be unaware of the studies in retina. Pessacq and Reissenweber [90] and Bar and Budi Santoso [91] observed capillary strands. Guseo and Gallyas [92] believed there were three types of intercapillary bridges; i) collapsed capillaries, ii) protrusions of endothelial cells surrounded by BM, and iii) pericyte processes. They believed that string vessels might participate in capillary growth in newborns, with pericyte processes serving as trails for endothelial cell protrusions. In adults, they thought they might be involved in regressive processes in the brain as a consequence of a disease or aging. In 1988, Leibnitz and Bar [93] studied string vessels in rat, mouse, cat, tree shrew, and rhesus monkey. They believed that string vessels were cells, a subtype of pericytes. Likely, they were seeing actual pericytes still present in BMs from destroyed capillaries. In fact, they noted a striking similarity between pericytes and string vessels. They believed that intercapillary bridges were involved in forming new capillaries.
Beginning in the 1990s, there were discoveries of string vessels in AD, and some suggested that they were increased [2,4,6–10]. We reported increased string vessels in AD compared to age-matchedcontrols [4]. More recently, we found increased numbers of string vessels in the hippocampus in mesial temporal sclerosis [94]. In contrast to the findings in AD and mesial temporal sclerosis, we found string vessels to be decreased in brain white matter in subjects with leukoaraiosis (LA) (manuscript in preparation). In the deep white matter, the site of most LA lesions, there was an age-related decline in string vessels. We have also found a decreased density of intact capillaries in the deep white matter [95, 96]. However, the decrease in string vessels was greater than the decrease in capillaries. This may be because early loss of capillaries in LA is followed in a few years by the disappearance of the string vessels. Most of the strings in LA lesions may be gone by the time of death, usually old age. Our interpretation is that LA begins in late middle age with the gradual loss of capillaries in the deep white matter and an attendant increase in string vessels. Over the years, there would be fewer and fewer capillaries left to die and form string vessels. Meanwhile, the string vessels would be gradually disappearing, so that by the time the subject dies there are few capillaries and few string vessels.
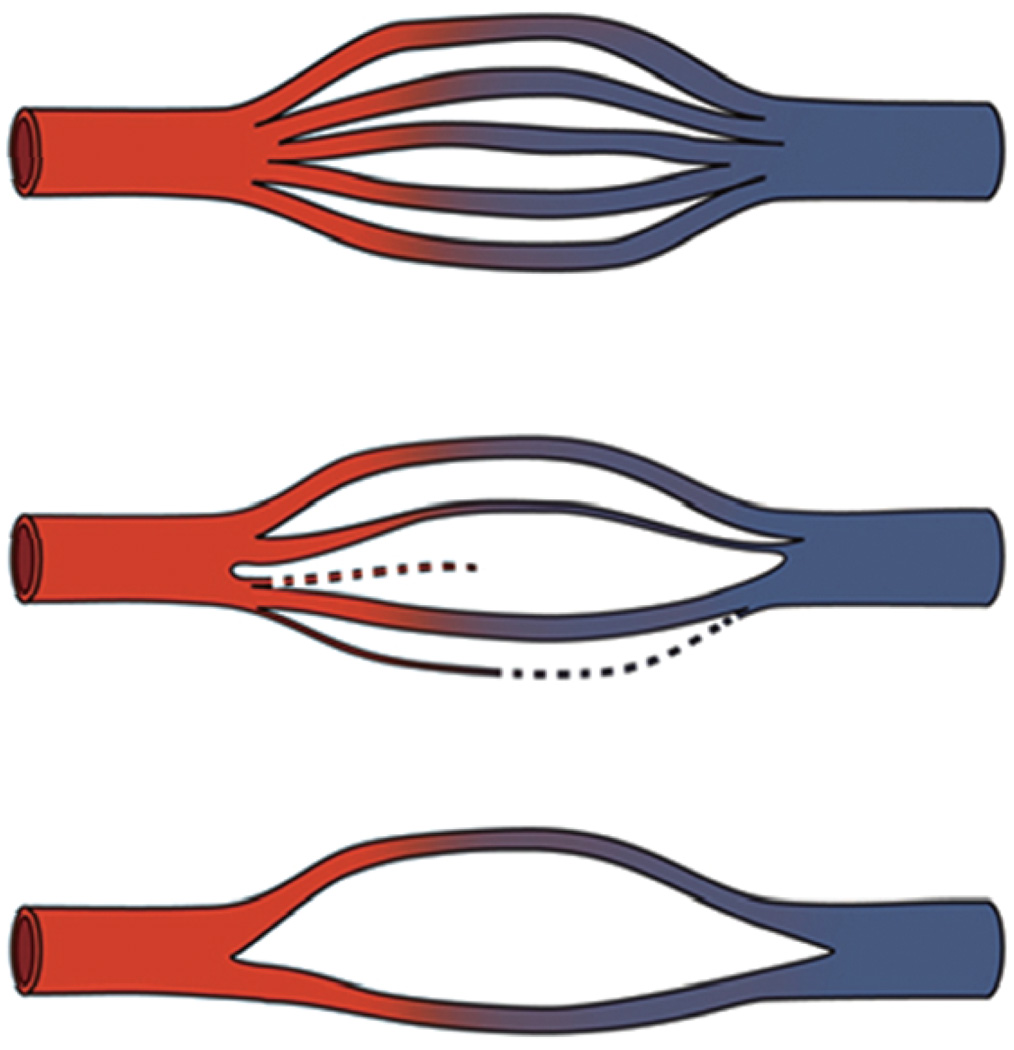
Regarding the extent of capillary loss, it is interesting to note that in LA there appears to be a minimum value for vascular density in the areas of spongeosis: vascular density appears to not go below a certain level. It might be that since there are no shunts in the brain, a certain minimum number of capillaries must remain functioning to carry blood from the arterioles to the veins (see Fig. 2),even through spongeotic white matter lesions. In this regard, it appears that LA lesions never develop into cavitating lesions, no matter how severe the LA. Perhaps the last remaining capillaries prevent further degeneration of the deep white matter to a final phase that would be characterized by cavitation.
Fig. 2.
Schematic drawing of string vessel development. Over time, a capillary network can lose capillaries, but it appears that some remain to carry the blood from the arterial side to the veins.
STRING VESSELS AND IRRADIATION
In one early study in 1933, Lyman et al. [97] investigated the effects of radiation on the brains of Pavlov’s dogs. He reported that connective tissue fibrils ran from one capillary to another. In 1966, Phillips [98] found that three months after irradiation of rat lung, there was loss of endothelial lining of the alveolar capillaries. Mast cells adhered to the BM and occluded the capillary lumen. Buds of new capillaries grew into the previous capillary space. The endothelial cells developed a lumen and a separate BM. In the 1980s, Irvine et al. [99,100] found that irradiated monkey retinas showed capillary closure with loss of capillary endothelial cells and then pericytes, leaving thin, acellular strands. There was also re-canalization of some vessels and lamellar reduplications of the BMs [100]. Archer and colleagues [27,31] reported the development of numerous string vessels in the retina after irradiation. Focal capillary closure was among the first changes found after irradiation. Archambeau et al. [26, 101] found that fifteen months after irradiation there was loss of endothelial cells from capillaries and formation of string vessels. In support of the idea that radiation damages capillaries, we [102, 103] and others have shown that the capillary densityis decreased in the brain after irradiation, and we suggested that brain irradiation could be used in animals as a model of vascular dementia [103, 104].
HEAVY METAL POISONING CAUSES BRAIN VASCULAR DAMAGE
Several studies of lead poisoning found vascular damage and string vessels in the brains of newborns. In 1967, Gabbiani and collaborators [105] found that the injection of lead, cadmium, mercury, indium, and thallium caused hemorrhages in the brains of newborn rats and rabbits, particularly in the cerebellum. Tang et al. [106] reported that lead caused segmental thinning and occlusion of capillaries, producing a “string collapse” effect. In 1968, Rosenblum and Johnson [107] showed that lead poisoning in mouse pups caused a 10-fold increase in intervascular strands and they were thought to represent atretic vessels from vascular regression. There was also vascular hyperpermeability, most prominently in the cerebellum, with edema and brain swelling. Thomas et al. [108] found perivascular edema, hemorrhages, endothelial cell degeneration, platelet thrombi, and collapsed, bloodless capillaries. In studies of lead poisoning in children and rat pups, in 1974, Clasen and coworkers [109] found petechial hemorrhages and intervascular strands in the brain. The strands were interpreted as arrested development of vessels. Vistica and Ahrens [110] found a few intervascular strands. Zook et al. [111] found edema, hemorrhages, endothelial cell damage, necrotic capillaries, thrombi, and intravascular strands (which were not in excess of those in controls). In 1985, Press [112] found hemorrhages, blood-brain barrier (BBB) breakdown, and degenerating endothelial cells, some of which were engulfed by macrophages. There are some interesting similarities with Norrie disease [89] which causes retinal hypovascularization, ischemia, and retinal hemorrhages. Aside from the retinal defects, there are also hemorrhages in the cerebellum, but not the cerebrum. This echoes the fact that lead poisoning has more severe effects on the developing vasculature of the cerebellum than the cerebrum. In this regard, it is significant that both the retinal and cerebellar vasculatures develop quite late.
BASEMENT MEMBRANE TUBES FROM NERVE REMNANTS
Closely similar to vascular BM tubes are nerve BM tubes. In 1974, Vracko [113] found that axons of peripheral nerves regrew along the BM tracts of old nerves. In 1983, Ide et al. [114] found that regenerating axons always grew within BM tubes left after destruction of mouse sciatic nerves. No axons grew outside of the empty tubes and the tubes were collapsed and pleated. In 1990, Giannini and Dyck [115] studied Schwann cell BM tubes and found that they showed deterioration at 4 weeks. Ochi et al. [116] found that by 8 weeks nerve regeneration was reduced. Gulati [117] found BM tubes to be remarkably durable, remaining for several months after macrophages had cleared the degenerated axons. Nerve regeneration was very good at 6 weeks and 3 months, significantly reduced at 6 months, and greatly reduced at 12 months. In 2002, Nguyen et al. [118] observed that Schwann cell BM tubes provided a structural and chemical pathway for re-innervation back to the original motor end-plates on muscle.
INVASION AND RE-CANALIZATION OF EMPTY BASEMENT MEMBRANE TUBES
Vracko, in 1972 [119] and 1974 [113], provided several important in sights into the nature of BM tubes. After infarction in skeletal muscle, some capillaries were reconstituted by proliferation and migration of endothelial cells along the inside of remaining tubes of vascular BM. Acellular BM tubes were found for up to 3 weeks after injury. A new layer of BM appeared along the portions of the plasma membrane of the invading endothelial cells, where they were not in close apposition with the old BM. For a time, there were two layers of BM. After multiple injuries there may be multiple concentric layers of BM, as is sometimes seen in the thickened BMs of capillaries in diabetes. Vracko [113] concluded that the BM maintains the special plan of the tissue aided by the polarity of the membrane and its specificity for cell types. It provides a physical substrate for growth and orientation. The BM may also affect the induction and restriction of cell proliferation. Cells repopulating a BM tube begin to multiply and continue to do so until the cell-free BM surface is covered. In injuries resulting in thickened BMs, it is not due to a thick homogenous widening of a single BM. Instead, the BM is thickened by sequential deposition of normally thick layers of BM by the new cell generations. However, where the plasma membrane is immediately apposed to the old BM, a new BM is not formed. The redundant old BM may be removed by fibroblast-like cells several weeks or months after the removal of cell debris is complete.
In 1975,Hamilton et al. [120] showedre-canalization of some BM tubes after ischemic damage to the retina of monkeys. Irvine and Wood [100] also reported re-canalization of BM tubes in the retina, and lamellar reduplications of the BMs. In 1989, Engerman [121] observed acellular retinal capillaries with ingrowths of glial processes. Archer [31] found that some residual BM tubes were invaded by glial cells, while others were re-canalized by new capillaries with a second BM. In 2006, Mancuso and colleagues [122] reported that inhibition of VEGF induced a reduction in tumor vessels, leaving empty sleeves of BM and associated pericytes. A high concentration of VEGF was bound to the BM tubes. After withdrawal of the VEGF inhibition, endothelial sprouts grew abundantly into the BM tubes, and by 7 days the tumors were fully re-vascularized. The BM tubes were reused. This process is very similar to axonal regrowth where tubes of Schwann cell BM, left after axon degeneration, provide a structural and chemical pathway for re-innervation back to the original motor endplates on muscle. Those BM tubes were found to be remarkably durable, remaining for several months after macrophages have cleared the degenerated axons.
PERICYTES IN CAPILLARY STABILITY
Although most of the vascular network has considerable stability throughout a lifetime, some capillaries disappear. Clark [18] directly observed capillary formation, regression, and remnants. In the modern era, studies of capillary formation and loss have moved on to the roles of pericytes, apoptosis, and molecular signals among various cells and the BM.
Pericytes have an important role in stabilizing capillaries. As early as 1963, Kuwabara and Cogan [65] noted that the presence of mural cells (pericytes) appeared to inhibit angiogenesis. In 1998, Benjamin and collaborators [123] reported that during retinal vascular development, there is a delay of several days between the formation of endothelial tubes and pericyte coverage. VEGF accelerated pericyte coating of the new vessels. In 2004, Baluk et al. [124] showed that after VEGF expression in airway epithelium, endothelial cells sprouted from venules in one day and sprouts were abundant at 3 to 5 days. At 7 days they had a pericyte coat and a thin and ragged BM. Surprisingly, a few pericytes bridged adjacent capillaries via long cellular processes. Within 3 days of VEGF withdrawal, half of the new vessels were gone. There was early stasis of blood flow, luminal occlusion, influx of inflammatory cells, and retraction and apoptosis of endothelial cells and pericytes. This left many empty sleeves of BM. Saint-Geniez and D’Amore [57] reported that endothelial cells recruit pericytes by secreting platelet derived growth factor (PDGF) that stimulates the proliferation of mesenchymal cells and their chemotaxis toward the endothelial cells. Those mesenchymal cells differentiated into pericytes.
In 2005, Armulik et al. [125] reported that pericytes stabilize nascent vasculature and pericyte coverage is highest in the CNS where they are thought to contribute to the formation of the BBB. Stenman and collaborators [126] found that Wnt signaling induced BBB maturation, and Daneman et al. [127] reported that the binding of Wnt ligands (expressed by neural progenitors) to Frizzled/LRP receptors on endothelial cells stabilizes β-catenin which translocates to the nucleus and modulates endothelial cell gene expression. These genes affect cell proliferation, differentiation, adhesion, morphogenesis, and BBB formation. In β-catenin mutants, the glut-1 transporter, a hallmark of adult BBB, was not expressed in CNS endothelial cells. Also, β-catenin itself is a component of endothelial cell adherens junctions. It was concluded by von Tell and colleagues [128] that it is likely that pericytes influence vessel stability by matrix deposition and promotion of endothelial cell differentiation and quiescence. They concluded that pericytes likely signal endothelial cells via the specialized invaginations named peg-socket contacts. Pericytes are recruited to developing vessels by PDGFR-β which is expressed by endothelial cells [128]. In the absence of pericytes, endothelial cells fail to form mature inter-endothelial junctions, and the capillaries leak. Furthermore, the luminal endothelial surface becomes highly irregular, with multiple processes and folds that can impair blood flow [128].
BASEMENT MEMBRANE COMPLEXITY
In studies of the BM, Folkman’s group [129] noted that during steroid-induced inhibition of angiogenesis in chick chorioallantoic membrane, there was BM dissolution accompanying the loss of the newly formed capillaries. Denekamp [130] noted that the formation of a capillary sprout involves localized dissolution of the BM, migration of the endothelial cells towards the angiogenic stimulus, and endothelial cell proliferation. In 2004, Inai et al. [131] described BMs after capillary loss as providing a ghost-like record and a potential scaffold for vessel regrowth. They found that vessel closure preceded endothelial cell degeneration, and they concluded that the loss of blood flow would eliminate the shear stress that helps maintain endothelial cell survival. Kalluri [132], in his review of BMs, described them as providing structural support, dividing tissues into compartments, and regulating cell behavior. He found that the vascular BM acts as microenvironment sensors for endothelial cells and pericytes. BMs are a highly crosslinked and insoluble material made up of about 50 proteins of which about 50%is collagen, especially type IV collagen. Other significant components include laminin, haparan-sulphate proteoglycans, and nidogen/entactin. The molecular composition of the BM is unique for each tissue, conferring tissue specificity. This specificity is achieved by the expression of different isoforms of these components. Type IV collagen and laminin each self assemble into sheet-like structures. Laminin polymers form on the cell surface and type IV collagen polymers associate with the laminin polymers via facilitation by nidogen/entactin. Type IV collagen is normally found only in the BM, but in pathogenesis it is found in fibrosis. The BM, in its assembled form, exposes various constituents to the endothelial cells, whereas during assembly or disassembly, endothelial cells can be exposed to different domains of these molecules, thus receiving different signals. Vascular BM components mediate angiogenesis with both pro- and anti-angiogenic factors. During degradation by matrix metalloproteinases, the BM releases growth factors such as VEGF, basic fibroblast growth factor (bFGF), and platelet-derived growth factor (PDGF).
ENDOTHELIAL CELL DESTRUCTION
Endothelial cell destruction during capillary loss occurs in specific ways. In studies of cyclic angiogenesis and blood vessel regression in the ovary, in 1996, Modlich et al. [133] found that capillary endothelial cells detached from the basement membrane singly or in sheets, and most endothelial cells became detached before undergoing apoptosis. Bartel and Lametschwandtner [134], studying regression of capillaries in tadpole epithelium, found that endothelial cells bulged into the lumen, almost plugging the vessel. Many endothelial cells were apoptotic. Endothelial cells and fragments were shed from the basement membrane and, along with erythrocytes, were found to occlude some capillaries. Sometimes macrophages penetrated the basement membrane and engulfed the endothelial cells. In 2003, Ishida et al. [135] reported that activated leukocytes prune the retinal vasculature during normal development and obliterate it in disease. T-cells and other leukocytes adhered to endothelial cells via leukocyte integrin CD18 interaction with endothelial cell ICAM-1. This caused leukocyte activation and FasL-mediated apoptosis of the endothelial cells in capillary segments. Gariano and Gardner [136] also found Fas ligand-mediated endothelial cell apoptosis during capillary loss.
VEGF AND ENDOTHELIAL CELL SURVIVAL
VEGF is the key molecule in capillary survival. VEGF expression is induced in response to hypoxia. This occurs via binding of the transcription factor hypoxia-inducible factor-1 (HIF-1) to the hypoxia responsive element (HRE), which is present on more than 50 target genes, including VEGF [57]. In 1995, Stone [137] reported that VEGF acts through paracrine signaling via the VEGF receptor (flk-1) on endothelial cells. Alon et al. [138] caused obliteration of newly formed capillaries by hyperoxygenation. This resulted in downregulation of VEGF by neuroglial cells with resultant apoptosis of endothelial cells, selectively in the new capillaries. These studies demonstrate the important principle that oxygen levels regulate vascular growth or regression via the endothelial cell survival factor VEGF. Arden [139] concluded that anoxic up-regulation of VEGF is the primary cause of diabetic retinopathy. Saint-Geniez and D’Amore [57] found that retinal astrocytes respond to hypoxia by secreting VEGF, which induces vascular proliferation and vascular permeability. Gariano and Gardner [136] reported that various VEGF receptors mediate discrete functions, such as proliferation, migration, guidance, survival, and permeability, and the same receptor can elicit different effects in endothelial cells depending on the location in the growing vessel. In the developing retina, they found that fine endothelial filipodia on vascular sprouts often extended along processes of an underlying network of astrocytes that secreted hypoxia-induced VEGF.
Adams and Alitalo [140] described how vascular sprouts contain tip and stalk endothelial cells. Endothelial cell tips are very similar to axonal growth cones. They noted that many signaling pathways are shared between the nervous system and the vasculature. For example, VEGFs are important growth factors for both endothelial cells and neural cells and semaphorins control both axon guidance and vascular patterning. Tip cells avoid fusing with incompatible parts of the vasculature that would result in arteriovenous shunts or connection with the lymphatic system. They reported that the lymphatic system originates from the embryonic veins, and terminal lymphatics lack a continuous BM, so they never contribute to the formation of string vessels.
Also important among the factors affecting capillary growth and regression are the Tie-2 receptors on endothelial cells and their angiopoietin ligands [141]. Tie signaling is modulated by crosstalk with different VEGFs and is involved in the stabilization and destabilization of existing vessels. In normoxic conditions, angiopoietin-1 is constitutively expressed by pericytes and occupies and activates the Tie-2 receptors on endothelial cells. This maintains the structural integrity of the capillaries [142]. Hypoxia causes the induction of angiopoietin-2 in endothelial cells and pericytes. Angiopoietin-2 then occupies the Tie-2 receptors, preventing angiopoietin-1 activation and causing the pericytes to move away from the capillaries and destabilize them. In the presence of VEGF, angiogenesis will be initiated, but in the absence of VEGF, the capillaries will undergo apoptotic regression [143].
FLUID SHEAR STRESS AND ENDOTHELIAL CELL SURVIVAL
Shear stress is a mechanical force caused by blood flow. It acts parallel to the vessel wall, creating a frictional shear force on the surface of the endothelium. In recent years it has become clear that shear stress stimulates endothelial cells and helps maintain capillaries. In 1996, an important observation was made that blood stasis can trigger synchronous apoptosis in all of the endothelial cells in a capillary segment [144]. This process was initiated when macrophage-mediated endothelial cell apoptosis caused the death of an endothelial cell and caused it to project into the lumen and restricted the blood flow. It was subsequently reported that blockage of capillary flow prevented endothelial cell access to the survival factor VEGF, whereas hypoxia was not a significant factor [145]. Dimmeler et al. [146] observed that fluid shear stress alters the morphology and function of endothelial cells and inhibits growth factor withdrawal-induced apoptosis of endothelial cells. Shear stress was found to accelerate endothelial wound closure by cell spreading and migration, but not by proliferation [147]. Endothelial cells lost their elongated orientation in low or disturbed flow patterns, and the intimal wall was more easily denuded. In a review, Resnick and collaborators [148] reported that reduced blood flow is correlated with endothelial cell loss and apoptosis; VCAM-1 is down-regulated in vessels undergoing normal blood flow; and blood flow modulates vascular tone. Vessel closure was noted to precede endothelial cell degeneration[131], and the authors concluded that the loss of blood flow would eliminate the shear stress that helps maintain endothelial cell survival. In 2005, Tzima et al. [149] found that blood flow caused shear stress on endothelial cells and this stress was transmitted through the cell cytoskeleton to cell-cell and cell-matrix adhesions. Platelet endothelial cell adhesion molecule (PECAM-1), a cell-cell junction molecule, was found to be a direct transducer of the mechanical force. PECAM-1 was also involved in the alignment of endothelial cells in the direction of blood flow. The alignment of endothelial cells in the direction of blood flow was influenced by localized α4 integrin phosphorylation which informs the endothelial cell about the direction of blood flow [150]. In 2009, Chen and Tzima [151] reported that PECAM-1 is involved in flow-induced monocyte adhesion to endothelium. Adamo and coworkers [152] reported on another effect of shear stress. It promotes embryonic hematopoiesis through increasing the expression of Runx1 and nitric oxide (NO) production. Interestingly, VEGF is also known to induce NO production [153]. In addition, it was found that blood flow induces an increase in the numbers of hematopoietic stem cells (HSCs) through NO production in the endothelium and HSCs in zebrafish [153]. The functionally relevant isoform was neuronal NO synthase (nnos, nos1), which is highly related to endothelial nos (enos, nos3). There was no effect by nos2 (inducible nos, inos). They also found that NO may initiate arterial specification and maintain arterial identity as an ongoing flow-dependent process.
CONCLUSIONS
String vessels have been shown to begin as empty BM tubes, after endothelial cell destruction. Several names for these structures appear in the literature, so an attempt at unifying the terminology may be useful. When the capillary first loses its endothelial cells, it becomes an empty BM tube. This clear and descriptive name may be preferable to acellular capillary, because such tubes no longer carry blood. The empty BM tube soon collapses into a string of collagen, leaving a trace of the destroyed capillary. At this stage it could well be described as a collagen strand, but the name string vessel may perhaps be better because it adds the information that this collagen strand is the remnant of a vessel. One caveat with the term empty BM tube is that destroyed nerves also leave empty BM tubes. For this reason, the name string vessel might be a good general term for the structures left after capillary destruction. The term empty BM tube still has a useful place; it is a good description of a subset of string vessels.
Many of the causes and mechanisms of capillary loss, particularly in the CNS, have been detailed, giving insight into the life and death of capillaries. One key element of string vessels is that they provide evidence of capillary loss, evidence that can be useful in understanding some aspects of brain and retina pathology. Another feature of string vessels is their potential for facilitating the reconstitution of the destroyed capillary. However, string vessels eventually degrade and disappear. Until they are degraded, they can provide a structural scaffold, replete with signaling molecules. This can be a problem in tumor control, but it might be useful for recovery from capillary loss in CNS pathology. Thus, string vessels have the potential to be involved in therapeutic manipulations in tumor control and CNS pathology.
In regards to AD in particular and neuropathology in general, string vessels illustrate when and where there is vascular destruction at the capillary level. The detailed mechanisms that lead to capillary loss in various neurodegenerative processes varies with each particular disease, but we may be able to gain some insights into these processes in examining the final common pathway resulting in capillary loss. For example, capillary loss and string vessels are most prominent in the deep white matter in the early phases of leukoaraiosis and spread outward toward the cortex. Later, string vessels are rarer in the deep white matter. This gives us clues about the development of this pathology. Detailed studies of string vessels in AD in different brain regions and at different times may also provide additional information about this disease process.
One aspect of capillary loss that may be of particular interest in AD and other neurodegenerative diseases is that of a possible age-related diminishment of the capacity for angiogenesis in the brain. It has been reported that the responsiveness of HIF-1 to hypoxia wanes with age [154,155]. There is also an age-dependent defect in VEGF expression associated with reduced HIF-1 activity [156]. These types of defects may be responsible for the reported age-related decline in the capacity for cerebral angiogenesis [157,158]. Furthermore, age-related changes in HIF-1 function are associated with neuronal loss in rat brain [159]. Thus, there may be an age-related failure of recovery from hypoxia-induced bouts of capillary loss in AD, leukoaraiosis, other neurodegenerative diseases, or simply aging. Such a failure of capillary regeneration could lead to neuron loss or dysfunction and it may be an important part of the so-called loss of brain reserve that has been proposed to occur with aging.
ACKNOWLEDGMENTS
I thank Dixon Moody, Venkata Challa, and Clara Thore for scientific contributions during our studies of string vessels and Patricia Wood and Carolyn Cox for technical assistance. This work was supported by NIH grants NS20618, NS36780, and CA113321.
Footnotes
The author’s disclosure is available online (http://www.j-alz.com/disclosures/view.php?id=395).
REFERENCES
- 1.Cammermeyer J. A comparative study of intervascular connective tissue strands in the central nervous system. J Comp Neurol. 1960;114:189–208. doi: 10.1002/cne.901140206. [DOI] [PubMed] [Google Scholar]
- 2.Buée L, Hof PR, Bouras C, Delacourte A, Perl DP, Morrison JH, Fillit HM. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol. 1994;87:469–480. doi: 10.1007/BF00294173. [DOI] [PubMed] [Google Scholar]
- 3.Challa VR, Thore CR, Moody DM, Brown WR, Anstrom JA. A three-dimensional study of brain string vessels using celloidin sections stained with anti-collagen antibodies. J Neurol Sci. 2002;203–204:165–167. doi: 10.1016/s0022-510x(02)00284-8. [DOI] [PubMed] [Google Scholar]
- 4.Challa VR, Thore CR, Moody DM, Anstrom JA, Brown WR. Increase of white matter string vessels in Alzheimer’s disease. J Alzheimers Dis. 2004;6:379–383. doi: 10.3233/jad-2004-6404. [DOI] [PubMed] [Google Scholar]
- 5.Kalaria RN. The blood-brain barrier and cerebral microcirculation in Alzheimer disease. Cerebrovasc Brain Metab Rev. 1992;4:226–260. [PubMed] [Google Scholar]
- 6.Kalaria RN, Kroon SN. Expression of leukocyte antigen CD34 by brain capillaries in Alzheimer’s disease and neurologically normal subjects. Acta Neuropathol. 1992;84:606–612. doi: 10.1007/BF00227737. [DOI] [PubMed] [Google Scholar]
- 7.Kalaria RN, Hedera P. Differential degeneration of the cerebral microvasculature in Alzheimer’s disease. Neuroreport. 1995;6:477–480. doi: 10.1097/00001756-199502000-00018. [DOI] [PubMed] [Google Scholar]
- 8.McGeer PL, Zhu SG, Dedhar S. Immunostaining of human brain capillaries by antibodies to very late antigens. J Neuroimmunol. 1990;26:213–218. doi: 10.1016/0165-5728(90)90003-6. [DOI] [PubMed] [Google Scholar]
- 9.Perlmutter LS, Chui HC, Saperia D, Athanikar J. Microangiopathy and the colocalization of heparan sulfate proteoglycan with amyloid in senile plaques of Alzheimer’s disease. Brain Res. 1990;508:13–19. doi: 10.1016/0006-8993(90)91111-s. [DOI] [PubMed] [Google Scholar]
- 10.Perlmutter LS, Chui HC. Microangiopathy, the vascular basement membrane and Alzheimer’s disease: a review. Brain Res Bull. 1990;24:677–686. doi: 10.1016/0361-9230(90)90007-m. [DOI] [PubMed] [Google Scholar]
- 11.Ramon y, Cajal S. Histology of the nervous system of man and vertebrates. New York: Translated from the French by Swanson N, Swanson L. Oxford University Press; 1995. [Google Scholar]
- 12.Henle FGJ. Ueber die Ausbreitung des Epithelium im menschlichen Korper. Archiv für Anatomie, Physiologie und wissenschaftliche Medicin [Mullers] 1838:103–128. [Google Scholar]
- 13.Robertson WF. On a new method of obtaining a black reaction in certain tissue elements of the central nervous system (platinum method) Scottish Med Surg J. 1899;4:23–30. [Google Scholar]
- 14.Tilton RG, Hoffmann PL, Kilo C, Williamson JR. Pericyte degeneration and basement membrane thickening in skeletal muscle capillaries of human diabetics. Diabetes. 1981;30:326–334. doi: 10.2337/diab.30.4.326. [DOI] [PubMed] [Google Scholar]
- 15.Tilton RG, Faller AM, Burkhardt JK, Hoffmann PL, Kilo C, Williamson JR. Pericyte degeneration and acellular capillaries are increased in the feet of human diabetic patients. Diabetologia. 1985;28:895–900. doi: 10.1007/BF00703132. [DOI] [PubMed] [Google Scholar]
- 16.Plenk H. Uber argyrophilen Fasern (Gitterfasern) und ihre Bildungszellen. Erg Anat. 1927;27:302–412. [Google Scholar]
- 17.Bohle A, Herfarth C. Zur Frage eines intercapillaren Bindegewebes im Glomerulum der Niere des Menschen. Vir-chows Arch Pathol Anat. 1958;331:573–590. doi: 10.1007/BF00955228. [DOI] [PubMed] [Google Scholar]
- 18.Clark ER. Studies on the growth of blood vessels in the tail of the frog larva by observation and experiment on the living animal. Am J Anat. 1918;23:37–88. [Google Scholar]
- 19.Sandison JC. Observations on the growth of blood vessels as seen in the transparent chamber introduced into the rabbit’s ear. Am J Anat. 1928;41:475–496. [Google Scholar]
- 20.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–H576. doi: 10.1152/ajpheart.00133.2005. [DOI] [PubMed] [Google Scholar]
- 21.McDonald DM, Choyke PL. Imaging of angiogenesis: from microscope to clinic. Nat Med. 2003;9:713–725. doi: 10.1038/nm0603-713. [DOI] [PubMed] [Google Scholar]
- 22.Binswanger O, Schaxel J. Beitrage zur normalen und pathologischen Anatomie der Arterien des Gehirns. Eur Arch Psychiatry Clin Neurosci. 1917;58:141–187. [Google Scholar]
- 23.Biggart JH. Pathology of the Nervous System. Edinburgh: E and S Livingstone; 1936. [Google Scholar]
- 24.Kuwabara T, Carrolll JM, Cogan DG. Retinal vascular patterns. III. Age, hypertension, absolute glaucoma, injury. Arch Ophthalmol. 1961;65:708–716. doi: 10.1001/archopht.1961.01840020710019. [DOI] [PubMed] [Google Scholar]
- 25.Reinecke RD, Kuwabara T, Cogan DG, Weis DR. Retinal vascular patterns. V. Experimental ischemia of the cat eye. Arch Ophthalmol. 1962;67:470–475. doi: 10.1001/archopht.1962.00960020470015. [DOI] [PubMed] [Google Scholar]
- 26.Archambeau JO, Mao XW, McMillan PJ, Gouloumet VL, Oeinck SC, Grove R, Yonemoto LT, Slater JD, Slater JM. Dose response of rat retinal microvessels to proton dose schedules used clinically: a pilot study. Int J Radiat Oncol Biol Phys. 2000;48:1155–1166. doi: 10.1016/s0360-3016(00)00754-9. [DOI] [PubMed] [Google Scholar]
- 27.Archer DB, Amoaku WM, Gardiner TA. Radiation retinopathy-clinical, histopathological, ultrastructural and experimental correlations. Eye (Lond) 1991;5(Pt 2):239–251. doi: 10.1038/eye.1991.39. [DOI] [PubMed] [Google Scholar]
- 28.Prados M, Swank RL. Vascular and interstitial cell changes in thiamin-deficient animals. Arch Neurol Psychiatry. 1942;47:626–644. [Google Scholar]
- 29.Ramon y, Cajal S. Contribution à la connaissance de la nevroglie cérébrale et cérebelleuse dans la paralysie générale progressive. Trab Lab Invest Biol Univ Madrid. 1925;23:157–216. [Google Scholar]
- 30.Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, We-instein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 31.Archer DB. Doyne Lecture. Responses of retinal and choroidal vessels to ionising radiation. Eye (Lond) 1993;7(Pt 1):1–13. doi: 10.1038/eye.1993.3. [DOI] [PubMed] [Google Scholar]
- 32.Cammermeyer J. Cerebral intervascular strands of connective tissue as routes of transportation. Anat Rec. 1965;151:251–259. doi: 10.1002/ar.1091510306. [DOI] [PubMed] [Google Scholar]
- 33.Kohner EM, Henkind P. Correlation of fluorescein angiogram and retinal digest in diabetic retinopathy. Am J Ophthalmol. 1970;69:403–414. doi: 10.1016/0002-9394(70)92273-7. [DOI] [PubMed] [Google Scholar]
- 34.DeVenecia G, Davis M, Engerman R. Clinicopathologic correlations in diabetic retinopathy. I. Histology and fluorescein angiography of microaneurysms. Arch Ophthalmol. 1976;94:1766–1773. doi: 10.1001/archopht.1976.03910040540013. [DOI] [PubMed] [Google Scholar]
- 35.Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol. 1919;52:457–474. doi: 10.1113/jphysiol.1919.sp001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss HR. Measurement of cerebral capillary perfusion with a fluorescent label. Microvasc Res. 1988;36:172–180. doi: 10.1016/0026-2862(88)90017-9. [DOI] [PubMed] [Google Scholar]
- 37.Villringer A, Them A, Lindauer U, Einhaupl K, Dirnagl U. Capillary perfusion of the rat brain cortex. An in vivo confocal microscopy study. Circ Res. 1994;75:55–62. doi: 10.1161/01.res.75.1.55. [DOI] [PubMed] [Google Scholar]
- 38.Gobel U, Theilen H, Kuschinsky W. Congruence of total and perfused capillary network in rat brains. Circ Res. 1990;66:271–281. doi: 10.1161/01.res.66.2.271. [DOI] [PubMed] [Google Scholar]
- 39.Hudetz AG, Feher G, Kampine JP. Heterogeneous autoregulation of cerebrocortical capillary flow: evidence for functional thoroughfare channels? Microvasc Res. 1996;51:131–136. doi: 10.1006/mvre.1996.0015. [DOI] [PubMed] [Google Scholar]
- 40.Pawlik G, Rackl A, Bing RJ. Quantitative capillary topography and blood flow in the cerebral cortex of cats: an in vivo microscopic study. Brain Res. 1981;208:35–58. doi: 10.1016/0006-8993(81)90619-3. [DOI] [PubMed] [Google Scholar]
- 41.Vetterlein F, Demmerle B, Bardosi A, Gobel U, Schmidt G. Determination of capillary perfusion pattern in rat brain by timed plasma labeling. Am J Physiol. 1990;258(1 Pt 2):H80–H84. doi: 10.1152/ajpheart.1990.258.1.H80. [DOI] [PubMed] [Google Scholar]
- 42.Williams JL, Shea M, Jones SC. Evidence that heterogeneity of cerebral blood flow does not involve vascular recruitment. Am J Physiol. 1993;264(5 Pt 2):H1740–H1743. doi: 10.1152/ajpheart.1993.264.5.H1740. [DOI] [PubMed] [Google Scholar]
- 43.Zoccoli G, Lucchi ML, Andreoli E, Lenzi P, Franzini C. Density of perfused brain capillaries in the aged rat during the wake-sleep cycle. Exp Brain Res. 2000;130:73–77. doi: 10.1007/s002219900219. [DOI] [PubMed] [Google Scholar]
- 44.Zweifach BW. Biologic properties of vascular endothelium. Angiology. 1961;12:507–510. [Google Scholar]
- 45.Tilton RG, Kilo C, Williamson JR, Murch DW. Differences in pericyte contractile function in rat cardiac and skeletal muscle microvasculatures. Microvasc Res. 1979;18:336–352. doi: 10.1016/0026-2862(79)90042-6. [DOI] [PubMed] [Google Scholar]
- 46.Kelley C, D’Amore P, Hechtman HB, Shepro D. Mi-crovascular pericyte contractility in vitro: comparison with other cells of the vascular wall. J Cell Biol. 1987;104:483–490. doi: 10.1083/jcb.104.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das A, Frank RN, Weber ML, Kennedy A, Reidy CA, Mancini MA. ATP causes retinal pericytes to contract in vitro. Exp Eye Res. 1988;46:349–362. doi: 10.1016/s0014-4835(88)80025-3. [DOI] [PubMed] [Google Scholar]
- 48.Tilton RG. Capillary pericytes: perspectives and future trends. J Electron Microsc Tech. 1991;19:327–344. doi: 10.1002/jemt.1060190308. [DOI] [PubMed] [Google Scholar]
- 49.Engerman RL, Pfaffenbach D, Davis MD. Cell turnover of capillaries. Lab Invest. 1967;17:738–743. [PubMed] [Google Scholar]
- 50.Tannock IF, Hayashi S. The proliferation of capillary endothelial cells. Cancer Res. 1972;32:77–82. [PubMed] [Google Scholar]
- 51.Hirst DG, Denekamp J, Travis EL. The response of mesenteric blood vessels to irradiation. Radiat Res. 1979;77:259–275. [PubMed] [Google Scholar]
- 52.Stewart FA, Denekamp J, Hirst DG. Proliferation kinetics of the mouse bladder after irradiation. Cell Tissue Kinet. 1980;13:75–89. doi: 10.1111/j.1365-2184.1980.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 53.Hirst DG, Denekamp J, Hobson B. Proliferation studies of the endothelial and smooth muscle cells of the mouse mesentery after irradiation. Cell Tissue Kinet. 1980;13:91–104. doi: 10.1111/j.1365-2184.1980.tb00452.x. [DOI] [PubMed] [Google Scholar]
- 54.Hobson B, Denekamp J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br J Cancer. 1984;49:405–413. doi: 10.1038/bjc.1984.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharma NK, Gardiner TA, Archer DB. A morphologic and autoradiographic study of cell death and regeneration in the retinal microvasculature of normal and diabetic rats. Am J Ophthalmol. 1985;100:51–60. doi: 10.1016/s0002-9394(14)74982-7. [DOI] [PubMed] [Google Scholar]
- 56.Kuwabara T, Cogan DG. Studies of retinal vascular patterns. I. Normal architecture. Arch Ophthalmol. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- 57.Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- 58.Ashton N. Arteriolar involvement in diabetic retinopathy. Br J Ophthalmol. 1953;37:282–292. doi: 10.1136/bjo.37.5.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashton N, Pedler C. Studies on developing retinal vessels: IX. Reaction of endothelial cells to oxygen. Br J Ophthalmol. 1962;46:257–276. doi: 10.1136/bjo.46.5.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ashton N. Studies of the retinal capillaries in relation to diabetic and other retinopathies. Br J Ophthalmol. 1963;47:521–538. doi: 10.1136/bjo.47.9.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wolter JR. The human optic papilla; a demonstration of new anatomic and pathologic findings. Am J Ophthalmol. 1957;44(4, Part 2):48–65. [PubMed] [Google Scholar]
- 62.Wolter JR. Diabetic retinopathy. Am J Ophthalmol. 1961;51:1123–1141. doi: 10.1016/0002-9394(61)91802-5. [DOI] [PubMed] [Google Scholar]
- 63.Wolter JR. The nature of capillary microaneurysms in diabetic retinopathy. Diabetes. 1962;11:126–131. [PubMed] [Google Scholar]
- 64.Cogan DG, Toussaint D, Kuwabara T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- 65.Kuwabara T, Cogan DG. Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch Ophthalmol. 1963;69:492–502. doi: 10.1001/archopht.1963.00960040498013. [DOI] [PubMed] [Google Scholar]
- 66.Cogan DG. Development and senescence of the human retinal vasculature. Trans Ophthalmol Soc U K. 1963;83:465–489. [PubMed] [Google Scholar]
- 67.Latker CH, Kuwabara T. Regression of the tunica vasculosa lentis in the postnatal rat. Invest Ophthalmol Vis Sci. 1981;21:689–699. [PubMed] [Google Scholar]
- 68.Cogan DG, Kuwabara T. Comparison of retinal and cerebral vasculature in trypsin digest preparations. Br J Ophthalmol. 1984;68:10–12. doi: 10.1136/bjo.68.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kornzweig AL, Eliasoph I, Feldstein M. Retinal vasculature in the aged. Bull N Y Acad Med. 1964;40:116–129. [PMC free article] [PubMed] [Google Scholar]
- 70.Kornzweig AL, Eliasoph I, Feldstein M. The retinal vasculature in macular degeneration. Arch Ophthalmol. 1966;75:326–333. doi: 10.1001/archopht.1966.00970050328005. [DOI] [PubMed] [Google Scholar]
- 71.Kornzweig AL, Eliasoph I, Feldstein M. Selective atrophy of the radial peripapillary capillaries in chronic glaucoma. Arch Ophthalmol. 1968;80:696–702. doi: 10.1001/archopht.1968.00980050698002. [DOI] [PubMed] [Google Scholar]
- 72.Henkind P, De Oliveira LF. Development of retinal vessels in the rat. Invest Ophthalmol. 1967;6:520–530. [PubMed] [Google Scholar]
- 73.Glatt HJ, Henkind P. Aging changes in the retinal capillary bed of the rat. Microvasc Res. 1979;18:1–17. doi: 10.1016/0026-2862(79)90014-1. [DOI] [PubMed] [Google Scholar]
- 74.Ausprunk DH, Falterman K, Folkman J. The sequence of events in the regression of corneal capillaries. Lab Invest. 1978;38:284–294. [PubMed] [Google Scholar]
- 75.Wang JL, Toida K, Uehara Y. The tunica vasculosa lentis; an expedient system for studying vascular formation and regression. J Electron Microsc (Tokyo) 1990;39:46–49. [PubMed] [Google Scholar]
- 76.Schroder S, Palinski W, Schmid-Schonbein GW. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139:81–100. [PMC free article] [PubMed] [Google Scholar]
- 77.Lang R, Lustig M, Francois F, Sellinger M, Plesken H. Apoptosis during macrophage-dependent ocular tissue remodelling. Development. 1994;120:3395–3403. doi: 10.1242/dev.120.12.3395. [DOI] [PubMed] [Google Scholar]
- 78.Los LI, van Luyn MJ, Nieuwenhuis P. Vascular remnants in the rabbit vitreous body. I. Morphological characteristics and relationship to vitreous embryonic development. Exp Eye Res. 2000;71:143–151. doi: 10.1006/exer.2000.0864. [DOI] [PubMed] [Google Scholar]
- 79.Kern TS, Engerman RL. Vascular lesions in diabetes are distributed non-uniformly within the retina. Exp Eye Res. 1995;60:545–549. doi: 10.1016/s0014-4835(05)80069-7. [DOI] [PubMed] [Google Scholar]
- 80.Mizutani M, Kern TS, Lorenzi M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest. 1996;97:2883–2890. doi: 10.1172/JCI118746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Pathol. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, Kern TS, Adamis AP. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 83.Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Ball S, Berkowitz BA, Kern TS. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007;50:1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]
- 84.Zheng L, Gong B, Hatala DA, Kern TS. Retinal ischemia and reperfusion causes capillary degeneration: similarities to diabetes. Invest Ophthalmol Vis Sci. 2007;48:361–367. doi: 10.1167/iovs.06-0510. [DOI] [PubMed] [Google Scholar]
- 85.Zhang JZ, Xi X, Gao L, Kern TS. Captopril inhibits capillary degeneration in the early stages of diabetic retinopathy. Curr Eye Res. 2007;32:883–889. doi: 10.1080/02713680701584123. [DOI] [PubMed] [Google Scholar]
- 86.Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, Kern TS, Grant MB. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–967. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Otani A, Kinder K, Ewalt K, Otero FJ, Schimmel P, Friedlander M. Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis. Nat Med. 2002;8:1004–1010. doi: 10.1038/nm744. [DOI] [PubMed] [Google Scholar]
- 88.Dorrell MI, Otani A, Aguilar E, Moreno SK, Friedlander M. Adult bone marrow-derived stem cells use R-cadherin to target sites of neovascularization in the developing retina. Blood. 2004;103:3420–3427. doi: 10.1182/blood-2003-09-3012. [DOI] [PubMed] [Google Scholar]
- 89.Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pessacq TP, Reissenweber NJ. Structural aspects of vasculogenesis in the central nervous system. 3. Morphology of glial cells in the course of blood vessel formation. Acta Anat (Basel) 1972;81:556–569. doi: 10.1159/000143787. [DOI] [PubMed] [Google Scholar]
- 91.Bar T, Budi Santoso AW. Identification of pericytes in the central nervous system by silver staining of the basal lamina. Cell Tissue Res. 1984;236:491–493. doi: 10.1007/BF00214255. [DOI] [PubMed] [Google Scholar]
- 92.Guseo A, Gallyas F. Intercapillary bridges and the development of brain capillaries. In: Cervâos-Navarro J, editor. Pathology of cerebral microcirculation. Berlin: de Gruyter; 1974. pp. 448–453. [Google Scholar]
- 93.Leibnitz L, Bar B. A blood capillaries-bridging cell type in adult mammalian brains. J Hirnforsch. 1988;29:367–375. [PubMed] [Google Scholar]
- 94.Mott RT, Thore CR, Moody DM, Glazier SS, Ellis TL, Brown WR. Reduced ratio of afferent to total vascular density in mesial temporal sclerosis. J Neuropathol Exp Neurol. 2009;68:1147–1154. doi: 10.1097/NEN.0b013e3181b9d75f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only inthe lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–66. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moody DM, Thore CR, Anstrom JA, Challa VR, Lange-feld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology. 2004;233:883–890. doi: 10.1148/radiol.2333020981. [DOI] [PubMed] [Google Scholar]
- 97.Lyman RS, Kupalov PS, Scholz W. Effect of roentgen rays on the central nervous system: Results of large doses on the brains of adult dogs. Arch Neurol Psychiatry. 1933;29:56–87. [Google Scholar]
- 98.Phillips TL. An ultrastructural study of the development of radiation injury in the lung. Radiology. 1966;87:49–54. doi: 10.1148/87.1.49. [DOI] [PubMed] [Google Scholar]
- 99.Irvine AR, Alvarado JA, Wara WM, Morris BW, Wood IS. Radiation retinopathy: an experimental model for the ischemic - proliferative retinopathies. Trans Am Ophthalmol Soc. 1981;79:103–122. [PMC free article] [PubMed] [Google Scholar]
- 100.Irvine AR, Wood IS. Radiation retinopathy as an experimental model for ischemic proliferative retinopathy and rubeosis iridis. Am J Ophthalmol. 1987;103:790–797. doi: 10.1016/s0002-9394(14)74395-8. [DOI] [PubMed] [Google Scholar]
- 101.Mao XW, Archambeau JO, Kubinova L, Boyle S, Petersen G, Grove R. Quantification of rat retinal growth and vascular population changes after single and split doses of proton irradiation: translational study using stereology methods. Radiat Res. 2003;160:5–13. doi: 10.1667/rr3007. [DOI] [PubMed] [Google Scholar]
- 102.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–668. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 103.Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, Wheeler KT. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci. 2007;257:67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 104.Brown WR, Moody DM, Thore CR, Anstrom JA, Challa VR. Microvascular changes in the white mater in dementia. J Neurol Sci. 2009;283:28–31. doi: 10.1016/j.jns.2009.02.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gabbiani G, Baic D, Deziel C. Toxicity of cadmium for the central nervous system. Exp Neurol. 1967;18:154–160. doi: 10.1016/0014-4886(67)90037-4. [DOI] [PubMed] [Google Scholar]
- 106.Tang TT, Cyrus AE, McCreadie SR. Some hitherto undescribed morphologic changes in lead encephalopathy. Lab Invest. 1968;18:336. [Google Scholar]
- 107.Rosenblum WI, Johnson MG. Neuropathologic changes produced in suckling mice by adding lead to the maternal diet. Arch Pathol. 1968;85:640–648. [PubMed] [Google Scholar]
- 108.Thomas JA, Dallenbach FD, Thomas M. Considerations on the development of experimental lead encephalopathy. Virchows Arch A Pathol Pathol Anat. 1971;352:61–74. doi: 10.1007/BF00549764. [DOI] [PubMed] [Google Scholar]
- 109.Clasen RA, Hartmann JF, Starr AJ, Coogan PS, Pandolfi S, Laing I, Becker R, Hass GM. Electron microscopic and chemical studies of the vascular changes and edema of lead encephalopathy. A comparative study of the human and experimental disease. Am J Pathol. 1974;74:215–240. [PMC free article] [PubMed] [Google Scholar]
- 110.Vistica DT, Ahrens FA. Microvascular effects of lead in the neonatal rat. II. An ultrastructural study. Exp Mol Pathol. 1977;26:139–154. doi: 10.1016/0014-4800(77)90073-9. [DOI] [PubMed] [Google Scholar]
- 111.Zook BC, London WT, Wilpizeski CR, Sever JL. Experimental lead paint poisoning in nonhuman primates. III. Pathologic findings. J Med Primatol. 1980;9:343–360. doi: 10.1159/000460164. [DOI] [PubMed] [Google Scholar]
- 112.Press MF. Lead-induced permeability changes in immature vessels of the developing cerebellar microcirculation. Acta Neuropathol. 1985;67:86–95. doi: 10.1007/BF00688128. [DOI] [PubMed] [Google Scholar]
- 113.Vracko R. Basal lamina scaffold-anatomy and significance for maintenance of orderly tissue structure. Am J Pathol. 1974;77:314–346. [PMC free article] [PubMed] [Google Scholar]
- 114.Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983;288:61–75. doi: 10.1016/0006-8993(83)90081-1. [DOI] [PubMed] [Google Scholar]
- 115.Giannini C, Dyck PJ. The fate of Schwann cell basement membranes in permanently transected nerves. J Neuropathol Exp Neurol. 1990;49:550–563. doi: 10.1097/00005072-199011000-00002. [DOI] [PubMed] [Google Scholar]
- 116.Ochi M, Wakasa M, Ikuta Y, Kwong WH. Nerve regeneration in predegenerated basal lamina graft: the effect of duration of predegeneration on axonal extension. Exp Neurol. 1994;128:216–225. doi: 10.1006/exnr.1994.1130. [DOI] [PubMed] [Google Scholar]
- 117.Gulati AK. Peripheral nerve regeneration through short- and long-term degenerated nerve transplants. Brain Res. 1996;742:265–270. doi: 10.1016/s0006-8993(96)01012-8. [DOI] [PubMed] [Google Scholar]
- 118.Nguyen QT, Sanes JR, Lichtman JW. Pre-existing pathways promote precise projection patterns. Nat Neurosci. 2002;5:861–867. doi: 10.1038/nn905. [DOI] [PubMed] [Google Scholar]
- 119.Vracko R, Benditt EP. Basal lamina: the scaffold for orderly cell replacement. Observations on regeneration of injured skeletal muscle fibers and capillaries. J Cell Biol. 1972;55:406–419. doi: 10.1083/jcb.55.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hamilton AM, Marshall J, Kohner EM, Bowbyes JA. Retinal new vessel formation following experimental vein occlusion. Exp Eye Res. 1975;20:493–497. doi: 10.1016/0014-4835(75)90216-x. [DOI] [PubMed] [Google Scholar]
- 121.Engerman RL. Pathogenesis of diabetic retinopathy. Diabetes. 1989;38:1203–1206. doi: 10.2337/diab.38.10.1203. [DOI] [PubMed] [Google Scholar]
- 122.Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, Yao VJ, Inai T, Brooks P, Freimark B, Shalinsky DR, Hu-Lowe DD, McDonald DM. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Benjamin LE, Hemo I, Keshet E. A plasticity window for blood vessel remodelling is defined by pericyte coverage of the preformed endothelial network and is regulated by PDGF-B and VEGF. Development. 1998;125:1591–1598. doi: 10.1242/dev.125.9.1591. [DOI] [PubMed] [Google Scholar]
- 124.Baluk P, Lee CG, Link H, Ator E, Haskell A, Elias JA, McDonald DM. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071–1085. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 126.Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 127.Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 129.Ingber DE, Madri JA, Folkman J. A possible mechanism for inhibition of angiogenesis by angiostatic steroids: induction of capillary basement membrane dissolution. Endocrinology. 1986;119:1768–1775. doi: 10.1210/endo-119-4-1768. [DOI] [PubMed] [Google Scholar]
- 130.Denekamp J. Angiogenesis, neovascular proliferation and vascular pathophysiology as targets for cancer therapy. Br J Radiol. 1993;66:181–196. doi: 10.1259/0007-1285-66-783-181. [DOI] [PubMed] [Google Scholar]
- 131.Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 133.Modlich U, Kaup FJ, Augustin HG. Cyclic angiogen-esis and blood vessel regression in the ovary: blood vessel regression during luteolysis involves endothelial cell detachment and vessel occlusion. Lab Invest. 1996;74:771–780. [PubMed] [Google Scholar]
- 134.Bartel H, Lametschwandtner A. Regression of blood vessels in the ventral velum of Xenopus laevis Daudin during metamorphosis: light microscopic and transmission electron microscopic study. J Anat. 2000;197(Pt 2):157–166. doi: 10.1046/j.1469-7580.2000.19720157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ishida S, Yamashiro K, Usui T, Kaji Y, Ogura Y, Hida T, Honda Y, Oguchi Y, Adamis AP. Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med. 2003;9:781–788. doi: 10.1038/nm877. [DOI] [PubMed] [Google Scholar]
- 136.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 137.Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan-Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15(7 Pt 1):4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 139.Arden GB. Comments on “A new view of diabetic Retinopathy” by A.J. Barber. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:747–748. doi: 10.1016/j.pnpbp.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 140.Adams RH, Alitalo K. Molecular regulation of angio-genesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 141.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 142.Dore-Duffy P, LaManna JC. Physiologic angiodynam-ics in the brain. Antioxid Redox Signal. 2007;9:1363–1371. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- 143.LaManna JC, Chavez JC, Pichiule P. Structural and functional adaptation to hypoxia in the rat brain. J Exp Biol. 2004;207(Pt 18):3163–3169. doi: 10.1242/jeb.00976. [DOI] [PubMed] [Google Scholar]
- 144.Meeson A, Palmer M, Calfon M, Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122:3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- 145.Meeson AP, Argilla M, Ko K, Witte L, Lang RA. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development. 1999;126:1407–1415. doi: 10.1242/dev.126.7.1407. [DOI] [PubMed] [Google Scholar]
- 146.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 147.Albuquerque ML, Waters CM, Savla U, Schnaper HW, Flozak AS. Shear stress enhances human endothelial cell wound closure in vitro. Am J Physiol Heart Circ Physiol. 2000;279:H293–H302. doi: 10.1152/ajpheart.2000.279.1.H293. [DOI] [PubMed] [Google Scholar]
- 148.Resnick N, Einav S, Chen-Konak L, Zilberman M, Yahav H, Shay-Salit A. Hemodynamic forces as a stimulus for arteriogenesis. Endothelium. 2003;10:197–206. doi: 10.1080/10623320390246289. [DOI] [PubMed] [Google Scholar]
- 149.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 150.Goldfinger LE, Tzima E, Stockton R, Kiosses WB, Kinbara K, Tkachenko E, Gutierrez E, Groisman A, Nguyen P, Chien S, Ginsberg MH. Localized alpha4 integrin phosphorylation directs shear stress-induced endothelial cell alignment. Circ Res. 2008;103:177–185. doi: 10.1161/CIRCRESAHA.108.176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen Z, Tzima E. PECAM-1 is necessary for flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009;29:1067–1073. doi: 10.1161/ATVBAHA.109.186692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracia-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, García-Cardeña G, Daley GQ. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459:1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P, Dzierzak E, Zon LI. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Frenkel-Denkberg G, Gershon D, Levy AP. The function of hypoxia-inducible factor 1 (HIF-1) is impaired in senescent mice. FEBS Lett. 1999;462:341–344. doi: 10.1016/s0014-5793(99)01552-5. [DOI] [PubMed] [Google Scholar]
- 155.Chavez JC, LaManna JC. Hypoxia-inducible factor-1alpha accumulation in the rat brain in response to hypoxia and ischemia is attenuated during aging. Adv Exp Med Biol. 2003;510:337–341. doi: 10.1007/978-1-4615-0205-0_55. [DOI] [PubMed] [Google Scholar]
- 156.Rivard A, Berthou-Soulie L, Principe N, Kearney M, Curry C, Branellec D, Semenza GL, Isner JM. Age-dependent defect in vascular endothelial growth factor expression is associated with reduced hypoxia-inducible factor 1 activity. J Biol Chem. 2000;275:29643–29647. doi: 10.1074/jbc.M001029200. [DOI] [PubMed] [Google Scholar]
- 157.Black JE, Polinsky M, Greenough WT. Progressive failure of cerebral angiogenesis supporting neural plasticity in aging rats. Neurobiol Aging. 1989;10:353–358. doi: 10.1016/0197-4580(89)90048-1. [DOI] [PubMed] [Google Scholar]
- 158.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- 159.Rapino C, Bianchi G, Di Giulio C, Centurione L, Cacchio M, Antonucci A, Cataldi A. HIF-1alpha cytoplasmic accumulation is associated with cell death in old rat cerebral cortex exposed to intermittent hypoxia. Aging Cell. 2005;4:177–185. doi: 10.1111/j.1474-9726.2005.00161.x. [DOI] [PubMed] [Google Scholar]